Abstract
A prevailing question in the Ypt/Rab field is whether these conserved GTPases are specific to cellular compartments. The established role for Ypt1 and its human homolog Rab1 is in endoplasmic reticulum (ER)-to-Golgi transport. More recently these regulators were implicated also in autophagy. Two different TRAPP complexes, I and III, were identified as the guanine-nucleotide-exchange factors (GEFs) of Ypt1 in ER-to-Golgi transport and autophagy, respectively. Confusingly, Ypt1 and TRAPP III were also suggested to regulate endosome-to-Golgi transport, implying that they function at multiple cellular compartments, and bringing into question the nature of Ypt/Rab specificity. Recently, we showed that the role of TRAPP III and Ypt1 in autophagy occurs at the ER and that they do not regulate endosome-to-Golgi transport. Here, we discuss the significance of this conclusion to the idea that Ypt/Rabs are specific to cellular compartments. We postulate that Ypt1 regulates 2 alternative routes emanating from the ER toward the Golgi and the lysosome/vacuole. We further propose that the secretory and endocytic/lysosomal pathways intersect in 2 junctures, and 2 Ypts, Ypt1 and Ypt31, coordinate transport in the 2 intersections: Ypt1 links ER-to-Golgi and ER-to-autophagy transport, whereas Ypt31 links Golgi-to-plasma membrane (PM) transport with PM-to-Golgi recycling through endosomes.
Keywords: cellular recycling, ER-phagy, endosome-to-Golgi transport, GTPase, Rab, secretory pathway, TRAPP, Ypt1, Ypt31
Introduction
The conserved Ypt/Rab GTPases regulate all vesicle-mediated transport steps of the exocytic (secretory) and endocytic pathways, and are considered to be highly specific to cellular compartments.1 When stimulated by nucleotide exchangers termed GEFs, the GTP-bound Ypt/Rabs interact with their multiple downstream effectors. These effectors then mediate the multiple steps of vesicular transport, from vesicle formation and motility to their tethering and fusion with the acceptor compartment.2 Recently, Ypt/Rab GTPases have also emerged as candidates for coordination of intra-cellular transport steps.3 An open question in the field is the nature of Ypt/Rab specificity: Are they specific to a particular transport pathway and/or a cellular organelle?
In the exocytic pathway, cargo is transported from the ER, through the Golgi, to the plasma membrane (PM). Our previous work has established that in yeast, 2 Ypts regulate Golgi entry and exit: Ypt1 regulates ER-to-cis Golgi transport and the functional pair Ypt31/Ypt32 GTPases regulate trans Golgi-to-PM transport.4-6 The human functional homolog of Ypt1, Rab1, also regulates ER-to-Golgi transport.7,8 In the endocytic pathway, cargo is transported from the PM through endosomes to the lysosome, a major degradative compartment. A different set of Ypt/Rabs regulates transport between the endocytic compartments.
In addition to the unidirectional exocytic and endocytic pathways, cells have 2 different cellular recycling pathways. The first, autophagy, recycles parts of the cytoplasm, including cytoplasmic organelles, for reuse of their building blocks. In this pathway, a double membrane organelle, the autophagosome, shuttles cargo for degradation in the lysosome (vacuole in yeast). While the origin of the autophagosomal membrane is not completely established, at least one source is thought to be the ER.9,10 In selective autophagy, specific proteins or organelles are sent for degradation, whereas upon starvation, non-selective autophagy is induced. We and others have shown that Ypt1 and Rab1 regulate selective and non-selective autophagy.11-17 Moreover, we identified Atg11 as a Ypt1 effector specific for its role in selective autophagy.11
