Abstract
Background and Aims
Oxidative stress is a core abnormality responsible for disease progression in nonalcoholic fatty liver disease (NAFLD). By employing a highly sensitive liquid chromatography-tandem mass spectrometry (LC/MS/MS) approach we recently were able to define the circulating profile of bioactive lipid peroxidation products characteristic of patients with nonalcoholic steatohepatitis (NASH) and developed the OxNASH score for NASH diagnosis. The aims of this study were to assess the utility of OxNASH as a predictor of NASH and study the association between OxNASH and specific histological features of NAFLD.
Methods
Our cohort consisted of 122 patients undergoing liver biopsy for clinical suspicion of NAFLD. The NAFLD activity score (NAS) calculated for each patient. Levels of fatty acid oxidation products were quantified using stable isotope dilution LC/MS/MS and OxNASH was calculated.
Results
The mean age was 49.3 (± 11.6) years and the mean BMI was 31.5 (± 4.8) kg/m2. The majority of patients were Caucasian (82%) and 48% were female. OxNASH correlated with NAS and its individual histologic features (steatosis, inflammation, and ballooning. P <0.05) with the strongest association being with inflammation [rho (95% CI) = 0.40 (0.23–0.57), p < 0.001]. Furthermore, there was a correlation between the stage of fibrosis and OxNASH (p = 0.001). These associations remained statistically significant after adjusting for multiple confounders.
Conclusions
In adult patients with NAFLD, OxNASH correlates with histologic features of NASH and appears to be a promising noninvasive marker.
Keywords: Nonalcoholic steatohepatitis, oxidative stress, lipid peroxidation, noninvasive
Introduction
Nonalcoholic fatty liver disease (NAFLD) is by far the most common form of chronic liver disease in the United States affecting 1 in 3 adults and 1 in 10 children (5, 21). NAFLD is an umbrella term used to cover a wide spectrum of diseases ranging from simple steatosis, thought to be a benign condition in terms of liver-related morbidity, to the more aggressive form of nonalcoholic steatohepatitis (NASH), which can progress to cirrhosis and hepatocellular carcinoma (2, 6, 14). Oxidative stress (OS), characterized by an imbalance between pro- and anti-oxidant mechanisms, is thought to have a pivotal role in the pathogenesis of NAFLD and disease progression toward NASH and fibrosis (16, 22). Patients with NASH have increased levels of OS as compared with patients with simple steatosis (10, 11, 19). Interestingly, not all lipid peroxidation products are increased within the plasma of subjects with biopsy proven NASH, and a characteristic pattern of specific oxidation products of linoleic and arachidonic acids are observed in subjects with NASH (10). The recent demonstration that vitamin E supplementation can ameliorate histopathologic features of NASH in both adult and pediatric patients (13, 20) has highlighted the role of the oxidation hypothesis in disease progression to NASH.
Currently, the only reliable way to diagnose steatohepatitis or NASH is a liver biopsy, which is costly, invasive and associated with some morbidity (17). Furthermore, it is recommended that liver histology should be used as the primary endpoint in clinical trials for NASH treatment (18). The NASH clinical research network designed the NAFLD activity score (NAS), a semi-quantitative scoring system to be used for evaluating histological changes after therapeutic intervention trials (12). This approach, that requires a biopsy before initiation of treatment and another biopsy at the end of treatment, is clearly not suitable for daily clinical practice for a disease that affects an estimated 50 million Americans. Therefore, there is an urgent need for non-invasive markers that correlate with the severity of liver injury and can predict response to new therapeutic approaches that are being developed. In a previous study, we identified specific fatty acid oxidation products as potential novel systemic non-invasive (plasma) markers to identify NASH (10). The results demonstrated that specific oxidized fatty acids (oxFA) products are markedly increased in the blood of patients with NASH and are mainly the result of free radical mediated processes (10). Using a learning cohort and subsequent validation cohort, we developed the OxNASH score, which incorporates 13-hydroxy-octadecadienoic acid (13-HODE), an oxidation product of linoleic acid (LA). In the current study we aimed at further assess the utility of OxNASH as a predictor of NASH and specific histological features of liver damage in a large and well-characterized group of patients with the full-spectrum of NAFLD.
Methods
Patient characteristics
Our cohort consisted of 122 consecutive patients who were referred for liver biopsy to the Cleveland Clinic with clinical suspicion of NAFLD. The study was approved by the Cleveland Clinic Institutional Review Board, and all patients gave written informed consent prior to participation. Information regarding demographics, medical history, and medications were obtained by patient interview and confirmed by chart review. Race information was based on self report and the information used for analyses was pre-specified prior to the study. The clinical outcome data were verified by source documentation. Adult patients between the ages of 18–70 years inclusive were enrolled, and had less than 20 grams/day of alcohol consumption for males and less than 10 grams/day for females. Patients were excluded from the study if other liver diseases were detected by serologic, biopsy or imaging studies. Dyslipidemia was defined as having a triglyceride level of >150 mg/dL or having an HDL level of < 40 mg/dL in men and < 50 mg/dL in women.
