Abstract
While for a century therapeutics has been dominated by small molecules, i.e. organic chemicals of ~400Da absorbable via the gut, this is no longer the case. There are now a plethora of important medicines which are proteins and injectable, which have dramatically improved the therapy of many inflammatory diseases and of cancer. Most of these are monoclonal antibodies, some are receptor Ig Fc fusion proteins, others are cytokines or enzymes. The key to this new aspect of therapeutics has been the filling of unmet needs, and the consequent commercial success, which promoted further research and development. The first ‘biologic’ for a common disease, rheumatoid arthritis (RA), was a monoclonal antibody, infliximab, to human tumour necrosis factor (TNF). This was based on our work, which is described in this review, summarizing how TNF was defined as a good target in RA, how it was developed is described here, as well as future indications for anti-TNF and related agents. Biologics are now the fastest growing sector of therapeutics.
Keywords: anti-cytokine therapy, atherosclerosis, fibrosis, fractures, rheumatoid arthritis
Introduction
While much of medical therapy in the past was ‘empirical’, for example the use of low-dose methotrexate (MTX) in rheumatoid arthritis (RA), drugs of unknown mechanism are no longer being developed. The key now is defining and understanding therapeutic targets. In this review, the role of tumour necrosis factor (TNF) in disease, and how anti-TNF therapy changed the therapeutic landscape, in general, is discussed.
TNF is an important host defence molecule, which acts as one of the body’s important danger detection systems or ‘fire alarms’. TNF is the first cytokine to appear in the blood after any injury or stress, within minutes. Other pro-inflammatory mediators such as IL-1 or IL-6 appear much later, and there is evidence that much of the IL-1 or IL-6 depends on the prior release of TNF (1).
The importance of TNF in biology is highlighted by its discovery in multiple key processes. For example, the commonly used name ‘tumour necrosis factor’ depended on vascular activation and thrombosis, leading to necrosis of subcutaneous tumours in mice (2). It is not a great name, as subsequent work has shown that TNF is of importance in the generation of ovarian, skin, breast, colon and other cancers (3).
TNF is unusual that it activates biological effects in two distinct receptors. First, TNFR1, also known as p55, is a homotrimeric receptor present on all cells, and its cross-linking by TNF induces pro-inflammatory response. It activates NF-κB, mitogen-activated protein kinases as well as anti-apoptotic responses, and there is the consequent generation of other pro-inflammatory cytokines such as IL-1, IL-6, GM-CSF, etc. Thus TNFR1 ‘knockout’ mice have considerably reduced inflammatory responses and are protected from many diseases.
Second, TNFR2, also known as p75, is of much more restricted tissue distribution and activates different signalling pathways. It appears to be important for repair and homeostasis, and the cells with the highest expression of TNFR2 are Tregs of TNRF2. TNRF2 knockouts have augmented pathology, such as poor survival following coronary artery ligation.
It is thus one possibility that selective blockade of TNFR1 may be even more useful than blockade of TNF. This appears to be the case in mice, not just in the extreme knockouts, but also in therapy (4, 5).
In this article, how anti-TNF therapy was developed, how the anti-TNF antibodies are used therapeutically and prospects for the future are described. Currently, there are five approved anti-TNFs: (i) infliximab, a chimaeric IgG anti-human monoclonal (Remicade®); (ii) etanercept, a TNFR2 dimeric fusion protein, with an IgG1 Fc (Enbrel®); (iii) adalimumab, a fully human monoclonal antibody (mAb) (Humira®); (iv) golimumab, a fully human mAb (Simponi®) and (v) certolizumab, a PEGylated Fab fragment (Cimzia®).
However, many more are on the way as many ‘biosimilars’ are in development, and one of these biosimilars—known as ‘Remsima’, a mimic of infliximab—is already on sale in Europe.
Overview
When Ravinder Maini and Marc Feldmann led the first clinical trial of TNF blockade in any disease apart from sepsis, using cA2 mAb (now infliximab, Remicade®), the culmination of many years intensive research, we did not expect that the impact of its success would spread far beyond the treatment of severe RA.
In this review, we will summarize how we defined TNF as a good target and how anti-TNF therapy was optimized and then discuss the impact—scientifically, medically and commercially and on other diseases.
How was TNF defined as a good target for therapy?
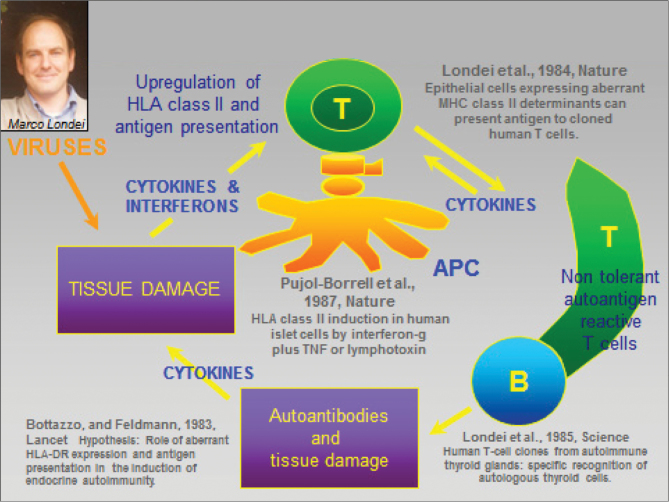
TNF is an important host defence molecule. It helps protect from many stresses including intracellular infection. Hence, it was not considered by most immunologists or rheumatologists to be an important part of the pathogenesis of autoimmune disease. However, on the basis of the hypothesis of Marc Feldmann in 1983 (6), published with Franco Bottazzo’s group (summarized in Fig. 1), and rapidly validated using Graves’ thyroid disease tissue, in 1985, Ravinder Maini and Marc Feldmann set about systematically exploring the role of cytokines in RA.