The second pathway recycles proteins from the PM, including machinery components of exocytosis, for multiple rounds of function, by transporting these proteins through endosomes to the Golgi. We have established that Ypt31/32, together with their recycling-specific effector Rcy1, regulate a leg of this PM recycling pathway: endosome-to-Golgi transport.18
The modular TRAPP complex acts as a GEF for Ypt1 and Ypt31/32. Whereas TRAPP II, which resides in the trans Golgi, is a GEF for Ypt31/32,16,17 TRAPP I acts as a GEF for Ypt1 in the ER-to-Golgi transport step and the Trs85-containing TRAPP III stimulates Ypt1 during autophagy.11,12,16,19
However, in addition to their role in autophagy, a number of studies implicated Ypt1 and the TRAPP III-specific subunit Trs85 in the regulation of another transport step: endosome-to-Golgi. This idea is based on 2 different observations. First, specific ypt1 mutations and deletion of TRS85 that are not defective in ER-to-Golgi transport cause intra-cellular accumulation of Snc1.20-22 GFP-tagged Snc1 is commonly used as a marker for endosome-to-Golgi transport,23 and the presence of intracellular Snc1 in ypt1 and trs85Δ mutant cells was interpreted as evidence for a block in this step. Second, Ypt1 was reported to localize to a late Golgi compartment based on co-localization of fluorescently tagged Ypt1 with Sec7.21,24
The idea that Ypt1 and TRAPP III regulate multiple transport steps emanating from different origins raised multiple questions: why 2 Ypts, Ypt1 and Ypt31/32, are needed for the regulation of endosome-to-Golgi, what defines Ypt1 specificity if it indeed regulates 3 transport steps in at least 2 cellular locations, and how can one GEF, TRAPP III, regulate transport in 2 different processes? In our most recent paper, we address these puzzling questions and provide support for the Ypt/Rab compartment specificity model.25
Here, we discuss our recent findings that dispute the idea that Ypt1 regulates endosome-to-Golgi transport and point to limitations of the argument that Ypt1 resides in the trans Golgi. Finally, we outline a new paradigm for coordination of the secretory pathway with 2 fundamentally different cellular recycling pathways, autophagy and PM recycling, by Ypt/Rab GTPases.
Rigorous Analysis is Needed for Establishing An Endsome-to-Golgi Block
GFP-tagged Snc1 has been extensively used as a marker for PM recycling.23 For example, we have previously used its accumulation in endosomes to implicate a role for Ypt31/32 in the regulation of endosome-to-Golgi transport.18 Snc1, a v-SNARE that plays an essential role in fusion of trans-Golgi vesicles with the PM, is recycled for multiple rounds of function from the PM to the trans-Golgi through endosomes. However, intra-cellular accumulation of GFP-tagged Snc1 cannot be used as the only evidence for a role in this step for the following reason: like any other protein with a trans-membrane domain, Snc1 is inserted into cellular membranes at the ER. Consequently, its intra-cellular accumulation can result from either a block in the exocytic pathway en route to the PM, or a block in its recycling back to the PM. For example, we showed that intra-cellular accumulation GFP-tagged Snc1 can serve as a marker for an ER-phagy block.25 Therefore, additional analyses should be used to distinguish between these 2 possibilities.
