Abstract
West Nile virus (WNV) is a mosquito-borne flavivirus that has become endemic in the United States. From 1999-2012, there have been 37,088 reported cases of WNV and 1,549 deaths, resulting in a 4.2% case-fatality rate. Despite development of effective WNV vaccines for horses, there is no vaccine to prevent human WNV infection. Several vaccines have been tested in preclinical studies and to date there have been 8 clinical trials, with promising results in terms of safety and induction of antiviral immunity. Although mass vaccination is unlikely to be cost-effective, implementation of a targeted vaccine program may be feasible if a safe and effective vaccine can be brought to market. Further evaluation of new and advanced vaccine candidates is strongly encouraged.
Keywords: West Nile virus, neurotropic, vaccination, epidemiology, flaviviruses, antibody, immunity, immunocompromised
Introduction
West Nile virus (WNV) is a member of the genus Flavivirus, which includes arthropod-borne viruses belonging to the Flaviviridae family. Besides WNV, there are several clinically significant human pathogens within this group including St. Louis encephalitis virus (SLEV), dengue virus (DENV), tick-borne encephalitis virus (TBEV), Japanese encephalitis virus (JEV), Murray Valley encephalitis virus (MVEV), and yellow fever virus (YFV) [1,2]. These single-stranded positive-sense RNA viruses have a relatively small genome of approximately 11 Kb and form enveloped mature infectious particles that are ~50 nm in diameter. The genomic RNA of flaviviruses contains a single open reading frame, which is translated into a large polyprotein that is processed by both cellular and viral proteases into 3 structural proteins (C; capsid, prM; premembrane, and Env; envelope) and 7 nonstructural proteins (NS1, NS2a, NS2b, NS3, NS4a, NS4b, and NS5).
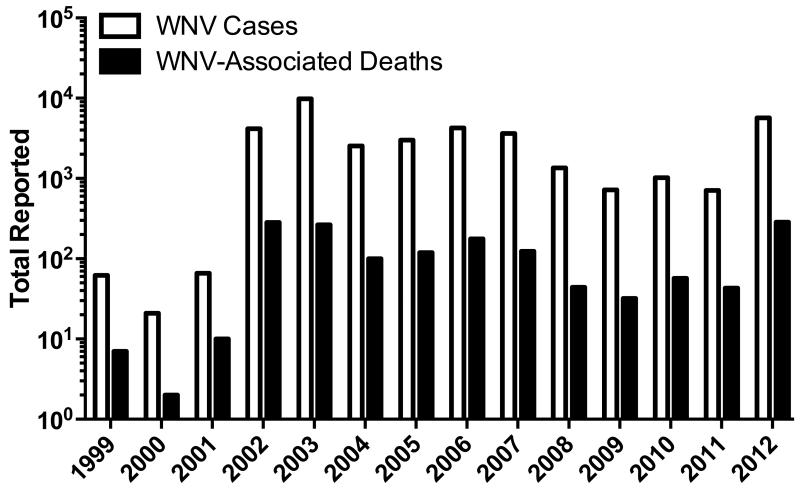
Following its initial discovery in Uganda in 1937, WNV was generally considered a minor public health threat, though sporadic outbreaks were occasionally noted [3]. During the 1990’s, more severe outbreaks with increased neuroinvasive disease were seen in North African and Southern European countries [3]. Neuroinvasive disease has also been the hallmark of WNV in the Western hemisphere, starting with a cluster of viral encephalitis cases in New York City in 1999 [2,4]. Since that time, the number of WNV cases has grown rapidly throughout the United States (Figure 1). Although there was hope that WNV would eventually decrease in incidence, epidemiology data suggests that WNV has become endemic throughout the continental United States, with periodic peaks and lulls in disease incidence. For example, after dropping to a post-endemic low of 720 reported WNV cases and 32 deaths in 2009, WNV jumped to 5,674 cases and a record 286 deaths in 2012.
Figure 1. Sustained prevalence of West Nile virus in the United States.
The annual number of reported WNV cases and associated deaths from 1999-2012 are shown. The highest number of deaths were reported in 2012, with 286 fatalities [201].
While a large number of flaviviruses represent important human pathogens, they are also distinguished by a number of successful vaccines to control disease. Licensed human vaccines for flaviviruses include formaldehyde-inactivated vaccines against TBEV and JEV as well as a live, attenuated vaccine against YFV. Although cellular immunity plays an important role in clearing primary WNV infection [5-9], memory CD8+ T cells are dispensable if high levels of antiviral antibody are present [7] and vaccine-induced memory T cells may not play a substantial role in controlling flavivirus infection in humans [10]. Moreover, a number of studies using passive immunization have shown that transfer of neutralizing antibodies to naïve animals is sufficient for protection against lethal WNV infection [11-15]. Accordingly, neutralizing antibody titers are generally correlated with protection against disease for licensed flavivirus vaccines. For example, YFV vaccine recipients with a serum antibody log neutralizing index (LNI) ≥ 0.7 are considered protected against clinical disease [401]. This was based on vaccination studies performed in rhesus macaques [16], and is an efficacy benchmark that has continued to be used in YFV clinical trials [17,18]. Similarly, the protective threshold for the JEV vaccine is correlated to neutralizing antibody titers, with a serum PRNT50 ≥ 1:10 considered protective by vaccine manufacturers [402] as well as a WHO recommendation panel [19]. While the TBEV vaccine does not have a specific, established level for achieving protective immunity, neutralizing antibody titers are still considered the key to vaccine efficacy [20]. Based on this track record with related flaviviruses, a WNV vaccine should be feasible and a large number of WNV vaccines are in various stages of development. Although several veterinary vaccines against WNV have been licensed for use in horses, a human vaccine is still not available. In this review, we will discuss the range of WNV vaccines that have been developed and tested at both the pre-clinical and clinical level. We will also review current challenges to vaccine licensure, including technical limitations in late-stage efficacy trials and concerns regarding cost-effectiveness, and propose alternate approaches towards development of a safe and effective WNV vaccine.
WNV epidemiology and surveillance
WNV is considered the most geographically widespread arbovirus in the world, reaching into every continent except for Antarctica [3]. The virus was first identified in Africa in 1937 from a patient experiencing fever, which later resolved without incident [21]. In the decades following this initial identification, sporadic rural outbreaks linked to WNV were recorded throughout the world, though reports of severe neurological disease were limited [22]. Starting in the 1990’s, more frequent and severe outbreaks were seen in countries bordering the Mediterranean Sea, with further movement of the virus north and west into countries such as Romania, Russia and Israel [3]. During this same time frame, WNV reached the shores of North America, starting with a cluster of viral encephalitis cases in Queens, New York in 1999 [2,4,23]. Sequencing of WNV RNA indicated close homology with an Israeli isolate of WNV [4] and the current theory is that WNV may have been introduced from Israel, with subsequent transmission to mosquito populations and spread into the local ecosystem [24]. Following this initial introduction, WNV spread across the continent, reaching the West Coast of the United States by 2003. The rapid spread of WNV in North America has been linked to the flyways of migratory fowl, with the virus able to take advantage of yearly migration routes for rapid transit and spread into local bird populations [23]. Other aspects attributed to the spread of WNV include a wide range of vertebrate hosts and mosquito species that can carry the virus [23], though the transmission cycle between Culex spp. of mosquitoes and various bird species is considered the primary enzootic maintenance pattern. Perhaps the most important aspect underlying increased virulence and spread are the genetic differences between strains of WNV. Presently, WNV is categorized into five lineages, though most isolates fall into the lineage 1 or lineage 2 categories [25]. Lineage 1 strains (such as WNV-NY99) are considered emerging diseases, generally associated with increased virulence in humans [25]. By comparison, lineage 2 strains of WNV (including the founding Uganda 1937 strain) are usually less severe, though recent outbreaks in Europe with pathogenic lineage 1 strains may modify this position [26]. This type of divergence within lineages is not unprecedented, with even the lineage 1 strain of WNV split into multiple clades (1a-1c) representing a broad spectrum of pathogenicity [27]. For example, while WNV-Kunjin (an Australian clade 1b) shows ~98% amino acid identity to WNV-NY99 (clade 1a) [5], WNV-Kunjin is highly attenuated in humans, typically resulting in either mild or clinically asymptomatic infections [28].
Since its introduction into North America in 1999, surveillance shows that WNV has now become endemic throughout the continent. In the United States, 37,088 cases have been reported from 1999-2012 (Figure 1) with 16,196 classified as neuroinvasive disease [201]. During this time frame there were 1,549 deaths associated with WNV, yielding a case-fatality rate of 4.2%. The impact of WNV has also extended north into Canada, with a total of 5,094 cases and 71 deaths (equaling an estimated 1.4% case fatality rate) reported from 2002-2012 [202]. After a peak in reported cases and deaths in the United States from 2002-2003, WNV activity generally waned from 2006-2011, reaching a low of 720 cases and 32 deaths in 2009 (Figure 1). However, 2012 saw an explosion in WNV incidence, with 5,674 reported cases and 286 deaths, the most WNV-associated fatalities on record in the United States (Figure 1). A similar spike in reported cases was also seen in Canada [202], suggesting that North America will continue to have WNV outbreaks into the foreseeable future. A rise in WNV incidence has also been observed recently in Southern Europe, with Greece experiencing a significant outbreak in 2010 [26]. Following this outbreak, the European Union, along with other groups, have begun efforts to improve surveillance across affected European countries and neighboring regions [203]. Surveillance from 2010-2012 demonstrated a total of 2,414 WNV cases with 127 associated deaths (Table 1) for a case-fatality rate of 5.3%, similar to the rate observed in the United States. While Figure 1 shows the number of reported cases and deaths in the United States, the overall disease burden is likely much higher. For instance, North Dakota reported ~1,300 cases of WNV from 1999-2008 [204] but seroprevalence studies indicate that >40,000 were infected in the same time frame [29], suggesting that at least 30 undiagnosed cases of WNV occur for every case reported. This is not unique to North Dakota; recent evidence suggests that across the United States there have been nearly 3 million WNV infections, resulting in an estimated 780,000 illnesses [30]. Based on these estimates, WNV outbreaks and disease incidence are far greater than previously realized and this could have profound consequences on the economic impact of WNV disease and cost-effectiveness calculations for a WNV vaccine [31] that were made prior to publication of this study in 2013 [30].
Table 1.
Surveillance of West Nile virus in the Southern Europe and neighboring countries from 2010-20121
| Country | National Surveillance System | Reported Cases |
Deaths | |
|---|---|---|---|---|
|
Southern
Europe |
Greece | Permanent | 523 | 62 |
| Romania | Seasonal | 83 | 7 | |
| Italy | Seasonal | 45 | 8 | |
| Bulgaria | Permanent (starting 2011) | 2 | 0 | |
| Spain | Permanent (specific areas) | 2 | 0 | |
| France | Permanent + seasonally enhanced | 0 | 0 | |
| Malta | Permanent (starting 2012) | 0 | 0 | |
|
| ||||
| The Balkans | Serbia | Seasonal (starting 2011) | 71 | 9 |
| Albania | Permanent | 50 | 0 | |
| Republic of Macedonia | Permanent | 10 | 0 | |
| Croatia | Permanent | 6 | 0 | |
| Kosovo | Permanent | 6 | 0 | |
| Bosnia-Herzegovina | None | 1 | 0 | |
| Montenegro | Permanent (starting 2012) | 1 | 0 | |
| Cyprus | Permanent | 0 | 0 | |
| Slovenia | Permanent | 0 | 0 | |
|
| ||||
|
North Africa
and the Middle East |
Israel | Permanent | 255 | 10 |
| Tunisia | Permanent + seasonally enhanced | 92 | 12 | |
| Turkey | Permanent | 65 | 13 | |
| Palestine | Permanent + seasonally enhanced | 3 | 0 | |
| Algeria | None | 1 | 0 | |
| Egypt | Unavailable | 0 | 0 | |
| Morocco | Permanent | 0 | 0 | |
| Jordan | Permanent | 0 | 0 | |
| Lebanon | None | 0 | 0 | |
| Libya | Permanent | 0 | 0 | |
| Syria | None | 0 | 0 | |
|
| ||||
|
Neighboring
regions |
Russia2 | Unavailable | 1,152 | 6 |
| Hungary | Unavailable | 26 | 0 | |
| Ukraine | Unavailable | 20 | 0 | |
|
| ||||
| Total | 2,414 | 127 | ||
Source: EpiSouth co-funded by the European Union DG SANCO/EAHC and DEVCO/EuropeAid [203].
Of cases reported in Russia, 40-74% occurred in the southwestern Volgograd oblast.
