Abstract
Patients with relapsed/refractory leukemias or advanced myelodysplastic syndrome (MDS) fare poorly following allogeneic hematopoietic cell transplant (HCT). We report prospective phase II study results of 29 patients given clofarabine 30 mg/m2/day i.v. × 5 days followed immediately by HCT conditioning while at the cytopenic nadir. A total of 15/29 patients (52%) were cytoreduced according to pre-defined criteria (cellularity < 20% and blasts < 10%). Marrow cellularity (P < 0.0001) and blast% (P = 0.03) were reduced. Toxicities were acceptable, with transient hyperbilirubinemia (48%) and gr3–4 infections (10%). In all, 28/29 proceeded to transplant; 27 received ATG or alemtuzumab. Post HCT, 180 day non-relapse mortality (NRM) was 7% (95% confidence interval (CI): 1–21), relapse was 29% (95% CI: 13–46) and OS was 71% (95% CI: 51–85), comparing favorably to published data for high-risk patients. Two-year graft vs host disease incidence was 40% (95% CI: 21–58) and 2 year OS was 31% (95% CI: 14–48). Disease at the nadir correlated with inferior OS after HCT (HR = 1.22 for each 10% marrow blasts, 95% CI: 1.02–1.46). For AML/MDS patients, there was a suggestion that successful cytoreduction increased PFS (330 vs 171 days, P = 0.3) and OS (375 vs 195 days, P = 0.31). Clofarabine used as a bridge to HCT reduces disease burden, is well tolerated, and permits high-risk patients to undergo HCT with acceptable NRM. Late relapses are common; thus, additional strategies should be pursued. NCT-00724009.
Keywords: allo-SCT, relapsed leukemia, AML, bridge therapy, myelodysplastic syndrome
INTRODUCTION
Allogeneic hematopoietic SCT (HCT) offers a curative approach to patients with high-risk or refractory AML, ALL, CML or myelodysplastic syndrome (MDS). Leukemia patients with primary refractory disease or uncontrolled relapse have a very poor prognosis,1 and those with active disease at HCT fare poorly regardless of regimen intensity.2,3 Relapsed/refractory patients typically receive multiple lines of salvage therapy in an attempt to achieve remission, because greater disease burden strongly predicts for poor outcomes after HCT.2–7 This approach leads to increased toxicity and may allow disease evolution, further compromising the opportunity to undergo HCT. Induction therapy followed immediately by transplant conditioning while still at the cytopenic nadir may circumvent these problems.8,9
Clofarabine, a purine nucleoside with activity against AML and MDS, is well tolerated in adults with the most common side effects being hematological toxicity, transient transaminitis and creatinine elevation.10–12 Recently, clofarabine was safely incorporated into HCT conditioning regimens with particular success in patients with active myeloid malignancies.13–16
We previously reported a retrospective review of the safety of initiating HCT conditioning during the hematological nadir following clofarabine induction. Importantly, clofarabine extrame-dullary toxicities were non-overlapping with those of conditioning regimens and patients were able to proceed to transplant.17 Based upon these observations we designed a prospective study of clofarabine induction followed by transplant conditioning for relapsed/refractory leukemia and MDS patients, to test the feasibility of proceeding to transplant and reducing disease burden.
MATERIALS AND METHODS
This IRB approved feasibility study took place at the University of Chicago Medical Center (ClinicalTrials.govIdentifier: NCT-00724009). Enrollment was from March 2008 to September 2010.
Study objectives
The primary endpoint was achievement of cytoreductive response, defined as a nadir (day 12 following initiation of clofarabine) BM biopsy with a cellularity of < 20% and a blasts < 10% of all cellular elements before initiation of HCT conditioning.17,18 To maintain uniformity, one hematopathologist reviewed all cases.
Secondary endpoints included day 100, day 180 and 1 year OS, PFS, non-relapse mortality (NRM), relapse rate; clofarabine toxicity; proportion proceeding to HCT; and whether BM cellularity and blast % after clofarabine predicts PFS or OS after HCT.
Patient eligibility
Patients with relapsed/refractory AML or ALL, MDS with >5% BM myeloblasts, or CML in accelerated or blast phase or resistant/intolerant to imatinib, dasatinib and/or nilotinib were eligible. All patients had an identified matched related donor, matched unrelated donor, mis-matched unrelated donor or suitable cord blood donor unit and were otherwise eligible and cleared, per institutional guidelines, to proceed to transplant. Peripheral blood progenitor cells were preferred using G-CSF mobilization. HLA matching was performed at HLA-A, HLA-B, HLA-C and HLA-DRB1 by high-resolution techniques. Criteria for cord blood matching followed standard criteria: low-resolution at HLA-A, and HLA-B and high-resolution at HLA-DRB1.
Eligibility required serum glutamic oxaloacetic transaminase (SGOT)/ serum glutamic pyruvic transaminase ≤2.5 × upper limit normal; alkaline phosphatase ≤2.5 × upper limit normal; serum bilirubin < 1.5 mg/dL; serum creatinine < 1.0 mg/dL or creatinine clearance > 60 mL/min; age≥/ = 18 years; ECOG performance status ≤/ = 2.19 All patients provided informed written consent.
Treatment schema
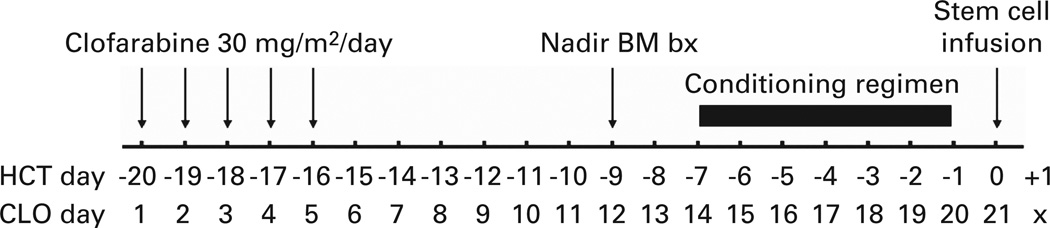
Clofarabine 30 mg/m2 was i.v. administered consecutively for 5 days, with dexamethasone 10mg i.v. administered 1.5 h before clofarabine. HCT conditioning was to begin at the hematological nadir, 12–21 days after clofarabine initiation (Figure 1). Conditioning regimens and GVHD prophylaxis for each patient were at the discretion of the treating physician in accordance with institutional guidelines based upon disease, patient and donor characteristics and were not dependent upon response or toxicity to clofarabine cytoreduction.15,20–23 Continuation to HCT after clofarabine was at the discretion of the treating physician. Post-transplant immunosuppression was tacrolimus from day −2 to day + 100 for matched related donor recipients and until day + 180 for unrelated adult donor recipients. For cord blood recipients, tacrolimus was from day −1 to day + 180 and mycophenolate mofetil from day − 3 to day + 56. The majority of patients received either ATG or alemtuzumab.