Fig. 1.
1983: a new hypothesis for autoimmunity (e.g. Graves’ disease).
This project was a natural collaboration, since both of us previously studied immune cell supernatants (7, 8) and were impressed by their potency. At the time, their molecular nature was unknown and—with the techniques available at the time—unknowable but the molecular biological revolution of the mid to late 1970s led to the cloning of cDNAs reflecting cytokine mRNAs and these tools permitted specific evaluation of cytokine production in diseased tissue sites. One of the most accessible tissue disease sites is the joints, just under the skin, and this facilitated our progress. Biopsies and larger operative samples and occasionally synovial fluid were available for research, and by miniaturization, it was possible to evaluate which pro-inflammatory cytokines were produced by cells in the RA joint as judged by their mRNA expression (9–11).
To our surprise, we found that all active RA synovium samples were producing all the pro-inflammatory cytokines that could be measured using tools available in the late 1980s (1987–89), for example IL-1, TNF, lymphotoxin, IL-6, GM-CSF, IFN-γ, etc. (9–11). This was puzzling as usually cytokines are ‘transiently’ expressed (a few hours to 1–2 days) and so their presence in all samples indicated continuous production, and hence dysregulation of their production.
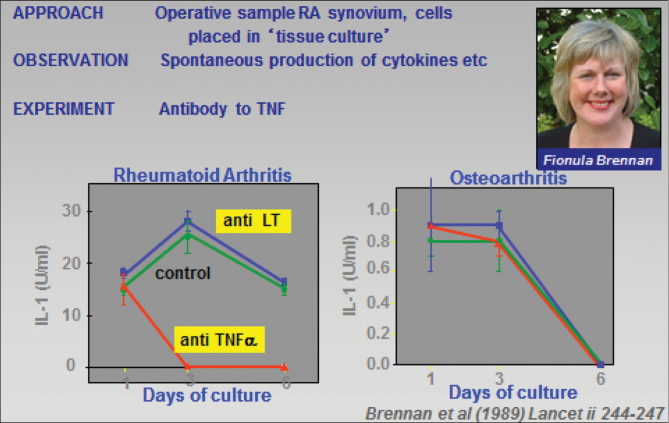
To explore this dysregulation, we set up a novel (at the time) culture system, which permitted all the cells that had been enzymatically extracted from the synovium to survive for a week in vitro and so enabled us to study the cytokine over-expression or dysregulation. Prior studies had focused on the fibroblast-like synoviocytes. Using neutralizing antibodies in vitro, we found that anti-TNF (generously given to us by Michael Shepard from Genentech) inhibited the synovial culture synthesis of other important pro-inflammatory cytokines—IL-1 (12), IL-6 and GM-CSF (13). This led to the novel concept that TNF was at the apex of a pro-inflammatory cytokine ‘cascade’.
This unexpected finding, in experiments performed by Fionula Brennan (12) (Fig. 2) when she was a young post-doctoral researcher, opened up the field: it solved the major dilemma; namely how could blocking a ‘single’ cytokine (TNF) clinically impact a complex inflammatory process (RA) with over-expression of a host of pro-inflammatory cytokines (IL-1, IL-6, TNF, GM-CSF, IFN-γ, etc.) which had overlapping biological effects, at least as assessed in vitro, a property known as ‘redundancy’.
Fig. 2.
The importance of TNF for IL-1 production in RA, revealed by analysis of cytokine regulation in the presence of anti-TNF or anti-lymphotoxin in vitro (12).
This established the first rationale for defining TNF as a target, which was confirmed by the amelioration of collagen-induced arthritis in mice, experiments performed by Richard Williams, a PhD student at the time with Ravinder Maini, using hamster anti-mouse-TNF mAbs, generously donated by Bob Schreiber (14).
Our major challenge was convincing sceptical companies that had already made monoclonal anti-TNF antibodies, in order to test Tony Cerami’s interesting concept that bacterial sepsis and septic shock (a major killer) could be treated by TNF blockade (15). That concept was not proven—perhaps the clinical trials were too technically challenging for the time (over 20 years ago); however, multiple companies had generated and tested anti-TNF biological therapeutics (‘biologicals’), mAbs or antibody-like fusion proteins.
We were not able to convince any of these companies in the UK, but when a small US biotechnology company, Centocor, hired my ex-student, Dr James N. Woody as Chief Scientific Officer, we had an ally who understood both the science and the medical implications. The crucial first proof of principle clinical trial was performed at Charing Cross Hospital, London, with Ravinder Maini and Marc Feldmann as Principal Investigators, with Centocor providing the ‘drug’, cA2, a chimaeric (mouse anti-TNF FAb linked to human IgG1) antibody developed from a hybridoma made in Jan Vilcek’s laboratory at New York University, and a small grant to the Kennedy Institute, which was then in London on the Charing Cross Hospital campus.
Centocor did not even provide their expert clinical group, which was at the time pre-occupied with anti-CD4 mAb therapy, so James Woody was the Centocor clinician in charge for the vital proof of principle trial, which succeeded dramatically and thus subsequently led to randomized, placebo-controlled trials and registration: an excellent, if perhaps not so common, example of effective academic–industrial interaction.
The proof of principle clinical trial, initially with 10 patients, was performed from May 1992 onwards. All responded well to 20mg kg−1 of Centocor’s anti-TNF mAb, now sold as infliximab, infused in several episodes over 2 weeks. The response was clinical as well as biochemical (e.g. reduced C-reactive protein) and was publically disclosed in September 1992, in a small conference in Arad, Israel, that Marc Feldmann helped organized, together with David Naor. This disclosure, 15 months before publication (16), was important for the patients, as it enabled other companies with already generated TNF inhibitors to refocus their efforts away from sepsis and into the treatment of RA. It also initiated interests from experts in related chronic inflammatory diseases—Crohn’s disease, psoriasis, ankylosing spondylitis, juvenile RA, etc.—to evaluate anti-TNF in their patients.
