Abstract
Silicon dioxide (SiO2) nanoparticles (NPs) have been widely used in the biomedical field, such as in drug delivery and gene therapy. However, little is known about the biological effects and potential hazards of SiO2. Herein, the colloidal SiO2 NPs with two different sizes (20 nm and 100 nm) and different charges (L-arginine modified: SiO2EN20[R], SiO2EN100[R]; and negative: SiO2EN20[−], SiO2EN100[−] were orally administered (750 mg/kg/day) in female C57BL/6 mice for 14 days. Assessments of immunotoxicity include hematology profiling, reactive oxygen species generation and their antioxidant effect, stimulation assays for B- and T-lymphocytes, the activity of natural killer (NK) cells, and cytokine profiling. In vitro toxicity was also investigated in the RAW 264.7 cell line. When the cellularity of mouse spleen was evaluated, there was an overall decrease in the proliferation of B- and T-cells for all the groups fed with SiO2 NPs. Specifically, the SiO2EN20(−) NPs showed the most pronounced reduction. In addition, the nitric oxide production and NK cell activity in SiO2 NP-fed mice were significantly suppressed. Moreover, there was a decrease in the serum concentration of inflammatory cytokines such as interleukin (IL)-1β, IL-12 (p70), IL-6, tumor necrosis factor-α, and interferon-γ. To elucidate the cytotoxicity mechanism of SiO2 in vivo, an in vitro study using the RAW 264.7 cell line was performed. Both the size and charge of SiO2 using murine macrophage RAW 264.7 cells decreased cell viability dose-dependently. Collectively, our data indicate that different sized and charged SiO2 NPs would cause differential immunotoxicity. Interestingly, the small-sized and negatively charged SiO2 NPs showed the most potent in vivo immunotoxicity by way of suppressing the proliferation of lymphocytes, depressing the killing activity of NK cells, and decreasing proinflammatory cytokine production, thus leading to immunosuppression.
Keywords: silicon dioxide, nanoparticle, immunotoxicity, oxidative stress, cytokines, immunosuppression
Introduction
The growing fabrication and characterization of silica nanoscale materials has received attention in biomedical research, such as in the development of biosensors, enzyme immobilization, controlled drug release and delivery, and cellular uptake.1–6 Since these various studies on nanomedicine have emerged, the medical applications of nanoparticles (NPs) were highlighted, especially for the development of anticancer agents. Mesoporous silica NPs (MSNs) attracted great attention in the last few decades for their wider plausible application in the emerging field of nanomedicine. MSNs are inorganic nanocarriers that are known to be highly stable in a physicochemical and biochemical context; thus, they are vitally important in the construction of anticancer medicine.6,7 Recently, these MSNs received United States Food and Drug Administration approval as inorganic carriers in nanomedicine, and they are considered one of the most promising inorganic nanobiomaterials.7,8 Their potential applications seem endless based on the unique physicochemical characteristics of this nanomaterial, such as varying sizes, shapes, chemical composition, and assembly.9 Meantime, the special physicochemical characteristics of silica posed concerns about their potential environmental and health implications.10 To date, animal exposure to colloidal silica confirmed liver damage11 and moderate to severe pulmonary inflammation and tissue damage, primarily induced by oxidative stress and apoptosis.12,13 The physicochemical properties play an important role in the toxic reaction of silica NPs. Small particles mean that there is a larger surface area, and this might indicate an increase in surface reactivity, which enables the NPs to interact with cell biomolecules.9 The altered surface charge of NPs provides a unique way to facilitate their uptake into the interior structure of the cells.14 However, most studies have focused on pulmonary and liver toxicity; very few studies have overlooked the toxicological effects of silica NPs on the immune response in vivo.15–17 In addition, the influence of NP properties (for example, size, surface charge) on their potential hazards to the biological system needs to be elucidated.
In this study, we investigated the potential immunotoxicity of colloidal silicon dioxide (SiO2) NPs with two different sizes (20 nm and 100 nm) and different charges (L-arginine modified: SiO2EN20[R], SiO2EN100[R]; and negative: SiO2EN20[−], SiO2EN100[−]) in mice and in the RAW 264.7 cell line. Accordingly, cytotoxicity was performed in vitro using the Cell Counting Kit-8 (CCK-8; Dojindo Molecular Technologies, Inc., Rockville, MD, USA). The primary indicators of immune toxicity were also assessed in vivo, such as body weight measurement and hematology profiles. The NP-induced oxidative effect was also examined with reactive oxygen species (ROS) generation, superoxide dismutase (SOD) activity, and intracellular levels of glutathione peroxidase (GPx). The cellularity of the spleen and analysis of the functional capacity of specific immune cells were evaluated by stimulation assays for B- and T-lymphocytes, and the activity of natural killer (NK) cells was examined. In addition, we also focused on the inflammatory responses induced by NPs; therefore, the concentration of cytokines was determined.
Materials and methods
Preparation of particle suspensions
Manufactured SiO2 NPs were purchased from E&B Nanotech Co, Ltd (Ansan, Republic of Korea). The colloidal SiO2 NPs were already coated with citrate, and they had these following characteristics: the size and zeta potential of 20 nm (SiO2EN20[−] and 100 nm (SiO2EN100[−]) are 20±2 nm; −19 mV and 90±13 nm; −40 mV, respectively. To shift the strong negative charge of SiO2 NPs to a positive direction, their surface was modified with L-arginine (R) (SiO2EN20[R], SiO2EN100[R]), as reported.18 After the surface modification with L-arginine, the size and zeta potential of 20 nm and 100 nm became: 20±2 nm; −9 mV (SiO2EN20[R]) and 92±9 nm; −22 mV (SiO2EN100[R]). Then, powdered particle suspensions were prepared by dispersing the particles in 1× phosphate buffered saline (PBS), and to prevent agglomeration and to ensure uniform suspension, particles were sonicated in an ultrasonic bath (Hielscher Ultrasonics GmbH, Teltow, Germany) for 5–10 minutes.
Cell culture
The RAW 264.7 mouse leukemic monocyte macrophage cell line (American Type Culture Collection, Manassas, VA, USA) was maintained in Dulbecco’s Modified Eagle’s Medium (HyClone; Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 10% (v/v) heat-activated fetal bovine serum (FBS) (HyClone; Thermo Fisher Scientific) and 1% (v/v) antibiotic-antimycotic (Gibco®; Life Technologies) at 37°C in a 5% CO2 incubator.
