Abstract
Nanoparticles (NPs) are used commercially in health and fitness fields, but information about the toxicity and mechanisms underlying the toxic effects of NPs is still very limited. The aim of this study is to investigate the toxic effect(s) of 100 nm negatively (ZnOAE100[−]) or positively (ZnOAE100[+]) charged zinc oxide (ZnO) NPs administered by gavage in Sprague Dawley rats, to establish a no observed adverse effect level, and to identify target organ(s). After verification of the primary particle size, morphology, hydrodynamic size, and zeta potential of each test article, we performed a 90-day study according to Organisation for Economic Co-operation and Development test guideline 408. For the 90-day study, the high dose was set at 500 mg/kg and the middle and low doses were set at 125 mg/kg and 31.25 mg/kg, respectively. Both ZnO NPs had significant changes in hematological and blood biochemical analysis, which could correlate with anemia-related parameters, in the 500 mg/kg groups of both sexes. Histopathological examination showed significant adverse effects (by both test articles) in the stomach, pancreas, eye, and prostate gland tissues, but the particle charge did not affect the tendency or the degree of the lesions. We speculate that this inflammatory damage might result from continuous irritation caused by both test articles. Therefore, the target organs for both ZnOAE100(−) and ZnOAE100(+) are considered to be the stomach, pancreas, eye, and prostate gland. Also, the no observed adverse effect level for both test articles was identified as 31.25 mg/kg for both sexes, because the adverse effects were observed at all doses greater than 125 mg/kg.
Keywords: zinc oxide nanoparticles, surface charge, 90-day oral dose toxicity, no observed adverse effect level
Introduction
Nanoparticles (NPs) are widely used in health and fitness fields such as cosmetics, clothing, personal care, sporting goods, and sunscreen products. Moreover, NPs are expected to be applied in the fields of diagnosis, imaging, and drug delivery. One of the most commonly used types of NPs is zinc oxide (ZnO) NPs.1 As ZnO NPs absorb ultraviolet light, they have been used in sunscreen products.2,3 In addition, ZnO NPs have been explored as photoconductive materials in electronics, including cellular phones and iPods.4,5 However, nanomaterials are associated with problems, including toxicity and their environmental impact. Furthermore, limited information is available about the toxicity and mechanisms underlying the toxic effects of NPs.
Because ZnO NPs are the most commonly utilized nanomaterials in various consumer products, many studies have shown the toxic effects of ZnO NPs in several experimental models, including cell lines, bacteria, nematodes, algae, plants, and fish.6,7 In particular, in vivo study is considered necessary to investigate the toxic effect of NPs in biological systems, which would stress the importance of local toxicity from the administration of NPs. Before evaluating the potential toxicity of NPs, it is important to understand how living organisms are exposed to them. Exposure can occur through the lung (inhalation), skin (dermal absorption), or digestive system (oral ingestion), as shown by a number of in vivo studies on the nanotoxicity of ZnO NPs.8,9 For example, after oral administration of 30 nm ZnO NPs for 14 days to mice, ZnO NPs significantly accumulated in the liver and caused oxidative stress mediated by DNA damage and apoptosis.10 Similarly, ZnO NPs caused impairment of mitochondria and cell membranes in rat kidneys after oral administration of ZnO NPs for 14 days.11 Repeated application through dermal routes for 28 days decreases the collagen level at the site of application, which may be induced by oxidative stress.12 These results suggest that nanotoxicity of ZnO NPs may be mediated by induction of oxidative stress similar to their in vitro toxic mechanisms. However, as these observations regarding nanotoxicity from short-term exposure studies are still limited, long-term exposure studies are required to determine the potential chronic toxicity of ZnO NPs. In spite of the importance of repeated toxicity studies, only a few in vivo studies have been performed to examine the toxicity of ZnO NPs through oral administration for 90 days.
It is well known that the toxicity of NPs may depend on their physicochemical properties, such as particle size, particle shape, surface area, and surface charge. For example, Pasupuleti et al13 reported differences in nanotoxicity between nanosized ZnO and microsized ZnO particles after 14-day oral administration to Sprague Dawley (SD) rats. They found that incidences of lesions in the liver, pancreas, heart, and stomach were higher in rats treated with low doses of NPs than in those treated with high doses; however, high doses of the microsized NPs caused more lesions than the low doses. In addition, Ho et al14 found that mass and surface area were effective metrics responsible for the toxicity of ZnO NPs through inhalation exposure. These results suggest that particle size and dose metrics are key concepts of nanotoxicology and need to be considered while evaluating the toxicity of manufactured NPs. Another critical factor for nanotoxicity is surface charge, especially ligands, which modify their surface and are considered to affect cellular responses to NPs. Some in vitro toxicity studies reported that positively charged NPs had higher cellular uptake and cytotoxicity.15,16 Recently, Yin et al17 reported that coated ZnO NPs, which produce differences in zeta potential, showed significant genotoxicity compared with uncoated ZnO NPs in in vitro systems. However, information regarding the charge effect of NPs on in vivo toxicity is very limited because of the complexity and dissolution property of in vivo systems.
In the present study, we performed a 90-day toxicity study of 100 nm ZnO NPs with different surface charges (negatively charged, ZnOAE100[−], and positively charged, ZnOAE100[+]) administered by gavage to SD rats to determine their no observed adverse effect level (NOAEL) and to identify target organs.
Materials and methods
Preparation of NPs
We purchased 100 nm ZnO NPs from American Elements (Los Angeles, CA, USA, Lot Number 1871511079–673). The surface charge was modified with coating reagents, citrate (for [−] charge) and L-serine (for [+] charge). For preparation of ZnOAE100(−), the sodium citrate (Sigma-Aldrich, St Louis, MO, USA, CAS No 6132–04–3) was dissolved in 20 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) (Sigma-Aldrich, CAS No 7365–45–9) buffer, which was set to pH 7 by using 1 M sodium carbonate (Na2CO3) (Duksan Pure Chemical Co Ltd, Daejeon, Korea, CAS No 497–19–8) solution. For preparation of ZnOAE100(+), the L-serine (Sigma-Aldrich, CAS No 56–45–1) was dissolved in 20 mM HEPES buffer, which was set to pH 6 by using 1 M Na2CO3 solution. The final concentration of sodium citrate and L-serine was 1 w/v% in 20 mM HEPES buffer solution. Before initiating this study, the physicochemical properties (including the primary particle size, morphology, hydrodynamic size, and zeta potential) of the two test articles were verified.18 The dose formulations were prepared once a day throughout the study. The formulated test articles was freshly homogenized by vortexing before administration.
Experimental animals and animal husbandry
Six-week-old Crl:CD(SD) specific pathogen-free rats, which are being used widely in safety evaluations, including repeated-dose toxicity studies and recommended animal species in Organisation for Economic Co-operation and Development test guideline 408,19 were obtained from Orient Bio Inc. (Gyeonggi-do, Korea). We used 25 animals of each sex for the dose range finding (DRF) study and 65 animals of each sex were used for the 90-day repeated-dose oral toxicity study. Animals were housed in stainless wire cages (270 W × 500 D × 200 H mm), two animals per cage, in the animal room, which was maintained at a temperature of 21.0°C–23.1°C, relative humidity of 42.6%–54.5%, with light hours from 8 am to 8 pm (150300 Lux), and ventilation frequency ten to 30 times per hour. Rodent feed and water were sterilized with 2.0 Mrad radiation and ultraviolet radiation, respectively, and both were available ad libitum. All animals were acclimated and quarantined for 8 days in an animal room of the health care institute. This study was performed in compliance with Good Laboratory Practice (GLP) and in accordance with the regulations for the care and use of laboratory animals in the Health Care Research Laboratory in the Korea Testing and Research Institute (Institutional Animal Care and Use Committee, 2010) based on the Animal Protection Act No 10995 (2011, complete revision).20
14-day DRF study
After acclimatization and quarantine, 25 male and female healthy animals selected for this study were randomly distributed into groups of approximately equal initial mean body weights. On the basis of the result of a preliminary acute oral pharmacokinetics absorption study (data not shown), in which no significant effect was observed in the 2,000 mg/kg group, the high dose for the 14-day study was set to 2,000 mg/kg body weight, and the middle and low doses were 1,000 mg/kg and 500 mg/kg body weight, respectively. Distilled water and a vehicle solution were administered to the negative and vehicle control groups, respectively. Vehicle control and all ZnO NP treatment groups consisted of five rats of each sex, and the negative control group consisted of two rats of each sex. Body weight ranges at dosing were 183.8–204.9 g for males and 145.8–162.2 g for females. The test articles were administered by oral gavage between 9 am and 12 midday, and the dose volume was 10 mL/kg for all groups. Clinical signs and body weight were observed throughout the 14-day experimental period, and gross findings were observed on the scheduled necropsy day.
90-day repeated oral toxicity study
After acclimatization and quarantine, 65 male and female healthy animals selected for this study were randomly distributed into groups of approximately equal initial mean body weights. Significant effects were observed at 1,000 mg/kg and 2,000 mg/kg body weight in the 14-day repeated-dose study (data not shown), and thus the high dose of this study was set to 500 mg/kg, and the middle and low doses were 125 mg/kg and 31.25 mg/kg body weight, respectively. Distilled water and a vehicle solution were administered to the negative control and vehicle control groups, respectively. Recovery was observed during the 2 weeks after the end of the administration in the 500 mg/kg, vehicle control, and negative control groups. Negative control, vehicle control, and high-dose groups consisted of 15 rats of each sex, and the low and middle dose groups consisted of ten rats of each sex. Body weight ranges at dosing were 181.2–196.1 g for males and 150.9–168.5 g for females. The test article was administered into the stomach by oral gavage. After examining the body weight just before the administration and once a week thereafter, the administration volume was calculated to be 10 mL/kg. The test article was administered 7 days/week once daily for 90 days. Test items included clinical observation, body weight, feed and water consumption, urinalysis, ophthalmological test, necropsy, organ weight, hematological and biochemical analysis, and histopathological observation.
Observation and examination items
Clinical signs
During the test, all animals were observed once daily after treatment for death or clinical signs of toxicity.
Feed and water consumption
Feed and water consumption was recorded daily after the starting date of treatment. Consumption was calculated from the differences between the supplied amounts and the remaining amounts measured the next day.
Urinalysis
Urinalysis was performed in five animals per group of both sexes during the last week of the administration using Bayer Diagnostics Multistix 10 SG strips (Siemens AG, Munich, Germany) and a Clinitek 500 urine analyzer (Siemens AG). Specific gravity; pH; leukocyte count; level of protein, glucose, ketone bodies, uroblilinogen, bilirubin, blood, and nitrite; and color were examined in urinalysis. Microscopic examination of urinary sediments was performed for the following items: urine amount, red blood cell, white blood cell, epithelial cell, cast, and crystal in the fresh urine. Urine was collected from five animals of each sex in the control and high-dose groups by keeping the animals in metabolic cages for 3 hours. The urine volume was measured using the urine collected for 24 hours.
Ophthalmological test
In the negative control and high-dose groups, the eyes were examined before grouping and during the last week of the experiment, and the ocular fundus was observed through a fundus camera (Genesis; Kowa, Tokyo, Japan) after the pupil was dilated using a mydriatic drug (atropine [Ocu-tropine]; Samil Pharm Co, Seoul, Korea).
Necropsy and organ weight
Necropsy examination was performed on moribund and dead animals, which were found immediately. After the blood samples were collected under deep anesthesia with isoflurane, the animal was killed by exsanguination. The external surface, all orifices, and all organs in the cranial cavity and thoracic and abdominal cavities and their contents were examined. Tissues were collected from all animals and preserved for microscopic examination, as described in the section “Histopathological examination”. After necropsy, the absolute and relative (organ-to-body-weight ratio) weights of the major organs were measured in all survivors when they were killed. The major organs included the liver, kidney, spleen, adrenal gland, testis, ovary, brain, pituitary gland, lung, heart, thymus, uterus, prostate, epididymis, and submaxillary gland.