Liver biopsy
Liver histology was assessed by an experienced histopathologist in a blinded manner to the clinical data. Biopsy length and the average number of portal tracts were recorded for each patient. Study subjects were divided into three groups based on their liver histology results according to Brunt’s criteria (7)–(i) Normal liver biopsy; (ii) hepatic steatosis and (iii) NASH. The NAFLD activity scoring system recently proposed by the NIDDK NASH Clinical Research Network (NASH CRN) was calculated for each patient (12). According to this scoring system, the degree of steatosis, liver injury and inflammatory activity are measured using a 9-point scale (steatosis: 0–3; lobular inflammation: 0–3 and ballooning: 0–3). The NAFLD activity score (NAS) is the unweighted sum of steatosis, lobular inflammation and hepatocellular ballooning score. The degree of fibrosis was measured using a 6-point scale (1a, b = zone 3 perisinusoidal fibrosis; 1c = portal fibrosis only; 2 = zone 3 and portal /periportal fibrosis; 3 = bridging fibrosis; 4= cirrhosis). Severity of fibrosis was defined as: Stage 1: no fibrosis or mild fibrosis; Stage 2: moderate fibrosis; Stage 3 and 4: severe fibrosis. The quality of liver biopsies was rated as follows: optimal quality: biopsy >2.5 cm length, more than 10 portal tracts and no fragmentation; good quality: biopsy >1.5 cm length, more than 6 portal tracts and no fragmentation; inadequate biopsy: <1.5 cm length, fewer than 6 portal tracts and fragmented. Only optimal and good quality samples were included in the study.
Sample collection and liquid chromatography online electrospray ionization tandem mass spectrometry (LC/MS/MS)
Whole blood was collected into ethylenediaminetetraacetic acid (EDTA) tubes from fasting patients. Blood was immediately placed on ice or within a refrigerator, and samples centrifuged at 3500 rpm for 10 minutes at 4°C within 2 hr of collection. Plasma was then immediately stored under conditions to minimize artificial oxidation (i.e. with antioxidant cocktail, under inert atmosphere) (24). Briefly, plasma was aliquoted into tubes containing butylated hydroxytoluene (100 μM final) and diethylenetriamine pentaacetic acid (100 μM final), overlaid with argon, snap frozen in liquid nitrogen, and stored at −80°C until analysis.
Levels of fatty acid oxidation products in plasma that are used to calculate the OxNASH score (including 13 HODE and LA) were quantified using LC/MS/MS as previously described (10). The OxNASH score was calculated for each patient and included the ratio of 13-HODE to LA, age, BMI and AST with values between 0 to 100.
Statistical analysis
Clinical diagnosis, histopathological diagnosis and mass spectrometry assays were performed by investigators in a blinded manner. Descriptive statistics were computed for all factors. These included means, standard deviations and percentiles for continuous factors and frequencies and percentages for categorical variables. Spearman correlation coefficients were used to evaluate correlations between OxNASH and continuous and ordinal clinical and histological factors. In addition, Student t-tests were used to assess associations between OxNASH ratio and several categorical variables such as presence of diabetes and hyperlipidemia. Analysis of variance (ANOVA) was used to compare OxNASH between subjects with normal biopsies, steatosis and NASH. In addition, analysis of covariance was performed to study the association between NAFLD and OxNASH while adjusting for potential confounders such as gender and hyperlipidemia. Receiver Operating Characteristics (ROC) analysis was performed to evaluate prediction accuracy of OxNASH for diagnosing NASH and each of its histological features. The areas under the ROC curves (AUC) and corresponding 95% confidence intervals (95% CI) are reported. A p<0.05 was considered statistically significant. All analyses were performed using SAS version 9.2 software (The SAS Institute, Cary, NC) and R version 2.10.1 (The R Foundation for Statistical Computing, Vienna, Austria) was used to construct all plots.
Results
Demographic, clinical, and histologic characteristics of the patients
The main clinical and laboratory data of our patient population are summarized in Table 1. The mean age was 49.3 (± 11.6) years, the mean BMI was 31.5 (± 4.8) kg/m2, the majority of patients were Caucasian (82%), and 48% were female. Twenty eight percent of the patients had clinical diabetes and 47.5% had hypertension. Other features of the metabolic syndrome were commonly present including dyslipidemia in 58% with median [interquartile range (IQR)] triglyceride level of 163 [114, 236] mg/dL and median (IQR) high-density lipoprotein cholesterol (HDL) level of 44 [38, 54] mg/dL. None of the patients were on anti-oxidant therapy with vitamin E or C at the time of measuring fatty acid oxidation products in plasma. Twenty one patients (17.2%) were on lipid lowering agents (statins).
Table 1.
Patient characteristics
| Factor | N = 122 |
|---|---|
| Female (%) | 58 (47.5) |
| Caucasian (%) | 100 (82.0) |
| Age (years) | 49.3 ± 11.6 |
| BMI (kg/m2) | 31.5 ± 4.8 |
| Diabetes (%) | 33 (27.5) |
| Dyslipidemia (%) | 70 (58.3) |
| Hypertension (%) | 56 (47.5) |
| AST (U/L) | 47.0 [34.0, 65.0] |
| ALT (U/L) | 59.0 [38.0, 97.0] |
| Albumin (g/dL) | 4.3 ± 0.4 |
| Platelets (× 103/μL) | 242.5 [200.5, 276.0] |
| Cholesterol (mg/dL) | 197.0 ± 42.7 |
| Triglycerides (mg/dL) | 163.0 [114.0, 236.0] |
| HDL (mg/dL) | 44.0 [38.0, 54.0] |
| LDL (mg/dL) | 109.0 [87.0, 135.4] |
| OxNASH | 58.0 [37.4, 79.0] |
Values presented as Mean ± SD, Median [P25, P75] or N (%)
The main liver biopsy features are summarized in Table 2. Fifty six patients (46%) had a histological diagnosis of NASH, 45 patients (37%) were classified as having hepatic steatosis, while 21 patients (17%) had no or minimal changes on liver biopsy and were classified as normal. Some degree of inflammation was present in 52 % of patients, ballooning in 47 %, and fibrosis in 46%. Fifteen patients (12.3%) had bridging fibrosis (stage 3) and six patients (4.9%) had cirrhosis (stage 4). Interface hepatitis or other features suggestive of autoimmune hepatitis (an exclusion criterion of enrollment) were not present in any of the cases. OxNASH scores were significantly higher in females (64 ± 24 vs. 52 ± 30; p=0.017) and subjects with dyslipidemia (63 ± 27 vs. 52 ±27; p=0.024) or hypertension (65 ± 22 vs. 53 ± 30; p=0.013).