Figure 1.
Treatment schema and estimated chronology. Relative days are presented in relationship to both clofarabine and transplant. Before therapy, donor arrangements were made and patients were scheduled to begin conditioning between 12 and 21 days from the first day of clofarabine initiation. Nadir BM biopsy was done 12 days after clofarabine initiation.
Study monitoring and statistical design
Based upon our retrospective data we hypothesized that 66% of patients would achieve a cytoreductive response, defined as a post clofarabine BM biopsy with < 20% cellularity and < 10% blasts,17 and an unacceptable result would be if only 40% achieved this. The one stage design required 27 patients to test the null hypothesis that the response is ≤40% against the alternative that it is ≥ 60% for a one-sided α of 0.10 and 90% power. To account for cumulative toxicity of clofarabine in conjunction with the HCT regimen, we utilized a toxicity monitoring plan based on Pocock-type boundaries.24 For this stopping threshold, we counted ≥Grade 3 toxicities preventing cell infusion within 30 days after clofarabine. Unacceptable toxicity was defined as 25% by day 30.
Secondary endpoints included analysis of the AML/MDS subset. Kaplan–Meier curves for PFS and OS were generated. Cumulative incidence curves for relapse, NRM and GVHD were generated. NRM was defined as death from any cause without evidence of relapse. Time to event analysis used the day of transplant as day 0. Patients were stratified by cytoreduced or non-cytoreduced and outcomes compared using Wilcoxon-test statistic. Toxicity associated with clofarabine was assessed prospectively using CTCAE v.3. Descriptive statistics were used for other endpoints. Complete donor chimerism was defined as > 95% donor cells in both the unfractionated and CD3 compartments as evaluated by variable number of tandem repeats methodology. Cytogenetics for AML patients at diagnosis were classified according to CALGB criteria.25
RESULTS
Patient characteristics
Twenty nine patients were enrolled. Patient characteristics are presented in Table 1. Only two did not meet high-risk CIBMTR classification: one had tyrosine kinase inhibitor resistant Ph + CML in CP1 with loss of cytogenetic response to imatinib, and failure to respond to nilotonib and dasatinib; the other had CML in CP2 with prior progression to blast phase while on imatinib and failed to respond to dasatinib. Patients were heavily pretreated and often refractory to prior therapy. No MDS patient treated with a hypomethylating agent responded (n = 6). Another MDS patient failed a lenalidomide-based regimen. All AML patients were relapsed or refractory. All AML patients received ara-C as part of their induction regimen. For the seven AML patients that previously achieved CR1 (n = 7), the median duration was 1 month (range < 1– 15 months) with the remaining 9 having primary induction failure. AML patients that achieved CR1 recieved high-dose ara-C consolidation. Salvage therapy containing ara-C was given to 10 AML patients at relapse or after failed standard 7 + 3 ara-C and anthracycline induction. Only two AML patients had achieved a CR1 > 12 months and both were transplanted in CR1 (1 auto and 1 allo). Myeloproliferative disorder patients included a JAK2 mutated primary myelofibrosis in accelerated phase managed only with corticosteroids before transplant.
Table 1.
Patient and transplant characteristics
| Patient characteristics (n = 29) |
Transplant characteristics (n = 28) |
||
|---|---|---|---|
| Gender | Patients proceeding to HCT | 28 | |
| Male | 16 | HCT within 30 days of clofarabine | 26 |
| Female | 13 | Days clofarabine to conditioning (median) | 14 |
| Median age, years | 51 | (range) | (11–68) |
| Range | (23–69) | Days clofarabine to HCT (median) | 21 |
| Disease/disease state | (Range) | (17–75) | |
| ALL | 2 | Donor | |
| REL1 | 1 | Matched related | 11 |
| REL2 | 1 | Matched unrelated | 12 |
| MPD | 3 | Haploidentical + cord blood | 5 |
| MDS | 8 | Source | |
| Therapy related MDS | 1 | Peripheral blood | 22 |
| Prior response to hypomethylator | 0 | BM | 1 |
| AML | 16 | Cord blood | 5 |
| PIF | 9 | Conditioning regimen: | |
| REL1 | 6 | CLofarabine/melphalan/alemtuzumab | 3 |
| REL2 | 1 | Fludarabine/BU/ alemtuzumab | 2 |
| Refractory to last therapy | 12 | Fludarabine/melphalan/ alemtuzumaba | 17 |
| Therapy related AML | 2 | Fludarabine/melphalan/ ATG | 5 |
| Antecedent MDS | 3 | TBI/etoposide | 1 |
| Marrow blast%, median (n = 16) | 40 | TBI/etoposide/alemtuzumab | 1 |
| Range | (7.5–86) | GVHD prophylaxis | |
| Peripheral blood blast%, median (n = 16) | 3.5 | Tacrolimus/alemtuzumaba | 23 |
| Range | (0–81) | Tacrolimus/MMF/ATG | 5 |
| Cytogenetic risk group at diagnosis | Tacrolimus/MMF | 1 | |
| Adverse | 16 | CMV serostatus | |
| Intermediate | 12 | Recipient and/or donor positive | 18 |
| Favorable | 0 | Recipient and donor negative | 10 |
| HCT-comorbidity index, range | (0–5) | Donor gender (adult only) | |
| Low (0) | 16 | Female | 8 |
| Intermediate (1–2) | 6 | Male | 15 |
| High (3 or more) | 7 | Median donor age (adult only) | 41 |
| Prior treatment | (Range) | (21–72) | |
| None | 2 | ||
| 1 Prior regimen | 10 | ||
| 2 Prior regimens | 9 | ||
| 3 Or more prior regimens | 8 |
Abbreviations: CMV = cytomegalovirus; HCT = hematopoietic cell transplant; MDS = myelodysplastic syndrome; MPD = myeloproliferative disorder; PIF = primary induction failure; REL1 = first relapse; REL2 = second relapse. REL1, REL2, PIF, chronic phase (CP), accelerated phase (AP), MPD, CML, conditioning regimens have all been previously reported and included: fludarabine 30 (FLU) mg/m2/day i.v. on day − 7 through − 3 + melphalan (MEL) 140 mg/m2 on day –2 + alemtuzumab (ALEM) (100mg total) on day − 7 through − 3; clofarabine (CLO) 30 mg/m2/day i.v. on day − 7 through − 3 + MEL 140 mg/m2 on day −2 + ALEM (100mg total) on day − 7 through − 3; FLU mg/m2/day i.v. on day − 7 through − 3 + BU 3.2 mg/kg/day i.v. on day −6 through −3 (BU) + ALEM (100mg total) on day − 7 through − 3; TBI 1200 cGY + etoposide 60 mg/kg i.v. on day − 3 ± ALEM (100 mg total) on day − 7 through −3 for ALL patients; or FLU on day − 7 to − 3 + MEL 70 mg/m2/day i.v. on day −3 to − 2 + rATG 1.5 mg/kg i.v. (6 mg/kg total) on day − 7, − 5, − 3, − 1 (flu/mel/ATG) for cord blood recipients. All cord blood units were supplemented with additional CD34-selected haplo-identical related adult peripheral blood stem cells.
