Abstract
Hermansky-Pudlak syndrome (HPS) is a genetic disorder characterized by oculocutaneous albinism, bleeding tendency and susceptibility to pulmonary fibrosis. No curative therapy is available. Genetic correction directed to the lungs, bone marrow and/or gastro-intestinal tract might provide alternative forms of treatment for the diseases multi-systemic complications. We demonstrate that lentiviral-mediated gene transfer corrects the expression and function of the HPS1 gene in patient dermal melanocytes, which opens the way to development of gene therapy for HPS.
1. Introduction
Hermansky-Pudlak syndrome (HPS) is an autosomal recessive disorder characterized by defective biogenesis of lysosome-related organelles (LROs) [1,2]. Nine genetic HPS subtypes have been defined with clinical manifestations that include hypopigmentation, bleeding diathesis, pulmonary fibrosis, granulomatous colitis and neutropenia. HPS-associated pulmonary fibrosis (HPS-PF) remains the most serious complication for patients affected with the HPS-1, −2 and −4 types of the disease [3]. Several lines of evidence suggest that alveolar type II pneumocytes (ATII) play a significant role in the susceptibility of HPS patients to PF [4,5]. The prognosis of HPS-PF is extremely poor and, currently, the only treatment is lung transplantation. Thus, the development of alternative forms of treatment for HPS-PF remains a high priority.
Genes mutated in HPS encode subunits of the biogenesis of lysosome-related organelles complexes (BLOCs) which participate in intracellular protein trafficking and vesicle biosynthesis. The HPS1/HPS4 protein complex, BLOC-3, functions as a Rab32 and Rab38 (Rab32/38) guanine nucleotide exchange factor [6]. In melanocytes, activated Rab32/38 is important for efficient transport of tyrosinase and tyrosinase related protein 1 (TYRP1) from early endosomes to melanosomes and likely provides the pathophysiologic basis of HPS-1 complications. In ATII cells, Rab38 contributes to maintaining lamellar body morphology and surfactant homeostasis [7,8].
Thus, correcting BLOC-3 function in ATII cells by HPS1 gene transfer may be an effective therapy for treating HPS1-PF. To begin to explore gene therapy strategies for HPS, we studied the effects of lentiviral (LV)-mediated gene correction in HPS-1 human dermal melanocytes (HDM) as an initial feasibility model.
2. Materials and methods
2.1. Cells
All HPS patient cells used in this study were homozygous for a 16-bp duplication in exon 15 of the HPS1 gene (c.1472_1487dup16) and cultured as previously described [9]. Patients were enrolled in protocol NCT00001456, “Clinical and Basic Investigations Into Hermansky-Pudlak Syndrome”, approved by the NHGRI Institutional Review Board, and gave written informed consent. All assays described below were performed in triplicate.
2.2. LV construction, production and transduction
We prepared two LV constructs: the LV-HPS1 therapeutic vector, expressing both the human HPS1 cDNA (GeneCopoeia, Germantown, MD) and the green fluorescent protein (GFP) reporter gene (Addgene Inc., Cambridge, MA) under the control of ubiquitously acting chromatin opening element (UCOE), and the LV-GFP control vector that expressed only GFP (Fig. 1A). Viral particles were produced as previously described [10]. HPS-1 HDM were exposed to LV-HPS1 or LV-GFP viral particles for two hours at a multiplicity of infection of 10.
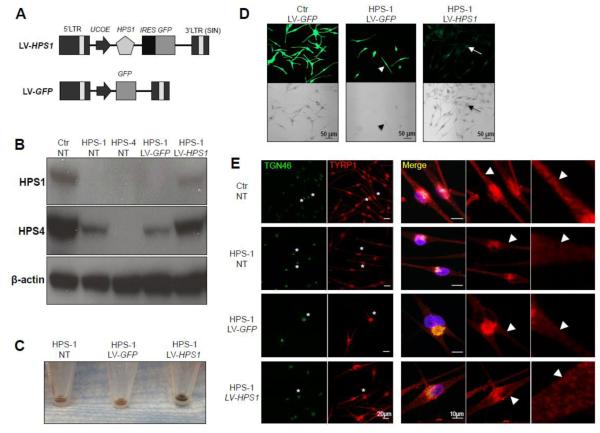
Figure 1. LV-HPS1 transduction corrects BLOC-3 function in HPS-1 human dermal melanocytes.
