Abstract
Aim
To develop a management strategy (rehabilitation programme) for postsurgical erectile dysfunction (ED) among men experiencing ED associated with treatment of prostate, bladder or rectal cancer that is suitable for use in a UK NHS healthcare context.
Methods
PubMed literature searches of ED management together with a survey of 13 experts in the management of ED from across the UK were conducted.
Results
Data from 37 articles and completed questionnaires were collated. The results discussed in this study demonstrate improved objective and subjective clinical outcomes for physical parameters, sexual satisfaction, and rates of both spontaneous erections and those associated with ED treatment strategies.
Conclusion
Based on the literature and survey analysis, recommendations are proposed for the standardisation of management strategies employed for postsurgical ED.
What's known
Following surgery for prostate, bladder or rectal cancers, loss of erections and cavernous tissue damage may result in significant reductions in penile length and circumference, and these changes have been shown to occur within the first few months of surgery. However, with the introduction of nerve-sparing (NS) surgery, erectile function (EF) can be preserved in a significant proportion of patients. Unfortunately, even with NS techniques, ED can still be a long-term and sometimes permanent complication for many patients
What's new
Currently, there are no UK-wide recommendations for postsurgical ED management strategies following treatment for prostate, bladder or rectal cancer. This study provides a brief overview of current strategies for postsurgical ED management and preservation of erectile function, based on a worldwide literature search. Literature review data are combined with recommendations from an expert panel – individuals who have used various strategies in their clinical practice – to propose evidence-based recommendations for standardised ED management that can be implemented effectively throughout the UK.
Introduction
Prostate cancer is the most common male cancer, accounting for 24% of all new cancer diagnoses 1. Bladder cancer is the fourth most common cancer in male gender in the UK 1. Colorectal cancer is the third most common cancer in the UK 1, with about 50% of patients surviving for more than 5 years after treatment 2. Radical prostatectomy (RP) for prostate cancer, radical cystectomy (RC) plus urinary diversion for bladder cancer and surgery for colorectal cancer invariably lead to postsurgical erectile dysfunction (ED) 3–10.
In addition to a loss of erections, cavernous tissue damage following RP may result in significant reductions in penile length and circumference, and these changes have been shown to occur within the first few months of surgery 11–13. However, with the introduction of nerve-sparing (NS) surgery, erectile function (EF) can be preserved in a significant proportion of men undergoing RP 14. Indeed, with the adoption of this technique, EF recovery rates between 60% and 85% have been reported in some centres 15–17. Unfortunately, many men have less favourable results 18 and ED can be a long-term and sometimes permanent complication, even when maximal cavernous NS techniques are applied 18.
Untreated ED has been associated with penile atrophy and further diminished EF 19. In RP, the removal of the prostate normally results in an almost obligatory period of neuropraxia of the nerves that govern the functional aspects of an erection. This situation may lead to a loss of daily and nocturnal erections resulting in persistent failure of cavernous oxygenation and secondary erectile tissue damage associated with the production of pro-apoptotic factors (i.e. loss of smooth muscle) and pro-fibrotic factors (i.e. an increase in collagen) within the corpora cavernosa 14.
Most men or couples who seek ED treatment after surgery for colorectal, bladder or prostate cancer report difficulty in maintaining sexual activity and intimate relationships. Despite the presence of partners in nearly half of patient consultations, involvement of the partner has been shown to be minimal. Overall, discussions of wider psychosexual concerns are marginalised in medical consultations, and there are limited opportunities for couples to discuss the impact of RP on sexual functioning 20. Preoperative assessment of a couple's readiness to engage in an ED rehabilitation programme is advisable 21. Patients want their partners to be included in the sexual rehabilitation process, but few institutions currently offer couple-based rehabilitation programmes 21. ED is an important cancer survivorship issue for men who have undergone RP and clinicians tend to underestimate patients' distress and desire for early treatment 22.
Finally, assessing comorbidities that affect EF is also important, particularly in the presence of cardiovascular disease (CVD) risk factors. Studies have reported an association between sexual behaviour and comorbidities, such as CVD 23. Continued sexual function also has health benefits. For example, the Caerphilly Cohort Study demonstrated a 50% reduction in cardiac death with more than two orgasms per week 24. ED has an impact on partners and relationships. Sexual dissatisfaction was found to be a risk factor for myocardial infarction in a case–control study of women, with premature ejaculation or inability to get an erection in the male partner being the major underlying cause 25. ED is also independently associated with depressive symptoms in prostate cancer up to 4 years post diagnosis, indicating long-lasting psychological impact 26.
Management of postsurgical ED consists of preventing and/or treating ED after pelvic surgery by countering postsurgical pathophysiological changes during the period of neural recovery. Management of ED after surgery involves the use of any medication or device to maximise recovery of EF. Treating ED is important for endothelial and smooth muscle protection, neuromodulation and reduction in corporal fibrosis 27–31. Psychological and sexual counselling/psychosexual therapy have important roles in improving any biomedical rehabilitation and management programme for postoperative EF impairment 14. This is particularly so where the man/couple exhibit increased levels of distress or limited coping strategies.
The goal of EF management strategy is restoration of assisted and non-assisted EF and prevention of structural changes.
The current options for postsurgical ED management in the UK include
Oral medications [phosphodiesterase type 5 inhibitors (PDE5-Is: sildenafil, vardenafil or tadalafil)]
Intracorporeal injections (ICI)
Intraurethral suppository containing alprostadil that dilates the penile blood vessels
Vacuum erection device (VED)
Sexual counselling
Pelvic floor exercises
Combinations of the above
Penile implant: malleable or inflatable as last resort.
Rationale for recommendations development
The International Consensus of Sexual Medicine (ICSM) Committee recommends that clinicians instruct patients on the essential elements of the pathophysiology of postoperative ED 32. However, currently, there are no specific recommendations regarding the optimal rehabilitation regimen 32 or any standard guidelines for improving EF following surgery. Guidelines from the British Society of Sexual Medicine recommend PDE5-Is (e.g. sildenafil, tadalafil, vardenafil) for first-line treatment of men who have had a prostatectomy, but combination therapy is not discussed 33. With a growing number of sexually active patients undergoing RP or surgery for other pelvic cancers, and improvements in cancer survival rates, the restoration of sexual function has become increasingly important to men, couples and clinicians. On the basis of data presented here, it is reasonable to discuss the implementation of an ED rehabilitation programme (also called EF Restoration programme) for patients undergoing surgery. The ED rehabilitation programme should help preserve EF in men undergoing prostate, rectal or bladder cancer surgery. The goal of the programme is to provide men with improved and earlier return of natural or assisted erections following surgery.
Methods
Literature analysis
A review of published literature (papers published within the last 10 years) was carried out to determine current management options for postsurgical ED and establish the evidence grade for each option. The studies identified and used in this literature analysis were graded using the Oxford Centre for Evidence-based Medicine – Levels of Evidence 34.
The literature search terms included various combinations of the following words: penile rehabilitation; erectile dysfunction/erectile function/sexual function + cancer/prostate/rectal/bladder; sexual dysfunction + cancer/prostate/bladder/rectal; erectile dysfunction + prostatectomy; phosphodiesterase type 5 inhibitor + cancer; vacuum erection + cancer; alprostadil + cancer; intracorporeal injections + cancer; erectile dysfunction/sexual function + diet/exercise.
Reviews (except systematic reviews), commentaries and animal studies were excluded. Any studies that did not utilise surgical methods for the treatment of prostate/bladder or rectal cancer were excluded. All studies analysed used at least one intervention for postsurgical ED management/penile rehabilitation for ED.
Clinician's survey
Recommendations from the group of authors were gained by conducting an email survey of current practice across England with 13 experts representing 10 organisations (listed at the end of this article). The survey investigated current practice in restoring or treating ED after surgery in prostate, bladder and rectal cancer patients, focusing on questions related to current clinical practice; scope of the problem in the UK and treatment approaches.
Results
PubMed search overview
The literature search identified 37 articles after applying the selection criteria. Both randomised and non-randomised studies were included (Table1).
Table 1.