These clinical studies followed, without the need for the elaborate pre-clinical evaluation, or the major difficulty in convincing industry that blocking a single cytokine, in a disease with many up-regulated cytokines, could possibly work. Following is easier than leading! But the effect of anti-TNF in late-stage RA patients treated with a single course of anti-TNF antibodies lasted 12–18 weeks, before all relapsed. It was hence not a cure.
Benefits of anti-TNF therapy in RA
RA is a life-long disease that can not only reduce quality of life, but also, if not well treated, reduce its duration, by 7 years. Would TNF blockade be durable, or would TNF as ‘driver’ of disease if blocked just be replaced by other signals, as often happens in cancer? The first evidence came from re-treating the patients in the open study discussed above; seven patients were re-treated several times after they had relapsed, with 10 mg kg−1 of infliximab. All responded well, but as expected from half the amount of the first dosing, the duration of benefit was less (17).
However, the effective treatment data showed that the critical role of TNF was lasting, and thus it encouraged both the formal randomized phase II double-blind efficacy trials and repeated dosing trials (18, 19). The latter showed that there was added benefit of co-therapy with low-dose MTX even, paradoxically, in patients who did not respond well or at all to MTX. Subsequent studies were able to document the marked joint-protection effects (20) of combined therapy of anti-TNF and MTX and led to approval of the various anti-TNF biological therapies, first etanercept (Enbrel®) in USA in 1998, infliximab (Remicade®) in 1999 and then adalimumab (Humira®) in 2002. Triggered by our work and its early disclosure, other companies had been successfully using their TNF inhibitors in arthritis clinical trials.
The joint-protection effects demonstrated that TNF blockade was not just an effective (and expensive) anti-inflammatory drug, but that it had a major impact on pathogenesis and disease mechanisms, influencing as documented in mechanism of action studies, all facets of disease (Fig. 4).
Studies on the mechanism of action of infliximab
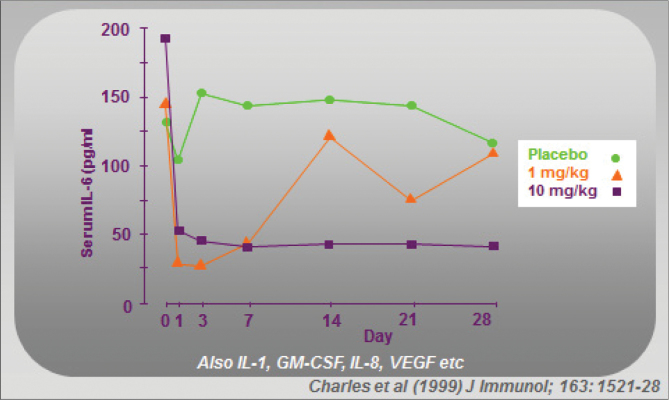
With the ‘TNF-dependent cascade’ concept, elucidated from synovial cell cultures that gave the first clue to the key role of TNF in RA, it was of interest to determine whether this cascade in fact occurred in vivo in patients with RA. Since IL-6 in the blood is bioactive, its rapid decline on day of treatment confirmed that a TNF-dependent cytokine cascade was indeed operating (Fig. 3 ) (21).
Fig. 3.
Mechanism of action of infliximab. The TNF-dependent cytokine cascade is inhibited (‘cytokine washout’) in vivo after administration of infliximab (1 or 10mg kg−1) to patients with RA (21).
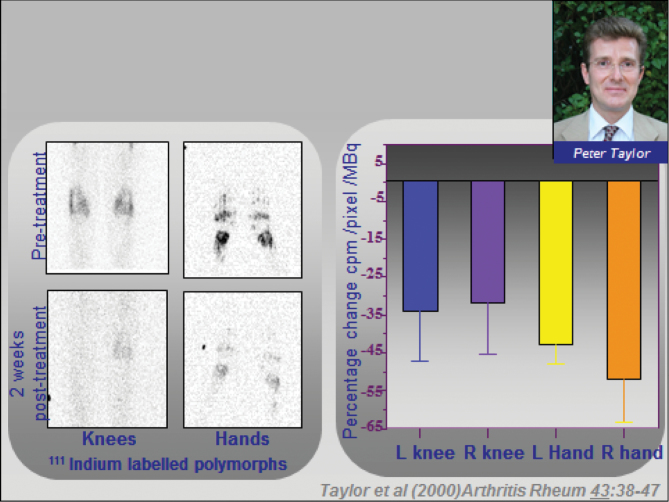
Very detailed mechanistic studies were performed, and all aspects of the disease were found to improve, including immune function, haematology, joint function, etc., summarized in Fig. 4. But perhaps the most important finding was obtained in a complex neutrophil radiolabelling study performed by Peter Taylor et al., which was that leucocyte trafficking into the joints was markedly reduced (22) by anti-TNF therapy (Fig. 5).
Fig. 4.
How cytokine washout by infliximab is translated into clinical benefit (6, 11, 12, 17, 18, 20, 22). Abbreviations: CCP, cyclic citrullinated peptide; MMPs, matrix metalloproteins; RF, rheumatoid factor; XR, X-ray radiography.
Fig. 5.
Mechanism of action of infliximab. There is reduced polymorphonuclear-leucocyte trafficking after infliximab therapy in patients with RA (22).
Current status of anti-TNF therapy
Anti-TNF therapy of RA is now standard of care. It typically follows after MTX, in patients who are not doing well. In the majority, 70–80%, it is used as combination therapy with MTX, driven by the fact that all the clinical trials of MTX plus all the anti-TNFs/anti-TNFR (infliximab, etanercept, adalimumab, golimumab and certolizumab) (23, 24) have demonstrated the additional benefit of the combination. In contrast, other ‘disease-modifying drugs’, apart from MTX, do not show this added benefit in RA (25).