Hematological analysis
The animals were fasted overnight before necropsy. Blood was collected from the abdominal aorta under anesthesia with isoflurane. We collected 3 mL of blood in a CBC (counting blood cells) bottle (ethylenediaminetetraacetic acid 3 K; Becton, Dickinson and Company, Franklin Lakes, NJ, USA) and analyzed it using an autohematoanalyzer (ADVIA120E; Siemens AG). Other blood was transferred into vacutainer tubes (sodium citrate 3.2%; Becton, Dickinson and Company) and centrifuged at 3,000 rpm for 10 minutes. The plasma was separated and used to determine clotting time by using a coagulometer (ACL 7000; Instrumentation Laboratory, Bedford, MA, USA). Hematological parameters were as follows: total leukocyte and differential leukocyte (neutrophil, lymphocyte, monocyte, eosinophil, and basophil) counts, total erythrocyte count, hemoglobin concentration, hematocrit (Ht) level, mean cell volume (MCV), mean cell hemoglobin (MCH), mean cell hemoglobin concentration (MCHC), reticulocyte, platelet, prothrombin time, and activated partial thromboplastin time.
Blood biochemical analysis
Blood was allowed to clot for 1 hour at room temperature and was centrifuged at 3,000 rpm for 10 minutes to collect serum for biochemical analysis using an automatic serum analyzer (HITACHI 7060; Hitachi, Tokyo, Japan) and an electrolyte analyzer (EasyLyte PLUS Na/K/Cl Analyzer; Medica Corp, Bedford, MA, USA). We analyzed the total protein (TP) level, albumin (Alb) level, albumin/globulin ratio, total bilirubin level, alkaline phosphatase level, aspartate aminotransferase level, alanine aminotransferase (ALT) level, creatinine level, blood urea nitrogen level, total cholesterol (T-Cho) level, triglyceride (TG) level, glucose (Glu) level, calcium (Ca) level, inorganic phosphorus (P) level, creatine kinase level, sodium level, potassium level, and chloride (Cl) level.
Histopathological examination
Internal organs from all animals were collected at necropsy and fixed in 10% neutral buffered formalin. The testis and epididymis were fixed in Bouin’s solution, and the eyes were fixed in Davidson’s solution. Histopathological examination was performed only in the tissues from negative control and high-dose groups. The internal organs examined were the liver, kidney, adrenal gland, heart, lung, pituitary gland, spleen, seminal vesicle, testis, ovary, epididymis, prostate gland, uterus, vagina, tongue, trachea, esophagus, thymus, thyroid gland, stomach, small and large intestine, urinary bladder, submandibular gland, eyeball, skin, pancreas, sternum, mammary gland, spinal cord, femur, mesenteric lymph node, and sciatic nerve.
Statistical analysis
Data on the body weights, feed and water consumption, hematological data, blood biochemical data, and organ weights were analyzed for homogeneity of variances using Levene’s test. One-way analysis of variance was performed to evaluate the significance of differences. If the variance was homogeneous and the significance of difference was confirmed, Scheffé’s multiple comparison test was performed as a post hoc test. If the variance was not homogeneous, the data were analyzed using Dunnett’s T3 test. Analysis of the data from recovery groups was performed using Student’s t-test. All analyses were performed using SPSS (version 19.0) software (IBM Corporation, Armonk, NY, USA).
Results
14-day DRF study
No moribund or dead animals were observed in the 14-day DRF study of ZnOAE100(−), but a dead animal was found in the group treated with ZnOAE100(+). The animals showed white feces, loss of body weight, and corneal opacity regardless of the surface charge of 100 nm ZnO NPs (data not shown). In addition, histopathological examination showed that the lesions of the stomach and spleen were related to the administration of both test articles (data not shown).
Based on these results, under the conditions of this study in which doses of 500 mg/kg, 1,000 mg/kg, and 2,000 mg/kg of ZnOAE100(−) or ZnOAE100(+) were repeatedly administered by gavage for 14 days in SD rats, the effects of test articles were observed in the 500 mg/kg group, and this was considered to represent toxicity as a result of test article administration.
90-day repeated-dose oral toxicity study
Mortality and clinical signs
No deaths were observed in the animals treated with 100 nm ZnO NPs with either surface charge. In addition, salivation and white feces were sporadically observed after administration of both test articles. For example, in the ZnOAE100(−) group, salivation was sporadically observed in the male rats receiving 125 mg/kg on days 35, 63, and 65; in the male rats receiving 500 mg/kg from days 13–63; and in the female rats receiving 500 mg/kg from day 12 to day 87. In addition, in the ZnOAE100(+) group, salivation was observed in the male rats receiving 125 mg/kg on days 19, 48, and 74; in the male rats receiving 500 mg/kg on days 6–80; and in the female rats receiving 500 mg/kg on days 18–47. White feces were observed in the 500 mg/kg groups of both test articles from day 2 until treatment completion in both sexes. Fur loss and scarring were observed in some animals in the male and female groups receiving 500 mg/kg of ZnOAE100(−), male rats receiving 125 mg/kg of ZnOAE100(+), and rats of both sexes receiving 500 mg/kg of ZnOAE100(+).
Body weight
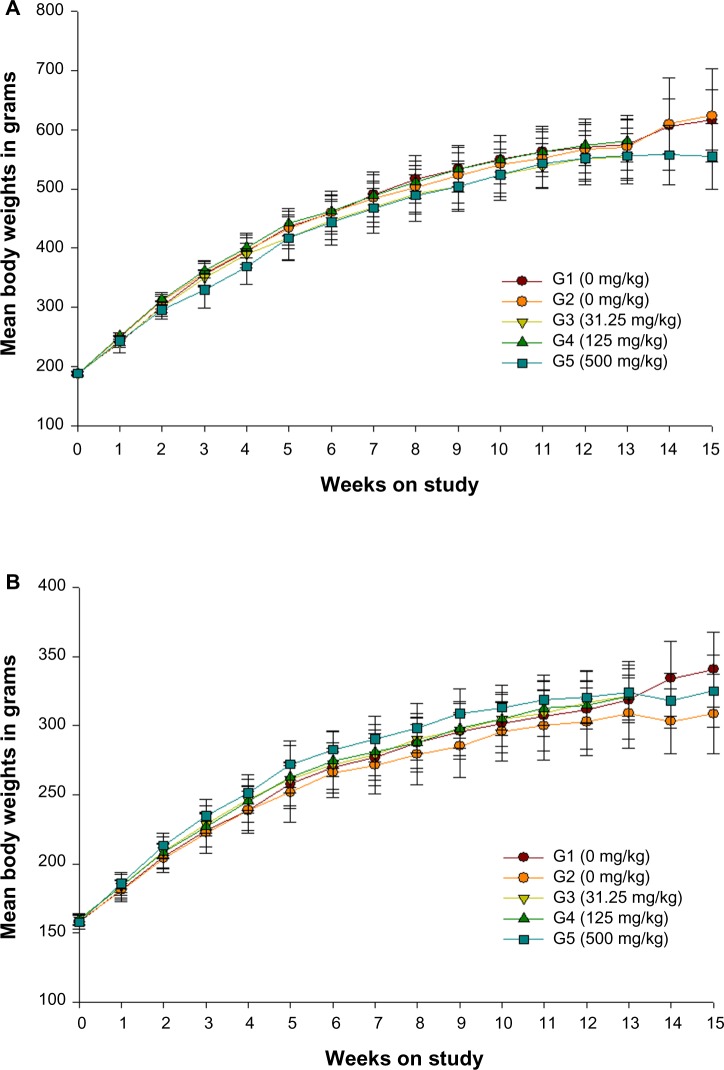
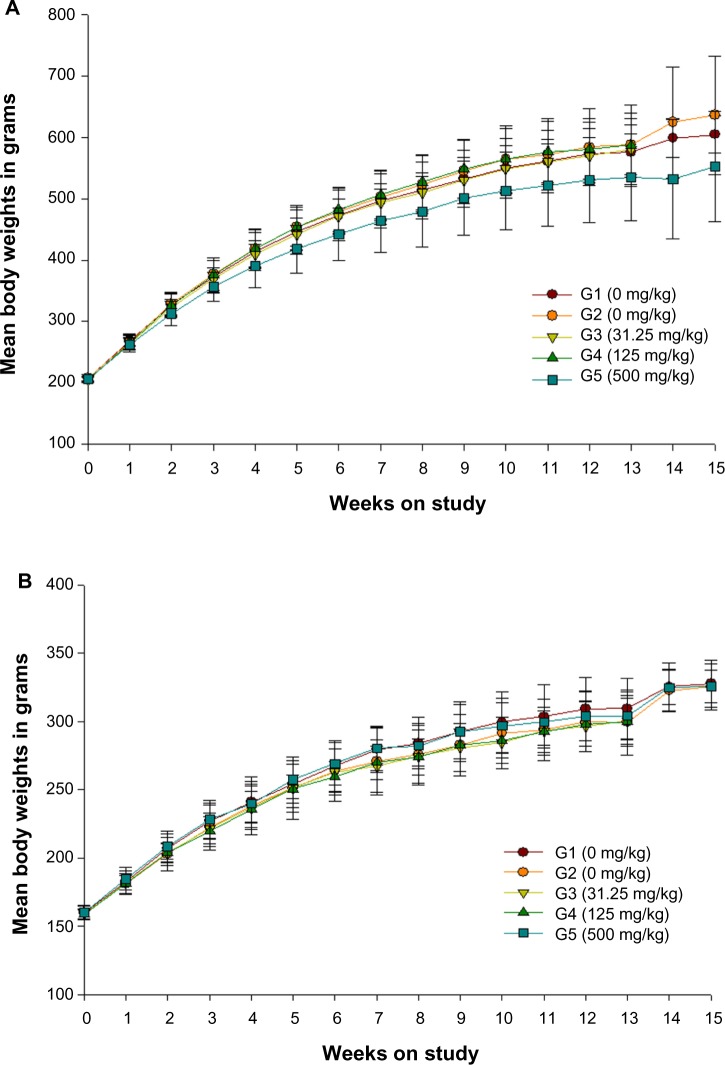
No statistically significant differences were observed between the treated rats and their respective control groups in both sexes (Figures 1 and 2).
Figure 1.
Growth curves for (A) male and (B) female rats administered ZnOAE100(−) by gavage for 90 days.a
Notes: aZnOAE100(−) was orally administered to Sprague Dawley rats at doses of 31.25 mg/kg, 125 mg/kg, and 500 mg/kg for 90 days. The results are presented as mean ± standard deviation.
Abbreviations: G1, negative control; G2, vehicle control, G3, 31.25 mg/kg treatment group; G4, 125 mg/kg treatment group; G5, 500 mg/kg treatment group; ZnO, zinc oxide; ZnOAE100(−), 100 nm negatively charged ZnO.
Figure 2.
Growth curves for (A) male and (B) female rats administered ZnOAE100(+) by gavage for 90 days.a
Notes: aZnOAE100(+) was orally administered to Sprague Dawley rats at doses of 31.25 mg/kg, 125 mg/kg, and 500 mg/kg for 90 days. The results are presented as mean ± standard deviation.
Abbreviations: G1, negative control; G2, vehicle control, G3, 31.25 mg/kg treatment group; G4, 125 mg/kg treatment group; G5, 500 mg/kg treatment group; ZnO, zinc oxide; ZnOAE100(+), 100 nm positively charged ZnO.
Feed consumption
Feed consumption was significantly increased in males receiving 31.25 mg/kg of ZnOAE100(−) at weeks 12 and 13; in females receiving 125 mg/kg of ZnOAE100(−) at weeks 1, 4, 5, 6, 7, 9, 10, 12, and 13; and in females receiving 500 mg/kg of ZnOAE100(−) at weeks 2–13. In females, feed consumption was increased in the 500 mg/kg group at weeks 1–8 and week 10 and at week 1 during the post-treatment recovery period (Table 1). In the study, feed consumption was significantly increased in males receiving 125 mg/kg of ZnOAE100(+) at weeks 5–9, 11, and 12, and those receiving 500 mg/kg of ZnOAE100(+) at weeks 3 and 5–13 and during both weeks of the post-treatment recovery period. In females, feed consumption decreased in the 31.25 mg/kg group at week 7 and increased in the 125 mg/kg group at weeks 7 and 10 and in the 500 mg/kg group at weeks 2, 4, 7–11, and 13 (Table 2).
Table 1.
