Abstract
Diabetic kidney disease (DKD) is the leading cause of ESRD. We conducted an open-label, prospective, randomized trial to determine whether pentoxifylline (PTF), which reduces albuminuria, in addition to renin-angiotensin system (RAS) blockade, can slow progression of renal disease in patients with type 2 diabetes and stages 3–4 CKD. Participants were assigned to receive PTF (1200 mg/d) (n=82) or to a control group (n=87) for 2 years. All patients received similar doses of RAS inhibitors. At study end, eGFR had decreased by a mean±SEM of 2.1±0.4 ml/min per 1.73 m2 in the PTF group compared with 6.5±0.4 ml/min per 1.73 m2 in the control group, with a between-group difference of 4.3 ml/min per 1.73 m2 (95% confidence interval [95% CI], 3.1 to 5.5 ml/min per 1.73 m2; P<0.001) in favor of PTF. The proportion of patients with a rate of eGFR decline greater than the median rate of decline (0.16 ml/min per 1.73 m2 per month) was lower in the PTF group than in the control group (33.3% versus 68.2%; P<0.001). Percentage change in urinary albumin excretion was 5.7% (95% CI, −0.3% to 11.1%) in the control group and −14.9% (95% CI, −20.4% to −9.4%) in the PTF group (P=0.001). Urine TNF-α decreased from a median 16 ng/g (interquartile range, 11–20.1 ng/g) to 14.3 ng/g (interquartile range, 9.2–18.4 ng/g) in the PTF group (P<0.01), with no changes in the control group. In this population, addition of PTF to RAS inhibitors resulted in a smaller decrease in eGFR and a greater reduction of residual albuminuria.
Keywords: albuminuria, chronic kidney disease, diabetic nephropathy, progression of chronic renal failure, inflammation
Diabetic kidney disease (DKD) is the most frequent cause of CKD, and ESRD in type 2 diabetes is considered a medical catastrophe.1 Strong evidence supports the blockade of the renin-angiotensin system (RAS), most commonly with angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin-receptors blockers (ARBs), as an established standard of care in these patients to reduce the risk of developing ESRD.2 However, these therapies do not provide complete renal protection,3 and diabetic patients continue to show a high renal risk, which is positively associated with residual albuminuria.4
Unfortunately, recent studies evaluating new strategies to delay the progression of diabetic nephropathy (DN) have had little success. Trials with pyridoxamine or sulodexide failed to detect a renoprotective effect in patients with type 2 diabetes and renal impairment.5,6 Meanwhile, studies with the endothelin antagonist avosentan,7 more recently the Bardoxolone Methyl Evaluation in Patients with Chronic Kidney Disease and Type 2 Diabetes: The Occurrence of Renal Events trial,8 and the Veterans Affairs Nephropathy in Diabetes trial,9 were prematurely stopped because of serious safety concerns. Therefore, there is a pressing need for innovative approaches and novel therapies to treat nephropathy in type 2 diabetes.
Inflammation is recognized as a cardinal factor in the pathogenesis and progression of DN, which has been considered an inflammatory disease.10 Thus, inflammatory molecules and pathways are new potential targets for the treatment of this complication.11,12
Pentoxifylline (PTF) is a methylxanthine derivate and nonspecific phosphodiesterase inhibitor clinically used to treat patients with occlusive peripheral vascular disorders for more than 30 years. In addition to its rheologic properties, PTF has anti-inflammatory, antiproliferative, and antifibrotic actions13–17 that have been associated with beneficial effects in experimental models of renal disease progression.18,19 We previously reported that PTF administration to diabetic patients resulted in the reduction of clinical markers of glomerular and tubulointerstitial injury.20,21 In addition, recent meta-analyses indicate that PTF may reduce proteinuria and that this drug could offer some beneficial effects on renal function in patients with DKD.22,23 Therefore, we conducted the present randomized trial to prospectively test the hypothesis that add-on PTF to maximized ACEI or ARB treatment provides additional benefits against renal disease progression in patients with type 2 diabetes who have stages 3–4 CKD.
Results
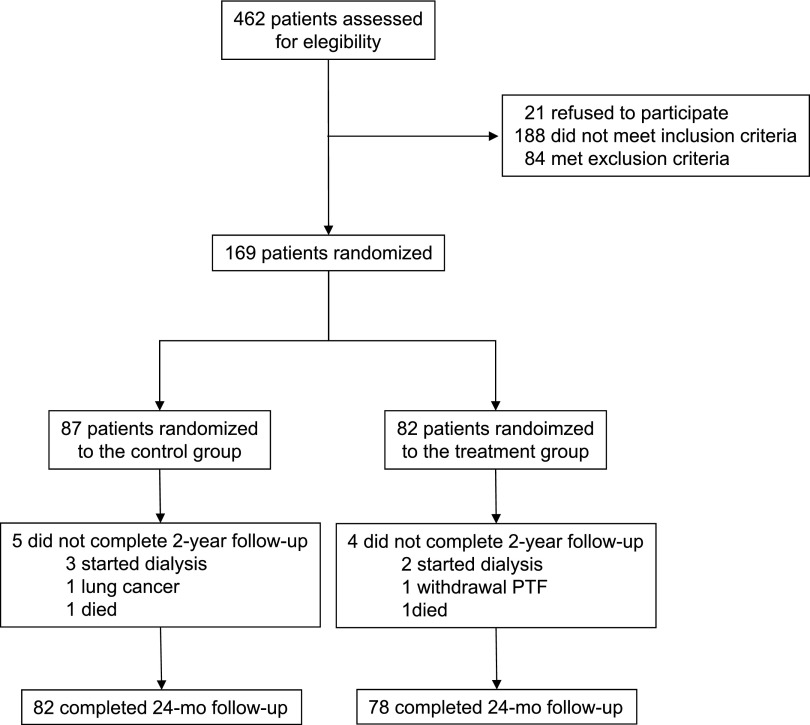
Figure 1 depicts the flow of participants in the trial. Of 462 patients initially screened, 169 were randomly assigned to a control or a treatment group. All patients were white, with a mean age of 69.8±9.2 years, a similar distribution by sex (53.8% men and 46.2% women), and a mean duration of diabetes of 15±3.4 years. The mean baseline eGFR was 37.4±12.1 ml/min per 1.73 m2, with 116 patients (68.6%) having stage 3 CKD (eGFR, 30–59 ml/min per 1.73 m2) and 53 (31.3%), stage 4 CKD (eGFR, 15–29 ml/min per 1.73 m2). The median urinary albumin excretion (UAE) was 1100 (interquartile range [IQR], 640–1800) mg/d; 154 patients (91.1%) had macroalbuminuria (UAE>300 mg/d) and 92 (54.4%) had a UAE>1000 mg/d. All patients were hypertensive, most had hyperlipidemia, and >40% had a medical history of coronary heart disease. Regarding medication use, all patients were under RAS blockade, and most received diuretics, statins, and aspirin. The mean doses of ACEIs and ARBs were similar in both groups: 16.8±4.7 mg/d and 102.7±83 mg/d in the control group versus 15.9±4.9 mg/d (P=0.41) and 106.2±82.9 mg/d (P=0.84) in the PTF group, respectively. Baseline demographic, clinical, and biochemical characteristics and concomitant treatments were balanced between the study groups (Table 1).