We have used 4 additional assays to conclusively determine a block in endosome-to-Golgi transport in ypt and TRAPP mutant cells. The first assay utilizes the Snc1 variant GFP-Snc1-PEM. Once reaching the PM, this version of GFP-tagged Snc1 cannot be internalized.23 Therefore, intra-cellular accumulation of Snc1-PEM cannot be caused by a PM recycling defect, and indicates that Snc1 never reached the PM. The second assay entails quantifying co-localization of intra-cellular GFP-tagged Snc1 or Snc1-PEM with endosomal and ER markers. In the third assay, which was used to support Snc1 accumulation in the ER, we assessed induction of UPR, a cellular response induced when proteins accumulate in the ER (and not in endosomes). In the fourth assay we used Kex2-YFP as a second marker for determining a block in the endosome-to-Golgi transport step. In wild-type cells, Kex2, a furin homolog, resides mostly in the trans Golgi, but it cycles between the trans Golgi and endosomes. When endosome-to-Golgi transport is blocked, Kex2 is shuttled from endosomes to the vacuole for degradation 26. A block in endosome-to-Golgi transport of Kex2-YFP can be determined either by the lower level of Kex2-YFP protein using immuno-blot analysis, or by the loss of its punctate staining using live-cell microscopy.18
Ypt31/32 and TRAPP II, but not Ypt1 and TRAPP III, regulate endosome-to-Golgi transport
We have used the 4 aforementioned assays to characterize the roles of Ypt1, Ypt31/32 and their GEFs, in endosome-to-Golgi transport. For Ypt1, we used 2 mutations specifically defective in autophagy and not in ER-to-Golgi transport: ypt1-1 (T40K) and ypt1-T40A. These 2 mutations were characterized independently as mutations that do not affect ER-to-Golgi transport.5,14,21 Accumulation of intra-cellular GFP-tagged Snc1 in ypt1-T40A mutant cells was previously used as evidence for an endosome-to-Golgi block.21 Using the 4 assays we showed that both mutations confer a block in autophagy, and the presence of intracellular GFP-tagged Snc1 in these mutant cells is due to a defect in ER-phagy, a process which is induced by over-expression of GFP-tagged Snc1.11,25
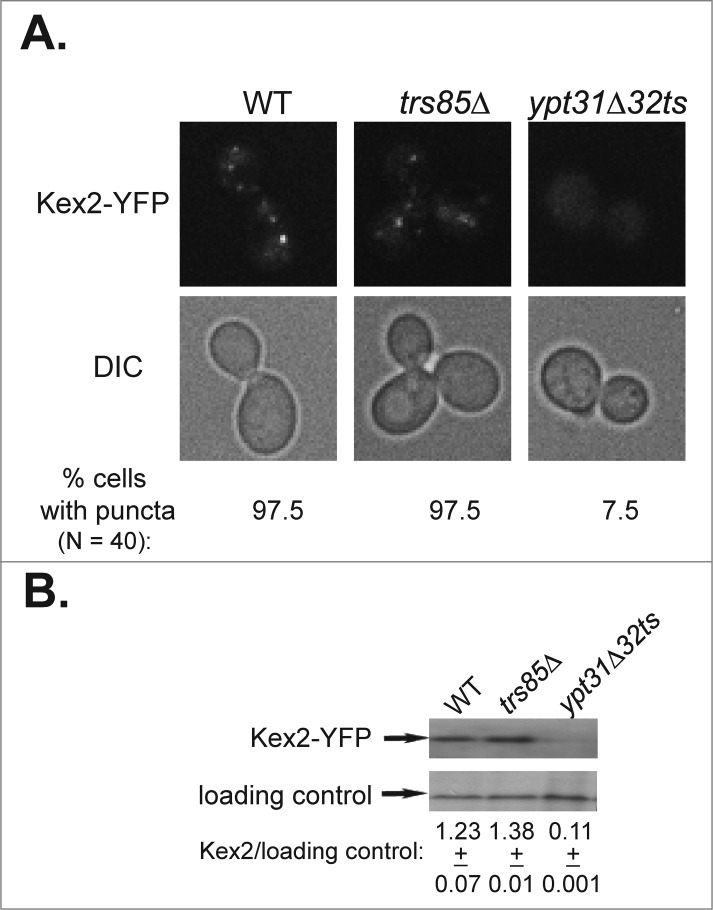
Likewise, we showed that trs85Δ mutant cells accumulate GFP-tagged Snc1 and Snc1-PEM in the ER and not in endosomes, and UPR is induced when GFP-tagged Snc1 is over-expressed.17,25 Additionally, we examined Kex2 as a marker for endosome-to-Golgi transport. Using the microscopic and the immuno-blot assays, we show that trs85Δ mutant cells are not defective in this transport step (Fig. 1).
Figure 1.
Endosome-to-Golgi transport of Kex2 requires Ypt31/32, but not Trs85. (A) Deletion of TRS85 does not affect the punctate distribution of the Golgi protein Kex2-YFP. Kex2 was tagged with YFP at its C-terminus in wild type, trs85Δ, and ypt31Δ/ypt32ts mutant cells. The intra-cellular distribution of Kex2-YFP was determined by live-cell microscopy. Shown from top to bottom: YFP, DIC, and% cells with puncta (N, number of cells visualized for each strain). In trs85Δ mutant cells, like in wild type cells, Kex2-YFP shows punctate distribution. As previously shown,18 in ypt31Δ/32ts mutant cells, which are defective in endosome-to-Golgi transport, Kex2-YFP is diffuse. (B) Deletion of TRS85 does not affect the level of Kex2-YFP. The level of Kex2-YFP protein in cells from panel A was determined using immuno-blot analysis and anti-GFP antibodies. From top to bottom: Kex2-YFP, loading control (G6PDH), and ratio of Kex2-YFP/loading control (+/−, STD). The level of Kex2-YFP in trs85Δ mutant cells is similar to that of the wild type, whereas it is lower in ypt31/32ts mutant cells, as previously shown.18 Results shown in this figure represent 2 independent experiments.