Includes patient that did not receive transplant.
Cytoreduction and feasibility of transplant
Transplant characteristics are presented in Table 1. RIC was used in the majority of patients (25/29, 86%) and most received PBSC allografts (22/29, 76%).
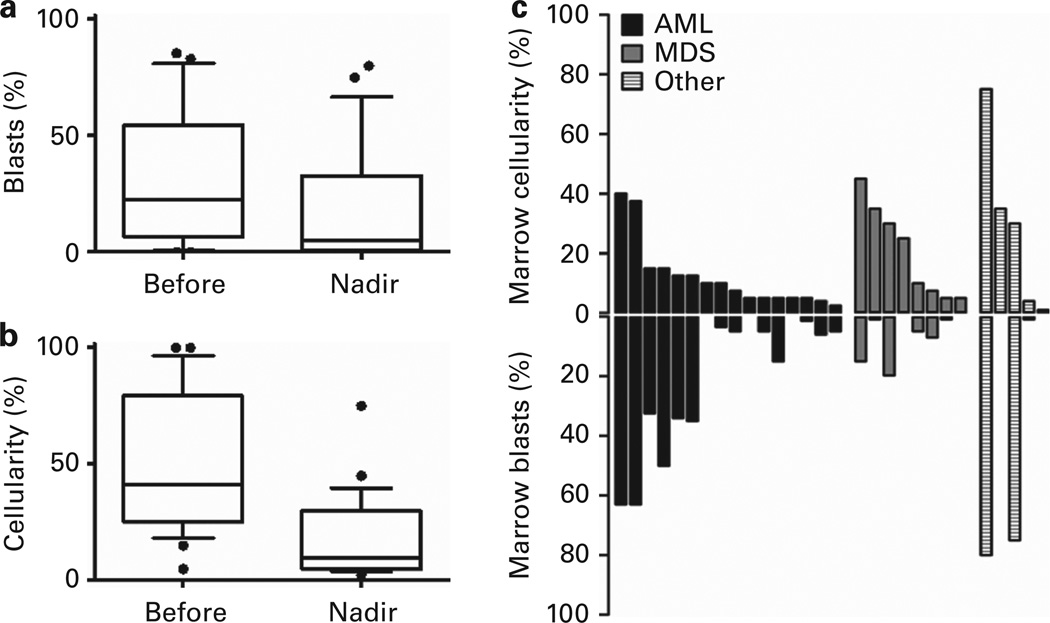
A cytoreductive response was achieved in 15/29 patients (52%), and 13/24 (54%) patients in the MDS/AML subset.17 BM cellularity and blast percentage before and after clofarabine are presented in Figure 2. Circulating blasts were present in 18/29 (62%) patients at the time of clofarabine and 7/29 patients (24%) at the time of conditioning.
Figure 2.
BM disease burden. BM disease burden is reduced after clofarabine. (a) The median BM blast percentage was 22% (mean = 30%, range 0–86%) before clofarabine and 5% (mean = 18%, range 0–75%) after clofarabine (P = 0.035). (b) The median BM cellularity was 41% (mean = 31%, range 5–100%) before clofarabine and 10% (mean = 17%, range 1–75%) after clofarabine (P < 0.0001). (c) Post clofarabine nadir BM cellularity and blasts by disease category. Each column represents a single patient’s result.
Twenty eight of 29 patients received an allogeneic transplant. One patient had conditioning initiated after cytoreduction, but died of sepsis before stem cell infusion. Conditioning was initiated at the nadir after one cycle of clofarabine for 26/28 patients: one patient developed infection necessitating delay and another had progressive disease. Both patients went on to receive HiDAC + mitoxantrone cytoreduction and underwent transplantation at the nadir.
Toxicity of clofarabine bridge and conditioning regimen
Toxicities are summarized in Table 2. SGOT and total bilirubin elevations were common but transient: all patients had normal total bilirubin on HCT day 0. Relative to clofarabine the median peak SGOT elevation was day 5 (range 3–31) and peak bilirubin was day 8 (range 2–31). There were no cases of veno-occlusive disease. Three patients developed neutropenia-associated infection following clofarabine but before conditioning: one aspergillus pneumonia responded to treatment and the patient was transplanted after delay; another developed a tunneled catheter infection requiring debridement; a third patient developed genital HSV, which responded to therapy. Nine patients had grade 3 or 4 infection after the start of conditioning therapy but before day + 12 after HCT. One patient with refractory AML underwent conditioning but died 1 day before stem cell infusion due to sepsis (grade 5 infection).