West Nile virus disease
Most cases of WNV infection are clinically inapparent but approximately 25% will present as West Nile fever (WNF) [32] and 1 in 150 to 1 in 250 will develop more severe West Nile neurotropic disease (WNND) [33,34]. WNF symptoms include fever of >38°C, general fatigue, headache, muscle pain, malaise and in some cases, gastrointestinal symptoms and rash [35,36]. In some patients, symptoms may last more than a month after disease onset [36,37]. WNND manifests as encephalitis, meningitis, or flaccid poliomyelitis-like paralysis that may result in respiratory failure [38-44]. Although the overall case-fatality rate is 4.2%, there is a 9.6% case-fatality rate among patients with WNND [45]. Unfortunately, neuroinvasive disease is likely to be under-reported since only 40% of meningitis or encephalitis patients are tested for WNV, even during well-publicized WNV outbreaks [46]. WNND is not only accompanied by a high rate of acute mortality [47] but survivors often experience long-term neurological dysfunction [43] with many requiring assistance with daily activities after hospital discharge [47,48]. Following recovery from WNV encephalitis, up to 77% of patients continue to have neurological complications including impaired gait, muscle weakness, hearing loss, and tremors lasting ≥3 years after infection [49]. Moreover, WNV survivors demonstrate a 2.5-fold to 3-fold higher age- and sex-adjusted mortality rate within the first 2 years following hospitalization compared to controls [50].
Although the main focus of WNV pathogenesis has been neurological complications, recent evidence suggests that chronic kidney disease (CKD) may be a previously underappreciated complication of WNV infection [51]. Persistent WNV infection has been described in several animal models [51] and WNV may be shed in the urine of infected Golden hamsters for up to 8 months [52,53]. A strain of WNV isolated from hamster urine at 274 days post-infection was found to have lost neurovirulence but induce persistent renal infection in mice [54]. In humans, WNV RNA has been detected in urine during the acute stages of infection [37,51,55] and one study found WNV RNA in urine from 25% (5/25) of patients between 1.6-6.7 years after infection [56]. However, another report was unable to identify WNV RNA among a cohort of 40 patients examined at >6 years post-infection [57]. Acute renal failure has been reported in WNV patients suffering from encephalitis [58,59] but CKD may be more common then previously thought; a recent study found that 40% of WNV patients had evidence of CKD within 4-9 years after infection and the presence of detectable WNV RNA in the urine was associated with more severe renal disease [60]. Although not confirmatory, this is consistent with a study that found 21% of deceased WNV patients had documented renal failure listed as a cause or underlying condition at the time of death [61]. While more studies are needed, if CKD proves to be an important clinical outcome of human WNV infection, then this could greatly alter the economic impact of WNV infection and further emphasize the need for development of a safe and effective vaccine.
Vaccines in pre-clinical development
Following the initial outbreak of WNV in the United States, a large number of vaccine candidates have been developed (Table 2). These approaches can be divided into several broad categories including DNA-based vaccines, live chimeric/recombinant vaccine constructs, live attenuated virus, and inactivated or subunit vaccines. DNA-vectored vaccines have offered the promise of rapid vaccine development through the power of modern genetic tools [62]. However, to date no DNA vaccines have been licensed for use in humans. DNA vaccines expressing the prM and Env proteins from WNV [63-65] or the domain III (DIII) region of the Env protein [66] have been developed and tested in both mice and horses. Other approaches to DNA vaccines have included constructs encoding for single-round infectious particles (SRIP) [67,68] or a full-length cDNA copy of the attenuated Kunjin strain of WNV [69]. Candidates expressing the prM and Env proteins elicited plaque reduction neutralization (PRNT) titers against WNV-NY99 in both mice (range; 1:320-1:640) and horses (range; 1:40-1:320) after a single dose [63] and a vaccine based on this technology was developed into a licensed veterinary vaccine (though later discontinued by Pfizer) and eventually pursued in human clinical trials.
Table 2.
Preclinical approaches to West Nile vaccine development
| Vaccine Approach | Animal Models | References |
|---|---|---|
| DNA-vectored vaccines | ||
|
| ||
| DNA plasmid expressing WNV prM and Env | Mice, Horses | [63-65] |
| DNA plasmid expressing WNV EDIII | Mice | [66] |
| Pseudo-infectious DNA vector (C deletion mutants) | Mice, Horses | [67,68] |
| DNA plasmid expressing the attenuated Kunjin strain of WNV | Mice | [69] |
|
| ||
| Live chimeric/recombinant vaccines | ||
|
| ||
| YFV-17D backbone expressing WNV prM/Env | Mice, Hamsters, Horses, NHP |
[70-72,74] |
| DV4 backbone expressing WNV PrM/Env | Mice, Geese, NHP | [75,76] |
| Canarypox vector expressing WNV PrM/Env | Cats, Dogs, Horses | [77,78] |
| Adenovirus vector expressing WNV C, prM, Env and NS1 proteins | Mice | [84] |
| WNV Env expressed by multiple VSV vectors | Mice | [83] |
| HIV-based vectors expressing WNV Env protein | Mice | [79,80] |
| Measles vector expressing WNV Env protein | Mice, NHP | [81,82] |
|
| ||
| Live attenuated or pseudo-infectious vaccines | ||
|
| ||
| Neutralizing MAb escape variant following serial passage in cell culture | Mice, Geese | [85] |
| Molecularly cloned lineage 2 WNV strain | Mice | [86] |
| Attenuating mutations in the glycosylation sites of WNV Env and NS1 | Mice | [87] |
| Attenuating point mutations in the WNV Env and 3′-UTR | Mice | [88] |
| Attenuating point mutations in the WNV NS2A or NS4B proteins | Mice | [89,90] |
| Pseudo-infectious WNV achieved through C protein deletion mutations | Mice, Hamsters, NHP | [91,92] |
|
| ||
| Recombinant subunit vaccines | ||
|
| ||
| WNV virus-like particle | Mice | [94] |
| Recombinant WNV Env protein | Mice, Horses, Birds, NHP |
[101,131,139- 143] |
| WNV EDIII constructs with different conjugate and adjuvant approaches | Mice | [98- 100,142,144] |
| Env peptide vaccine derived from the EDIII domain | Mice | [145] |
|
| ||
| Inactivated whole virus vaccines | ||
|
| ||
| Formalin-inactivated WNV | Mice, Geese, Hamsters, Horses |
[72,93,103-105] |
| Hydrogen peroxide (H2O2) inactivated WNV | Mice | [5,111] |
Chimeric/recombinant vaccines have been another active field for the development of WNV vaccines (Table 2). One well-studied approach uses the YFV-17D vector backbone expressing WNV prM and Env (ChimeriVax-WN, Sanofi), with testing performed across several animal species including mice, hamsters, horses, and non-human primates (NHP) [70-72]. The construct is based on the genetic backbone of the vaccine strain of YFV (YFV-17D) in which the YFV prM and Env genes have been replaced by the WNV-NY99 prM and Env proteins, with several point mutations engineered into the Env to reduce potential neurovirulence [70]. This vaccine platform (ChimeriVax™) has been the basis for a number of related flavivirus vaccine candidates including JEV and all four serotypes of DENV [73]. In preclinical studies of the ChimeriVax-WN candidate, vaccinated rhesus macaques reached average PRNT50 titers against homologous vaccine virus of 1:381 by 30 days post-vaccination, declining to 1:193 by day 63 [71]. Vaccinated mice demonstrated a low level of immunity, with neutralizing titers in the range of 1:20-1:37 at four weeks following vaccination. This vaccine has since moved into several clinical trials, and was licensed as a veterinary horse vaccine under the trade name, PreveNile® [74]. However, the horse vaccine was later recalled in 2010 after reports of increased adverse events in horses following vaccination [403]. An alternate chimeric flavivirus platform, using an attenuated DENV serotype 4 (DENV4) backbone expressing WNV prM and Env, has also been developed [75,76]. In NHP, peak PRNT60 titers against WNV-NY99 of 1:324 were observed at day 28 post-vaccination, dropping to 1:170 by day 42 [76]. A number of other live, attenuated recombinant virus vector vaccines have also been developed. A veterinary vaccine using a canarypox-based vector expressing WNV prM and Env has demonstrated efficacy in preventing viremia across several animal species including horses, cats and dogs [77,78]. An HIV-based lentiviral vector expressing the WNV Env protein was shown to induce a protective immune response in mice within 1 week of a single immunization [79]. A non-integrative version of this same vector system was later developed in an attempt to reduce safety concerns, and was also shown to induce protective immune responses in mice [80]. A WNV candidate based on an attenuated strain of the measles virus (Schwarz strain) expressing WNV Env was tested in both mice [81] and squirrel monkeys [82], with protection demonstrated against death or viremia, respectively. Similarly, a vesicular stomatitis virus (VSV) vaccine vector has also been used to express the WNV Env protein, with 90% protection achieved against lethal WNV challenge in mice following a two-dose, intranasal vaccination schedule [83]. A multi-antigen adenovirus-vectored vaccine expressing the WNV C, prM, Env, and NS1 proteins induced robust PRNT50 serum titers against WNV-NY99 (average = 1:2,816) following a two-dose vaccination schedule in mice [84]. While several of these approaches show promising results with regard to immunogenicity and protective efficacy in animal models, some practical constraints may limit their clinical utility. For vaccine approaches using lentiviral vectors [79,80] or VSV [83], can safety and biocontainment issues be adequately addressed? For candidates using adenovirus [84] and measles virus vectors [81,82], will pre-existing immunity in humans limit their use? These questions may need to be addressed if these vaccine platforms are to move forward into clinical development.
A number of preclinical studies have been reported using attenuated strains of WNV [85-90], created either through classic cell culture methods or targeted genetic mutations. For example, through a targeted deletion in the WNV C protein, one group has developed a WNV vaccine candidate that is limited to a single round of infection (RepliVAX WN, [91,92]). Using this approach, investigators were able to induce neutralizing antibodies and protective immune responses in mice, hamsters [91] and NHP [92]. Another group demonstrated that concurrent mutations in the Env and NS1 proteins were able to dramatically reduce the virulence of replication-competent WNV-NY99, increasing the 50% lethal dose (LD50) from 5 plaque forming units (PFU) with wild type virus, to >1,000,000 PFU in the attenuated vaccine candidate [87]. Despite these mutations, the attenuated virus could elicit high PRNT50 neutralizing responses against WNV-NY99 in mice (range; 1:320-1:2,560) with as little as a 103 PFU dose. Results such as these hold promise as an additional avenue for development of a WNV vaccine, but live viral vaccines have been somewhat unpredictable in the past and may pose potential regulatory concerns for the elderly and immunocompromised, representing two vulnerable populations with the greatest need for a safe and effective WNV vaccine.
In terms of protein vaccines, three main types have been developed including chemically-inactivated whole virus, virus-like particle (VLP), and recombinant WNV envelope subunit formulations (Table 2). The first successful veterinary vaccine, licensed in 2003, was a formaldehyde-inactivated preparation of WNV-NY99, shown to protect against WNV challenge [93]. Though this vaccine was not developed for human use, it has provided an important proof-of-principle for this class of non-replicating protein vaccines against WNV disease. Another early WNV vaccine candidate within this general class of vaccines was a VLP expressing the prM and Env proteins [94]. Vaccination elicited neutralizing antibody titers in mice, and although responses were relatively low (average; 1:37) they could be boosted with a monophosporyl lipid A (MPL, a detoxified form of LPS) and saponin-based liposomal adjuvant (average; 1:75) [94]. Several groups have developed vaccine candidates based on the DIII of the WNV Env protein, since this region of the Env has been shown to harbor potent neutralizing antibody epitopes in mice [95]. One caveat is that recent studies indicate that domain II (DII) is the immunodominant domain in humans following WNV infection [96,97]. Using a 13-kDa DIII recombinant protein for vaccination, mice mounted significant PRNT50 serum titers (1:1,000) against the lineage 2 WNV-Sarafend strain following three immunizations with 100 μg of antigen per dose [98]. Other groups have pursued vaccines based on VLP platforms that incorporate fusion proteins engineered to display the WNV Env DIII on their surface, but results have varied [99,100]. In one study using an HIV-based VLP, only 1/5 mice seroconverted (PRNT50 ≥ 1:10) against the lineage 1 WNV-Kunjin strain following vaccination [100]. Using a bacteriophage expression system, another DIII-based VLP vaccine was shown to induce neutralizing responses against WNV-NY99 in mice following a single immunization and partial protection against lethal WNV challenge (PRNT100 ~1:20-1:30, 60% survival), but higher neutralizing titers and full protective immunity (PRNT100 ~1:1,000, 100% survival) were achieved after three doses [99]. A recent report exploring the subunit WNV Env vaccine approach has provided further insight into the role of different adjuvants in this vaccine model system [101]. In this study, mice were immunized on days 0 and 28 with 10 μg of WNV Env protein alone, or adjuvanted with Matrix-M™, a saponin-based adjuvant. At 21 days after vaccination, PRNT90 titers (against the lineage 1 WNV Eg101 strain) in the Env group averaged ~1:250. By comparison, the Matrix-M formulated vaccine induced neutralizing antibody titers of ~1:8,000, an approximate 30-fold increase in immunogenicity. Additional studies demonstrated that Matrix-M was also ~4-fold more immunogenic than an aluminum hydroxide based adjuvant. It is possible that subunit WNV vaccines will require the use of advanced adjuvants in humans to elicit high immunogenicity, and although these approaches may bring additional regulatory complexity, the Matrix-M adjuvant looks very promising since it has been used in >10 million horses vaccinated with Equilis®Prequenza and has been shown to be safe and effective in a human influenza vaccine Phase I clinical trial [102].
