Abstract
Activation of the renin-angiotensin system (RAS) plays an essential role in the pathogenesis of CKD and cardiovascular disease. However, current anti-RAS therapy only has limited efficacy, partly because of compensatory upregulation of renin expression. Therefore, a treatment strategy to simultaneously target multiple RAS genes is necessary to achieve greater efficacy. By bioinformatics analyses, we discovered that the promoter regions of all RAS genes contained putative T-cell factor (TCF)/lymphoid enhancer factor (LEF)-binding sites, and β-catenin induced the binding of LEF-1 to these sites in kidney tubular cells. Overexpression of either β-catenin or different Wnt ligands induced the expression of all RAS genes. Conversely, a small-molecule β-catenin inhibitor ICG-001 abolished RAS induction. In a mouse model of nephropathy induced by adriamycin, either transient therapy or late administration of ICG-001 abolished established proteinuria and kidney lesions. ICG-001 inhibited renal expression of multiple RAS genes in vivo and abolished the expression of other Wnt/β-catenin target genes. Moreover, ICG-001 therapy restored expression of nephrin, podocin, and Wilms’ tumor 1, attenuated interstitial myofibroblast activation, repressed matrix expression, and inhibited renal inflammation and fibrosis. Collectively, these studies identify all RAS genes as novel downstream targets of Wnt/β-catenin. Our results indicate that blockade of Wnt/β-catenin signaling can simultaneously repress multiple RAS genes, thereby leading to the reversal of established proteinuria and kidney injury.
Keywords: renal fibrosis, CKD, renin angiotensin system, Wnt, β-catenin
Extensive studies over the last several decades have established that activation of the renin-angiotensin system (RAS) plays an essential role in the pathogenesis of CKD and cardiovascular disease.1–3 RAS consists of several key components, including angiotensinogen (AGT), renin, angiotensin-converting enzyme (ACE), angiotensin II type 1 receptor (AT1), and angiotensin II type 2 receptor (AT2). Many studies indicate that, after kidney injury, intrarenal RAS is markedly activated because of concurrent upregulation of multiple RAS genes.4,5 RAS activation contributes to kidney and cardiovascular injury through a range of mechanisms. In addition to regulating BP and hemodynamics,6,7 angiotensin II, the principal and active mediator of RAS, activates TGF-β1 and NF-κB signaling and directly promotes renal inflammation and fibrosis.8–10 Studies using both genetic and pharmacologic approaches have confirmed the relevance and importance of RAS activation in the development and progression of CKD and cardiovascular disease. However, current anti-RAS therapy using ACE inhibitors (ACEIs) or angiotensin II receptor blockers (ARBs) only displays limited efficacy, partly because of compensatory upregulation of renin expression.11–15 In this context, developing a strategy to simultaneously target multiple RAS genes is necessary and essential for the effective treatment of patients with chronic kidney and cardiovascular disorders.
Wnt/β-catenin signaling is an evolutionarily conserved developmental signaling cascade that plays a pivotal role in regulating organ development and tissue homeostasis.16–19 Upon activation by Wnts, β-catenin is stabilized and translocated into the nucleus, where it binds to the T-cell factor (TCF)/lymphoid enhancer-binding factor (LEF) family of transcription factors and then assembles a complex by recruiting the transcriptional coactivator cAMP response element-binding protein (CREB)-binding protein (CBP) to transactivate its target genes. Despite being relatively silent in normal adult kidneys, Wnt/β-catenin signaling is reactivated in a wide variety of CKDs, such as obstructive nephropathy, diabetic nephropathy, adriamycin (ADR) nephropathy, polycystic kidney disease, and chronic allograft nephropathy.20–24 Accordingly, blockade of this signal cascade by an assortment of approaches is able to prevent kidney injury and ameliorate proteinuria and fibrotic lesions in animal models of CKD.20,21,25 However, although both Wnt/β-catenin signaling and RAS are coincidently activated in diseased kidneys, whether they are directly related is completely unknown.
Through bioinformatics analyses, we have uncovered that all RAS genes contain putative TCF/LEF-binding sites (TBSs) in their promoter regions. This finding implies a possible connection between Wnt/β-catenin signaling and RAS activation. To test this hypothesis, we have investigated the regulation of RAS genes by Wnt/β-catenin both in vitro and in vivo. Using a small molecule (ICG-001) that inhibits β-catenin–mediated gene transcription,26,27 we have found that inhibition of Wnt/β-catenin signaling completely abolishes RAS activation. In a mouse model of ADR nephropathy, either transient therapy or late administration of ICG-001 is able to abolish established proteinuria and kidney lesions. Our results suggest that Wnt/β-catenin could be an unparalleled therapeutic target, because its inhibition could simultaneously repress all RAS genes.
Results
Multiple RAS Genes Are Novel Targets of Wnt/β-Catenin Signaling
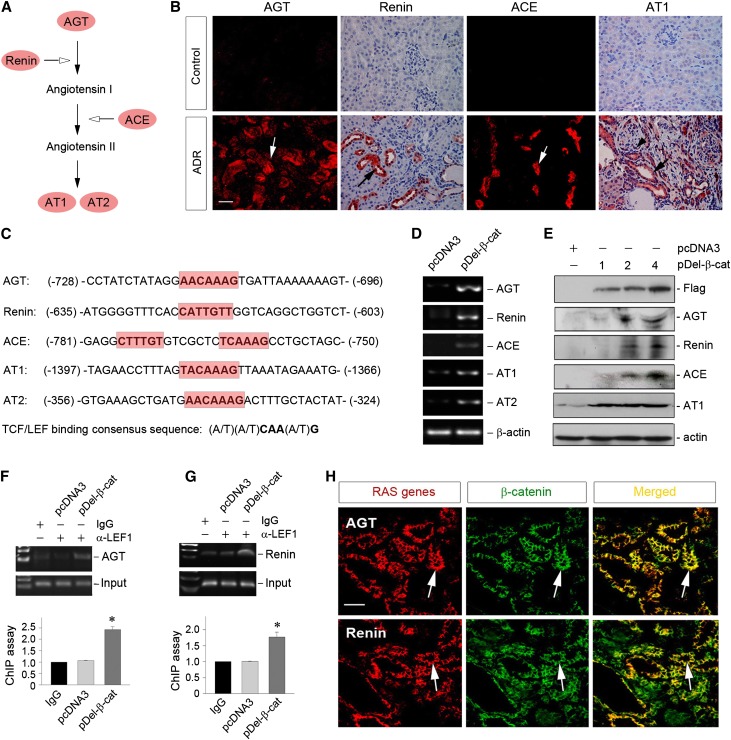
As shown in Figure 1A, RAS consists of five distinct genes, including AGT, renin, ACE, AT1, and AT2. In ADR nephropathy, multiple RAS genes were upregulated specifically in renal tubular epithelium (Figure 1B), underscoring a marked RAS activation in diseased kidneys. This pattern of simultaneous induction of multiple RAS genes in renal tubules suggests that there might be a common mechanism in controlling their expression. To examine this issue, we interrogated the regulatory regions of RAS gene promoters. As shown in Figure 1C, bioinformatics analyses revealed the presence of putative TBSs in the promoter regions of all RAS genes. There were perfect TBS consensus sequences in the promoter of AGT, renin, AT1, and AT2 genes (Figure 1C, highlighted in red). Although the TBS in the ACE promoter is imperfect because of a single base pair difference, there were two copies of these TBSs in close proximity separated by only five nucleotides in this gene (Figure 1C), which could cooperate with each other in mediating ACE induction by Wnt/β-catenin.
Figure 1.