Figure 1.
Flow diagram of participants in the study.
Table 1.
Baseline characteristics of participants in the trial
| Characteristic | Control Group (n=87) | Pentoxifylline Group (n=82) |
|---|---|---|
| Demographic characteristics | ||
| Age (yr) | 69.5±9.5 | 70.2±8.9 |
| Men (%) | 46 (52.8) | 45 (54.8) |
| Clinical characteristics | ||
| Known duration of diabetes (yr) | 14.8±3.5 | 15.3±3.2 |
| CKD stage 3, n (%) | 63 (72.4) | 53 (64.6) |
| CKD stage 4, n (%) | 24 (27.5) | 29 (35.3) |
| Body mass index (kg/m2) | 28.9±2.9 | 29.4±3 |
| Systolic BP (mmHg) | 141.8±8.4 | 142.2±9.4 |
| Diastolic BP (mmHg) | 86.4±7.7 | 86.5±8.5 |
| Biochemical variables | ||
| Hemoglobin A1c (%) | 7.2±0.7 | 7.3±0.7 |
| Cholesterol (mmol/L) | ||
| Total | 4.5±0.8 | 4.3±1.0 |
| LDL | 2.4±0.6 | 2.3±0.6 |
| HDL | 1.1±0.3 | 1.0±0.2 |
| Triglycerides (mmol/L) | 1.8±0.9 | 1.8±0.7 |
| Serum albumin (g/dl) | 4.04±0.30 | 4.01±0.25 |
| eGFR (ml/min per 1.73 m2) | 37.6±11.9 | 37.1±12.4 |
| UAE (mg/d) | 1000 (600–1800) | 1100 (689–2190) |
| UAE>1 g/d, n (%) | 43 (49.4) | 49 (59.7) |
| Urinary TNF-α (ng/g) | 16 (9.1–22) | 16 (11–20.1) |
| Medical history, n (%) | ||
| Hypertension | 87 (100) | 82 (100) |
| Hyperlipidemia | 84 (96) | 78 (95) |
| Coronary heart disease | 41 (47) | 35 (42) |
| Congestive heart disease | 18 (20) | 15 (18) |
| Stroke | 3 (3) | 2 (2) |
| Peripheral vascular disease | 23 (26) | 21 (25) |
| Concomitant medication use, n (%) | ||
| Insulin | 43 (47) | 40 (48) |
| ACEIs | 40 (46) | 32 (39) |
| ARBs | 47 (54) | 50 (61) |
| Diuretic | 70 (80) | 67 (82) |
| Calcium-channel blockers | 52 (59) | 45 (54) |
| β-Blocker | 40 (45) | 38 (46) |
| α-Blocker | 15 (17) | 17 (21) |
| Central-acting agents | 9 (10) | 4 (8) |
| Statins | 80 (92) | 74 (90) |
| Aspirin | 78 (89) | 71 (86) |
Data are n (%) or mean±SD except for UAE and urinary TNF-α excretion, which are expressed as median (IQR).
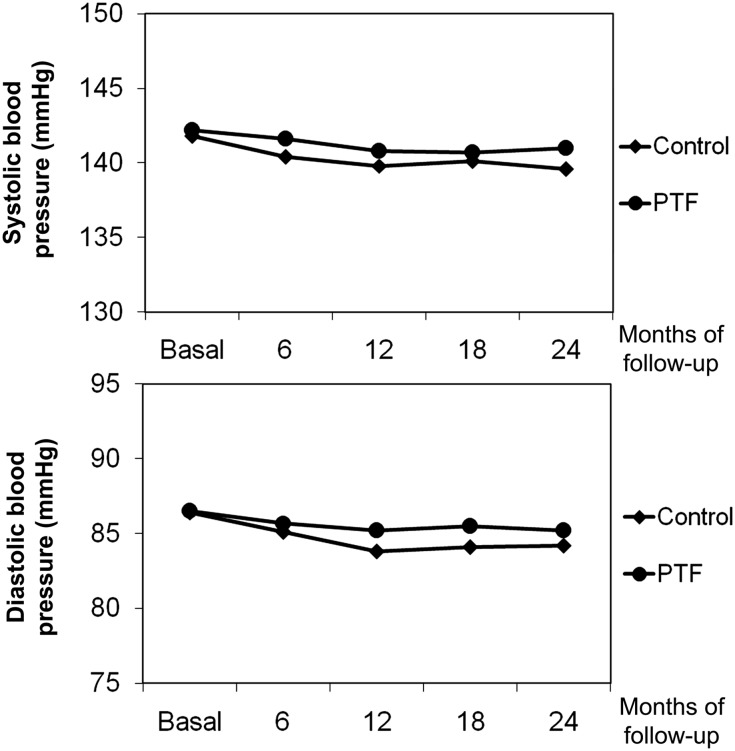
The groups did not differ for BP control, average hemoglobin A1c levels, or concomitant therapies at the various times of follow-up. BP did not significantly vary in either group during the study. BP at baseline averaged 141.8/86.4 mmHg in the control group and 142.2/86.5 mmHg in the PTF group; pulse pressure was 55.4 mmHg and 55.7 mmHg, respectively (P=0.78). At 1 year, the values averaged 139.8/83.8 mmHg in the control group and 140.8/85.2 mmHg in the PTF group; average pulse pressure was 55.9 mmHg and 55.6 mmHg, respectively (P=0.74). At the end of the study, the BP values were 139.6/84.2 mmHg and 141/85.2 mmHg, respectively, and pulse pressure was 55.4 mmHg and 55.7 mmHg, respectively (P=0.78) (Figure 2). Likewise, glycemic control did not differ between groups during follow-up. At baseline, average hemoglobin A1c level was 7.26%±0.71% in the control group and 7.34%±0.74% in the PTF group (P=0.48). The values were 7.18%±0.67% and 7.39%±1.04% (P=0.12), respectively, at 1 year and 7.25±0.69% and 7.40%±0.68% (P=0.16) at study end.
Figure 2.
Evolution of average systolic and diastolic BP at randomization (basal) and during follow-up in the control and PTF groups without any significant difference between groups.
The mean duration of follow-up for the overall participants was 23.6±1.7 months, with no differences between groups. Seventy-seven (95%) participants assigned to the PTF group completed the 2 years of follow-up, and 80 (98.7%) received the study drug for >18 months.