But not all patients do well on this combination. What happens next for these patients? In Europe and the USA, about half the patients get treated with another anti-TNF, half get treated with rituximab (26) (anti-CD20) and fewer get treated with abatacept (CTLA4–Ig fusion protein) (27) and tocilizumab (anti-IL-6R mAb) (28). In all these instances, about half respond significantly, meaning an ACR20 effect.
The sales of anti-TNF biologicals document the course of therapy, with adalimumab being the world’s best-selling medicine—global sales over $US 10 billion per year and total sales of the various anti-TNFs/anti-TNFR drugs exceeding $US 25 billion, globally making anti-TNFs the most profitable drug class, eclipsing status in financial terms in 2012. By comparison, the sales of abatacept and actemra are in the $US 1 billion range.
The arrival of successful anti-TNF therapy in RA—a common disease affecting ~1% of the population—together with use of antibodies such as herceptin (29), avastin (30) and rituximab in cancer has had dramatic impact on the development of therapies by pharmaceutical companies, which realize that biologicals, despite needing injections, can be highly successful. Six of the top 10 best-selling pharmaceuticals are now biologicals and now half of all clinical trials are of injectable proteins related to antibodies. There has been a major change in the therapeutic landscape.
What are the other indications for anti-TNF therapy?
With the demonstration by Peter Taylor and Ravinder Maini’s trial that infliximab reduces leucocyte trafficking (22), there is a clear rationale for anti-TNF therapy of many chronic local inflammatory diseases. Crohn’s disease, ulcerative colitis, psoriatic arthritis, psoriasis, ankylosing spondylitis and juvenile RA are now approved indications, and there is off-label use in Behcet’s syndrome and amyloidosis. Crohn’s disease was in fact the first approval for infliximab, despite the fact that clinical trials did not begin until after the success of infliximab in RA was disclosed. This was because of the misconception that it would be easier to get approval for Crohn’s, and so Centocor’s resources were diverted to prioritize Crohn’s. Immunex demonstrated that getting regulatory approval for an anti-TNF (etanercept) in RA was not difficult, due to efficacy.
There have been clinical trials of, e.g., etanercept, a TNFR IgG fusion protein in multiple sclerosis, which have not shown any benefit (31), and with reports of occasional demyelination during anti-TNF therapy and concerns that this might be mechanism related, it is not going to be reinvestigated (32). Clinical trials in Sjogren’s syndrome have not succeeded. Despite the increase of anti-DNA antibodies after anti-TNF in RA (33), there have been pilot studies infliximab in systemic lupus erythematosus nephritis, which have been moderately successful, but on extension, infections emerged (34).
Are there other possible clinical uses of anti-TNF?
Acute indications
The first proposed use of anti-TNF was in septic shock (19), in the acute and dramatic response to infection, with low blood pressure and shock. Despite a clear rationale in experimental models, if the anti-TNF was given very soon after disease induction, it was not possible to how convincing benefit of multiple anti-TNFs in any prospective clinical trial (35).
Retrospective analysis suggested benefit in patients who still had elevated cytokine levels, suggesting that the problem may be due to inability to treat patients soon enough unlike in mouse models (e.g. IL-6). With the monumental costs of this failure, and the disappointment, there has been an almost 25-year ‘moratorium’ on clinical trials in acute conditions. This is not a good situation, as cytokines are major acute-response modifiers, and there are many other good opportunities, for example in acute respiratory distress, burns, in severe influenza (36) or in ventilator-induced lung injury (37). Acute fractures will be discussed separately.
Post-operative cognitive dysfunction
Major surgery, especially heart surgery on bypass machines, has long been known to cause memory and other cognitive impairment (38). The mechanism is not fully understood, initial ‘obvious’ concepts of this being due to general anaesthesia do not appear credible as post-operative cognitive dysfunction (POCD) also occurs after regional (e.g. spinal) anaesthesia.
Mervyn Maze et al. have explored the possibility that POCD is due to ‘trauma’-induced neuroinflammation. This was first demonstrated in rats with splenic surgery (34) and then in mice with orthopaedic (fracture) surgery (39). Analysis of the brain showed hippocampal inflammation and cytokine up-regulation and, importantly, pre-treatment with IL-1R antagonist (40) or anti-TNF was shown to prevent POCD, in mice (41). Human trials have not been performed but are justified and possible if funding can be found.
Fibrosis of the hand: Dupuytren’s contracture
The pathogenesis of Dupuytren’s contracture has been carefully analysed by my colleague, Jagdeep Nanchahal, and his team. This research was fully performed exclusively using human tissues, as there is no animal model. The condition is limited to the palm of the hand and causes the fingers to irreversibly curl in, severely compromising hand function. Currently, there is no treatment for early disease and currently patients are treated late when there is deformity by surgical excision of the fibrotic cords or cutting them using a needle or Clostridial collagenase injection (42). Patients require prolonged rehabilitation therapy following surgery and the less-invasive techniques are associated with high recurrence rates.
Instead of targeting the end-stage collagen of the cords, we studied the molecular and cellular mechanism, especially in myofibroblasts, the cells that produce and contract the matrix as a result of the polymerization of intracellular α-smooth muscle actin (43). We were particularly intrigued that classically activated macrophages co-located with the myofibroblasts in the cellular nodules. Using a model of freshly disaggregated cells to identify the relevant cytokines, similar to the one we used to identify TNF as a therapeutic target in RA, we found that only TNF converted normal fibroblasts from the palm of patients with Dupuytren’s disease into myofibroblasts, whereas transforming growth factor-β1 converted all fibroblasts, irrespective of source. Myofibroblasts only retain their phenotype under tension and we studied the efficacy of cytokine inhibition on myofibroblasts in a 3D collagen matrix anchored at both ends. Anti-TNF resulted in reversal of the myofibroblast phenotype (44). Based on these findings, clinical trials of an anti-TNF are planned for patients with early Dupuytren’s disease.