Multiple genes of the RAS are direct targets of Wnt/β-catenin signaling. (A) Schematic presentation of the genes in the RAS. (B) Activation of intrarenal RAS in ADR nephropathy. The expression of RAS components, such as AGT, renin, ACE, and AT1, was assessed in the kidneys at 5 weeks after ADR injection. Frozen or paraffin kidney sections were used for immunostaining for RAS components. Arrows indicate positive staining. Scale bar, 50 µm. (C) Bioinformatics analyses revealed the presence of putative TBSs in the promoter regions of all human RAS genes. The sequences and positions of the putative TBS in multiple RAS genes are highlighted in red, whereas the TBS consensus sequence is given at the bottom of this panel. (D) Overexpression of β-catenin induced the expression of multiple RAS genes in kidney proximal tubular cells. HKC-8 cells were transfected with N-terminally truncated, FLAG-tagged, constitutively activated β-catenin expression vector (pDel-β-cat) or pcDNA3 empty vector for 24 hours. Cells were then analyzed for mRNA expression of multiple RAS genes. (E) Exogenous β-catenin induced protein expression of multiple RAS components in a dose-dependent fashion. HKC-8 cells were transfected with different amounts of pDel-β-cat plasmid as indicated (micrograms per well) or pcDNA3 empty vector (4 µg/well). Cell lysates were analyzed for protein expression of various RAS components by Western blotting. (F and G) ChIP assay showed that ectopic expression of β-catenin promoted the binding of LEF-1 to putative TBS in the promoter of RAS genes. HKC-8 cells transfected with either pcDNA3 or pDel-β-cat plasmids were subjected to ChIP analyses using specific anti–LEF-1 antibody. Increased binding of LEF-1 to putative TBS in (F) AGT and (G) renin gene promoters after β-catenin expression is shown. Representative ChIP assay (upper panels) and quantitative ChIP data (lower panels) are presented. *P<0.05 (n=3). (H) Colocalization of β-catenin and various RAS components in ADR nephropathy. Kidney cryosections were coimmunostained for AGT or renin (red) and β-catenin (green). Arrows indicate AGT (or renin) and β-catenin colocalization in the same tubule. Scale bar, 50 µm.
To test the functionality of these putative TBSs, we transfected human kidney proximal tubular epithelial cell (HKC-8) with N-terminally truncated, FLAG-tagged, constitutively activated β-catenin expression vector. As shown in Figure 1D, overexpression of β-catenin substantially induced the mRNA expression of all RAS genes, including AGT, renin, ACE, AT1, and AT2, which was illustrated by RT-PCR analysis. Similarly, exogenous β-catenin also induced protein expression of multiple RAS components in tubular epithelial cells in a dose-dependent fashion (Figure 1E). To further investigate whether Wnt/β-catenin regulates the transcription of RAS genes, we examined the binding of LEF-1 to the TBS identified in the promoters by chromatin immunoprecipitation (ChIP) assay. As shown in Figure 1, F and G, expression of constitutively activated β-catenin promoted LEF-1 binding to the TBS consensus sequences of RAS genes, such as AGT and renin, in HKC-8 cells. Consistently, in the fibrotic kidneys induced by ADR, β-catenin and various RAS components, such as AGT and renin, were colocalized in renal tubular epithelium (Figure 1H). Taken together, these results clearly suggest that Wnt/β-catenin directly controls the expression of multiple RAS genes both in vitro and in vivo.
Blockade of Wnt/β-Catenin Signaling Abolishes RAS Induction In Vitro
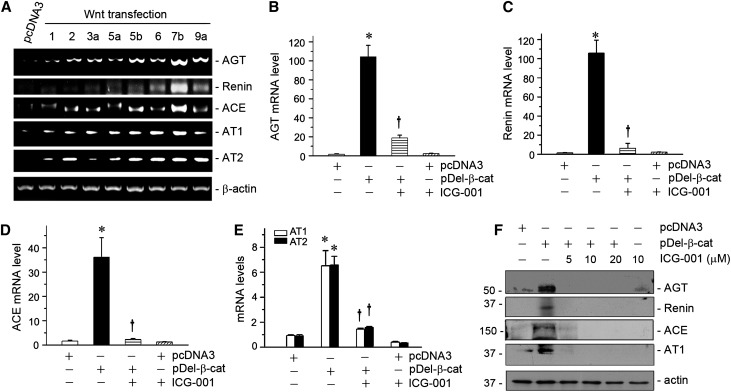
We further investigated the regulation of RAS genes by Wnt/β-catenin in kidney tubular epithelial cells by expressing various Wnt ligands. To this end, HKC-8 cells were transfected with the expression vectors encoding different Wnts. As shown in Figure 2A, ectopic expression of various Wnts, such as Wnt1, Wnt2, Wnt3a, Wnt5a, Wnt5b, Wnt6, Wnt7b, and Wnt9a, substantially induced AGT mRNA expression in HKC-8 cells. Similarly, various Wnts also stimulated the mRNA expression of renin, ACE, AT1, and AT2 (Figure 2A). These data indicate that Wnt/β-catenin signaling is able to induce all RAS genes in tubular epithelial cells in vitro.
Figure 2.
Wnts induce RAS expression, and small-molecule β-catenin inhibitor abolishes RAS induction in vitro. (A) Representative RT-PCR analyses showed that various Wnts induced the expression of RAS genes in tubular epithelial cells. HKC-8 cells were transfected with either pcDNA3 or the expression vectors for various Wnts as indicated for 24 hours. The mRNA expression of various RAS genes was analyzed by RT-PCR. (B–E) Small-molecule inhibitor ICG-001 abolished β-catenin–mediated RAS induction in vitro. HKC-8 cells were transfected with either pcDNA3 or N-terminally truncated, FLAG-tagged, constitutively activated β-catenin expression vector (pDel-β-cat) plasmid for 24 hours in the absence or presence of ICG-001 (10 µM). Quantitative real-time RT-PCR analyses showed the relative mRNA abundances of (B) AGT, (C) renin, (D) ACE, and (E) AT1 and AT2 after various treatments as indicated. Data are expressed as means±SEMs of three independent experiments. *P<0.05 versus pcDNA3 alone; †P<0.05 versus pDel-β-cat alone. (F) Representative Western blot analyses showed that ICG-001 abolished β-catenin–mediated RAS induction in tubular epithelial cells in vitro. HKC-8 cells were treated as indicated. Whole-cell lysates were immunoblotted for the protein expression of AGT, renin, ACE, and AT1, respectively.
We then assessed whether inhibition of Wnt/β-catenin signaling abolishes the induction of RAS genes. As shown in Figure 2, B–E, expression of constitutively activated β-catenin dramatically induced the mRNA expression of AGT, renin, ACE, AT1, and AT2, although the magnitude of induction was dissimilar for different RAS genes. Incubation of HKC-8 cells with ICG-001, a small-molecule inhibitor that represses β-catenin–mediated gene transcription in a CBP-dependent fashion,26,28 almost completely abolished the induction of all RAS genes (Figure 2, B–E). Consistently, ICG-001 also inhibited the protein induction of AGT, renin, ACE, and AT1 in HKC-8 cells (Figure 2F). These results indicate that blockade of Wnt/β-catenin signaling represses the expression of multiple RAS genes in vitro.
Blockade of Wnt/β-Catenin Signaling Ameliorates an Established Proteinuria and Kidney Injury
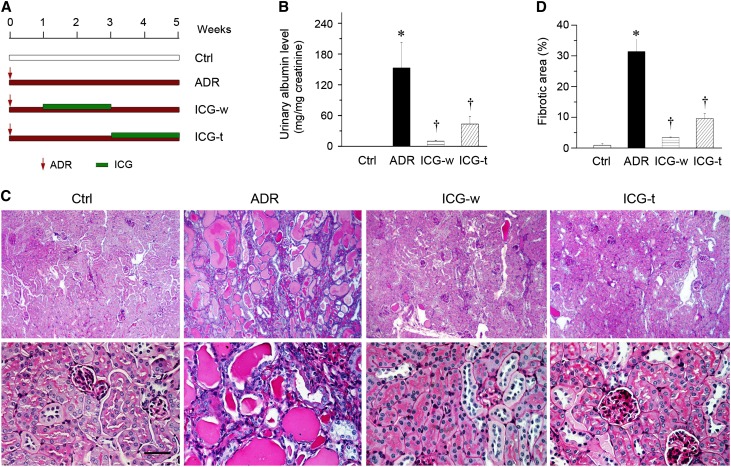
The ability of blocking Wnt/β-catenin signaling by ICG-001 to repress RAS genes in vitro prompted us to assess its therapeutic efficacy on an established kidney disease in vivo. To this end, we used a mouse model of ADR nephropathy, a model of human FSGS characterized by initial podocyte damage and proteinuria and subsequent tubulointerstitial inflammation and fibrosis,29 by using two different treatment protocols. As shown in Figure 3A, in the withdrawal protocol (ICG-w), ICG-001 was administered at 7 days after ADR injection, a time point when robust albuminuria and glomerular injury were established in this model.20,30 ICG-001 treatment lasted for 2 weeks and then stopped. The experiments were terminated at 5 weeks after ADR injection. In the therapeutic protocol (ICG-t), ICG-001 was given at 3 weeks after ADR injection (Figure 3A). As illustrated in Figure 3B, albuminuria was significantly reduced in both ICG-w and ICG-t protocols. Morphologic injury, which was shown by periodic acid–Schiff staining (Figure 3C), was clearly evident in the kidneys at 5 weeks after ADR injection, which was characterized by glomerular sclerotic lesions, tubular dilation with expanded lumen loaded with proteins, interstitial inflammation, and matrix deposition. Administration of ICG-001 by both ICG-w and ICG-t protocols largely ameliorated these morphologic lesions (Figure 3, C and D). These data suggest that ICG-001 is able to reverse an established proteinuria and kidney injury in ADR nephropathy.
