Summary
A major challenge in biodiversity conservation is to facilitate viable populations of large apex predators in ecosystems where they were recently driven to ecological extinction due to resource conflict with humans.
Monetary compensation for losses of livestock due to predation is currently a key instrument to encourage human–carnivore coexistence. However, a lack of quantitative estimates of livestock losses due to predation leads to disagreement over the practice of compensation payments. This disagreement sustains the human–carnivore conflict.
The level of depredation on year‐round, free‐ranging, semi‐domestic reindeer by large carnivores in Fennoscandia has been widely debated over several decades. In Norway, the reindeer herders claim that lynx and wolverine cause losses of tens of thousands of animals annually and cause negative population growth in herds. Conversely, previous research has suggested that monetary predator compensation can result in positive population growth in the husbandry, with cascading negative effects of high grazer densities on the biodiversity in tundra ecosystems.
We utilized a long‐term, large‐scale data set to estimate the relative importance of lynx and wolverine predation and density‐dependent and climatic food limitation on claims for losses, recruitment and population growth rates in Norwegian reindeer husbandry.
Claims of losses increased with increasing predator densities, but with no detectable effect on population growth rates. Density‐dependent and climatic effects on claims of losses, recruitment and population growth rates were much stronger than the effects of variation in lynx and wolverine densities.
Synthesis and applications. Our analysis provides a quantitative basis for predator compensation and estimation of the costs of reintroducing lynx and wolverine in areas with free‐ranging semi‐domestic reindeer. We outline a potential path for conflict management which involves adaptive monitoring programmes, open access to data, herder involvement and development of management strategy evaluation (MSE) models to disentangle complex responses including multiple stakeholders and individual harvester decisions.
Keywords: depredation, human–carnivore conflict, MODIS, onset of spring, plant productivity, predator compensation, Rangifer
Short abstract
Our analysis provides a quantitative basis for predator compensation and estimation of the costs of reintroducing lynx and wolverine in areas with free‐ranging semi‐domestic reindeer. We outline a potential path for conflict management which involves adaptive monitoring programmes, open access to data, herder involvement and development of management strategy evaluation (MSE) models to disentangle complex responses including multiple stakeholders and individual harvester decisions.
Introduction
Ecologically functional apex predator communities are crucial for the maintenance of intact ecosystems and may also be important for services that modern societies rely upon (Estes et al. 2011). This recognition has led to some notable campaigns to restore large carnivores into areas where persecution drove them to ecological extinction only a few decades ago. The recovery of large carnivores in terrestrial ecosystems is nonetheless one of the most controversial wildlife management actions of present times. Controversies include the impact of predators on ungulates and resulting competition with hunters over game, and conflicts with livestock producers and pastoralists (Dickman, Macdonald & Macdonald 2011; Hebblewhite 2011). To encourage human–carnivore coexistence, financial instruments have been established world‐wide to compensate for negative effects of large carnivores on a local scale (Dickman, Macdonald & Macdonald 2011). However, predator compensation may have unintended side effects such as providing an incentive for pastoralists to increase stock size that in turn might have negative effects on other ecosystem components (Bulte & Rondeau 2005).
Reindeer herding is a circumpolar activity, and in Norway, Sweden and Finland, about half the land area is utilized for year‐round grazing. The Sami reindeer herders of Norway share their ranges with wildlife including Eurasian lynx Lynx lynx L. and wolverine Gulo gulo L. Semi‐domesticated reindeer Rangifer tarandus L. are a main prey for both lynx and wolverine (van Dijk et al. 2008; Mattisson et al. 2011), which are perceived as the two most significant predators on reindeer in Norway. To reduce human–carnivore conflicts, predators are controlled through hunting quotas and culling by the Norwegian Environment Agency to keep population sizes and their spatial distributions within politically determined limits. In the period 2000–2012, the average annual number of registered lynx family groups was 30·0 (SD = 7·6) within the reindeer herding area, and lynx accounted for 39% of the documented losses of semi‐domesticated reindeer (number of documented cases: n = 2430, Norwegian Environment Agency website). The average annual number of registered reproducing wolverines was 26·5 (SD = 7·0), and wolverine accounted for 32% of the documented losses (n = 1954). Among the other predators of reindeer, the golden eagle Aquila chrysaetos L. accounted for 27% (n = 1664) of the documented losses, while brown bear Ursus arctos L. and wolf Canis lupus L. accounted for <2% of the total losses. Wolves are prevented from establishing within the reindeer herding area. Recent monitoring of brown bear suggests that c. 6 females reproduce annually in Norway, while monitoring data for golden eagle are lacking. In 2011, c. 57 thousand calves and c. 18 thousand adult reindeer were claimed to have been lost due to the above‐mentioned carnivores. The Norwegian Environment Agency gave monetary compensation for only one out of four reindeer claimed to be lost to predation, leading to a compensation of about 68·7 million Norwegian kroner (c. €8·4 million, Norwegian Environment Agency website) and substantial controversy between reindeer herders and management authorities over the magnitude of losses due to large carnivores. In comparison, c. 43 thousand calves and c. 20 thousand adults of a population of c. 240 thousand individuals prior to calving were slaughtered, leading to a meat production income of c. €12·7 million that year.