Table 2.
Toxicities
| Toxicity assessmenta (n = 29) | ||||||
|---|---|---|---|---|---|---|
| Total toxicity (n = 29) | Clofarabine to conditioning (n = 29) |
Conditioning to day + 12 (n = 29) | ||||
| Grade 1–2 | Grade 3–4 | Grade 1–2 | Grade 3–4 | Grade 1–2 | Grade 3–4 | |
| Renal (creatinine) | 9 (31%) | 1 (3%) | 2 (7%) | 0 | 9 (31%) | 1 (3%) |
| Hepatic (total bilirubin) | 14 (48%) | 3 (10%) | 12 (41%) | 2 (7%) | 8 (28%) | 0 |
| Hepatic (SGOT) | 14 (48%) | 9 (31%) | 14 (48%) | 7 (24%) | 17 (59%) | 3 (10%) |
| Cardiac | 2 (7%) | 0 | 0 | 0 | 2 (7%) | 0 |
| Skin | 1 (3%) | 0 | 1 (3%) | 0 | 0 | 0 |
| Infection | 0 | 12 (41%)b | 0 | 3 (10%) | 0 | 10 (34%)b |
All toxicities calculated by CTCAE v.3 grading criteria expressed as % of patients experiencing the maximum toxicity level achieved during the indicated time period.
Includes one grade 5 from sepsis on day − 1.
Transplant outcomes
Median days to engraftment were 11 (95% confidence interval (CI): 10–12) for neutrophils and 22 (95% CI: 18–35) for platelets. At day 30 after transplant, 27/28 patients remained alive. One patient relapsed on day + 28, the remaining 26 patients had morphological CR by day + 30 BM biopsy. Of these 26, 25 had an evaluable BM cytogenetic samples showing reemergence of a normal karyotype.25 Day + 30 chimerism results for patients in remission were: 100% (n = 26) unsorted complete donor chimerism and 88% (n = 23) CD3 complete donor chimerism.
Three HCT patients died before day 100; one in remission from sepsis and two of progressive disease. The 100 day cumulative incidence of NRM was 3.6%. For all patients receiving clofarabine, 2/29 died in remission before HCT day + 100.
The 100 day, 6-month and 1-year cumulative incidence of grade 2–4 acute GVHD were 21% (95% CI: 9–38), 29% (95% CI: 13–46) and 35% (95% CI: 18–54). There were no cases of grade 3–4 acute GVHD before day 100. Two late onset acute GVHD cases occurred after day 100, making the 100 day, 6-month and 1-year cumulative incidence of grade 3–4 acute GVHD were 0%, 4% (95% CI: 0–16) and 7% (95% CI: 1–21). The cumulative incidence of chronic GVHD was 7% (95% CI: 1–21%) at 1 year and 7% (95% CI: 1–21) at 2 years. Of the three patients with chronic GVHD, two were mild and one moderate by NIH consensus criteria.
Survival analysis
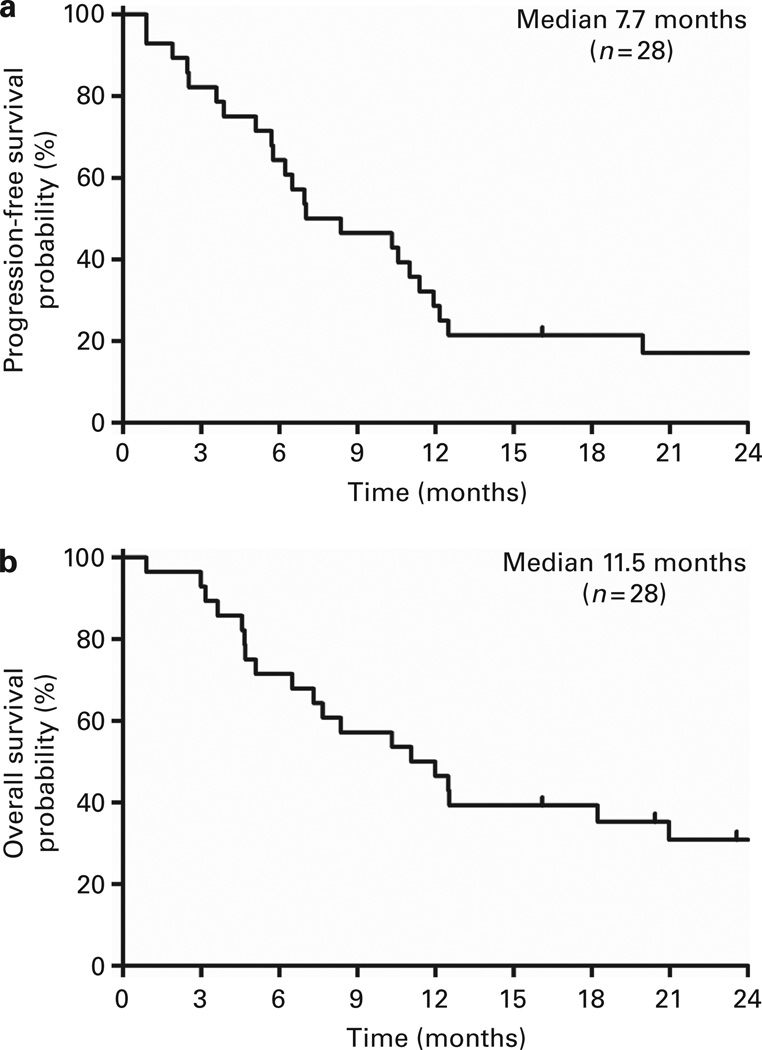
With a median follow-up of 854 days after HCT for 9 survivors, median PFS was 211 days (95% CI: 171–342) and median OS was 332 days (95% CI: 195–629) (Figure 3). For AML and MDS patients (n = 23), median PFS was 251 days (95% CI: 173–358) and median OS was 373 days (95% CI: > 220). The median days to relapse, by competing risk analysis, were 330 days (95% CI: 173–599) for all patients, and 330 days (95% CI: 187–599) for AML/MDS patients. Outcomes at day 100, 6 months and 1 year are summarized in Table 3. The Kaplan–Meier estimate for two OS was 31% (95% CI: 14– 48) for all patients and 33% (95% CI: 14–52) for AML/MDS patients.