Fracture repair
Fractures are a major clinical problem, with 2% of the population suffering a fracture per year. High-energy fractures in young people are limb-threatening injuries, whilst with an aging population, the numbers of fragility fractures in osteoporotic bone are increasing dramatically. The most severe are fractures of the neck of the femur, which result in permanent disability in 50% and have a mortality rate of >20% in the first year (45).
Jagdeep Nanchahal and his team, studying human bone fragments in vitro, found that only supernatants from fracture fragments but not surgically cut bone specimens led to the differentiation of mesenchymal stromal cells (MSC) into bone-producing cells. This important observation led them to hypothesize that the inflammatory cytokines produced by the trauma may be key in initiating the reparative process. They found that TNF is the most potent of the cytokines in leading to osteogenic differentiation of the MSC (46). Local administration of TNF at the fracture site in murine models of normal and osteoporotic bone resulted in acceleration of physiological fracture repair, suggesting that local TNF therapy might be beneficial.
Cardiovascular disease
The role of TNF in heart failure was tested with etanercept and infliximab in clinical trials that did not succeed and paradoxically had increased mortality (47, 48). The reasons for this are not fully understood, but in the rationale for anti-TNF therapy in heart failure was non-convincing. In patients with RA, the use of anti-TNF therapy clearly reduces the incidence of heart failure and cardiovascular disease (CVD) events (49, 50). Further studies on cytokine blockade in atherosclerosis and CVD are warranted.
Claudia Monaco et al. have made the important discovery that Toll-like receptor 2 (TLR2) was the major driver of cytokine production in human plaque cultures (51). This leads to the highly intriguing question: what are the important TLR2 agonists involved? Are they all extrinsic danger signals? If so, which? Could there be an interaction with intrinsic (e.g. infectious) danger signals?
Conclusion
Anti-TNF therapy has led to major progress not only for patients with RA but also for patients with other chronic inflammatory diseases, such as Crohn’s disease, ulcerative colitis, psoriasis, psoriatic arthritis, ankylosing spondylitis and juvenile RA. These are common diseases, for example RA affects ~1% of the western population, and while anti-TNF therapy is restricted to the more severe patients, the combined sales of anti-TNF have grown progressively and it is now the best-selling pharmaceutical drug class, with sales of over $US 25 billion per year. But while this is a very rosy picture for the pharmaceutical industry, it is not so good for the patients. There are at least three problems.
First, it is worth pointing out that the monumental sales (over $US 25 billion) are driven by the monumental costs to health care systems, of ~$25000–30000 per patient per year. So actually sales reflect ~1 million patients at any one time. That is a small fraction of those who could potentially benefit, so the cost is leading to rationing. Might lower prices lead to greater total sales, following Adam Smith’s well-known ‘bell-shaped’ curve relating costs and sales? (52).
Second, there is considerable evidence now that the benefit of treating early is so much greater than treating late. This is most clearly documented from the Dutch BeST series of clinical trial papers, where therapy was instigated within a month (53). The acronym BeST is very apt—it actually stands for Behandel Strategieën treatment strategies but they are very informative clinical trials. The degree of response is greater if treatment is initiated very early, in this trial, a median of 2 weeks (interquartile range 1–5 weeks after diagnosis). Reported response rates are higher than the typical late-treatment patients who have had to wait until they failed one or two (in the UK) disease-modifying anti-rheumatic drugs, and the magnitude of response in terms of those attaining remission or low disease activity is greater. For example, in BeST, 71% of these patients with infliximab and MTX had low disease-activity scores (DAS ≤ 2.4) after treatment (53). More importantly, over half the patients (56%) treated early with anti-TNF plus MTX who responded well (DAS < 2.4) could be taken off anti-TNF and kept stably on 10-mg MTX (54). Furthermore, joint damage was halted or prevented in those given anti-TNF early (53, 54).
Third, it is also clear that for the majority of patients, who are not treated very early, anti-TNF plus MTX still leaves an unmet medical need: it leaves symptoms such as joint pain, fatigue, as well as signs, joint swelling, etc.
As it seems likely that very early treatment, as in the BeST trial, will not be feasible in most countries including the USA, the most likely useful strategy is to develop combination therapy to get closer to a cure, especially of systemic symptoms. Combination therapy in RA currently has a mixed to bad reputation; but can we use combinations to get closer to a cure?
The combination trials (in late-stage RA) of etanercept [anti-TNFR2 (55) plus anakinra (IL-1R antagonist) or etanercept plus abatacept (CTLA4–Ig) (56)] have paradoxically not led to any increased clinical benefit and disappointingly had markedly increased of rates of serious infection. So, many in the Pharmaceutical Industry do not consider combination therapy feasible in light of these data.
However, we think a solution is possible. Infectious risk can be minimized if we learn the lessons of the failed trials. It is not helpful to diminish a given type of biological response too much—etanercept (targeting TNF) and anakinra (targeting IL-1) or these are too similar in their actions. Perhaps that is also the case for etanercept plus abatacept.
Medications to be combined with anti-TNF plus MTX need to target distinct pathogenic mechanisms that then would not augment the risk of infection. These other targets could include angiogenesis, such as by inhibiting vascular endothelial growth factor as extensively studied by our colleague Ewa Paleolog (57), or inhibiting fibroblast-like synoviocytes (58) or inhibiting antigen-specific immunity, such as by targeting the peptidyl arginine deiminase enzymes that generate the autoantigens in RA (59, 60).
Importantly, before we reconsider how to optimize combination therapy, we need to understand how to monitor the human immune response in much greater detail. This is becoming possible, through the pioneering work of Mark Davis in Stanford, CA, USA, whose group has made significant progress in analysing human immune function (61). No too long! But knowing the marked benefits of combination therapy in lethal diseases such as HIV, acute myeloid leukaemia and chronic lymphocytic leukaemia, it is probably the best way to get closer to a cure.
