(See the major articles by Lichtner et al on pages 178–86 and Johnson et al on pages 187–96.) 10.1093/infdis/jiu417 10.1093/infdis/jiu424
Cytomegalovirus (CMV) is a complex virus that continues to contribute to morbidity in a range of patients. These include solid organ and stem cell transplant recipients, in whom the virus is associated with both direct effects, such as CMV hepatitis [1] and gastrointestinal disease, and indirect effects, including acute rejection, reduced long-term graft function, accelerated posttransplantation vascular disease, and, in some cases, death [2]. In addition, CMV remains an important cause of congenital disease, leading to a range of sequelae, including microcephaly, mental retardation, and sensorineural hearing loss [3]. In human immunodeficiency virus (HIV)–infected individuals, CMV was once one of the most feared pathogens because it led to sight-threatening retinitis in patients whose CD4+ T-cell counts dropped below 50 cells/µL, and despite anti-CMV therapy, recurrences of infection were frequent and often due to drug-resistant strains of CMV [4, 5]. Fortunately, in HIV-infected populations with access to effective antiretroviral therapy (ART), the incidence of CMV retinitis is now very rare, and CMV disease in HIV patients has become a distant memory for many physicians.
Given the strong association of CMV with inflammation [6] and a skewing of the T-cell immune response [7], it is noteworthy that in the early days of the HIV epidemic, CMV was suggested as a cofactor for HIV disease progression. A seminal article in this area was published in 1989 by Webster et al [8]. This study investigated 108 HIV type 1 (HIV-1)–infected hemophiliac men whose date of HIV seroconversion was accurately known; the men were then stratified according to their CMV immunoglobulin G (IgG) status. Individuals with prior CMV infection, as evidenced by serological findings, were 2.5 times as likely to progress to an AIDS-defining disease, compared with CMV-seronegative individuals, and survival analysis revealed that the risk increased approximately 2 years after seroconversion. At the time this study was published, it created some controversy, not least because a mechanistic basis for the accelerating effects of CMV on HIV disease progression was not readily available. Nevertheless, a further publication from the same group in 1995 showed that after 13 years of follow-up, the risks of CMV positivity on progression to AIDS, death, and a CD4+ T-cell count of 50 cells/µL were 2.28, 2.42, and 2.34, respectively, although the risks were influenced by patient age [9]. Interestingly, a double-blind, placebo-controlled study of high-dose acyclovir (800 mg 4 times daily) in patients with AIDS who had CD4+ T-cell counts of <150 cells/µL blood showed no effect on the incidence of CMV disease between the arms but found a significant survival benefit to those patients exposed to acyclovir (the odds ratio decreased from 0.39 to 0.23; P = .018) [10]. This survival effect was also observed in a meta-analysis of 8 trials of high-dose acyclovir involving 2947 patient-years of follow-up [11].
In the intervening years, especially with the advent of effective ART, interest in the CMV cofactor hypothesis for HIV progression has waned, even though studies in groups where ART is not widely available have shown that CMV DNAemia in HIV-infected mothers is a risk factor for 2-year mortality in both mothers and their infants (adjusted hazard ratio, 4.3) [12]. It is, thus, timely that the article by Lichtner et al (submitted) in this issue of The Journal of Infectious Diseases revisits the issue of CMV coinfection in a large cohort of HIV-infected individuals (n = 6111) participating in the Italian Cohort of Antiretroviral Naive Patients (ICONA) study. This study was set up in 1997 as an Italian multicenter observational study of HIV-1–infected individuals, and the power of such cohorts is beautifully demonstrated by the findings presented in their current report. Although CMV seroprevalence was high, at 83.8%, this study provided a sufficient number of CMV IgG–negative individuals to address questions relating to the influence of CMV seropositivity on the progression of HIV disease and the occurrence of specific HIV diseases. It was noteworthy that CMV seropositivity had little effect on the progression to an AIDS-defining event. However, when the authors investigated time to a severe non-AIDS event or non-AIDS death, a different story emerged. In this category, CMV seropositivity was associated with a statistically significant increase in events (from 6.2% to 8.9%), and it remained a significant increased risk in Cox regression models (hazard ratio, 1.53). The study went on to investigate whether CMV seropositivity was associated with an increased incidence of specific non-AIDS diseases, and the authors showed that only the incidence of cardiocerebral vascular diseases appeared to be elevated in patients with prior CMV infection. CMV has been extensively linked with cardiovascular disease in patients without HIV infection [13, 14], in both nontransplantion and posttransplantion populations, and the ability of CMV to alter the size and shape of the immune system may be relevant in the context of chronic activation of the vascular endothelium and the inflammatory response. Of note is the recent demonstration that CMV replication and a heightened T-cell immune response in patients with common variable immune deficiency disease leads to a range of inflammatory diseases [15].