Inactivated whole virus vaccines represent another important class of vaccine candidates (Table 2). The first licensed veterinary vaccine was based on this approach, using a formalin-inactivated crude viral harvest of WNV-NY99, formulated with a squalene-based adjuvant, to induce protective immunity in horses [93] as well as other animal models [72]. A formalin-inactivated vaccine based on a pathogenic lineage 1 strain of WNV (ISR98) has also been described, and was shown to be protective in a goose challenge model [103]. Two other groups have developed formalin-inactivated vaccines, both based on the virulent WNV-NY99 strain of virus [104,105]. In one study, mice immunized with a two-dose schedule (1 μg per dose, alum-adjuvanted) achieved virus neutralizing titers of ~1:250 against WNV-NY99 at two weeks after final vaccination and were protected from intranasal challenge with virulent WNV [105]. In a second study, mice were also given a two-dose schedule of experimental, non-adjuvanted vaccine provided by the Research Foundation for Microbial Diseases of Osaka University (BIKEN, Osaka, Japan) [104]. At 4-weeks post-boost, vaccinated mice demonstrated a neutralizing titer of 1:70 (WNV-NY99) and were protected against lethal WNV challenge. From a clinical perspective, one concern for these formalin-inactivated WNV vaccine candidates is that they are based on pathogenic strains of WNV. The use of highly pathogenic strains of virus for inactivated vaccines creates logistical issues associated with the handling of BSL3 pathogens during large-scale cGMP manufacturing, in addition to safety concerns if complete inactivation is not achieved. For instance, one of the worst vaccine-related tragedies in the United States came from the improper inactivation of virulent poliovirus during vaccine manufacturing in 1955 (i.e., “The Cutter Incident”) [106]. This resulted in 120,000 doses of vaccine that contained live poliovirus and resulted in 40,000 children who were infected, 56 who developed paralytic poliomyelitis, and 5 children died [107-109]. While modern manufacturing practices ensure the safety of the inactivated polio vaccine (IPV), there has still been a push to further increase the safety margin of IPV by switching from current virulent poliovirus strains to attenuated virus vaccine strains [110]. As an alternative to traditional formaldehyde-based vaccines, a novel hydrogen peroxide (H2O2) inactivation approach has been developed to produce a first-generation whole-virus vaccine against WNV [5,111]. Mice immunized with two 10 μg doses of H2O2-inactivated WNV formulated with aluminum hydroxide plus MPL demonstrated high serum neutralizing titers, with PRNT50 values reaching 1:14,400 against WNV-NY99, and vaccinated mice showed complete protection against lethal WNV challenge [111]. Using this same H2O2 inactivation platform, a single aluminum hydroxide-adjuvanted 10 μg dose of WNV vaccine (using H2O2-inactivated WNV-Kunjin) induced PRNT50 titers against WNV-Kunjin of 1:6,958 at 90 days post-immunization [5]. Following two immunizations, the H2O2-WNV vaccine demonstrated 90% protection in a robust intracranial challenge model involving 1,000,000 times the LD90 for WNV-NY99 [5]. In light of these promising preclinical results, Najít Technologies, Inc. has recently produced a clinical lot of H2O2-inactivated WNV vaccine and plans to initiate a Phase I clinical trial in 2014.
Vaccines in clinical development
DNA vectored vaccines
Since the introduction of WNV into the United States in 1999, significant research efforts have been expended to create a viable vaccine for disease prevention in humans. To date, there have been eight published clinical trials assessing safety and immunogenicity across multiple vaccine platforms (Table 3). One of the first candidates to reach the clinic was a single-plasmid, recombinant DNA vaccine, VRC-WNVDNA017-00-VP, encoding the WNV prM and Env [112]. The vaccine consisted of a closed, circular plasmid DNA vector incorporating a cytomegalovirus (CMV) promoter, with the WNV-NY99 prM and Env coding sequences expressed downstream from a modified JEV signal sequence. Vaccine material was provided by Vical (San Diego, CA), with the National Institute of Allergy and Infectious Diseases (NIAID) sponsoring the clinical trial. The trial was performed as an open-label Phase I study in healthy subjects aged 18-50 years old. The vaccine was administered at 4 mg per dose intramuscularly using a needle-free injection system on days 0, 28, and 56. Fifteen vaccinees were enrolled, with a total of 12 vaccinees completing the entire 3-dose regimen. The most common side effects were limited to local injection site reactions, with no reports of serious adverse events. All vaccinees that completed the full 3-dose regimen demonstrated seroconversion by serum ELISA titers but PRNT50 titers were variable and generally low. At week 12 (approximately 1 month following the final immunization) PRNT50 titers ranged from 1:16 - 1:128, with a group geometric mean of 1:50. By comparison, subjects infected with live WNV demonstrate a PRNT50 of about 1:1,400 at 1 year following infection [111]. An alternative WNV reporter-virus particle (RVP) neutralization assay was also utilized to assess immunogenicity, with RVP neutralization titers ranging from 1:100–1:1,000. The reason for the differences between the two assays is uncertain, though studies using WNV-specific monoclonal antibodies (MAb) indicate that the maturation state of the virus used in the RVP assay may play a role [113]. In other vaccine models, international serum standards have proven useful for bridging the results obtained from different research groups [114] and it may be worthwhile to standardize WNV neutralizing assays with a defined target virus (e.g., WNV-NY99), a highly characterized reference serum standard, and perhaps a standard approach to presenting the neutralizing titers (e.g., PRNT50, PRNT60 or PRNT90) to further aid in comparisons of immunogenicity within the field. Since WNV-NY99 represents a BSL3 infectious agent and it is not always feasible for organizations to accommodate this level of biosafety, another option might be to use WNV-Kunjin as a reference standard since it is a closely related Lineage 1 strain of WNV but can be handled under BSL2 containment. However, if WNV-Kunjin is to be used for these purposes, then a bridging study comparing neutralizing antibody responses of WNV-Kunjin to a virulent strain of WNV, such as WNV-NY99, may also be needed.
Table 3.
West Nile virus vaccine clinical trials
| Sponsor & Phase |
Vaccine Approach | Seroconversion1 | Neutralizing Titer (Range)2 |
Ref. |
|---|---|---|---|---|
| DNA-vectored vaccines | ||||
|
| ||||
| Vical/NIAID Phase I |
DNA plasmid expressing WNV prM/Env |
96.6-100% | 50 (16-128) | [112,115] |
|
| ||||
| Live chimeric/recombinant vaccines | ||||
|
| ||||
| Sanofi Phase I |
YFV-17D backbone expressing WNV prM/Env |
100% | 11,3923 | [70] |
| Sanofi Phase II |
YFV-17D backbone expressing WNV prM/Env |
95.4-97.3% | 3,309 (1,727-6,342) 3 | [121,122] |
| NIAID Phase I |
DV4 backbone expressing WNV prM/Env |
75-89% | 161 (8-1530) | [120] |
|
| ||||
| Recombinant, subunit vaccines | ||||
|
| ||||
| Hawaii Biotech Phase I |
Recombinant WNV Env protein |
100% | ~10-1004 | [301] |
Seroconversion definitions may vary between trials. From each clinical trial the group with the highest reported response is highlighted in the table.
Peak geometric mean titers (GMT) with range are given for neutralizing antibody responses as determined by PRNT, when available.
In an effort to improve immunogenicity, a modified DNA plasmid construct incorporating an additional regulatory element from the human T cell leukemia virus type 1 (HTLV-1), in conjunction with the previously used CMV promoter, was tested in a Phase I clinical trial [115]. The clinical protocol closely matched the previous study [112] in terms of vaccination dose and booster regimen, but included both a young (ages; 18-50) and older (ages; 51-65) cohort, with 15 subjects enrolled per group. As with the prior DNA construct, side effects were mild and generally limited to the site of injection. Neutralizing antibody titers against RVP indicated a trend toward higher antibody responses with the modified vector, although this was not statistically significant. At 12 weeks (~1 month following the final dose), RVP-based seroconversion was demonstrated in 28/29 (96.6%) subjects. However, PRNT50 serum titers against WNV were not directly assessed in this second clinical trial, limiting the ability to make comparisons to other clinical studies. Both DNA vaccine candidates are similar to a veterinary horse vaccine formerly produced by Wyeth’s Ft. Dodge Animal Health division (West Nile-Innovator DNA) that was licensed by the USDA in 2005. However, this veterinary vaccine has since been discontinued following Pfizer’s acquisition of Wyeth in 2009 [116]. Further development of the WNV DNA vaccine platform is unclear, with the most recent human clinical trial completed in 2007, and the results published in 2011 [115]. In general, DNA vaccines have suffered from concerns over immunogenicity and potential safety issues such as DNA integration into the genome [117]. However, in this instance the WNV DNA vaccine was able to induce a measurable WNV-specific immune response and was well-tolerated without any serious adverse events [118]. Improvements in DNA delivery technologies are continuing to occur [119] and these innovations, combined with this promising vaccine candidate, may offer a path towards further development of a successful WNV-specific DNA vaccine.
Live, attenuated chimeric/recombinant vaccines
To date, two live, attenuated chimeric flavivirus vaccine candidates have been tested in humans [70,120-122]. The first WNV chimeric vaccine to enter clinical trials, ChimeriVax-WN02, was originally developed by Acambis, with testing continued by Sanofi Pasteur following their acquisition of Acambis in 2008. In an initial Phase I study, subjects were immunized with either 103 (n=15) or 105 (n=30) PFU of ChimeriVax-WN02, as well as 5 control subjects who received the standard YFV-17D vaccine [70]. Viremia, as measured by the area under the curve (AUC), was significantly higher with the lower dose (312 vs. 173 PFU/mL per day), a trend that has since been observed in other chimeric flavivirus vaccines [120]. Peak antibody titers for both the 103 and 105 PFU dose groups were recorded at 21 days post-vaccination, reaching 1:11,392 and 1:6,241 respectively, with 100% seroconversion in both groups. These titers fell to 1:1,218 and 1:1,280 by day 28. At 12 months, 97% (35/36) of tested subjects remained seropositive, with antibody titers of ~1:600 in both dose groups. One caveat is that the experimental approach for determining PRNT50 titers was based on neutralizing homologous vaccine virus (ChimeriVax-WN) rather than a wild-type strain of WNV. Using a similar vaccine approach, others have indicated that differences in the target virus used in the neutralization assays may have a substantial impact on the outcome of PRNT titers when assessing clinical samples [120]. In addition, prior studies in NHP vaccinated with chimeric DENV or JEV constructs demonstrated that PRNT50 titers ranged from about 2- to 64-fold lower when using wild type strains of target virus as compared to the homologous chimeric vaccine construct [123,124]. It is unclear if the WNV-specific neutralizing titers described in the ChimeriVax-WN Phase I trial would likewise be reduced if a strain of WNV was used instead of ChimeriVax-WN vaccine virus in the PRNT assay, but this highlights the need for consensus in the WNV vaccine field in terms of how immunogenicity and neutralizing assays should be measured in clinical studies.
Following the Phase I results, two Phase II studies were performed using a plaque purified derivative of ChimeriVax-WN02, which had been developed in an effort to increase attenuation and reduce viremia in vaccinated subjects [121,122]. The first of these trials was divided into two parts, assessing safety and immunogenicity between adult (part 1, ages, 18-40) and older patient populations (part 2, ages, 41-64 or ≥65). In part 1, a total of 112 subjects were enrolled, receiving 3.7×103, 3.7×104 or 3.7×105 PFU of ChimeriVax-WN02, or placebo. In part 2, 96 subjects were enrolled and received only the 3.7×105 PFU dose or placebo. As with the Phase I trial, the vaccine was generally well tolerated across dosages and age groups. Peak viremia was reduced in comparison to the Phase I study, though lower doses of virus still resulted in higher levels of viremia. In part 2 of the study, increased viremia was shown to be associated with advanced age. AUC measurements demonstrated a value of 181 PFU/mL per day in those ≥65 years of age, compared to 115 PFU/mL per day in those aged 41-64 [125]. Although there were no severe adverse events observed in this small study, this result indicates that close monitoring of viremia should be continued in the future since the elderly represent the primary target population for a successful WNV vaccine. Seroconversion rates in both parts 1 and 2 reached >95% by day 28 regardless of dose or age group. Immunogenicity (as judged by neutralization against the homologous vaccine virus) increased with virus dose in part 1 and group PRNT50 titers reached 1:3,309 by day 28 after vaccination with the highest dose of virus (3.7×105 PFU). In part 2 (3.7×105 dose only, subjects aged ≥41 years), PRNT50 titers peaked at day 28, but only ranged between 1:883-1:965. It is unclear why there was a difference (~3.5-fold) in peak serum antibody titers between Part 1 and Part 2 of this clinical study, though part 2 was limited to older subjects. Subjects in part 2 were followed for up to 1 year, at which point neutralizing titers had declined to an average of 1:116. A second Phase II study focused on subjects ≥50 years of age and was performed to collect additional safety and immunogenicity data in this older demographic [122]. In this study, a total of 479 subjects were enrolled and received vaccine at 4×103, 4×104 or 4×105 PFU/dose, or placebo. At 28 days post-vaccination, seroconversion rates ranged from 92-95%, increasing slightly with each dose level. PRNT50 titers at day 28 were not statistically different between vaccine groups and averaged between 1:600-1:688, somewhat lower than that observed in elderly subjects in the prior trial [121]. Additional immunogenicity time points were not assessed. While peak viremia remained lower compared to the Phase I study (prior to further cell culture attenuation of the chimeric vaccine virus), AUC measurements were similar, ranging from 234-309 PFU/ml per day across all dose groups [125]. Why viremia levels increased in this clinical trial compared to the prior Phase II study is unclear, though the demographics of the study population were more narrowly focused on older individuals. Further development of this WNV vaccine is uncertain since this program was suspended by Sanofi Pasteur after acquisition of Acambis in 2008 [126].