Figure 3.
Kaplan–Meier curves from the time of transplant. (a) Kaplan–Meier plot of PFS for transplanted patients and (b) OS for transplanted patients, in months from the time of transplant.
Table 3.
Outcomes after transplant
| Outcomes after allo-SCT | ||||
|---|---|---|---|---|
| Entire cohort (n = 28) | AML/MDS (n = 23) | |||
| % | 95% CI | % | 95% CI | |
| Relapse risk | ||||
| 100 Days | 14% | 4–30 | 13% | 3–30 |
| 6 Months | 29% | 13–46 | 26% | 10–45 |
| 1 Year | 54% | 36–74 | 61% | 36–78 |
| Non-relapse mortality | ||||
| 100 Days | 4% | 0–16 | 0% | |
| 6 Months | 7% | 1–21 | 4% | 0–19 |
| 1 Year | 18% | 6–34 | 13% | 3–30 |
| PFS | ||||
| 100 Days | 82% | 62–92 | 87% | 65–96 |
| 6 Months | 64% | 44–79 | 70% | 47–84 |
| 1 Year | 25% | 11–42 | 26% | 11–45 |
| OS | ||||
| 100 Days | 89% | 70–96 | 96% | 79–99 |
| 6 Months | 71% | 51–85 | 78% | 55–90 |
| 1 Year | 46% | 28–63 | 52% | 31–70 |
Abbreviations: CI = confidence interval; MDS = myelodysplastic syndrome.
Median PFS for cytoreduced patients compared with those not achieving cytoreduction was 251 (range 27–1433) vs 171 days (range 27–916) (P = 0.4); median OS was 360 (range 27–1433) vs 195 (range 90–916) days (P = 0.4). Subset analysis of AML and MDS patients revealed median PFS for cytoreduced vs non-cytoreduced patients was 330 (range 116–1433) vs 171 (range 27–897) days (P = 0.3) and median OS was 375 (range 137–1433) vs 195 (range 90–897) days (P = 0.31).
Prognostic factors
The pre-clofarabine BM blast percentage did not predict post-transplant OS (HR = 0.9, 95% CI: 0.74–1.09). Each 10% increase in blasts present in the post clofarabine BM biopsy was associated with decreased OS following transplant (HR = 1.22, 95% CI: 1.02– 1.46). The following factors: age > 50 years; poor-risk cytogenetics; and circulating blasts, did not predict achievement of cytoreduction, OS or PFS in the entire cohort or the AML/MDS subset. Lack of an HLA-matched related donor did not predict for OS or PFS.
DISCUSSION
This trial demonstrates that clofarabine induction 2 weeks before initiation of transplant conditioning is a tolerable and effective bridge strategy allowing allogeneic HCT in those patients with persistent disease. The majority of all patients (52%), and the AML/ MDS subset (54%), achieved the primary endpoint of cytoreduction.17 Clofarabine reduced marrow blast% (P = 0.03) and cellularity (P < 0.0001). All patients proceeded to conditioning, with only one death before allograft infusion and one non-relapse death before day + 100.
The lack of cytoreduction in a substantial minority may relate to highly resistant disease and/or insufficient clofarabine dose intensity. Clofarabine has efficacy against AML at 40 mg/m2 daily × 5. The dose in this trial was 30 mg/m2 daily × 5 based on studies showing the lower dose has activity in older adults with AML.11 These cytoreduction results are consistent with published overall response rates of 47% for clofarabine + cytarabine compared with 23% for cytarabine + placebo induction for relapsed/refractory AML.26
Renal, skin and cardiac toxicities attributable to clofarabine were minimal and acceptable. Transaminase and bilirubin elevations were common yet transient: all patients had a normal bilirubin, and 4% had grade 3–4 SGOT elevation on the day of transplant. No patients developed veno-occlusive disease. The majority received T-cell depletion, which likely contributed to tolerability by minimizing early acute GVHD.
Successful cytoreduction did not clearly improve clinical outcomes and the results do not support a practice of canceling transplant if cytoreduction is not achieved as the study was not powered to show clinical differences. However, there was quantitatively better survival (with wide CIs) for patients successfully cytoreduced, particularly in the MDS/AML subset.
Our study is limited in the ability to make definitive recommendations for a number of reasons including the small sample size, patient and disease heterogeneity, and variability in transplant characteristics. The inclusion of ALL and CML patients weakens conclusions surrounding cytoreduction and clinical outcomes, as the efficacy of clofarabine for treatment of these diseases is less clear than in AML or MDS (Figure 2c). The inconsistency of conditioning regimens, donor sources, GVHD prophylaxis and high degree of in vivo T-cell depletion all introduce additional variables and prevent reliable comparison of our study with controlled transplant trials of relapsed/refractory AML and high-risk MDS patients.9,16 Interpretation of transplant outcomes is also limited by the fact that two patients did not proceed directly to HCT after clofarabine (due to lack of effective cytoreduction) and both achieved cytoreduction with alternative therapy before HCT. Nevertheless, this prospective trial confirms the feasibility and potential therapeutic benefit of the clofarabine cytoreduction approach by allowing the majority of high-risk MDS/AML patients to proceed to transplant with variable graft sources and conditioning regimens.