Table 2.
Histologic features and pathologist diagnosis
| Factor | N = 122 |
|---|---|
| Fibrosis (0–4) | |
| 0 | 66 (54.1) |
| 1 | 25 (20.5) |
| 2 | 10 (8.2) |
| 3 | 15 (12.3) |
| 4 | 6 (4.9) |
| Steatosis (0–3) | |
| None | 23 (19.0) |
| 1–33% | 39 (32.2) |
| 34–66% | 43 (35.5) |
| >66% | 16 (13.2) |
| Inflammation (0–3) | |
| No/minimal | 58 (47.9) |
| Mild | 34 (28.1) |
| Moderate | 26 (21.5) |
| Severe | 3 (2.5) |
| Ballooning (0–2) | |
| None | 62 (52.1) |
| Few | 29 (24.4) |
| Many | 28 (23.5) |
| NAS (0–8) | 2.0 [1.0, 5.0] |
| NAS diagnosis | |
| Normal (0) | 23 (19.3) |
| Steatosis (1–2) | 37 (31.1) |
| Borderline (3–4) | 20 (16.8) |
| NASH (5–8) | 39 (32.8) |
| Pathologist diagnosis | |
| Normal | 21 (17.2) |
| Simple Steatosis | 45 (36.9) |
| NASH | 56 (45.9) |
Values presented as Median [P25, P75] or N (%)
OxNASH score predicts NASH on liver biopsy
OxNASH scores were highest in patients with a histological diagnosis of NASH according to the Brunt’s criteria. Indeed, the levels of OxNASH were significantly higher in the NASH group (median of 69.7 [63.1, 76.4]) compared to both patients with steatosis (50.8 [43.4, 58.1]) or normal biopsy (45.4 [34.6, 56.2]), p < 0.0001 (Figure 1a). When we analyzed the association between the OxNASH score with known laboratory abnormalities seen in patients with NAFLD, we found that there were no significant correlations between the OxNASH score and alanine aminotransferase (ALT), albumin, platelet count and fasting lipid levels (Table 3).
Figure 1. Correlation between OxNASH and NASH histology.
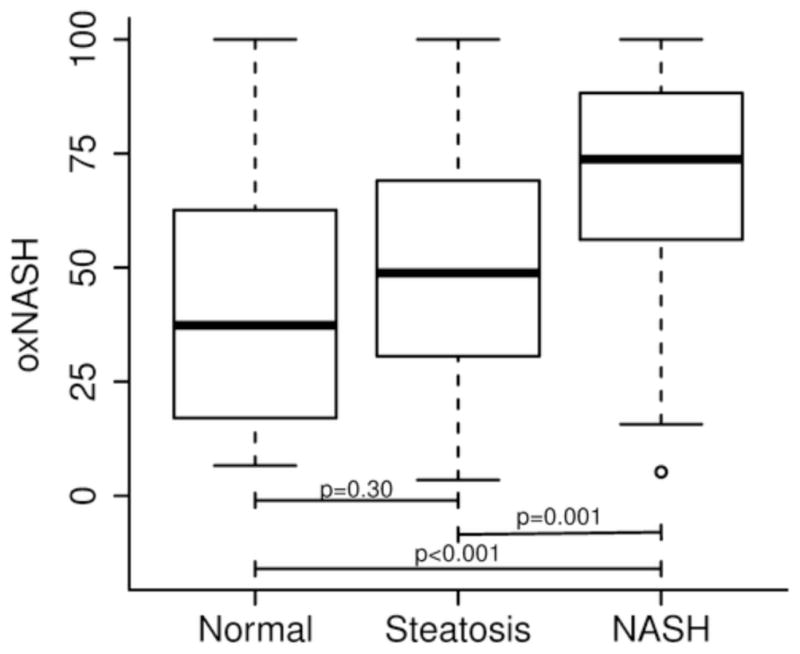
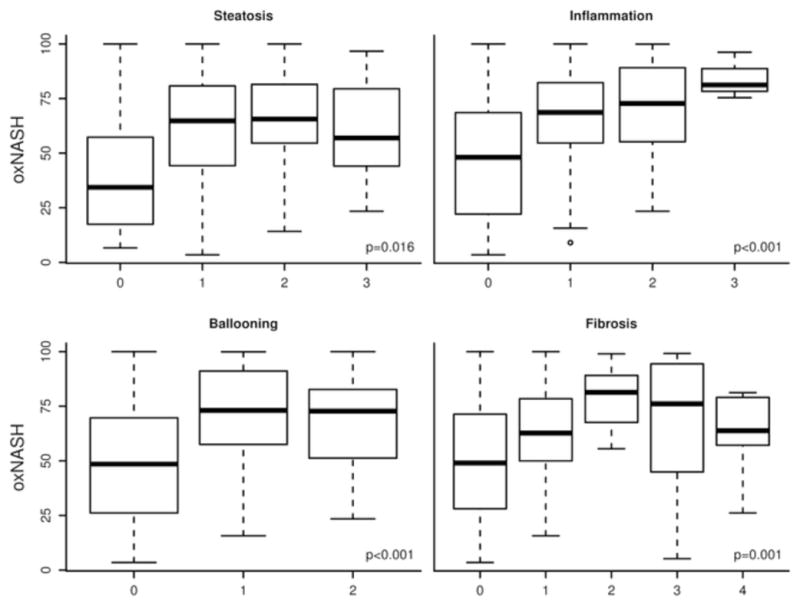
The OxNASH score was significantly higher in patients with NASH compared to those with steatosis or normal biopsy (figure 1a). The OxNASH score also correlated with the individual histological features of NASH with the strongest correlation being between OxNASH and inflammation (figure 1b).