Feed consumption for rats in the 90-day gavage study of negatively charged 100 nm zinc oxide nanoparticles*
| Negative control | Vehicle control | 31.25 mg/kg | 125 mg/kg | 500 mg/kg | |
|---|---|---|---|---|---|
| n# | 15 | 15 | 10 | 10 | 15 |
| Male | |||||
| Week | |||||
| 1 | 30.2±1.9 | 31.5±1.7 | 32.4±1.6 | 32.7±0.5b | 29.3±3.0 |
| 2 | 34.3±1.9 | 31.8±4.4 | 34.4±1.2 | 35.3±1.4 | 36.6±2.3c |
| 3 | 34.4±1.3 | 35.6±2.9 | 35.6±3.0 | 36.4±1.8 | 38.6±3.4b |
| 4 | 33.7±1.6 | 33.0±1.6 | 34.2±2.5 | 35.8±1.8d | 39.5±2.4b,d |
| 5 | 31.9±1.9 | 32.8±3.1 | 32.9±2.0 | 36.5±1.8b,d | 34.9±2.3a |
| 6 | 33.5±1.4 | 34.1±3.4 | 36.2±2.7 | 35.4±0.6b | 41.5±2.7b,d |
| 7 | 32.9±2.0 | 29.6±2.4 | 31.7±1.3 | 33.9±2.9c | 35.2±4.4d |
| 8 | 31.3±1.9 | 31.0±2.2 | 31.5±2.4 | 32.5±1.3 | 35.4±3.5b,d |
| 9 | 31.4±1.2 | 29.9±1.6 | 31.9±2.0 | 32.5±1.8 | 34.5±3.5d |
| 10 | 31.2±1.4 | 30.9±2.0 | 32.3±2.6 | 34.7±2.8a,c | 37.3±3.4b,d |
| 11 | 31.3±0.9 | 30.6±1.3 | 32.5±2.6 | 31.0±2.5 | 33.2±2.8c |
| 12 | 24.4±4.0 | 26.1±3.9 | 29.1±2.3a | 27.7±3.5 | 31.0±3.6b,c |
| 13 | 25.4±3.1 | 26.8±2.4 | 29.9±3.5a | 30.8±1.0b,d | 31.0±4.5b,c |
| 14 | 32.7±1.1 | 29.3±0.8 | 31.9±2.2 | ||
| 15 | 29.3±1.3 | 28.7±0.9 | 29.8±1.4 | ||
| Female | |||||
| Week | |||||
| 1 | 20.6±1.2 | 20.1±2.5 | 20.0±1.7 | 21.6±1.6 | 22.8±1.8b,d |
| 2 | 23.9±1.6 | 21.7±3.3 | 23.1±1.5 | 24.0±2.0 | 26.7±2.2b,d |
| 3 | 22.4±1.4 | 22.6±1.9 | 24.4±1.0 | 24.5±1.5 | 27.1±2.9b,d |
| 4 | 24.0±1.4 | 22.5±2.4 | 23.3±2.6 | 25.0±2.5 | 26.5±2.5d |
| 5 | 21.5±2.7 | 21.7±2.4 | 22.5±3.6 | 20.6±2.7 | 25.0±1.5b,d |
| 6 | 22.8±2.9 | 22.9±2.3 | 23.2±5.8 | 24.1±1.9 | 28.1±2.9b,d |
| 7 | 22.5±2.8 | 21.8±2.5 | 22.8±2.2 | 23.8±1.7 | 26.1±2.6b,d |
| 8 | 22.8±1.5 | 22.2±2.3 | 22.6±1.4 | 23.9±1.1 | 25.7±1.7b,d |
| 9 | 20.3±2.8 | 20.8±2.1 | 19.2±3.7 | 22.4±2.4 | 23.0±2.7 |
| 10 | 21.6±3.0 | 20.7±3.1 | 22.3±2.6 | 22.9±2.1 | 25.0±3.2a,d |
| 11 | 22.1±2.1 | 20.6±2.7 | 20.0±3.6 | 21.5±2.0 | 21.9±2.4 |
| 12 | 19.0±2.7 | 18.6±3.7 | 18.4±2.6 | 19.2±4.5 | 21.5±3.0 |
| 13 | 20.3±2.0 | 19.6±2.0 | 19.8±1.2 | 20.9±2.7 | 21.8±3.1 |
| 14 | 17.5±0.5 | 21.1±1.3 | 19.7±1.0a | ||
| 15 | 18.8±0.9 | 17.6±4.3 | 22.0±4.0 | ||
Notes:
Data are given as mean ± standard deviation (g/rat/day);
number of animals. Significantly different from negative control (aP<0.05, bP<0.01) and vehicle control (cP<0.05, dP<0.01) by Scheffé’s test.
Table 2.
Feed consumption for rats in the 90-day gavage study of positively charged 100 nm zinc oxide nanoparticles*
| Negative control | Vehicle control | 31.25 mg/kg | 125 mg/kg | 500 mg/kg | |
|---|---|---|---|---|---|
| n# | 15 | 15 | 10 | 10 | 15 |
| Male | |||||
| Week | |||||
| 1 | 30.9±1.1 | 31.0±2.8 | 31.0±2.2 | 31.9±1.0 | 31.4±1.7 |
| 2 | 32.5±1.0 | 33.0±2.2 | 32.0±2.2 | 34.3±2.5 | 33.9±3.1 |
| 3 | 32.1±2.1 | 32.3±4.1 | 32.5±2.6 | 34.3±5.4 | 36.5±2.9b,c |
| 4 | 31.8±5.0 | 33.3±3.6 | 33.3±3.7 | 35.5±1.3 | 35.1±4.8 |
| 5 | 32.5±1.3 | 33.3±3.8 | 31.9±2.1 | 37.2±1.6b,d | 36.0±1.8b |
| 6 | 31.3±2.2 | 35.7±6.4 | 31.3±2.2 | 35.5±2.8a | 38.0±4.9b |
| 7 | 29.9±1.6 | 29.8±2.9 | 29.7±1.3 | 33.5±0.6b,d | 33.8±2.9b,d |
| 8 | 29.7±2.1 | 28.8±2.1 | 31.1±2.7 | 34.3±1.4b,d | 34.2±3.2b,d |
| 9 | 31.9±1.5 | 33.0±4.3 | 33.1±2.1 | 36.9±1.3b,c | 35.2±2.6b |
| 10 | 32.8±2.1 | 33.6±3.9 | 33.1±2.2 | 34.3±4.1 | 37.7±3.1b,c |
| 11 | 30.2±1.0 | 30.1±2.5 | 30.9±2.1 | 33.4±2.4a,d | 34.8±2.2b,d |
| 12 | 27.5±1.9 | 28.6±3.3 | 30.3±3.6 | 30.9±1.9b | 35.2±4.1b,d |
| 13 | 27.4±1.4 | 28.0±2.2 | 27.1±3.2 | 31.2±4.5 | 31.7±4.2a |
| 14 | 29.6±0.0 | 30.4±3.6 | 31.6±0.5b | ||
| 15 | 26.6±0.1 | 29.7±0.4 | 30.3±0.3b | ||
| Female | |||||
| Week | |||||
| 1 | 20.3±2.5 | 20.8±1.2 | 20.5±2.4 | 20.7±1.2 | 20.5±1.7 |
| 2 | 22.9±1.4 | 21.7±2.0 | 22.4±1.2 | 21.1±2.1 | 24.4±2.1d |
| 3 | 21.7±1.7 | 22.0±3.0 | 20.1±1.5 | 20.0±1.9 | 23.4±2.2 |
| 4 | 22.9±3.0 | 22.1±2.5 | 23.0±2.3 | 22.4±2.2 | 26.5±2.3b,d |
| 5 | 22.9±3.6 | 23.1±2.1 | 22.6±2.2 | 21.0±2.4 | 24.6±1.7 |
| 6 | 23.4±1.3 | 22.0±1.6 | 22.4±2.4 | 22.4±2.7 | 23.8±3.4 |
| 7 | 22.8±1.9 | 21.0±1.6 | 19.6±2.0b | 23.3±1.5b | 23.8±1.6d |
| 8 | 20.3±2.4 | 18.8±2.2 | 19.5±2.4 | 19.0±1.3 | 24.4±1.9b,d |
| 9 | 21.7±2.9 | 21.9±1.3 | 22.4±3.5 | 22.0±3.8 | 25.3±2.7a,d |
| 10 | 22.7±2.1 | 21.5±1.2 | 21.9±4.8 | 23.8±0.8d | 24.4±1.4d |
| 11 | 20.9±1.8 | 19.9±2.2 | 19.9±2.6 | 19.2±1.0 | 23.4±2.7d |
| 12 | 19.0±2.6 | 18.1±3.3 | 19.7±2.2 | 18.0±4.3 | 21.2±1.1 |
| 13 | 18.2±2.0 | 19.5±2.8 | 20.7±3.4 | 19.4±1.4 | 23.8±2.1b,d |
| 14 | 23.1±3.4 | 20.5±1.7 | 23.2±0.2 | ||
| 15 | 19.2±0.6 | 19.2±1.5 | 19.8±0.8 | ||
Notes:
Data are given as mean ± standard deviation (g/rat/day);
number of animals. Significantly different from negative control (aP<0.05, bP<0.01) and vehicle control (cP<0.05, dP<0.01) by Scheffé’s test.
Water consumption
Water consumption significantly increased in males receiving 125 mg/kg of ZnOAE100(−) at weeks 3, 8, 10, 11, 12, and 13 and in males receiving 500 mg/kg of ZnOAE100(−) at weeks 1, 2, 3, and 5–13. Water consumption increased in females receiving 31.25 mg/kg and 125 mg/kg of ZnOAE100(−) at week 4 and in females receiving 500 mg/kg of ZnOAE100(−) at weeks 1, 3, 4, 8, and 9 and during both weeks of the post-treatment period (Table 3). Water consumption significantly increased in males receiving 31.25 mg/kg and 125 mg/kg of ZnOAE100(+) at week 3 and in females receiving 500 mg/kg of ZnOAE100(+) at weeks 1–4, 6, and 10 and during both weeks of the post-treatment recovery period. In females, water consumption increased in the 31.25 mg/kg group at week 3, in the 125 mg/kg group at weeks 3 and 6, and in the 500 mg/kg group at weeks 2, 4, 6, and 9 (Table 4).
Table 3.
Water consumption for rats in the 90-day gavage study of negatively charged 100 nm zinc oxide nanoparticles*
| Negative control | Vehicle control | 31.25 mg/kg | 125 mg/kg | 500 mg/kg | |
|---|---|---|---|---|---|
| n# | 15 | 15 | 10 | 10 | 15 |
| Male | |||||
| Week | |||||
| 1 | 40.9±2.1 | 41.4±1.5 | 39.2±15.7 | 40.9±16.5 | 50.3±6b,d |
| 2 | 46.9±4.2 | 44.8±9.0 | 41.0±15.5 | 42.8±12.0 | 55.9±4.4b,d |
| 3 | 47.1±7.2 | 43.0±5.4 | 38.9±12.8 | 45.2±14.9c | 55.7±8.1a,d |
| 4 | 42.0±7.7 | 46.0±6.8 | 38.0±12.6 | 42.8±12.8 | 48.3±10.0 |
| 5 | 46.9±8.2 | 42.7±4.9 | 36.5±12.3 | 36.8±11.9 | 54.1±7.2d |
| 6 | 46.3±10.2 | 46.8±9.5 | 41.5±15.4 | 49.3±15.7 | 46.6±11.5 |
| 7 | 48.4±8.4 | 43.1±8.7 | 36.3±13.2 | 44.0±15.4 | 55.7±14.4 |
| 8 | 48.2±7.9 | 43.9±11.6 | 37.8±13.0 | 48.9±18.0c | 60.8±12.2a,d |
| 9 | 47.9±6.3 | 42.4±11.0 | 41.3±16.2 | 45.8±13.1 | 56.4±10.7d |
| 10 | 43.8±7.7 | 41.4±10.9 | 36.5±12.4 | 45.3±14.9c | 55.6±10.3a,d |
| 11 | 42.4±3.4 | 36.0±6.6 | 34.3±11.3 | 39.0±11.9d | 44.9±6.3d |
| 12 | 38.2±7.6 | 32.7±10.7 | 31.0±10.3 | 38.7±12.5d | 41.5±6.3 |
| 13 | 40.4±2.3 | 40.0±8.5 | 37.8±14.8 | 42.9±14b,c | 50.0±10.2a |
| 14 | 46.8±9.4 | 33.7±2.7 | 39.2±1.9 | ||
| 15 | 47.6±7.7 | 30.1±4.7 | 48.2±9.3 | ||
| Female | |||||
| Week | |||||
| 1 | 32.5±5.1 | 31.6±2.8 | 25.8±10.9 | 31.2±12.1 | 39.1±2.9c |
| 2 | 33.2±5.5 | 33.9±7.5 | 28.4±9.4 | 32.5±10 | 38.3±10.8 |
| 3 | 32.9±5.3 | 34.5±5.4 | 30.9±9.3 | 31.8±8.7 | 45.8±5.0b,d |
| 4 | 31.8±4.2 | 38.5±6.8 | 35.5±13.3b | 33.7±8.6a | 38.8±4.9b |
| 5 | 32.0±10.7 | 35.0±4.9 | 27.5±8.2 | 27.7±7.7 | 38.8±5.4 |
| 6 | 40.7±12.2 | 34.8±9.9 | 27.5±8.6 | 29.2±9.9 | 39.9±11.8 |
| 7 | 39.9±14.2 | 36.4±5.7 | 32.5±11.7 | 35.2±10.1 | 48.5±17.5 |
| 8 | 39.6±6.4 | 41.9±6.5 | 36.8±12 | 37.7±11.1 | 46.7±5.1a |
| 9 | 35.6±8.6 | 38.0±7.6 | 31.7±10.6 | 37.8±10.9 | 46.3±5.1b,c |
| 10 | 34.6±5.1 | 36.5±8.2 | 31.9±12.2 | 36.7±11.7 | 42.4±6.6 |
| 11 | 32.9±3.1 | 33.0±6.6 | 28.3±8.3 | 29.8±8.1 | 32.4±4.9 |
| 12 | 39.4±17.3 | 28.8±4.2 | 23.2±6.2 | 31.4±13.1 | 31.1±7.2 |
| 13 | 33.8±5.8 | 35.3±3.9 | 33.6±15.6 | 37.0±14.3 | 38.2±4.7 |
| 14 | 23.3±2.2 | 30.6±4.6 | 38.0±3.9b | ||
| 15 | 28.4±1.8 | 33.0±11.3 | 36.8±2.1b | ||
Notes:
Data are given as mean ± standard deviation (g/rat/day);
number of animals. Significantly different from negative control (aP<0.05, bP<0.01) and vehicle control (cP<0.05, dP<0.01) by Scheffé’s test.