Funding
Arthritis Research Campaign, Centocor Inc.; The Medical Research Council; the Wellcome Trust and the Nuffield Foundation .
Conflict of interest statement: The authors declared no conflict of interests.
References
- 1. Fong Y., Tracey K. J., Moldawer L. L., et al. 1989. Antibodies to cachectin/tumor necrosis factor reduce interleukin 1 beta and interleukin 6 appearance during lethal bacteremia. J. Exp. Med. 170:1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Carswell E. A., Old L. J., Kassel R. L., Green S., Fiore N., Williamson B. 1975. An endotoxin-induced serum factor that causes necrosis of tumors. Proc. Natl Acad. Sci. USA 72:3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Naylor M. S., Stamp G. W., Foulkes W. D., Eccles D., Balkwill F. R. 1993. Tumor necrosis factor and its receptors in human ovarian cancer. Potential role in disease progression. J. Clin. Invest. 91:2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Xanthoulea S., Pasparakis M., Kousteni S., et al. 2004. Tumor necrosis factor (TNF) receptor shedding controls thresholds of innate immune activation that balance opposing TNF functions in infectious and inflammatory diseases. J. Exp. Med. 200:367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. McCann F. E., Perocheau D. P., Ruspi G., et al. 2014. Selective tumor necrosis factor receptor I blockade is antiinflammatory and reveals an immunoregulatory role for tumor necrosis factor receptor II in collagen-induced arthritis. Arthritis Rheum. 66:2728. [DOI] [PubMed] [Google Scholar]
- 6. Bottazzo G. F., Pujol-Borrell R., Hanafusa T., Feldmann M. 1983. Hypothesis: role of aberrant HLA-DR expression and antigen presentation in the induction of endocrine autoimmunity. Lancet ii:1115. [DOI] [PubMed] [Google Scholar]
- 7. Maini R. N., Bryceson A. D., Wolstencroft R. A., Dumonde D. C. 1969. Lymphocyte mitogenic factor in man. Nature 224:43. [DOI] [PubMed] [Google Scholar]
- 8. Feldmann M., Basten A. 1972. Specific collaboration between T and B lymphocytes across a cell impermeable membrane in vitro . Nat. New Biol. 237:13. [DOI] [PubMed] [Google Scholar]
- 9. Buchan G. S., Barrett K., Fujita T., Taniguchi T., Maini R. N., Feldmann M. 1988. Detection of activated T cell products in the rheumatoid joint using cDNA probes to interleukin 2, IL-2 receptor and interferon γ. Clin. Exp. Immunol. 71:295. [PMC free article] [PubMed] [Google Scholar]
- 10. Buchan G., Barrett K., Turner M., Chantry D., Maini R. N., Feldmann M. 1988. Interleukin-1 and tumour necrosis factor mRNA expression in rheumatoid arthritis: prolonged production of IL-1 alpha. Clin. Exp. Immunol. 73:449. [PMC free article] [PubMed] [Google Scholar]
- 11. Feldmann M., Brennan F. M., Maini R. N. 1996. Role of cytokines in rheumatoid arthritis. Annu. Rev. Immunol. 14:397. [DOI] [PubMed] [Google Scholar]
- 12. Brennan F. M., Chantry D., Jackson A., Maini R. N., Feldmann M. 1989. Inhibitory effect of TNF alpha antibodies on synovial cell interleukin-1 production in rheumatoid arthritis. Lancet 2:244. [DOI] [PubMed] [Google Scholar]
- 13. Haworth C., Brennan F. M., Chantry D., Turner M., Maini R. N., Feldmann M. 1991. Expression of granulocyte-macrophage colony-stimulating factor (GM-CSF) in rheumatoid arthritis: regulation by tumor necrosis factor-alpha. Eur. J. Immunol. 21:2575. [DOI] [PubMed] [Google Scholar]
- 14. Williams R. O., Feldmann M., Maini R. N. 1992. Anti-tumor necrosis factor ameliorates joint disease in murine collagen-induced arthritis. Proc. Natl Acad. Sci. USA 89:9784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Beutler B., Milsark I. W., Cerami A. C. 1985. Passive immunization against cachectin/tumor necrosis factor protects mice from lethal effect of endotoxin. Science 229:869. [DOI] [PubMed] [Google Scholar]
- 16. Elliott M. J., Maini R. N., Feldmann M., et al. 1993. Treatment of rheumatoid arthritis with chimeric monoclonal antibodies to tumor necrosis factor alpha. Arthritis Rheum. 36:1681. [DOI] [PubMed] [Google Scholar]
- 17. Elliott M. J., Maini R. N., Feldmann M., et al. 1994. Repeated therapy with monoclonal antibody to tumour necrosis factor alpha (cA2) in patients with rheumatoid arthritis. Lancet 344:1125. [DOI] [PubMed] [Google Scholar]
- 18. Elliott M. J., Maini R. N., Feldmann M., et al. 1994. Randomised double-blind comparison of chimeric monoclonal antibody to tumour necrosis factor alpha (cA2) versus placebo in rheumatoid arthritis. Lancet 344:1105. [DOI] [PubMed] [Google Scholar]
- 19. Maini R. N., Breedveld F. C., Kalden J. R., et al. 1998. Therapeutic efficacy of multiple intravenous infusions of anti-tumor necrosis factor alpha monoclonal antibody combined with low-dose weekly methotrexate in rheumatoid arthritis. Arthritis Rheum. 41:1552. [DOI] [PubMed] [Google Scholar]
- 20. Lipsky P. E., van der Heijde D. M. F. M., St Clair E. W., et al. 2000. Infliximab and methotrexate in the treatment of RA. N. Engl. J. Med. 343:1594. [DOI] [PubMed] [Google Scholar]
- 21. Charles P., Elliott M. J., Davis D., et al. 1999. Regulation of cytokines and acute phase proteins following TNFα blockade in rheumatoid arthritis. J. Immunol. 163:1521. [PubMed] [Google Scholar]