Table 3.
Correlations between clinical and histologic factors and oxNASH
| Factor | rho (95% CI) | p-value |
|---|---|---|
| ALT (U/L) | 0.12 (−0.06,0.30) | 0.18 |
| Albumin (g/dL) | −0.03 (−0.22,0.17) | 0.79 |
| Platelet (× 103/μL) | −0.03 (−0.22,0.16) | 0.76 |
| Cholesterol (mg/dL) | 0.09 (−0.09,0.27) | 0.31 |
| Triglycerides (mg/dL) | 0.13 (−0.05,0.31) | 0.16 |
| HDL (mg/dL) | −0.04 (−0.22,0.14) | 0.64 |
| LDL (mg/dL) | 0.08 (−0.10,0.26) | 0.4 |
| Steatosis | 0.22 (0.04,0.40) | 0.016 |
| Ballooning | 0.35 (0.18,0.52) | <0.001 |
| Inflammation | 0.40 (0.23,0.57) | <0.001 |
| Fibrosis | 0.29 (0.12,0.47) | 0.001 |
| NAS | 0.36 (0.19,0.53) | <0.001 |
rho: Spearman’s correlation coefficient; CI: confidence interval; p-value tests null hypothesis of rho=0; bold p-values are statistically significant.
OxNASH score correlates with NAFLD activity score (NAS) and its individual histological features
In order to further study the potential links between the OxNASH measurements with liver damage in patients with NAFLD we examined the associations between OxNASH and the NAS as well as with each individual histological feature of liver damage. OxNASH scores significantly correlated with NAS and specific histological features including steatosis, inflammation, and ballooning; p <0.05 for all (Figure 1b and Table 3). The strongest association was with inflammation [rho (95% CI) = 0.40 (0.23–0.57), p < 0.001] followed by ballooning [rho (95% CI) = 0.35 (0.18–0.52), p < 0.001]. Furthermore, there was a statistically significant positive correlation between the stage of fibrosis and OxNASH (p = 0.001) (Figure 1b). Plasma 13-HODE levels showed similar pattern, with significant correlations [rho (95% CI)] noted with each of the histopathologic features of NAS: inflammation [0.22(0.04–0.39), p=0.017; fibrosis [0.17(0.01–0.35), p=0.06], steatosis [0.126(0.08–0.44), p=0.004], ballooning [0.20(0.02–0.38), p=0.029]. The associations between oxNASH or 13-HODE and these histopahologic features remained statistically significant after adjusting for multiple confounders including gender, dyslipidemia, the use of statins, diabetes and hypertension. More importantly, when patients were divided into tertiles according to their OxNASH score, patients in the upper tertile for OxNASH were almost eight-fold more likely to have inflammation and ballooning (Figure 2a and 2b) and five-fold more likely to have any fibrosis (F 1–4), p < 0.001 than those on the bottom tertile (Figure 2c). We also evaluated the accuracy of the OxNASH score for predicting the presence of each histological feature on liver biopsy as shown in Figure 3. The AUC was highest for inflammation 0.730 [95% CI (0.637, 0.823)]; followed by ballooning 0.723 [95% CI (0.630, 0.816)]; steatosis 0.705 [95% CI (0.570, 0.840)]; and fibrosis 0.673 [95% CI (0.577, 0.770)]. Different cutoff points for oxNASH for Prediction of histologic features of NAFLD are provided in Table 4 with their corresponding sensitivity, specificity, positive predictive value, and negative predictive value.
Figure 2. Risk of having inflammation, ballooning, and fibrosis based on OxNASH tertiles.