Cell viability assay
Approximately 1×105 of RAW 264.7 cells were seeded into each well of 96-well plates. After reaching 70% confluence, the culture medium in the plate was replaced with a freshly prepared 100 μL of SiO2 NP suspension, which was diluted to appropriate concentrations (10 μg/mL, 20 μg/mL, 40 μg/mL, 80 μg/mL, 160 μg/mL, 320 μg/mL, and 640 μg/mL) with the culture medium, and the cells were incubated for 24 hours at 37°C in a 5% CO2 incubator. Cells not treated with SiO2 NPs served as a control in the experiment. Afterwards, a commercially available CCK-8 was used to evaluate the cytotoxic effect of SiO2 NPs. Then, 10 μL of the CCK-8 solution was added to each well and incubated at 37°C for 1 hour. Colored supernatants were measured at 450 nm by a DTX-880 multimode microplate reader (Beckman Coulter, Inc., Brea, CA, USA). All experiments were performed in triplicate.
Animals and exposure
For this experiment, 6-week-old female C57BL/6 (number [n]=5) mice weighing 18±2 g were purchased from Orient Bio Inc. (Seongnam, Republic of Korea) and were maintained at 22°C±2°C and 40%–60% humidity under a 12:12 hours light–dark cycle. The mice were acclimatized for 1 week and randomly assigned to one of five groups: normal control (purified water alone); or one of four experimental groups treated with four types of SiO2 NPs (SiO2EN20[R], SiO2EN100[R], SiO2EN20[−], and SiO2EN100[−]), respectively. All of the animals were orally administered 750 mg/kg of an SiO2 NP suspension daily for 2 continuous weeks. During the administration periods, the animals’ body weight was recorded. The animal use protocol for this experiment was approved by the Institutional Animal Care and Use Committee (IACUC) of Wonju College of Medicine, Yonsei University Wonju Campus (Wonju-si, Gangwon-do, Republic of Korea).
Analysis of blood samples
Animals were sacrificed following the 2-week oral administration of SiO2 NPs. Their blood was collected from the retro-orbital venous plexus, and the total white blood cell (WBC) count was collected in tubes coated with an anticoagulant. The blood was mixed with an automatic mixer for 5 minutes, and the WBCs and their differential counts (such as neutrophils, lymphocytes, monocytes, eosinophils, and basophils) were determined by an automatic blood analyzer (Hemavet® HV950 FS; Drew Scientific, Erba Diagnostics, Inc., Dallas, Texas, USA).
Preparation of splenocytes
For the isolation of splenocytes, spleens were aseptically removed from recently sacrificed mice and the tissue was transferred to a tube containing 1× PBS on ice. Splenocyte suspensions were prepared by blandly squeezing the spleen between the frosted ends of the two sterile microscope slides into a 100 mm tissue culture grade Petri dish. The slides were rinsed at regular intervals with 1× PBS. The cells were formed into a single suspension using a pipette. After that, the cell suspensions were filtered through a cell strainer. Next, the cell suspensions were centrifuged at 1,500 rpm at 4°C for 5 minutes to produce pellets. For the optimal lysis of erythrocytes, the pellets were resuspended in 5 mL of red blood cell lysis buffer and incubated on ice for 5 minutes with occasional shaking. The reaction was stopped by diluting the lysis buffer with 25 mL of 1× PBS. Thereafter, cells were spun (1,500 rpm at 4°C for 5 minutes), and the supernatant was carefully removed. The pellet was then washed two times in 1× PBS and resuspended in Roswell Park Memorial Institute (RPMI)-1640 supplemented with 3% FBS and 1% antibiotic–antimycotic. Cells were then counted. The viability of the cells used in all the experiments was higher than 95%, as measured by the trypan blue exclusion method (Sigma-Aldrich, St Louis, MO, USA).
Immune cell proliferation
Single cell suspensions of lymphocytes from the spleen were prepared. Splenocytes were counted in a hemocytometer, and 1×105 cells per well were suspended in a 96-well flat-bottom plate in 100 μL of RPMI-1640 supplemented with 10% heat-activated FBS and 1% antibiotics. Thereafter, cells were treated with 2.5 mg/mL of Concanavalin A (Con-A) (Sigma-Aldrich) and 1 mg/mL lipopolysaccharide (LPS) (Sigma-Aldrich) with 1 μL/well. The treated cells were incubated at 37°C in a humidified atmosphere under 5% CO2 for 2 hours. Cell proliferation was determined using a CCK-8 kit according to the manufacturer’s instructions. Absorbance was measured at 450 nm by a DTX-880 multimode microplate reader.
NK cytotoxicity assay
The murine lymphoma cancer cell line, YAC-1 (American Type Culture Collection), as target cells were cocultured with NK-enriched murine splenocytes. The percentage of target cells killed by effector NK cells was determined. Briefly, various effector cell dilutions were prepared: 1×105 cells/well for 100:1; 5×104 cells/well for 50:1; and 2.5×104 cells/well for 25:1. The target cells (1×103 cells/well) were then cocultured with the different effector cell dilutions prepared in a 96-well plate and incubated for 6 hours at 37°C in a humidified incubator with 5% CO2. The CCK-8 was conducted according to the manufacturer’s instructions. A special highly water soluble tetrazolium salt (WST-8) (2-[2-methoxy-4-nitrophenyl]-3[4-nitrophenyl]-5[2-4disulfophenyl]-2H-tetrazolium, monosodium salt) was added to the culture. WST-8 is reduced by dehydrogenase activities in cells to give the orange formazan dye, which is soluble in the culture media. The amount of the formazan dye generated by dehydrogenase in cells is directly proportional to the number of living cells. After 1 hour of incubation with the WST-8 solution, the cell suspension was then colorimetrically measured at 450 nm by a DTX-880 multimode microplate reader, and the number of live cells at the different ratios was determined.
Measurement of nitric oxide (NO) production
The nitrite (NO2−) present in the supernatant of splenocytes was used as an indicator of nitric oxide (NO) production using the Griess reagent (Promega Corporation, Fitchburg, WI, USA). The assay relies on measuring nitrite (NO2−), one of the primary, stable, and nonvolatile breakdown products of NO. Briefly, 50 μL of the splenocyte supernatant was mixed with an equal volume of Griess reagent in a 96-well microtiter plate and incubated at room temperature for 15 minutes. The absorbance was read at 540 nm using a DTX-880 multimode microplate reader. The NO2− concentration was calculated by comparison with the representative NO2− standard curve generated by serial two-fold dilutions of sodium nitrate.
Intracellular ROS detection
2′,7′-Dichlorodihydrofluorescein diacetate (DCFH-DA) was used to detect the intracellular ROS level. DCFH-DA can enter into cells as a fluorescent probe, and be acetylated to form DCFH (2′,7′ dichlorofluorescin) trapped in the cells. The oxidation of DCFH (nonfluorescent) by the intracellular ROS transforms it to DCF (dichlorofluorescein) (fluorescent). The fluorescent intensity of DCF can indicate intracellular ROS quantity. The spleens from mice were collected, and splenocytes were prepared as described in the “Preparation of splenocytes” section. Splenocytes (1×106 cells) were incubated with 10 μmol/L of DCFH-DA for 30 minutes at 37°C in the dark. After washing twice with PBS, the splenocytes were analyzed by a DTX-880 multimode microplate reader at an excitation wavelength of 488 nm and an emission wavelength of 525 nm.