These same 4 rigorous assays were used to confirm a role for Ypt31/3218,25 and the TRAPP II-specific subunit Trs13017,25 in PM recycling and not in ER-phagy.
In addition, functional grouping of Ypt1 with TRAPP III and Ypt31/32 with TRAPP II was supported also by suppression analysis. Specifically, overexpression of Ypt1, but not Ypt31, suppresses the growth, Snc1 accumulation and autophagy phenotypes of trs85Δ mutant cells.11,17 In contrast over-expression of Ypt31, but not Ypt1, suppresses the growth, Snc1 accumulation and autophagy phenotypes of trs130ts mutant cells.17,27-30
Our evidence, that accumulation of intracellular Snc1 in ypt1 and trs85Δ mutant cells reflects an ER-phagy block and not an endosome-to-Golgi transport block, undermines the main support for a role for Ypt1 and TRAPP III in this transport step. Moreover, we have 3 reservations for using the co-localization of fluorescently-tagged Ypt1 with Sec7 as support for its role in endosome-to-Golgi transport.21 First, the functionality of mCherry-Ypt1 or GFP-Ypt1 and their level of expression were not shown.21,24 These 2 criteria are important for making conclusions about Ypt1 localization. Second, co-localization of Ypt1 with other late Golgi markers is needed before a conclusion about its localization to this compartment can be made, especially because YPT1 and SEC7 interact genetically.31 Lastly, the presence of a protein in a compartment downstream of where it functions cannot be taken as evidence for its function in the later compartment. Therefore, the jury is still out on both the localization of Ypt1 to a late Golgi compartment and the relevance of such localization to its function.
Based on our cumulative evidence, we conclude that Ypt1 and Trs85-containing TRAPP III are not required for endosome-to-trans Golgi transport, and the regulation of this transport step is reserved for the Ypt31/32 functional pair with Trs130-containing TRAPP II.
Coordination of the exocytic and cellular recycling pathways by Ypt/Rabs
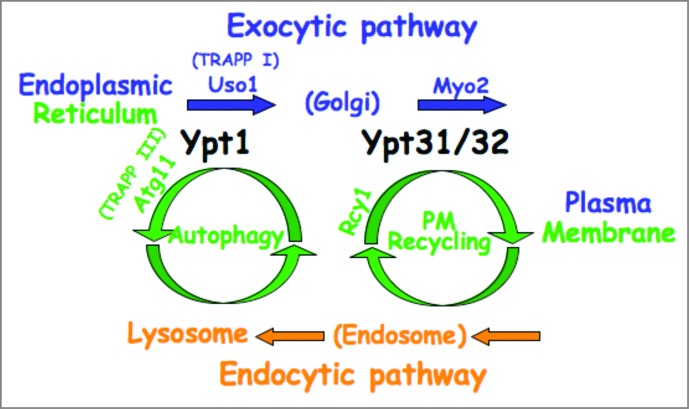
We propose a new way to think about trafficking inside cells: In our view, the exocytic pathway intersects with 2 cellular recycling pathways that connect it with endocytic compartments: at the early stage it intersects with autophagy, and at a later stage with PM recycling. Based on our collective data, we propose that a single Ypt/Rab regulates each juncture: Ypt1 in the first and Ypt31/32 in the second (Fig. 2).
Figure 2.
Model for coordination of 2 intersections of the exocytic and cellular recycling pathways by Ypt/Rab GTPases. We propose that the exocytic and endocytic pathways connect through 2 intersections with cellular recycling pathways: the early secretory pathway intersects with lysosomes via autophagy, whereas the late secretory pathway intersects with endosomes via PM recycling. We further postulate that Ypt1 with its autophagy-specific effector Atg11 coordinates trafficking through the first juncture, and Ypt31/32 with its PM-recycling effector Rcy1 through the second juncture.11,18 These Ypts also have secretory pathway-specific effectors: e.g., Uso1 and Myo2 for Ypt1 and Ypt31/32, respectively.37,38 For Ypt1, 2 different GEF complexes, TRAPP I and TRAPP III, regulate ER-to-Golgi transport and autophagy, respectively.