(A) Schematic representation of the lentiviral vectors. LTR, long terminal repeat; UCOE, ubiquitously acting chromatin opening element; HPS1, Hermansky-Pudlak syndrome 1 open reading frame cDNA (GenBank accession number NM_000195); IRES, internal ribosome entry site; GFP, enhanced green fluorescent protein open reading frame cDNA; SIN, self-inactivating.
(B) Immunoblots of cell lysates from healthy donor (Ctr) and HPS-1 human dermal melanocytes (HPS-1) and HPS-4 fibroblasts (HPS-4). The cells were either non-transduced (NT) or transduced with LV-GFP or LV-HPS1. The results show the restoration of both HPS1 and HPS4 proteins in HPS-1 melanocytes transduced with LV-HPS1.
(C) Effects of gene transfer on HPS-1 melanocyte macroscopic pigmentation. HPS-1 melanocytes were either non-transduced (NT) or transduced with LV-GFP or LV-HPS1 and cultured for 15 days. Cell pellets after 15 days of culturing showed significantly increased pigmentation in LV-HPS1-transduced cells, indicating correction of BLOC-3 function.
(D) Fluorescent (to visualize GFP expression in transduced cells, green) and brightfield (to visualize pigmentation, black) images of healthy control (Ctr) and HPS-1 patient melanocytes transduced with LV-GFP or LV-HPS1. LV-GFP transduced HPS-1 cells lacked pigmentation (arrowheads), while HPS-1 cells transduced with LV-HPS1 showed increased pigmentation (arrows) with a similar intensity and distribution as in control cells.
(E) Corrected BLOC-3 function results in trafficking of the melanogenic enzyme TYRP1 to early endosomes/melanosomes. BLOC-3 function is evaluated by immunofluorescence analysis of TYRP1 expression and localization (red, visualized by a MEL5 mouse anti-TYRP1 antibody; BioLegend). Trans-Golgi network (TGN, green) is visualized by a sheep anti-TGN antibody (AbD Serotec). TYRP1 in Ctr melanocytes (Ctr NT) is localized in the TGN area, as well as in an endosomal, punctate distribution into the dendrites (arrowheads). HPS-1 melanocytes (HPS-1 NT) showed TYRP1 localization to the TGN, but the dendritic endosomal TYRP1 localization appeared markedly decreased (arrowheads), suggesting a BLOC-3 defect. Transduction of HPS-1 cells with LV-HPS1 increased dendritic endosomal expression of TYRP1 (arrowheads), indicating improvement/correction of BLOC-3 function. Transduction of HPS-1 cells with LV-GFP did not correct TYRP1 distribution and/or BLOC-3 function. The perinuclear regions of selected cells (asterisks) were cropped and enlarged (right panels) to better visualize the perinuclear dendritic regions of the cells.
All cell images were taken at the same intensity and magnification settings per experiment on a Zeiss LSM510 META confocal microscope using 20X (Figure D) or 40X (Figure E) objective.
2.3. Quantitative real-time PCR
Genome-integrated vector sequences were detected using previously described methods [11] and by quantitative PCR analysis (using universal probe #67: 5’-TGCTGGAG-3’ (Roche, Indianapolis, IN,) and primers: 5’-CCATGCCGAGAGTGATCC-3’ (forward) and 5’-GAAGCGCGATCACATGGT-3’ (reverse)).