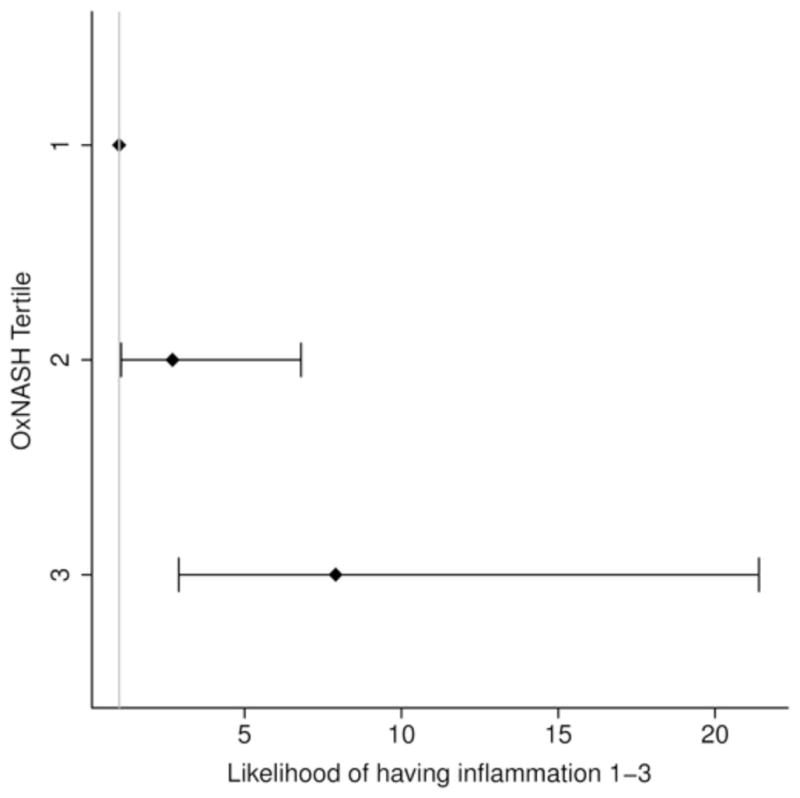
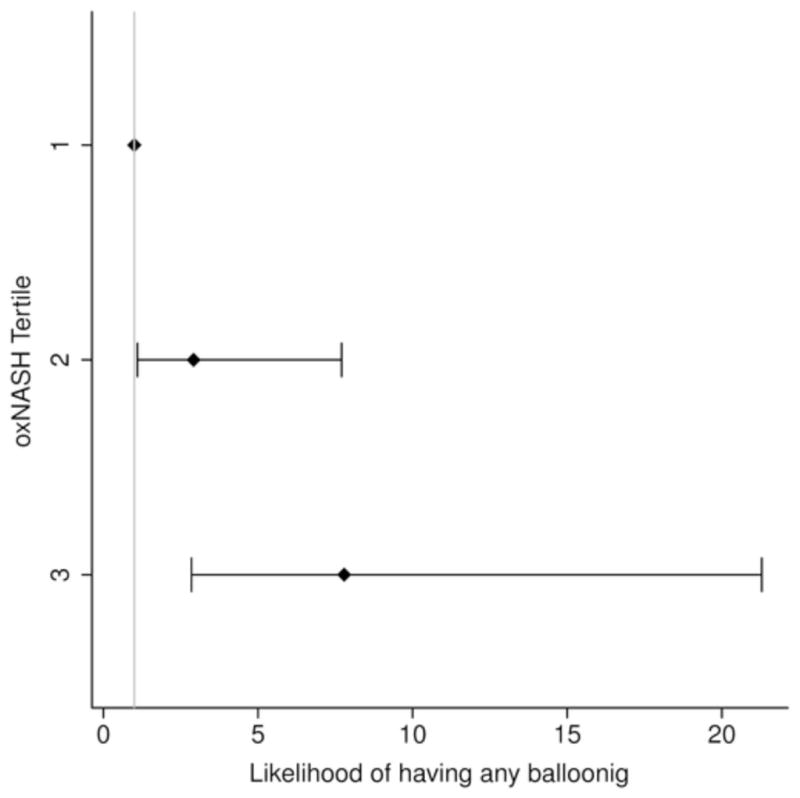
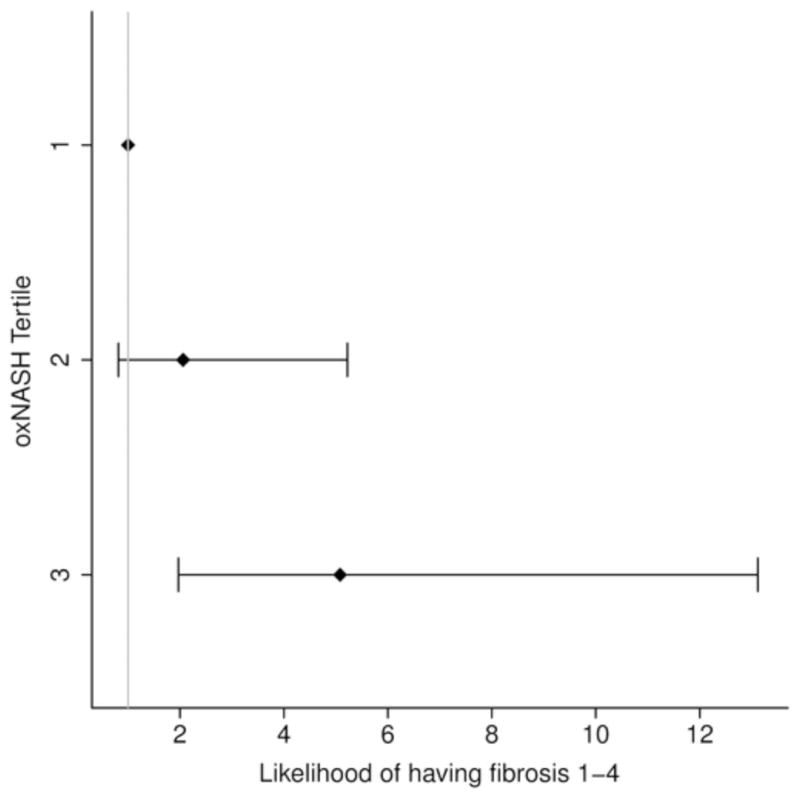
Patients in the upper tertile for OxNASH were almost eight folds more likely to have inflammation and ballooning (2a and 2b) and five folds more likely to have any fibrosis (2c).