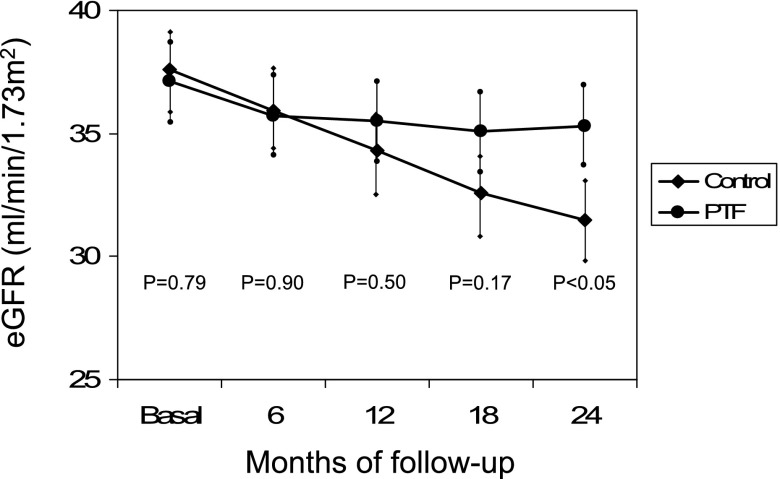
The evolution of the eGFR in both groups is presented in Figure 3. Table 2 compares the change in eGFR between both groups at each time point during the study. There was a significant overall between-group difference for changes in the primary endpoint (P<0.001) from baseline to the end of the study. The eGFR decreased by a mean±SEM of 2.1±0.4 ml/min per 1.73 m2 in patients treated with PTF versus 6.5±0.4 ml/min per 1.73 m2 in the control group, which resulted in a significant mean difference of 4.3 ml/min per 1.73 m2 (95% confidence interval [95% CI], 3.1 to 5.5 ml/min per 1.73 m2) (P<0.001), in favor of PTF. The monthly rate of decline in eGFR was 0.27±0.18 ml/min per 1.73 m2 per month in the control group versus 0.08±0.14 ml/min per 1.73 m2 per month in the PTF group (P<0.001). Figure 4 shows the mean percentage change in eGFR from baseline to the end of the study. Analysis of all measurements collected across visits showed that the difference between groups regarding the loss of eGFR reached statistical significance from the first year and was sustained thereafter (Table 2).
Figure 3.
Evolution of the mean eGFR at randomization (basal) and during follow-up in the control and PTF groups. Difference of the mean eGFR between groups show statistical significance after 24 months of follow-up. Vertical bars represent the SD. P values are for the comparison of the PTF group versus the control group.
Table 2.
Changes from baseline in eGFR and albuminuria at follow-up visits by study group
| Variable | Control Group | PTF Group | P Value between Groups |
|---|---|---|---|
| eGFR (ml/min/1.73 m2) | |||
| Mean baseline±SD | 37.6±11.9 | 37.1±12.4 | |
| Least-square mean change±SEM (95% CI) per follow-up period | |||
| 6 mo | −1.7±0.1 (−2.1 to −1.4) | −1.4±0.1 (−1.7 to −1.0) | 0.1 |
| 12 mo | −3.4±0.3 (−4.1 to −2.8) | −1.2±0.3 (−1.9 to -0.6) | <0.001 |
| 18 mo | −5.3±0.3 (−6.1 to −4.5) | −1.7±0.4 (−2.5 to -0.9) | <0.0001 |
| 24 mo | −6.5±0.4 (−7.3 to −5.6) | −2.1±0.4 (−3.0 to -1.2) | <0.0001 |
| UAE | |||
| Median baseline (IQR) (mg/d) | 1000 (600–1800) | 1100 (689–2190) | |
| Least-square mean percentage change per follow-up period±SEM (95% CI) | |||
| 6 mo | 1.4±1.1 (−0.8 to 3.8) | −10.6±1.2 (−13.0 to -8.2) | <0.001 |
| 12 mo | 4.9±2.8 (−0.7 to 10.6) | −13.0±2.9 (−18.8 to −7.2) | <0.0001 |
| 18 mo | 4.9±2.6 (−0.3 to 10.1) | −14.8±2.7 (−20.1 to −9.4) | <0.0001 |
| 24 mo | 5.7±2.7 (−0.3 to 11.1) | −14.9±2.7 (−20.4 to −9.4) | <0.0001 |
| Patients per follow-up period (n) | |||
| 6 mo | 87 | 81 | |
| 12 mo | 85 | 81 | |
| 18 mo | 84 | 79 | |
| 24 mo | 82 | 78 |
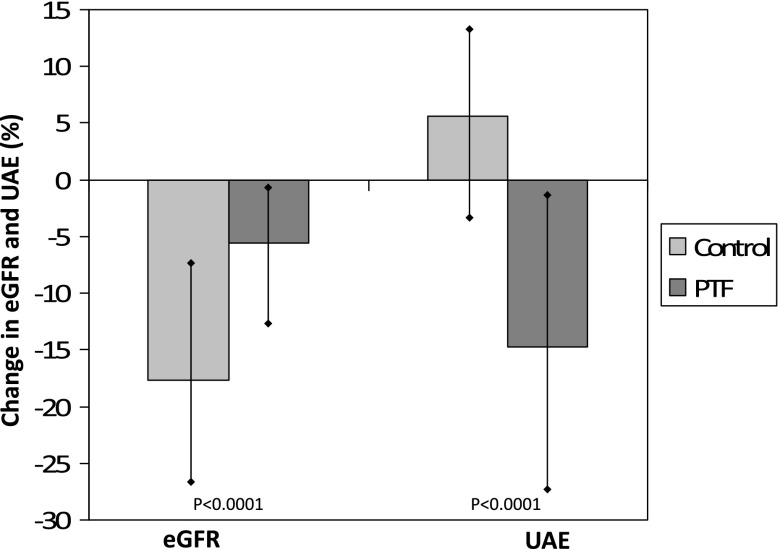
Figure 4.
Change in eGFR and UAE from baseline to the end of the study. Patients in the control group presented a significantly higher reduction of UAE and a lower decrease in the eGFR. Vertical bars represent 95% CIs. P values are for the comparison of the PTF group versus the control group.
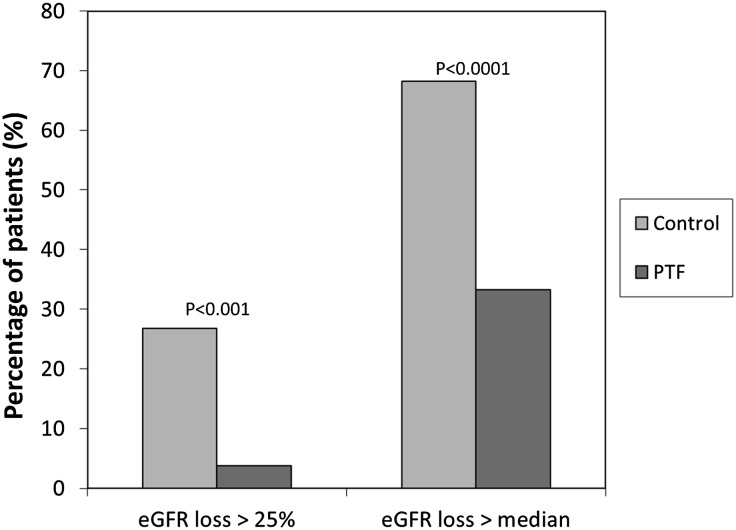
The proportion of patients with a reduction of eGFR>25% with respect to baseline was lower in the PTF group (3.8% [3 of 78]) than in the control group (26.8% [22 of 82]) (P<0.001). In the overall group, the median rate of decline in eGFR per month was 0.16 ml/min per 1.73 m2. Patients were analyzed according to this threshold, and thus, the percentage of patients with a reduction in eGFR greater than this value was significantly lower among patients treated with PTF (33.3% [26 of 78]) compared with this percentage in the control group (68.2% [56 of 82]) (P<0.001) (Figure 5).