For each intersection we showed a recycling-specific ypt mutant phenotype, and identified a recycling-specific Ypt effector. For the first intersection, we characterized 2 ypt1 mutations defective specifically in autophagy and identified Atg11 as an autophagy-specific effector of Ypt1.11,25 Atg11 is a coiled-coil protein suggested to function as a tether in autophagy.32 For the second intersection, we showed a PM-recycling phenotype for ypt31Δ/32ts under permissive growth conditions that do not block Golgi exit, and identified Rcy1 as a PM recycling-specific effector of Ypt31/32.18 Rcy1 is a F-box protein that regulates ubiquitination of recycling cargo.33
We propose the following 3 principles as a new paradigm of coordination of the secretory pathway with 2 different cellular recycling pathways by Ypt/Rabs (Fig. 2). First, we suggest a “division of labor” for 2 Ypts: Ypt1 acts on the ER (or ER vesicles) to regulate their delivery to the right pathway, whereas Ypt31/32 act at the trans Golgi to regulate Golgi exit and entry from endosomes. This proposal is in agreement with a view of Ypt/Rabs as compartment specific regulators.1
Second, we propose a single Ypt/Rab can regulate different transport steps that involve the same compartment.25 For example, at the ER, Ypt1 regulates both ER-to-Golgi and ER-to-autophagy, wher eas at the trans-Golgi, Ypt31/32 regulate Golgi-to-PM and endosome-to-trans-Golgi. We reason that in each case, coordination of 2 alternative steps by a single Ypt/Rab is more efficient then regulation of the 2 steps by multiple Ypt/Rabs.
Third, we propose that the mechanism allowing Ypt/Rabs to function in different transport steps is assembly of different Ypt/Rab GTPase modules, where each module includes a module-specific effector and perhaps a module-specific GEF. For example, Ypt1 can assemble 2 different modules: To deliver cargo from the ER to the cis Golgi, the GEF is the TRAPP I complex,16,34,35 and an effector is Uso1.36,37 To shuttle cargo for degradation in the lysosome, Ypt1 assembles with TRAPP III GEF and the Atg11 effector.11,12 Ypt31/32 can also assemble in 2 modules. While it is not known yet if there are 2 different GEFs for each step, we know effectors specific for each step: Myo2 and Sec2 serve as effectors for the Golgi-to-PM step, and Rcy1 is an effector for the endosome-to-Golgi transport step.18,38,39
Future perspectives
At the mechanistic level, the hypothesis proposed above implies that, depending on the cargo, a single Ypt/Rab can assemble into different modules to shuttle membrane carriers from one compartment to different destinations. In addition to the GTPase, such modules contain a destination-specific effector, and maybe destination-specific GEF. This hypothesis entails that activated Ypt/Rabs are not free to interact with their effectors randomly, but that depending on the cargo and the GEF, they interact with specific effectors. While intriguing, this idea remains to be tested.
All the cellular processes discussed here are important for human health and disease. Defects in the secretory pathway can cause deficiencies in secretion of essential substances, like insulin in diabetes, or presentation of receptors or channels on the plasma membrane, e.g., megalin in renal disease.40,41 As in secretion, defects in recycling processes are also significant in human health. Failure to shuttle an excess of mis-folded membrane proteins for degradation in the vacuole by ER-phagy can have implications on neurodegenerative diseases.43 Defects in PM recycling can cause defects in the secretory pathway as well as in PM homeostasis, thereby contributing to an array of human diseases.44 Furthermore, the major players discussed here are conserved from yeast to humans. Therefore, we propose that coordination of cellular recycling processes with the secretory pathway by Ypt/Rabs is also conserved, and its mechanism is very likely important in human disease as well. Future studies in mammalian cells should test this idea and determine the mechanistic details of Ypt/Rab GTPase module organization.
Materials and Methods
TRS85 was deleted in wild type cells in which Kex2 was tagged at its C-terminus with YFP (NSY970) to construct trs85Δ Kex2-YFP (NSY1605). Wild type (NSY970), ypt31Δ/32ts (NSY972),18 and trs85Δ (NSY1605, this study) were used for Kex2-YFP microscopy and protein level analyses as previously described.18
Acknowledgments
We thank D Taussig and AU Hain for critical reading of the manuscript.