Although long-term outcomes remain poor, 6-month and 1-year OS (46%) and PFS (25%) rates compare favorably with previously published results for patients with active disease receiving HCT.3,6,27 Many of these patients would not be widely recommended for transplant due to high-risk features beyond active disease: median age 51 years; 61% lacked a matched related donor; a majority with poor-risk cytogenetic features and circulating blasts at clofarabine initiation; 21% were intermediate and 24% high risk by the HCT-CI score; and the median relapsed/refractory AML pre-transplant risk score was 2 (n = 15, range 0–5) indicating an estimated 1 year OS of 25% for AML patients.4
Future trials in relapsed/refractory leukemia patients should consider primary endpoints such as cytoreduction or the likelihood of proceeding to transplant.28,29 Alternative chemotherapeutics with non-overlapping toxicities to common transplant conditioning regimens must be validated for tolerable cytoreduction. Interestingly, the combination of ara-C and clofarabine led to higher rates of remission for relapsed/ refractory AML patients at the time of HCT as compared with ara-C alone, although the effectiveness at cytoreduction remains uncharacterized.26,30
The low 6-month relapse rate (28.6%), combined with the low NRM and minimal GVHD, indicates that this platform is a strong base from which to investigate post HCT maintenance interventions. Chronic GVHD is known to be associated with protection from relapse,31 and the low incidence of cGVHD with the clofarabine bridge is likely associated with in vivo T depletion (alemtuzumab = 23, ATG = 5).32 T-cell depletion remains controversial as enhanced tolerance and reduced GVHD comes at the expense of relapse without clearly improving survival.32,33 However, high disease risk also predisposes to higher NRM and our series affirms high-tolerance and low GVHD risks.
Building upon this platform should allow for development of GVL with permissible GVHD. DLI is associated with GVL and prolonged survival for patients relapsing after HCT,34 although efficacy for AML/MDS is limited to patients having > 6 months remission duration after HCT.35 A strategy of planned DLI may induce GVL for patients with minimal or undetectable disease.36,37 An alternative, and possibly synergistic, strategy would be to initiate an immunomodulator, hypomethylating agent or molecularly targeted drug between 100 days and 6 months after HCT.38–41
In conclusion, this novel bridge approach allows for a short interval from salvage to transplant (median 21 days from clofarabine initiation to transplant), minimizes progression or failure to respond as ineligibility criteria for transplant (27/29 patients proceeded to conditioning at the nadir after 1 cycle of clofarabine), and avoids attrition of transplant eligible patients due to toxicity and morbidity associated with repeated induction therapy (1 patient died in remission before day 100 after transplant). Clofarabine cytoreduction immediately before allograft conditioning is well tolerated and permits high-risk patients to proceed to transplant with low NRM, making it an ideal platform for testing post-transplant maintenance strategies.
ACKNOWLEDGEMENTS
This study, investigator initiated trial, was supported by Genzyme/Sanofi-Aventis and the University of Chicago Comprehensive Cancer Center (Biostatistics Core Facility and Cancer Clinical Trials Office, CCSG, CA14599).
Footnotes
Presented in part at the ASCO annual meeting 2010 (abstract no. 6537) and the ASH annual meeting 2011(abstract no. 496) NCT-00724009.
CONFLICT OF INTEREST
FLL has received honoraria from Genzyme/Sanofi-Aventis; RL is a compensated consultant for Sanofi-Aventis; KvB has received research support from Genzyme/ Sanofi-Aventis; trial support for this investigator initiated study provided by Genzyme/Sanofi-Aventis.
REFERENCES
- 1.Craddock C, Tauro S, Moss P, Grimwade D. Biology and management of relapsed acute myeloid leukaemia. Br J Haematol. 2005;129:18–34. doi: 10.1111/j.1365-2141.2004.05318.x. [DOI] [PubMed] [Google Scholar]
- 2.Sierra J, Storer B, Hansen JA, Martin PJ, Petersdorf EW, Woolfrey A, et al. Unrelated donor marrow transplantation for acute myeloid leukemia: an update of the Seattle experience. Bone Marrow Transplant. 2000;26:397–404. doi: 10.1038/sj.bmt.1702519. [DOI] [PubMed] [Google Scholar]
- 3.Kebriaei P, Kline J, Stock W, Kasza K, Le Beau MM, Larson RA, et al. Impact of disease burden at time of allogeneic stem cell transplantation in adults with acute myeloid leukemia and myelodysplastic syndromes. Bone Marrow Transplant. 2005;35:965–970. doi: 10.1038/sj.bmt.1704938. [DOI] [PubMed] [Google Scholar]
- 4.Duval M, Klein JP, He W, Cahn JY, Cairo M, Camitta BM, et al. Hematopoietic stem-cell transplantation for acute leukemia in relapse or primary induction failure. J Clin Oncol. 2010;28:3730–3738. doi: 10.1200/JCO.2010.28.8852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Warlick ED, Cioc A, Defor T, Dolan M, Weisdorf D. Allogeneic stem cell transplantation for adults with myelodysplastic syndromes: importance of pretransplant disease burden. Biol Blood Marrow Transplant. 2009;15:30–38. doi: 10.1016/j.bbmt.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 6.Castro-Malaspina H, Jabubowski AA, Papadopoulos EB, Boulad F, Young JW, Kernan NA, et al. Transplantation in remission improves the disease-free survival of patients with advanced myelodysplastic syndromes treated with myeloablative T cell-depleted stem cell transplants from HLA-identical siblings. Biol Blood Marrow Transplant. 2008;14:458–468. doi: 10.1016/j.bbmt.2008.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scott BL, Storer B, Loken MR, Storb R, Appelbaum FR, Deeg HJ. Pretransplantation induction chemotherapy and posttransplantation relapse in patients with advanced myelodysplastic syndrome. Biol Blood Marrow Transplant. 2005;11:65–73. doi: 10.1016/j.bbmt.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 8.Platzbecker U, Thiede C, Fussel M, Geissler G, Illmer T, Mohr B, et al. Reduced intensity conditioning allows for up-front allogeneic hematopoietic stem cell transplantation after cytoreductive induction therapy in newly-diagnosed high-risk acute myeloid leukemia. Leukemia. 2006;20:707–714. doi: 10.1038/sj.leu.2404143. [DOI] [PubMed] [Google Scholar]