Figure 3.
Small-molecule inhibitor ICG-001 ameliorates an established proteinuria and kidney injury in ADR nephropathy. (A) Diagram shows the experimental design. Arrows indicate the starting point of ADR injection. Green bars indicate ICG-001 treatment. (B) Urinary albumin levels in mice at 5 weeks after ADR injection. Urinary albumin was expressed as milligrams per milligram creatinine. (C) Representative micrographs show kidney injury at 5 weeks after ADR injection in different groups of mice as indicated. Images of periodic acid–Schiff staining with different magnifications are shown. Scale bar, 50 µm. (D) Quantitative determination of kidney fibrotic lesions in different groups. Ctrl, control. *P<0.05 versus normal controls; †P<0.05 versus ADR alone (n=5–6).
Blockade of Wnt/β-Catenin Signaling Abolishes RAS Induction In Vivo
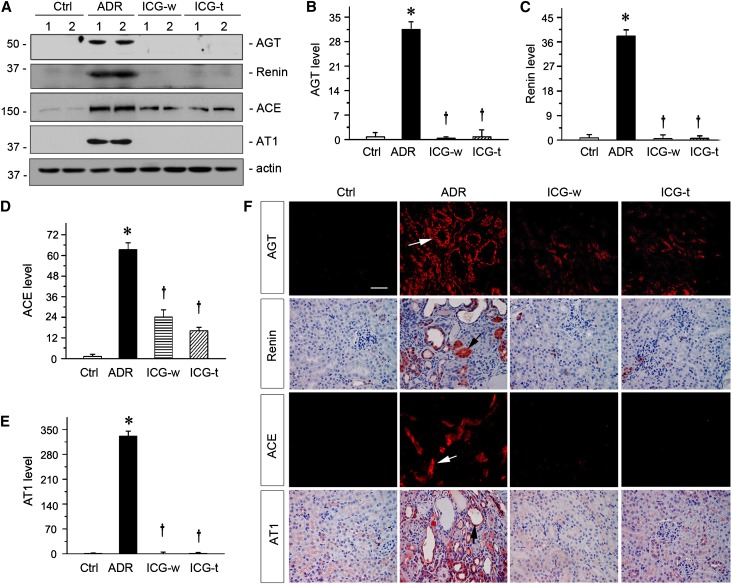
We next examined whether ICG-001 abolishes RAS induction in ADR nephropathy in vivo. Western blot analyses of kidney tissue homogenates showed a marked induction of major components of RAS, such as AGT, renin, ACE, and AT1, in ADR nephropathy compared with controls (Figure 4A). However, inhibition of Wnt/β-catenin signaling by ICG-001 significantly abrogated intrarenal induction of RAS genes in vivo (Figure 4, A–E). ICG-001 therapy in both ICG-w and ICG-t protocols showed similar efficacy in terms of inhibition of RAS genes (Figure 4, A–E). Similarly, ICG-001 also repressed the mRNA expression of various RAS genes, which were assessed by RT-PCR (data not shown). We also examined the expression and localization of RAS components in different groups by immunostaining. As shown in Figure 4F, RAS proteins, such as AGT, renin, ACE, and AT1, were predominantly induced in renal tubular epithelium in ADR nephropathy (Figure 4F, arrows). Consistently, ICG-001 in both ICG-w and ICG-t protocols also suppressed renal induction of RAS proteins. These data show that blockade of Wnt/β-catenin signaling by ICG-001 markedly represses the expression of multiple RAS genes in vivo and is accompanied by alleviation of kidney injury.
Figure 4.
Inhibition of β-catenin signaling by ICG-001 abolished RAS induction in ADR nephropathy. (A) Representative Western blot analyses revealed that ICG-001 abolished RAS induction in ADR nephropathy. Kidney lysates from different groups as indicated were immunoblotted with antibodies against AGT, renin, ACE, AT1, and actin. Numbers 1 and 2 represent different animals in a given group. (B–E) Graphic presentations of the relative abundances of (B) AGT, (C) renin, (D) ACE, and (E) AT1 in different groups as indicated. *P<0.05 versus normal controls; †P<0.05 versus ADR (n=5–6). (F) Representative micrographs showed RAS components in different groups as indicated. Kidney sections were stained with different antibodies against AGT, renin, ACE, and AT1. Arrows indicate positive tubules. Ctrl, control. Scale bar, 50 µm.
Blockade of Wnt/β-Catenin Signaling Restores Podocyte Integrity
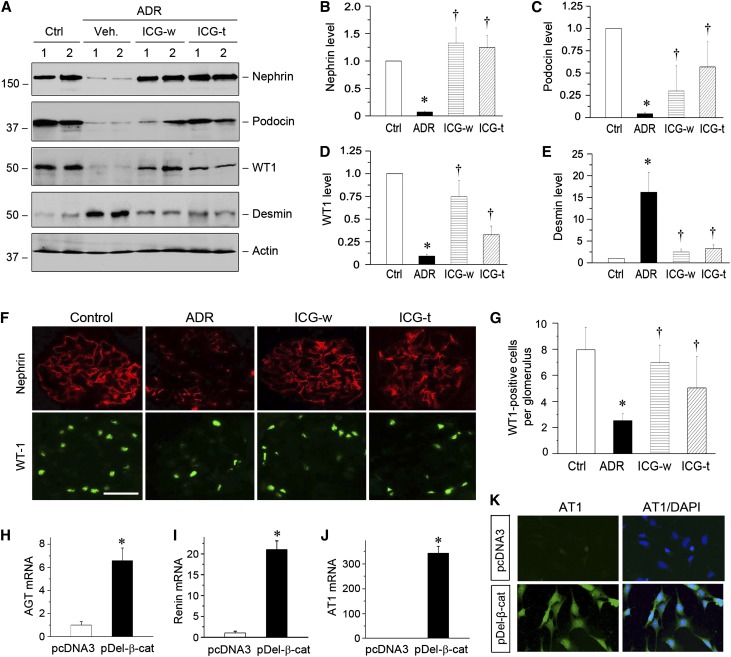
Because ADR nephropathy is characterized by initial podocyte injury and proteinuria,29 we next studied the effect of ICG-001 on podocyte integrity. As shown in Figure 5A, renal expression of podocyte-specific proteins, such as nephrin, podocin, and Wilms’ tumor 1 (WT1), was markedly reduced at 5 weeks after ADR injection. However, either transient treatment in the ICG-w protocol or late administration of ICG-001 in the ICG-t protocol completely restored nephrin expression (Figure 5, A and B). ICG-001 also significantly restored renal expression of podocin and WT1 (Figure 5, A, C, and D). Interestingly, ADR induced renal expression of desmin, a podocyte injury marker, which was largely inhibited by ICG-001 treatment (Figure 5E). Immunofluorescence staining also showed that ICG-001 restored nephrin and WT1 expression in both ICG-w and ICG-t protocols (Figure 5, F and G). These results are consistent with a previous report that Wnt/β-catenin signaling plays a critical role in podocyte injury and proteinuria in ADR nephropathy.31
Figure 5.