Table 4.
Water consumption for rats in the 90-day gavage study of positively charged 100 nm zinc oxide nanoparticles*
| Negative control | Vehicle control | 31.25 mg/kg | 125 mg/kg | 500 mg/kg | |
|---|---|---|---|---|---|
| n# | 15 | 15 | 10 | 10 | 15 |
| Male | |||||
| Week | |||||
| 1 | 30.2±1.9 | 31.5±1.7 | 32.4±1.6 | 32.7±0.5b | 29.3±3.0 |
| 2 | 34.3±1.9 | 31.8±4.4 | 34.4±1.2 | 35.3±1.4 | 36.6±2.3c |
| 3 | 34.4±1.3 | 35.6±2.9 | 35.6±3.0 | 36.4±1.8 | 38.6±3.4b |
| 4 | 33.7±1.6 | 33.0±1.6 | 34.2±2.5 | 35.8±1.8d | 39.5±2.4b,d |
| 5 | 31.9±1.9 | 32.8±3.1 | 32.9±2.0 | 36.5±1.8b,d | 34.9±2.3a |
| 6 | 33.5±1.4 | 34.1±3.4 | 36.2±2.7 | 35.4±0.6b | 41.5±2.7b,d |
| 7 | 32.9±2.0 | 29.6±2.4 | 31.7±1.3 | 33.9±2.9c | 35.2±4.4d |
| 8 | 31.3±1.9 | 31.0±2.2 | 31.5±2.4 | 32.5±1.3 | 35.4±3.5b,d |
| 9 | 31.4±1.2 | 29.9±1.6 | 31.9±2.0 | 32.5±1.8c | 34.5±3.5d |
| 10 | 31.2±1.4 | 30.9±2.0 | 32.3±2.6 | 34.7±2.8a,c | 37.3±3.4b,d |
| 11 | 31.3±0.9 | 30.6±1.3 | 32.5±2.6 | 31.0±2.5 | 33.2±2.8c |
| 12 | 24.4±4.0 | 26.1±3.9 | 29.1±2.3a | 27.7±3.5 | 31.0±3.6b,c |
| 13 | 25.4±3.1 | 26.8±2.4 | 29.9±3.5a | 30.8±1.0b,d | 31.0±4.5b,c |
| 14 | 32.7±1.1 | 29.3±0.8 | 31.9±2.2 | ||
| 15 | 29.3±1.3 | 28.7±0.9 | 29.8±1.4 | ||
| Female | |||||
| Week | |||||
| 1 | 20.6±1.2 | 20.1±2.5 | 20.0±1.7 | 21.6±1.6 | 22.8±1.8b,d |
| 2 | 23.9±1.6 | 21.7±3.3 | 23.1±1.5 | 24.0±2.0 | 26.7±2.2b,d |
| 3 | 22.4±1.4 | 22.6±1.9 | 24.4±1.0 | 24.5±1.5 | 27.1±2.9b,d |
| 4 | 24.0±1.4 | 22.5±2.4 | 23.3±2.6 | 25.0±2.5 | 26.5±2.5d |
| 5 | 21.5±2.7 | 21.7±2.4 | 22.5±3.6 | 20.6±2.7 | 25.0±1.5b,d |
| 6 | 22.8±2.9 | 22.9±2.3 | 23.2±5.8 | 24.1±1.9 | 28.1±2.9b,d |
| 7 | 22.5±2.8 | 21.8±2.5 | 22.8±2.2 | 23.8±1.7 | 26.1±2.6b,d |
| 8 | 22.8±1.5 | 22.2±2.3 | 22.6±1.4 | 23.9±1.1 | 25.7±1.7b,d |
| 9 | 20.3±2.8 | 20.8±2.1 | 19.2±3.7 | 22.4±2.4 | 23.0±2.7 |
| 10 | 21.6±3.0 | 20.7±3.1 | 22.3±2.6 | 22.9±2.1 | 25.0±3.2a,d |
| 11 | 22.1±2.1 | 20.6±2.7 | 20.0±3.6 | 21.5±2.0 | 21.9±2.4 |
| 12 | 19.0±2.7 | 18.6±3.7 | 18.4±2.6 | 19.2±4.5 | 21.5±3.0 |
| 13 | 20.3±2.0 | 19.6±2.0 | 19.8±1.2 | 20.9±2.7 | 21.8±3.1 |
| 14 | 17.5±0.5 | 21.1±1.3 | 19.7±1.0a | ||
| 15 | 18.8±0.9 | 17.6±4.3 | 22.0±4.0 | ||
Notes:
Data are given as mean ± standard deviation (g/rat/day);
number of animals. Significantly different from negative control (aP<0.05, bP<0.01) and vehicle control (cP<0.05, dP<0.01) by Scheffé’s test.
Urinalysis
No statistically significant changes were observed between the groups treated with ZnOAE100(−) and ZnOAE100(+) and the controls in both sexes.
Necropsy finding
Multifocal light brown colored change was observed in both kidneys and multifocal dark red color change was also observed in the left kidney in the male negative control group. Reduced left seminal vesicle, multifocal black spots on the liver, light brown color change in the head of the spleen, and reduced size of the testis and epididymis were observed in the male vehicle control group. Light yellowish granule-shaped mass in the ventral prostate, reduced right testis, reduced and light brown color change and adhesion with surrounding tissue in the left kidney, enlarged right kidney, red color change in the margin of the right kidney, and dark red color change in the right lateral lobe of the liver were observed in males receiving 500 mg/kg of ZnOAE100(−). The smaller pituitary gland was observed in females.
The size of the left testis and the left thyroid gland was decreased in the male negative control group. Swollen and yellowish color change in the intestine, light brown color change in the kidney, adhesion to surrounding tissue in the left kidney, and red color change and enlarged submaxillary lymph node were observed (one each) in the female negative control group. In the male vehicle control, reduction in the size of the prostate gland and yellow color change in the right lateral lobe of the liver were observed (one each). In addition, adhesion to surrounding tissues in the spleen, dark red color change in the lung, and light brown color change in the left kidney were observed in one animal each in the female vehicle control group. In the group receiving 31.25 mg/kg of ZnOAE100(−), reduced left seminal vesicle and light brown color change in the right kidney were observed in males and females, respectively. In males receiving 125 mg/kg, light yellowish granule-shaped masses were observed in the ventral prostate in three animals, light brown color change in the liver in two, reduced testis and epididymis in one, and reduced right seminal vesicle in one. In the female 125 mg/kg group, one animal had a light yellow color change in the stomach. The male 500 mg/kg group had light yellowish granule-shaped masses in the ventral prostate in 13, light brown color change in both kidneys in two, light brown color change in both adrenal glands in one, and cyst at the margin of the right kidney in one. One animal in the female 500 mg/kg group showed a red color change in the left lobe in the liver and a light brown color change in the rest of the liver and kidney.
Organ weight
Compared with the control groups, the females in the recovery group receiving 500 mg/kg of ZnOAE100(−) showed an increase in the absolute weight of the submandibular gland. Compared with the control groups, the group of females receiving 500 mg/kg of ZnOAE100(+) study showed a significant increase in the absolute and relative weight of the liver (Table 5). The males in the 500 mg/kg group showed a significant increase in the relative adrenal gland weight, and the absolute spleen weight decreased during the post-treatment recovery period (Table 6).
Table 5.
Organ weight and organ-weight-to-body-weight ratios of main groups in the 90-day gavage study of 100 nm zinc oxide nanoparticles*
| Negative control | Vehicle control | 31.25 mg/kg | 125 mg/kg | 500 mg/kg | |
|---|---|---|---|---|---|
| n# | 10 | 10 | 10 | 10 | 10 |
| ZnOAE100(−) | |||||
| Male | |||||
| Necropsy body weight | 539.8±41.8 | 529.3±41.2 | 526.0±38.6 | 550.0±34.5 | 525.5±46.6 |
| Submaxillary gland | |||||
| Absolute (g) | 0.82±0.08 | 0.77±0.08 | 0.79±0.08 | 0.86±0.08 | 0.87±0.13 |
| Relative (%) | 0.15±0.02 | 0.15±0.01 | 0.15±0.02 | 0.16±0.01 | 0.17±0.02a |
| ZnOAE100(+) | |||||
| Female | |||||
| Necropsy body weight | 286.1±21.9 | 273.3±18.4 | 284.0±17.9 | 283.9±15.8 | 272.3±13.0 |
| Liver | |||||
| Absolute (g) | 7.22±0.45 | 6.67±0.54 | 6.92±0.53 | 7.11±0.80 | 7.58±0.53c |
| Relative (%) | 2.53±0.15 | 2.44±0.11 | 2.44±0.09 | 2.50±0.21 | 2.52±0.10a,d |
Notes:
Organ weights (absolute weights) and body weights are given in g. Organ-weight-to-body-weights (relative weights) are given as mg/g (organ weights/body weight) (mean ± standard deviation);
number of animals. Significantly different from negative control (aP<0.05) and vehicle control (cP<0.05, dP<0.01) by Scheffé’s test.
Abbreviations: ZnO, zinc oxide; ZnOAE100(−), 100 nm negatively charged ZnO; ZnOAE100(+), 100 nm positively charged ZnO.
Table 6.
Organ weight and organ-weight-to-body-weight ratios of recovery groups in the 90-day gavage study of 100 nm positively charged zinc oxide nanoparticles*
| Negative control | Vehicle control | 500 mg/kg | |
|---|---|---|---|
| n# | 5 | 5 | 5 |
| Male | |||
| Necropsy body weight | 575.9±31.8 | 606.1±18.4 | 519.3±90.5 |
| Adrenal gland | |||
| Absolute (g) | 0.052±0.012 | 0.067±0.011 | 0.059±0.005 |
| Relative (%) | 0.009±0.0017 | 0.011±0.0013 | 0.012±0.0013a |
| Spleen | |||
| Absolute (g) | 0.84±0.08 | 0.78±0.14 | 0.66±0.05a |
| Relative (%) | 0.15±0.02 | 0.13±0.02 | 0.13±0.02 |
Notes:
Organ weights (absolute weights) and body weights are given in g. Organ-weight-to-body-weights (relative weights) are given as mg/g (organ weights/body weight) (mean ± standard deviation);
number of animals. Data are given as mean ± standard deviation. Significantly different from negative control (aP<0.05) by Scheffé’s test.