Study and patient characteristics of articles selected for literature review
| Study | Level of evidence | No. of patients | Study design | Regimen | Start of treatment (after RP) | Duration/follow up |
|---|---|---|---|---|---|---|
| Conservative management | ||||||
| Campbell et al. (Cochrane review) 35 | 1A | 1937 | Systematic review | Effects of conservative management for urinary incontinence after prostatectomy (sexual rehabilitation was included but not main focus) | Varied | Varied |
| Intracorporeal injections | ||||||
| Montorsi et al. 36 | 1B | 30 | RCT | ICI three times per week for 12 weeks vs. no treatment | 1 month post surgery | 6 months |
| Mulhall et al. 37 | 3B | 132 (58 active treatment vs. 74 in no treatment group) | Non-randomised study | Trimix/Bimix three times per week vs. no treatment or as needed (nonresponders switched to sildenafil) | 4 months post surgery | 18 months |
| Yiou et al. 38 | 4 | 87 | Uncontrolled cohort study | 2.5 μg intracavernous alprostadil injection | 1 month | 12 months |
| Intraurethral PGE-1/Alprostadil | ||||||
| Costabile et al. 39 | 2B | 384 | Controlled clinical trial | Intraurethral PGE-1 initially then active drug vs. placebo | ≤ 3 months after surgery | 3 months (follow up) |
| Gontero et al. 40 | 4 | 76 | Randomised study | 20 mg prostaglandin E1 injection | 1–12 months | 12 months |
| Raina et al. 41 | 4 | 91 | Case comparison study | MUSE (125 or 250 μg three times per week) vs. on-demand treatment | 3 weeks | 6 months |
| Raina et al. 42 | 4 | 54 | Case series (single cohort) | Transurethral alprostadil (PGE1) (MUSE) four times per month | 3 weeks | 6 months |
| Vacuum erection device | ||||||
| Köhler et al. 43 | 1B | 28 | RCT | Daily VED (10 min), early vs. delayed after RP | 1 month after RP vs. 6 months after RP | 6 months |
| Raina et al. 44 | 1B | 109 | RCT | VED twice a week vs. no treatment | 1 month | 9 months |
| Phosphodiesterase type 5 inhibitor (PDE5i) | ||||||
| Aydogdu et al. 45 | 1B | 65 | RCT | Tadalafil vs. No treatment | 1 week | 6–12 months |
| Bannowsky 46 | 1B | 43 | RCT | Nightly sildenafil 25 mg/day vs. control (no sildenafil) | After catheter removal (7–14 days) | 12 months |
| Brock et al. 47 | 1B | 440 | RCT | Vardenafil, 10 and 20 mg vs. placebo | 6 months–5 years post surgery | 3 months |
| McCullough 48 | 1B | 139 | RCT | Intraurethral alprostadil or oral sildenafil citrate (50–100 mg) | Within 1 month | 9 months |
| Montorsi 49 | 1B | 628 | RCT | Placebo, nightly vardenafil, or on demand vardenafil | 14 days | 12 months |
| Montorsi et al. 50 | 1B | 303 | RCT | Tadalafil 20 mg, on demand vs. placebo | 12–48 months | 3 months |
| Mosbah et al. 51 | 1B | 18 | RCT | Sildenafil twice per week to be shifted to ICI of PGE1 in not responders | 2 and 6 months post surgery | 6 months |
| Nehra et al. 52 | 1B | 440 | RCT | Placebo, 10 mg vardenafil or 20 mg vardenafil | 4 weeks | 3 months |
| Pace et al. 53 | 1B | 40 | RCT | Flexible-dose sildenafil vs. no treatment | 14 days | 6 months |
| Padma-Nathan et al. 54 | 1B | 76 | RCT | Sildenafil, 50 or 100 mg, daily and nightly | 4 weeks | 9 months followed by 8 week drug free period |
| Rectal cancer: Lindsey 55 | 1B | 32 | RCT | Sildenafil vs. placebo | 5.6 years | 1 month |
| Briganti et al. 56 | 2B | 435 | Single cohort | Test the effects of: No treatment after surgery On-demand PDE5i Daily use of PDE5i (every day or every other) | Varied | 36 months |
| Müller 57 | 2C | 92 | Retrospective database analysis | Sildenafil or ICI therapy (if oral therapy failed) | Within 12 months | 18 months |
| Schwartz et al. 58 | 3B | 40 | Case control study | 50 mg sildenafil and 100 mg sildenafil every other night | At catheter removal | 6 months |
| Briganti et al. 59 | 4 | 435 | Single cohort | PDE5-I treatment schedule (daily and on-demand) | Varied | 3–6 months (treatment) 36 month (follow up) |
| Mulhall 60 | 4 | 48 | Single cohort | Sildenafil, and if unsuccessful, then ICI | < 6 months (early) vs. ≥ 6 months after RP (delayed) | 24 months |
| Zippe et al. 61 | 4 | 49 | Survey | Sildenafil – varied | Immediately; 6 and ≥ 12 months | 12 months |
| NS radical cystectomy: El-Bahnasawy et al. 62 | 4 | 100 | Open-label, study | 50 and 100 mg sildenafil | 80.7 months | 3 months |
| Rectal cancer: Nishizawa 10 | 4 | 207 | Single cohort uncontrolled study | On demand treatment with sildenafil | 3 months | 12 months |
| Combination therapy | ||||||
| Titta et al. 63 | 1B | 57 | RCT | Sexual counselling + PGE1-ICI therapy vs. only ICI | ∼1 month | 18 months |
| Mydlo et al. 64 | 2B | 34 | Retrospective study | Sildenafil or vardenafil. After a max of eight doses treated with ICI therapy using 15 or 20 mg alprostadil + oral therapy | Varied | 7 months |
| Yassin et al. 65 | 3B | 69 | Controlled study, non-randomised | Sildenafil, 25 mg three times per week or tadalafil, 5 mg, twice weekly + VED twice daily | 11 days | 3 months |
| Basal et al. 66 | 4 | 203 | Retrospective case series | Review of patients on penile rehab | Varied | 15–72 months |
| Kimura et al. 67 | 4 | 676 | Case series | PDE5i + VED – review of factors affecting rehab | N/A | N/A |
| Lee et al. 68 | 4 | 77 | Case series | Sildenafil citrate or tadalafil three times per week | Varied | 8 months |
| Moskovic et al. 69 | 4 | 29 | Case series | Sildenafil 25 mg nightly + 250 μg alprostadil suppository three times per week. At 1 month, additional daily use of VED (10 min/day) + 100 mg of sildenafil on demand prior to sexual attempt | Prior to surgery and 3 days after RP | 6 months |
| Nandipati et al. 70 | 4 | 22 | Uncontrolled study | Sildenafil 50 mg/day at discharge + PGE1-4 μg or low-dose Trimix (20 U) two to three times per week | At hospital discharge | 12 months |
RP, radical prostatectomy, RCT, randomised controlled trial; VED, vacuum erection device; ICI, intracorporeal injection.
Studies and patient characteristics from the literature analysis
Of the 7725 patients included in the 37 selected studies (Table1), 1937 were from the Cochrane review of conservative treatment and 5788 from other studies involving active treatment(s). All selected studies enrolled ≥ 15 patients. Most of the studies were non-randomised, uncontrolled studies. Postintervention follow up ranged from 12 weeks to ≥ 6 years (Table1).
Presurgical assessment
Literature analysis
Partner involvement
There was a paucity of studies that included couple/partner assessment. An overview of these studies is summarised in Table2.
Table 2.
Studies that assessed partners pre- and post pelvic surgery
| Study | Investigations | No. of patients |
|---|---|---|
| Claes et al. 72 | Erection Hardness Score (EHS) International Index of Erectile Function (IIEF) Self-esteem And Relationship (SEAR) questionnaire Sexual Life Quality Questionnaire (mSLQ-QOL) | 447 |
| Davison 21 | IIEF Miller Social Intimacy Scale (MSIS) | 143 |
| El-Meliegy et al. 77 | Partner Relationship Questionnaire | 493 |
| Jiann et al. 73 | Female Sexual Function Index (FSFI) IIEF | 2159 |
| Moskovic et al. 69 | Compliance with intracorporeal injections Sexual Health Inventory for Men (SHIM) Female partners completed the Female Sexual Function Index (FSFI) | 29 |
| Neese et al. 76 | Survey | 320 |
| Polito et al. 78 | IIEF | 430 |
| Rubio-Aurioles et al. 71 | Partner Relationship Questionnaire | 511 |
| Sato et al. 79 | Patients' sexual function, sexual bother and expectations for postoperative sexual life | 162 |
| Schover et al. 74 | Sexual Self-Schema Scale-Male Version IIEF Urinary and bowel symptom scales from the Los Angeles Prostate Cancer Index Short-Form Health Survey | 2636 |
| Shindel et al. 75 | IIEF FSFI | 1134 |
The literature analysis demonstrated that patients who had partners with a negative sexual attitude lost sexual motivation up to 1 year after surgery 71. However, patients with cooperative partners maintained sexual motivation 71,72 and demonstrated improvements in measures of sexual function and quality of life 72. In addition, ED of the male partner was a significant risk factor for female sexual difficulty 73 and compliance with/dropout rates from rehabilitation protocols were related to partner preferences and preoperative female sexual function 69,73,74. Overall, there was a correlation between sexual dysfunction in female and male partners 75,76.
Clinician Survey findings
Table3 outlines the baseline assessments of ED before surgery carried out by the clinicians surveyed
Table 3.
Survey findings of baseline assessments of EF before surgery
| Assessment | No. of responders |
|---|---|
| IIEF/SHIM | 6 |
| Verbal sexual history take only | 5 |
| Surgical nurse assessment (depends on nurse availability) | 1 |
| Partner consultation | 1 |
EF, erectile function; IIEF/SHIM, International Index of Erectile Function/Sexual Health Inventory for Men.
The assessment usually involved
Discussion with patient.
Biomedical components, including the disease, treatment, current medications, current medical history, previous medical and surgical history and medication history.
Psychological factors (sexual self-esteem/confidence), relationship issues and any issues of a social context that impact on sexuality or that are affected by the sexual dysfunction, e.g. reduced penile size, loss of ejaculation.
IIEF/SHIM/verbal – can patients have penetrative intercourse?
Most clinicians stated that partners were not always involved in assessment unless the patient consented to include them. However, patients were encouraged to take their partner to appointments in the ED clinic. ED assessment in clinical practice was generally driven by the urologist or surgeon and the multidisciplinary team.
Length of time from surgery to assessment and treatment
According to the literature analysis, the length of time from surgery to treatment varied from 1 week post surgery to ≥ 12 months. However, improved outcomes were observed with earlier treatment (within 1 month) of surgery or onset of ED especially with VED 43,44; PDE5-I 32,58; combination therapy 70.
In the survey analysis, four participants stated that early intervention was likely to lead to better results and improved return of EF and therefore would assess the patient at catheter removal or up to 10 days postoperatively. However, the interval from surgery to ED treatment varied in clinical practice and ED was assessed and managed up to 3 months after surgery in some cases. Re-assessment usually occurred at 8 weeks, 3 months and at 3 monthly intervals thereafter, usually coinciding with the men's cancer treatment review schedule.
Very few participants assessed ED presurgery, although most recognised the need to do so. All participants assessed patients for comorbidities and concurrent drug therapy, which may affect ED and response to ED management. Testosterone levels were not routinely checked in clinical practice unless there was a clear indication to do this.
Current available management strategies for postsurgical ED
Current management strategies for ED are summarised in Table4.
Table 4.
Current management strategies for postsurgical ED from literature analysis
| Strategy | No. of publications | Total no. of patients | Results | Adverse events |
|---|---|---|---|---|
| Conservative management | 1 | 1937 | No significant benefit from pelvic floor exercises for ED 35 | N/A |
| Psychosexual counselling | 1 | 57 | Significant improvements in IIEF scores when combined with ICI injections 63 | |
| Intracorporeal injections | 5 | 382 | Significant self-reported recovery of spontaneous erection sufficient for satisfactory sexual intercourse 36,37,40 Significant improvements in IIEF scores 37,38,63 and EHS 38 Significant improvements in response to PDE5-I 37,63 | Prolonged erection/penile nodule (n = 3) 36,63 Painful erections (n = 10) – pain decreased over time in one study 38,63 |
| Intraurethral PGE-1/Alprostadil | 3 | 529 | Achieved erections sufficient for intercourse 39,41,42 Significant improvements in SHIM 41,42 Self-reported recovery of spontaneous erection sufficient for satisfactory sexual intercourse 41 | Urethral pain/burning 39,41,42 |
| Vacuum erection device | 2 | 137 | Significant improvements in IIEF scores, especially with early use 43,44 No significant changes in penile length 43 Improvements in smooth muscle content 58 Self-reported return of natural erections sufficient for vaginal intercourse with early use 44 | Difficulties in using the device 80 |
| Phosphodiesterase type 5 inhibitor (PDE5-I) | 18 | 4977 | Preserved penile length 45 Significant improvements in IIEF with on demand and daily therapy 47–50,52–57,62 No difference in terms of EF recovery was found between patients receiving on-demand vs. daily PDE5-I 56 Higher efficacy with daily therapy vs. on-demand PDE5-I in patients with intermediate risk of ED 56 Treatment schedule correlates with ED risk (higher risk patients benefit from daily therapy) 56 Erections sufficient for successful penetration 50–52,62 Recovery of unassisted erections 51,53,54,57 Significant differences between early vs. delayed therapy 58,60 Responsiveness to PDE5-I may persist for 8 h post dose in men with mild-to-moderate ED 48 | Headache, flushing and rhinitis with PDE5-Is 47,50,52 Dyspepsia and myalgia with tadalafil 50 |
| Combination therapy | Return of spontaneous erections 69Significant improvements in SHIM scores 64,70 Early therapy associated with improved outcomes 70 Early combination therapy with PDE5-I allowed lower dose of intracavernous injections 70 Erections sufficient for intercourse 65 |
ED, erectile dysfunction; ICI, Intracorporeal injection; IIEF, International Index of Erectile Function; EHS, Erection Hardness Score; PDE5-I, phosphodiesterase type 5 inhibitor; SHIM, Sexual Health Inventory for Men.