ICG-001 restores podocyte integrity in ADR nephropathy. (A) ICG-001 restores podocyte-specific proteins and inhibits desmin expression. Kidney lysates were immunoblotted with specific antibodies against nephrin, podocin, WT1, desmin, and actin. Numbers 1 and 2 represent different animals in a given group as indicated. (B–E) Graphic presentations of (B) nephrin, (C) podocin, (D) WT1, and (E) desmin expressions in different groups as indicated. *P<0.05 versus normal controls; †P<0.05 versus ADR alone (n=5–6). (F) Representative micrographs showing glomerular nephrin and WT1 expression. Frozen kidney tissue sections were stained with nephrin (red) and WT1 (green). (G) Graphic presentation shows the numbers of WT1-positive podocytes per glomerular cross-section. *P<0.05 versus normal controls; †P<0.05 versus ADR alone (n=5–6). (H–J) Expression of active β-catenin induces mRNA expression of RAS genes in vitro. Mouse podocytes were transiently transfected with N-terminally truncated, FLAG-tagged, constitutively activated β-catenin expression vector (pDel-β-cat) or control pcDNA3. The mRNA expression of (H) AGT, (I) renin, and (J) AT1 was assessed by quantitative RT-PCR. *P<0.05 (n=3). (K) Expression of active β-catenin induces AT1 protein expression in cultured podocytes. Mouse podocytes after transfection were immunostained for AT1 protein (green). Cell nuclei were visualized with DAPI staining (blue). Ctrl, control; DAPI, 4′,6-diamidino-2-phenylindole; Veh, vehicle.
To investigate whether Wnt/β-catenin directly induces RAS activation in podocytes, we examined the expression of RAS genes in cultured podocytes in vitro. As shown in Figure 5, H–J, expression of β-catenin dramatically induced the mRNA expression of AGT, renin, and AT1 in podocytes but not ACE and AT2. Similarly, AT1 protein was induced in podocytes after β-catenin expression (Figure 5K). We also observed that AGT and AT1 proteins were induced in the glomeruli in ADR nephropathy, which was colocalized with α-actinin-4 (data not shown). These data suggest that RAS activation in situ by Wnt/β-catenin could be a potential mechanism leading to podocyte injury and proteinuria.
Inhibition of the Wnt/β-Catenin/RAS Axis Inhibits Fibrosis-Related Genes and Reduces Renal Fibrosis
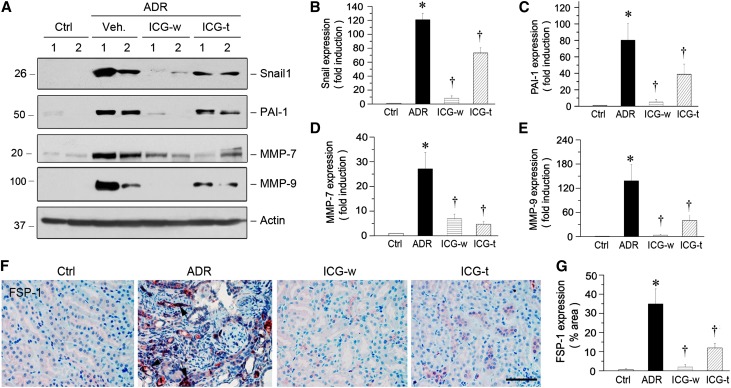
We next examined the expression of several key fibrosis-related genes in ADR nephropathy. As shown in Figure 6A, renal expression of Snail1, PAI-1, MMP-7, and MMP-9 was markedly induced at 5 weeks after ADR injection. Administration of ICG-001 in the withdrawal protocol almost completely abolished the induction of these genes. Delayed administration of ICG-001 at 3 weeks after ADR injection also inhibited, to a lesser extent, renal expression of these genes (Figure 6A). Quantification of these results is presented in Figure 6, B–E. ICG-001 also abolished the induction of FSP1, another direct target gene of β-catenin that is also known as S100A4 protein,32 in diseased kidneys (Figure 6, F and G).
Figure 6.
ICG-001 inhibits renal expression of the Wnt/β-catenin target genes in ADR nephropathy. (A) Western blot analyses of renal expressions of the Wnt/β-catenin target genes in different groups. (B–E) Graphic representations of (B) Snail1, (C) PAI-1, (D) MMP-7, and (E) MMP-9 expressions in different groups as indicated. *P<0.05 versus normal controls; †P<0.05 versus ADR alone (n=5–6). (F) Representative micrographs show FSP-1 expression in different groups. Paraffin sections were stained with anti–FSP-1 antibody. Arrows indicate FSP-1–positive tubules. (G) Graphic presentation shows FSP-1 staining in different groups. Ctrl, control; Veh, vehicle. *P<0.05 versus normal controls; †P<0.05 versus ADR alone (n=5–6).
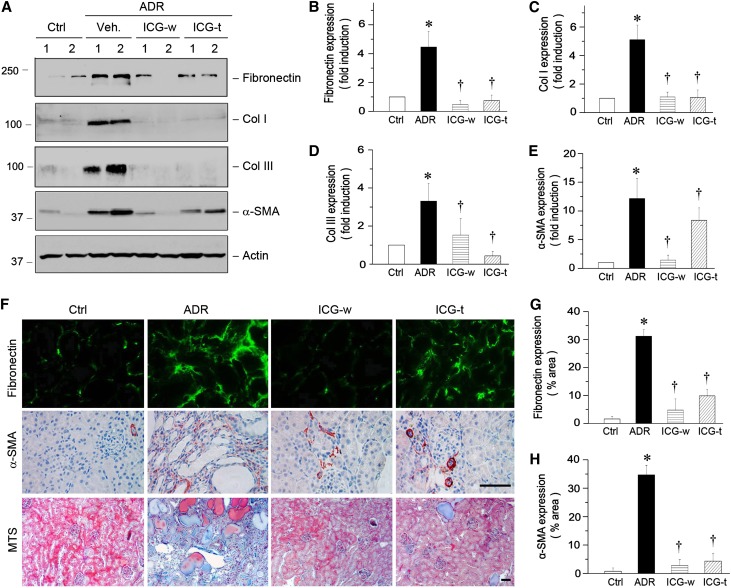
We also investigated the therapeutic effects of inhibition of the Wnt/β-catenin/RAS axis by ICG-001 on renal fibrotic lesions. As shown in Figure 7A, renal expression of major interstitial matrix components, including fibronectin and types I and III collagen, was markedly induced at 5 weeks after ADR injection. Administration of ICG-001 in either the withdrawal or therapeutic protocol almost completely abolished the induction of these matrix genes (Figure 7, A–D). Similarly, ICG-001 also largely inhibited the expression of α-smooth muscle actin (α-SMA), the molecular signature of renal myofibroblasts (Figure 7, A and E). Similar results were obtained when kidney tissues were assessed by immunostaining (Figure 7, F–H). Furthermore, Masson's Trichrome staining also revealed significant collagen deposition in the kidneys at 5 weeks after ADR injection, and administration of ICG-001 in both protocols ameliorated renal deposition of collagens (Figure 7F).
Figure 7.
ICG-001 inhibits matrix gene expression and reduces renal fibrosis in ADR nephropathy. (A) Western blot analyses show the expression of multiple fibrosis-related genes. Kidney lysates were immunoblotted with specific antibodies against fibronectin, types I and III collagen, α-SMA, and actin. (B–E) Graphic representations of (B) fibronectin, (C) collagen I, (D) collagen III, and (E) α-SMA expressions in different groups as indicated. *P<0.05 versus normal controls; †P<0.05 versus ADR alone (n=5–6). (F) Representative micrographs show fibronectin and α-SMA protein expression and collagen deposition in different groups. Frozen kidney tissue sections were stained with antifibronectin antibody (green), whereas paraffin sections were used for α-SMA and Masson's Trichrome staining. Scale bar, 50 µm. (G and H) Graphic presentation shows fibronectin and α-SMA expression in different groups. Ctrl, control; MTS, Masson's Trichrome Staining; Veh, vehicle. *P<0.05 versus normal controls; †P<0.05 versus ADR alone (n=5–6).