Hematological analysis
The MCV and Hb levels were significantly lower in male rats receiving 125 mg/kg ZnOAE100(−) than in control rats. Although the Hb, MCV, MCH, and MCHC levels were significantly lower, the number of eosinophils was significantly greater in the male and female rats receiving 500 mg/kg of ZnOAE100(−) than in the control groups. In addition, total erythrocyte count significantly decreased in male rats receiving 500 mg/kg of ZnOAE100(−). The number of eosinophils was significantly higher in males receiving 125 mg/kg of ZnOAE100(+) than in the control group. Similar to the findings observed with ZnOAE100(−), ZnOAE100(+) significantly decreased the Hb, MCV, MCH, and MCHC levels in the males and females receiving 500 mg/kg compared with those in the control groups. Compared with the control group, the group of male rats receiving 500 mg/kg ZnOAE100(+) showed significantly decreased Ht level and prothrombin time but significantly increased thrombocyte counts. The total white blood cell and thrombocyte counts were significantly increased in the female 500 mg/kg group compared with those in the control group (Table 7).
Table 7.
Hematology data of main groups in the 90-day gavage study of 100 nm zinc oxide nanoparticles*
| Negative control | Vehicle control | 31.25 mg/kg | 125 mg/kg | 500 mg/kg | |
|---|---|---|---|---|---|
| n# | 10 | 10 | 10 | 10 | 10 |
| ZnOAE100(−) | |||||
| Male | |||||
| Eosinophils (%) | 1.20±0.4 | 1.40±0.6 | 1.50±0.5 | 1.80±0.6 | 2.60±0.9b,d |
| Total erythrocyte (106 cells/μL) | 8.30±0.26 | 8.53±0.31 | 8.47±0.33 | 8.57±0.31 | 9.29±0.52b,d |
| Hemoglobin (g/dL) | 14.4±0.4 | 14.5±0.6 | 14.1±0.5 | 14.1±0.4 | 12.8±0.8b,d |
| Ht (%) | 44.4±1.4 | 44.9±1.8 | 44.1±1.5 | 44.1±1.5 | 41.7±1.8b,d |
| MCV (fL) | 53.4±1.2 | 52.6±1.3 | 52.1±2.0 | 51.5±1.5a | 45.0±3.6b,d |
| MCH (pg) | 17.3±0.4 | 17.0±0.4 | 16.7±0.7 | 16.5±0.5a | 13.9±1.4b,d |
| MCHC (g/dL) | 32.4±0.2 | 32.3±0.2 | 32.0±0.4 | 32.0±0.4 | 30.8±0.8b,d |
| PT (seconds) | 15.7±0.7 | 15.7±0.8 | 15.1±0.7 | 15.2±0.5 | 14.3±1.0b,d |
| Female | |||||
| Eosinophils (%) | 1.60±0.6 | 1.90±0.8 | 1.90±0.6 | 2.30±1.3 | 3.10±1.3a |
| Hemoglobin (g/dL) | 13.9±0.8 | 14.0±0.3 | 13.9±0.6 | 13.8±0.8 | 12.9±0.8c |
| MCV (fL) | 54.2±1.5 | 54.7±1.5 | 54.7±1.8 | 54.3±1.6 | 50.2±2.7b,d |
| MCH (pg) | 17.7±0.5 | 17.9±0.6 | 17.8±0.6 | 17.6±0.7 | 15.7±1.0b,d |
| MCHC (g/dL) | 32.6±0.5 | 1.85±0.49 | 32.5±0.6 | 32.3±0.5 | 31.4±0.8b,d |
| ZnOAE100(+) | |||||
| Male | |||||
| Hemoglobin (g/dL) | 14.3±0.7 | 14.5±0.6 | 14.4±0.4 | 14.0±0.5 | 12.5±0.9b,d |
| Ht (%) | 44.4±2.0 | 45.3±1.6 | 44.5±1.5 | 43.8±1.6 | 40.8±2.3b,d |
| MCV (fL) | 51.9±1.0 | 52.2±1.8 | 51.2±1.5 | 50.5±1.8 | 46.1±2.4b,d |
| MCH (pg) | 16.7±0.6 | 16.7±0.6 | 16.6±0.5 | 16.1±0.7 | 14.1±1.0b,d |
| MCHC (g/dL) | 32.3±0.5 | 32.1±0.5 | 32.4±0.6 | 31.9±0.3 | 30.7±0.6b,d |
| PLT (103 cells/μL) | 1,019±104 | 1,045±112 | 1,070±113 | 1,054±112 | 1,248±228a |
| PT (seconds) | 15.5±0.7 | 15.7±0.7 | 15.5±1.0 | 15.4±1.0 | 14.3±0.6b,d |
| Female | |||||
| Total leukocyte count (103 cells/μL) | 3.02±0.91 | 3.22±0.7 | 3.12±0.83 | 3.53±0.96 | 6.11±1.61b,d |
| MCV (fL) | 55.2±3.0 | 54.3±1.7 | 53.9±1.5 | 53.9±1.5 | 48.2±3.8b,d |
| MCH (pg) | 18.1±0.8 | 17.9±0.6 | 17.7±0.5 | 17.7±0.5 | 15.0±1.4b,d |
| MCHC (g/dL) | 32.8±0.4 | 32.9±0.5 | 32.8±0.3 | 32.8±0.3 | 30.9±1.4b,d |
| PLT (103 cells/μL) | 1,056±132 | 1,078±101 | 1,158±112 | 1,158±112 | 1,296±225b,d |
Notes:
Data are given as mean ± standard deviation;
number of animals. Significantly different from negative control (aP<0.05, bP<0.01) and vehicle control (cP<0.05, dP<0.01) by Scheffé’s test.
Abbreviations: Ht, hematocrit; MCH, mean cell hemoglobin; MCHC, mean cell hemoglobin concentration; MCV, mean cell volume; PLT, platelet; PT, prothrombin time; ZnO, zinc oxide; ZnOAE100(−), 100 nm negatively charged ZnO; ZnOAE100(+), 100 nm positively charged ZnO.
During the post-treatment recovery period, total erythrocyte counts significantly increased, regardless of the surface charges of ZnO NPs, but monocyte counts, MCV, and MCH significantly decreased in the 500 mg/kg groups in both sexes compared with those in the control groups. In addition, neutrophil counts and activated partial thromboplastin time significantly increased, but lymphocyte counts, Hb level, and MCHC significantly decreased in the male rats receiving 500 mg/kg of ZnOAE100(−) compared with those in the control groups (Table 8).
Table 8.
Hematology data of recovery groups in the 90-day gavage study of 100 nm zinc oxide nanoparticles*
| Negative control | Vehicle control | 500 mg/kg | |
|---|---|---|---|
| n# | 5 | 5 | 5 |
| ZnOAE100(−) | |||
| Male | |||
| Neutrophils (%) | 15.2±3.6 | 18.8±2.6 | 23.5±4.9a |
| Lymphocytes (%) | 79.6±3.7 | 75.6±3.8 | 69.5±6.8a |
| Total erythrocyte (106 cells/μL) | 8.44±0.44 | 8.20±0.23 | 9.28±0.61a,d |
| Hemoglobin (g/dL) | 14.3±0.5 | 13.5±0.3 | 13.3±0.5a |
| MCV (fL) | 52.2±1.6 | 51.6±0.5 | 47.8±2.2b,d |
| MCH (pg) | 16.9±0.7 | 16.5±0.1 | 14.4±1.1b,d |
| MCHC (g/dL) | 32.5±0.4 | 31.9±0.4 | 30.1±1.1b,d |
| APTT (seconds) | 18.8±1.1 | 18.2±1.7 | 20.6±0.7c |
| Female | |||
| Monocytes (%) | 3.4±0.6 | 2.7±0.5 | 2.0±0.3b |
| Total erythrocyte (106 cells/μL) | 7.63±0.48 | 7.45±0.20 | 8.24±0.38c |
| MCV (fL) | 55.8±2.1 | 54.3±1.1 | 50.8±2.0b,c |
| MCH (pg) | 18.2±0.6 | 17.9±0.5 | 16.3±1.0b,c |
| ZnOAE100(+) | |||
| Male | |||
| Total erythrocyte (106 cells/μL) | 8.35±0.27 | 8.28±0.19 | 9.40±0.48b,d |
| MCV (fL) | 53.1±1.1 | 52.9±1.2 | 48.8±1.6b,d |
| MCH (pg) | 17.4±0.3 | 17.1±0.4 | 14.8±0.8b,d |
| MCHC (g/dL) | 32.7±0.3 | 32.3±0.3 | 30.3±1.1b,d |
| Female | |||
| Total erythrocyte (106 cells/μL) | 7.38±0.67 | 7.52±0.30 | 8.18±0.31d |
| MCH (pg) | 18.5±0.7 | 17.8±0.9 | 16.4±0.7b,c |
| MCHC (g/dL) | 32.9±0.5 | 32.1±0.4 | 31.2±0.5b,c |
Notes:
Data are given as mean ± standard deviation;
number of animals. Significantly different from negative control (aP<0.05, bP<0.01) and vehicle control (cP<0.05, dP<0.01) by Scheffé’s test.
Abbreviations: APTT, activated partial thromboplastin time; MCH, mean cell hemoglobin; MCHC, mean cell hemoglobin concentration; MCV, mean cell volume; ZnO, zinc oxide; ZnOAE100(−), 100 nm negatively charged ZnO; ZnOAE100(+), 100 nm positively charged ZnO.
Blood biochemical analysis
Compared with the control groups, the group of male rats receiving 31.25 mg/kg of ZnOAE100(−) showed significantly decreased blood urea nitrogen levels and those receiving 125 mg/kg showed significantly decreased Alb levels. ALP and P levels were significantly higher in the male group of rats receiving 500 mg/kg of ZnOAE100(−). In the female 500 mg/kg ZnOAE100(−) group, the levels of TP, Alb, and Glu significantly decreased, but the Cl level significantly increased compared with those in the control groups. Similarly, in the male 500 mg/kg group of ZnOAE100(+), the levels of TP, Alb, and Glu significantly decreased, but the P level significantly increased compared with those in the control groups. In addition, compared with the control groups, the female 500 mg/kg group showed significantly decreased levels of TP, Alb, T-Cho, and Ca but significantly increased levels of ALP, creatine kinase, and Cl. TP and Ca levels were significantly lower in the female 31.25 mg/kg group, and TP level was significantly lower in the female 125 mg/kg group than that in the control groups (Table 9).
Table 9.
Blood biochemistry data of main groups in the 90-day gavage study of 100 nm zinc oxide nanoparticles*
| Negative control | Vehicle control | 31.25 mg/kg | 125 mg/kg | 500 mg/kg | |
|---|---|---|---|---|---|
| n# | 10 | 10 | 10 | 10 | 10 |
| ZnOAE100(−) | |||||
| Male | |||||
| TP (g/dL) | 2.9±0.3 | 6.0±0.2 | 6.0±0.3 | 5.6±0.3 | 5.4±0.4b,d |
| Alb (g/dL) | 2.3±0.1 | 2.5±0.1 | 2.4±0.1 | 2.3±0.1c | 2.2±0.1a,d |
| AP (U/L) | 264±40 | 319±144 | 325±59 | 284±49 | 445±131a |
| P (mg/dL) | 6.7±0.5 | 6.7±0.4 | 6.6±0.6 | 6.9±0.7 | 8.2±1.3b,d |
| Female | |||||
| TP (g/dL) | 6.7±0.3 | 6.5±0.4 | 6.6±0.4 | 6.1±0.3 | 5.6±0.4b,d |
| Alb (g/dL) | 2.9±0.2 | 2.9±0.2 | 3.0±0.2 | 2.8±0.2 | 2.6±0.1b,c |
| Glu (mg/dL) | 156±16 | 137±14 | 145±21 | 139±13 | 126±23a |
| Cl (mmol/L) | 107.7±1.9 | 107.6±1.9 | 108.8±1.6 | 108.5±1.7 | 110.0±1.1c |
| ZnOAE100(+) | |||||
| Male | |||||
| TP (g/dL) | 6.2±0.4 | 6.2±0.3 | 6.1±0.2 | 5.8±0.2 | 5.4±0.4b,d |
| Alb (g/dL) | 2.5±0.2 | 2.4±0.1 | 2.5±0.1 | 2.4±0.1 | 2.3±0.1a |
| Glu (mg/dL) | 173±43 | 154±18 | 155±23 | 148±10 | 130±12a |
| P (mg/dL) | 6.6±0.6 | 6.5±0.6 | 7.0±0.6 | 6.8±0.7 | 7.6±0.6b,d |
| Female | |||||
| TP (g/dL) | 7.1±0.4 | 6.6±0.4 | 6.5±0.3a | 6.3±0.4b | 5.6±0.4b,d |
| Alb (g/dL) | 3.2±0.3 | 2.9±0.2 | 2.8±0.2 | 2.9±0.2 | 2.5±0.2b,d |
| AP (U/L) | 159±39 | 173±49 | 189±53 | 178±54 | 349±70b,d |
| T-Cho (mg/dL) | 82±12 | 79±13 | 71±13 | 70±20 | 62±12b |
| Ca (mg/dL) | 10.8±0.3 | 10.3±0.4 | 10.3±0.3c | 10.5±0.2 | 10.4±0.3c |
| CK (IU/L) | 300±142 | 427±204 | 512±286 | 437±167 | 795±360b |
Notes:
Data are given as mean ± standard deviation;
number of animals. Significantly different from negative control (aP<0.05, bP<0.01) and vehicle control (cP<0.05, dP<0.01) by Scheffé’s test.