Treatment regimens for nerve-sparing vs. non-nerve-sparing surgery
Clinician survey findings of strategies employed in clinical practice for managing ED associated with NS and non-NS surgery are summarised in Table5.
Table 5.
Survey analysis of treatment strategies for nerve-sparing vs. non-nerve-sparing surgery
| Nerve-sparing | Non-nerve-sparing surgery |
|---|---|
| First-line strategy used | First-line |
| PDE5-I once daily/on demand +/− VED | Sexual and relationship therapy + VED or VED + ICI/Intraurethral alprostadil |
| Second-line strategy used | Exclude PDE5-Is, all other treatments may work |
| ICI/intraurethral alprostadil (probably second line and ICI third line) | |
| Third-line strategy used Penile prosthesis | Second-line Penile prosthesis |
VED, vacuum erection device; ICI, intracorporeal injection; PDE5-I, phosphodiesterase type 5 inhibitor.
Treatment algorithm
A typical treatment algorithm employed in clinical practice and provided by most of the survey participants for NS prostatectomy and for non-NS surgery is shown in Figure1A, B, respectively. The algorithms shown are mainly based on locally agreed practice.
Figure 1.
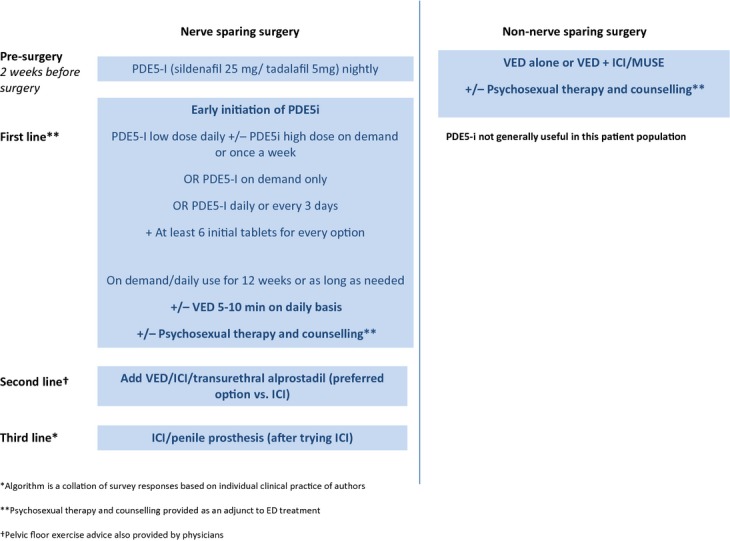
Erection Restoration Algorithm. *Algorithm is a collation of survey responses based on individual clinical practice of authors. **Psychosexual therapy and counselling provided as an adjunct to ED treatment. Pelvic floor exercise advice also provided by physicians
Advantages and disadvantages for each postsurgical ED management strategy
The advantages and disadvantages for each postsurgical ED management strategy are summarised in Table6.
Table 6.
Advantages and disadvantages for each postsurgical ED management strategy
| Post-surgical ED management strategy | Advantage | Disadvantage |
|---|---|---|
| Oral medications (PDE5-I) | Easy to take Acceptable to most patients and partners Generally good response Safe – Good tolerance generally Does not interfere with foreplay | Side effects, e.g. muscle aches, headaches Some patients will need to take the drug on at least 8–12 occasions to achieve a reliable response Compliance Treatment failure Cost Un-licensed indication for daily dosing in ED rehabilitation programme |
| Vacuum erection device | Avoids medication – may be more acceptable to some men Non-invasive Safe – no systemic effects or medical toxicity Cost-effective Simple to use Can be effective but depends on patient acceptability | Uncomfortable, clumsy, mechanical Erection not natural looking Commitment to learn Skilled instructor needed Slow response Partner acceptance If used for penetration: altered penile sensations Can be painful |
| Intraurethral suppository | Relatively easy to use and learn Rapid onset May not be effective for all patients No needles Painless to insert Well-tolerated Local treatment | Not helpful for most men Can be difficult to insert Urethral burning |
| Intracorporeal injections | More natural looking erection Quick administration and result Usually effective – direct drug delivery | Compliance Psychological – not acceptable to all men or their partners Requires motivated patient Good manual dexterity needed Skilled instructor needed Patient fear of priapism Pain and bruising, recommended precautions |
| Combination strategy | May be helpful for those with incomplete erections with PDE5-I alone Easy to use Problems as above with the differing combinations therefore additive Seems to be the most effective, allows early resumption Works on all aspects of postoperative ED | Need for two separate interventions Patient commitment Expensive and time consuming Not always available on the NHS |
ED, erectile dysfunction; PDE5-I, phosphodiesterase type 5 inhibitor.
All participants agreed that combination therapy was the most cost-effective therapy, although not always available to the patients. The most effective combination depended on patient and partner needs. However, the commonest favoured combination was VED + PDE5-I (daily or/and on demand).
Duration of treatment
In the literature analysis, the duration of treatment lasted from 6 months to ≥ 6 years.
According to the survey findings, the duration of any treatment can range from 3 months until the patient no longer needs the treatment. The duration of time for any treatment may depend on various factors including patient acceptance, need for treatment, side effects or simply access to and cost of the treatment.
There is no clear evidence of the optimal length of treatment with daily dosing in the literature and in clinical practice; this varies from patient to patient. Hence, the decision to stop treatment must be individualised and strict or arbitrary time limits are inappropriate.
Discussion
The literature search identified 37 articles after applying the selection criteria. Both randomised and non-randomised studies were used. However, it must be noted that most studies identified were non-randomised or controlled studies.
Goals of postsurgical ED management/restoring EF
All the participants of the clinician survey agreed that the goals for postsurgical ED management should be
Restoration of normal, assisted or non-assisted EF
Achievement of an erection that is satisfactory for the patient for masturbation or penetrative sexual purposes
Achievement of patient-agreed sexual recovery goals
○ Establish the patient's expectations/recovery goals
○ Establish the couple's expectations/recovery goals
○ Be realistic when managing patient/couples' expectations
Improvement of specific ED-related quality of life issues, e.g. stress/depression
Provision of ongoing support for the man/couple relating to the sexual consequences of their cancer treatment
Maintenance of blood flow to the penis and maximisation of the potential for cavernosal nerve repair to enable natural penetrative EF, prevent penile shortening, endothelial damage and promote re-oxygenation of tissue.
Predictive factors
The recovery of EF is a multifactorial process that depends on many variables 81,82. These include
Patient and partner age – older age is associated with ED
Preoperative EF (including preoperative use of PDE5-I – better preoperative function is associated with better outcomes
Comorbidity status at the time of surgery (fewer comorbidities are associated with lower risk of ED)
Surgical technique (plan for NS surgical technique: unilateral/bilateral or not)
Prostate-specific antigen level (lower levels associated with better outcomes)
Lower cancer grade/risk category associated with better outcomes
African American race/ethnicity and lower body mass index are associated with better EF
Testosterone levels – normal levels are important for recovery of EF
Assessment of EF
The results from the literature analysis and the survey support the finding that the partner's sexual function should also be assessed as part of any ED management programme because partners may require intervention if they are to support rehabilitation efforts for their spouses 21.
The ICSM 2001 recommends that clinicians discuss ED prevalence rates, the pathophysiology of ED after RP and the predictors of EF recovery. They also propose that validated instruments and published cut-offs for normal function should be used for baseline and subsequent assessment, and that sexual rehabilitation and its potential benefits should be discussed with patients 60.
A list of some of the tools available for clinicians to assess baseline sexual function and track the progress of patients (and partners) over therapy is shown in Table7. However, a detailed assessment is not necessarily carried out in each case. In the clinician survey, most participants confirmed that they evaluate patients presurgery via verbal assessment or through IIEF/SHIM questionnaires.
Table 7.
Overview of some commonly used sexual function assessment tools
| Survey name | Abbreviation | Number of items | Domains | Estimated time to complete (min) |
|---|---|---|---|---|
| International index of erectile function 83 | IIEF | 15 | Erectile function Orgasmic function Sexual desire Intercourse satisfaction Overall sexual satisfaction | 3 |
| Sexual health inventory in men 84 | SHIM (IIEF-5) | 5 | Erectile function Intercourse satisfaction | 1 |
| Erection hardness scale 85,86 | EHS | 1 | Assessment of erection hardness | 1 |
According to the clinician survey findings, most participants would evaluate patients presurgery via verbal assessment or through IIEF/SHIM questionnaires [although many did not carry out ED assessment presurgery (n = 6)]. The survey showed that assessment can begin before surgery or at catheter removal (up to 3 months after surgery).
Inclusion of other assessments, e.g. biomedical tests, depended on the clinician's speciality. Involvement of partners at assessment, although recommended in the literature, depended on patient agreement in clinical practice.
Assessing the partners' sexual function is important in managing ED. Satisfactory preoperative female partner sexual function has been shown to correlate with greater patient compliance with the localised component of the EF rehabilitation programme 69. There is a paucity of data from research in same sex partnerships. However, sexual health assessment for partners should also be performed to ensure that the proposed couple recovery programme is appropriate.
There is a significant correlation between postoperative sexual life of patients and their partners. Partners' cooperative attitude might contribute to maintaining patients' sexual desire and motivation. Survey data show that patients wish their partners to be included in the sexual rehabilitation process, but few institutions currently offer couple-based rehabilitation programmes 21. Although intimacy levels of couples are high preoperatively, there is a need to prospectively determine how these levels are impacted by changes in sexual function postoperatively. Baseline assessment of a couple's sexual function and willingness to participate in an EF management programme should ideally be performed preoperatively 21.
Once ED management is initiated, the clinicians surveyed recommend that re-assessment should occur regularly (at least every 3 months).
Management of ED post surgery
The restoration of EF usually involves proactive and early management strategies to
Minimise severity/duration of ED
Improve cavernosal oxygenation
Promote endothelial protection
Prevent/minimise cavernosal structure changes
The ICSM Committee has listed five different types of rehabilitative approaches: (i) phosphodiesterase type 5 inhibitors (PDE5-Is), (ii) ICIs, (iii) intraurethral alprostadil, (iv) VED, and (v) neuromodulatory agents, although no specific recommendation was given regarding the optimal rehabilitation regimen 60.
Current strategies
Current strategies for managing ED in the UK include
PDE5 inhibitors
ICI
Intraurethral alprostadil
VED
Psychosexual therapy
Physiotherapy (pelvic floor exercises)
Penile implants/prosthesis
Combinations of the above
Oral therapy – PDE5-Is
PDE5-Is commonly used for treating ED post surgery include sildenafil, tadalafil and vardenafil. In men with ED, postbilateral NS RP, sildenafil, vardenafil and tadalafil are all equally effective treatments, although most of the trials conducted to date have been performed with sildenafil 49,50,87. Furthermore, PDE5-Is are not currently licensed for use in men for ED rehabilitation programmes.