- 9.Schmid C, Schleuning M, Schwerdtfeger R, Hertenstein B, Mischak-Weissinger E, Bunjes D, et al. Long-term survival in refractory acute myeloid leukemia after sequential treatment with chemotherapy and reduced-intensity conditioning for allogeneic stem cell transplantation. Blood. 2006;108:1092–1099. doi: 10.1182/blood-2005-10-4165. [DOI] [PubMed] [Google Scholar]
- 10.Kantarjian H, Gandhi V, Cortes J, Verstovsek S, Du M, Garcia-Manero G, et al. Phase 2 clinical and pharmacologic study of clofarabine in patients with refractory or relapsed acute leukemia. Blood. 2003;102:2379–2386. doi: 10.1182/blood-2003-03-0925. [DOI] [PubMed] [Google Scholar]
- 11.Faderl S, Ravandi F, Huang X, Garcia-Manero G, Ferrajoli A, Estrov Z, et al. qA randomized study of clofarabine versus clofarabine plus low-dose cytarabine as front-line therapy for patients aged 60 years and older with acute myeloid leukemia and high-risk myelodysplastic syndrome. Blood. 2008;112:1638–1645. doi: 10.1182/blood-2007-11-124602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burnett AK, Russell NH, Kell J, Dennis M, Milligan D, Paolini S, et al. European development of clofarabine as treatment for older patients with acute myeloid leukemia considered unsuitable for intensive chemotherapy. J Clin Oncol. 2010;28:2389–2395. doi: 10.1200/JCO.2009.26.4242. [DOI] [PubMed] [Google Scholar]
- 13.Martin MG, Uy GL, Procknow E, Stockerl-Goldstein K, Cashen A, Westervelt P, et al. Allo-SCT conditioning for myelodysplastic syndrome and acute myeloid leukemia with clofarabine, cytarabine and ATG. Bone Marrow Transplant. 2009;44:13–17. doi: 10.1038/bmt.2008.423. [DOI] [PubMed] [Google Scholar]
- 14.Andersson BS, Valdez BC, de Lima M, Wang X, Thall PF, Worth LL, et al. Clofarabine +/− fludarabine with once daily i.v. busulfan as pretransplant conditioning therapy for advanced myeloid leukemia and MDS. Biol Blood Marrow Transplant. 2011;17:893–900. doi: 10.1016/j.bbmt.2010.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Besien K, Stock W, Rich E, Odenike O, Godley LA, O’Donnell PH, et al. Phase I–II Study of clofarabine-melphalan-alemtuzumab conditioning for allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2012;18:913–921. doi: 10.1016/j.bbmt.2011.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Magenau J, Tobai H, Pawarode A, Braun T, Peres E, Reddy P, et al. Clofarabine and busulfan conditioning facilitates engraftment and provides significant antitumor activity in nonremission hematologic malignancies. Blood. 2011;118:4258–4264. doi: 10.1182/blood-2011-06-358010. [DOI] [PubMed] [Google Scholar]
- 17.Locke FL, Artz A, Rich E, Zhang Y, van Besien K, Stock W. Feasibility of clofarabine cytoreduction before allogeneic transplant conditioning for refractory AML. Bone Marrow Transplant. 2010;45:1692–1698. doi: 10.1038/bmt.2010.32. [DOI] [PubMed] [Google Scholar]
- 18.Kern W, Haferlach T, Schoch C, Loffler H, Gassmann W, Heinecke A, et al. Early blast clearance by remission induction therapy is a major independent prognostic factor for both achievement of complete remission and long-term outcome in acute myeloid leukemia: data from the German AML Cooperative Group (AMLCG) 1992 Trial. Blood. 2003;101:64–70. doi: 10.1182/blood-2002-02-0532. [DOI] [PubMed] [Google Scholar]
- 19.Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–655. [PubMed] [Google Scholar]
- 20.van Besien K, Artz A, Smith S, Cao D, Rich S, Godley L, et al. Fludarabine, melphalan, and alemtuzumab conditioning in adults with standard-risk advanced acute myeloid leukemia and myelodysplastic syndrome. J Clin Oncol. 2005;23:5728–5738. doi: 10.1200/JCO.2005.15.602. [DOI] [PubMed] [Google Scholar]
- 21.O’Donnell PH, Artz AS, Undevia SD, Pai RK, Del Cerro P, Horowitz S, et al. Phase I study of dose-escalated busulfan with fludarabine and alemtuzumab as conditioning for allogeneic hematopoietic stem cell transplant: reduced clearance at high doses and occurrence of late sinusoidal obstruction syndrome/veno-occlusive disease. Leuk Lymphoma. 2010;51:2240–2249. doi: 10.3109/10428194.2010.520773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sobecks RM, Daugherty CK, Hallahan DE, Laport GF, Wagner ND, Larson RA. A dose escalation study of total body irradiation followed by high-dose etoposide and allogeneic blood stem cell transplantation for the treatment of advanced hematologic malignancies. Bone Marrow Transplant. 2000;25:807–813. doi: 10.1038/sj.bmt.1702230. [DOI] [PubMed] [Google Scholar]
- 23.Liu H, Rich ES, Godley L, Odenike O, Joseph L, Marino S, et al. Reduced-intensity conditioning with combined haploidentical and cord blood transplantation results in rapid engraftment, low GVHD, and durable remissions. Blood. 2011;118:6438–6445. doi: 10.1182/blood-2011-08-372508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ivanova A, Qaqish BF, Schell MJ. Continuous toxicity monitoring in phase II trials in oncology. Biometrics. 2005;61:540–545. doi: 10.1111/j.1541-0420.2005.00311.x. [DOI] [PubMed] [Google Scholar]
- 25.Byrd JC, Mrozek K, Dodge RK, Carroll AJ, Edwards CG, Arthur DC, et al. Pretreatment cytogenetic abnormalities are predictive of induction success, cumulative incidence of relapse, and overall survival in adult patients with de novo acute myeloid leukemia: results from Cancer and Leukemia Group B (CALGB 8461) Blood. 2002;100:4325–4336. doi: 10.1182/blood-2002-03-0772. [DOI] [PubMed] [Google Scholar]