Antioxidant endogenous enzyme activities
The activity of SOD and GPx were measured using a BioVision kit (BioVision, Inc., Milpitas, CA, USA). Splenocyte suspensions were lysed in ice-cold radioimmunoprecipitation assay buffer, the crude cell lysate was centrifuged at 14,000 rpm for 5 minutes at 4°C, and the cell debris was discarded. The supernatant was then checked for protein concentration using the Bradford method.19 Thereafter, supernatant was used to measure the activities of different antioxidant enzymes (SOD, GPx) according to the manufacturer’s instructions (BioVision, Inc., Milpitas, CA, USA).
Multiplex cytokine analysis
Serum levels of selected cytokines, such as interleukin (IL)-1β, IL-6, IL-10, IL-12p70, tumor necrosis factor (TNF)-α, and interferon (IFN)-γ, were measured using a Luminex bead-based suspension array system (Bio-Rad Laboratories, Hercules, CA, USA). This Luminex-based multiplexed system combines the principle of a sandwich immunoassay with fluorescent bead-based technology. Briefly, each set of premixed beads coated with the target antibodies was added to the well, and it was incubated with the sample in a 96-well round-bottomed microtiter plate to react with specific analytes. Then, premixed detection antibodies were added to the wells followed by a fluorescently labeled reporter molecule that specifically binds the analyte. Standard curves for each cytokine were generated using the standard control concentrations provided in the kit. Each step requires a specific incubation time, with shaking at room temperature and washing steps. All washes were performed using a Bio-Plex® Pro wash station (Bio-Rad Laboratories). Finally, the samples were then read using the Bio-Plex® suspension array reader, and the raw fluorescence data were analyzed using the Bio-Plex® Manager™ software using five-parameter logistic fitting (Bio-Rad Laboratories).
Statistical analysis
For the cell viability assay, the half maximal effective concentration (EC50) was calculated via nonlinear regression using GraphPad Prism version 5.0 software (GraphPad Software, Inc., La Jolla, CA, USA). For the remaining in vivo analysis, data values were expressed as the mean ± standard deviation. The mean values among the groups were analyzed and compared using one-way analysis of variance followed by a subsequent multiple comparison test (Tukey) with the GraphPad Prism version 5.0 software package. Differences were considered statistically significant at P<0.05, P<0.01, and P<0.001.
Results
Cytotoxicity assay
After exposure for 24 hours at varying doses of SiO2, murine macrophage RAW 264.7 cell viability detected by the CCK-8 assay resulted in an explicit dose-dependent reduction (Figure 1). Also, size- and electrostatic charge-dependent cytotoxicity of SiO2 NPs were found. The EC50 values after 24 hours of exposure were 71.10 ug/mL (SiO2EN20[−]), 211.4 ug/mL (SiO2EN100[−]), 233.2 ug/mL (SiO2EN20[R]), and 833.6 ug/mL (SiO2EN100[R]).
Figure 1.
Effect of differently sized and electrostatic charged SiO2 NPs on the viability of murine macrophage RAW 264.7 cells.
Notes: Cells were incubated with indicated concentrations of colloidal SiO2 NPs: (A) SiO2EN20(−); (B) SiO2EN100(−); (C) SiO2EN20(R); and (D) SiO2N100(R) for 24 hours. Cells not treated with SiO2 NPs served as the control in the experiment. A commercially available cell viability assay Cell Counting Kit-8 was used to evaluate the cytotoxicity effect of the SiO2 NPs according to manufacturer’s instructions (Dojindo Molecular Technologies, Inc., Rockville, MD, USA). Data are presented as the mean ± standard deviation. *P<0.05; **P<0.01; ***P<0.001 versus control (0 μg/mL).
Abbreviations: SiO2EN(−), negatively charged silicon dioxide; SiO2EN(R), silicon dioxide modified with L-arginine; SiO2, silicon dioxide; NPs, nanoparticles.
Body weight measurement
Body weight gain or loss may indicate an indirect immunotoxic effect. To evaluate the toxicity of SiO2 NPs, mice were fed 750 mg/kg of SiO2 every day for 2 weeks, and their body weight condition was monitored every 5 days for 2 weeks. After 2 weeks of feeding with SiO2 NPs, the groups SiO2EN20(−) and SiO2EN100(R) showed significant elevated body weight (P<0.05) compared to the nontreated group (NC). However, the SiO2EN20(R)-fed group showed significant depressions in body weight (P<0.05) compared to the NC. Further, the SiO2EN100(−)-fed group showed no obvious difference in body weight compared to the NC (Figure 2).
Figure 2.
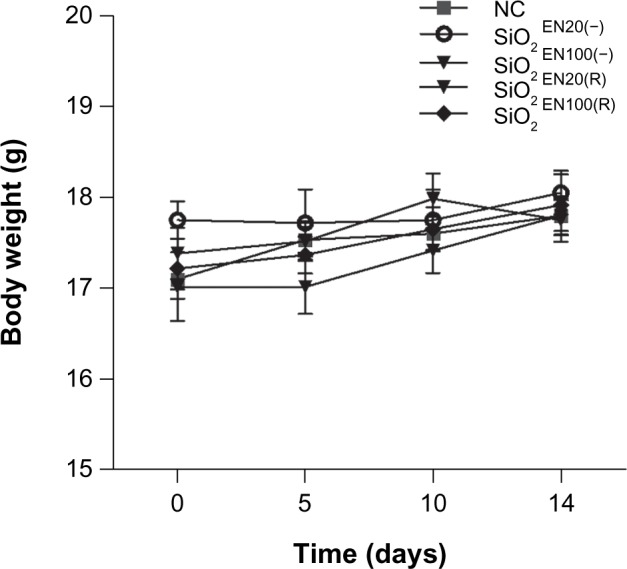
The effect on body weight of SiO2 NPs fed to mice.
Notes: C57BL/6 mice were treated with varying sizes (20 nm, 100 nm) and charges (SiO2EN[R]; negative, SiO2EN[−]) of 750 mg/kg colloidal SiO2 NPs for 14 days. Body weight was measured all throughout the experiment period, and it served as one of the primary indicators of SiO2 NP immune toxicity. Data are presented as the mean ± standard deviation; n=5.
Abbreviations: NC, normal control; SiO2EN(−), negatively charged silicon dioxide; SiO2EN(R), silicon dioxide modified with L-arginine; SiO2, silicon dioxide; NP, nanoparticle; n, number.