A multicentre, placebo-controlled, 12-week, parallel arm randomised trial of 440 men compared placebo with vardenafil, 10 and 20 mg. At 12 weeks, improved erections (based on GAQ) were reported by 65.2% and 59.4% of patients on 20 and 10 mg vardenafil, respectively, and by only 12.5% of patients on placebo (p < 0.0001) 47. Tadalafil 20 mg administered as needed for 12 weeks was well tolerated and was effective in the treatment of ED when initiated 12–48 months following prostatectomy 50. The duration of action following 100 mg sildenafil may persist for 8 h post dose in men with mild-to-moderate ED 48.
McCullough et al. have reported the results of a prospective, randomised, open-label, multicentre study comparing nightly intraurethral alprostadil and oral sildenafil in men with preoperative fully normal EF who underwent NS RP 48. Both nightly treatments were started ≥ 1 month after surgery, at the catheter removal visit, and continued for nine consecutive months. At months 3 and 6, there was a 7–14% difference in IIEF scores in favour of IUA, but the differences were not significant. Mean IIEF scores were not significantly different in the two groups at the end of the study (IUA 15.28 vs. sildenafil 17.65). Overall, there were no statistically significant differences between the two groups in terms of IIEF-EF domain scores and intercourse success rates 48.
Pace et al. investigated EF recovery of patients treated by early penile rehabilitation therapy (14 days post surgery) with sildenafil vs. a no treatment control group 53. The treatment was initiated 14 days after surgery. Significant differences in IIEF mean scores improvements were observed with sildenafil vs. control group at 24 weeks. The authors concluded that PDE5-Is should be offered early after RP to support recovery of EF. A study by Mulhall et al. also suggested a benefit from early pharmacological rehabilitation with sildenafil after RP 60. Early institution of tadalafil therapy after RP also appeared to preserve EF and speed the return to sexual intercourse 88.
Current data show that sildenafil 89,90, tadalafil 50,89 and vardenafil 47,89,52 taken when needed may improve ED post RP. On-demand treatment with vardenafil during a 3-month period significantly improved intercourse satisfaction, orgasmic function and overall satisfaction with sexual experience 52.
Daily therapy has shown similar benefits 91. Bedtime sildenafil (50–100 mg daily) administration for nine consecutive months, beginning 1 month postoperatively, results in a greater return to baseline EF and improvements in spontaneous erections 54. Bannowsky et al. further demonstrated that sildenafil was effective in cases of early postoperative nocturnal erections 46. Montorsi et al. showed that sildenafil taken nightly enhances nocturnal penile tumescence after RP 92.
Briganti et al. 59, in a large contemporary series of patients treated by high-volume surgeons, showed that the 3-year EF recovery rates were significantly higher in patients who used postoperative PDE5-Is compared with patients who did not use any postoperative PDE5-Is (73% and 37%; p < 0.001), regardless of the class of risk to which patients belonged according to their own preoperative characteristics. However, EF recovery rates were not significantly different according to the PDE5-I treatment schedule (daily compared with on-demand) after NSRP. In addition, a randomised, double-blind, double-dummy, multicenter, parallel group study compared 9-month 10-mg nightly dosing of vardenafil and flexible-dose on-demand vardenafil (starting at 10 mg, with the option to titrate to 5 or 20 mg) in patients who had a NSRP 49, showed that nightly dosing with vardenafil did not have any effect beyond that of on-demand use.
Another study that tested the effect of penile rehabilitation according to preoperative patient characteristics in 435 patients demonstrated that although there was no difference in terms of EF recovery between patients receiving on-demand vs. daily PDE5-I; in patients with low or high risk of ED risk of ED, daily therapy showed significantly higher efficacy for the EF recovery rate compared with the on-demand PDE5-I administration schedule in patients with intermediate risk of ED 56.
Early high-dose therapy with sildenafil may preserve the smooth muscle content within the corpora cavernosa in humans 58. In addition, tadalafil rehabilitation is effective in preserving penile size especially in the early postoperative period after surgery 45.
In a study designed to assess the efficacy and safety of sildenafil for managing ED following RC, sildenafil therapy significantly improved the ability to achieve and maintain an erection, with a dose-dependent response 62.
A study, carried out to determine the extent of male sexual dysfunction after surgical treatment of rectal cancer and to examine the outcome of postoperative treatment with sildenafil in 207 patients, demonstrated that on-demand sildenafil was effective in improving ED 10. A double-blind placebo-controlled study aimed to determine the efficacy of sildenafil after rectal excision for cancer or inflammatory bowel disease in 32 patients. In this study, sildenafil completely reversed or satisfactorily improved postproctectomy ED in 79% of patients 55.
In summary, the likelihood of response to PDE5-Is increases with time, and has been shown to be ineffective in the first 6–9 months post RP in some studies 93. The response rates to sildenafil after RP depend on age, dosage, interval to start of therapy and degree of cavernous nerve damage 93. Although younger patients with good preoperative EF may experience good EF recovery rates even without any treatment, using PDE5-Is after surgery further improves their functional outcomes 14,94. Treating patients immediately postoperatively is important and may lead to better long-term results in terms of either EF recovery or ED treatment 14. Furthermore, early use of sildenafil after RP may preserve intracorporeal smooth muscle content 53,58,60. The literature analysis and survey results suggest that PDE5-Is should be offered early after RP to allow the recovery of EF 58. PDE5-Is could be initiated as early as the removal of the catheter or during the very first month after surgery 14.
Although daily use of PDE5-Is has shown better efficacy data vs. on-demand use in patients with intermediate risk of ED 56, high-quality RCTs are lacking, which compare on-demand vs. daily use of PDE5-Is. Studies emphasise that early management of ED prior to penile fibrosis development is important 14. There is evidence for a protective role with prolonged dosage of a PDE5-I for preventing cavernosal damage in animal models 27,29,30,95,96. For example, both sildenafil and tadalafil decrease numbers of apoptotic cavernous smooth muscle and endothelial cells and increase numbers of kinases associated with cellular survival in animal models 28,96. Early high-dose use of PDE5-Is has shown to preserve smooth muscle content within the corpora cavernosa in humans and they may also help preserve penile size 45,58,97. Long-term use of PDE5-Is post cavernosal nerve resection has been shown to prevent the alterations in corporal histology induced by cavernosal nerve damage 20,96.
Specific counselling on ED treatment modalities and re-education of patients could promote higher initial acceptance rate and a reduction in the postoperative discontinuation rate for PDE5-Is 98.
Intracorporeal injection
Montorsi et al. compared regular administration of alprostadil ICIs vs. no injection therapy postoperatively in a total of 30 patients. The patients were randomized to alprostadil injections three times per week for 12 weeks (15 patients) or observation without any erectogenic treatment (15 patients). Six months post RP, spontaneous sexual erections were reported in significantly more men who had undergone routine ICI compared with those who did not 36. In the alprostadil group, eight patients (67%) reported the recovery of spontaneous erection sufficient for satisfactory sexual intercourse, compared with three patients (20%) in the control group (p < 0.01) 36. In a prospective non-randomised study, Mulhall et al. evaluated the postoperative outcome of men with functional preoperative erections who underwent either NS or non-NS RP and were provided with early postoperative oral sildenafil (within 6 months after RP) 37. Non-responders were switched to ICI (TriMix) three times a week or on-demand use 4 months after surgery. At 18 months after RP, all patients who had used TriMix did not report either pain or prolonged erections (priapism) 37. Furthermore, the injections significantly improved the proportion of patients responding to sildenafil (patients who followed the protocol = 64% vs. patients who did not follow the protocol = 24%, p < 0.001). These data indicate that a pharmacological penile rehabilitation protocol results in higher rates of spontaneous functional erections and erectogenic drug response after RP 37.
In a prospective study, Yiou et al. assessed the safety and efficacy of intracavernosal alprostadil (2.5 mg of alprostadil twice a week) in a cohort of 87 men who underwent laparoscopic NS RP and started self-injection treatment 1 month after surgery 38. The alprostadil dose was titrated up until the patients were able to achieve an erection hard enough to allow vaginal penetration; it was also suggested that patients attempt intercourse as often as possible. 11% of patients discontinued because of painful erections. Among those who continued the treatment, pain intensity during erection and pain scores significantly decreased over time (i.e. between 6 and 12 months after RP). In contrast, pain scales did not correlate significantly with any of the sexual scores at 12 months, suggesting that the adverse impact of pain diminished over time 38.
Montorsi et al. published a study supporting use of ICIs of alprostadil three times weekly for 12 weeks after RP with those using no treatment. The study showed that early postoperative administration of alprostadil injections significantly increased the recovery rate of spontaneous erections after NS radical retropubic prostatectomy 36.
In men who received a non-nerve-sparing RP (NNSRP), Gontero et al. showed a trend towards a progressively decreasing erectile response with time from the operation 40. A total of 73 patients received prostaglandin E1 injections at 1, 2–3, 4–6 and 7–12 months postoperatively. A significantly higher proportion of patients who received the injections in the first 3 months after surgery had erections hard enough for penetration compared with patients who received the injections at 4–12 months after surgery. However, injection given in postoperative month 1 provided the best response rate but with significant complications and poor patient compliance. Gontero et al. concluded that patients who received ICI ≤ 3 months after RP achieved an erection sufficient for sexual intercourse; after that period of time, the chances of an acceptable response to alprostadil decreased 40. The authors further suggested that 3 months post surgery is a reasonable compromise to start treatment in terms of effectiveness and patient compliance 40.
Intracorporeal injection is usually the treatment of choice for patients in whom PDE5-Is are relatively ineffective 14. The timing for starting ICI, like any other ED intervention, should be accurately defined 14. ICI can often cause penile discomfort, which affects patient compliance 14.
Intraurethral alprostadil
Costabile et al. evaluated erectile response rates to intraurethral PGE-1 beginning at least 3 months after RP 39. Approximately 70% of treated men developed erections sufficient for intercourse. The responders were then randomised into a 3-month home trial with either PGE-1 or placebo. Approximately 57% of the patients in the PGE-1 group had erections sufficient for intercourse vs. 6.6% in the placebo group 39.
Zippe et al. conducted a prospective non-randomised study on the early use of intraurethral alprostadil after RP in 91 patients who received intraurethral alprostadil three times/week for the first 6 weeks after RP vs. control group (no intraurethral alprostadil). At 6 months, 74% of the patients resumed sexual activity, 53% had natural erections sufficient for vaginal penetration without intraurethral alprostadil and 47% continued to use intraurethral alprostadil as an adjuvant treatment for successful intercourse. In the control group, only 11% of patients at 6 months achieved natural erections sufficient for satisfactory sexual intercourse 61. The intraurethral alprostadil discontinuation rate was 32% 61. Raina et al. evaluated 54 patients from a single surgical series who used intraurethral alprostadil 6 months after RP 42. Overall, 55% of patients achieved and maintained erections sufficient for intercourse, 48% continued long-term therapy with a mean use of 2.3 years (average usage of four times per month) and there was a 61% spousal satisfaction rate 42. The intraurethral alprostadil discontinuation rate was 52% 42.
The most common reasons for discontinuation of intraurethral alprostadil were insufficient erections, switch to other ED therapies, natural return of erections, and urethral pain and burning 42,61.
Intraurethral PGE-1/alprostadil has been shown to be an effective therapy for post-RP ED, especially when treatment is initiated early 39. Early intraurethral alprostadil therapy following RP is associated with increased frequency of sexual activity, and increased incidence of natural erections sufficient for intercourse 39,61.