Figure 5.
Percentage of patients with an eGFR reduction greater than the median decline observed in the overall group and >25% with regard to baseline according to study group. In both cases, the percentage of subjects was significantly lower in the PTF group. P values are for the comparison between groups.
After 24 months of follow-up, the median UAE increased from 1000 (IQR, 600–1800) mg/d to 1117 (IQR, 584–1762) mg/d (P=0.02) in the control group compared with a reduction from 1100 (IQR, 689–2190) mg/d to 973 (IQR, 574–1780) mg/d (P<0.001) in the PTF group. Therefore, the UAE experienced a mean percentage increase of 5.7% (95% CI, −0.3% to 11.1%) in the control group versus a mean percentage reduction of −14.9% (95% CI, −20.4% to −9.4%) in patients treated with PTF (Figure 4). This resulted in a mean difference at the end of the study of 20.6% (95% CI, 28.3% to 12.9%) between groups in favor of PTF (P<0.001). The differences between groups regarding the change in UAE were statistically significant from the sixth month (Table 2). At baseline, the percentage of patients within the control group with a UAE>1 g/d was 49.4%, with a similar proportion at the end of the study. On the contrary, in the PTF group, the number of patients with a UAE>1 g/d decreased from 49 (59.7%) to 34 (43.5%). Correlation analysis showed that in both the control and PTF groups there was a significant inverse relationship between the change in the eGFR and the variation in UAE (r=−0.74 and r=−0.81, respectively; P<0.01).
Median urinary TNF-α concentration at baseline was 16 (IQR, 10–20.1) ng/g in the overall group. This variable was positively related to the magnitude of UAE (r=0.38; P<0.01). At the end of the study, urine TNF-α decreased from 16 (IQR, 11–20.1) to 14.3 (IQR, 9.2–18.4) ng/g in patients treated with PTF (P<0.01), with no significant changes in the control group. The mean percentage variation after 2 years was 5.1% (95% CI, 1.4% to 8.8%; P=0.07) in the control group and −11.5% (95% CI, −16.4% to −6.6%; P<0.01) in the PTF group. There was no significant correlation between changes in urine TNF-α with variations of eGFR or UAE in the control group. On the contrary, in patients treated with PTF, the reduction in urinary TNF-α concentration was directly correlated with the change in UAE (r=0.62; P<0.01) and inversely correlated with the variation in eGFR (r=−0.66; P<0.01).
Five patients initiated dialysis during the study (three in the control group and two in the PTF group). One patient in each group died. Cardiovascular and cerebrovascular events and the number of hospitalizations did not differ between groups (Table 3). Nine episodes of AKI were recorded during the study: four in the control group and five in the PTF group. All were related to intercurrent events (four, heart failure; two, gastroenteritis; one, myocardial infarction; one, revascularization process; one, pneumonia). The most frequent adverse effects in patients treated with PTF were gastrointestinal symptoms (abdominal discomfort, flatus, dyspepsia, nausea, and vomiting), which were significantly more frequent than in the control group (21.9% versus 10.3%; P=0.03). In most cases these symptoms were mild and disappeared during the first month without further medical assistance. In one case PTF was withdrawn.
Table 3.
Summary of adverse events in study participants
| Adverse Events | Control Group (n=87) | PTF Group (n=82) | P Value |
|---|---|---|---|
| Hospitalization episodes | 32 (36.7) | 24 (29.2) | 0.29 |
| Cardiovascular events | |||
| Myocardial infarction | 2 (2.3) | 1 (1.2) | 0.59 |
| Stroke | 2 (2.3) | 1 (1.2) | 0.59 |
| Heart failure | 4 (4.5) | 3 (3.6) | 0.75 |
| Revascularization | 5 (5.7) | 2 (2.4) | 0.28 |
| Syncope | 1 (1.1) | 0 | |
| Noncardiovascular events | |||
| ESRD | 3 (5.7) | 2 (2.4) | 0.60 |
| AKI | 4 (4.5) | 5 (6.0) | 0.31 |
| Digestive symptoms | 9 (10.3) | 18 (21.9) | 0.03 |
| Hemorrhoid bleed | 2 (2.3) | 1 (1.2) | 0.59 |
| Pneumonia | 1 (1.1) | 2 (2.4) | 0.52 |
| Eye disorders | 3 (5.7) | 3 (3.6) | 0.94 |
| Malignant neoplasms | 1 (1.1) | 0 | |
Unless otherwise noted, values are expressed as the number (percentage) of patients. Revascularization indicates peripheral and cardiac angioplasty, and cardiac bypass procedures.
Discussion
The present study shows that treatment with PTF for 24 months led to a slowing of the rate of progression of nephropathy among patients with type 2 diabetes who had stages 3–4 CKD and were receiving standard medical care and the maximum recommended dosage of ACEIs or ARBs. This beneficial effect was reflected by a significant mean difference of 4.3 ml/min per 1.73 m2 in the reduction of eGFR between the study groups in favor of PTF, as well as a halving of the percentage of patients with a decline in eGFR greater than the median value in the PTF group compared with the control group. The difference in the reduction of eGFR between the groups showed a trend at 6 months and reached statistical significance after 1 year, suggesting that a long period of PTF treatment is necessary to protect renal function. In addition, our results show that PTF provided an additive antiproteinuric effect in patients with type 2 diabetes and residual proteinuria, with a mean difference of 20.6% in favor of PTF respect to the control group. Unlike that observed for eGFR, the difference in the evolution of albuminuria between the groups was significant from the sixth month. We interpret these results as demonstrating that addition of PTF to maximum-dosage blockade of the RAS was renoprotective in patients with type 2 diabetes and advanced CKD.
Previous clinical studies that have evaluated the effect of PTF on renal function in such patients are scarce. Diskin et al.24 and Lin et al.25 reported a smaller decrease in renal function and a trend toward stabilization of eGFR in patients treated with PTF compared with the control groups. Perkins et al.,26 in a 1-year pilot, double-blind, randomized, placebo-controlled trial of 40 patients, found that the rate of eGFR decrease was significantly lower in the PTF group, with a difference in mean values of −5.1 ml/min per 1.73 m2 per year. Finally, in a recent randomized trial, Goicoechea et al.27 studied renal disease progression as a secondary objective and observed that mean eGFR significantly increased 2.5 ml/min per 1.73 m2 after 12 months in the PTF group, compared with a mean 5.2 ml/min per 1.73 m2 reduction in the control group. However, the conclusions of those studies were limited by several factors, including the analysis of kidney function as a secondary objective, the inadequate sample size, the heterogeneity of primary renal disease, and incomplete follow-up. By contrast, the present study was designed to evaluate renal disease progression as the primary outcome, with adequate power, sample size, and follow-up time, and the results provide new evidence on the beneficial effects of PTF on DKD progression.