Inhibition of the Wnt/β-Catenin/RAS Axis Inhibits Renal Inflammation
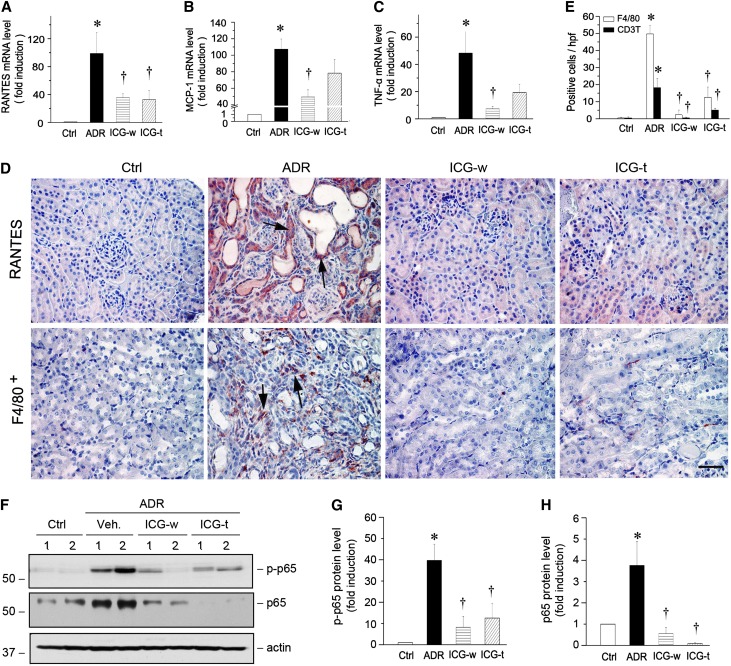
Interstitial infiltration of inflammatory cells is one of the characteristic features in ADR nephropathy. As shown in Figure 8, A–C, major proinflammatory chemokines, including regulated upon activation, normal T cell expressed and secreted (RANTES), MCP-1, and TNF-α, were markedly induced in the kidneys at 5 weeks after ADR injection. However, inhibition of the Wnt/β-catenin/RAS axis by treatment with ICG-001 suppressed the expression of these chemokines. Immunohistochemical staining also showed a marked induction of RANTES protein, predominantly in renal tubular epithelium (Figure 8D), and ICG-001 largely abolished its induction. Consistent with this chemokine expression, significant infiltration of F4/80-positive macrophages (Figure 8D) as well as CD3-positive T cells (data not shown) was observed in the interstitium of the diseased kidneys, which was largely prevented by administration of ICG-001 as well (Figure 8, D and E).
Figure 8.
ICG-001 inhibits inflammatory cytokines expression and reduces renal infiltration of macrophages in ADR nephropathy. (A–C) Quantitative real-time RT-PCR shows renal mRNA levels of (A) RANTES, (B) MCP-1, and (C) TNF-α in different groups. *P<0.05 versus normal controls; †P<0.05 versus ADR alone (n=5–6). (D) Representative micrographs show renal expression and localization of RANTES and F4/80 antigen in different groups. Paraffin-embedded kidney sections were stained with RANTES and F4/80 antibodies. Arrows indicate positive staining. Scale bar, 50 µm. (E) Graphic presentation of F4/80+ macrophages and CD3+ T cells in the kidney sections. Data are presented as the numbers of positive cells per high-power field (hpf). *P<0.05 versus normal controls; †P<0.05 versus ADR alone (n=5–6). (F) Western blot analyses show the active phosphorylated p65 (p-p65) and total p65 expressions in vivo. (G and H) Graphic presentations show the (G) p-p65 and (H) p65 protein abundances in different groups. Relative protein levels over the controls (fold induction) are reported. Ctrl, control; Veh, vehicle. *P<0.05 versus normal controls; †P<0.05 versus ADR alone (n=5–6).
We also investigated the expression and activation of p65 NF-κB, a key transcription factor involved in regulating inflammation. As shown in Figure 8, F–H, both phosphorylated active p65 and total p65 were significantly induced in the kidneys at 5 weeks after ADR injection. ICG-001 treatment abolished p65 induction and its activation (Figure 8, F–H). Therefore, consistent with the predominant role of RAS activation in promoting renal inflammation,8 inhibition of the Wnt/β-catenin/RAS axis by ICG-001 is able to repress NF-κB signaling and reduce renal inflammation in ADR nephropathy in vivo.
Discussion
In the clinical setting, patients with CKD are often diagnosed well before they reach end stage kidney failure; however, no currently available treatment is effective in reversing or halting the progressive loss of kidney function.11,33 This dilemma underscores the need for identifying novel targets for therapeutic intervention. Recently, increasing evidence suggests that Wnt/β-catenin plays a crucial role in the pathogenesis and progression of CKD.21–23,34 As a key transcription regulator, β-catenin integrates major fibrogenic signal pathways and controls the transcription of numerous profibrotic genes.31,35,36 In this study, we have uncovered that all components of the entire RAS, including AGT, renin, ACE, AT1, and AT2, are novel downstream targets of Wnt/β-catenin. Consistently, blockade of Wnt/β-catenin signaling by a small-molecule inhibitor ICG-001 effectively represses RAS activation both in vitro and in vivo and reverses established proteinuria and kidney injury in ADR nephropathy. These results indicate that blockade of pathogenic Wnt/β-catenin could simultaneously target multiple genes of RAS, thereby leading to the reversal of an established CKD.
Given the importance of RAS activation in the pathogenesis of kidney and cardiovascular diseases, the mainstay of current therapy for CKD patients is anti-RAS through either ACEIs or ARBs.1,37–39 The drawback of this therapy is, at least partially, the compensatory upregulation of renin expression.14,15 Renin, as a highly selective aspartic protease, converts AGT to angiotensin I, the first and rate-limiting step in RAS activation. However, renin and its precursor, prorenin, can bind to a specific plasma membrane receptor, (pro)renin receptor (PRR), and transduce unique intracellular signals.40–42 It has been shown that (pro)renin binding to PRR provokes a rapid activation of mitogen-activated protein kinase and promotes matrix gene expression in an angiotensin II–independent fashion.41,43,44 Furthermore, PRR is also an integral component of the Wnt receptor complex, and it augments Wnt signaling in a renin-independent manner.45,46 Therefore, it is conceivable that some of the pathogenic effects of RAS components are independent of angiotensin II, which could account for the limited efficacy of current therapy through ACEIs and ARBs. In view of the fact that multiple RAS genes are upregulated in diseased kidneys (Figure 1), it is critical and essential to develop a strategy to simultaneously repress their expression.
The most novel finding in this study is that multiple RAS genes are direct downstream targets of Wnt/β-catenin signaling. This conclusion is authenticated by several lines of evidence. First, all RAS genes harbor consensus sequences of the TBSs in their promoter regions, and β-catenin promotes LEF-1 binding to these sites (Figure 1). Second, either β-catenin or different Wnt ligands induces the expression of multiple RAS genes in tubular epithelial cells (Figures 1 and 2) and podocytes (Figure 5). Third, inhibition of Wnt/β-catenin signaling by ICG-001 abolishes RAS induction in vitro and in vivo and reverses proteinuria and kidney injury (Figures 2–4). The fact that Wnt/β-catenin targets multiple RAS genes suggests that the intrarenal regulation of RAS genes is controlled by a shared common mechanism. Of note, this kind of RAS regulation is most likely to be exclusively operated under pathologic conditions, in which Wnt/β-catenin is activated after kidney injury. The finding that Wnt/β-catenin controls the expression of multiple RAS genes provides novel and mechanistic insights into how Wnt/β-catenin contributes to the development of CKD. These studies also underscore that, unlike current anti-RAS therapy through ACEIs or ARBs, targeting Wnt/β-catenin alone, in theory, could simultaneously repress multiple components of RAS, including AGT, renin, ACE, and AT1 (Figure 4). In that regard, this “one stone killing multiple birds” strategy by inhibiting Wnt/β-catenin is at least equivalent to (if not better than) combinational therapies with ACEI and ARB plus renin inhibitor.47
The therapeutic efficacy of blockade of Wnt/β-catenin by ICG-001 is quite impressive, which results in a virtually complete inhibition of RAS (such as AGT, renin, ACE, and AT1) and reversal of an established proteinuria and kidney injury in mice (Figures 3 and 4). ICG-001 not only restored podocyte integrity (Figure 5) and inhibited renal fibrosis (Figure 7) but also suppressed NF-κB signaling (Figure 8). This finding is consistent with the pivotal role of RAS activation in promoting renal inflammation. Although there is a putative TBS in the p65 NF-κB promoter as well, overexpression of β-catenin did not induce p65 expression in HKC-8 cells (data not shown). Therefore, the downregulation of p65 protein after ICG-001 treatment (Figure 8) could be the consequence of a blunted RAS activation and reduced kidney injury rather than the direct inhibition of a possible β-catenin–mediated p65 expression.