- 22. Taylor P. C., Peters A. M., Paleolog E., et al. 2000. Reduction of chemokine levels and leukocyte traffic to joints by tumor necrosis factor alpha blockade in patients with rheumatoid arthritis. Arthritis Rheum. 43:38. [DOI] [PubMed] [Google Scholar]
- 23. Weinblatt M. E., Kremer J. M., Bankhurst A. D., et al. 1999. A trial of etanercept, a recombinant tumor necrosis factor receptor:Fc fusion protein, in patients with rheumatoid arthritis receiving methotrexate. N. Engl. J. Med. 340:253. [DOI] [PubMed] [Google Scholar]
- 24. Weinblatt M. E., Keystone E. C., Furst D. E., et al. 2003. Adalimumab, a fully human anti-tumor necrosis factor alpha monoclonal antibody, for the treatment of rheumatoid arthritis in patients taking concomitant methotrexate: the ARMADA trial. Arthritis Rheum. 48:35. [DOI] [PubMed] [Google Scholar]
- 25. Combe B., Codreanu C., Fiocco U., et al. 2009. Efficacy, safety and patient-reported outcomes of combination etanercept and sulfasalazine versus etanercept alone in patients with rheumatoid arthritis: a double-blind randomised 2-year study. Ann. Rheum. Dis. 68:1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Keystone E., Emery P., Peterfy C. G., et al. 2009. Rituximab inhibits structural joint damage in patients with rheumatoid arthritis with an inadequate response to tumour necrosis factor inhibitor therapies. Ann. Rheum. Dis. 68:216. [DOI] [PubMed] [Google Scholar]
- 27. Genovese M. C., Becker J. C., Schiff M., et al. 2005. Abatacept for rheumatoid arthritis refractory to tumor necrosis factor alpha inhibition. N. Engl. J. Med. 353:1114. [DOI] [PubMed] [Google Scholar]
- 28. Emery P., Keystone E., Tony H. P., et al. 2008. IL-6 receptor inhibition with tocilizumab improves treatment outcomes in patients with rheumatoid arthritis refractory to anti-tumour necrosis factor biologicals: results from a 24-week multicentre randomised placebo-controlled trial. Ann. Rheum. Dis. 67:1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shepard H. M., Jin P., Slamon D. J., Pirot Z., Maneval D. C. 2008. Herceptin. Handb. Exp. Pharmacol. 181:183. [DOI] [PubMed] [Google Scholar]
- 30. Hurwitz H., Fehrenbacher L., Novotny W., et al. 2004. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N. Engl. J. Med. 350:2335. [DOI] [PubMed] [Google Scholar]
- 31. The Lenercept Multiple Sclerosis Study Group and The University of British Columbia MS/MRI Analysis Group. 1999. TNF neutralization in MS: results of a randomized, placebo-controlled multicenter study. Neurology 53:457. [PubMed] [Google Scholar]
- 32. Gregory A. P., Dendrou C. A., Attfield K. E., et al. 2012. TNF receptor 1 genetic risk mirrors outcome of anti-TNF therapy in multiple sclerosis. Nature 488:508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Charles P. J., Smeenk R. J., De Jong J., Feldmann M., Maini R. N. 2000. Assessment of antibodies to double-stranded DNA induced in rheumatoid arthritis patients following treatment with infliximab, a monoclonal antibody to tumor necrosis factor alpha: findings in open-label and randomized placebo-controlled trials. Arthritis Rheum. 43:2383. [DOI] [PubMed] [Google Scholar]
- 34. Aringer M., Graninger W. B., Steiner G., Smolen J. S. 2004. Safety and efficacy of tumor necrosis factor alpha blockade in systemic lupus erythematosus: an open-label study. Arthritis Rheum. 50:3161. [DOI] [PubMed] [Google Scholar]
- 35. Abraham E., Anzueto A., Gutierrez G., et al. 1998. Double-blind randomised controlled trial of monoclonal antibody to human tumour necrosis factor in treatment of septic shock. NORASEPT II Study Group. Lancet 351:929. [PubMed] [Google Scholar]
- 36. Hussell T., Pennycook A., Openshaw P. J. 2001. Inhibition of tumor necrosis factor reduces the severity of virus-specific lung immunopathology. Eur. J. Immunol. 31:2566. [DOI] [PubMed] [Google Scholar]
- 37. Wilson M. R., Choudhury S., Takata M. 2005. Pulmonary inflammation induced by high-stretch ventilation is mediated by tumor necrosis factor signaling in mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 288:L599. [DOI] [PubMed] [Google Scholar]
- 38. Moller J. T., Cluitmans P., Rasmussen L. S., et al. 1998. Long-term postoperative cognitive dysfunction in the elderly ISPOCD1 study. ISPOCD investigators. International Study of Post-Operative Cognitive Dysfunction. Lancet 351:857. [DOI] [PubMed] [Google Scholar]
- 39. Wan Y., Xu J., Ma D., Zeng Y., Cibelli M., Maze M. 2007. Postoperative impairment of cognitive function in rats: a possible role for cytokine-mediated inflammation in the hippocampus. Anesthesiology 106:436. [DOI] [PubMed] [Google Scholar]
- 40. Terrando N., Rei Fidalgo A., Vizcaychipi M., et al. 2010. The impact of IL-1 modulation on the development of lipopolysaccharide-induced cognitive dysfunction. Crit. Care 14:R88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Terrando N., Monaco C., Ma D., Foxwell B. M., Feldmann M., Maze M. 2010. Tumor necrosis factor-alpha triggers a cytokine cascade yielding postoperative cognitive decline. Proc. Natl Acad. Sci. USA 107:20518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hurst L. C., Badalamente M. A., Hentz V. R., et al. 2009. Injectable collagenase clostridium histolyticum for Dupuytren’s contracture. N. Engl. J. Med. 361:968. [DOI] [PubMed] [Google Scholar]