The mechanisms of the renoprotective effect of PTF are unknown, although it is possible to speculate about some possibilities. The first one is reduction of residual proteinuria, a critical risk factor for progression of renal impairment.28 Most studies have shown a beneficial decrease of urinary protein excretion when PTF is added to RAS blockers, an effect that is higher in patients with overt proteinuria than in those with microalbuminuria.22 In our trial, baseline residual UAE was in the range of macroalbuminuria and decreased by 14.9% after 2 years of PTF administration, with a significant mean difference of 20.6% between groups in favor of PTF. In addition, other studies have reported that PTF treatment stopped the decrease in eGFR independently of its antiproteinuric properties, suggesting additional protective effects on kidney function.26 Another possibility is a putative effect on arachidonic acid metabolism. In an early study, Donadio et al.29 reported that the administration of a platelet-inhibitor regimen of dipyridamole and aspirin in patients with type 1 diabetes mellitus and established nephropathy may stabilize renal function by reducing the production of thromboxanes by platelets or renal tissue. Later studies showed that the administration of dipyridamole alone or combined with aspirin to patients with type 2 diabetes and DN resulted in 14.8% and 37.3% reductions in proteinuria, respectively.30 A study has shown that PTF does not affect the arachidonic acid–induced thromboxane formation by human platelets.31 However, an experimental study using an isolated perfused kidney model demonstrated that PTF had a protective renal effect during sepsis by modulating arachidonic acid metabolism within the kidney.32 There is a strong biologic rationale for inflammation, oxidative stress, and fibrosis, to be critical risk factors for renal disease progression in DKD.33–35 Experimental research has shown that PTF diminishes renal tissue damage through beneficial anti-inflammatory, antioxidant, and antifibrotic effects,17,36,37 resulting in attenuation of kidney disease progression. This outcome is maximized after combination with RAS blockers.18 This experimental evidence has been translated into the clinical level, where the favorable actions of PTF administration on UAE and renal function in diabetic patients have been related to improvements in markers of inflammation, fibrosis, and oxidative stress.21,25,27,38,39 In the present study, urinary TNF-α decreased by 11.5% after PTF administration, which was directly correlated with the change in UAE and inversely correlated with the variation in the eGFR. Previous studies with PTF have found similar results regarding urinary TNF-α,21,25 as well as other inflammatory molecules, such as monocyte chemoattractant protein-1 and IL-6.25,40 The present trial could not completely determine whether urinary TNF-α decrease was part of the reduction in proteinuria or was a special effect of PTF. However, in a previous study we found that UAE was directly and independently associated with urinary TNF-α excretion, with no correlation between serum and urinary TNF-α, suggesting an intrarenal production of this cytokine.41 Moreover, preceding studies found a significant reduction in urinary TNF-α levels in patients with DKD who received PTF, with a positive and significant correlation between the change in albuminuria and the change in urinary TNF-α.21,25 Again, no significant relationship was observed between serum and urinary levels of this cytokine, indicating that TNF-α is produced within the kidneys and that PTF administration is associated with a modulation in its production and urinary excretion.
Adverse events were consistent with the known safety profile of PTF obtained from a wide clinical experience for >30 years in patients with vascular disease, with and without diabetes and renal function impairment. The most common secondary effects were transient, self-limited digestive symptoms that disappeared during the first month. In one case PTF was withdrawn, and in five patients the dosage could not be increased to 1200 mg/d because of digestive intolerance. The schedule of PTF administration based on an initial 1-month period at half-dosage (600 mg/d), the use of an extended-release formulation, and the administration with food are potential factors that could positively influence tolerability.
Our study was a randomized, prospective trial, performed under usual clinical practice conditions. The study groups were well balanced, and patients received the maximum doses of RAS inhibitors before starting treatment with PTF. However, some limitations should be considered. First, this study was not designed in a double-blinded fashion, and the open-label design has inherent bias. Nevertheless, the main study outcomes were based on laboratory measurements, which were performed blinded to the study group allocation of patients. On the other hand, because this study was an independent clinical trial (as a result of limited resources), a placebo was not used in the control group. We do not think these features played a relevant part in a comparison of the study groups. However, we recognize that the lack of a placebo control, and subsequently the lack of a potential placebo effect, is a weakness. Thus, this limitation may underlie the present results, and we acknowledge that without a placebo control it is possible that we could have not detected a significant difference in the PTF versus the control group.
Second, the single-center design also represents a limitation, and, as with any other single-center study, reproducibility and generalizability of this report will require further validation by a double-blind, placebo-controlled, adequately powered, multicenter trial.
Third, the primary outcome was assessed by measuring eGFR; therefore, the use of more accurate methods for determination of the GFR would be important. In addition, the use of eGFR as an endpoint may be a potential limitation because ideally, progression to ESRD would be the endpoint. However, progression to ESRD, or even the doubling of serum creatinine (which is accepted by the US Food and Drug Administration as a surrogate endpoint for the development of kidney failure in clinical trials), is a late event in CKD and takes a long time to develop, with important costs and resources necessary to achieve adequate power for such endpoints. Thus, there is interest in considering alternative endpoints to shorten the duration of clinical trials and extend their application to earlier stages of CKD. In addition, GFR estimates provide a substantial improvement over the measurement of serum creatinine alone in the clinical assessment of kidney function; the reciprocal relationship between GFR and serum creatinine levels makes it difficult for clinicians to appreciate the level and rate of change in GFR by simply monitoring serum creatinine levels.
Fourth, although all patients received dietary counseling during the study, we did not evaluate the potential role of dietary factors in the results, especially with regard to protein intake.
Finally, all patients in the present study were white. Because African-American and Indo-Asian patients have a higher risk of diabetic renal disease progression, our results might not be extrapolated to these ethnic groups. However, a previous study that included a large proportion of African-American participants showed a positive effect of PTF on the rate of eGFR decrease compared with placebo.26
In conclusion, patients with type 2 diabetes and stages 3–4 CKD who are under standard care with RAS blockers and received treatment with PTF had a smaller decrease in eGFR and a higher reduction of residual UAE, which is consistent with a potential renoprotective effect of PTF in this population. Our study indicates that PTF could be an effective therapeutic option for treatment of DKD in type 2 diabetes. However, our study is not definitive and is still hypothesis-generating with regard to renal outcomes. Therefore, PTF should not be considered part of clinical practice without more definitive trials (large-scale, adequately powered, multicenter, prospective, placebo-controlled studies, with definitive endpoints on efficacy and safety) to demonstrate with the maximum grade of evidence the renoprotective properties of PTF in this population.
Concise Methods
Study Design
The PREDIAN (Pentoxifylline for Renoprotection in Diabetic Nephropathy) study42 is an independent, investigator-initiated trial without any commercial interest. This single-center, open-label, prospective, randomized controlled clinical trial was conducted in a university teaching hospital and a major regional tertiary care hospital in Santa Cruz de Tenerife, Spain. The Institutional Review Board and Ethics Committee approved the protocol. The study was performed in accordance with the Declaration of Helsinki, the European Union Clinical Trial Directive (2001/20/EC), and Good Clinical Practice, and it was approved by the Spanish Agency of Medicines and Sanitary Products (Spanish Ministry of Health and Social Policy). This trial was registered on the European Union Drug Regulating Authorities Clinical Trials (EudraCT #2007–005985–10). All participants provided written informed consent before study entry.