- 26.Faderl S, Wetzler M, Rizzieri D, Schiller G, Jagasia M, Stuart R, et al. Clofarabine plus cytarabine compared with cytarabine alone in older patients with relapsed or refractory acute myelogenous leukemia: results from the CLASSIC I Trial. J Clin Oncol. 2012;30:2492–2499. doi: 10.1200/JCO.2011.37.9743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Lima M, Couriel D, Thall PF, Wang X, Madden T, Jones R, et al. Once-daily intravenous busulfan and fludarabine: clinical and pharmacokinetic results of a myeloablative, reduced-toxicity conditioning regimen for allogeneic stem cell transplantation in AML and MDS. Blood. 2004;104:857–864. doi: 10.1182/blood-2004-02-0414. [DOI] [PubMed] [Google Scholar]
- 28.Eom KS, Min WS, Kim HJ, Cho BS, Choi SM, Lee DG, et al. FLANG salvage chemotherapy is an effective regimen that offers a safe bridge to transplantation for patients with relapsed or refractory acute myeloid leukemia. Med Oncol. 2011;28(Suppl 1):S462–S470. doi: 10.1007/s12032-010-9653-6. [DOI] [PubMed] [Google Scholar]
- 29.Appelbaum FR, Rosenblum D, Arceci RJ, Carroll WL, Breitfeld PP, Forman SJ, et al. End points to establish the efficacy of new agents in the treatment of acute leukemia. Blood. 2007;109:1810–1816. doi: 10.1182/blood-2006-08-041152. [DOI] [PubMed] [Google Scholar]
- 30.Buchholz S, Dammann E, Stadler M, Krauter J, Beutel G, Trummer A, et al. Cytoreductive treatment with clofarabine/ara-C combined with reduced-intensity conditioning and allogeneic stem cell transplantation in patients with high-risk, relapsed, or refractory acute myeloid leukemia and advanced myelodysplastic syndrome. Eur J Haematol. 2012;88:52–60. doi: 10.1111/j.1600-0609.2011.01703.x. [DOI] [PubMed] [Google Scholar]
- 31.Valcarcel D, Martino R, Caballero D, Martin J, Ferra C, Nieto JB, et al. Sustained remissions of high-risk acute myeloid leukemia and myelodysplastic syndrome after reduced-intensity conditioning allogeneic hematopoietic transplantation: chronic graft-versus-host disease is the strongest factor improving survival. J Clin Oncol. 2008;26:577–584. doi: 10.1200/JCO.2007.11.1641. [DOI] [PubMed] [Google Scholar]
- 32.van Besien K, Kunavakkam R, Rondon G, De Lima M, Artz A, Oran B, et al. Fludarabine-melphalan conditioning for AML and MDS: alemtuzumab reduces acute and chronic GVHD without affecting long-term outcomes. Biol Blood Marrow Transplant. 2009;15:610–617. doi: 10.1016/j.bbmt.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Soiffer RJ, Lerademacher J, Ho V, Kan F, Artz A, Champlin RE, et al. Impact of immune modulation with anti-T-cell antibodies on the outcome of reduced-intensity allogeneic hematopoietic stem cell transplantation for hematologic malignancies. Blood. 2011;117:6963–6970. doi: 10.1182/blood-2011-01-332007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schmid C, Labopin M, Nagler A, Bornhauser M, Finke J, Fassas A, et al. Donor lymphocyte infusion in the treatment of first hematological relapse after allogeneic stem-cell transplantation in adults with acute myeloid leukemia: a retrospective risk factors analysis and comparison with other strategies by the EBMT Acute Leukemia Working Party. J Clin Oncol. 2007;25:4938–4945. doi: 10.1200/JCO.2007.11.6053. [DOI] [PubMed] [Google Scholar]
- 35.Levine JE, Braun T, Penza SL, Beatty P, Cornetta K, Martino R, et al. Prospective trial of chemotherapy and donor leukocyte infusions for relapse of advanced myeloid malignancies after allogeneic stem-cell transplantation. J Clin Oncol. 2002;20:405–412. doi: 10.1200/JCO.2002.20.2.405. [DOI] [PubMed] [Google Scholar]
- 36.Marks DI, Lush R, Cavenagh J, Milligan DW, Schey S, Parker A, et al. The toxicity and efficacy of donor lymphocyte infusions given after reduced-intensity conditioning allogeneic stem cell transplantation. Blood. 2002;100:3108–3114. doi: 10.1182/blood-2002-02-0506. [DOI] [PubMed] [Google Scholar]
- 37.Yan CH, Liu DH, Liu KY, Xu LP, Liu YR, Chen H, et al. Risk stratification-directed donor lymphocyte infusion could reduce relapse of standard-risk acute leukemia patients after allogeneic hematopoietic stem cell transplantation. Blood. 2012;119:3256–3262. doi: 10.1182/blood-2011-09-380386. [DOI] [PubMed] [Google Scholar]
- 38.Jabbour E, Cortes J, Kantarjian H, Giralt S, Andersson BS, Giles F, et al. Novel tyrosine kinase inhibitor therapy before allogeneic stem cell transplantation in patients with chronic myeloid leukemia: no evidence for increased transplant-related toxicity. Cancer. 2007;110:340–344. doi: 10.1002/cncr.22778. [DOI] [PubMed] [Google Scholar]
- 39.Platzbecker U, Wermke M, Radke J, Oelschlaegel U, Seltmann F, Kiani A, et al. Azacitidine for treatment of imminent relapse in MDS or AML patients after allogeneic HSCT: results of the RELAZA trial. Leukemia. 2012;26:381–389. doi: 10.1038/leu.2011.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schroeder T, Czibere A, Kroger N, Platzbecker U, Bug G, Uharek L, et al. Phase II study of azacitidine (Vidaza(R), Aza) and donor lymphocyte infusions (DLI) As first salvage therapy in patients with acute myeloid leukemia (AML) or myelodysplastic syndromes (MDS) relapsing after allogeneic hematopoietic stem cell transplantation (allo-SCT): Final results from the AZARELA-trial (NCT-00795548) ASH Annu Meet Abstr. 2011;118:656. [Google Scholar]
- 41.Bashey A, Medina B, Corringham S, Pasek M, Carrier E, Vrooman L, et al. CTLA4 blockade with ipilimumab to treat relapse of malignancy after allogeneic hematopoietic cell transplantation. Blood. 2009;113:1581–1588. doi: 10.1182/blood-2008-07-168468. [DOI] [PMC free article] [PubMed] [Google Scholar]