Another chimeric flavivirus vaccine based on a DENV4 backbone vector expressing the WNV prM and Env proteins has also completed early-phase clinical testing [120] (Table 3). The vaccine, WN/DEN4Δ30, originated from the NIH Laboratory of Infectious Diseases and had previously been tested for safety and efficacy in preclinical animal studies [75,76]. The backbone was developed as a DENV4 vaccine candidate (rDEN4Δ30) attenuated through a 30-nucleotide deletion in the 3′ untranslated region (UTR) of the viral genome [127], which was then further engineered to express the WNV-NY99 prM and Env. Two cGMP lots were produced, with the second lot modified to contain additional non-coding point mutations in an effort to increase virus production from cell culture, though the amino acid sequence of the polyprotein did not differ between lots [128]. A total of 82 subjects, aged 18-50, were enrolled into the study, which was divided into three subcutaneous dose levels (103, 104 and 105 PFU/dose) with 20 vaccine recipients per dose, and a total of 22 placebo controls. For the low dose groups (103 and 104 PFU/dose) only a single dose was administered, whereas the 105 high dose group received a booster immunization at 6 months. The vaccine was found to be safe and well tolerated, with no statistically significant differences in injection site or systemic adverse events between vaccine and placebo groups. rDEN4Δ30 viremia was detected in 8 subjects, generally starting within 1-2 weeks post-vaccination, and lasting for 1-3 days. Interestingly, the lower doses of virus led to a higher percentage of viremic subjects (16-20%), while the 105 dose resulted in detectable viremia in only 5% of subjects. This continued the trend of an inverse relationship between virus dose and subsequent viremia levels previously established with the YFV-17D-based ChimeriVax platform [129]. Levels of viremia appeared to mirror seroconversion rates, with a range of 74-75% seroconversion in the two low dose groups (≥4-fold rise in serum PRNT60 at day 28 or 42), compared to only 55% seroconversion in the 105 high dose group by 42 days after vaccination (note; 2 more subjects also seroconverted by study day 180). Group geometric mean PRNT60 titers also varied with dose. At the 103 dose level, PRNT60 titers peaked at day 42 with an average of 1:161 (range; 1:8-1:1,530), while the 104 dose demonstrated a peak of 117 (range; 1:5-1:3,218) on day 28. By day 180, the 103 and 104 group titers had dropped to 76 (range; <1:5-1:290) and 35 (range; <1:5-1:232) respectively. The 105 high dose group peaked at day 28 with a group average PRNT60 of only 44 (range; 1:18-1:183), which dropped to 15 (range; <1:5-1:120) by day 180. Boosting of the 105 group on day 180 increased the rate of seroconversion to 89%, with a concomitant increase in average neutralizing antibody titers to 57 (range; 1:17-1:134). The design of clinical studies enrolling older subjects (>50 years of age) is underway [120], indicating that this vaccine will continue to be evaluated as a novel approach to WNV vaccination.
Recombinant, subunit vaccines
The only WNV subunit vaccine candidate that has been tested in clinical trials is WNV-80E, a recombinant form of the WNV-NY99 Env protein produced in Drosophila S2 cells. This vaccine was developed by Hawaii Biotech, and is based on a truncated WNV Env protein formulated with aluminum hydroxide. Preclinical studies in multiple species including mice, birds and NHP, demonstrated the induction of WNV-specific neutralizing responses after vaccination [130,131]. In a Phase I clinical trial, a total of 24 subjects were enrolled to assess immunogenicity and safety [301]. Subjects were divided into 4 groups, receiving 5, 15 or 50 g of WNV-80E adjuvanted with aluminum hydroxide, or 50 μg of vaccine without adjuvant. Immunizations were performed intramuscularly at weeks 0, 4 and 8 for a total of 3 inoculations per subject. The vaccine was well tolerated, with most side effects limited to injection site reactions. At 4 weeks following primary immunization, PRNT50 titers against WNV were negative (<1:10) in all groups. Following the second dose of vaccine, the aluminum hydroxide-adjuvanted 15 μg and 50 μg doses elicited average PRNT50 titers ranging between 1:10-1:100. After the third immunization, mean titers in these two groups increased but appeared to remain within the range of 1:10-1:100. The low dose group (5 μg) reached seropositive status (PRNT50 ≥1:10), but only after receiving a third dose of vaccine. The Phase I clinical trial of this WNV vaccine ended in 2009 but further clinical development appears to have stalled. While WNV-specific immunogenicity seems to be generally low, the use of advanced adjuvant systems (such as saponin derivatives [101]) may improve vaccine potency and are worth exploring in future studies.
Challenges for future clinical development and licensure
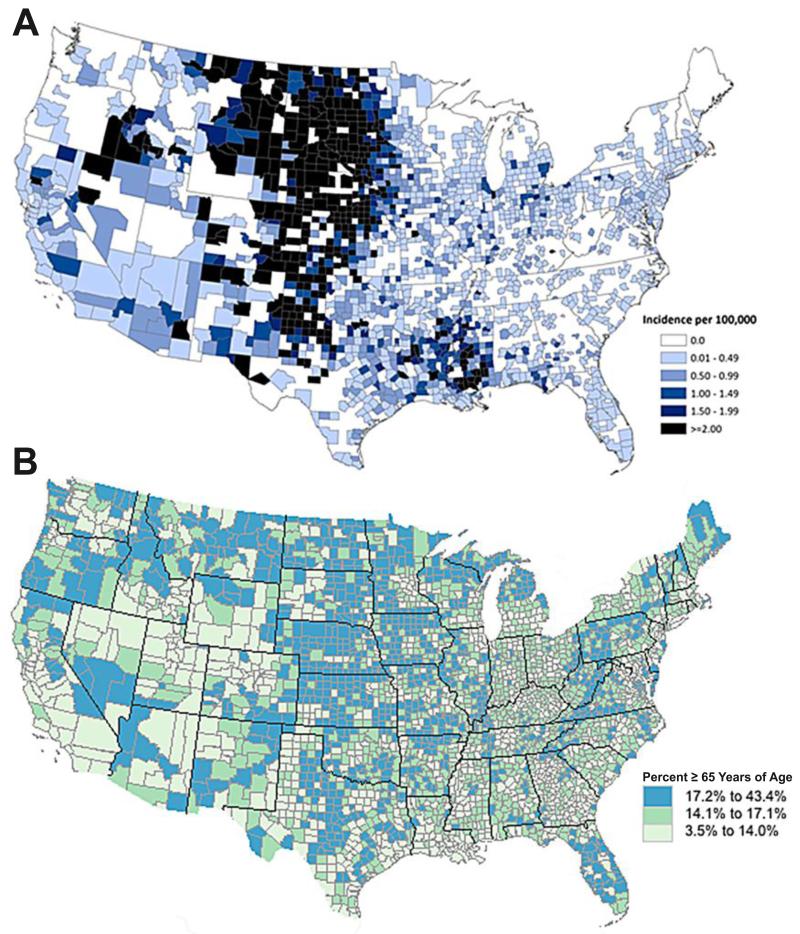
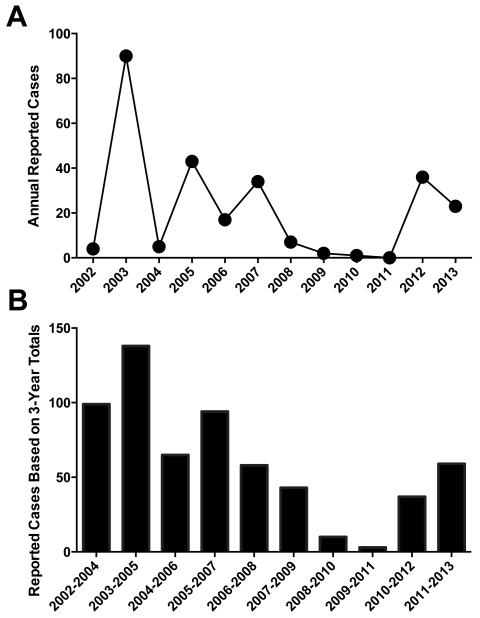
Before a vaccine can be licensed for commercial use, it must be shown to be both safe and effective at reducing the disease that it is designed to prevent. One of the biggest hurdles in reaching licensure for a human WNV vaccine is the limited feasibility to perform field efficacy trials. Although WNV is endemic throughout the continental United States, the relatively low incidence and sporadic nature of WNV outbreaks poses challenges for study design and implementation. However, with over a decade of detailed epidemiology available from active WNV surveillance programs, it may be possible to identify long-term trends in WNV outbreaks, and look for “hot spots” of more sustained or predictable WNV activity. While some periodicity of WNV incidence has been found in states such as California and Texas, the overall incidence tends to be low (<1 case per 100,000). In contrast, Midwestern states have relatively high incidence of WNV disease (Figure 2A) and an older population at risk for WNV infection (Figure 2B). For example, South Dakota has recorded an average incidence of ~20 cases of WNV per 100,000 since 2002, resulting in one of the highest rates in the nation [204]. When focusing locally at the county level, northeastern Brown County, SD demonstrated an annual incidence of ~60 cases per 100,000 during that same time frame, and a total number of 262 reported WNV cases despite a small population size of ~37,000 (Figure 3A). One complicating factor is that even in these endemic locations, WNV disease activity varies greatly from year to year. However, if a systematic effort is put forth to test the efficacy of an advanced WNV vaccine, then it may be possible to vaccinate an at-risk population and monitor WNV disease activity over the course of 2-3 seasons or until enough WNV cases have accumulated to provide statistical significance between vaccine and placebo groups. Using Brown County, SD as an example, if the cumulative number of reported WNV cases is calculated for each overlapping 3-year period from 2002-2013, then for 8/10 (80%) of these blocks of time there were between 37 to 138 reported cases of WNV (Figure 3B). By performing an efficacy trial at more than one location with historically high WNV incidence, the risk of failing to identify a statistically significant decrease in disease incidence may be further mitigated. Albeit logistically challenging, this long-term approach to disease monitoring during a WNV Phase III field trial may provide the feasibility necessary to determine vaccine efficacy and move an effective vaccine closer to licensure.
Figure 2. Maps of West Nile virus neuroinvasive disease incidence and age distribution in the United States.
(A) The average annual incidence of human West Nile virus neuroinvasive disease in the United States, 1999-2012, provided by the national ArboNET surveillance system conducted by the Centers for Disease Control and Prevention [201]. (B) Distribution of Americans aged 65 years and older based on the 2010 Census.
Figure 3. High WNV disease burden in localized regions of the United States.
The annual number of reported cases for WNV (A) or the total number of cases for each overlapping 3-year period (B) are shown for Brown County (population size ~37,000), South Dakota from 2002-2013 [204]. Reported WNV cases declined during 2008-2011, but a substantial increase was observed in 2012 and 2013, indicating that relatively high incident, recurrent WNV may be likely to continue in certain at-risk geographic areas.
As an alternative to field efficacy trials, some have suggested that the FDA Animal Rule may provide a route for licensure [125]. The Animal Rule was put forward to allow FDA evaluation of drug effectiveness based on evidence from appropriate animal studies in instances where human efficacy studies are considered unethical or unfeasible (21 CFR 601 Subpart H, 21 CFR 314 Subpart I for New Drugs). However, the Animal Rule appears to have been primarily implemented in the context of bioterrorism threats wherein no other practical alternative exists [205]. Since initial publication of the Animal Rule in 2002, two drug products for the treatment of nerve gas poisoning have been approved following this mechanism, but no vaccines have advanced to licensure [205]. As noted by the FDA, the Animal Rule does not provide a short-cut to licensure, and may actually take much longer than standard clinical testing [132]. As another method to supporting innovations in medicine, the FDA has recently developed its Advancing Regulatory Science initiative, which has been suggested as an avenue for WNV vaccine development [125], though how this would work in practice remains to be seen.
Will a vaccine against West Nile virus be cost-effective?
Outbreaks of WNV cause considerable morbidity, mortality and disease-associated economic loss. Although the economic impact of a successful vaccination program should not be the sole determinant involved in making public health decisions, it is nevertheless an important parameter in determining overall feasibility. If the financial cost to a society is reduced through decreased disease burden, there will be more support for implementing a particular vaccine policy rather than when there is little or no cost advantage. In 2002, there were 4,156 reported cases of WNV in the U.S. (Figure 1), with 329 cases identified in Louisiana [133]. The estimated cost associated with these 329 cases was $20.1M (i.e., $61,094/case) [133] and if applied to all 4,156 cases, this would suggest a cost of $254M in 2002 dollars. Albeit optimistic, if we assume that the earliest date that a vaccine could be commercially available is in 5 years (e.g., 2019), then a similarly sized outbreak in 2019 would have an estimated economic impact of $356M after adjusting 2% per year to 2019 dollars. Even though the outbreaks in 2006 and 2012 were larger then that encountered in 2002 (Figure 1), over the past 10 years (2003-2012), the average annual number of reported cases of WNV in the U.S. is 3,278. Based on this average number of annual WNV cases, the total financial impact of WNV in 2019 would be estimated at $281M.