Funding Statement
This research was supported by grant GM-45444 from NIH to N Segev.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Zerial M, McBride H. Rab proteins as membrane organizers. Nat rev 2001; 2:107-7; PMID:; http://dx.doi.org/10.1038/35052055 [DOI] [PubMed] [Google Scholar]
- 2.Segev N. Ypt and Rab GTPases: insight into functions through novel interactions. Curr opin cell biol 2001; 13:500-11; PMID:; http://dx.doi.org/10.1016/S0955-0674(00)00242-8 [DOI] [PubMed] [Google Scholar]
- 3.Segev N. Coordination of intracellular transport steps by GTPases. Semincell dev biol 2011; 22:33-8; PMID:; http://dx.doi.org/10.1016/j.semcdb.2010.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jedd G, Mulholland J, Segev N. Two new Ypt GTPases are required for exit from the yeast trans-Golgi compartment. J cell biol 1997; 137:563-80; PMID:; http://dx.doi.org/10.1083/jcb.137.3.563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jedd G, Richardson C, Litt R, Segev N. The Ypt1 GTPase is essential for the first two steps of the yeast secretory pathway. J cell biol 1995; 131:583-90; PMID:; http://dx.doi.org/10.1083/jcb.131.3.583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Segev N. Mediation of the attachment or fusion step in vesicular transport by the GTP-binding Ypt1 protein. Science (New York, NY 1991; 252:1553-6. [DOI] [PubMed] [Google Scholar]
- 7.Haubruck H, Prange R, Vorgias C, Gallwitz D. The ras-related mouse ypt1 protein can functionally replace the YPT1 gene product in yeast. EMBO J 1989; 8:1427-32; PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pind SN, Nuoffer C, McCaffery JM, Plutner H, Davidson HW, Farquhar MG, Balch WE. Rab1 and Ca2+ are required for the fusion of carrier vesicles mediating endoplasmic reticulum to Golgi transport. J cell biol 1994; 125:239-52; PMID:; http://dx.doi.org/10.1083/jcb.125.2.239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tooze SA, Yoshimori T. The origin of the autophagosomal membrane. Nat Cell Biol 2010; 12:831-5; PMID: [DOI] [PubMed] [Google Scholar]
- 10.Yang Z, Klionsky DJ. An overview of the molecular mechanism of autophagy. Curr Top Microbiol Immunol 2009; 335:1-32; PMID:; http://dx.doi.org/10.1007/978-3-642-00302-8_1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lipatova Z, Belogortseva N, Zhang XQ, Kim J, Taussig D, Segev N. Regulation of selective autophagy onset by a YptRab GTPase module. Proc Nat Acad Scie U S A 2012; 109:6981-6; PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lynch-Day MA, Bhandari D, Menon S, Huang J, Cai H, Bartholomew CR, Brumell JH, Ferro-Novick S, Klionsky DJ. Trs85 directs a Ypt1 GEF, TRAPPIII, to the phagophore to promote autophagy. Proc Nat Acad Sci U S A 2010; 107:7811-6; PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pinar M, Pantazopoulou A, Penalva MA. Live-cell imaging of aspergillus nidulans autophagy: RAB1 dependence, golgi independence and ER involvement. Autophagy 2013; 9:1024-43; PMID:; http://dx.doi.org/10.4161/auto.24483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Segev N, Botstein D. The ras-like yeast YPT1 gene is itself essential for growth, sporulation, and starvation response. Mol Cell Biol 1987; 7:2367-77; PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zoppino FC, Militello RD, Slavin I, Alvarez C, Colombo MI. Autophagosome formation depends on the small GTPase Rab1 and functional ER exit sites. Traffic (Copenhagen, Denmark) 2010; 11:1246-61; PMID:; http://dx.doi.org/10.1111/j.1600-0854.2010.01086.x [DOI] [PubMed] [Google Scholar]
- 16.Morozova N, Liang Y, Tokarev AA, Chen SH, Cox R, Andrejic J, Lipatova Z, Sciorra VA, Emr SD, Segev N. TRAPPII subunits are required for the specificity switch of a Ypt-Rab GEF. Nat Cell Biol 2006; 8:1263-9; PMID: [DOI] [PubMed] [Google Scholar]