Abbreviations: Alb, albumin; AP, alkaline phosphatase; Ca, calcium; CK, creatine kinase; Cl, chloride; Glu, glucose; P, inorganic phosphorus; T-Cho, total cholesterol; TP, total protein; ZnO, zinc oxide; ZnOAE100(−), 100 nm negatively charged ZnO; ZnOAE100(+), 100 nm positively charged ZnO.
During the post-treatment recovery period of the group treated with ZnOAE100(−), the levels of aspartate aminotransferase, ALT, and TG significantly decreased in the male 500 mg/kg group. In the female 500 mg/kg group, T-Cho and TG levels significantly decreased, but the ALT level markedly increased compared with that in the control groups. In addition, in the ZnOAE100(+) group, P level increased in the male 500 mg/kg group, and ALP level and albumin/globulin ratio significantly increased in the female 500 mg/kg group compared with those in the control groups (Table 10).
Table 10.
Blood biochemistry data of recovery groups in the 90-day gavage study of 100 nm zinc oxide nanoparticles*
| Negative control | Vehicle control | 500 mg/kg | |
|---|---|---|---|
| n# | 5 | 5 | 5 |
| ZnOAE100(−) | |||
| Male | |||
| ASP (U/L) | 157±48 | 134±16 | 98±23a |
| ALP (U/L) | 44±10 | 33±5 | 26±4b |
| TG (mg/dL) | 86±19 | 113±14 | 60±26d |
| Female | |||
| AP (U/L) | 124±12 | 137±30 | 219±51b |
| T-Cho (mg/dL) | 94±10 | 88±18 | 72±9a |
| TG (mg/dL) | 50±12 | 30±17 | 22±9a |
| ZnOAE100(+) | |||
| Male | |||
| P (mg/dL) | 6.1±0.3 | 6.1±0.5 | 7.3±0.9a,c |
| Female | |||
| A/G ratio | 0.8±0.1 | 0.8±0.0 | 0.9±0.1a |
| AP (U/L) | 164±18 | 119±30 | 164±23c |
Notes:
Data are given as mean ± standard deviation;
number of animals. Significantly different from negative control (aP<0.05, bP<0.01) and vehicle control (cP<0.05, dP<0.01) by Scheffé’s test.
Abbreviations: A/G ratio, albumin/globulin ratio; ALP, alanine aminotransferase; AP, alkaline phosphatase; ASP, aspartate aminotransferase; T-Cho, total cholesterol; TG, triacylgycerol; P, inorganic phosphorus; ZnO, zinc oxide; ZnOAE100(−), 100 nm negatively charged ZnO; ZnOAE100(+), 100 nm positively charged ZnO.
Histopathological observation
Regardless of the surface charge of ZnO NPs, squamous cell hyperplasia and vacuolation in nonglandular stomach, intracytoplasmic hyaline droplet, submucosal edema and inflammation, eosinophilic chief cell, and mucous cell hyperplasia in glandular stomach were observed in the 500 mg/kg groups of both sexes (Figures 3 and 4). Acinar cell apoptosis and chronic inflammation in the pancreas, retinal atrophy in the eye, and suppurative inflammation in the prostate gland were also observed in the 500 mg/kg groups of both sexes (Figures 5–7). As significant lesions in the stomach, pancreas, eye, and prostate gland were observed in the 500 mg/kg groups, these organs were also examined in the 31.25 mg/kg and 125 mg/kg groups. In the stomach, most lesions observed in the 500 mg/kg group were observed in the 31.25 mg/kg and 125 mg/kg groups and they exhibited dose dependency. The suppurative inflammatory lesion in the prostate gland, which was observed in the 500 mg/kg group, was also observed in the 31.25 mg/kg and 125 mg/kg groups. The lesion of retinal atrophy in the eye was observed in only one animal in the male 125 mg/kg group. However, in the pancreas, most lesions observed in the 500 mg/kg group were not observed in the 31.25 mg/kg and 125 mg/kg groups (data not shown).
Figure 3.
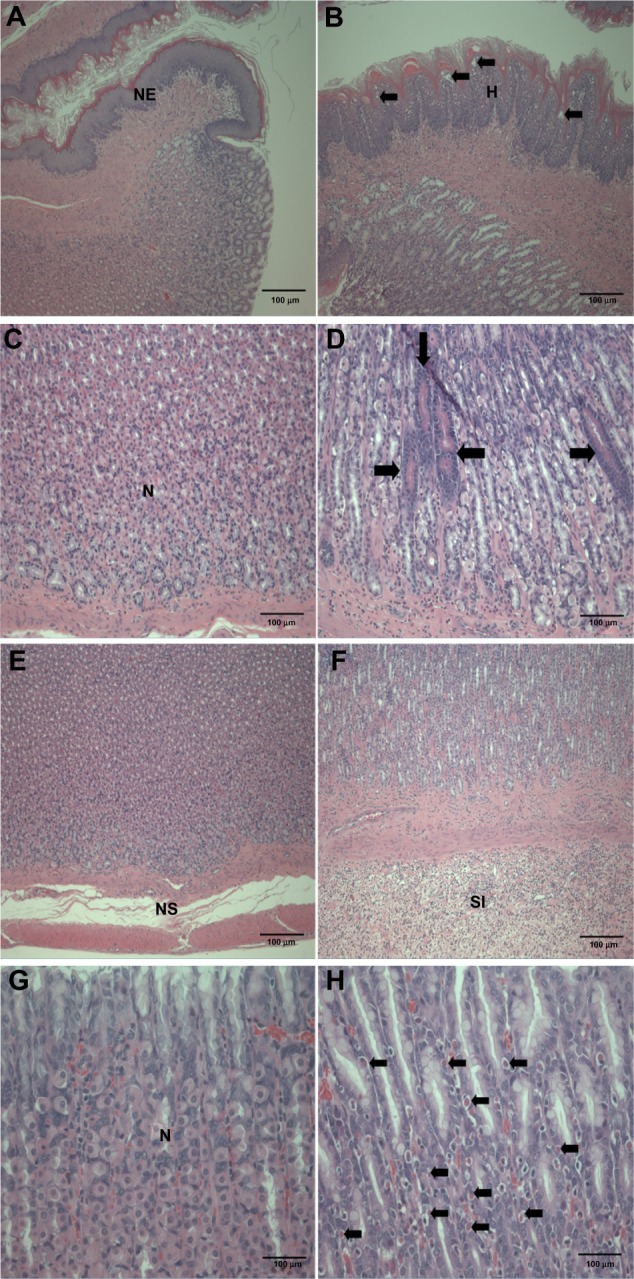
Histopathological changes in the stomach after treatment with ZnOAE100(−) at a dose of 500 mg/kg for 90 days. Stomach sections were stained with hematoxylin and eosin. (A) Control for limiting ridge. (C, E and G) Control for mucosa in glandular stomach. (B, D, F and H) 500 mg/kg treatment groups.a
Notes: aArrows in (B) represent squamous cell vacuolation, in (D) show eosinophilic chief cells, in (F) indicate acinar cell apoptosis, and in (H) represent intracytoplasmic hyaline droplets.
Abbreviations: H, squamous cell hyperplasia; N, normal mucosa; NE, normal epithelium; NS, normal submucosa; SI, submucosal edema and inflammatory cell infiltration; ZnO, zinc oxide, ZnOAE100(−), 100 nm negatively charged ZnO.
Figure 4.
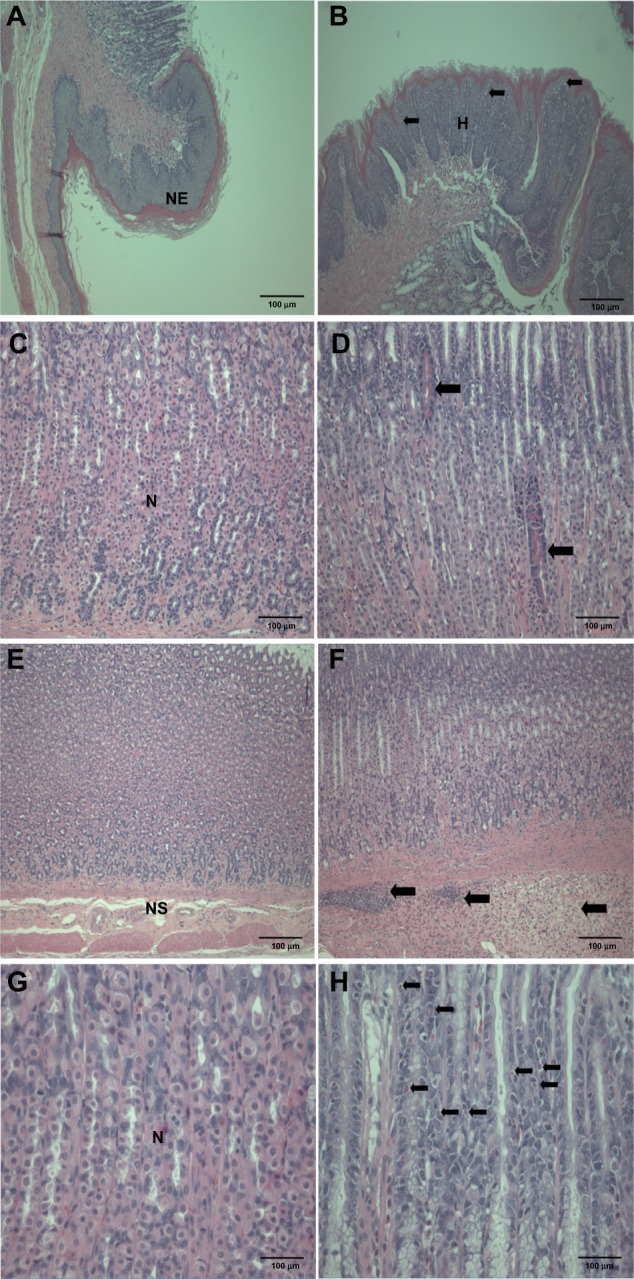
Histopathological changes in the stomach after treatment with ZnOAE100(+) at a dose of 500 mg/kg for 90 days. Stomach sections were stained with hematoxylin and eosin. (A) Control for limiting ridge. (C, E and G) Control for mucosa in glandular stomach. (B, D, F and H) 500 mg/kg treatment groups.a
Note: aArrows in (B) represent squamous cell vacuolation, in (D) show eosinophilic chief cells, in (F) indicate submucosal edema and inflammatory cell infiltration, and in (H) represent intracytoplasmic hyaline droplets.
Abbreviations: H, squamous cell hyperplasia; N, normal mucosa; NE, normal epithelium; NS, normal submucosa; ZnO, zinc oxide; ZnOAE100(+), 100 nm positively charged ZnO.
Figure 5.