2.4. Western blotting
Protein cell lysates were immunoblotted using anti-HPS1 and anti-HPS4 polyclonal antibodies (Proteintech Group, Inc., Chicago, IL) and anti-β-actin monoclonal antibody as loading control. Western blots were quantified using the ImageJ 1.32 software (National Institutes of Health, MD) after densitometric scanning of the films. The intensity of HPS1 and HPS4 protein was normalized to that of β-actin.
2.5. Immunofluorescence microscopy
Immunofluorescence microscopy was performed as described [9], using a mouse monoclonal antibody against TYRP1 obtained from BioLegend (Dedham, MA, USA), a sheep polyclonal antibody against TGN46 from AbD Serotec (Raleigh, NC, USA), and the DAPI nuclear stain (Vector Laboratories, Burlingame, CA, USA). All imaging was performed on a Zeiss 510 META confocal laser-scanning microscope (Carl Zeiss, Thornwood, NY, USA).
3. Results and discussion
3.1. Efficacy of HPS1 expression
Fluorescence-activated cell sorting (FACS) analysis and quantitative real-time PCR were performed to evaluate efficiency of expression of the lentiviral constructs 15 days after transduction. FACS analysis showed that lentiviral gene transfer in HPS-1 HDM resulted in 98.8% (LV-GFP) and 50.0% (LV-HPS1) GFP-positive cell populations (data not shown). Quantitative PCR analysis showed that cells transduced with the LV-HPS1 and LV-GFP vectors carried 4.3-13.3 and 28.9-30.5 vector copies per cell, respectively (data not shown).
By western blot analysis, the HPS1 protein (~79 kDa) was detected in lanes containing extracts from non-transduced (NT) HDM from a healthy donor (Ctr) and in LV-HPS1-transduced HPS-1 HDM, while it was barely detectable in extracts from non-transduced (NT) and LV-GFP-transduced HPS-1 HDM (Fig. 1B). Quantification of HPS1 protein expression by ImageJ 1.32 software showed that HPS1 protein expression in LV-HPS1 transduced HPS-1 HDM was 52.2% of the level expressed in HDM from a healthy donor. Because LV-HPS1 transduction did not restore HPS1 protein expression to normal levels in HPS-1 HDM, it was important to obtain evidence of the functional effects of the gene correction. HPS1 and HPS4 proteins are stable in BLOC-3, but when either is absent the other is either absent or greatly reduced [12]. We therefore tested the stability of the BLOC-3 complex by evaluating HPS4 protein expression, which was significantly reduced in NT and LV-GFP-transduced HPS-1 HDM. Importantly, however, LV-HPS1-transduced HPS-1 HDM showed HPS4 protein increased to 89.3% of the level expressed in control HDM (Fig. 1B). Therefore, in addition to restoring HPS1 expression, LV-mediated HPS1 gene transfer resulted in the correction of BLOC-3 formation, as indicated by regeneration of HPS4 expression in HPS-1 HDM.
3.3. Assessing the restoration of BLOC-3 function
To determine whether BLOC-3 function was restored in LV-HPS1-transduced HPS-1 HDM, we assessed melanocyte pigmentation. Fifteen days after transduction, pelleted LV-HPS1-transduced HPS-1 HDM showed significantly increased pigmentation, compared to NT or LV-GFP-transduced HPS1 HDM (Fig. 1C). Similarly, confocal microscopy showed increased pigmentation in LV-HPS1-transduced HPS-1 HDM (arrows), while LV-GFP transduced HPS-1 HDM (arrow heads) lacked pigmentation (Fig. 1D).
Lastly, we assessed intracellular localization of the melanogenic enzyme TYRP1 by immunofluorescence in HPS-1 HDM transduced with LV-GFP or LV-HPS1 (Fig. 1E). In control cells (Ctr NT), TYRP1 was localized to the Golgi region as well as to endosomes (likely early stage melanosomes) outside the Golgi region (arrow heads). In HPS-1 HDM (HPS-1 NT), however, TYRP1 remained mostly in the trans-Golgi region and was markedly decreased in the endosomes in the dendritic regions (arrow heads). In contrast, in LV-HPS1-transduced HPS-1 HDM (HPS-1 LV-HPS1), TYRP1 appeared to regain dendritic endosomal localization, similar to control cells (Fig. 1E).