Vacuum erection device
Raina et al., in a non-randomised study of 109 patients at the Cleveland Clinic, evaluated the use of early VED after prostatectomy (1 month after surgery). They demonstrated that 23% of patients compliant with VED treatment complained of decreased penile length and girth as compared with 85% who were non-compliant 44. This was consistent with a previous study, which assessed the efficacy of VED, daily for 9 months, following RP 44. The study had demonstrated that early use of VED following RP facilitates early sexual intercourse, early patient/spouse sexual satisfaction and potentially an earlier return of natural erections sufficient for vaginal penetration 44. Köhler et al. looked at early (starting 1 month after prostatectomy) vs. late (starting 6 months after prostatectomy) usage of the VED. The VED was used for a total of 5 months after RP, and showed an improvement in IIEF scores and stretched penile length in the early usage group 43.
In summary, the literature analysis has shown that initiating the use of a VED protocol at 1 month after RP can improve early sexual function and help preserve penile length 43. The literature analysis shows that early use of VED (within 1 month of surgery) can lead to significant improvements in IIEF and sexual function 43,44. In these studies, the VED was effective at restoring erections and maintaining penile size when therapy was initiated at 1 month or between 2 and 8 weeks (mean 3.9 weeks) after surgery 43,44. Therefore, 4–8 weeks may be considered an appropriate postoperative recovery period, and VED can possibly be initiated after this time. VEDs are often effective, low-cost treatment options for ED. They are cost-effective compared with PDE5-Is 99. However, there are no head-to-head trials examining VED vs. oral therapy.
Psychosexual therapy and counselling
In a prospective study, a cohort of 57 patients who completed the IIEF 1 month after RP were randomised to either treatment with ICI only or ICI-focused sexual counselling for 18 months 63. At the end of the study protocol, the analyses showed that men who received sexual counselling coupled with ICI therapy reported the best quality in all IIEF domain, the lowest discontinuation rate and the highest degree of couple's satisfaction compared with men who did not receive any formal counselling 63. Sexual counselling reduced patients' feeling of lack of sexual spontaneity, dissatisfaction and fear of needles 63.
Psychological and sexual counselling may also play an important role in improving any rehabilitation and management programme of postoperative EF impairment 14. Many studies have shown that sexual counselling contributes to better biomedical treatment efficacy, patient acceptance and compliance 14,40,63,99–101.
Pelvic floor exercise
A Cochrane review, which assessed the effects of ‘conservative’ management for urinary incontinence after prostatectomy, concluded that there was no evidence of benefit for ED with pelvic floor muscle training 35.
Combination therapy
Mydlo et al. retrospectively reviewed 34 men after RP with subsequent ED 64. Patients were titrated on sildenafil or vardenafil to maximum doses. All had a suboptimal response and were then treated with ICI therapy using 15 or 20 mg alprostadil. EF was improved in 22 (68%) patients after ICI therapy. On follow up, 36% of these patients used ICI therapy only intermittently, instead of regularly, as they felt that this was adequate to generate good results 64.
Nandipati et al. evaluated early combination therapy with ICI therapy with alprostadil and oral sildenafil vs. low-dose TriMix (papaverine, phentolomine and PGE-1) vs. low-dose PGE-1 after RP in 22 patients 70. Sildenafil 50 mg/day was started at the time of hospital discharge. ICI therapy with alprostadil or low-dose TriMix was started within 3 weeks or at catheter removal. This therapy was to be attempted two to three times weekly. The patients were followed up for up to 12 months every 3 months. After a mean follow up of 6 (3–8) months, 21/22 (96%) patients were sexually active. Approximately 45% were sexually active in the injection-only group vs. 50% with combination therapy. Early ICIs following RP may facilitate early sexual intercourse, patient satisfaction and potentially earlier return of natural erections. A previous report by the same author also evaluating the role of intracavernosal alprostadil (PGE1) combined with sildenafil in stimulating early recovery of spontaneous erections following RP showed that the combination regimen assisted in early sexual activity and satisfaction 70. Furthermore, early combination therapy with sildenafil allowed a lower dose of ICIs, minimising penile discomfort 70,101.
Yessin et al. reported on combination therapy with VED and PDE5-I for early penile rehabilitation following NSRP 65. The patients were started on sildenafil, 25 mg, three times per week or tadalafil, 5 mg, twice weekly with VED being used twice daily. This combination therapy was started 11 days after RP and continued for 3 months. Overall, 56% of the patients on combination therapy obtained erections sufficient for intercourse. Patients on sildenafil reported higher success rates than those on tadalafil (78% vs. 64%) 65.
Moskovic et al. instructed their patients to take 25 mg of sildenafil nightly, as well as to use 250-μg alprostadil urethral suppositories three times per week, even beginning 1 week prior to surgery 69. Patients had to restart 25 mg of sildenafil nightly 3 days after NSRP with the addition of 250-μg alprostadil urethral suppositories three times per week once the catheter was removed. One month after RP, the patients were also instructed to use a VED at least 10 min/day, encouraged to engage in sexual activity and to use 100 mg of sildenafil on demand prior to sexual attempts. Of 29 patients originally included, 3 and 6 of the 24 patients who did not discontinue the programme (10.3% and 25%, respectively) had reported a return to natural unassisted EF sufficient for penetration at 3- and 6-month assessment, respectively. Preoperative female partner sexual function was correlated with greater patient compliance with the localised component of the EF rehabilitation programme.
A recent study, by Basal et al., demonstrated that penile rehabilitation programmes were effective for men after RP, especially ones that include PDE5-Is 66. The study demonstrated that programmes that incorporated PDE5-Is, either alone or with VEDs, were beneficial. The investigators examined the preoperative SHIM scores for 203 men, who were divided into three groups based on their degree of ED, group 1 (SHIM = 8–16), group 2 (SHIM = 17–21) and group 3 (SHIM = 22–25). The participants then underwent four types of penile rehabilitation programmes: PDE5-Is only, VED only, combined PDE5-I and VED therapies, and no therapy. The mean EF recovery periods for groups 1, 2 and 3 were 15.44, 12.31 and 8.73 months (p < 0.05). Only PDE5-Is or the combination of PDE5-Is and VED use had a beneficial effect on EF recovery periods (p < 0.05). Using PDE5-Is and VED together provided the best result, but there was no difference between PDE5-Is and a VED when used alone (p ≥ 0.05) 66.
A study carried out in 18 patients to compare early vs. late penile rehabilitation in patients after radical cysto-prostatectomy utilised sildenafil twice weekly to be changed to ICI of PGE1 (prostaglandins) if not responding. The treatments were started at 2nd and 6th month postoperatively. Results demonstrated that early compared with delayed erectile rehabilitation brings forward the natural healing time of potency and maintains nerve-assisted erection 51.
Combination therapy can include ICI with PDE5-I, or VED and PDE5-I. Data from published studies demonstrate that a combination regimen of alprostadil and PDE5-I can assist in early sexual activity and satisfaction 70. Furthermore, early combination therapy with PDE5-I allows a lower dose of ICIs to be used, minimising penile discomfort 70,101. Data showed that early use of PDE5-Is with VED was also associated with significant benefits in sexual function 66,69.
Erection restoration algorithm based on clinician survey findings
The erection restoration algorithm generally used in clinical practice by survey participants for NS prostatectomy is summarised in Figure1. In general, first-line treatment consisted of early use of PDE5-Is (daily +/− on demand) +/− VED, followed by the addition of intraurethral suppositories/ICI and implant if these strategies failed. Some participants stated that they initiate PDE5-I before surgery (if pre-existing problems identified at presurgical assessment) or at catheter removal to improve outcomes. However, for NNSRP patients, most participants agreed that PDE5-I drugs are not useful. For NNSRP, VED was generally the treatment of choice +/− ICI/intraurethral alprostadil and sexual counselling was recommended.
Although one participant recommended VED + PDE5-I for second-line use in NNSRP, most participants recommended excluding PDE5-Is for NNSRP. In general, VED is the treatment of choice +/− ICI/intraurethral alprostadil and sexual counselling was recommended for these patients.
Use of psychosexual therapy/pelvic floor exercises with the treatments described in Figure1 depends on patient preference. Most participants agreed that the treatment schedule does not vary significantly for bladder, prostate or rectal cancer patients. However, for patients undergoing bladder surgery, presurgery ED is worse (patients are older) and recovery of EF is not optimal; therefore, a more intensive regime may be required.
Table8 provides an Erection Restoration Algorithm from the authors for the management of erectile dysfunction after surgery.
Table 8.
Key recommendations for ED rehabilitation programme
| Key recommendations for ED rehabilitation programme |
|---|
| Preoperative recommendations |
| Preoperative discussion with the patient and their partner of the impact of surgery on sexual functioning and components of the proposed ED rehabilitation programme |
| The partner's sexual function should be assessed as part of any ED management programme pre and postoperatively because partners may require medical/psychosexual therapy if they are to support rehabilitation efforts for their partners |
| Preoperative assessment of a couple's readiness to engage in a ED rehabilitation programme is advisable |
| Preoperative assessment of comorbidities or concurrent medications, which would affect sexual function |
Pre-operative assessment should include:
|
| Postoperative recommendations |
| Discuss the implementation of an ED rehabilitation programme with patients and partners postoperatively |
| Re-assess sexual function at catheter removal or up to 10 days post surgery |
| Treatment algorithm |
| See Figure1 for treatment algorithm recommendations for nerve-sparing prostatectomy, rectal surgery and an algorithm for non-nerve-sparing surgery |
| Consider first-line treatment with combination therapy Combination therapy is usually the most cost-effective therapy (generally, PDE5-I + VED) |
| Consider daily PDE5-I therapy in patients with nerve-sparing surgery, especially during initial (early) management (although level 1 evidence is lacking for superiority of on-demand vs. daily treatment) In patients with low or high risk of ED, daily therapy showed significantly higher efficacy for the EF recovery rate compared with the on-demand PDE5-I administration schedule in patients with intermediate risk of ED Early high-dose PDE5-I may preserve the smooth muscle content within the corpora cavernosa |
| Add intraurethral alprostadil/ICI followed by discussion of penile implants if these initial treatment strategies fail |
| For non-nerve-sparing procedures, VED is generally the treatment of choice +/− ICI intraurethral alprostadil |
| VED is a useful adjunct to medication and facilitates early sexual activity where drugs alone are not effective |
| Treatment initiation |
| Consider initiating treatment preferably as soon as any catheter is removed and definitely within first 3 months of surgery |
| In some cases, PDE5-I can be initiated before surgery (if pre-existing problems are identified at presurgical assessment) or at catheter removal to improve outcomes |
| Psychosexual therapy and psychological counselling |
| Recommend psychosexual therapy or psychological counselling for patient and partner pre and postoperatively: Psychosexual therapy and counselling contribute to better biomedical treatment efficacy, patient acceptance and compliance |
| Encourage partner support of rehabilitation programme through ongoing psychosexual therapy and counselling for the couple and unless contraindicated, include partners in all decision-making processes |
| Psychosexual therapy and psychological counselling are a useful adjunct to ED rehabilitation treatments |
| Re-assessment |
| Once ED management is initiated, re-assess at regular intervals post treatment (re-assessment schedule can coincide with cancer review schedule), e.g. 8 weeks, 3 months and at 6 months |
| Treatment duration |
| Recommend to try one strategy at least on eight occasions before switching to another strategy unless the patient experiences adverse events warranting an early switch |
| Individualise duration of treatment for each patient as strict limits are inappropriate in clinical practice The duration of any treatment can range from 3 months until the patient no longer needs the treatment |
ED, erectile dysfunction; IIEF, International Index of Erectile Function; SHIM, Sexual Health Inventory for Men; PDE5-I, phosphodiesterase type 5 inhibitor; VED, vacuum erection device; ICI, intracorporeal injection.