White blood cells (WBCs) and their differential count
Total WBC counts are used to evaluate the severity of inflammation. The altered WBC counts may be indicative of direct or indirect effects of the NPs on cellular proliferation. The WBCs of mice fed orally with SiO2EN100(−) for 14 days showed depressed counts, which were most prominent on the differential count of lymphocytes (Table 2). Further, the SiO2EN20(−)-fed group showed slight decreases in WBC count and each type of WBC: lymphocytes and eosinophils. Moreover, the SiO2EN100(R)-fed group showed no obvious change in WBC count compared to the NC group (Table 1). However, the SiO2EN20(R)-fed group showed an elevated WBC count for each type of WBC.
Table 2.
Total WBC and type of WBC counts in negatively charged SiO2 NP-fed mice
| WBC and members | NC | SiO2EN20(−) | SiO2EN100(−) |
|---|---|---|---|
| Total WBC, ×109/L | 6.047±0.797 | 5.623±0.737 | 5.087±1.423 |
| Neutrophil, ×109/L | 0.762±0.247 | 0.690±0.220 | 0.775±0.327 |
| Lymphocyte, ×109/L | 5.060±0.721 | 4.768±0.526 | 4.138±1.057 |
| Monocyte, ×109/L | 0.172±0.056 | 0.153±0.041 | 0.137±0.031 |
| Eosinophil, ×109/L | 0.038±0.041 | 0.010±0.011 | 0.025±0.052 |
| Basophil, ×109/L | 0.010±0.011 | 0.000±0.000 | 0.008±0.012 |
Notes: Data are the mean ± standard deviation; n=5.
Abbreviations: WBC, white blood cell; SiO2, silicon dioxide; NP, nanoparticle; NC, normal control; SiO2EN(−), negatively charged silicon dioxide.
Table 1.
Total WBC and type of WBC counts in L-arginine surface modified SiO2 NP-fed mice
| WBC and members | NC | SiO2EN20(R) | SiO2EN100(R) |
|---|---|---|---|
| Total WBC, ×109/L | 4.030±0.600 | 4.843±1.525 | 4.017±1.452 |
| Neutrophil, ×109/L | 0.673±0.129 | 0.828±0.362 | 0.568±0.191 |
| Lymphocyte, ×109/L | 3.258±0.565 | 3.800±1.127 | 3.270±1.190 |
| Monocyte, ×109/L | 0.092±0.025 | 0.160±0.072 | 0.162±0.087 |
| Eosinophil, ×109/L | 0.003±0.005 | 0.040±0.083 | 0.003±0.005 |
| Basophil, ×109/L | 0.002±0.004 | 0.015±0.023 | 0.002±0.004 |
Notes: Data are the mean ± standard deviation; n=5.
Abbreviations: WBC, white blood cell; SiO2, silicon dioxide; NP, nanoparticle; NC, normal control; SiO2EN(R), silicon dioxide modified with L-arginine; n, number.
Stimulation assays for B- and T- cell lymphocytes
All immune responses are mediated by lymphocytes.20 There are two main classes of lymphocytes: B-lymphocytes (B-cells) and T-lymphocytes (T-cells). Con-A and bacterial LPS are known to preferentially stimulate T-lymphocytes and B-lymphocytes, respectively. Stimulation assays performed on spleen-cell suspensions have been used as immune toxicity indicators. Splenocytes of mice treated with SiO2EN20(−) showed significantly reduced B-cell (P<0.01) and slightly reduced T-cell counts (Figure 3A and B). SiO2EN20(R) and SiO2EN100(R) also showed significant reductions in B-cell (P<0.001) and T-cell counts (P<0.05; P<0.001, respectively). On the other hand, splenocytes from SiO2EN100(−) mice showed significantly elevated B-cell (P<0.05) and T-cell counts (P<0.001) (Figure 3A and B).
Figure 3.
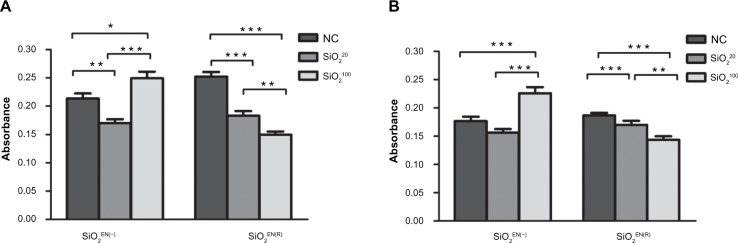
The effect on primary lymphocyte proliferation of SiO2 NPs fed to mice.
Notes: C57BL/6 mice were treated with varying sizes (20 nm, 100 nm) and charges (SiO2EN[R]; negative, SiO2EN[−]) of 750 mg/kg colloidal SiO2 NPs for 14 days. At the end of the treatment period, single-cell suspensions were made from spleens of mice, and the proliferation of (A) B-cells and (B) T-cells were assessed. Data are presented as the mean ± standard deviation; n=5. *P<0.05, **P<0.01, and ***P<0.001 indicate significant differences when tested with ANOVA. Tukey’s test was used for post hoc tests.
Abbreviations: NC, normal control; SiO2, silicon dioxide; SiO2EN(−), negatively charged silicon dioxide; SiO2EN(R), silicon dioxide modified with L-arginine; NP, nanoparticle; n, number; ANOVA, analysis of variance.
NO production
NO is a reactive molecule that reacts with ROS to produce reactive nitrogen species; moreover, it is recognized as a mediator and regulator of immune responses.21 NO has various physiological and pathophysiological responses, depending on its relative concentration.22 Here, we measured the level of NO after exposure to SiO2 NPs. Our results showed that both SiO2EN20(−) and SiO2EN100(−) showed significant decreases in NO production compared to the NC (Figure 4A). However, there was no substantial difference in the NO level in SiO2EN20(R) when compared to the NC group.
Figure 4.
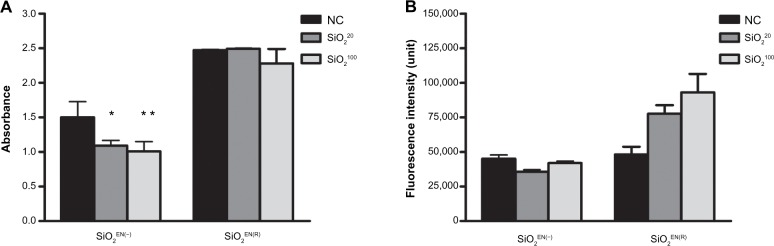
The effect on NO and ROS production of SiO2 NPs fed to mice.
Notes: C57BL/6 mice were treated with varying sizes (20 nm, 100 nm) and charges (SiO2EN(R); negative, SiO2EN(−)) of 750 mg/kg colloidal SiO2 NPs for 14 days. The supernatant of splenocytes from mice were harvested and (A) NO2− accumulation was assessed using the Griess colorimetric assay according to manufacturer’s instructions (Promega Corporation, Fitchburg, WI, USA), and (B) intracellular ROS accumulation was detected by fluorescent probe 2′,7′-dichlorodihydrofluorescein diacetate using a fluorescence microplate reader. Data are presented as the mean ± standard deviation; n=5. *P<0.05, and **P<0.01 indicate significant differences when tested with ANOVA. Tukey’s test was used for post hoc tests.