In contrast to these rough estimates of cost, a formal analysis of the cost-effectiveness of WNV vaccination was performed in 2006 [31] and this study is frequently used as the basis for estimating the costs associated with more recent WNV outbreaks [134,135]. Using simulations and sensitivity analysis, the health care costs per case of WNV were estimated at $36,000 (range; $20,000 to $59,000/case) in 2004 dollars [31]. If adjusted to 2019 dollars ($48,451/case) and an estimated incidence of WNV at 3,278 cases/year, then the direct societal cost of WNV outbreaks would be approximately $159M/year. The Zohrabian et al. study [31] concluded that universal vaccination against WNV would be unlikely to result in societal monetary savings and this has led many to believe that development of a WNV vaccine will not be feasible. However, this model was based on several assumptions including the implementation of mass vaccination of 100 million people with a vaccine regimen costing $100/person. Results from their sensitivity analysis indicated that the probability that a WNV vaccine would provide societal cost savings changed from 0% to 76% as the cost of vaccination decreased from $150 to $10. Since it may be challenging for a new vaccine to have an appreciable profit margin when initially launched at a price of $10/vaccinee, commercial enthusiasm for development of a safe and effective WNV vaccine has subsequently waned.
One key parameter for determining economic cost is loss of productivity due to death or short-term and long-term disability [31]. However, since WNV disease disproportionally afflicts the aged population, there is consequently a lower base productivity profile and decreased lifespan potential. Together, this indicates that diseases that target aged and/or low-income populations will simply not have the same financial impact that is associated with diseases of younger people or individuals from high-income communities based on mathematical modeling estimates alone. The lack of a more humanitarian component to cost-effectiveness projections is a challenging issue, especially in cases in which cost-effectiveness is borderline or not cost-effective despite providing a mechanism to reduce clinical disease burden [136]. Another key point with the Zohrabian study [31] is that it is based only on direct WNV-related health care costs and does not take into account the costs of WNV surveillance, vector control, and outbreak prevention and response costs, which can be large [133,135]. Others have also argued that cost-benefit analyses should include the more “intangible” value of a successful WNV vaccine program by taking into account the broader economic costs associated with the impact of WNV outbreaks on travel, tourism, and local economic growth [137]. Further studies that incorporate these factors will be important for gauging the full economic impact of WNV outbreaks in the U.S. and abroad.
Instead of implementing mass vaccination, a more feasible and cost-effective approach to preventing WNV outbreaks in the U.S. might be to perform targeted vaccination of the populations at greatest risk for WNV disease (Figure 2). For instance, although it may be unlikely for a vaccine manufacturer to develop a new WNV vaccine for 100 million people at an initial price of $10/each [31], there is higher potential for commercialization of a vaccine developed to provide targeted immunization to 10 million people at a price of $50-100/each and this would still provide a favorable cost-benefit ratio. A targeted vaccine campaign could be designed based on vaccinating specific regions with the highest WNV disease incidence or the highest total number of reported WNV cases. Alternatively, the targeted vaccine campaign could be based on age, with the elderly representing the most at-risk population. WNV activity has been reported in all 48 contiguous states but the risk of contracting WNND varies substantially both between states and even within each state when monitored at the county level (Figure 2A). Interestingly, the Midwestern states and individual counties with the highest incidence of WNND are also enriched for an older population of people ≥65 years of age (Figure 2B), representing the group that bears the most severe short-term and long-term WNV disease manifestations and WNV-associated mortality. The low population density in the regions most greatly impacted by WNV provides further support for the approach of a targeted regional vaccine program instead of implementing mass vaccination of the nation at large, which will invariably include large populations at low historical risk for WNV disease. There are 8 states (CO, LA, MI, MT, NE, ND, SD, and WY) with an incidence of WNND of >1/100,000 (annual incidence from 1999-2012; Figure 2A). Based on 2012 Census estimates (Source: www.census.gov), there are approximately 24.6 million people living in these states, with a subpopulation of 2.3 million people over the age of 65 who could be the focus of targeted vaccination. Alternatively, instead of basing a targeted vaccine campaign only on states or counties with the highest disease incidence, one could focus vaccine programs in states/counties with the highest total number of reported WNV cases. In this case, there are 8 states (AZ, CA, CO, IL, LA, MI, OH, and TX) that have had between 604 and 2,357 reported cases of WNND from 1999-2012 and account for more than half of all reported WNV neurotropic disease in the U.S. [201]. The total population of these states is 115 million, making mass vaccination a potentially challenging prospect from a cost-effectiveness perspective. On the other hand, if focused only on the aged population, then a nation-wide vaccine plan could be implemented. In 2012, approximately 40.5 million people in the U.S. were ≥65 years of age and with a targeted vaccine developed for 10 million aged individuals, it would be possible to vaccinate one-fourth of the people in this age group who are at the highest risk for exposure and complications from WNV infection. Alternatively, in 2012 there were approximately 102 million people at ≥50 years of age and another approach would be to vaccinate 10% of this age group who are at greatest risk for WNV infection. By using a targeted vaccine program aimed at the most at-risk populations across the nation at either the state or county level, a safe and effective WNV vaccine could sharply reduce disease burden and mortality while still providing substantial societal cost savings.
Expert commentary
West Nile Virus is an emerging/reemerging pathogen that has become endemic in the continental United States, and appears to be on the rise in Southern Europe and neighboring regions. While several promising WNV vaccines have been evaluated in clinical trials over the last decade, a licensed human vaccine remains elusive. One concern for future vaccine development is the feasibility of performing Phase III efficacy trials for a zoonotic disease like WNV that is known for sporadic outbreaks that are often difficult to predict. However, with detailed WNV epidemiologic data from across the US, it may be possible to identify locations for potential vaccine trials, especially in Midwestern regions with relatively low population density but high WNV disease incidence. Although universal WNV vaccination is unlikely to be cost-effective, further studies are needed to determine if targeted vaccine campaigns focused on at-risk age groups or geographical regions will provide a favorable cost:benefit ratio, especially in light of recent evidence indicating that almost 3 million Americans have likely been infected with WNV and most cases continue to go unreported. The recognition of much larger WNV incidence, coupled with increasing evidence for long-term disability and decreased quality of life among WNV survivors, further indicates a compelling need for a safe and effective WNV vaccine.
Five-year view
The next 5 years may be pivotal in terms of the continued successful development of a safe and effective vaccine against West Nile virus. Several vaccine products are in various stages of clinical development and new vaccine technologies are still entering the pipeline. A targeted vaccine program has the potential to be cost-effective and there is renewed interest in WNV vaccine technology, especially since the outbreak of 2012 represented not only the largest outbreak since 2003, but also resulted in the most WNV-associated deaths on record. Despite the continued dedication to WNV-related research spanning the last 15 years, several key challenges remain. For instance, a WNV vaccine will likely represent a “niche market” compared to other blockbuster vaccine products and steps may be needed to incentivize industry leaders to move forward with further clinical development. Another important challenge to these efforts will be to identify a feasible path forward for conducting Phase III efficacy trials or implementing the FDAs “Animal Rule” to demonstrate efficacy when human efficacy studies are not ethical or feasible [132]. Due to the sporadic nature of WNV outbreaks and many mitigating factors (e.g., bird density/diversity, urban/agricultural landscape, temperature, rainfall, human population density/socioeconomics) [33,138], further advances in WNV epidemiology and outbreak prediction may be necessary in order to quickly implement local vaccine trials in areas either undergoing an early-stage WNV outbreak or those with a high likelihood of an upcoming WNV outbreak. Through the combined and collaborative efforts of WNV epidemiologists, virologists, vaccine manufacturers, state and county health officials, and regulatory agencies, it is possible that a safe and effective vaccine against West Nile virus can become a reality and provide protection to the vulnerable populations within our communities that need it most.
Key issues.
Based on more than a decade of surveillance in the United States, it is expected that WNV will continue to threaten vulnerable populations for the foreseeable future.
The severity of WNV disease is associated with advanced age and often results in long-term health issues including potentially severe neurological sequelae and a higher mortality rate after recovery from acute infection. More studies are needed to determine if WNV infection is linked to chronic kidney disease.
Several early-stage vaccine clinical trials have been completed, but none have advanced to licensure.
Reference standards for performing WNV-specific neutralization assays, including highly characterized serum standards and reference strains of WNV, should be considered.
Due to the sporadic nature of WNV outbreaks, concerns remain regarding the feasibility of Phase III vaccine field efficacy trials. However, this may be mitigated, at least in part, by maintaining intense surveillance efforts, performing trials in locations of high/continued WNV incidence, and monitoring for vaccine efficacy over a prolonged period of time (possibly 1 to 3 years).
A formal cost-benefit analysis of targeted WNV vaccination should be performed, preferably including not only direct health care costs but also costs associated with WNV surveillance, prevention, and outbreak response.
Acknowledgements
We thank Andrew Townsend for excellent graphical design and assistance. This project was funded in part with federal funds from the National Institute of Allergy and Infectious Diseases, U01 AI082196 (to MKS), R44 AI079898 (to MKS and IJA), R01 AI098723 (to MKS) and Oregon National Primate Research Center grant, 8P51 OD011092-53 (to MKS).
Footnotes
Financial & competing interests disclosure
OHSU, Dr. Slifka, and Dr. Amanna have a financial interest in Najít Technologies, Inc., a company that is developing a new West Nile virus vaccine based on a hydrogen peroxide-based inactivation approach. This potential individual and institutional conflict of interest has been reviewed and managed by OHSU. No writing assistance was utilized in the production of this manuscript.