Summary
Erectile function after RP depends on several factors that must be taken into account for adequate patient stratification and therapy. There are several options for managing ED post NS and non-NS RP. The treatment duration may range from 3 months to ≥ 6 years. Delaying the start of rehabilitation for ED is associated with poorer outcomes for EF.
Early ED rehabilitation can hopefully improve the return of spontaneous erections; facilitate early sexual intercourse, early patient/spousal sexual satisfaction, and potentially an earlier return of natural/unassisted erections sufficient for vaginal penetration.
PDE5-Is were considered first-line therapy by most clinicians. Early use of VED following RP also facilitates early sexual intercourse, early patient/spousal sexual satisfaction, and maintenance of penile length/girth and, potentially, an earlier return of natural erections. Psychological and sexual therapy and counselling emerges as an important adjunct to any rehabilitation programme and treatment of postoperative ED.
Acknowledgments
Funding for the development of this clinical guidance was provided by Prostate Cancer UK. The authors would also like to acknowledge the following organizations for supporting and endorsing this publication: the British Society for Sexual Medicine (BSSM), the British Uro-oncology Group (BUG), Macmillan and the Sexual Advice Association (SAA). The authors would also like to acknowledge Right Angle Communications for editorial assistance.
Author contributions
All authors took part in the survey for physicians, reviewed and edited the publication, which was produced by Mike Kirby and Isabel White with editorial assistance from Sabah Al-Lawati at Right Angle.
References
- 1.Cancer Research UK. www.cancerresearchuk.org/prod…common/…/documents/…/018070.pdf. (accessed October 2013)
- 2.Cancer Research UK. 2010. Bowel cancer statistics – key facts http://www.cancerresearchuk.org/cancer-info/cancerstats/keyfacts/bowel-cancer/ (accessed October 2013)
- 3.Koga F, Kihara K. Selective bladder preservation with curative intent for muscle-invasive bladder cancer: a contemporary review. Int J Urol. 2012;19(5):388–401. doi: 10.1111/j.1442-2042.2012.02974.x. [DOI] [PubMed] [Google Scholar]
- 4.Nandipati KC, Raina R, Agarwal A, et al. Erectile dysfunction following radical retropubic prostatectomy: epidemiology, pathophysiology and pharmacological management. Drugs Aging. 2006;23:101–17. doi: 10.2165/00002512-200623020-00002. [DOI] [PubMed] [Google Scholar]
- 5.Mulhall JP, Morgentaler A. Penile rehabilitation should become the norm for radical prostatectomy patients. J Sex Med. 2007;4:538–43. doi: 10.1111/j.1743-6109.2007.00486.x. [DOI] [PubMed] [Google Scholar]
- 6.Fitch MI, Miller D, Sharir S, McAndrew A. Radical cystectomy for bladder cancer: a qualitative study of patient experiences and implications for practice. Can Oncol Nurs J. 2010;20(4):177–87. doi: 10.5737/1181912x204177181. [DOI] [PubMed] [Google Scholar]
- 7.Ellis R, Smith A, Wilson S, Warmington S, Ismail T. The prevalence of erectile dysfunction in post-treatment colorectal cancer patients and their interests in seeking treatment: a cross-sectional survey in the West Midlands. J Sex Med. 2010;7:1488–96. doi: 10.1111/j.1743-6109.2009.01461.x. [DOI] [PubMed] [Google Scholar]
- 8.Pietrangeli A, Pugliese P, Perrone M, Sperduti I, Cosimelli M, Jandolo B. Sexual dysfunction following surgery for rectal cancer – a clinical and neurophysiological study. J Exp Clin Cancer Res. 2009;17(28):128. doi: 10.1186/1756-9966-28-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kasparek MS, Hassan I, Cima RR, Larson DR, Gullerud RE, Wolff BG. Long-term quality of life and sexual and urinary function after abdominoperineal resection for distal rectal cancer. Dis Colon Rectum. 2012;55(2):147–54. doi: 10.1097/DCR.0b013e31823d2606. [DOI] [PubMed] [Google Scholar]
- 10.Nishizawa Y, Ito M, Saito N, Suzuki T, Sugito M, Tanaka T. Male sexual dysfunction after rectal cancer surgery. Int J Colorectal Dis. 2011;26(12):1541–8. doi: 10.1007/s00384-011-1247-z. [DOI] [PubMed] [Google Scholar]
- 11.Rogers CG, Trock BP, Walsh PC. Preservation of accessory pudendal arteries during radical retropubic prostatectomy: surgical technique and results. Urology. 2004;64:148–51. doi: 10.1016/j.urology.2004.02.035. [DOI] [PubMed] [Google Scholar]
- 12.Munding M, Wessells H, Dalkin B. Pilot study of changes in stretched penile length 3 months after radical retropubic prostatectomy. Urology. 2001;58:567–9. doi: 10.1016/s0090-4295(01)01270-5. [DOI] [PubMed] [Google Scholar]
- 13.Savoie M, Kim SS, Soloway M. A prospective study measuring penile length in men treated with radical prostatectomy for prostate cancer. J Urol. 2003;169:1462–5. doi: 10.1097/01.ju.0000053720.93303.33. [DOI] [PubMed] [Google Scholar]
- 14.Salonia A, Burnett AL, Graefen M, et al. Prevention and management of postprostatectomy sexual dysfunctions. Part 1: choosing the right patient at the right time for the right surgery. Eur Urol. 2012;62:273–86. doi: 10.1016/j.eururo.2012.04.046. [DOI] [PubMed] [Google Scholar]
- 15.Walsh PC, Marschke P, Ricker D, Burnett AL. Patient-reported urinary continence and sexual function after anatomic radical prostatectomy. Urology. 2000;55:58–61. doi: 10.1016/s0090-4295(99)00397-0. [DOI] [PubMed] [Google Scholar]
- 16.Rabbani F, Stapleton AM, Kattan MW, et al. Factors predicting recovery of erections after radical prostatectomy. J Urol. 2000;164:1929–34. [PubMed] [Google Scholar]
- 17.Kundu SD, Roehl KA, Eggener SE, et al. Potency, continence and complications in 3,477 consecutive radical retropubic prostatectomies. J Urol. 2004;172:2227–31. doi: 10.1097/01.ju.0000145222.94455.73. [DOI] [PubMed] [Google Scholar]
- 18.Tutolo M, Briganti A, Suardi N, et al. Optimizing postoperative sexual function after radical prostatectomy. Ther Adv Urol. 2012;4(6):347–65. doi: 10.1177/1756287212450063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Albaugh JA. Addressing and managing erectile dysfunction after prostatectomy for prostate cancer. Urol Nurs. 2010;30(3):167–77. [PubMed] [Google Scholar]
- 20.Forbat L, White I, Marshall-Lucette S, Kelly D. Discussing the sexual consequences of treatment in radiotherapy and urology consultations with couples affected by prostate cancer. BJU Int. 2012;109:98–103. doi: 10.1111/j.1464-410X.2011.10257.x. [DOI] [PubMed] [Google Scholar]
- 21.Davison BJ, Matthew A, Elliott S, Breckon E, Griffin S. Assessing couples' preferences for postoperative sexual rehabilitation before radical prostatectomy. BJU Int. 2012;110(10):1529–35. doi: 10.1111/j.1464-410X.2012.11083.x. [DOI] [PubMed] [Google Scholar]
- 22.Chartier-Kastler E, Amar E, Chevallier D, et al. Does management of erectile dysfunction after radical prostatectomy meet patients' expectations? Results of a national survey (REPAIR) by the French Urological Association. J Sex Med. 2008;5(3):693–704. doi: 10.1111/j.1743-6109.2007.00743.x. [DOI] [PubMed] [Google Scholar]
- 23.Nehra A, Jackson G, Miner M, et al. The Princeton III consensus recommendations for the management of erectile dysfunction and cardiovascular disease. Mayo Clin Proc. 2012;87(8):766–78. doi: 10.1016/j.mayocp.2012.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith DG, Frankel S, Yarnell J. Sex and death: are they related? Findings from the Caerphilly cohort study. BMJ. 1997;315:1641. doi: 10.1136/bmj.315.7123.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abramov LA. Sexual life and frigidity among women developing acute myocardial infarction. Psychosom Med. 1976;38:418–25. doi: 10.1097/00006842-197611000-00006. [DOI] [PubMed] [Google Scholar]
- 26.Nelson CJ, Mulhall JP, Roth AJ. The association between erectile dysfunction and depressive symptoms in men treated for prostate cancer. J Sex Med. 2011;8(2):560–6. doi: 10.1111/j.1743-6109.2010.02127.x. [DOI] [PubMed] [Google Scholar]
- 27.Ferrini MG, Davila HH, Kovanecz I, Sanchez SP, Gonzalez-Cadavid NF, Rajfer J. Vardenafil prevents fibrosis and loss of corporal smooth muscle that occurs after bilateral cavernosal nerve resection in the rat. Urology. 2006;68:429–35. doi: 10.1016/j.urology.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 28.Mulhall JP, Müller A, Donohue JF, et al. The functional and structural consequences of cavernous nerve injury are ameliorated by sildenafil citrate. J Sex Med. 2008;5:1126–36. doi: 10.1111/j.1743-6109.2008.00794.x. [DOI] [PubMed] [Google Scholar]
- 29.Sirad F, Hlaing S, Kovanecz I, et al. Sildenafil promotes smooth muscle preservation and ameliorates fibrosis through modulation of extracellular matrix and tissue growth factor gene expression after bilateral cavernosal nerve resection in the rat. J Sex Med. 2011;8:1048–60. doi: 10.1111/j.1743-6109.2010.02195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ozden E, O¨ztu¨rk B, Kosan M, et al. Effect of sildenafil citrate on penile weight and physiology of cavernous smooth muscle in a post-radical prostatectomy model of erectile dysfunction in rats. Urology. 2011;77:761.e1–7. doi: 10.1016/j.urology.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 31.Kovanecz I, Rambhatla A, Ferrini MG, et al. Chronic daily tadalafil prevents the corporal fibrosis and veno-occlusive dysfunction that occurs after cavernosal nerve resection. BJU Int. 2008;101:203–10. doi: 10.1111/j.1464-410X.2007.07223.x. [DOI] [PubMed] [Google Scholar]