- 17.Zou S, Liu Y, Zhang XQ, Chen Y, Ye M, Zhu X, Yang S, Lipatova Z, Liang Y, Segev N. Modular TRAPP complexes regulate intracellular protein trafficking through multiple YptRab GTPases in Saccharomyces cerevisiae. Genetics 2012; 191:451-60; PMID:; http://dx.doi.org/10.1534/genetics.112.139378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen SH, Chen S, Tokarev AA, Liu F, Jedd G, Segev N. Ypt3132 GTPases and their novel F-box effector protein Rcy1 regulate protein recycling. Mol biol Cell 2005; 16:178-92; PMID:; http://dx.doi.org/10.1091/mbc.E04-03-0258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cai Y, Chin HF, Lazarova D, Menon S, Fu C, Cai H, Sclafani A, Rodgers DW, De La Cruz EM, Ferro-Novick S, Reinisch KM. The structural basis for activation of the Rab Ypt1p by the TRAPP membrane-tethering complexes. Cell 2008; 133:1202-13; PMID:; http://dx.doi.org/10.1016/j.cell.2008.04.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Montpetit B, Conibear E. Identification of the novel TRAPP associated protein Tca17. Traffic (Copenhagen, Denmark) 2009; 10:713-23; PMID:; http://dx.doi.org/10.1111/j.1600-0854.2009.00895.x [DOI] [PubMed] [Google Scholar]
- 21.Sclafani A, Chen S, Rivera-Molina F, Reinisch K, Novick P, Ferro-Novick S. Establishing a role for the GTPase Ypt1p at the late Golgi. Traffic (Copenhagen, Denmark) 2010; 11:520-32; PMID:; http://dx.doi.org/10.1111/j.1600-0854.2010.01031.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shirahama-Noda K, Kira S, Yoshimori T, Noda T. TRAPPIII is responsible for vesicular transport from early endosomes to Golgi, facilitating Atg9 cycling in autophagy. J Cell Sci 2013; 126:4963-73; PMID:; http://dx.doi.org/10.1242/jcs.131318 [DOI] [PubMed] [Google Scholar]
- 23.Lewis MJ, Nichols BJ, Prescianotto-Baschong C, Riezman H, Pelham HR. Specific retrieval of the exocytic SNARE Snc1p from early yeast endosomes. Mol biol Cell 2000; 11:23-38; PMID:; http://dx.doi.org/10.1091/mbc.11.1.23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suda Y, Kurokawa K, Hirata R, Nakano A. Rab GAP cascade regulates dynamics of Ypt6 in the Golgi traffic. Proc Nat Acad Sci USA 2013; 110:18976-81; PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lipatova Z, Shah AH, Kim JJ, Mulholland JW, Segev N. Regulation of ER-phagy by a YptRab GTPase module. Mol biol Cell 2013; 24:3133-44; PMID:; http://dx.doi.org/10.1091/mbc.E13-05-0269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brickner JH, Fuller RS. SOI1 encodes a novel, conserved protein that promotes TGN-endosomal cycling of Kex2p and other membrane proteins by modulating the function of two TGN localization signals. J Cell Biol 1997; 139:23-36; PMID:; http://dx.doi.org/10.1083/jcb.139.1.23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sciorra VA, Audhya A, Parsons AB, Segev N, Boone C, Emr SD. Synthetic genetic array analysis of the PtdIns 4-kinase Pik1p identifies components in a Golgi-specific Ypt31rab-GTPase signaling pathway. Mol biol Cell 2005; 16:776-93; PMID:; http://dx.doi.org/10.1091/mbc.E04-08-0700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamamoto K, Jigami Y. Mutation of TRS130, which encodes a component of the TRAPP II complex, activates transcription of OCH1 in Saccharomyces cerevisiae. Curr genet 2002; 42:85-93; PMID:; http://dx.doi.org/10.1007/s00294-002-0336-5 [DOI] [PubMed] [Google Scholar]
- 29.Zhang CJ, Bowzard JB, Greene M, Anido A, Stearns K, Kahn RA. Genetic interactions link ARF1, YPT3132 and TRS130. Yeast (Chichester, England) 2002; 19:1075-86; PMID:; http://dx.doi.org/10.1002/yea.903 [DOI] [PubMed] [Google Scholar]