Figure 3. Accuracy of OxNASH for predicting the presence of histological features of NASH on liver biopsy.
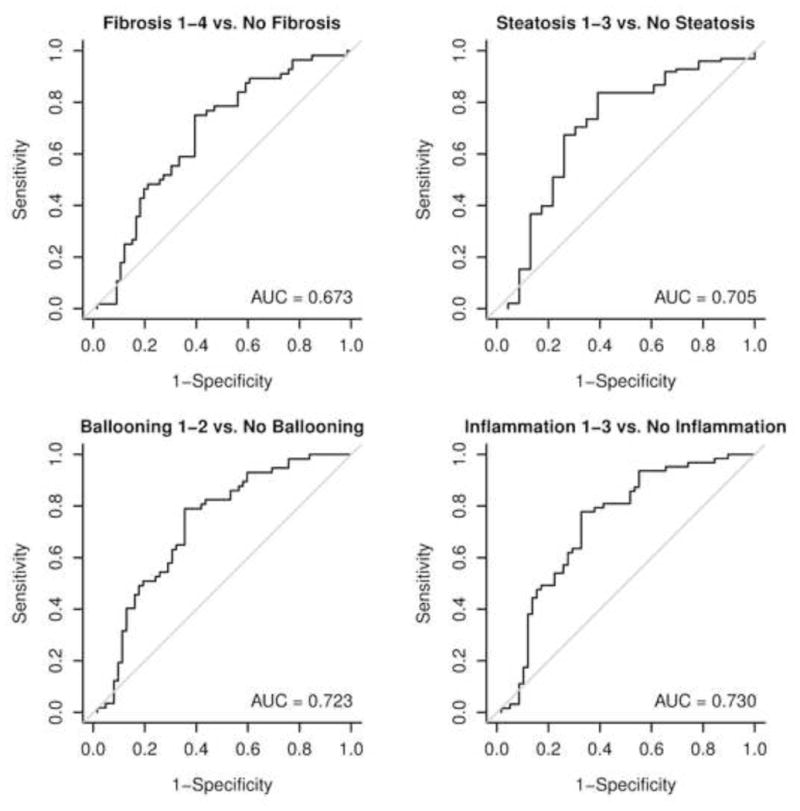
The area under the ROC curve (AUC) was highest for the prediction of inflammation followed by ballooning, steatosis and fibrosis.
Table 4.
Different cutoff points for oxNASH for Prediction of histologic features of NAFLD
| Outcome | oxNASH cutoff point | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|---|
| Fibrosis | 54.6 | 75.0 | 60.6 | 61.8 | 74.1 |
| Steatosis | 39.6 | 83.7 | 60.9 | 90.1 | 46.7 |
| Ballooning | 55.2 | 79.0 | 64.5 | 67.2 | 76.9 |
| Inflammation | 54.6 | 77.8 | 67.2 | 72.1 | 73.6 |
Discussion
The principal findings of this study relate to the utility of the OxNASH score for the non-invasive diagnosis of NASH. The results demonstrate that OxNASH correlates with individual histological features of disease severity in NASH, in particular with the degree of inflammatory activity in the liver.
OS has been established as a key factor in the development of NAFLD and the progression to NASH. OS can result from increased oxidation of intrahepatic fatty acids by mitochondria and other hepatocellular pro-oxidant pathways. The characteristic signature of specific LA oxidation products (e.g. 13-HODE) as being increased in NASH also argues for specificity in oxidation pathways being involved in the evolution of simple steatosis into NASH (10). Enhanced oxidant stress can lead to depletion of endogenous antioxidant defense mechanisms such as vitamin E and glutathione, perhaps accounting for the mechanism through which vitamin E provides improvement in histopathologic features in subjects with NASH (13, 20). Enhanced OS may also promote enhanced insulin resistance and the synthesis of several inflammatory cytokines via the upregulation of nuclear factor κβ, which may help further promote hepatocyte injury and further lipid peroxidation (9). Several small studies demonstrated either increased OS or reduced anti-oxidant capacity in NAFLD patients (15); however, the results were inconclusive in terms of the correlation with histological features of NASH. Chalasani et al. indirectly measured systemic lipid peroxidation in patients with NASH and age-, gender-, and BMI-matched controls by showing that both oxidized LDL and thiobarbituric acid-reacting substances were significantly higher in the NASH group (8). On the other hand, Baskol et al investigated the levels of the anti-oxidant enzyme paraoxonase 1 and found significantly lower activity in their small (n=23) NASH cohort (3). While recent LC/MS/MS studies by our group have shown that levels of serum paraoxonase activity are inversely correlated with multiple systemic indices of oxidative stress in humans (4), the studies by Baskol and colleagues observed no correlations between the level of this enzyme and the grade or stage of NAFLD. A decrease in the hepatic tissue levels of superoxide dismutase, catalase and glutathione peroxidase, and the antioxidant capacity of plasma has been shown in patients with NASH
A major weakness of the design of most antioxidant intervention studies has been the lack of concomitant assessments of systemic measures of OS because of the lack of validated OS measures that are associated with liver histology (1). The OxNASH score correlated with the major histological features of NASH making it an attractive candidate for use in future studies to stratify patients according to their OS status and to potentially monitor response to treatment avoiding the need for repeated liver biopsies and their potential complications. Our group has recently demonstrated the feasibility of using oxFA levels to assess response to treatment in patients with NASH (23). In a randomized placebo-controlled therapeutic trial, treatment of NASH with pentoxifylline was associated with significant reduction in specific oxFA compared to placebo linked to improvement in NASH histological features and disease activity.