Unlike the vast majority of the studies reported in the literature, in which therapy typically commences before or concurrently with injury,38,48 this study used both therapeutic and withdrawal protocols (Figure 3A). In particular, the withdrawal protocol imitates a scenario in a clinical situation in which patients are diagnosed with CKD and treated with therapeutic agent for a short duration, and then, the treatment is stopped and no longer needed. Of interest, transient treatment with a single agent (ICG-001) for 2 weeks is sufficient for almost complete reversal of established proteinuria and kidney lesions, underscoring the remarkable therapeutic efficacy of blocking Wnt/β-catenin. It should be stressed that proteinuria and kidney injury occur very early in this model, and at the time of ICG-001 administration (1 week after ADR injection), robust proteinuria and glomerular damage are already established, which was previously reported.20,30 Therefore, the action of ICG-001 is capable of reversing established kidney disease but not through preventing the initial injury. Consistent with this notion, delayed administration of ICG-001 at 3 weeks after ADR injection (Figure 3A), a time point when renal glomerular and interstitial lesions are prominent, is also effective in ameliorating proteinuria and kidney fibrotic lesions.
The results in this study, for the first time, show that β-catenin acts as a master regulator that directly dictates the expression of all genes in the entire RAS. In this context, Wnt/β-catenin and RAS are intimately linked to constitute a pathologic axis that plays a crucial role in the pathogenesis of CKD. Of note, β-catenin is positioned at the crossroad of multiple signal pathways in CKD, because numerous pathogenic cues can lead to its activation. Apart from Wnt, β-catenin can also be activated by integrin-linked kinase and TGF-β1 as well as Klotho deprivation.30,48–50 Taken together, it is clear that multiple pathogenic signaling converges on β-catenin/RAS, making it an unparalleled target for therapeutic intervention. Whether inhibition of β-catenin by ICG-001 also affects BP and systematic RAS warrants additional investigation, perhaps by using different animal models including chronic angiotensin II infusion.
In summary, we have shown that multiple RAS genes are novel downstream targets of Wnt/β-catenin signaling. Therefore, inhibition of Wnt/β-catenin is able to simultaneously repress all RAS genes and reverse an established proteinuria and progressive CKD. This study provides a novel and mechanistic linkage between Wnt/β-catenin signaling and RAS activation in the pathogenesis of CKD. Although more studies are needed, these results offer a proof of principle that targeted inhibition of pathogenic Wnt/β-catenin would completely eradicate intrarenal RAS activation and could represent an effective strategy for the treatment of CKD patients in the clinical settings.
Concise Methods
Animal Models
A mouse model of podocyte injury and proteinuria was established by intravenous injection of ADR as described previously.20 Male BALB/c mice weighing 20–22 g were obtained from Harlan Sprague–Dawley (Indianapolis, IN). The detailed experimental design is presented in Figure 3A. Four groups of mice were used: (1) normal control (n=5), (2) ADR mice injected with vehicle (n=6), (3) ADR mice treated with ICG-001 using ICG-w (n=6), and (4) ADR mice treated with ICG-001 using ICG-t (n=6). ADR (doxorubicin hydrochloride; Sigma-Aldrich, St. Louis, MO) was administered by a single intravenous injection at 10 mg/kg body wt. ICG-001–phosphate, which competes with β-catenin for binding to CBP and prevents β-catenin/CBP complex formation,28,51 was synthesized at the University of Southern California as previously described,28 dissolved in PBS, and administered by daily intraperitoneal injection at 5 mg/kg body wt. At 5 weeks after ADR injection, all mice were euthanized. Urine and kidney tissues were collected for various analyses. All animal studies were performed by use of the procedures approved by the Institutional Animal Care and Use Committee at the University of Pittsburgh.
Urinary Albumin and Creatinine Assay
Urine albumin was measured by using a mouse Albumin ELISA Quantitation Kit according to the manufacturer’s protocol (Bethyl Laboratories, Inc., Montgomery, TX). Urine creatinine was determined by a routine procedure as described previously.31 Urinary albumin was standardized to urine creatinine and expressed as milligrams per milligram urine creatinine.
Histology and Immunohistochemical Staining
Paraffin-embedded mouse kidney sections (3-µm thickness) were prepared by a routine procedure.20,52 Sections were stained with hematoxylin-eosin and periodic acid–Schiff reagent by standard protocol. Kidney sections were also subjected to Masson's Trichrome staining for assessing collagen deposition and fibrotic lesions. Quantification of the fibrotic area was carried out by a computer-aided, point-counting technique as described previously.26 Immunohistochemical staining was performed using routine protocol.20 Antibodies used were mouse anti–α-SMA (A2547; Sigma-Aldrich), rabbit polyclonal anti–FSP-1 (S100A4) (A5114; DAKO, Carpinteria, CA), rabbit polyclonal anti–β-catenin (ab15180; Abcam, Inc., Cambridge, MA), mouse monoclonal anti-RANTES (10R-R121A; Fitzgerald Industries International, Concord, MA), affinity-purified anti-mouse F4/80 antigen (14–4801; eBioscience, San Diego, CA), mouse monoclonal anti-CD3 (sc-20047; Santa Cruz Biotechnology, Santa Cruz, CA), rabbit monoclonal anti-ACE (ab75762; Abcam, Inc.), goat polyclonal anti-renin (sc-27320; Santa Cruz Biotechnology), goat polyclonal anti-AGT (sc-7419; Santa Cruz Biotechnology), and rabbit polyclonal anti-AT1 receptor (ab15552; EMD Millipore, Billerica, MA).
Immunofluorescence Staining and Confocal Microscopy
Kidney cryosections or cells cultured on coverslips were fixed with 3.7% paraformaldehyde for 15 minutes at room temperature. After blocking with 10% donkey serum for 30 minutes, the slides were immunostained with primary antibodies against AGT (Santa Cruz Biotechnology), ACE (Abcam, Inc.), renin (Santa Cruz Biotechnology), β-catenin (610154; BD Transduction Laboratories, San Jose, CA), AT1 (sc-1173; Santa Cruz Biotechnology), WT1 (sc-192; Santa Cruz Biotechnology), nephrin (20R-NP002; Fitzgerald Industries International), and fibronectin (F3648; Sigma-Aldrich). Slides were viewed under a Nikon Eclipse E600 microscope or Leica TCS-SL confocal microscope equipped with a digital camera.
Western Blot Analyses
Protein expression was analyzed by Western blot analysis as described previously.26 The primary antibodies used were anti-AGT (Santa Cruz Biotechnology), anti-renin (Santa Cruz Biotechnology), anti-ACE (Abcam, Inc.), anti-AT1 receptor (EMD Millipore), anti-FLAG (F4042; Sigma-Aldrich), anti-nephrin (Fitzgerald Industries International), anti-podocin (sc-22298; Santa Cruz Biotechnology), anti-WT1 (Santa Cruz Biotechnology), anti-desmin (D1033; Sigma-Aldrich), anti-fibronectin (Sigma-Aldrich), rabbit polyclonal anti–collagen I (234167; EMD Millipore), anti–collagen III (234189; EMD Millipore), anti–α-SMA (Sigma-Aldrich), anti-Snail1 (ab17732; Abcam, Inc.), anti–PAI-1 (sc-5297; Santa Cruz Biotechnology), anti–MMP-7 (3801; Cell Signaling Technologies), anti–MMP-9 (M5177; Sigma-Aldrich), anti–β-catenin (610154; BD Transduction Laboratories), anti-phospho–NF-κB p65 (Ser536) (3036; Cell Signaling Technology), anti-p65 (3034; Cell Signaling Technologies), anti-RANTES (Fitzgerald Industries International), and anti-actin (MAB1501; EMD Millipore).