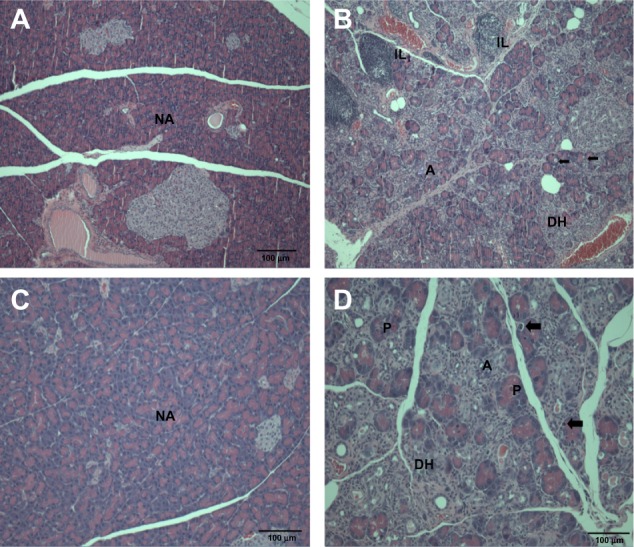
Histopathological changes in the pancreas after treatment with (A and B) ZnOAE100(−) and (C and D) ZnOAE100(+) at a dose of 500 mg/kg for 90 days. The pancreas sections were stained with hematoxylin and eosin. (A and C) Control for acinar cell. (B and D) 500 mg/kg treatment groups.a
Notes: aArrows in (B and D) represent acinar cell apoptosis in the pancreas.
Abbreviations: A, chronic inflammation; DH, ductular hyperplasia; IL, interstitial lymphoid cell infiltration; NA, normal acinar cell; P, prominent acinar cell; ZnO, zinc oxide; ZnOAE100(−), 100 nm negatively charged ZnO; ZnOAE100(+), 100 nm positively charged ZnO.
Figure 7.
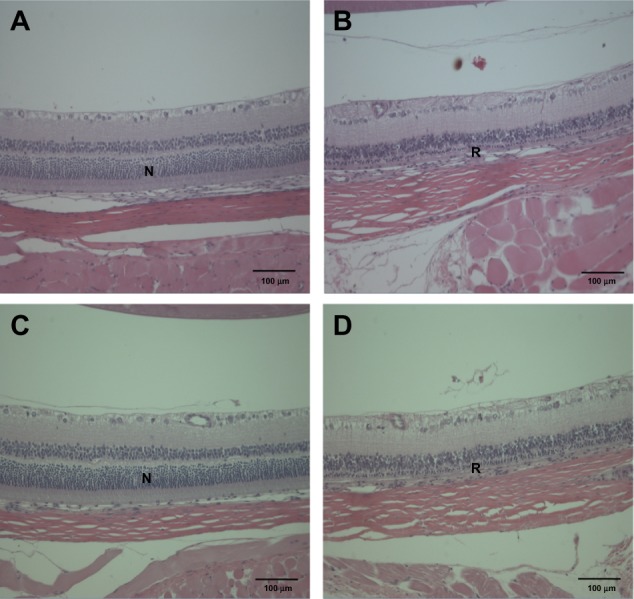
Histopathological changes in the eye after treatment with (A and B) ZnOAE100(−) and (C and D) ZnOAE100(+) at a dose of 500 mg/kg for 90 days. Eye sections were stained with hematoxylin and eosin. (A and C) Control for the eye. (B and D) 500 mg/kg treatment groups.
Abbreviations: N, normal eye; R, retinal atrophy; ZnO, zinc oxide; ZnOAE100(−), 100 nm negatively charged ZnO; ZnOAE100(+), 100 nm positively charged ZnO.
Discussion
The present study was performed (the repeated-dose 90-day oral toxicity study of two types of 100 nm ZnO NPs, ZnOAE100[−] and ZnOAE100[+]) to investigate different toxic effect(s) of surface charge and to determine the NOAEL and target organs in SD rats.
Two test articles, ZnOAE100(−) and ZnOAE100(+), were administered by gavage in male and female SD rats at the doses of 500 mg/kg, 1,000 mg/kg, and 2,000 mg/kg for 14 days in a DRF study. Because significant changes in the 500 mg/kg groups were observed only in the treatment groups, these changes were considered to be treatment related. On the basis of the DRF studies of both test articles, 31.25 mg/kg, 125 mg/kg, and 500 mg/kg were selected as the three dose levels for the 90-day repeated study.
Repeated oral administration of ZnOAE100(−) or ZnOAE100(+) in the 90-day toxicity study did not result in death at any dose. Clinical signs, including salivation, fur loss, and scarring, were observed in the 125 mg/kg and 500 mg/kg groups of ZnOAE100(−) and ZnOAE100(+), and white feces were commonly observed in groups treated with 500 mg/kg of test articles. However, these signs are not considered to be treatment related because they did not show consistent dose dependency during the experimental period and occurred sporadically. No significant changes were observed in body weight between the treatment groups and their respective control groups, regardless of the surface charge. Compared with the control groups, the female 500 mg/kg groups of ZnOAE100(−) and ZnOAE100(+) showed an increase in the absolute weights of the submandibular gland and liver, respectively. In addition, the relative adrenal gland weight and absolute spleen weight significantly changed in the male 500 mg/kg groups during the post-treatment recovery period. A significant increase in feed consumption was observed in the males receiving 31.25 mg/kg and 125 mg/kg of ZnOAE100(−) and in the females receiving 500 mg/kg of ZnOAE100(−), and in both sexes receiving 125 mg/kg and 500 mg/kg of ZnOAE100(+). In addition, a large increase was observed in water consumption in both the male and female 125 mg/kg and 500 mg/kg groups of ZnOAE100(−) and ZnOAE100(+) compared with that in the control groups. However, these changes in feed and water consumption did not show any dose dependency and were observed sporadically. Therefore, these are not considered to be toxic effects, because they did not relate to changes in body weight and organ weight.
The changes in hematological and blood biochemical analysis were commonly observed in the 500 mg/kg groups of both sexes of ZnOAE100(−) and ZnOAE100(+) compared with in their respective control groups. MCV, MCH, and MCHC significantly decreased after administration of both test articles, including the 2-week recovery period. TP and Ab levels also decreased. These significant decreases in hematological and blood biochemical molecules are considered to be related to the administration of ZnOAE100(−) or ZnOAE100(+). Recently, Yan et al11 reported that a high zinc diet resulted in iron deficiency anemia and this zinc-induced anemia was related to a decrease in the levels of Hb, Ht, MCV, and MCH, which was similar to the findings observed in our study. Similarly, 13-week oral administration of 40 nm ZnO NPs in SD rats induced significant changes in anemia-related parameters such as Hb levels, Ht levels, MCV, and MCH.21 Overdosing of metallic zinc or zinc salt in humans resulted in hemorrhaging, which reduced critical blood markers, and finally it led to gastrointestinal damage.22 Moreover, long-term supplementation of zinc in humans can cause anemia, which results from copper deficiency.23–26 On the basis of results from previous studies and those obtained in our study, it could be suggested that long-term exposure to ZnO NPs might lead to anemia.
Histopathological examination showed prominent changes in the stomach, eye, and pancreas compared with those in the negative and vehicle control groups. Squamous cell hyperplasia and vacuolation in nonglandular stomach and intracytoplasmic hyaline droplet, submucosal edema and inflammatory cell infiltration, and mucous cell hyperplasia in glandular stomach were considered to be treatment related because these changes were dose dependent and/or appeared prominently compared with those in the negative and vehicle controls. However, because these changes in the stomach induced by ZnOAE100(−) or ZnOAE100(+) tended to return to normal during the recovery period, we speculated that they were less likely to cause functional disturbances. Similar to our results, the results of acute toxicity studies of nanosized ZnO powders showed that oral administration of ZnO NPs caused slight pathological damages in the stomach in mice.27,28 Another 14-day oral toxicity study of 20 nm ZnO NPs in SD rats showed that the NPs induced inflammatory cell infiltration in the stomach.13 As compared with our observation, these short-term studies showed that histopathological changes in the stomach were occurring only in serosa or submucosa layer not in other layers. However, we found various lesions in the overall area of the stomach, such as granular stomach, fore stomach, and limiting ledge of the stomach; thus, we speculated that these changes were induced by continuous oral administration of ZnO NPs.
In addition, we found acinar cell apoptosis and chronic inflammation in the pancreas in the male and female groups receiving 500 mg/kg of both test articles. These lesions in the pancreas were resolved during the recovery period, but they are considered to be toxicologically significant because they seemed to be severe enough to induce functional abnormalities. In relation to our observation, oral administration of 40 nm ZnO NPs for 13 weeks in rats caused pancreatitis associated with focal lymphocyte infiltration and mild acinar cell apoptosis.20 Chronic inflammatory cells in the pancreas were found in mice treated with 20 nm and 120 nm ZnO NPs in a 14-day oral toxicity study.28 We also observed the suppurative inflammation in the prostate gland in males receiving 500 mg/kg and retinal atrophy in the eye in both sexes receiving 500 mg/kg of both test articles. In particular, lesions in the eye are considered to be caused by treatment of the test articles similar to that observed in the incidence and severity in recovery groups. These lesions in the prostate gland and the eye were not observed in the 14-day DRF study, and have never been reported in any other in vivo studies of oral administration of ZnO NPs. In relation to inflammation in the prostate gland, only Khan et al29 reported that a two-generation reproductive toxicity study of ZnCl2 in rats showed the reduction of prostate weight and induction of reproductive tract lesions. Recently, some in vitro studies reported that ZnO NPs led to cell damage and induced apoptosis or necrosis in rat retinal ganglion cells.30,31 It is known that lesions are usually mixtures of acute and chronic changes in the repeated-dose toxicity studies, as the articles were treated daily for a long period. In general, acute lesions mean changes induced within a few days after the administration, such as cellular degeneration, apoptosis, necrosis, and acute inflammation, which are infiltrated mainly with polymorphic nuclear cells. On the other hand, chronic lesions include chronic inflammation, fibrosis, and proliferative lesions such as hyperplasia and neoplasia. Chronic inflammation usually shows infiltration of mononuclear cells, fibroblasts, and fibrocytes. Moreover, these chronic lesions are observed in our histopathological examination. Therefore, considering results from our previous studies, we speculated that inflammatory damages in the pancreas, prostate gland, and eye resulted from continuous irritation caused by both test articles, ZnOAE100(−) or ZnOAE100(+). Although not many in vivo studies of ZnO NPs have been performed, some studies have reported that nanotoxicity of ZnO NPs might result from oxidative stress. For example, oral administration of 30 nm ZnO NPs resulted in an accumulation of ZnO and induced oxidative stress, which was mediated by DNA damage and apoptosis.10 After ZnO NPs were administrated orally to rats for 14 days, they impaired mitochondria and cellular membranes in the kidneys.14 These results suggest that one of the leading causes of these histopathological changes was oxidative stress induced by ZnO NP treatment.
As compared with our observations of ZnO NPs according to surface charge, we did not find any difference in the tendency and degree of the lesions. As the solubility of ZnO NPs is one of the important factors in this toxicity study, it should be verified whether the coated ZnO NPs remained in NP form in the course of the study or dissociated in the gastric conditions. Paek et al31 reported that ZnO NPs coated with L-serine and citrate, which have the same physicochemical properties as the test articles in the present study, were partially dissolved in gastric fluid (about 13%–14%), and it was not affected by their size and charge. Moreover, they showed that the behavior of oral zinc ions (ZnCl2) was different from that of oral ZnO NPs with different surface charge. Thus, it might be suggested that pH buffering resulted in partial dissolution of ZnO NPs, and it did not significantly affect the nanoparticulate property of ZnO NPs. As regards the toxic effect of surface chemistry of ZnO NPs, only a few studies have been reported. For example, as compared with poly(methacrylic acid)-coated ZnO NPs and uncoated ZnO NPs with zeta potential of about −40 mV and +4 mV, respectively, poly(methacrylic acid)-coated NPs showed higher genotoxicity than uncoated NPs in viable cells.17 However, Steuer et al33 reported that thrombus formation in human blood, which stimulates blood coagulation, was independent of surface charge of ZnO NPs. In addition, the same independent effect of charge was reported by Paek et al.32 They showed that there was no significant difference in tissue distribution of negatively and positively charged ZnO NPs in SD rats. Thus, as several physicochemical properties, including solubility, surface charge, and modified ligand, could influence nanotoxicity, further mechanism(s) studies about the toxic effects of ZnO NPs based on their physicochemical properties should be performed in the future.