Our study has several strengths including the inclusion of a large number of consecutive patients with biopsy-proven NAFLD with the full spectrum of disease ranging from simple steatosis to NASH. We assured the quality of liver biopsies by excluding any biopsy that was fragmented, < 1.5 cm in length or had < 6 portal tracts. Importantly, the plasma samples analyzed in our study were collected and processed under conditions designed to prevent artificial oxidation of lipids during both storage and analysis. Limitations to our study include referral bias as our patients were seen at a large tertiary care medical center which may limit the applicability of our results in community-based practices. Another limitation is the cross-sectional design which only allowed the determination of OxNASH scores at one time point without the ability to longitudinally assess the changes in OxNASH over time. We did not have data on the dietary intake of our subjects in the days leading to their liver biopsy and differences in their consumption of omega-3 fatty acids may have contributed to differences in the OxNASH score. Finally, the specimen collection and analysis of the lipid oxidation products is specialized and not easily transferable to the wider population.
In conclusion, the current study introduces the OxNASH as a potential prognostic indicator of multiple histopathological correlates of NASH and adverse prognosis. As a measure that relies on specific LA oxidation products, oxNASH may provide a gauge with which to monitor OS within the spectrum of NAFLD that correlates with the adverse histologic features of the disease and with disease activity or grade as assessed by NAS. The inclusion of measures of systemic OS and non-invasive markers of disease status is highly encouraged in the design of new clinical trials for NASH (18) and the OxNASH may be an ideal candidate for this purpose. Further studies are needed to assess the changes in OxNASH scores during the natural history of NAFLD and in therapeutic clinical trials in order to establish its utility for monitoring disease progression and response to treatment.
Acknowledgments
This work was supported by National Institutes of Health grants P01 HL076491, 1P20HL113452, P01HL098055, DK076852, AA017748, and P01 HL087018-020001, SLH is also supported by a gift from the Leonard Krieger Fund.
Footnotes
Disclosures: Dr. Hazen report being listed as co-inventor on pending and issued patents held by the Cleveland Clinic relating to diagnostics. Dr. Hazen reports having been paid as a consultant or speaker for the following companies: Abbott, Cleveland Heart Lab, Esperion, Lilly, Liposcience Inc., Merck & Co., Inc., and Pfizer Inc. Dr. Hazen reports receiving research funds from Abbott, Cleveland Heart Lab, Liposcience Inc., and Pfizer Inc. Dr. Hazen reports having the right to receive royalty payments for inventions or discoveries related to diagnostics or therapeutics from the companies.
Conflict of Interest: none to declare.
Author Contributions:
NA: Study concept and design; acquisition of data; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content.
MB: Acquisition of data; analysis and interpretation of data; critical revision of the manuscript for important intellectual content.
LY: Acquisition of data; analysis and interpretation of data; critical revision of the manuscript for important intellectual content.
RL: Analysis and interpretation of data; statistical analysis; critical revision of the manuscript for important intellectual content.
YC: Acquisition of data; analysis and interpretation of data; critical revision of the manuscript for important intellectual content.
RZ: Acquisition of data; analysis and interpretation of data; critical revision of the manuscript for important intellectual content.
TMC: Study concept and design; analysis and interpretation of data; critical revision of the manuscript for important intellectual content.
AEF: Study concept and design; acquisition of data; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content; obtained funding.
SLH: Study concept and design; acquisition of data; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content; obtained funding.