Real-Time RT-PCR
Total RNA isolation and quantitative RT-PCR or real-time RT-PCR were carried out by the procedures described previously.52 Briefly, first-strand cDNA synthesis was carried out by using a Reverse Transcription System Kit according to the instructions of the manufacturer (Promega, Madison, WI). Real-time RT-PCR was performed on an ABI StepOnePlus Real-Time PCR System (Applied Biosystems, Foster City, CA) as described previously.48 The PCR reaction mixture in a 25-µl volume contained 12.5 µl 2× SYBR Green PCR Master Mix (Applied Biosystems), 5 µl diluted reverse-transcription product (1:10), and 0.5 µM sense and antisense primer sets. The sequences of the primer pairs used in RT-PCR or quantitative RT-PCR were given in Supplemental Table 1. PCR reaction was run by using standard conditions. Real-time PCR was performed using a Plantinum SYBR Green qPCR SuperMix-UDG Kit (Invitrogen). After sequential incubations at 50°C for 2 minutes and 95°C for 10 minutes, respectively, the amplification protocol consisted of 50 cycles of denaturing at 95°C for 15 seconds, annealing, and extension at 60°C for 60 seconds. The standard curve was made from series dilutions of template cDNA. The mRNA levels of various genes were calculated after normalizing with β-actin.
Cell Culture and Treatment
HKC-8 were provided by Lorraine Racusen (Johns Hopkins University, Baltimore, MD). Mouse podocytes were provided by Peter Mundel (Massachusetts General Hospital, Boston, MA). Cell culture was carried out according to the procedures described previously26 and transfected with different Wnts-expressing vectors or N-terminally truncated, FLAG-tagged, constitutively activated β-catenin expression vector plasmid by Lipofectamine 2000 (Invitrogen) according to the manufacturer’s protocol. In some experiments, cells were incubated with ICG-001 at 10 μM. Total RNA was extracted for RT-PCR analyses. In addition, whole-cell lysates were prepared and subjected to Western blot analyses.
ChIP Assay
A ChIP assay was performed to analyze the interactions of TCF/LEF-1 and putative TBS in the promoters of human RAS genes. This assay was carried out essentially according to the protocols specified by the manufacturer (Upstate Biotechnology). Briefly, after various treatments as indicated, HKC-8 cells were cross-linked with 1% formaldehyde and then resuspended in SDS lysis buffer containing protease inhibitors. The chromatin solution was sonicated, and the supernatant was diluted 10-fold. An aliquot of total diluted lysate was used for total genomic DNA as input DNA control. The anti–LEF-1 (17–604; EMD Millipore) was added and incubated at 4°C overnight followed by incubation with protein A-agarose for 1 hour. The precipitates were washed, and chromatin complexes were eluted. After reversal of the cross-linking at 65°C for 4 hours, the DNA was purified, and ChIP samples were used as a template for PCR. Primer sets encompass regions of different RAS promoters containing putative TBS. The sequences of primers used for ChIP assay are given in Supplemental Table 1. PCR samples were analyzed by electrophoresis on a 2.0% agarose gel.
Statistical Analyses
All data examined were expressed as means±SEMs. Statistical analyses of the data were carried out using SigmaStat software (Jandel Scientific, San Rafael, CA). Comparison between groups was made using one-way ANOVA followed by a Newman–Kuels test. P<0.05 was considered significant.
Disclosures
None.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grants DK064005 and DK091239, 973 Program Grant 2012CB517700, and National Science Foundation of China Grants 81130011 and 81370839. L.Z. was supported by National Science Foundation of China Grant 81370014. R.J.T. was supported by American Heart Association Fellow-to-Faculty Transition Grant 13FTF16990086.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Antagonism of Canonical Wnt/β-Catenin Signaling: Taking RAS Blockade to the Next Level?,” on pages 3–5.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2014010085/-/DCSupplemental.
References
- 1.Ruggenenti P, Cravedi P, Remuzzi G: Mechanisms and treatment of CKD. J Am Soc Nephrol 23: 1917–1928, 2012 [DOI] [PubMed] [Google Scholar]
- 2.Santos PC, Krieger JE, Pereira AC: Renin-angiotensin system, hypertension, and chronic kidney disease: Pharmacogenetic implications. J Pharmacol Sci 120: 77–88, 2012 [DOI] [PubMed] [Google Scholar]
- 3.Crowley SD, Coffman TM: Recent advances involving the renin-angiotensin system. Exp Cell Res 318: 1049–1056, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cao W, Zhou QG, Nie J, Wang GB, Liu Y, Zhou ZM, Hou FF: Albumin overload activates intrarenal renin-angiotensin system through protein kinase C and NADPH oxidase-dependent pathway. J Hypertens 29: 1411–1421, 2011 [DOI] [PubMed] [Google Scholar]
- 5.Freundlich M, Quiroz Y, Zhang Z, Zhang Y, Bravo Y, Weisinger JR, Li YC, Rodriguez-Iturbe B: Suppression of renin-angiotensin gene expression in the kidney by paricalcitol. Kidney Int 74: 1394–1402, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Crowley SD, Gurley SB, Herrera MJ, Ruiz P, Griffiths R, Kumar AP, Kim HS, Smithies O, Le TH, Coffman TM: Angiotensin II causes hypertension and cardiac hypertrophy through its receptors in the kidney. Proc Natl Acad Sci U S A 103: 17985–17990, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gurley SB, Riquier-Brison AD, Schnermann J, Sparks MA, Allen AM, Haase VH, Snouwaert JN, Le TH, McDonough AA, Koller BH, Coffman TM: AT1A angiotensin receptors in the renal proximal tubule regulate blood pressure. Cell Metab 13: 469–475, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen S, Ge Y, Si J, Rifai A, Dworkin LD, Gong R: Candesartan suppresses chronic renal inflammation by a novel antioxidant action independent of AT1R blockade. Kidney Int 74: 1128–1138, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Reiser J, Mundel P: Dual effects of RAS blockade on blood pressure and podocyte function. Curr Hypertens Rep 9: 403–408, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Frimodt-Møller M, Høj Nielsen A, Strandgaard S, Kamper AL: Feasibility of combined treatment with enalapril and candesartan in advanced chronic kidney disease. Nephrol Dial Transplant 25: 842–847, 2010 [DOI] [PubMed] [Google Scholar]
- 11.Hou FF, Zhang X, Zhang GH, Xie D, Chen PY, Zhang WR, Jiang JP, Liang M, Wang GB, Liu ZR, Geng RW: Efficacy and safety of benazepril for advanced chronic renal insufficiency. N Engl J Med 354: 131–140, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Nakamura T, Fujiwara N, Kawagoe Y, Sugaya T, Ueda Y, Koide H: Effects of telmisartan and enalapril on renoprotection in patients with mild to moderate chronic kidney disease. Eur J Clin Invest 40: 790–796, 2010 [DOI] [PubMed] [Google Scholar]
- 13.Müller DN, Luft FC: Direct renin inhibition with aliskiren in hypertension and target organ damage. Clin J Am Soc Nephrol 1: 221–228, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Tan X, He W, Liu Y: Combination therapy with paricalcitol and trandolapril reduces renal fibrosis in obstructive nephropathy. Kidney Int 76: 1248–1257, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Z, Zhang Y, Ning G, Deb DK, Kong J, Li YC: Combination therapy with AT1 blocker and vitamin D analog markedly ameliorates diabetic nephropathy: Blockade of compensatory renin increase. Proc Natl Acad Sci U S A 105: 15896–15901, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clevers H, Nusse R: Wnt/β-catenin signaling and disease. Cell 149: 1192–1205, 2012 [DOI] [PubMed] [Google Scholar]
- 17.Angers S, Moon RT: Proximal events in Wnt signal transduction. Nat Rev Mol Cell Biol 10: 468–477, 2009 [DOI] [PubMed] [Google Scholar]