- 43. Verjee L. S., Midwood K., Davidson D., Eastwood M., Nanchahal J. 2010. Post-transcriptional regulation of alpha-smooth muscle actin determines the contractile phenotype of Dupuytren’s nodular cells. J. Cell. Physiol. 224:681. [DOI] [PubMed] [Google Scholar]
- 44. Verjee L. S., Verhoekx J. S. N., Chan J. K. K., et al. 2013. Unraveling the signaling pathways promoting fibrosis in Dupuytren’s disease reveals TNF as a therapeutic target. Proc. Natl Acad. Sci. USA 110:E928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mundi S., Pindiprolu B., Simunovic N., Bhandari M. 2014. Similar mortality rates in hip fracture patients over the past 31 years. Acta Orthop. 85:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Glass G. E., Chan J. K., Freidin A., Feldmann M., Horwood N. J., Nanchahal J. 2011. TNF-alpha promotes fracture repair by augmenting the recruitment and differentiation of muscle-derived stromal cells. Proc. Natl Acad. Sci. USA 108:1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chung E. S., Packer M., Lo K. H., Fasanmade A. A., Willerson J. T. 2003. Randomized, double-blind, placebo-controlled, pilot trial of infliximab, a chimeric monoclonal antibody to tumor necrosis factor-alpha, in patients with moderate-to-severe heart failure: results of the anti-TNF Therapy Against Congestive Heart Failure (ATTACH) trial. Circulation 107:3133. [DOI] [PubMed] [Google Scholar]
- 48. Mann D. L., McMurray J. J., Packer M., et al. 2004. Targeted anticytokine therapy in patients with chronic heart failure: results of the Randomized Etanercept Worldwide Evaluation (RENEWAL). Circulation 109:1594. [DOI] [PubMed] [Google Scholar]
- 49. Jacobsson L. T., Turesson C., Gülfe A., et al. 2005. Treatment with tumor necrosis factor blockers is associated with a lower incidence of first cardiovascular events in patients with rheumatoid arthritis. J. Rheumatol. 32:1213. [PubMed] [Google Scholar]
- 50. Wolfe F., Michaud K. 2004. Heart failure in rheumatoid arthritis: rates, predictors, and the effect of anti-tumor necrosis factor therapy. Am. J. Med. 116:305. [DOI] [PubMed] [Google Scholar]
- 51. Monaco C., Gregan S. M., Navin T. J., Foxwell B. M., Davies A. H., Feldmann M. 2009. Toll-like receptor-2 mediates inflammation and matrix degradation in human atherosclerosis. Circulation 120:2462. [DOI] [PubMed] [Google Scholar]
- 52. Smith A, ed. 1776. An Inquiry Into the Nature and Cause of the Wealth of Nations. W. Strahan and T. Cadell, London, UK. [Google Scholar]
- 53. Goekoop-Ruiterman Y. P., de Vries-Bouwstra J. K., Allaart C. F., et al. 2005. Clinical and radiographic outcomes of four different treatment strategies in patients with early rheumatoid arthritis (the BeSt study): a randomized, controlled trial. Arthritis Rheum. 52:3381. [DOI] [PubMed] [Google Scholar]
- 54. Ban der Bijl A. E., Goekoop-Ruiterman Y. P., de Vries-Bouwstra J. K., et al. 2007. Infliximab and methotrexate as induction therapy in patients with early rheumatoid arthritis. Arthritis Rheum. 56:2129. [DOI] [PubMed] [Google Scholar]
- 55. Genovese M. C., Cohen S., Moreland L., et al. 2004. Combination therapy with etanercept and anakinra in the treatment of patients with rheumatoid arthritis who have been treated unsuccessfully with methotrexate. Arthritis Rheum. 50:1412. [DOI] [PubMed] [Google Scholar]
- 56. Weinblatt M., Schiff M., Goldman A., et al. 2007. Selective costimulation modulation using abatacept in patients with active rheumatoid arthritis while receiving etanercept: a randomised clinical trial. Ann. Rheum. Dis. 66:228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Miotla J., Maciewicz R., Kendrew J., Feldmann M., Paleolog E. 2000. Treatment with soluble VEGF receptor reduces disease severity in murine collagen-induced arthritis. Lab. Invest. 80:1195. [DOI] [PubMed] [Google Scholar]
- 58. Vanniasinghe A. S., Manolios N., Schibeci S., et al. 2014. Targeting fibroblast-like synovial cells at sites of inflammation with peptide targeted liposomes results in inhibition of experimental arthritis. Clin. Immunol. 151:43. [DOI] [PubMed] [Google Scholar]
- 59. Foulquier C., Sebbag M., Clavel C., et al. 2007. Peptidyl arginine deiminase type 2 (PAD-2) and PAD-4 but not PAD-1, PAD-3, and PAD-6 are expressed in rheumatoid arthritis synovium in close association with tissue inflammation. Arthritis Rheum. 56:3541. [DOI] [PubMed] [Google Scholar]
- 60. Maresz K. J., Hellvard A., Sroka A., et al. 2013. Porphyromonas gingivalis facilitates the development and progression of destructive arthritis through its unique bacterial peptidylarginine deiminase (PAD). PLoS Pathog. 9:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Su L. F., Han A., McGuire H. M., Furman D., Newell E. W., Davis M. M. 2013. The promised land of human immunology. 2014. Cold Spring Harb. Symp. Quant. Biol. 78:203. [DOI] [PMC free article] [PubMed] [Google Scholar]