References
- 1.Alkhouri N, Feldstein AE. The TONIC trial: A step forward in treating pediatric nonalcoholic fatty liver disease. Hepatology (Baltimore, Md. 2012 Apr;55(4):1292–1295. doi: 10.1002/hep.24767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ascha MS, Hanouneh IA, Lopez R, Tamimi TA, Feldstein AF, Zein NN. The incidence and risk factors of hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. Hepatology (Baltimore, Md. 2010 Jun;51(6):1972–1978. doi: 10.1002/hep.23527. [DOI] [PubMed] [Google Scholar]
- 3.Baskol M, Baskol G, Deniz K, Ozbakir O, Yucesoy M. A new marker for lipid peroxidation: serum paraoxonase activity in non-alcoholic steatohepatitis. Turk J Gastroenterol. 2005 Sep;16(3):119–123. [PubMed] [Google Scholar]
- 4.Bhattacharyya T, Nicholls SJ, Topol EJ, Zhang R, Yang X, Schmitt D, et al. Relationship of paraoxonase 1 (PON1) gene polymorphisms and functional activity with systemic oxidative stress and cardiovascular risk. Jama. 2008 Mar 19;299(11):1265–1276. doi: 10.1001/jama.299.11.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology (Baltimore, Md. 2004 Dec;40(6):1387–1395. doi: 10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- 6.Brunt EM. Pathology of nonalcoholic steatohepatitis. Hepatol Res. 2005 Oct;33(2):68–71. doi: 10.1016/j.hepres.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 7.Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander-Tetri BA, Bacon BR. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. The American journal of gastroenterology. 1999 Sep;94(9):2467–2474. doi: 10.1111/j.1572-0241.1999.01377.x. [DOI] [PubMed] [Google Scholar]
- 8.Chalasani N, Deeg MA, Crabb DW. Systemic levels of lipid peroxidation and its metabolic and dietary correlates in patients with nonalcoholic steatohepatitis. The American journal of gastroenterology. 2004 Aug;99(8):1497–1502. doi: 10.1111/j.1572-0241.2004.30159.x. [DOI] [PubMed] [Google Scholar]
- 9.Edmison J, McCullough AJ. Pathogenesis of non-alcoholic steatohepatitis: human data. Clinics in liver disease. 2007 Feb;11(1):75–104. ix. doi: 10.1016/j.cld.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 10.Feldstein AE, Lopez R, Tamimi TA, Yerian L, Chung YM, Berk M, et al. Mass spectrometric profiling of oxidized lipid products in human nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Journal of lipid research. 2010 Oct;51(10):3046–3054. doi: 10.1194/jlr.M007096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.George J, Pera N, Phung N, Leclercq I, Yun Hou J, Farrell G. Lipid peroxidation, stellate cell activation and hepatic fibrogenesis in a rat model of chronic steatohepatitis. Journal of hepatology. 2003 Nov;39(5):756–764. doi: 10.1016/s0168-8278(03)00376-3. [DOI] [PubMed] [Google Scholar]
- 12.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology (Baltimore, Md. 2005 Jun;41(6):1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 13.Lavine JE, Schwimmer JB, Van Natta ML, Molleston JP, Murray KF, Rosenthal P, et al. Effect of vitamin E or metformin for treatment of nonalcoholic fatty liver disease in children and adolescents: the TONIC randomized controlled trial. Jama. 2011 Apr 27;305(16):1659–1668. doi: 10.1001/jama.2011.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, McCullough AJ. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999 Jun;116(6):1413–1419. doi: 10.1016/s0016-5085(99)70506-8. [DOI] [PubMed] [Google Scholar]
- 15.Miller MH, Ferguson MA, Dillon JF. Systematic review of performance of non-invasive biomarkers in the evaluation of non-alcoholic fatty liver disease. Liver Int. 2011 Apr;31(4):461–473. doi: 10.1111/j.1478-3231.2011.02451.x. [DOI] [PubMed] [Google Scholar]
- 16.Oliveira CP, da Costa Gayotto LC, Tatai C, Della Bina BI, Janiszewski M, Lima ES, et al. Oxidative stress in the pathogenesis of nonalcoholic fatty liver disease, in rats fed with a choline-deficient diet. Journal of cellular and molecular medicine. 2002 Jul-Sep;6(3):399–406. doi: 10.1111/j.1582-4934.2002.tb00518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rockey DC, Caldwell SH, Goodman ZD, Nelson RC, Smith AD. Liver biopsy. Hepatology (Baltimore, Md. 2009 Mar;49(3):1017–1044. doi: 10.1002/hep.22742. [DOI] [PubMed] [Google Scholar]
- 18.Sanyal AJ, Brunt EM, Kleiner DE, Kowdley KV, Chalasani N, Lavine JE, et al. Endpoints and clinical trial design for nonalcoholic steatohepatitis. Hepatology (Baltimore, Md. 2011 Jul;54(1):344–353. doi: 10.1002/hep.24376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sanyal AJ, Campbell-Sargent C, Mirshahi F, Rizzo WB, Contos MJ, Sterling RK, et al. Nonalcoholic steatohepatitis: association of insulin resistance and mitochondrial abnormalities. Gastroenterology. 2001 Apr;120(5):1183–1192. doi: 10.1053/gast.2001.23256. [DOI] [PubMed] [Google Scholar]
- 20.Sanyal AJ, Chalasani N, Kowdley KV, McCullough A, Diehl AM, Bass NM, et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. The New England journal of medicine. 2010 May 6;362(18):1675–1685. doi: 10.1056/NEJMoa0907929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schwimmer JB, Deutsch R, Kahen T, Lavine JE, Stanley C, Behling C. Prevalence of fatty liver in children and adolescents. Pediatrics. 2006 Oct;118(4):1388–1393. doi: 10.1542/peds.2006-1212. [DOI] [PubMed] [Google Scholar]
- 22.Videla LA, Rodrigo R, Araya J, Poniachik J. Oxidative stress and depletion of hepatic long-chain polyunsaturated fatty acids may contribute to nonalcoholic fatty liver disease. Free radical biology & medicine. 2004 Nov 1;37(9):1499–1507. doi: 10.1016/j.freeradbiomed.2004.06.033. [DOI] [PubMed] [Google Scholar]
- 23.Zein CO, Lopez R, Kirwan JP, Yerian LM, McCullough AJ, Hazen SL, et al. Pentoxifylline decreases oxidized lipid products in nonalcoholic steatohepatitis: New evidence on the potential therapeutic mechanism. Hepatology (Baltimore, Md. 2012 Apr 13; doi: 10.1002/hep.25778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang R, Shen Z, Nauseef WM, Hazen SL. Defects in leukocyte-mediated initiation of lipid peroxidation in plasma as studied in myeloperoxidase-deficient subjects: systematic identification of multiple endogenous diffusible substrates for myeloperoxidase in plasma. Blood. 2002 Mar 1;99(5):1802–1810. [PubMed] [Google Scholar]