- 32.Mulhall JP, Bella AJ, Briganti A, McCullough A, Brock G. Erectile function rehabilitation in the radical prostatectomy patient. J Sex Med. 2010;7:1687–98. doi: 10.1111/j.1743-6109.2010.01804.x. [DOI] [PubMed] [Google Scholar]
- 33.British Society for Sexual Medicine. Guidelines on the management of erectile dysfunction. http://www.eguidelines.co.uk/eguidelinesmain/guidelines/summaries/obstetrics_gynaecology_urology/bssm_ed.php#.UiOEB-I1ipo (accessed September 2013) [DOI] [PubMed]
- 34.Oxford Centre for Evidence-based Medicine – Levels of Evidence. http://www.cebm.net/index.aspx?o = 1025 (accessed September 2013)
- 35.Campbell SE, Glazener CM, Hunter KF, Cody JD, Moore KN. Conservative management for postprostatectomy urinary incontinence. Cochrane Database Syst Rev. 2012;13:CD001843. doi: 10.1002/14651858.CD001843.pub4. [DOI] [PubMed] [Google Scholar]
- 36.Montorsi F, Guazzoni G, Strambi LF, et al. Recovery of spontaneous erectile function after nerve-sparing radical retropubic prostatectomy with and without early intracavernous injections of alprostadil: results of a prospective, randomized trial. J Urol. 1997;158:1408–10. [PubMed] [Google Scholar]
- 37.Mulhall J, Land S, Parker M, et al. The use of an erectogenic pharmacotherapy regimen following radical prostatectomy improves recovery of spontaneous erectile function. J Sex Med. 2005;2:532–54. doi: 10.1111/j.1743-6109.2005.00081_1.x. discussion 540–542. [DOI] [PubMed] [Google Scholar]
- 38.Yiou R, Cunin P, de la Taille A, et al. Sexual rehabilitation and penile pain associated with intracavernous alprostadil after radical prostatectomy. J Sex Med. 2011;8:575–82. doi: 10.1111/j.1743-6109.2010.02002.x. [DOI] [PubMed] [Google Scholar]
- 39.Costabile RA, Spevak M, Fishman IJ, et al. Efficacy and safety of transurethral alprostadil in patients with erectile dysfunction following radical prostatectomy. J Urol. 1998;160:1325–8. [PubMed] [Google Scholar]
- 40.Gontero P, Fontana F, Bagnasacco A, et al. Is there an optimal time for intracavernous prostaglandin E1 rehabilitation following nonnerve sparing radical prostatectomy? Results from a hemodynamic prospective study. J Urol. 2003;169:2166–9. doi: 10.1097/01.ju.0000064939.04658.15. [DOI] [PubMed] [Google Scholar]
- 41.Raina R, Pahlajani G, Agarwal A, Zippe CD. The early use of transurethral alprostadil after radical prostatectomy potentially facilitates an earlier return of erectile function and successful sexual activity. BJU Int. 2007;100(6):1317–21. doi: 10.1111/j.1464-410X.2007.07124.x. [DOI] [PubMed] [Google Scholar]
- 42.Raina R, Agarwal A, Zaramo CE, Ausmundson S, Mansour D, Zippe CD. Long-term efficacy and compliance of MUSE for erectile dysfunction following radical prostatectomy: SHIM (IIEF-5) analysis. Int J Impot Res. 2005;17(1):86–90. doi: 10.1038/sj.ijir.3901284. [DOI] [PubMed] [Google Scholar]
- 43.Köhler TS, Pedro R, Hendlin K, et al. A pilot study on the early use of the vacuum erection device after radical retropubic prostatectomy. BJU Int. 2007;100(4):858–62. doi: 10.1111/j.1464-410X.2007.07161.x. [DOI] [PubMed] [Google Scholar]
- 44.Raina R, Agarwal A, Ausmundson S, et al. Early use of vacuum constriction device following radical prostatectomy facilitates early sexual activity and potentially earlier return of erectile function. Int J Impot Res. 2006;18(1):77–81. doi: 10.1038/sj.ijir.3901380. [DOI] [PubMed] [Google Scholar]
- 45.Aydogdu O, Gokce MI, Burgu B, Baltacı S, Yaman O. Tadalafil rehabilitation therapy preserves penile size after bilateral nerve sparing radical retropubic prostatectomy. Int Braz J Urol. 2011;37(3):336–44. doi: 10.1590/s1677-55382011000300007. [DOI] [PubMed] [Google Scholar]
- 46.Bannowsky A, Schulze H, van der Horst C, Hautmann S, Jünemann KP. Recovery of erectile function after nerve-sparing radical prostatectomy: improvement with nightly low-dose sildenafil. BJU Int. 2008;101(10):1279–83. doi: 10.1111/j.1464-410X.2008.07515.x. [DOI] [PubMed] [Google Scholar]
- 47.Brock G, Nehra A, Lipshultz LI, et al. Safety and efficacy of vardenafil for the treatment of men with erectile dysfunction after radical retropubic prostatectomy. J Urol. 2003;170(4 Pt 1):1278–83. doi: 10.1097/01.ju.0000086947.00547.49. [DOI] [PubMed] [Google Scholar]
- 48.McCullough AR, Hellstrom WG, Wang R, Lepor H, Wagner KR, Engel JD. Recovery of erectile function after nerve sparing radical prostatectomy and penile rehabilitation with nightly intraurethral alprostadil versus sildenafil citrate. J Urol. 2010;183:2451–6. doi: 10.1016/j.juro.2010.01.062. [DOI] [PubMed] [Google Scholar]
- 49.Montorsi F, Brock G, Lee J, et al. Effect of nightly versus on-demand vardenafil on recovery of erectile function in men following bilateral nerve-sparing radical prostatectomy. Eur Urol. 2008;54(4):924–31. doi: 10.1016/j.eururo.2008.06.083. [DOI] [PubMed] [Google Scholar]
- 50.Montorsi F, Nathan HP, McCullough A, et al. Tadalafil in the treatment of erectile dysfunction following bilateral nerve sparing radical retropubic prostatectomy: a randomized, double-blind, placebo controlled trial. J Urol. 2004;172(3):1036–41. doi: 10.1097/01.ju.0000136448.71773.2b. [DOI] [PubMed] [Google Scholar]
- 51.Mosbah A, El Bahnasawy M, Osman Y, Hekal IA, Abou-Beih E, Shaaban A. Early versus late rehabilitation of erectile function after nerve-sparing radical cystoprostatectomy: a prospective randomized study. J Sex Med. 2011;8(7):2106–11. doi: 10.1111/j.1743-6109.2010.02046.x. [DOI] [PubMed] [Google Scholar]
- 52.Nehra A, Grantmyre J, Nadel A, Thibonnier M, Brock G. Vardenafil improved patient satisfaction with erectile hardness, orgasmic function and sexual experience in men with erectile dysfunction following nerve sparing radical prostatectomy. J Urol. 2005;173:2067–71. doi: 10.1097/01.ju.0000158456.41788.93. [DOI] [PubMed] [Google Scholar]
- 53.Pace G, Del Rosso A, Vicentini C. Penile rehabilitation therapy following radical prostatectomy. Disabil Rehabil. 2010;32(14):1204–8. doi: 10.3109/09638280903511594. [DOI] [PubMed] [Google Scholar]
- 54.Padma-Nathan H, McCullough AR, Levine LA, et al. Study Group. Randomized, double-blind, placebo-controlled study of postoperative nightly sildenafil citrate for the prevention of erectile dysfunction after bilateral nerve-sparing radical prostatectomy. Int J Impot Res. 2008;20(5):479–86. doi: 10.1038/ijir.2008.33. [DOI] [PubMed] [Google Scholar]
- 55.Lindsey I, George B, Kettlewell M, Mortensen N. Randomized, double-blind, placebo-controlled trial of sildenafil (Viagra) for erectile dysfunction after rectal excision for cancer and inflammatory bowel disease. Dis Colon Rectum. 2002;45(6):727–32. doi: 10.1007/s10350-004-6287-9. [DOI] [PubMed] [Google Scholar]
- 56.Briganti A, Di Trapani E, Abdollah F, et al. Choosing the best candidates for penile rehabilitation after bilateral nerve-sparing radical prostatectomy. J Sex Med. 2012;9(2):608–17. doi: 10.1111/j.1743-6109.2011.02580.x. [DOI] [PubMed] [Google Scholar]
- 57.Müller A, Parker M, Waters BW, Flanigan RC, Mulhall JP. Penile rehabilitation following radical prostatectomy: predicting success. J Sex Med. 2009;6(10):2806–12. doi: 10.1111/j.1743-6109.2009.01401.x. [DOI] [PubMed] [Google Scholar]
- 58.Schwartz EJ, Wong P, Graydon RJ. Sildenafil preserves intracorporeal smooth muscle after radical retropubic prostatectomy. J Urol. 2004;171(2 Pt 1):771–4. doi: 10.1097/01.ju.0000106970.97082.61. [DOI] [PubMed] [Google Scholar]
- 59.Briganti A, Gallina A, Suardi N, et al. Predicting erectile function recovery after bilateral nerve sparing radical prostatectomy: a proposal of a novel preoperative risk stratification. J Sex Med. 2010;7(7):2521–31. doi: 10.1111/j.1743-6109.2010.01845.x. [DOI] [PubMed] [Google Scholar]
- 60.Mulhall JP, Parker M, Waters BW, Flanigan R. The timing of penile rehabilitation after bilateral nerve-sparing radical prostatectomy affects the recovery of erectile function. BJU Int. 2010;105(1):37–41. doi: 10.1111/j.1464-410X.2009.08775.x. [DOI] [PubMed] [Google Scholar]
- 61.Zippe C, Nandipati K, Agarwal A, Raina R. Sexual dysfunction after pelvic surgery. Int J Impot Res. 2006;18(1):1–18. doi: 10.1038/sj.ijir.3901353. [DOI] [PubMed] [Google Scholar]
- 62.El-Bahnasawy MS, Ismail T, Elsobky E, Alzalouey EI, Bazeed MA. Prognostic factors predicting successful response to sildenafil after radical cystoprostatectomy. Scand J Urol Nephrol. 2008;42(2):110–5. doi: 10.1080/00365590701571563. [DOI] [PubMed] [Google Scholar]
- 63.Titta M, Tavolini IM, Moro FD, Cisternino A, Bassi P. Sexual counseling improved erectile rehabilitation after non-nervesparing radical retropubic prostatectomy or cystectomy – results of a randomized prospective study. J Sex Med. 2006;3:267–73. doi: 10.1111/j.1743-6109.2006.00219.x. [DOI] [PubMed] [Google Scholar]
- 64.Mydlo JH, Viterbo R, Crispen P. Use of combined intracorporal injection and a phosphodiesterase-5 inhibitor therapy for men with a suboptimal response to sildenafil and/or vardenafil monotherapy after radical retropubic prostatectomy. BJU Int. 2005;95(6):843–6. doi: 10.1111/j.1464-410X.2005.05413.x. [DOI] [PubMed] [Google Scholar]
- 65.Yassin AA, Haidar A, Saad F. Favorable effects of early “penile rehabilitation” following nerve sparing radical prostatectomy. J Sex Med. 2006;3:407. [Google Scholar]