Participants
All patients were receiving usual care appropriate to their individual profile according to routine clinical practice, with emphasis on the targets of clinical practice guidelines, including a goal to achieve hemoglobin A1c levels <7% and an LDL cholesterol level <100 mg/dl. Regarding BP, the general target was <130 mmHg systolic and <80 mmHg diastolic, except for patients with cardiovascular disease, in whom the BP target was <140 mmHg systolic and <90 mmHg diastolic. The single-center design ensured the adherence to the guidelines. Management was done without specific limitations, except for the ACEI/ARB combination and the use of aldosterone or renin inhibitors, none of which were permitted. The specific inclusion criteria were age >40 years; clinical diagnosis of type 2 diabetes mellitus (according to the American Diabetes Association definition) and DN; diabetes duration≥8 years; CKD stages 3–4; UAE>30 mg/24 hours; stable renal function, defined as a variability in serum creatinine <15% with regard to the baseline value in a previous test done 4–8 weeks before initiation of the study; presence of diabetic retinopathy; therapy with ACEI or ARB at the maximal recommended dosage for >6 months; and ability to give informed consent. Exclusion criteria included type 1 diabetes; nondiabetic kidney disease; history of chronic inflammatory, immunologic, or tumoral disease; acute inflammatory or infectious intercurrent episode in the previous 3 months; institutionalization; receipt of immunotherapy or immunosuppressive treatment; previous therapy with PTF; treatment with combination ACEI/ARB treatment or aldosterone antagonists or direct renin inhibitors; systolic BP≥180 mmHg or diastolic BP≥110 mmHg; glycated hemoglobin>10%; pregnancy, breast-feeding, plans to become pregnant, or sexually active and not using birth control (women); and patient refusal.
Randomization and Procedures
Participants were randomly assigned following simple randomization procedures (computerized random numbers) to a control group to a group treated with PTF (treatment group). Allocation was concealed by enclosing assignments in sequentially numbered, opaque, sealed, and stapled envelopes, which were opened only after the enrolled participants completed all baseline assessments and it was time to allocate the intervention.
Baseline assessments for trial participants included medical history, laboratory analyses, and current medications. Patients randomly assigned to the treatment group started PTF, 600 mg daily (extended-release tablets), for 1 month, at which point the dose was increased to 600 mg twice daily. Medication adherence was assessed at each follow-up visit by direct questioning. Moreover, patients completed a daily record of pills taken or missed for ACEIs and ARBs in the control group and ACEIs/ARBs and PTF in the study group. Patients were considered adherent to the medication if <10% of the tablets were missed over each 6-month follow-up period. Adherence was >95% in both groups. Participants were followed up with clinical visits every 3 or 6 months according to the stage of CKD. The planning of visits was identical for the two groups; therefore, the patients in the PTF group were seen the same number of times as patients in the control group. BP, adverse events, concomitant drug therapies, treatment adherence, and blood chemistry were assessed at every visit. A 24-hour urine collection was obtained every 6 months. Likewise, for the assessment of the evolution of eGFR, this variable was computed at 6-months intervals, with an accepted range for measurement of ±14 days at each follow-up time.
Blood samples were drawn in the morning after an 8- to 12-hour overnight fast. The eGFR was calculated using the four-variable Modification of Diet in Renal Disease study equation.43 Serum creatinine was measured by an enzymatic method that has been standardized against isotope dilution mass spectrometry (CREA Plus; Roche Diagnostic, Mannheim, Germany), without changes during the study. The coefficients of variation (within-run and between-run) were <1.5%. Daily internal and external quality control was performed, and calibration of the serum creatinine assay did not change throughout the study period.
All participants were requested to provide 24-hour urine specimens. Study staff instructed patients on how to collect the two 24-hour urine collections, and written standardized instructions were given. In addition, patients were called the day before the scheduled urine collection to remind them about the procedure and avoid potential errors. Participants were asked to first empty their bladder, discard the urine, and record this time and date on the collection-container label as the start time and date. Then, they were asked to collect all urine into the container for a complete 24-hour period, with the final urine sample at the same time of day as the start time, collecting the last sample at that time even if the patient does not feel the urge to urinate, and emptying the bladder completely. The urine container was delivered to the laboratory the same day the collection was finished. Assessment of total 24-hour urinary creatinine excretion was used to evaluate the completeness of sample collection. In addition to the 24-hour UAE, a urine albumin-to-creatinine ratio using the concentrations of albumin and creatinine from the 24-hour urine collection was calculated to ensure the collection accuracy. A correlation analysis between the 24-hour UAE and the albumin-to-creatinine ratio for each time point showed correlation coefficients between 0.81 and 0.92 (P<0.001).
UAE was quantified by immunoturbidimetry (Tina-quant Albumin Assay; Roche Diagnostic). The intra-assay coefficient of variation was <4%. Regarding analytical sensitivity and specificity, the limit of blank was 2 mg/dl and the limit of detection was 3 mg/dl. Urinary concentration of TNF-α was measured in duplicate by immunoenzymatic ELISA method (Human TNF-α Quantikine HS ELISA; R&D Systems, Minneapolis, MN) in a DSXTM 4 Plate ELISA Processor (Vitro SA, Spain). Sensitivity of the assay was 0.19 pg/ml, and the intra-assay and interassay coefficients of variation were <8.8% and 10.5%, respectively. Urinary TNF-α levels were normalized to urinary creatinine levels to control for variations in the urine flow rate. All laboratory measurements were performed blinded to patients’ characteristics and group assignment for the duration of the study.
Outcome Measures
The primary outcome measure was progression of DKD, which was assessed by change from baseline in the eGFR with PTF compared with the control group after 2 years of follow-up. We measured the difference in eGFR between the PTF and the control group every 6 months after baseline and analyzed the rates of decline in eGFR (ml/min per 1.73 m2 per month). Secondary outcomes included the percentage of patients with a reduction in the eGFR of ≥25%, the percentage of patients with an eGFR decline greater than the median rate of decline in eGFR per month, and the changes from baseline in the UAE. A tertiary outcome was to assess the effect of PTF on the urinary excretion of TNF-α and its relationship with changes in eGFR and UAE.
Sample Size Calculation
The mean rate of decline of eGFR on the expected population to be recruited in the present trial, based on previous studies in patients with type 2 diabetes under RAS blockade,44 was assumed to be 0.45±0.36 ml/min per 1.73 m2 per month. The study was powered to detect a 35% difference in the change from baseline in the eGFR between the PTF and the control groups. A total trial size of 168 patients provided 80% power to detect the expected difference in change in eGFR at a two-tailed 5% level of significance, allowing for a 5% dropout rate.
Statistical Analyses
Continuous variables are reported as means±SDs, except for UAE and TNF-α, which are expressed as medians and interquartile ranges. Categorical data are presented as absolute values and percentages. Baseline comparisons were performed by independent t test or by the chi-squared test or Fisher exact test. Data were analyzed using an intention-to-treat principle, defined as participants who met all the inclusion criteria, met none of the exclusion criteria, had at least one dose of the study drug, and had one or more postrandomization measurement of eGFR. A repeated-measures analysis of covariance model was used to compare groups on their mean change in eGFR and UAE, with treatment group as a factor and baseline eGFR and UAE as covariates. Least-square means with SEMs and two-sided 95% CIs for differences between groups were estimated. To compare the percentage of patients with a reduction in eGFR greater than the median rate of decline observed in the study for the overall group, or >25% the baseline value, as well as the proportion of patients with an UAE>1 g/d, the differences between groups were assessed with the Fisher exact test. Correlations between change in eGFR and UAE, and variations in urinary TNF-α, were analyzed by the Spearman rank-order test. P<0.05 was considered to indicate a statistically significant difference. Calculations were computed with Statistica 7.1 software (StatSoft, Inc., Tulsa, OK).