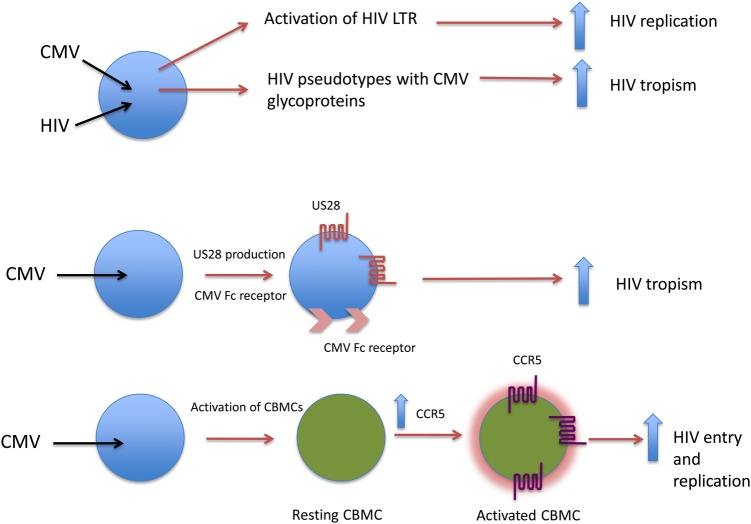
Many potential mechanisms have been put forward to explain how CMV could act as a cofactor for HIV disease progression [16]. These included direct coinfection of cells, leading to enhanced HIV replication; facilitating HIV uptake through the Fc receptor of CMV, providing alternative coreceptor pathways for virus entry; transactivation of the long terminal repeat; and viral pseudotype formation. Laboratory evidence for each of these mechanisms has been obtained, although the precise contribution that one or multiple pathways play in vivo remains elusive. In the latest addition to this list of potential mechanisms, an article by Johnson et al (submitted) in this issue of the Journal addresses this question in the context of the elevated frequency of in utero transmission of HIV-1 among mothers with ongoing or primary CMV infection. This study concentrated on using cord blood mononuclear cells (CBMCs), which the authors show have a relatively reduced ability to support HIV-1 replication in the absence of stimulatory signals. Flow cytometry found that these CBMCs had a lower fraction of CD45RO+ T memory cells (TCM), but this compartment could be increased when the CBMCs were stimulated with mitogens. Of particular interest was the observation that increases in the size of the TCM compartment also led to upregulation of CCR5 but not CXCR4, with the former reaching levels that were comparable to CCR5 expression in stimulated adult peripheral blood mononuclear cells. Given the central role of the chemokine coreceptors in HIV entry, low levels of CCR5, as observed in unstimulated CBMCs, likely mean that these cells are relatively refractory to HIV infection, whereas upregulation of CCR5 after stimulation would allow a significant elevation in HIV infection of these cells. On the basis of the evidence from in vivo studies that CMV infection enhances in utero transmission of HIV, the authors then proceeded to stimulate CBMCs by using a coculture method with CMV-infected adult macrophages. Of note, the coculture stimulation only led to a moderate increase in proliferation but resulted in a substantial increase in the TCM population, coupled with high levels of CCR5 expression. It was when the authors assessed the HIV susceptibility of these cells that the dramatic effects of CMV stimulation were revealed. Impressively, replication kinetics of HIV in the CMV-stimulated CBMCs was enhanced over that for cells treated with phytohemagglutinin and interleukin 2, and overall levels of gag and env messenger RNA were 607-fold and 421-fold greater, respectively, than levels for unstimulated CBMCs. A summary of these data and other potential mechanisms for CMV enhancement of HIV replication are shown in Figure 1.
Figure 1.
Potential mechanisms by which cytomegalovirus (CMV) might enhance human immunodeficiency virus (HIV) replication. Direct coinfection in cells such as macrophages could lead to enhanced HIV replication, through transactivation of the HIV long terminal repeat (LTR), or to enhanced HIV tropism, through pseudotype formation. Alternatively, CMV infection can lead to the production of the CMV G-coupled receptor US28 or its Fc receptor, leading to entry of HIV into cells that are normally not permissive. The bottom panel captures the data presented by Johnson et al (submitted), whereby CMV infection of macrophages leads to the activation of resting cord blood mononuclear cells, resulting in upregulation of CCR5, enhanced HIV entry, and increased HIV replication. Abbreviation: CBMC, cord blood mononuclear cell.
Together, these 2 articles should reinvigorate the interest in CMV as a cofactor for HIV disease progression and, indeed, mother-to-child transmission of HIV. The challenge remains to understand which of the various mechanisms are truly operational in vivo, including those that have been implicated in cardiovascular disease. The exciting availability of new anti-CMV drugs in the pipeline [17] may open up new opportunities for reinvestigating the effects of long-term inhibition of CMV replication in patients with HIV infection on end points such as cardiocerebral vascular diseases and HIV transmission from mother to infant.
Note
Potential conflicts of interest. Author certifies no potential conflicts of interest.