Abbreviations: NC, normal control; SiO2, silicon dioxide; SiO2EN(−), negatively charged silicon dioxide; SiO2EN(R), silicon dioxide modified with L-arginine; NP, nanoparticle; NO, nitric oxide; ROS, reactive oxygen species; NO2−, nitrate; n, number; ANOVA, analysis of variance.
ROS production and enzyme activities
Metal oxides mediate toxicity through oxidative stress.23 The potential of SiO2 NPs, as well as the influence of NP size and charge, in inducing oxidative stress was examined by measuring the ROS generation and the antioxidant activity of SOD and GPx levels in mice. We found that SiO2EN20(−) and SiO2EN100(−)-fed mice showed a slight decrease in ROS production, while SiO2EN20(R)- and SiO2EN100(R)-fed mice showed increased production of ROS compared to the NC group (Figure 4B). Furthermore, both the size and electrostatic charge of SiO2 had no effect on intracellular activities of SOD (Figure 5A), and they had little effect on GPx (Figure 5B). There was a slight increase in the activity of SiO2EN20(R)- and SiO2EN100(R)-fed mice, which can be correlated to the slight decrease in ROS production among these groups.
Figure 5.

The effect on antioxidant activity of SiO2 NPs fed to mice.
Notes: C57BL/6 mice were treated with varying sizes (20 nm, 100 nm) and charges (SiO2EN[R]; negative, SiO2EN[−]) of 750 mg/kg colloidal SiO2 NPs for 14 days. Spleens from mice were isolated aseptically and made into single-cell suspension splenocytes. (A) SOD activity and (B) GPx activity were measured in splenocyte lysate by an SOD activity assay kit and by a GPx activity colorimetric assay kit, according to the manufacturer’s instructions (BioVision, Inc., Milpitas, CA, USA). Data are presented as the mean ± standard deviation; n=5.
Abbreviations: SOD, superoxide dismutase; NC, normal control; SiO2, silicon dioxide; SiO2EN(−), negatively charged silicon dioxide; SiO2EN(R), silicon dioxide modified with L-arginine; GPx, glutathione peroxidase; n, number.
NK cell activity
NK cells are activated in response to cytokine production.24 NK cells were incubated with target cells (YAC-1) at different ratios. In particular, SiO2EN20(R) and SiO2EN20(−) showed the most significant reduction (P<0.001) in the killing activity of the effector cell (NK), while SiO2EN100(R) and SiO2EN100(−) showed comparable activity with the NC group (Table 3).
Table 3.
NK cell activity in SiO2 NP-fed mice
| Effector/target ratio | NK cell activity (% mean ± SD)
|
||||
|---|---|---|---|---|---|
| NC | SiO2EN20(−) | SiO2EN100(−) | SiO2EN20(R) | SiO2EN100(R) | |
| 100:1 | 76.61±3.622 | 46.95±6.117* | 71.12±19.99 | 55.04±4.845** | 82.50±4.560 |
| 50:1 | 58.11±1.691 | 40.45±3.662 | 48.87±10.36 | 45.39±1.263 | 58.96±3.361 |
| 25:1 | 53.12±2.229 | 37.51±1.1160 | 42.91±2.325 | 46.07±0.842 | 54.71±2.899 |
Notes: Data are presented as the mean ± SD; n=5. Nonparametric Tukey’s multiple comparison test (*P<0.01; **P<0.001 versus NC).
Abbreviations: NK, natural killer; SiO2, silicon dioxide; NP, nanoparticle; SD, standard deviation; NC, normal control; SiO2EN(−), negatively charged silicon dioxide; SiO2EN(R), silicon dioxide modified with L-arginine; n, number.
Cytokine analysis
Exposure to NPs can affect the production of inflammatory and T helper (Th)1-type cytokines. Varying the size and surface charge of SiO2 NPs differentially influenced cytokine production in the serum. Exposure with SiO2EN20(−), SiO2EN100(−), and SiO2EN100(R) led to suppressed production of cytokines, namely IL-1β, TNF-α, IL-12p70, IL-6, and IFN-γ (Table 4). Conversely, SiO2EN20(R) showed slight increases in IFN-γ. In addition, there were unaltered levels of Th1 cytokine (IL-12p70) and proinflammatory cytokine (TNF-α) among the group exposed to SiO2EN20(R), which was in reference to the NC group (Table 4).
Table 4.
Serum cytokine concentration in mice exposed to SiO2
| Cytokine | NC | SiO2EN20(R) | SiO2EN100(R) | SiO2EN20(−) | SiO2EN100(−) |
|---|---|---|---|---|---|
| IL-1β | 473.54±277.32 | 279.72±130.19 | 255.95±130.19 | 287.81±260.52 | 220.30±118.85 |
| IL-6 | 1.798±1.111 | 1.515±0.408 | 1.265±0.187 | 1.905±2.242 | 1.315±0.358 |
| IL-10 | 41.993±15.239 | 42.192±6.610 | 30.587±8.558 | 38.268±5.874 | 36.087±7.070 |
| IL-12p70 | 13.880±3.625 | 13.148±3.848 | 9.062±2.948* | 6.702±1.997** | 6.713±1.369** |
| IFN-γ | 0.747±0.324 | 0.930±0.241 | 0.692±0.176 | 0.508±0.146 | 0.655±0.167 |
| TNF-α | 122.54±54.341 | 120.60±52.055 | 75.060±23.715 | 102.57±64.786 | 46.717±21.941* |
Notes: Data are presented as the mean ± standard deviation; n=5. Nonparametric Tukey’s multiple comparison test (*P<0.05; **P<0.001 versus NC).
Abbreviations: SiO2, silicon dioxide; NC, normal control; SiO2EN(−), negatively charged silicon dioxide; SiO2EN(R), silicon dioxide modified with L-arginine; IL, interleukin; IFN, interferon; TNF, tumor necrosis factor; n, number.