- 30.Zou S, Chen Y, Liu Y, Segev N, Yu S, Liu Y, Min G, Ye M, Zeng Y, Zhu X, Hong B, Bjorn LO, Liang Y, Li S, Xie Z. Trs130 participates in autophagy through GTPases Ypt3132 in Saccharomyces cerevisiae. Traffic (Copenhagen, Denmark) 2013; 14:233-46; PMID:; http://dx.doi.org/10.1111/tra.12024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jones S, Jedd G, Kahn RA, Franzusoff A, Bartolini F, Segev N. Genetic interactions in yeast between Ypt GTPases and Arf guanine nucleotide exchangers. Genetics 1999; 152:1543-56; PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Backues SK, Klionsky DJ. Atg11: a Rab-dependent, coiled-coil membrane protein that acts as a tether for autophagy. Autophagy 2012; 8:1275-8; PMID:; http://dx.doi.org/10.4161/auto.21153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen SH, Shah AH, Segev N. Ypt3132 GTPases and their F-Box effector Rcy1 regulate ubiquitination of recycling proteins. Cell Log 2011; 1:21-31; PMID:; http://dx.doi.org/10.4161/cl.1.1.14695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jones S, Newman C, Liu F, Segev N. The TRAPP complex is a nucleotide exchanger for Ypt1 and Ypt3132. Mol Biol Cell 2000; 11:4403-11; PMID:; http://dx.doi.org/10.1091/mbc.11.12.4403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang W, Sacher M, Ferro-Novick S. TRAPP stimulates guanine nucleotide exchange on Ypt1p. J Cell Biol 2000; 151:289-96; PMID:; http://dx.doi.org/10.1083/jcb.151.2.289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Allan BB, Moyer BD, Balch WE. Rab1 recruitment of p115 into a cis-SNARE complex: programming budding COPII vesicles for fusion. Science (New York, NY 2000; 289:444-8; http://dx.doi.org/10.1126/science.289.5478.444 [DOI] [PubMed] [Google Scholar]
- 37.Cao X, Ballew N, Barlowe C. Initial docking of ER-derived vesicles requires Uso1p and Ypt1p but is independent of SNARE proteins. EMBO J 1998; 17:2156-65; PMID:; http://dx.doi.org/10.1093/emboj/17.8.2156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lipatova Z, Tokarev AA, Jin Y, Mulholland J, Weisman LS, Segev N. Direct interaction between a myosin V motor and the Rab GTPases Ypt3132 is required for polarized secretion. Mol Biol Cell 2008; 19:4177-87; PMID:; http://dx.doi.org/10.1091/mbc.E08-02-0220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ortiz D, Medkova M, Walch-Solimena C, Novick P. Ypt32 recruits the Sec4p guanine nucleotide exchange factor, Sec2p, to secretory vesicles; evidence for a Rab cascade in yeast. J Cell Biol 2002; 157:1005-5; PMID:; http://dx.doi.org/10.1083/jcb.200201003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Doyle ME, Egan JM. Pharmacological agents that directly modulate insulin secretion. Pharmacol Rev 2003; 55:105-31; PMID:; http://dx.doi.org/10.1124/pr.55.1.7 [DOI] [PubMed] [Google Scholar]
- 41.Vicinanza M, Di Campli A, Polishchuk E, Santoro M, Di Tullio G, Godi A, Levtchenko E, De Leo MG, Polishchuk R, Sandoval L, Marzolo MP, De Matteis MA. OCRL controls trafficking through early endosomes via PtdIns4,5P(2)-dependent regulation of endosomal actin. EMBO J 2011; 30:4970-85; PMID:; http://dx.doi.org/10.1038/emboj.2011.354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Farinha CM, Matos P, Amaral MD. Control of cystic fibrosis transmembrane conductance regulator membrane trafficking: not just from the endoplasmic reticulum to the Golgi. FEBS J 2013; 280:4396-06; PMID:; http://dx.doi.org/10.1111/febs.12392 [DOI] [PubMed] [Google Scholar]
- 43.Deegan S, Saveljeva S, Gorman AM, Samali A. Stress-induced self-cannibalism: on the regulation of autophagy by endoplasmic reticulum stress. Cell Mol Life Sci 2012; 70(14):2425-41; PMID:; http://dx.doi.org/10.1007/s00018-012-1173-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li X, DiFiglia M. The recycling endosome and its role in neurological disorders. Progress Neurobiol 2012; 97:127-41; PMID:; http://dx.doi.org/10.1016/j.pneurobio.2011.10.002 [DOI] [PubMed] [Google Scholar]