Conclusion
In this study, 100 nm ZnO NPs with different surface charges (ZnOAE100[−] and ZnOAE100[+]) at doses of 31.25 mg/kg, 125 mg/kg, and 500 mg/kg were repeatedly administered by gavage for 90 days in SD rats. The effects induced by ZnO NPs included salivation, white feces, statistically significant changes in feed and water consumption, and hematological analysis after the administration. In addition, histopathological findings included squamous cell hyperplasia and vacuolation in nonglandular stomach, intracytoplasmic hyaline droplet, submucosal edema, inflammatory cell infiltration and mucous cell hyperplasia in the glandular stomach, chronic inflammation and acinar cell apoptosis in the pancreas, suppurative inflammation in the prostate gland, and retinal atrophy in the eye. Thus, the target organs for the test articles are considered to be the stomach, pancreas, eye, and prostate gland. Significant toxic effects were observed in both sexes at doses greater than 125 mg/kg; therefore, the NOAEL of both test articles, ZnOAE100(−) and ZnOAE100(+), was considered to be 31.5 mg/kg for both sexes.
Figure 6.
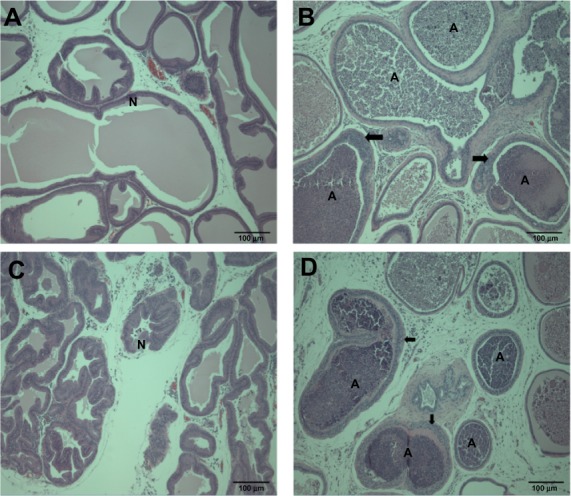
Histopathological changes in the prostate gland after treatment with (A and B) ZnOAE100(−) and (C and D) ZnOAE100(+) at a dose of 500 mg/kg for 90 days. Prostate gland sections were stained with hematoxylin and eosin. (A and C) Control for the prostate gland tubule. (B and D) 500 mg/kg treatment groups.a
Note: aArrows in (B and D) show tubular hyperplasia in the prostate gland.
Abbreviations: A, suppurative inflammation, N, normal tubule; ZnO, zinc oxide; ZnOAE100(−), 100 nm negatively charged ZnO; ZnOAE100(+), 100 nm positively charged ZnO.
Acknowledgments
This research was supported by a grant (10182MFDS991) from the Ministry of Food and Drug Safety in 2011. In addition, this study was supported by the Research-Driven Hospital Project of Korea University Anam Hospital accredited by the Korean Government Ministry of Health and Welfare 2013.
Footnotes
Disclosures
The authors report no conflicts of interest in this work.
References
- 1.Osmond MJ, McCall MJ. Zinc oxide nanoparticles in modern sunscreens: an analysis of potential exposure and hazard. Nanotoxicology. 2010;4(1):15–41. doi: 10.3109/17435390903502028. [DOI] [PubMed] [Google Scholar]
- 2.Osman IF, Baumgartner A, Cemeli E, Fletcher JN, Anderson D. Genotoxicity and cytotoxicity of zinc oxide and titanium dioxide in HEp-2 cells. Nanomedicine (Lond) 2010;5(8):1193–1203. doi: 10.2217/nnm.10.52. [DOI] [PubMed] [Google Scholar]
- 3.Becheri A, Dürr M, Lo Nostro P, Baglioni P. Synthesis and characterization of zinc oxide nanoparticles: application to textiles as UV-absorbers. J Nanopart Res. 2008;10(4):679–689. [Google Scholar]
- 4.Lin CC, Lin WH, Li YY. Synthesis of ZnO nanowires and their applications as an ultraviolet photodetector. J Nanosci Nanotechnol. 2009;9(5):2813–2819. doi: 10.1166/jnn.2009.008. [DOI] [PubMed] [Google Scholar]
- 5.Jin Y, Wang J, Sun B, Blakesley JC, Greenham NC. Solution-processed ultraviolet photodetectors based on colloidal ZnO nanoparticles. Nano Lett. 2008;8(6):1649–1653. doi: 10.1021/nl0803702. [DOI] [PubMed] [Google Scholar]
- 6.Heng BC, Zhao X, Xiong S, Ng KW, Boey FY, Loo JS. Toxicity of zinc oxide (ZnO) nanoparticles on human bronchial epithelial cells (BEAS-2B) is accentuated by oxidative stress. Food Chem Toxicol. 2010;48(6):1762–1766. doi: 10.1016/j.fct.2010.04.023. [DOI] [PubMed] [Google Scholar]
- 7.Lee S, Pie J-E, Kim Y-R, Lee H, Son S, Kim M-K. Effects of zinc oxide nanoparticles on gene expression profile in human keratinocytes. Mol Cell Toxicol. 2012;8(2):113–118. [Google Scholar]
- 8.Choi S-J, Lee J, Jeong J, Choy J-H. Toxicity evaluation of inorganic nanoparticles: considerations and challenges. Mol Cell Toxicol. 2013;9(3):205–210. [Google Scholar]
- 9.Jang Y, Lee E, Park Y-H, et al. The potential for skin irritation, phototoxicity, and sensitization of ZnO nanoparticles. Mol Cel Toxicol. 2012;8(2):171–177. [Google Scholar]
- 10.Sharma V, Singh P, Pandey AK, Dhawan A. Induction of oxidative stress, DNA damage and apoptosis in mouse liver after sub-acute oral exposure to zinc oxide nanoparticles. Mutat Res. 2012;745(1–2):84–91. doi: 10.1016/j.mrgentox.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 11.Yan G, Huang Y, Bu Q, et al. Zinc oxide nanoparticles cause nephrotoxicity and kidney metabolism alterations in rats. J Environ Sci Health A Tox Hazard Subst Environ Eng. 2012;47(4):577–588. doi: 10.1080/10934529.2012.650576. [DOI] [PubMed] [Google Scholar]
- 12.Surekha P, Kishore AS, Srinivas A, et al. Repeated dose dermal toxicity study of nano zinc oxide with Sprague–Dawley rats. Cutan Ocul Toxicol. 2012;31(1):26–32. doi: 10.3109/15569527.2011.595750. [DOI] [PubMed] [Google Scholar]
- 13.Pasupuleti S, Alapati S, Ganapathy S, Anumolu G, Pully NR, Prakhya BM. Toxicity of zinc oxide nanoparticles through oral route. Toxicol Ind Health. 2012;28(8):675–686. doi: 10.1177/0748233711420473. [DOI] [PubMed] [Google Scholar]
- 14.Ho M, Wu KY, Chein HM, Chen LC, Cheng TJ. Pulmonary toxicity of inhaled nanoscale and fine zinc oxide particles: mass and surface area as an exposure metric. Inhal Toxicol. 2011;23(14):947–956. doi: 10.3109/08958378.2011.629235. [DOI] [PubMed] [Google Scholar]
- 15.Yu B, Zhang Y, Zheng W, Fan C, Chen T. Positive surface charge enhances selective cellular uptake and anticancer efficacy of selenium nanoparticles. Inorganic chemistry. 2012;51(16):8956–8963. doi: 10.1021/ic301050v. [DOI] [PubMed] [Google Scholar]
- 16.Nagy A, Steinbruck A, Gao J, Doggett N, Hollingsworth JA, Iyer R. Comprehensive analysis of the effects of CdSe quantum dot size, surface charge, and functionalization on primary human lung cells. ACS Nano. 2012;6(6):4748–4762. doi: 10.1021/nn204886b. [DOI] [PubMed] [Google Scholar]
- 17.Yin H, Casey PS, McCall MJ, Fenech M. Effects of surface chemistry on cytotoxicity, genotoxicity, and the generation of reactive oxygen species induced by ZnO nanoparticles. Langmuir. 2010;26(19):15399–15408. doi: 10.1021/la101033n. [DOI] [PubMed] [Google Scholar]
- 18.Kim K-M, Kim T-H, Kim H-M, et al. Colloidal behaviors of ZnO nanoparticles in various aqueous media. Toxicol Environ Health Sci. 2012;4(2):121–131. [Google Scholar]
- 19.Organisation for Economic Co-operation and Development . Test No 408: Repeated Dose 90-Day Oral Toxicity Study in Rodents. Paris, France: OECD Publishing; [DOI] [Google Scholar]
- 20.Ministry of Agriculture, Food and Rural Affairs . Animal Protection Act. Republic of Korea; Korea Legislation Research Institute; 2011. [Accessed November 10, 2014]. Available from: http://elaw.klri.re.kr/eng_service/lawView.do?hseq=29131&lang=ENG. [Google Scholar]
- 21.Seok SH, Cho WS, Park JS, et al. Rat pancreatitis produced by 13-week administration of zinc oxide nanoparticles: biopersistence of nanoparticles and possible solutions. J Appl Toxicol. 2013;33(10):1089–1096. doi: 10.1002/jat.2862. [DOI] [PubMed] [Google Scholar]
- 22.Nriagu J. Zinc toxicity in humans. Encyclopedia of Environmental Health. 2011:801–807. [Google Scholar]
- 23.Plum LM, Rink L, Haase H. The essential toxin: impact of zinc on human health. Int J Environ Res Public Health. 2010;7(4):1342–1365. doi: 10.3390/ijerph7041342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van Campen DR. Copper interference with the intestinal absorption of zinc-65 by rats. J Nutr. 1969;97(1):104–108. doi: 10.1093/jn/97.1.104. [DOI] [PubMed] [Google Scholar]
- 25.Fiske DN, McCoy HE, III, Kitchens CS. Zinc-induced sideroblastic anemia: report of a case, review of the literature, and description of the hematologic syndrome. Am J Hematol. 1994;46(2):147–150. doi: 10.1002/ajh.2830460217. [DOI] [PubMed] [Google Scholar]
- 26.Sandstead HH. Requirements and toxicity of essential trace elements, illustrated by zinc and copper. Am J Clin Nutr. 1995;61(Suppl 3):621s–624s. doi: 10.1093/ajcn/61.3.621S. [DOI] [PubMed] [Google Scholar]
- 27.Wang B, Feng WY, Wang TC, et al. Acute toxicity of nano- and micro-scale zinc powder in healthy adult mice. Toxicol Lett. 2006;161(2):115–123. doi: 10.1016/j.toxlet.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 28.Wang B, Feng W, Wang M, et al. Acute toxicological impact of nano- and submicro-scaled zinc oxide powder on healthy adult mice. J Nanopart Res. 2008;10:263–276. [Google Scholar]
- 29.Khan AT, Graham TC, Ogden L, et al. A two-generational reproductive toxicity study of zinc in rats. J Environ Sci Health B. 2007;42(4):403–415. doi: 10.1080/03601230701312795. [DOI] [PubMed] [Google Scholar]
- 30.Guo D, Bi H, Wu Q, Wang D, Cui Y. Zinc oxide nanoparticles induce rat retinal ganglion cell damage through bcl-2, caspase-9 and caspase-12 pathways. J Nanosci Nanotechnol. 2013;13(6):3769–3777. doi: 10.1166/jnn.2013.7169. [DOI] [PubMed] [Google Scholar]
- 31.Guo D, Bi H, Liu B, Wu Q, Wang D, Cui Y. Reactive oxygen species-induced cytotoxic effects of zinc oxide nanoparticles in rat retinal ganglion cells. Toxicol In Vitro. 2013;27(2):731–738. doi: 10.1016/j.tiv.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 32.Paek HJ, Lee YJ, Chung HE, et al. Modulation of the pharmacokinetics of zinc oxide nanoparticles and their fates in vivo. Nanoscale. 2013;5(23):11416–11427. doi: 10.1039/c3nr02140h. [DOI] [PubMed] [Google Scholar]
- 33.Steuer H, Krastev R, Lembert N. Metallic oxide nanoparticles stimulate blood coagulation independent of their surface charge. J Biomed Mater Res B Appl Biomater. 2013 Dec 18; doi: 10.1002/jbm.b.33051. Epub. [DOI] [PubMed] [Google Scholar]