Discussion
There is an almost unanimous opinion about the potential toxicity of NP exposure, and it includes ROS generation, proinflammatory responses, and cell death.25,26 However, most of the studies on NPs have focused on lung and liver toxicity, such that the toxic effect of NPs on the immune system is poorly documented.15,16 In addition, the influence of NP size and electrostatic charge on potential immunotoxicity remains to be elucidated. The present study addressed the immunotoxicity of different sizes and electrostatic charges of SiO2 NPs that were fed to mice for 14 days (orally administered at doses of 750 mg/kg/day). In particular, our study shows that immune dysfunction from exposure to the varying sizes and electrostatic charges of SiO2 would lead to immunosuppression. This was evidenced by suppressing the proliferation of lymphocytes, by depressing the killing activity of NK cells, and by decreasing inflammatory cytokine production. In addition, our data showed that different sizes and charges of SiO2 NPs could lead to differential immunotoxicity in vivo. To clarify this, we first investigated the immune function in mouse spleen lymphocytes. Mitogens such as Con-A and LPS are used to stimulate T-cells and B-cells, respectively, and to assess immune function. We found that the proliferations of B- and T-cells were reduced when mice were fed with SiO2 NPs (SiO2EN20[R], SiO2EN20[−]2, and SiO2EN100[−]). This is parallel to the decreased lymphocyte count in the blood, as shown in Table 1 and Table 2. Our results are similar to the findings of Lee et al,27 who demonstrated that the intraperitoneally administered 100 nm colloidal silica NPs showed no increase in their proliferative responses to the lymphocyte mitogens. Since lymphocytes play pivotal roles in the immune response, altered B- and T-cell proliferations might culminate in the dysregulation of the immune response.28 This result might suggest that SiO2 NPs, specifically the groups fed negatively charged NPs, may show a decrease in the white pulp component of the spleen, as there is a significant decrease in lymphocyte count.
Cumulative data showed that oxidative stress is another toxic mechanism of NPs.17,29 Oxidative stress and NO are closely linked to inflammatory responses. NO is implicated in phagocytosis, as well as in the pathogenesis of inflammation.30,37 The impaired production of NO leads to undesired effects, such as inflammation and tissue damage.30 Thus, the abnormal production of NO, such as increases or decreases upon exposure to silica NPs, may induce a proinflammatory response.30 ROS is known to stimulate NO production. Of note, there is the decreased generation of ROS in negatively charged SiO2EN20(−) and SiO2EN100(−)-fed mice; therefore, there was decreased NO generation in these given groups. Further, increased production of ROS in SiO2EN20(R)- and SiO2EN100(R)-fed mice led to increased NO generation, as compared to the SiO2EN20(−)- and SiO2EN100(−)-fed groups. The NP surface charge influenced the capacity of ROS production.31,32 L-arginine-coated SiO2 NPs are capable of inducing intracellular ROS; however, the decreased ROS production among the mice that were fed negatively charged SiO2 NPs might be due to earlier or later ROS production that occurred in these groups. In addition, the decreased ROS generation among mice fed negatively charged SiO2EN20(−) and SiO2EN100(−) may be due to the leakage of the fluorescent product from the cell due to membrane damage, as there is a higher tendency of cytotoxicity on negative charge, as shown by in vitro viability. Size and charge influence the cytotoxicity of engineered nanomaterials.25,32 When comparing the cytotoxicity of particle size and surface charge, we found that negatively charged NPs tended to be higher in toxicity, and 20 nm NPs were the most toxic in a murine macrophage cell line (RAW 264.7) (see Figure 1). In line with our results, the reported data showed that phagocytic cells preferentially interacted with negatively charged particles.33 Therefore, the higher toxicity of the negatively charged SiO2EN20(−) is due, in part, to the stronger interaction with the macrophage cells. Given this, the in vitro cytotoxicity and in vivo ROS production showed that negatively charged SiO2 NPs are more toxic than their negative counterparts. To further prove our findings, we selected other immunotoxicity parameters, such as cytokine profiling and NK cell killing activity.
Any invading pathogens can trigger an inflammatory response, which involves the secretion of inflammation-induced mediators such as cytokines.34 In an inflammatory response, activated immune cells recognize the NPs by their unique physicochemical characteristics, such as their surface charge and surface properties, thus inducing the cytokines to attract more cells to eradicate the NPs.20 Electrostatic charges of the NPs are important parameters in an inflammatory response. As noted by Tan et al,35 cationic (positively charged) engineered nanomaterials, such as liposomes, induced the secretion of cytokines, such as TNF, IL-12, and IFN-γ. In relation to this, mice fed L-arginine surface modified NPs (SiO2EN20[R] and SiO2EN100[R]) showed induced secretion of cytokines such as IL-12, IFN-γ, and TNF-α, as compared to the anionic NP (negatively charged SiO2EN20[−] and SiO2EN100[−])-fed mice. Consistent with these results, the SiO2EN20(R)-fed group showed increased WBC production, as compared to the negatively charged (SiO2EN20[−] and SiO2EN100[−])-fed mice (which are both in reference to their corresponding normal control group WBC levels). With respect to the relationship between the WBC level and each type of WBC (such as lymphocytes and monocytes) with inflammation, activated WBCs trigger the secretion of cytokines.36 Schwentker et al37 reported that NO could affect the expression and activity of cytokines. Further, particle size and surface area are also important parameters that affect in vivo bioreactivity.38 NPs are predictive in stimulating cytokine production, specifically the ultrafine particles.39 Our study showed that induction of cytokine production is clearly seen in SiO2EN20(R)-fed mice. NO production was low in negatively charged SiO2EN20(−)-fed mice. In parallel, the secretion of cytokines (IL-1β, TNF-α, IL-12p70, and IFN-γ) in negatively charged SiO2EN20(−) and SiO2EN100(−)-fed mice was repressed. Of these cytokines, IL-12p70 (SiO2EN20[−], SiO2EN100[−]) and TNF-α (SiO2EN100[−]) were significantly decreased in concentration, as compared to the levels found among the SiO2EN20(R) and SiO2EN100(R)-fed mice and among the mice in the NC group. Negatively charged SiO2EN20(−)-fed mice showed the least secretion of IFN-γ among all of the groups. These two cytokines (IL-12p70 and IFN-γ) are involved in the activation of NK cells, which were found in decreased concentrations in mice fed with negatively charged SiO2EN20(−) NPs. In addition, the decreased proliferation of B-cells in negatively charged SiO2EN20(−)-fed mice also supports our findings, where B-cells also function to secrete cytokines such as IL-1, IL-10, IL-6, IFN-γ, and TNF-α which, in turn, activates the antigen-presenting cells.40 Physicochemical properties such as the size and charge of NPs determine their immunotoxicity, and they may also enhance their biological reactivity.41 The biological activity of NPs increases as the particle size decreases.42 However, this trend can be altered by a small change in the particle’s surface charge. NPs with positively charged surfaces could be more easily up-taken due to the attractive interaction to the negative cell membrane.43 Despite using varying sizes and electrostatic charges of NPs in this study, other factors need to be considered as well, such as the different exposure periods (chronic or acute) and the different dosages (low, medium, and high) of the NPs, in order to fully discuss, in detail, the potential immunotoxicity of SiO2. In addition, our study focused on the spleen, as it was suggested by other studies that the spleen is one of the major target organs for toxicity.44,45 Moreover, the spleen is also involved in the initiation of immune responses, such that lymphocyte proliferation make take place in this given organ.27 However, to fully elucidate the biosafety of SiO2 NPs in particular, the immune system and other target organs, such as the lymph nodes and liver, need to be tested. While this paper provides evidence on SiO2 immunotoxicity, the underlying mechanism still needs to be elucidated.