References
- 1.Go YY, Balasuriya UB, Lee CK. Zoonotic encephalitides caused by arboviruses: transmission and epidemiology of alphaviruses and flaviviruses. Clin Exp Vaccine Res. 2014;3(1):58–77. doi: 10.7774/cevr.2014.3.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roehrig JT. West nile virus in the United States - a historical perspective. Viruses. 2013;5(12):3088–3108. doi: 10.3390/v5123088. • •This review provides a candid, first-hand account and historical perspective on the actions taken to characterize and control WNV after introduction to the United States in 1999
- 3.Kramer LD, Styer LM, Ebel GD. A global perspective on the epidemiology of West Nile virus. Annu Rev Entomol. 2008;53:61–81. doi: 10.1146/annurev.ento.53.103106.093258. [DOI] [PubMed] [Google Scholar]
- 4.Lanciotti RS, Roehrig JT, Deubel V, et al. Origin of the West Nile virus responsible for an outbreak of encephalitis in the northeastern United States. Science. 1999;286(5448):2333–2337. doi: 10.1126/science.286.5448.2333. • Performed full length genomic sequencing of WNV-NY99 and discovered that it was most closely related to an Israeli strain identified in 1998 that also caused avian fatalities
- 5.Pinto AK, Richner JM, Poore EA, et al. A Hydrogen Peroxide-Inactivated Virus Vaccine Elicits Humoral and Cellular Immunity and Protects against Lethal West Nile Virus Infection in Aged Mice. J Virol. 2013;87(4):1926–1936. doi: 10.1128/JVI.02903-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sitati EM, Diamond MS. CD4+ T-cell responses are required for clearance of West Nile virus from the central nervous system. J Virol. 2006;80(24):12060–12069. doi: 10.1128/JVI.01650-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shrestha B, Ng T, Chu HJ, Noll M, Diamond MS. The relative contribution of antibody and CD8(+) T cells to vaccine immunity against West Nile encephalitis virus. Vaccine. 2008;26(16):2020–2033. doi: 10.1016/j.vaccine.2008.02.009. • Provides insight into the supplementary role of vaccine-induced WNV-specific CD8+ T cells; CD8+ T cells contribute to the antiviral response when neutralizing antibody levels are limited, but are dispensible if antibody levels achieve a protective threshold
- 8.Netland J, Bevan MJ. CD8 and CD4 T cells in west nile virus immunity and pathogenesis. Viruses. 2013;5(10):2573–2584. doi: 10.3390/v5102573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brien JD, Uhrlaub JL, Nikolich-Zugich J. Protective capacity and epitope specificity of CD8(+) T cells responding to lethal West Nile virus infection. Eur J Immunol. 2007;37(7):1855–1863. doi: 10.1002/eji.200737196. [DOI] [PubMed] [Google Scholar]
- 10.Amanna IJ, Slifka MK. Wanted, dead or alive: new viral vaccines. Antiviral Res. 2009;84(2):119–130. doi: 10.1016/j.antiviral.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ben-Nathan D, Lustig S, Tam G, Robinzon S, Segal S, Rager-Zisman B. Prophylactic and therapeutic efficacy of human intravenous immunoglobulin in treating West Nile virus infection in mice. J Infect Dis. 2003;188(1):5–12. doi: 10.1086/376870. [DOI] [PubMed] [Google Scholar]
- 12.Oliphant T, Engle M, Nybakken GE, et al. Development of a humanized monoclonal antibody with therapeutic potential against West Nile virus. Nat Med. 2005;11(5):522–530. doi: 10.1038/nm1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Engle MJ, Diamond MS. Antibody prophylaxis and therapy against West Nile virus infection in wild-type and immunodeficient mice. J Virol. 2003;77(24):12941–12949. doi: 10.1128/JVI.77.24.12941-12949.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kreil TR, Eibl MM. Pre- and postexposure protection by passive immunoglobulin but no enhancement of infection with a flavivirus in a mouse model. J Virol. 1997;71(4):2921–2927. doi: 10.1128/jvi.71.4.2921-2927.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thibodeaux BA, Garbino NC, Liss NM, et al. A humanized IgG but not IgM antibody is effective in prophylaxis and therapy of yellow fever infection in an AG129/17D-204 peripheral challenge mouse model. Antiviral Res. 2012;94(1):1–8. doi: 10.1016/j.antiviral.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mason RA, Tauraso NM, Spertzel RO, Ginn RK. Yellow fever vaccine: direct challenge of monkeys given graded doses of 17D vaccine. Appl Microbiol. 1973;25(4):539–544. doi: 10.1128/am.25.4.539-544.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Belmusto-Worn VE, Sanchez JL, McCarthy K, et al. Randomized, double-blind, phase III, pivotal field trial of the comparative immunogenicity, safety, and tolerability of two yellow fever 17D vaccines (Arilvax and YF-VAX) in healthy infants and children in Peru. Am J Trop Med Hyg. 2005;72(2):189–197. [PubMed] [Google Scholar]
- 18.Monath TP, Nichols R, Archambault WT, et al. Comparative safety and immunogenicity of two yellow fever 17D vaccines (ARILVAX and YF-VAX) in a phase III multicenter, double-blind clinical trial. Am J Trop Med Hyg. 2002;66(5):533–541. doi: 10.4269/ajtmh.2002.66.533. [DOI] [PubMed] [Google Scholar]
- 19.Hombach J, Solomon T, Kurane I, Jacobson J, Wood D. Report on a WHO consultation on immunological endpoints for evaluation of new Japanese encephalitis vaccines, WHO, Geneva, 2-3 September, 2004. Vaccine. 2005;23(45):5205–5211. doi: 10.1016/j.vaccine.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 20.Orlinger KK, Hofmeister Y, Fritz R, et al. A tick-borne encephalitis virus vaccine based on the European prototype strain induces broadly reactive cross-neutralizing antibodies in humans. J Infect Dis. 2011;203(11):1556–1564. doi: 10.1093/infdis/jir122. [DOI] [PubMed] [Google Scholar]
- 21.Smithburn KC, Hughes TP, Burke AW, Paul JH. A neurotropic virus isolated from the blood of a native of Uganda. Am J Trop Med Hyg. 1940;20:471–492. [Google Scholar]
- 22.Hayes CG. West Nile virus: Uganda, 1937, to New York City, 1999. Ann N Y Acad Sci. 2001;951:25–37. doi: 10.1111/j.1749-6632.2001.tb02682.x. [DOI] [PubMed] [Google Scholar]
- 23.Gubler DJ. The continuing spread of West Nile virus in the western hemisphere. Clin Infect Dis. 2007;45(8):1039–1046. doi: 10.1086/521911. [DOI] [PubMed] [Google Scholar]
- 24.Giladi M, Metzkor-Cotter E, Martin DA, et al. West Nile encephalitis in Israel, 1999: the New York connection. Emerg Infect Dis. 2001;7(4):659–661. doi: 10.3201/eid0704.010410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mackenzie JS, Williams DT. The zoonotic flaviviruses of southern, south-eastern and eastern Asia, and Australasia: the potential for emergent viruses. Zoonoses Public Health. 2009;56(6-7):338–356. doi: 10.1111/j.1863-2378.2008.01208.x. [DOI] [PubMed] [Google Scholar]
- 26.Papa A, Politis C, Tsoukala A, et al. West Nile virus lineage 2 from blood donor, Greece. Emerg Infect Dis. 2012;18(4):688–689. doi: 10.3201/eid1804.110771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.May FJ, Davis CT, Tesh RB, Barrett AD. Phylogeography of West Nile virus: from the cradle of evolution in Africa to Eurasia, Australia, and the Americas. J Virol. 2011;85(6):2964–2974. doi: 10.1128/JVI.01963-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prow NA. The changing epidemiology of kunjin virus in australia. Int J Environ Res Public Health. 2013;10(12):6255–6272. doi: 10.3390/ijerph10126255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carson PJ, Borchardt SM, Custer B, et al. Neuroinvasive disease and West Nile virus infection, North Dakota, USA, 1999-2008. Emerg Infect Dis. 2012;18(4):684–686. doi: 10.3201/eid1804.111313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Petersen LR, Carson PJ, Biggerstaff BJ, Custer B, Borchardt SM, Busch MP. Estimated cumulative incidence of West Nile virus infection in US adults, 1999-2010. Epidemiol Infect. 2013;141(3):591–595. doi: 10.1017/S0950268812001070. • •Estimates a remarkably high incidence of WNV disease in the United States, revealing that WNV infection is commonly underreported
- 31.Zohrabian A, Hayes EB, Petersen LR. Cost-effectiveness of West Nile virus vaccination. Emerg Infect Dis. 2006;12(3):375–380. doi: 10.3201/eid1203.050782. • Provides a formal cost-benefit analysis of universal WNV vaccination based on directly related health care costs associated with WNV morbidity and mortality
- 32.Zou S, Foster GA, Dodd RY, Petersen LR, Stramer SL. West Nile fever characteristics among viremic persons identified through blood donor screening. J Infect Dis. 2010;202(9):1354–1361. doi: 10.1086/656602. [DOI] [PubMed] [Google Scholar]
- 33.Petersen LR, Carson PJ, Biggerstaff BJ, Custer B, Borchardt SM, Busch MP. Estimated cumulative incidence of West Nile virus infection in US adults, 1999-2010. Epidemiol Infect. 2013;141:591–595. doi: 10.1017/S0950268812001070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mostashari F, Bunning ML, Kitsutani PT, et al. Epidemic West Nile encephalitis, New York, 1999: results of a household-based seroepidemiological survey. Lancet. 2001;358(9278):261–264. doi: 10.1016/S0140-6736(01)05480-0. [DOI] [PubMed] [Google Scholar]
- 35.Hayes EB, Sejvar JJ, Zaki SR, Lanciotti RS, Bode AV, Campbell GL. Virology, pathology, and clinical manifestations of West Nile virus disease. Emerg Infect Dis. 2005;11(8):1174–1179. doi: 10.3201/eid1108.050289b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Watson JT, Pertel PE, Jones RC, et al. Clinical characteristics and functional outcomes of West Nile Fever. Ann Intern Med. 2004;141(5):360–365. doi: 10.7326/0003-4819-141-5-200409070-00010. [DOI] [PubMed] [Google Scholar]
- 37.Barzon L, Pacenti M, Franchin E, et al. Clinical and virological findings in the ongoing outbreak of West Nile virus Livenza strain in northern Italy, July to September 2012. Euro Surveill. 2012;17(36):20260. [PubMed] [Google Scholar]
- 38.Hayes EB, Gubler DJ. West Nile virus: epidemiology and clinical features of an emerging epidemic in the United States. Annu Rev Med. 2006;57:181–194. doi: 10.1146/annurev.med.57.121304.131418. [DOI] [PubMed] [Google Scholar]
- 39.Sejvar JJ, Haddad MB, Tierney BC, et al. Neurologic manifestations and outcome of West Nile virus infection. JAMA. 2003;290(4):511–515. doi: 10.1001/jama.290.4.511. [DOI] [PubMed] [Google Scholar]
- 40.Sejvar JJ, Leis AA, Stokic DS, et al. Acute flaccid paralysis and West Nile virus infection. Emerg Infect Dis. 2003;9(7):788–793. doi: 10.3201/eid0907.030129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jeha LE, Sila CA, Lederman RJ, Prayson RA, Isada CM, Gordon SM. West Nile virus infection: a new acute paralytic illness. Neurology. 2003;61(1):55–59. doi: 10.1212/01.wnl.0000073617.08185.0a. [DOI] [PubMed] [Google Scholar]
- 42.Li J, Loeb JA, Shy ME, et al. Asymmetric flaccid paralysis: a neuromuscular presentation of West Nile virus infection. Ann Neurol. 2003;53(6):703–710. doi: 10.1002/ana.10575. [DOI] [PubMed] [Google Scholar]
- 43.Sejvar JJ. The long-term outcomes of human West Nile virus infection. Clin Infect Dis. 2007;44(12):1617–1624. doi: 10.1086/518281. [DOI] [PubMed] [Google Scholar]
- 44.Sejvar JJ, Bode AV, Marfin AA, et al. West Nile virus-associated flaccid paralysis. Emerg Infect Dis. 2005;11(7):1021–1027. doi: 10.3201/eid1107.040991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lindsey NP, Staples JE, Lehman JA, Fischer M. Surveillance for human West Nile virus disease - United States, 1999-2008. MMWR Surveill Summ. 2010;59(2):1–17. [PubMed] [Google Scholar]
- 46.Weber IB, Lindsey NP, Bunko-Patterson AM, et al. Completeness of West Nile virus testing in patients with meningitis and encephalitis during an outbreak in Arizona, USA. Epidemiol Infect. 2012;140(9):1632–1636. doi: 10.1017/S0950268811002494. [DOI] [PubMed] [Google Scholar]
- 47.Emig M, Apple DJ. Severe West Nile virus disease in healthy adults. Clin Infect Dis. 2004;38(2):289–292. doi: 10.1086/380458. [DOI] [PubMed] [Google Scholar]
- 48.Pepperell C, Rau N, Krajden S, et al. West Nile virus infection in 2002: morbidity and mortality among patients admitted to hospital in southcentral Ontario. CMAJ. 2003;168(11):1399–1405. [PMC free article] [PubMed] [Google Scholar]
- 49.Voelker R. Effects of West nile virus may persist. JAMA. 2008;299(18):2135–2136. doi: 10.1001/jama.299.18.2135. [DOI] [PubMed] [Google Scholar]
- 50.Green MS, Weinberger M, Ben-Ezer J, et al. Long-term Death Rates, West Nile virus epidemic, Israel, 2000. Emerg Infect Dis. 2005;11(11):1754–1757. doi: 10.3201/eid1111.040941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Barzon L, Pacenti M, Palu G. West Nile virus and kidney disease. Expert Rev Anti Infect Ther. 2013;11(5):479–487. doi: 10.1586/eri.13.34. • An extensive review of WNV infection of the kidney and the potential risk for acute and chronic kidney disease
- 52.Tesh RB, Siirin M, Guzman H, et al. Persistent West Nile virus infection in the golden hamster: studies on its mechanism and possible implications for other flavivirus infections. J Infect Dis. 2005;192(2):287–295. doi: 10.1086/431153. [DOI] [PubMed] [Google Scholar]
- 53.Tonry JH, Xiao SY, Siirin M, Chen H, da Rosa AP, Tesh RB. Persistent shedding of West Nile virus in urine of experimentally infected hamsters. Am J Trop Med Hyg. 2005;72(3):320–324. [PubMed] [Google Scholar]