Disclosures
None.
Acknowledgments
This independent, noncommercial clinical trial was funded by the Institute of Health Carlos III (ISCIII) (Spanish Ministry of Economy and Competitiveness, and Spanish Ministry of Health, Social Services and Equality; project number E07/90021). Research activity by J.F.N.G. is supported by Programa de Intensificación de la Actividad Investigadora (ISCIII/Comunidad Autónoma de Canarias) and he is national coordinator of the GEENDIAB (Spanish Group for the Study of Diabetic Nephropathy, RETIC/REDinREN/RD12/0021/0019, ISCIII).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Ritz E, Rychlík I, Locatelli F, Halimi S: End-stage renal failure in type 2 diabetes: A medical catastrophe of worldwide dimensions. Am J Kidney Dis 34: 795–808, 1999 [DOI] [PubMed] [Google Scholar]
- 2.Vejakama P, Thakkinstian A, Lertrattananon D, Ingsathit A, Ngarmukos C, Attia J: Reno-protective effects of renin-angiotensin system blockade in type 2 diabetic patients: A systematic review and network meta-analysis. Diabetologia 55: 566–578, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barnett AH, Bain SC, Bouter P, Karlberg B, Madsbad S, Jervell J, Mustonen J, Diabetics Exposed to Telmisartan and Enalapril Study Group : Angiotensin-receptor blockade versus converting-enzyme inhibition in type 2 diabetes and nephropathy. N Engl J Med 351: 1952–1961, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Eijkelkamp WB, Zhang Z, Remuzzi G, Parving HH, Cooper ME, Keane WF, Shahinfar S, Gleim GW, Weir MR, Brenner BM, de Zeeuw D: Albuminuria is a target for renoprotective therapy independent from blood pressure in patients with type 2 diabetic nephropathy: Post hoc analysis from the Reduction of Endpoints in NIDDM with the Angiotensin II Antagonist Losartan (RENAAL) trial. J Am Soc Nephrol 18: 1540–1546, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Packham DK, Wolfe R, Reutens AT, Berl T, Heerspink HL, Rohde R, Ivory S, Lewis J, Raz I, Wiegmann TB, Chan JC, de Zeeuw D, Lewis EJ, Atkins RC, Collaborative Study Group : Sulodexide fails to demonstrate renoprotection in overt type 2 diabetic nephropathy. J Am Soc Nephrol 23: 123–130, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lewis EJ, Greene T, Spitalewiz S, Blumenthal S, Berl T, Hunsicker LG, Pohl MA, Rohde RD, Raz I, Yerushalmy Y, Yagil Y, Herskovits T, Atkins RC, Reutens AT, Packham DK, Lewis JB, Collaborative Study Group : Pyridorin in type 2 diabetic nephropathy. J Am Soc Nephrol 23: 131–136, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mann JF, Green D, Jamerson K, Ruilope LM, Kuranoff SJ, Littke T, Viberti G, ASCEND Study Group : Avosentan for overt diabetic nephropathy. J Am Soc Nephrol 21: 527–535, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Zeeuw D, Akizawa T, Audhya P, Bakris GL, Chin L, Christ-Schmidt H, Goldsberry A, Houser M, Krauth M: LambersHeerspink HJ, McMurray JJ, Meyer CJ, Parving HH, Remuzzi G, Toto R, Vaziri ND, Wanner C, Wittes J, Wrolstad D, Chertow GM, for the BEACON Trial Investigators: Bardoxolone methyl in type 2 diabetes and stage 4 chronic kidney disease. N Engl J Med 369: 2492–2503, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fried LF, Emanuele N, Zhang JH, Brophy M, Conner TA, Duckworth W, Leehey DJ, McCullough PA, O’Connor T, Palevsky PM, Reilly RF, Seliger SL, Warren SR, Watnick S, Peduzzi P, Guarino P, VA NEPHRON-D Investigators : Combined angiotensin inhibition for the treatment of diabetic nephropathy. N Engl J Med 369: 1892–1903, 2013 [DOI] [PubMed] [Google Scholar]
- 10.Tuttle KR: Linking metabolism and immunology: Diabetic nephropathy is an inflammatory disease. J Am Soc Nephrol 16: 1537–1538, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Navarro-González JF, Mora-Fernández C, Muros de Fuentes M, García-Pérez J: Inflammatory molecules and pathways in the pathogenesis of diabetic nephropathy. Nat Rev Nephrol 7: 327–340, 2011 [DOI] [PubMed] [Google Scholar]
- 12.Wada J, Makino H: Inflammation and the pathogenesis of diabetic nephropathy. Clin Sci (Lond) 124: 139–152, 2013 [DOI] [PubMed] [Google Scholar]
- 13.Doherty GM, Jensen JC, Alexander HR, Buresh CM, Norton JA: Pentoxifylline suppression of tumor necrosis factor gene transcription. Surgery 110: 192–198, 1991 [PubMed] [Google Scholar]
- 14.Voisin L, Breuillé D, Ruot B, Rallière C, Rambourdin F, Dalle M, Obled C: Cytokine modulation by PX differently affects specific acute phase proteins during sepsis in rats. Am J Physiol 275: R1412–R1419, 1998 [DOI] [PubMed] [Google Scholar]
- 15.Strutz F, Heeg M, Kochsiek T, Siemers G, Zeisberg M, Müller GA: Effects of pentoxifylline, pentifylline and gamma-interferon on proliferation, differentiation, and matrix synthesis of human renal fibroblasts. Nephrol Dial Transplant 15: 1535–1546, 2000 [DOI] [PubMed] [Google Scholar]
- 16.Abdel-Salam OM, Baiuomy AR, El-Shenawy SM, Arbid MS: The anti-inflammatory effects of the phosphodiesterase inhibitor pentoxifylline in the rat. Pharmacol Res 47: 331–340, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Dávila-Esqueda ME, Martínez-Morales F: Pentoxifylline diminishes the oxidative damage to renal tissue induced by streptozotocin in the rat. Exp Diabesity Res 5: 245–251, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin SL, Chen YM, Chien CT, Chiang WC, Tsai CC, Tsai TJ: Pentoxifylline attenuated the renal disease progression in rats with remnant kidney. J Am Soc Nephrol 13: 2916–2929, 2002 [DOI] [PubMed] [Google Scholar]
- 19.Yagmurlu A, Boleken ME, Ertoy D, Ozsan M, Gokcora IH, Dindar H: Preventive effect of pentoxifylline on renal scarring in rat model of pyelonephritis. Urology 61: 1037–1041, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Navarro JF, Mora C, Muros M, Maca M, Garca J: Effects of pentoxifylline administration on urinary N-acetyl-beta-glucosaminidase excretion in type 2 diabetic patients: A short-term, prospective, randomized study. Am J Kidney Dis 42: 264–270, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Navarro JF, Mora C, Muros M, García J: Additive antiproteinuric effect of pentoxifylline in patients with type 2 diabetes under angiotensin II receptor blockade: A short-term, randomized, controlled trial. J Am Soc Nephrol 16: 2119–2126, 2005 [DOI] [PubMed] [Google Scholar]