Conclusion
Collectively, our data indicate that different sized and charged SiO2 NPs would cause differential in vivo immunotoxicity. Interestingly, the mice fed with the negatively charged SiO2 NPs exhibited higher cytotoxicity and in vivo immunotoxicity than their L-arginine modified charged counterparts by way of suppressing the proliferation of the lymphocytes, depressing the killing activity of NK cells, and decreasing inflammatory cytokine production, thus leading to immunosuppression. This is the first account of immunosuppression of differently sized and charged SiO2 NPs.
Acknowledgments
This research was supported by a grant (10182MFDS991) from the Ministry of Food and Drug Safety, 2012. This work was supported by the National Research Foundation of Korea Grant funded by the Korean Government (NRF-2013R1A1A2012665).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Barik TK, Sahu B, Swain V. Nanosilica-from medicine to pest control. Parasitol Res. 2008;103(2):253–258. doi: 10.1007/s00436-008-0975-7. [DOI] [PubMed] [Google Scholar]
- 2.Tallury P, Payton K, Santra S. Silica-based multimodal/multifunctional nanoparticles for bioimaging and biosensing applications. Nanomedicine (Lond) 2008;3(4):579–592. doi: 10.2217/17435889.3.4.579. [DOI] [PubMed] [Google Scholar]
- 3.Coll C, Mondragón L, Martínez-Máñez R, et al. Enzyme-mediated controlled release systems by anchoring peptide sequences on mesoporous silica supports. Angew Chem Int Ed Engl. 2011;50(9):2138–2140. doi: 10.1002/anie.201004133. [DOI] [PubMed] [Google Scholar]
- 4.Vivero-Escoto JL, Slowing II, Trewyn BG, Lin VS. Mesoporous silica nanoparticles for intracellular controlled drug delivery. Small. 2010;6(18):1952–1967. doi: 10.1002/smll.200901789. [DOI] [PubMed] [Google Scholar]
- 5.Chu Z, Huang Y, Tao Q, Li Q. Cellular uptake, evolution, and excretion of silica nanoparticles in human cells. Nanoscale. 2011;3(8):3291–3299. doi: 10.1039/c1nr10499c. [DOI] [PubMed] [Google Scholar]
- 6.He Q, Gao Y, Zhang L, et al. A pH-responsive mesoporous silica nanoparticles-based multi-drug delivery system for overcoming multi-drug resistance. Biomaterials. 2011;32(30):7711–7720. doi: 10.1016/j.biomaterials.2011.06.066. [DOI] [PubMed] [Google Scholar]
- 7.He Q, Shi J. MSN anti-cancer nanomedicines: chemotherapy enhancement, overcoming of drug resistance, and metastasis inhibition. Adv Mater. 2014;26(3):391–411. doi: 10.1002/adma.201303123. [DOI] [PubMed] [Google Scholar]
- 8.Benezra M, Penate-Medina O, Zanzonico PB, et al. Multimodal silica nanoparticles are effective cancer-targeted probes in a model of human melanoma. J Clin Invest. 2011;121(7):2768–2780. doi: 10.1172/JCI45600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maurer-Jones MA, Lin YS, Haynes CL. Functional assessment of metal oxide nanoparticle toxicity in immune cells. ACS Nano. 2010;4(6):3363–3373. doi: 10.1021/nn9018834. [DOI] [PubMed] [Google Scholar]
- 10.Colvin VL. The potential environmental impact of engineered nanomaterials. Nat Biotechnol. 2003;21(10):1166–1170. doi: 10.1038/nbt875. [DOI] [PubMed] [Google Scholar]
- 11.Isoda K, Tetsuka E, Shimizu Y, Saitoh K, Ishida I, Tezuka M. Liver injury induced by thirty- and fifty-nanometer-diameter silica nanoparticles. Biol Pharm Bull. 2013;36(3):370–375. doi: 10.1248/bpb.b12-00738. [DOI] [PubMed] [Google Scholar]
- 12.Kaewamatawong T, Shimada A, Okajima M, et al. Acute and subacute pulmonary toxicity of low dose of ultrafine colloidal silica particles in mice after intratracheal instillation. Toxicol Pathol. 2006;34(7):958–965. doi: 10.1080/01926230601094552. [DOI] [PubMed] [Google Scholar]
- 13.Kaewamatawong T, Kawamura N, Okajima M, Sawada M, Morita T, Shimada A. Acute pulmonary toxicity caused by exposure to colloidal silica: particle size dependent pathological changes in mice. Toxicol Pathol. 2005;33(7):743–749. doi: 10.1080/01926230500416302. [DOI] [PubMed] [Google Scholar]
- 14.Rasmussen JW, Martinez E, Louka P, Wingett DG. Zinc oxide nanoparticles for selective destruction of tumor cells and potential for drug delivery applications. Expert Opin Drug Deliv. 2010;7(9):1063–1077. doi: 10.1517/17425247.2010.502560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ahmad J, Ahamed M, Akhtar MJ, et al. Apoptosis induction by silica nanoparticles mediated through reactive oxygen species in human liver cell line HepG2. Toxicol Appl Pharm. 2012;259(2):160–168. doi: 10.1016/j.taap.2011.12.020. [DOI] [PubMed] [Google Scholar]
- 16.Akhtar MJ, Ahamed M, Kumar S, et al. Nanotoxicity of pure silica mediated through oxidant generation rather than glutathione depletion in human lung epithelial cells. Toxicology. 2010;276(2):95–102. doi: 10.1016/j.tox.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 17.Limbach LK, Wick P, Manser P, Grass RN, Bruinink A, Stark WJ. Exposure of engineered nanoparticles to human lung epithelial cells: influence of chemical composition and catalytic activity on oxidative stress. Environ Sci Technol. 2007;41(11):4158–4163. doi: 10.1021/es062629t. [DOI] [PubMed] [Google Scholar]
- 18.Kim KM, Kim HM, Choi MH, et al. Colloidal properties of surface coated colloidal silica nanoparticles in aqueous and physiological solutions. Sci Adv Mater. 2014 In Press. [Google Scholar]
- 19.Kruger NJ. The Bradford Method for Protein Quantitation. In: Walker JM, editor. The Protein Protocols Handbook. 3rd edition. Humana Press; 2009. pp. 17–24. [Google Scholar]
- 20.Dobrovolskaia MA, McNeil SE. Immunological properties of engineered nanomaterials. Nat Nanotechnol. 2007;2(8):469–478. doi: 10.1038/nnano.2007.223. [DOI] [PubMed] [Google Scholar]
- 21.Vaziri ND, Ni Z, Oveisi F, Liang K, Pandian R. Enhanced nitric oxide inactivation and protein nitration by reactive oxygen species in renal insufficiency. Hypertension. 2002;39(1):135–141. doi: 10.1161/hy0102.100540. [DOI] [PubMed] [Google Scholar]