- 54.Saxena V, Xie G, Li B, et al. A hamster-derived West Nile virus isolate induces persistent renal infection in mice. PLoS Negl Trop Dis. 2013;7(6):e2275. doi: 10.1371/journal.pntd.0002275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Barzon L, Pacenti M, Franchin E, et al. Excretion of West Nile virus in urine during acute infection. J Infect Dis. 2013;208(7):1086–1092. doi: 10.1093/infdis/jit290. [DOI] [PubMed] [Google Scholar]
- 56.Murray K, Walker C, Herrington E, et al. Persistent infection with West Nile virus years after initial infection. J Infect Dis. 2010;201(1):2–4. doi: 10.1086/648731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gibney KB, Lanciotti RS, Sejvar JJ, et al. West nile virus RNA not detected in urine of 40 people tested 6 years after acute West Nile virus disease. J Infect Dis. 2011;203(3):344–347. doi: 10.1093/infdis/jiq057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brener ZZ, Harbord NB, Zhuravenko I, et al. Acute renal failure in a patient with West Nile viral encephalitis. Nephrol Dial Transplant. 2007;22(2):662–663. doi: 10.1093/ndt/gfl599. [DOI] [PubMed] [Google Scholar]
- 59.Huang C, Slater B, Rudd R, et al. First Isolation of West Nile virus from a patient with encephalitis in the United States. Emerg Infect Dis. 2002;8(12):1367–1371. doi: 10.3201/eid0812.020532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nolan MS, Podoll AS, Hause AM, Akers KM, Finkel KW, Murray KO. Prevalence of Chronic Kidney Disease and Progression of Disease Over Time among Patients Enrolled in the Houston West Nile Virus Cohort. PLoS One. 2012;7(7):e40374. doi: 10.1371/journal.pone.0040374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lindsey NP, Sejvar JJ, Bode AV, Pape WJ, Campbell GL. Delayed mortality in a cohort of persons hospitalized with West Nile virus disease in Colorado in 2003. Vector Borne Zoonotic Dis. 2012;12(3):230–235. doi: 10.1089/vbz.2011.0721. [DOI] [PubMed] [Google Scholar]
- 62.Li L, Saade F, Petrovsky N. The future of human DNA vaccines. J Biotechnol. 2012;162(2-3):171–182. doi: 10.1016/j.jbiotec.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Davis BS, Chang GJ, Cropp B, et al. West Nile virus recombinant DNA vaccine protects mouse and horse from virus challenge and expresses in vitro a noninfectious recombinant antigen that can be used in enzyme-linked immunosorbent assays. J Virol. 2001;75(9):4040–4047. doi: 10.1128/JVI.75.9.4040-4047.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ishikawa T, Takasaki T, Kurane I, Nukuzuma S, Kondo T, Konishi E. Co-immunization with West Nile DNA and inactivated vaccines provides synergistic increases in their immunogenicities in mice. Microbes Infect. 2007;9(9):1089–1095. doi: 10.1016/j.micinf.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 65.Anwar A, Chandrasekaran A, Ng ML, Marques E, August JT. West Nile premembrane-envelope genetic vaccine encoded as a chimera containing the transmembrane and cytoplasmic domains of a lysosome-associated membrane protein: increased cellular concentration of the transgene product, targeting to the MHC II compartment, and enhanced neutralizing antibody response. Virology. 2005;332(1):66–77. doi: 10.1016/j.virol.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 66.Ramanathan MP, Kutzler MA, Kuo YC, et al. Coimmunization with an optimized IL15 plasmid adjuvant enhances humoral immunity via stimulating B cells induced by genetically engineered DNA vaccines expressing consensus JEV and WNV E DIII. Vaccine. 2009;27(32):4370–4380. doi: 10.1016/j.vaccine.2009.01.137. [DOI] [PubMed] [Google Scholar]
- 67.Seregin A, Nistler R, Borisevich V, et al. Immunogenicity of West Nile virus infectious DNA and its noninfectious derivatives. Virology. 2006;356(1-2):115–125. doi: 10.1016/j.virol.2006.07.038. [DOI] [PubMed] [Google Scholar]
- 68.Chang DC, Liu WJ, Anraku I, et al. Single-round infectious particles enhance immunogenicity of a DNA vaccine against West Nile virus. Nat Biotechnol. 2008;26(5):571–577. doi: 10.1038/nbt1400. [DOI] [PubMed] [Google Scholar]
- 69.Hall RA, Nisbet DJ, Pham KB, Pyke AT, Smith GA, Khromykh AA. DNA vaccine coding for the full-length infectious Kunjin virus RNA protects mice against the New York strain of West Nile virus. Proc Natl Acad Sci U S A. 2003;100(18):10460–10464. doi: 10.1073/pnas.1834270100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Monath TP, Liu J, Kanesa-Thasan N, et al. A live, attenuated recombinant West Nile virus vaccine. Proc Natl Acad Sci U S A. 2006;103(17):6694–6699. doi: 10.1073/pnas.0601932103. • The first clinical trial of a WNV vaccine in humans
- 71.Arroyo J, Miller C, Catalan J, et al. ChimeriVax-West Nile virus live-attenuated vaccine: preclinical evaluation of safety, immunogenicity, and efficacy. J Virol. 2004;78(22):12497–12507. doi: 10.1128/JVI.78.22.12497-12507.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tesh RB, Arroyo J, Travassos Da Rosa AP, Guzman H, Xiao SY, Monath TP. Efficacy of killed virus vaccine, live attenuated chimeric virus vaccine, and passive immunization for prevention of West Nile virus encephalitis in hamster model. Emerg Infect Dis. 2002;8(12):1392–1397. doi: 10.3201/eid0812.020229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Guy B, Guirakhoo F, Barban V, Higgs S, Monath TP, Lang J. Preclinical and clinical development of YFV 17D-based chimeric vaccines against dengue, West Nile and Japanese encephalitis viruses. Vaccine. 2010;28(3):632–649. doi: 10.1016/j.vaccine.2009.09.098. [DOI] [PubMed] [Google Scholar]
- 74.Long MT, Gibbs EP, Mellencamp MW, et al. Efficacy, duration, and onset of immunogenicity of a West Nile virus vaccine, live Flavivirus chimera, in horses with a clinical disease challenge model. Equine Vet J. 2007;39(6):491–497. doi: 10.2746/042516407X217416. [DOI] [PubMed] [Google Scholar]
- 75.Pletnev AG, Claire MS, Elkins R, Speicher J, Murphy BR, Chanock RM. Molecularly engineered live-attenuated chimeric West Nile/dengue virus vaccines protect rhesus monkeys from West Nile virus. Virology. 2003;314(1):190–195. doi: 10.1016/s0042-6822(03)00450-1. [DOI] [PubMed] [Google Scholar]
- 76.Pletnev AG, Swayne DE, Speicher J, Rumyantsev AA, Murphy BR. Chimeric West Nile/dengue virus vaccine candidate: preclinical evaluation in mice, geese and monkeys for safety and immunogenicity. Vaccine. 2006;24(40-41):6392–6404. doi: 10.1016/j.vaccine.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 77.Karaca K, Bowen R, Austgen LE, et al. Recombinant canarypox vectored West Nile virus (WNV) vaccine protects dogs and cats against a mosquito WNV challenge. Vaccine. 2005;23(29):3808–3813. doi: 10.1016/j.vaccine.2005.02.020. [DOI] [PubMed] [Google Scholar]
- 78.Siger L, Bowen RA, Karaca K, et al. Assessment of the efficacy of a single dose of a recombinant vaccine against West Nile virus in response to natural challenge with West Nile virus-infected mosquitoes in horses. Am J Vet Res. 2004;65(11):1459–1462. doi: 10.2460/ajvr.2004.65.1459. [DOI] [PubMed] [Google Scholar]
- 79.Iglesias MC, Frenkiel MP, Mollier K, Souque P, Despres P, Charneau P. A single immunization with a minute dose of a lentiviral vector-based vaccine is highly effective at eliciting protective humoral immunity against West Nile virus. J Gene Med. 2006;8(3):265–274. doi: 10.1002/jgm.837. [DOI] [PubMed] [Google Scholar]
- 80.Coutant F, Frenkiel MP, Despres P, Charneau P. Protective antiviral immunity conferred by a nonintegrative lentiviral vector-based vaccine. PLoS One. 2008;3(12):e3973. doi: 10.1371/journal.pone.0003973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Despres P, Combredet C, Frenkiel MP, Lorin C, Brahic M, Tangy F. Live measles vaccine expressing the secreted form of the West Nile virus envelope glycoprotein protects against West Nile virus encephalitis. J Infect Dis. 2005;191(2):207–214. doi: 10.1086/426824. [DOI] [PubMed] [Google Scholar]
- 82.Brandler S, Marianneau P, Loth P, et al. Measles vaccine expressing the secreted form of West Nile virus envelope glycoprotein induces protective immunity in squirrel monkeys, a new model of West Nile virus infection. J Infect Dis. 2012;206(2):212–219. doi: 10.1093/infdis/jis328. [DOI] [PubMed] [Google Scholar]
- 83.Iyer AV, Pahar B, Boudreaux MJ, et al. Recombinant vesicular stomatitis virus-based west Nile vaccine elicits strong humoral and cellular immune responses and protects mice against lethal challenge with the virulent west Nile virus strain LSU-AR01. Vaccine. 2009;27(6):893–903. doi: 10.1016/j.vaccine.2008.11.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Schepp-Berglind J, Luo M, Wang D, et al. Complex adenovirus-mediated expression of West Nile virus C, PreM, E, and NS1 proteins induces both humoral and cellular immune responses. Clin Vaccine Immunol. 2007;14(9):1117–1126. doi: 10.1128/CVI.00070-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lustig S, Olshevsky U, Ben-Nathan D, et al. A live attenuated West Nile virus strain as a potential veterinary vaccine. Viral Immunol. 2000;13(4):401–410. doi: 10.1089/vim.2000.13.401. [DOI] [PubMed] [Google Scholar]
- 86.Yamshchikov G, Borisevich V, Seregin A, et al. An attenuated West Nile prototype virus is highly immunogenic and protects against the deadly NY99 strain: a candidate for live WN vaccine development. Virology. 2004;330(1):304–312. doi: 10.1016/j.virol.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 87.Whiteman MC, Li L, Wicker JA, et al. Development and characterization of non-glycosylated E and NS1 mutant viruses as a potential candidate vaccine for West Nile virus. Vaccine. 2010;28(4):1075–1083. doi: 10.1016/j.vaccine.2009.10.112. [DOI] [PubMed] [Google Scholar]
- 88.Yu L, Robert Putnak J, Pletnev AG, Markoff L. Attenuated West Nile viruses bearing 3′SL and envelope gene substitution mutations. Vaccine. 2008;26(47):5981–5988. doi: 10.1016/j.vaccine.2008.08.064. [DOI] [PubMed] [Google Scholar]
- 89.Liu WJ, Wang XJ, Clark DC, Lobigs M, Hall RA, Khromykh AA. A single amino acid substitution in the West Nile virus nonstructural protein NS2A disables its ability to inhibit alpha/beta interferon induction and attenuates virus virulence in mice. J Virol. 2006;80(5):2396–2404. doi: 10.1128/JVI.80.5.2396-2404.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wicker JA, Whiteman MC, Beasley DW, et al. A single amino acid substitution in the central portion of the West Nile virus NS4B protein confers a highly attenuated phenotype in mice. Virology. 2006;349(2):245–253. doi: 10.1016/j.virol.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 91.Widman DG, Ishikawa T, Fayzulin R, Bourne N, Mason PW. Construction and characterization of a second-generation pseudoinfectious West Nile virus vaccine propagated using a new cultivation system. Vaccine. 2008;26(22):2762–2771. doi: 10.1016/j.vaccine.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 92.Widman DG, Ishikawa T, Giavedoni LD, et al. Evaluation of RepliVAX WN, a single-cycle flavivirus vaccine, in a non-human primate model of West Nile virus infection. Am J Trop Med Hyg. 2010;82(6):1160–1167. doi: 10.4269/ajtmh.2010.09-0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ng T, Hathaway D, Jennings N, Champ D, Chiang YW, Chu HJ. Equine vaccine for West Nile virus. Dev Biol (Basel) 2003;114:221–227. • Pivotal efficacy and field safety trial results for the first licensed veterinary vaccine against WNV
- 94.Qiao M, Ashok M, Bernard KA, et al. Induction of sterilizing immunity against West Nile Virus (WNV), by immunization with WNV-like particles produced in insect cells. J Infect Dis. 2004;190(12):2104–2108. doi: 10.1086/425933. [DOI] [PubMed] [Google Scholar]
- 95.Diamond MS, Pierson TC, Fremont DH. The structural immunology of antibody protection against West Nile virus. Immunol Rev. 2008;225:212–225. doi: 10.1111/j.1600-065X.2008.00676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Throsby M, Geuijen C, Goudsmit J, et al. Isolation and characterization of human monoclonal antibodies from individuals infected with West Nile Virus. J Virol. 2006;80(14):6982–6992. doi: 10.1128/JVI.00551-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Vogt MR, Moesker B, Goudsmit J, et al. Human monoclonal antibodies against West Nile virus induced by natural infection neutralize at a postattachment step. J Virol. 2009;83(13):6494–6507. doi: 10.1128/JVI.00286-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chu JH, Chiang CC, Ng ML. Immunization of flavivirus West Nile recombinant envelope domain III protein induced specific immune response and protection against West Nile virus infection. J Immunol. 2007;178(5):2699–2705. doi: 10.4049/jimmunol.178.5.2699. [DOI] [PubMed] [Google Scholar]