- 66.Basal S, Wambi C, Acikel C, Gupta M, Badani K. Optimal strategy for penile rehabilitation after robot-assisted radical prostatectomy based on preoperative erectile function. BJU Int. 2013;111(4):658–65. doi: 10.1111/j.1464-410X.2012.11487.x. [DOI] [PubMed] [Google Scholar]
- 67.Kimura M, Caso JR, Bañez LL, et al. Predicting participation in and successful outcome of a penile rehabilitation programme using a phosphodiesterase type 5 inhibitor with a vacuum erection device after radical prostatectomy. BJU Int. 2012;110:E931–8. doi: 10.1111/j.1464-410X.2012.11168.x. [DOI] [PubMed] [Google Scholar]
- 68.Lee DJ, Cheetham P, Badani KK. Penile rehabilitation protocol after robot-assisted radical prostatectomy: assessment of compliance with phosphodiesterase type 5 inhibitor therapy and effect on early potency. BJU Int. 2010;105(3):382–8. doi: 10.1111/j.1464-410X.2009.08820.x. [DOI] [PubMed] [Google Scholar]
- 69.Moskovic DJ, Mohamed O, Sathyamoorthy K, et al. The female factor: predicting compliance with a post-prostatectomy erectile preservation program. J Sex Med. 2010;7(11):3659–65. doi: 10.1111/j.1743-6109.2010.02014.x. [DOI] [PubMed] [Google Scholar]
- 70.Nandipati K, Raina R, Agarwal A, Zippe CD. Early combination therapy: intracavernosal injections and sildenafil following radical prostatectomy increases sexual activity and the return of natural erections. Int J Impot Res. 2006;18(5):446–51. doi: 10.1038/sj.ijir.3901448. [DOI] [PubMed] [Google Scholar]
- 71.Rubio-Aurioles E, Reyes LA, Borregales L, Cairoli C, Sorsaburu S. A 6 month, prospective, observational study of PDE5 inhibitor treatment persistence and adherence in Latin American men with erectile dysfunction. Curr Med Res Opin. 2013;29(6):695–706. doi: 10.1185/03007995.2013.791262. [DOI] [PubMed] [Google Scholar]
- 72.Claes HI, Andrianne R, Opsomer R, Albert A, Patel S, Commers K. The HelpED study: agreement and impact of the erection hardness score on sexual function and psychosocial outcomes in men with erectile dysfunction and their partners. J Sex Med. 2012;9(10):2652–63. doi: 10.1111/j.1743-6109.2012.02883.x. [DOI] [PubMed] [Google Scholar]
- 73.Jiann BP, Su CC, Tsai JY. Is female sexual function related to the male partners' erectile function? J Sex Med. 2013;10(2):420–9. doi: 10.1111/j.1743-6109.2012.03007.x. [DOI] [PubMed] [Google Scholar]
- 74.Schover LR, Fouladi RT, Warneke CL, et al. The use of treatments for erectile dysfunction among survivors of prostate carcinoma. Cancer. 2002;95(11):2397–407. doi: 10.1002/cncr.10970. [DOI] [PubMed] [Google Scholar]
- 75.Shindel A, Quayle S, Yan Y, Husain A, Naughton C. Sexual dysfunction in female partners of men who have undergone radical prostatectomy correlates with sexual dysfunction of the male partner. J Sex Med. 2005;2(6):833–41. doi: 10.1111/j.1743-6109.2005.00148.x. [DOI] [PubMed] [Google Scholar]
- 76.Neese LE, Schover LR, Klein EA, Zippe C, Kupelian PA. Finding help for sexual problems after prostate cancer treatment: a phone survey of men's and women's perspectives. Psychooncology. 2003;12(5):463–73. doi: 10.1002/pon.657. [DOI] [PubMed] [Google Scholar]
- 77.El-Meliegy A, Rabah D, Al-Mitwalli K, et al. A 6-month, prospective, observational study of PDE5 inhibitor treatment persistence and adherence in Middle Eastern and North African men with erectile dysfunction. Curr Med Res Opin. 2013;29(6):707–17. doi: 10.1185/03007995.2013.791263. [DOI] [PubMed] [Google Scholar]
- 78.Polito M, d'Anzeo G, Conti A, Muzzonigro G. Erectile rehabilitation with intracavernous alprostadil after radical prostatectomy: refusal and dropout rates. BJU Int. 2012;110(11 Pt C):E954–7. doi: 10.1111/j.1464-410X.2012.11484.x. [DOI] [PubMed] [Google Scholar]
- 79.Sato Y, Tanda H, Nakajima H, et al. Dissociation between patients and their partners in expectations for sexual life after radical prostatectomy. Int J Urol. 2013;20(3):322–8. doi: 10.1111/iju.12022. [DOI] [PubMed] [Google Scholar]
- 80.Manktelow B, Dann S. Kent Oncology Centre Survey Report. 2009.
- 81.Briganti A, Capitanio U, Chun FK, et al. Prediction of sexual function after radical prostatectomy. Cancer. 2009;115(13 Suppl):3150–9. doi: 10.1002/cncr.24349. [DOI] [PubMed] [Google Scholar]
- 82.Alemozaffar M, Regan MM, Cooperberg MR, et al. Prediction of erectile function following treatment for prostate cancer. JAMA. 2011;306(11):1205–14. doi: 10.1001/jama.2011.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rosen RC, Riley A, Wagner G, Osterloh IH, Kirkpatrick J, Mishra A. The international index of erectile function (IIEF): a multidimensional scale for assessment of erectile dysfunction. Urology. 1997;49:822–30. doi: 10.1016/s0090-4295(97)00238-0. [DOI] [PubMed] [Google Scholar]
- 84.Cappelleri JC, Rosen RC. The sexual health inventory for men (SHIM): a 5-year review of research and clinical experience. Int J Impot Res. 2005;17:307–19. doi: 10.1038/sj.ijir.3901327. [DOI] [PubMed] [Google Scholar]
- 85.Mulhall JP, Goldstein I, Bushmakin AG, Cappelleri JC, Hvidsten K. Validation of the erection hardness score. J Sex Med. 2007;4:1626–34. doi: 10.1111/j.1743-6109.2007.00600.x. [DOI] [PubMed] [Google Scholar]
- 86.Rosen RC, Cappelleri JC, Smith MD, Lipsky J, Pena BM. Development and evaluation of an abridged, 5-item version of the international index of erectile function (IIEF-5) as a diagnostic tool for erectile dysfunction. Int J Impot Res. 1999;11:319–26. doi: 10.1038/sj.ijir.3900472. [DOI] [PubMed] [Google Scholar]
- 87.Nandipati KC, Raina R, Agarwal A, Zippe CD. Sildenafil, tadalafil, and vardenafil are equally effective treatments for erectile dysfunction after bilateral nerve sparing prostatectomy. Fertil Steril. 2005;84(Suppl. 1):S134. abstract presented at the 61st Annual American Society of Reproductive Medicine meeting: October 19, 2005. Montreal, Quebec] [Google Scholar]
- 88.Stefaniak H, Kang J, Kshirsagar A, et al. Tadalafil therapy beginning postoperative day one preserves erectile function after radical prostatectomy – preliminary results. J Am Coll Surg. 2005;201(3):S94. [Google Scholar]
- 89.Hatzimouratidis K, Burnett AL, Hatzichristou D, McCullough AR, Montorsi F, Mulhall JP. Phosphodiesterase type 5 inhibitors in postprostatectomy erectile dysfunction: a critical analysis of the basic science rationale and clinical application. Eur Urol. 2009;55:334–47. doi: 10.1016/j.eururo.2008.10.028. [DOI] [PubMed] [Google Scholar]
- 90.Montorsi F, McCullough A. Efficacy of sildenafil citrate in men with erectile dysfunction following radical prostatectomy: a systematic review of clinical data. J Sex Med. 2005;2:658–67. doi: 10.1111/j.1743-6109.2005.00117.x. [DOI] [PubMed] [Google Scholar]
- 91.Padma-Nathan H, McCullough AR, Levine LA, et al. Study Group. Randomized, double-blind, placebo-controlled study of postoperative nightly sildenafil citrate for the prevention of erectile dysfunction after bilateral nerve-sparing radical prostatectomy. Int J Impot Res. 2008;20(5):479–86. doi: 10.1038/ijir.2008.33. [DOI] [PubMed] [Google Scholar]
- 92.Montorsi F, Maga T, Strambi LF, et al. Sildenafil taken at bedtime significantly increases nocturnal erections: results of a placebo-controlled study. Urology. 2000;56(6):906–11. doi: 10.1016/s0090-4295(00)00841-4. [DOI] [PubMed] [Google Scholar]
- 93.Meuleman EJ, Mulders PF. Erectile function after radical prostatectomy: a review. Eur Urol. 2003;43(2):95–101. doi: 10.1016/s0302-2838(02)00546-8. [DOI] [PubMed] [Google Scholar]
- 94.Gallina A, Ferrari M, Suardi N, et al. Erectile function outcome after bilateral nerve sparing radical prostatectomy: which patients may be left untreated? J Sex Med. 2012;9:903–8. doi: 10.1111/j.1743-6109.2011.02622.x. [DOI] [PubMed] [Google Scholar]
- 95.Kovanecz I, Rambhatla A, Ferrini M, et al. Long-term continuous sildenafil treatment ameliorates corporal veno-occlusive dysfunction (CVOD) induced by cavernosal nerve resection in rats. Int J Impot Res. 2008;20:202–12. doi: 10.1038/sj.ijir.3901612. [DOI] [PubMed] [Google Scholar]
- 96.Lysiak JJ, Yang SK, Klausner AP, et al. Tadalafil increases Akt and extracellular signal-regulated kinase 1/2 activation, and prevents apoptotic cell death in the penis following denervation. J Urol. 2008;179:779e785. doi: 10.1016/j.juro.2007.09.021. [DOI] [PubMed] [Google Scholar]
- 97.Briganti A, Fabbri F, Salonia A, et al. Preserved postoperative penile size correlates well with maintained erectile function after bilateral nerve-sparing radical retropubic prostatectomy. Eur Urol. 2007;52(3):702–7. doi: 10.1016/j.eururo.2007.03.050. [DOI] [PubMed] [Google Scholar]
- 98.Salonia A, Gallina A, Zanni G, et al. Acceptance of and discontinuation rate from erectile dysfunction oral treatment in patients following bilateral nerve-sparing radical prostatectomy. Eur Urol. 2008;53:564–70. doi: 10.1016/j.eururo.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 99.Wang R. Penile rehabilitation after radical prostatectomy: where do we stand and where are we going? J Sex Med. 2007;4(4 Pt 2):1085–97. doi: 10.1111/j.1743-6109.2007.00482.x. [DOI] [PubMed] [Google Scholar]
- 100.Sadovsky R, Basson R, Krychman M, et al. Cancer and sexual problems. J Sex Med. 2010;7:349–73. doi: 10.1111/j.1743-6109.2009.01620.x. [DOI] [PubMed] [Google Scholar]
- 101.Bronner G, Shefi S, Raviv G. Sexual dysfunction after radical prostatectomy: treatment failure or treatment delay? J Sex Marital Ther. 2010;36:421–9. doi: 10.1080/0092623X.2010.510777. [DOI] [PubMed] [Google Scholar]