- 22.McCormick BB, Sydor A, Akbari A, Fergusson D, Doucette S, Knoll G: The effect of pentoxifylline on proteinuria in diabetic kidney disease: A meta-analysis. Am J Kidney Dis 52: 454–463, 2008 [DOI] [PubMed] [Google Scholar]
- 23.Shan D, Wu HM, Yuan QY, Li J, Zhou RL, Liu GJ: Pentoxifylline for diabetic kidney disease. Cochrane Database Syst Rev 2: CD006800, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Diskin CJ, Stokes TJ, Dansby LM, Radcliff L, Carter TB: Will the addition of pentoxifylline reduce proteinuria in patients with diabetic glomerulosclerosis refractory to maximal doses of both an angiotensin-converting enzyme inhibitor and an angiotensin receptor blocker? J Nephrol 20: 410–416, 2007 [PubMed] [Google Scholar]
- 25.Lin SL, Chen YM, Chiang WC, Wu KD, Tsai TJ: Effect of pentoxifylline in addition to losartan on proteinuria and GFR in CKD: A 12-month randomized trial. Am J Kidney Dis 52: 464–474, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Perkins RM, Aboudara MC, Uy AL, Olson SW, Cushner HM, Yuan CM: Effect of pentoxifylline on GFR decline in CKD: A pilot, double-blind, randomized, placebo-controlled trial. Am J Kidney Dis 53: 606–616, 2009 [DOI] [PubMed] [Google Scholar]
- 27.Goicoechea M, García de Vinuesa S, Quiroga B, Verdalles U, Barraca D, Yuste C, Panizo N, Verde E, Muñoz MA, Luño J: Effects of pentoxifylline on inflammatory parameters in chronic kidney disease patients: A randomized trial. J Nephrol 25: 969–975, 2012 [DOI] [PubMed] [Google Scholar]
- 28.Ivory SE, Packham DK, Reutens AT, Wolfe R, Rohde RD, Lewis J, Atkins RC, Collaborative Study Group : Residual proteinuria and eGFR predict progression of renal impairment within 2 years in type 2 diabetic patients with nephropathy who are receiving optimal treatment with angiotensin receptor blockers. Nephrology (Carlton) 18: 516–524, 2013 [DOI] [PubMed] [Google Scholar]
- 29.Donadio JV, Jr, Ilstrup DM, Holley KE, Romero JC: Platelet-inhibitor treatment of diabetic nephropathy: A 10-year prospective study. Mayo Clin Proc 63: 3–15, 1988 [DOI] [PubMed] [Google Scholar]
- 30.Khajehdehi P, Roozbeh J, Mostafavi H: A comparative randomized and placebo-controlled short-term trial of aspirin and dipyridamole for overt type-2 diabetic nephropathy. Scand J Urol Nephrol 36: 145–148, 2002 [DOI] [PubMed] [Google Scholar]
- 31.Matzky R, Darius H, Schrör K: The release of prostacyclin (PGI2) by pentoxifylline from human vascular tissue. Arzneimittelforschung 32: 1315–1318, 1982 [PubMed] [Google Scholar]
- 32.Krysztopik RJ, Matheson PJ, Spain DA, Garrison RN, Wilson MA: Lazaroid and pentoxifylline suppress sepsis-induced increases in renal vascular resistance via altered arachidonic acid metabolism. J Surg Res 93: 75–81, 2000 [DOI] [PubMed] [Google Scholar]
- 33.Kelly KJ, Dominguez JH: Rapid progression of diabetic nephropathy is linked to inflammation and episodes of acute renal failure. Am J Nephrol 32: 469–475, 2010 [DOI] [PubMed] [Google Scholar]
- 34.Stanton RC: Oxidative stress and diabetic kidney disease. Curr Diab Rep 11: 330–336, 2011 [DOI] [PubMed] [Google Scholar]
- 35.Kanasaki K, Taduri G, Koya D: Diabetic nephropathy: The role of inflammation in fibroblast activation and kidney fibrosis. Front Endocrinol (Lausanne) 4: 7, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Navarro JF, Milena FJ, Mora C, León C, García J: Renal pro-inflammatory cytokine gene expression in diabetic nephropathy: Effect of angiotensin-converting enzyme inhibition and pentoxifylline administration. Am J Nephrol 26: 562–570, 2006 [DOI] [PubMed] [Google Scholar]
- 37.Sun HK, Lee YM, Han KH, Kim HS, Ahn SH, Han SY: Phosphodiesterase inhibitor improves renal tubulointerstitial hypoxia of the diabetic rat kidney. Korean J Intern Med 27: 163–170, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rodriguez-Morán M, González-González G, Bermúdez-Barba MV, Medina de la Garza CE, Tamez-Pérez HE, Martínez-Martínez FJ, Guerrero-Romero F: Effects of pentoxifylline on the urinary protein excretion profile of type 2 diabetic patients with microproteinuria: A double-blind, placebo-controlled randomized trial. Clin Nephrol 66: 3–10, 2006 [DOI] [PubMed] [Google Scholar]
- 39.Maiti R, Agrawal NK, Dash D, Pandey BL: Effect of Pentoxifylline on inflammatory burden, oxidative stress and platelet aggregability in hypertensive type 2 diabetes mellitus patients. Vascul Pharmacol 47: 118–124, 2007 [DOI] [PubMed] [Google Scholar]
- 40.Leyva-Jiménez R, Rodríguez-Orozco AR, Ortega-Pierres LE, Ramírez-Enríquez J, Gómez-García A, Alvarez-Aguilar C: [Effect of pentoxifylline on the evolution of diabetic nephropathy]. Med Clin (Barc) 132: 772–778, 2009 [DOI] [PubMed] [Google Scholar]
- 41.Navarro JF, Mora C, Maca M, Garca J: Inflammatory parameters are independently associated with urinary albumin in type 2 diabetes mellitus. Am J Kidney Dis 42: 53–61, 2003 [DOI] [PubMed] [Google Scholar]
- 42.Navarro-González JF, Muros M, Mora-Fernández C, Herrera H, Meneses B, García J: Pentoxifylline for renoprotection in diabetic nephropathy: The PREDIAN study. Rationale and basal results. J Diabetes Complications 25: 314–319, 2011 [DOI] [PubMed] [Google Scholar]
- 43.Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, Kusek JW, Van Lente F, Chronic Kidney Disease Epidemiology Collaboration : Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med 145: 247–254, 2006 [DOI] [PubMed] [Google Scholar]
- 44.Lewis EJ, Hunsicker LG, Clarke WR, Berl T, Pohl MA, Lewis JB, Ritz E, Atkins RC, Rohde R, Raz I, Collaborative Study Group : Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med 345: 851–860, 2001 [DOI] [PubMed] [Google Scholar]