- 18.Schmidt-Ott KM, Barasch J: WNT/beta-catenin signaling in nephron progenitors and their epithelial progeny. Kidney Int 74: 1004–1008, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.White BD, Nguyen NK, Moon RT: Wnt signaling: It gets more humorous with age. Curr Biol 17: R923–R925, 2007 [DOI] [PubMed] [Google Scholar]
- 20.He W, Kang YS, Dai C, Liu Y: Blockade of Wnt/β-catenin signaling by paricalcitol ameliorates proteinuria and kidney injury. J Am Soc Nephrol 22: 90–103, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.He W, Dai C, Li Y, Zeng G, Monga SP, Liu Y: Wnt/β-catenin signaling promotes renal interstitial fibrosis. J Am Soc Nephrol 20: 765–776, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.von Toerne C, Schmidt C, Adams J, Kiss E, Bedke J, Porubsky S, Gretz N, Lindenmeyer MT, Cohen CD, Gröne HJ, Nelson PJ: Wnt pathway regulation in chronic renal allograft damage. Am J Transplant 9: 2223–2239, 2009 [DOI] [PubMed] [Google Scholar]
- 23.Zhou T, He X, Cheng R, Zhang B, Zhang RR, Chen Y, Takahashi Y, Murray AR, Lee K, Gao G, Ma JX: Implication of dysregulation of the canonical wingless-type MMTV integration site (WNT) pathway in diabetic nephropathy. Diabetologia 55: 255–266, 2012 [DOI] [PubMed] [Google Scholar]
- 24.Heikkilä E, Juhila J, Lassila M, Messing M, Perälä N, Lehtonen E, Lehtonen S, Sjef Verbeek J, Holthofer H: β-Catenin mediates adriamycin-induced albuminuria and podocyte injury in adult mouse kidneys. Nephrol Dial Transplant 25: 2437–2446, 2010 [DOI] [PubMed] [Google Scholar]
- 25.Surendran K, Schiavi S, Hruska KA: Wnt-dependent beta-catenin signaling is activated after unilateral ureteral obstruction, and recombinant secreted frizzled-related protein 4 alters the progression of renal fibrosis. J Am Soc Nephrol 16: 2373–2384, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Hao S, He W, Li Y, Ding H, Hou Y, Nie J, Hou FF, Kahn M, Liu Y: Targeted inhibition of β-catenin/CBP signaling ameliorates renal interstitial fibrosis. J Am Soc Nephrol 22: 1642–1653, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Henderson WR, Jr., Chi EY, Ye X, Nguyen C, Tien YT, Zhou B, Borok Z, Knight DA, Kahn M: Inhibition of Wnt/β-catenin/CREB binding protein (CBP) signaling reverses pulmonary fibrosis. Proc Natl Acad Sci U S A 107: 14309–14314, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Emami KH, Nguyen C, Ma H, Kim DH, Jeong KW, Eguchi M, Moon RT, Teo JL, Kim HY, Moon SH, Ha JR, Kahn M: A small molecule inhibitor of β-catenin/CREB-binding protein transcription [corrected]. Proc Natl Acad Sci U S A 101: 12682–12687, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pippin JW, Brinkkoetter PT, Cormack-Aboud FC, Durvasula RV, Hauser PV, Kowalewska J, Krofft RD, Logar CM, Marshall CB, Ohse T, Shankland SJ: Inducible rodent models of acquired podocyte diseases. Am J Physiol Renal Physiol 296: F213–F229, 2009 [DOI] [PubMed] [Google Scholar]
- 30.Kang YS, Li Y, Dai C, Kiss LP, Wu C, Liu Y: Inhibition of integrin-linked kinase blocks podocyte epithelial-mesenchymal transition and ameliorates proteinuria. Kidney Int 78: 363–373, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dai C, Stolz DB, Kiss LP, Monga SP, Holzman LB, Liu Y: Wnt/β-catenin signaling promotes podocyte dysfunction and albuminuria. J Am Soc Nephrol 20: 1997–2008, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stein U, Arlt F, Walther W, Smith J, Waldman T, Harris ED, Mertins SD, Heizmann CW, Allard D, Birchmeier W, Schlag PM, Shoemaker RH: The metastasis-associated gene S100A4 is a novel target of beta-catenin/T-cell factor signaling in colon cancer. Gastroenterology 131: 1486–1500, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Vilayur E, Harris DC: Emerging therapies for chronic kidney disease: What is their role? Nat Rev Nephrol 5: 375–383, 2009 [DOI] [PubMed] [Google Scholar]
- 34.Nelson PJ, von Toerne C, Gröne HJ: Wnt-signaling pathways in progressive renal fibrosis. Expert Opin Ther Targets 15: 1073–1083, 2011 [DOI] [PubMed] [Google Scholar]
- 35.He W, Tan R, Dai C, Li Y, Wang D, Hao S, Kahn M, Liu Y: Plasminogen activator inhibitor-1 is a transcriptional target of the canonical pathway of Wnt/β-catenin signaling. J Biol Chem 285: 24665–24675, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.He W, Tan RJ, Li Y, Wang D, Nie J, Hou FF, Liu Y: Matrix metalloproteinase-7 as a surrogate marker predicts renal Wnt/β-catenin activity in CKD. J Am Soc Nephrol 23: 294–304, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zeisberg M, Neilson EG: Mechanisms of tubulointerstitial fibrosis. J Am Soc Nephrol 21: 1819–1834, 2010 [DOI] [PubMed] [Google Scholar]
- 38.Boor P, Ostendorf T, Floege J: Renal fibrosis: Novel insights into mechanisms and therapeutic targets. Nat Rev Nephrol 6: 643–656, 2010 [DOI] [PubMed] [Google Scholar]
- 39.Liu Y: Cellular and molecular mechanisms of renal fibrosis. Nat Rev Nephrol 7: 684–696, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gonzalez AA, Luffman C, Bourgeois CR, Vio CP, Prieto MC: Angiotensin II-independent upregulation of cyclooxygenase-2 by activation of the (Pro)renin receptor in rat renal inner medullary cells. Hypertension 61: 443–449, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nguyen G, Muller DN: The biology of the (pro)renin receptor. J Am Soc Nephrol 21: 18–23, 2010 [DOI] [PubMed] [Google Scholar]
- 42.Nguyen G: Renin and prorenin receptor in hypertension: What’s new? Curr Hypertens Rep 13: 79–85, 2011 [DOI] [PubMed] [Google Scholar]
- 43.Feldt S, Batenburg WW, Mazak I, Maschke U, Wellner M, Kvakan H, Dechend R, Fiebeler A, Burckle C, Contrepas A, Jan Danser AH, Bader M, Nguyen G, Luft FC, Muller DN: Prorenin and renin-induced extracellular signal-regulated kinase 1/2 activation in monocytes is not blocked by aliskiren or the handle-region peptide. Hypertension 51: 682–688, 2008 [DOI] [PubMed] [Google Scholar]
- 44.Huang Y, Border WA, Noble NA: Functional renin receptors in renal mesangial cells. Curr Hypertens Rep 9: 133–139, 2007 [DOI] [PubMed] [Google Scholar]
- 45.Hermle T, Saltukoglu D, Grünewald J, Walz G, Simons M: Regulation of Frizzled-dependent planar polarity signaling by a V-ATPase subunit. Curr Biol 20: 1269–1276, 2010 [DOI] [PubMed] [Google Scholar]
- 46.Cruciat CM, Ohkawara B, Acebron SP, Karaulanov E, Reinhard C, Ingelfinger D, Boutros M, Niehrs C: Requirement of prorenin receptor and vacuolar H+-ATPase-mediated acidification for Wnt signaling. Science 327: 459–463, 2010 [DOI] [PubMed] [Google Scholar]
- 47.Locatelli F, Del Vecchio L, Cavalli A: Inhibition of the renin-angiotensin system in chronic kidney disease: A critical look to single and dual blockade. Nephron Clin Pract 113: c286–c293, 2009 [DOI] [PubMed] [Google Scholar]
- 48.Li Y, Tan X, Dai C, Stolz DB, Wang D, Liu Y: Inhibition of integrin-linked kinase attenuates renal interstitial fibrosis. J Am Soc Nephrol 20: 1907–1918, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang D, Dai C, Li Y, Liu Y: Canonical Wnt/β-catenin signaling mediates transforming growth factor-β1-driven podocyte injury and proteinuria. Kidney Int 80: 1159–1169, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhou L, Li Y, Zhou D, Tan RJ, Liu Y: Loss of Klotho contributes to kidney injury by derepression of Wnt/β-catenin signaling. J Am Soc Nephrol 24: 771–785, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Eguchi M, Nguyen C, Lee SC, Kahn M: ICG-001, a novel small molecule regulator of TCF/β-catenin transcription. Med Chem 1: 467–472, 2005 [DOI] [PubMed] [Google Scholar]
- 52.Ding H, Zhou D, Hao S, Zhou L, He W, Nie J, Hou FF, Liu Y: Sonic hedgehog signaling mediates epithelial-mesenchymal communication and promotes renal fibrosis. J Am Soc Nephrol 23: 801–813, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.