These data indicate that restoration of HPS1 protein expression by LV-HPS1 transduction in HPS-1 HDM corrected BLOC-3 function, likely through activation of Rab32/38 and correction of endosomal transport of TYRP1, leading to restored melanin production.
4. Conclusion
Our results show that lentiviral-mediated gene transfer resulted in effective HPS1 gene expression in HPS-1 HDM, restoration of BLOC-3 function, and correction of pigmentation in these cells.
Because viral vector-mediated gene transfer has resulted in serious adverse events of insertional oncogenesis in clinical trials that used constructs based on murine gamma-retroviruses [13], we decided to use a lentiviral-based gene transfer vector in the current study, as a safer alternative for potential future clinical use [14]. Envisionable gene therapy approaches that would benefit HPS will have to target the lung, a task that has proven challenging. However, significant progress has been made in recent years with the development of lentiviral vectors that efficiently transduce lung tissue [15].
In this context, our in vitro studies represent a promising first step toward the development of in vivo gene therapy strategies for Hermansky-Pudlak syndrome.
Highlights.
A lentiviral-mediated gene transfer system was developed for Hermansky-Pudlak syndrome (HPS).
HPS1 protein expression was restored in human dermal melanocytes from HPS patients after lentiviral-mediated gene HPS1 gene transfer.
Expression of HPS4 protein was also reconstituted in HPS dermal melanocytes indicating the restored stability of BLOC-3 in gene-corrected cells.
The results are promising regarding the feasibility of gene therapy approaches for HPS.
Acknowledgements
The authors thank Dr. Adrian J. Thrasher for providing the pHR’SINcPPT construct and the 2.6-kb fragment of UCOE sequence from the human HNRPA2B1-CBX locus (A2UCOE), and Dr. Inder Verma for the pMD.G, pCMVDR8.91 plasmids. This research was supported by the Intramural Research Program of the National Human Genome Research Institute, National Institutes of Health, Bethesda, MD, USA (ZIA HG000122) and the UNIL-CHUV grant no. CGRB 29583.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors have no conflicts of interest to declare.
References
- [1].Gahl WA, Huizing M, Syndrome Hermansky-Pudlak. GeneReviews at GeneTests: Medical Genetics Information Resource [database online] (Original 2002; Updated 2004, March 2007, September 2012). Copyright, University of Washington, Seattle, 1997-2010. Available at http://www.genetests.org.
- [2].Huizing M, Helip-Wooley A, Westbroek W, Gunay-Aygun M, Gahl WA. Disorders of Lysosome-related Organelle Biogenesis: Clinical and Molecular Genetics. Annu. Rev. Genomics Hum. Genet. 2008;9:359–386. doi: 10.1146/annurev.genom.9.081307.164303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Gochuico BR, Huizing M, Golas GA, Scher CD, Tsokos M, Denver SD, Frei-Jones MJ, Gahl WA. Interstitial Lung Disease and Pulmonary Fibrosis in Hermansky-Pudlak Syndrome Type 2, an AP-3 Complex Disease. Mol. Med. 2012;18:56–64. doi: 10.2119/molmed.2011.00198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Nakatani Y, Nakamura N, Sano J, Inayama Y, Kawano N, Yamanaka S, Miyagi Y, Nagashima Y, Ohbayashi C, Mizushima M, Manabe T, Kuroda M, Yokoi T, Matsubara O. Interstitial pneumonia in Hermansky-Pudlak syndrome: significance of florid foamy swelling/degeneration (giant lamellar body degeneration) of type-2 pneumocytes. Virchows Arch. 2000;437:304–313. doi: 10.1007/s004280000241. [DOI] [PubMed] [Google Scholar]