The author has submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Fishman JA, Rubin RH. Infection in organ-transplant recipients. N Engl J Med. 1998;338:1741–51. doi: 10.1056/NEJM199806113382407. [DOI] [PubMed] [Google Scholar]
- 2.Fishman JA, Emery V, Freeman R, et al. Cytomegalovirus in transplantation—challenging the status quo. Clin Transplant. 2007;21:149–58. doi: 10.1111/j.1399-0012.2006.00618.x. [DOI] [PubMed] [Google Scholar]
- 3.Manicklal S, Emery VC, Lazzarotto T, Boppana SB, Gupta RK. The "silent" global burden of congenital cytomegalovirus. Clin Microbiol Rev. 2013;26:86–102. doi: 10.1128/CMR.00062-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cytomegalovirus (CMV) culture results, drug resistance, and clinical outcome in patients with AIDS and CMV retinitis treated with foscarnet or ganciclovir. Studies of Ocular Complications of AIDS (SOCA) in collaboration with the AIDS Clinical Trial Group. J Infect Dis. 1997;176:50–8. doi: 10.1086/514039. [DOI] [PubMed] [Google Scholar]
- 5.Bowen EF, Emery VC, Wilson P, et al. Cytomegalovirus polymerase chain reaction viraemia in patients receiving ganciclovir maintenance therapy for retinitis. AIDS. 1998;12:605–11. doi: 10.1097/00002030-199806000-00009. [DOI] [PubMed] [Google Scholar]
- 6.Jeffery HC, Söderberg-Naucler C, Butler LM. Human cytomegalovirus induces a biphasic inflammatory response in primary endothelial cells. J Virol. 2013;87:6530–5. doi: 10.1128/JVI.00265-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lachmann R, Bajwa M, Vita S, et al. Polyfunctional T cells accumulate in large human cytomegalovirus-specific T cell responses. J Virol. 2012;86:1001–9. doi: 10.1128/JVI.00873-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Webster A, Lee CA, Cook DG, et al. Cytomegalovirus infection and progression towards AIDS in haemophiliacs with human immunodeficiency virus infection. Lancet. 1989;2:63–6. doi: 10.1016/s0140-6736(89)90312-7. [DOI] [PubMed] [Google Scholar]
- 9.Sabin CA, Phillips AN, Lee CA, Janossy G, Emery V, Griffiths PD. The effect of CMV infection on progression of human immunodeficiency virus disease is a cohort of haemophilic men followed for up to 13 years from seroconversion. Epidemiol Infect. 1995;114:361–72. doi: 10.1017/s095026880005799x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Youle MS, Gazzard BG, Johnson MA, et al. Effects of high-dose oral acyclovir on herpesvirus disease and survival in patients with advanced HIV disease: a double-blind, placebo-controlled study. European-Australian Acyclovir Study Group [published correction appears in AIDS 1994; 8:859] AIDS. 1994;8:641–9. doi: 10.1097/00002030-199405000-00010. [DOI] [PubMed] [Google Scholar]
- 11.Ioannidis JP, Collier AC, Cooper DA, et al. Clinical efficacy of high-dose acyclovir in patients with human immunodeficiency virus infection: a meta-analysis of randomized individual patient data. J Infect Dis. 1998;178:349–59. doi: 10.1086/515621. [DOI] [PubMed] [Google Scholar]
- 12.Slyker JA, Lohman-Payne BL, Rowland-Jones SL, et al. The detection of cytomegalovirus DNA in maternal plasma is associated with mortality in HIV-1-infected women and their infants. AIDS. 2009;23:117–24. doi: 10.1097/QAD.0b013e32831c8abd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Potena L, Holweg CT, Chin C, et al. Acute rejection and cardiac allograft vascular disease is reduced by suppression of subclinical cytomegalovirus infection. Transplantation. 2006;82:398–405. doi: 10.1097/01.tp.0000229039.87735.76. [DOI] [PubMed] [Google Scholar]
- 14.Terrazzini N, Bajwa M, Vita S, et al. A novel cytomegalovirus-induced regulatory-type T-cell subset increases in size during older life and links virus-specific immunity to vascular pathology. J Infect Dis. 2014;209:1382–92. doi: 10.1093/infdis/jit576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marashi SM, Raeiszadeh M, Workman S, et al. Inflammation in common variable immunodeficiency is associated with a distinct CD8(+) response to cytomegalovirus. J Allergy Clin Immunol. 2011;127:1385–93.e4. doi: 10.1016/j.jaci.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Griffiths PD. CMV as a cofactor enhancing progression of AIDS. J Clin Virol. 2006;35:489–92. doi: 10.1016/j.jcv.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 17.Chemaly RF, Ullmann AJ, Stoelben S, et al. AIC246 Study Team. Letermovir for cytomegalovirus prophylaxis in hematopoietic-cell transplantation. N Engl J Med. 2014;370:1781–9. doi: 10.1056/NEJMoa1309533. [DOI] [PubMed] [Google Scholar]