- 22.Thomas DD, Ridnour LA, Isenberg JS, et al. The chemical biology of nitric oxide: implications in cellular signaling. Free Radic Biol Med. 2008;45(1):18–31. doi: 10.1016/j.freeradbiomed.2008.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Manke A, Wang L, Rojanasakul Y. Mechanisms of nanoparticle-induced oxidative stress and toxicity. BioMed Res Int. 2013;2013:942916. doi: 10.1155/2013/942916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cooper MA, Elliot JM, Keyel PA, Yang L, Carrero JA, Yokoyama WM. Cytokine-induced memory-like natural killer cells. Proc Natl Acad Sci U S A. 2009;106(6):1915–1919. doi: 10.1073/pnas.0813192106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang H, Liu C, Yang D, Zhang H, Xi Z. Comparative study of cytotoxicity, oxidative stress and genotoxicity induced by four typical nanomaterials: the role of particle size, shape and composition. J Appl Toxicol. 2009;29(1):69–78. doi: 10.1002/jat.1385. [DOI] [PubMed] [Google Scholar]
- 26.Nel A, Xia T, Mädler L, Li N. Toxic potential of materials at the nanolevel. Science. 2006;311(5761):622–627. doi: 10.1126/science.1114397. [DOI] [PubMed] [Google Scholar]
- 27.Lee S, Kim MS, Lee D, et al. The comparative immunotoxicity of mesoporous silica nanoparticles and colloidal silica nanoparticles in mice. Int J Nanomedicine. 2013;8:147–158. doi: 10.2147/IJN.S39534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuhrt D, Faith SA, Leone A, et al. Evidence of early B-cell dysregulation in simian immunodeficiency virus infection: rapid depletion of naïve and memory B-cell subsets with delayed reconstitution of the naïve B-cell population. J Virol. 2010;84(5):2466–2476. doi: 10.1128/JVI.01966-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shvedova AA, Castranova V, Kisin ER, et al. Exposure to carbon nanotube material: assessment of nanotube cytotoxicity using human keratinocyte cells. J Toxicol Environ Health A. 2003;66(20):1909–1926. doi: 10.1080/713853956. [DOI] [PubMed] [Google Scholar]
- 30.Sharma JN, Al-Omran A, Parvathy SS. Role of nitric oxide in inflammatory diseases. Inflammopharmacology. 2007;15(6):252–259. doi: 10.1007/s10787-007-0013-x. [DOI] [PubMed] [Google Scholar]
- 31.Fröhlich E. The role of surface charge in cellular uptake and cytotoxicity of medical nanoparticles. Int J Nanomedicine. 2012;7:5577–5591. doi: 10.2147/IJN.S36111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Park YH, Bae HC, Jang Y, et al. Effect of the size and surface charge of silica nanoparticles on cutaneous toxicity. Molecular and Cellular Toxicol. 2013;9(1):67–74. [Google Scholar]
- 33.Tomita Y, Rikimaru-Kaneko A, Hashiguchi K, Shirotake S. Effect of anionic and cationic n-butylcyanoacrylate nanoparticles on NO and cytokine production in Raw 264.7 cells. Immunopharmacol Immunotoxicol. 2011;33(4):730–737. doi: 10.3109/08923973.2011.565345. [DOI] [PubMed] [Google Scholar]
- 34.Janeway CA Jr, Travers P, Walport M, Shlomchik Mark J, editors. Immunobiology: The Immune System in Health and Disease. 5th edition. New York: Garland Science; 2001. Induced innate responses to infection. [Google Scholar]
- 35.Tan Y, Li S, Pitt BR, Huang L. The inhibitory role of CpG immunostimulatory motifs in cationic lipid vector-mediated transgene expression in vivo. Hum Gene Ther. 1999;10(13):2153–2161. doi: 10.1089/10430349950017149. [DOI] [PubMed] [Google Scholar]
- 36.Lacy P, Stow JL. Cytokine release from innate immune cells: association with diverse membrane trafficking pathways. Blood. 2011;118(1):9–18. doi: 10.1182/blood-2010-08-265892. [DOI] [PubMed] [Google Scholar]
- 37.Schwentker A, Vodovotz Y, Weller R, Billiar TR. Nitric oxide and wound repair: role of cytokines? Nitric Oxide. 2002;7(1):1–10. doi: 10.1016/s1089-8603(02)00002-2. [DOI] [PubMed] [Google Scholar]
- 38.Nel AE, Mädler L, Velegol D, et al. Understanding biophysicochemical interactions at the nano-bio interface. Nat Mater. 2009;8(7):543–557. doi: 10.1038/nmat2442. [DOI] [PubMed] [Google Scholar]
- 39.Monteiller C, Tran L, MacNee W, et al. The pro-inflammatory effects of low-toxicity low-solubility particles, nanoparticles and fine particles, on epithelial cells in vitro: the role of surface area. Occup Environ Med. 2007;64(9):609–615. doi: 10.1136/oem.2005.024802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Godeau B. B-cell depletion in immune thrombocytopenia. Semin Hematol. 2013;50(Suppl 1):S75–S82. doi: 10.1053/j.seminhematol.2013.03.014. [DOI] [PubMed] [Google Scholar]
- 41.De Jong WH, Borm PJ. Drug delivery and nanoparticles: applications and hazards. Int J Nanomedicine. 2008;3(2):133–149. doi: 10.2147/ijn.s596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oberdorster G. Significance of particle parameters in the evaluation of exposure-dose-response-response relationships of inhaled particles. Inhal Toxicol. 1996;8(Suppl):73–89. [PubMed] [Google Scholar]
- 43.Chen L, Mccrate JM, Lee JC, Li H. The role of surface charge on the uptake and biocompatibility of hydroxyapatite nanoparticles with osteoblast cells. Nanotechnology. 2011;22(10):105708. doi: 10.1088/0957-4484/22/10/105708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nishimori H, Kondoh M, Isoda K, Tsunoda S, Tsutsumi Y, Yagi K. Histological analysis of 70-nm silica particles-induced chronic toxicity in mice. Eur J Pharm Biopharm. 2009;72(3):626–629. doi: 10.1016/j.ejpb.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 45.Oberdörster G. Safety assessment for nanotechnology and nanomedicine: concepts of nanotoxicology. J Intern Med. 2010;267(1):89–105. doi: 10.1111/j.1365-2796.2009.02187.x. [DOI] [PubMed] [Google Scholar]