- [5].Young LR, Gulleman PM, Bridges JP, Weaver TE, Deutsch GH, Blackwell TS, McCormack FX. The alveolar epithelium determines susceptibility to lung fibrosis in Hermansky-Pudlak syndrome. Am. J. Respir. Crit. Care Med. 2012;186:1014–1024. doi: 10.1164/rccm.201207-1206OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Gerondopoulos A, Langemeyer L, Liang JR, Linford A, Barr FA. BLOC-3 mutated in Hermansky-Pudlak syndrome is a Rab32/38 guanine nucleotide exchange factor. Curr. Biol. 2012;22:2135–2139. doi: 10.1016/j.cub.2012.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Wasmeier C, Romao M, Plowright L, Bennett DC, Raposo G, Seabra MC. Rab38 and Rab32 control post-Golgi trafficking of melanogenic enzymes. J. Cell Biol. 2006;175:271–281. doi: 10.1083/jcb.200606050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Zhang L, Yu K, Robert KW, Debolt KM, Hong N, Tao JQ, Fukuda M, Fisher AB, Huang S. Rab38 targets to lamellar bodies and normalizes their sizes in lung alveolar type II epithelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2011;301:L461–477. doi: 10.1152/ajplung.00056.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Cullinane AR, Curry JA, Golas G, Pan J, Carmona-Rivera C, Hess RA, White JG, Huizing M, Gahl WA. A BLOC-1 mutation screen reveals a novel BLOC1S3 mutation in Hermansky-Pudlak Syndrome type 8. Pigment Cell Melanoma Res. 2012;25:584–591. doi: 10.1111/j.1755-148X.2012.01029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Levasseur DN, Ryan TM, Pawlik KM, Townes TM. Correction of a mouse model of sickle cell disease: lentiviral/antisickling beta-globin gene transduction of unmobilized, purified hematopoietic stem cells. Blood. 2003;102:4312–4319. doi: 10.1182/blood-2003-04-1251. [DOI] [PubMed] [Google Scholar]
- [11].Kim YT, Kim YS, Larochelle A, Renaud G, Wolfsberg TG, Adler R, Donahue RE, Hematti P, Hong BK, Roayaei J, Akagi K, Riberdy JM, Nienhuis AW, Dunbar CE, Persons DA. Sustained high-level polyclonal hematopoietic marking and transgene expression 4 years after autologous transplantation of rhesus macaques with SIV lentiviral vector-transduced CD34+ cells. Blood. 2009;113:5434–5443. doi: 10.1182/blood-2008-10-185199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Nazarian R, Huizing M, Helip-Wooley A, Starcevic M, Gahl WA, Dell'angelica EC. An immunoblotting assay to facilitate the molecular diagnosis of Hermansky-Pudlak syndrome. Mol. Genet. Metab. 2008;93:134–144. doi: 10.1016/j.ymgme.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Knight S, Collins M, Takeuchi Y. Insertional mutagenesis by retroviral vectors: current concepts and methods of analysis. Curr. Gene Ther. 2013;13:211–227. doi: 10.2174/1566523211313030006. [DOI] [PubMed] [Google Scholar]
- [14].Montini E, Cesana D, Schmidt M, Sanvito F, Ponzoni M, Bartholomae C, Sergi L. Sergi, Benedifient C, Ambrosi A, Di Serio C. Hematopoietic stem cell gene tansfer in a tumor-prone mouse model uncovers low genotoxicity of lentiviral vector integration. Nat. Biotechnol. 2006;24:687–696. doi: 10.1038/nbt1216. M. [DOI] [PubMed] [Google Scholar]
- [15].Griesenbach U, Alton EW. Expert opinion in biological therapy: update on developments in lung gene transfer. Expert Opin. Biol. Ther. 2013;13:345–360. doi: 10.1517/14712598.2013.735656. [DOI] [PubMed] [Google Scholar]