- 99.Spohn G, Jennings GT, Martina BE, et al. A VLP-based vaccine targeting domain III of the West Nile virus E protein protects from lethal infection in mice. Virol J. 2010;7:146. doi: 10.1186/1743-422X-7-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chua AJ, Vituret C, Tan ML, et al. A novel platform for virus-like particle-display of flaviviral envelope domain III: induction of Dengue and West Nile virus neutralizing antibodies. Virol J. 2013;10:129. doi: 10.1186/1743-422X-10-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Magnusson SE, Karlsson KH, Reimer JM, et al. Matrix-M adjuvanted envelope protein vaccine protects against lethal lineage 1 and 2 West Nile virus infection in mice. Vaccine. 2013 doi: 10.1016/j.vaccine.2013.12.030. [DOI] [PubMed] [Google Scholar]
- 102.Cox RJ, Pedersen G, Madhun AS, et al. Evaluation of a virosomal H5N1 vaccine formulated with Matrix M adjuvant in a phase I clinical trial. Vaccine. 2011;29(45):8049–8059. doi: 10.1016/j.vaccine.2011.08.042. [DOI] [PubMed] [Google Scholar]
- 103.Samina I, Havenga M, Koudstaal W, et al. Safety and efficacy in geese of a PER.C6-based inactivated West Nile virus vaccine. Vaccine. 2007;25(49):8338–8345. doi: 10.1016/j.vaccine.2007.09.055. [DOI] [PubMed] [Google Scholar]
- 104.Lim CK, Takasaki T, Kotaki A, Kurane I. Vero cell-derived inactivated West Nile (WN) vaccine induces protective immunity against lethal WN virus infection in mice and shows a facilitated neutralizing antibody response in mice previously immunized with Japanese encephalitis vaccine. Virology. 2008;374(1):60–70. doi: 10.1016/j.virol.2007.12.021. [DOI] [PubMed] [Google Scholar]
- 105.Orlinger KK, Holzer GW, Schwaiger J, et al. An inactivated West Nile Virus vaccine derived from a chemically synthesized cDNA system. Vaccine. 2010;28(19):3318–3324. doi: 10.1016/j.vaccine.2010.02.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Brown F. Review of accidents caused by incomplete inactivation of viruses. Dev Biol Stand. 1993;81:103–107. [PubMed] [Google Scholar]
- 107.Nathanson N, Langmuir AD. The Cutter Incident. Poliomyelitis Following Formaldehyde-Inactivated Poliovirus Vaccination in the United States during the Spring of 1955. I. Background. Am J Hyg. 1963;78:16–28. doi: 10.1093/oxfordjournals.aje.a120327. [DOI] [PubMed] [Google Scholar]
- 108.Nathanson N, Langmuir AD. The Cutter Incident. Poliomyelitis Following Formaldehyde-Inactivated Poliovirus Vaccination in the United States during the Spring of 1955. Ii. Relationship of Poliomyelitis to Cutter Vaccine. Am J Hyg. 1963;78:29–60. doi: 10.1093/oxfordjournals.aje.a120328. [DOI] [PubMed] [Google Scholar]
- 109.Nathanson N, Langmuir AD. The Cutter incident. Poliomyelitis following formaldehyde-inactivated poliovirus vaccination in the United States during the Spring of 1955. II. Relationship of poliomyelitis to Cutter vaccine. 1963. Am J Epidemiol. 1995;142(2):109–140. doi: 10.1093/oxfordjournals.aje.a117611. discussion 107-108. [DOI] [PubMed] [Google Scholar]
- 110.Thomassen YE, van ’t Oever AG, van Oijen MG, Wijffels RH, van der Pol LA, Bakker WA. Next generation inactivated polio vaccine manufacturing to support post polio-eradication biosafety goals. PLoS One. 2013;8(12):e83374. doi: 10.1371/journal.pone.0083374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Amanna IJ, Raue HP, Slifka MK. Development of a new hydrogen peroxide-based vaccine platform. Nat Med. 2012;18(6):974–979. doi: 10.1038/nm.2763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Martin JE, Pierson TC, Hubka S, et al. A West Nile virus DNA vaccine induces neutralizing antibody in healthy adults during a phase 1 clinical trial. J Infect Dis. 2007;196(12):1732–1740. doi: 10.1086/523650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Nelson S, Jost CA, Xu Q, et al. Maturation of West Nile virus modulates sensitivity to antibody-mediated neutralization. PLoS Pathog. 2008;4(5):e1000060. doi: 10.1371/journal.ppat.1000060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Anderson SG, Skegg J. The international standard for anti-smallpox serum. Bull World Health Organ. 1970;42(4):515–523. [PMC free article] [PubMed] [Google Scholar]
- 115.Ledgerwood JE, Pierson TC, Hubka SA, et al. A West Nile virus DNA vaccine utilizing a modified promoter induces neutralizing antibody in younger and older healthy adults in a phase I clinical trial. J Infect Dis. 2011;203(10):1396–1404. doi: 10.1093/infdis/jir054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Cranenburgh R. DNA Vaccine Delivery. BioPharm International, Supplement. 2011 Oct;:s12–s18. [Google Scholar]
- 117.Diamond MS. Emerging infectious diseases of the 21st century. Springer; New York: 2009. SpringerLink (Online service). West Nile encephalitis virus infection viral pathogenesis and the host immune response; p. xix.p. 485.p. 416. [Google Scholar]
- 118.Petersen LR, Roehrig JT. Flavivirus DNA vaccines--good science, uncertain future. J Infect Dis. 2007;196(12):1721–1723. doi: 10.1086/523655. [DOI] [PubMed] [Google Scholar]
- 119.Sardesai NY, Weiner DB. Electroporation delivery of DNA vaccines: prospects for success. Curr Opin Immunol. 2011;23(3):421–429. doi: 10.1016/j.coi.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Durbin AP, Wright PF, Cox A, et al. The live attenuated chimeric vaccine rWN/DEN4Delta30 is well-tolerated and immunogenic in healthy flavivirus-naive adult volunteers. Vaccine. 2013;31(48):5772–5777. doi: 10.1016/j.vaccine.2013.07.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Biedenbender R, Bevilacqua J, Gregg AM, Watson M, Dayan G. Phase II, randomized, double-blind, placebo-controlled, multicenter study to investigate the immunogenicity and safety of a West Nile virus vaccine in healthy adults. J Infect Dis. 2011;203(1):75–84. doi: 10.1093/infdis/jiq003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Dayan GH, Bevilacqua J, Coleman D, Buldo A, Risi G. Phase II, dose ranging study of the safety and immunogenicity of single dose West Nile vaccine in healthy adults >/= 50 years of age. Vaccine. 2012;30(47):6656–6664. doi: 10.1016/j.vaccine.2012.08.063. [DOI] [PubMed] [Google Scholar]
- 123.Guirakhoo F, Pugachev K, Arroyo J, et al. Viremia and immunogenicity in nonhuman primates of a tetravalent yellow fever-dengue chimeric vaccine: genetic reconstructions, dose adjustment, and antibody responses against wild-type dengue virus isolates. Virology. 2002;298(1):146–159. doi: 10.1006/viro.2002.1462. [DOI] [PubMed] [Google Scholar]
- 124.Monath TP, Levenbook I, Soike K, et al. Chimeric yellow fever virus 17D-Japanese encephalitis virus vaccine: dose-response effectiveness and extended safety testing in rhesus monkeys. J Virol. 2000;74(4):1742–1751. doi: 10.1128/jvi.74.4.1742-1751.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Dayan GH, Pugachev K, Bevilacqua J, Lang J, Monath TP. Preclinical and clinical development of a YFV 17 D-based chimeric vaccine against West Nile virus. Viruses. 2013;5(12):3048–3070. doi: 10.3390/v5123048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kaiser J. Public health. Outbreak pattern stymies vaccine work. Science. 2012;337(6098):1030. doi: 10.1126/science.337.6098.1030. [DOI] [PubMed] [Google Scholar]
- 127.Durbin AP, Karron RA, Sun W, et al. Attenuation and immunogenicity in humans of a live dengue virus type-4 vaccine candidate with a 30 nucleotide deletion in its 3′-untranslated region. Am J Trop Med Hyg. 2001;65(5):405–413. doi: 10.4269/ajtmh.2001.65.405. [DOI] [PubMed] [Google Scholar]
- 128.Laassri M, Bidzhieva B, Speicher J, Pletnev AG, Chumakov K. Microarray hybridization for assessment of the genetic stability of chimeric West Nile/dengue 4 virus. J Med Virol. 2011;83(5):910–920. doi: 10.1002/jmv.22033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Monath TP, Guirakhoo F, Nichols R, et al. Chimeric live, attenuated vaccine against Japanese encephalitis (ChimeriVax-JE): phase 2 clinical trials for safety and immunogenicity, effect of vaccine dose and schedule, and memory response to challenge with inactivated Japanese encephalitis antigen. J Infect Dis. 2003;188(8):1213–1230. doi: 10.1086/378356. [DOI] [PubMed] [Google Scholar]
- 130.Lieberman MM, Clements DE, Ogata S, et al. Preparation and immunogenic properties of a recombinant West Nile subunit vaccine. Vaccine. 2007;25(3):414–423. doi: 10.1016/j.vaccine.2006.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lieberman MM, Nerurkar VR, Luo H, et al. Immunogenicity and protective efficacy of a recombinant subunit West Nile virus vaccine in rhesus monkeys. Clin Vaccine Immunol. 2009;16(9):1332–1337. doi: 10.1128/CVI.00119-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Burns DL. Licensure of vaccines using the Animal Rule. Curr Opin Virol. 2012;2(3):353–356. doi: 10.1016/j.coviro.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 133.Zohrabian A, Meltzer MI, Ratard R, et al. West Nile virus economic impact, Louisiana, 2002. Emerg Infect Dis. 2004;10(10):1736–1744. doi: 10.3201/eid1010.030925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Barber LM, Schleier JJ, 3rd, Peterson RK. Economic cost analysis of West Nile virus outbreak, Sacramento County, California, USA, 2005. Emerg Infect Dis. 2010;16(3):480–486. doi: 10.3201/eid1603.090667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Murray KO, Ruktanonchai D, Hesalroad D, Fonken E, Nolan MS. West Nile virus, Texas, USA, 2012. Emerg Infect Dis. 2013;19(11):1836–1838. doi: 10.3201/eid1911.130768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Stephens DS, Ahmed R, Orenstein WA. Vaccines at what price? Vaccine. 2014 doi: 10.1016/j.vaccine.2013.12.066. • Questions the principles that underlie vaccine cost-effectivness calculations and asks if other factors, including the moral obligation to reduce the incidence of disease, should play a larger role
- 137.Martina BE, Koraka P, Osterhaus AD. West Nile Virus: is a vaccine needed? Curr Opin Investig Drugs. 2010;11(2):139–146. [PubMed] [Google Scholar]
- 138.Petersen LR, Brault AC, Nasci RS. West Nile virus: review of the literature. JAMA. 2013;310(3):308–315. doi: 10.1001/jama.2013.8042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wang T, Anderson JF, Magnarelli LA, Wong SJ, Koski RA, Fikrig E. Immunization of mice against West Nile virus with recombinant envelope protein. J Immunol. 2001;167(9):5273–5277. doi: 10.4049/jimmunol.167.9.5273. [DOI] [PubMed] [Google Scholar]
- 140.Ledizet M, Kar K, Foellmer HG, et al. A recombinant envelope protein vaccine against West Nile virus. Vaccine. 2005;23(30):3915–3924. doi: 10.1016/j.vaccine.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 141.Demento SL, Bonafe N, Cui W, et al. TLR9-targeted biodegradable nanoparticles as immunization vectors protect against West Nile encephalitis. J Immunol. 2010;185(5):2989–2997. doi: 10.4049/jimmunol.1000768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Oliphant T, Nybakken GE, Austin SK, et al. Induction of epitope-specific neutralizing antibodies against West Nile virus. J Virol. 2007;81(21):11828–11839. doi: 10.1128/JVI.00643-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Jarvi SI, Hu D, Misajon K, Coller BA, Wong T, Lieberman MM. Vaccination of captive nene (Branta sandvicensis) against West Nile virus using a protein-based vaccine (WN-80E) J Wildl Dis. 2013;49(1):152–156. doi: 10.7589/2011-12-363. [DOI] [PubMed] [Google Scholar]
- 144.McDonald WF, Huleatt JW, Foellmer HG, et al. A West Nile virus recombinant protein vaccine that coactivates innate and adaptive immunity. J Infect Dis. 2007;195(11):1607–1617. doi: 10.1086/517613. [DOI] [PubMed] [Google Scholar]
- 145.Gershoni-Yahalom O, Landes S, Kleiman-Shoval S, et al. Chimeric vaccine composed of viral peptide and mammalian heat-shock protein 60 peptide protects against West Nile virus challenge. Immunology. 2010;130(4):527–535. doi: 10.1111/j.1365-2567.2010.03251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Websites
- 201.CDC . WNV Statistics & Maps. CDC; [Accessed January 30 2014]. 2014. [Online] Available: http://www.cdc.gov/westnile/statsMaps/ [Google Scholar]
- 202.PHAC [Accessed January 19 2014];Maps & Stats - West Nile virus - Public Health Agency of Canada. 2013 Online. Available: http://www.phac-aspc.gc.ca/wnv-vwn/index-eng.php.
- 203.EPISOUTH . Network for Communicable Disease Control in Southern Europe and Mediterranean Countries. EpiSouth; [Accessed January 19 2014]. 2014. Online. Available: http://www.episouth.org/home.php. [Google Scholar]
- 204.USGS . USGS West Nile Virus Maps. USGS; [Accessed January 16 2014]. 2014. Online. Available: http://diseasemaps.usgs.gov/wnv_historical.html. [Google Scholar]
- 205.FDA [Accessed January 16 2014];Countering Bioterrorism Questions and Answers. 2012 Online. Available: http://www.fda.gov/BiologicsBloodVaccines/SafetyAvailability/ProductSecurity/ucm110322.htm.
Patent Applications
- 301.Coller BA, Pai V, Weeks-Levy C, Ogata S. United States Patent Application No. US20120141520 A1: Recombinant subunit West Nile virus vaccine for protection of human subjects. 2012. [Google Scholar]
Vaccine Package Inserts/Recall Notices
- 401.YF-VAX Package Insert. Aventis Pasteur. Swiftwater, PA: 2005. [Google Scholar]
- 402.IXIARO Package Insert. Intercell-AG. Vienna: 2013. [Google Scholar]
- 403.Whitehead B. Updated recall notice PreveNile® West Nile Vaccine. Intervet Schering-Plough Animal Health; 2010. [Google Scholar]