The Norwegian scheme for compensation is linked to the herders' claims and ability to document losses. The usefulness of this compensation system is debated. Small calves lost shortly after birth are extremely difficult to find, thereby hindering documentation of cause of mortality, and this may lead to a negative bias in compensation payment. Conversely, it is alleged that claims are generally inflated, and generous compensation for losses has been linked to increased reindeer population growth rates (Næss et al. 2011) and overabundance on the tundra (Hausner et al. 2011). These resulting demographic trends have further been associated with negative cascading effects on herbs (Bråthen et al. 2007), Salix shrubs (Ims et al. 2007), willow ptarmigan (Henden et al. 2011) and arctic foxes (Killengreen et al. 2011). Thus, there is a pressing need to identify a compensation scheme that motivates for coexistence among reindeer pastoralist and large carnivores and that concurrently motivates ecologically sustainable stock sizes (cf. Bulte & Rondeau 2005).
The relative magnitude of top‐down regulation by apex predators and bottom‐up food limitation due to density dependence and stochastic variation in climate on ungulate populations has been vigorously discussed during the last century, and it is now well established that ungulates are affected by both processes (reviewed in Sæther 1997). The challenge in terms of estimating the impact of large carnivores on livestock is to disentangle the relative role of the various factors affecting reproduction and mortality (Hebblewhite 2011). Detailed information pertaining to the size of the lynx and wolverine populations plus detailed official statistics of the reindeer industry in Norway offer a unique opportunity to quantify the relative importance of food limitation and predation on recruitment, population dynamics and losses of reindeer.
Here, we evaluate the hypothesis that increased population sizes of lynx and wolverine lead to increased losses (mortality), lower reproductive output and lower population growth rates of reindeer. We had a priori knowledge that food limitation, determined by stochastic variation in climatic conditions, and reindeer population densities are important factors affecting recruitment, deaths and population dynamics (Tveraa et al. 2003, 2013; Bårdsen & Tveraa 2012). Therefore, our aim was to estimate the relative importance of food limitation and lynx and wolverine population sizes for losses, recruitment and population dynamics of reindeer. The presence of a strong impact of food limitation suggests that a potential ‘win‐win’ state for both humans and carnivores, leading to reduced human–carnivore conflict, does exist in the study system. We describe obstacles and approaches towards this reconciliatory state.
Materials and methods
Reindeer husbandry system
Semi‐domesticated reindeer are free ranging year‐round and gathered a few times a year for marking the annual recruitment of calves, slaughtering and, in some populations, herding between summer and winter pastures in the autumn and spring. Reindeer husbandry utilizes about 140 000 km2 of the Norwegian land area, including some major islands (Fig. 1). The total number of reindeer of each sex and age class (calves or older) is counted annually during round‐ups in winter. Early recruitment is registered during calf marking in summer or autumn, and slaughtering takes place in autumn and winter between September and March. The herders report by 31 March each year, data on total number of reindeer of each sex and age class in the herd, number of calves marked, number of animals slaughtered of each sex and age class, and total losses of calves and adults since previous reporting date. There are no independent estimates of population sizes and demography, but management authorities do control counts of herd sizes at nonsystematic intervals to assure that herders report correct herd sizes. An extensive subsidy system (e.g. Hausner et al. 2011) ensures that most animals (c. 90%) are slaughtered at government‐approved slaughter houses which forward information regarding age, sex and carcass mass of every individual slaughtered to the Reindeer Husbandry Administration. We removed from the analysis reindeer populations with year‐round pastures on islands because lynx and wolverine do not breed there, leaving reindeer populations utilizing 128 000 km2 in ten regions for further investigation (Fig. 1). Temporal trends in the size of the female populations, body masses of calves, number of calves and adults claimed lost, early recruitment and population growth rates within regions are given in Fig. S1 (Supporting Information).
Figure 1.
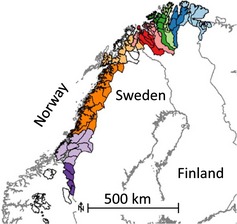
Map of Fennoscandia (Norway, Sweden, Finland) and overview of the study area with the 10 regions in which data were aggregated highlighted in different colours. Solid black lines indicate the various reindeer herding districts.
Lynx and wolverine population sizes
The size of the breeding population of lynx is based on observations of family groups (females with kittens) reported to the management authorities. Observations include reports from the general public, farmers, herders, hunters, etc. and structured transect line surveys on snow (Tovmo & Brøseth 2011; Brøseth & Tovmo 2012). All observations are checked by local carnivore contacts from the State Nature Inspectorate, and distance rules, based on known territory size and maximum daily displacement of radiomarked individuals, are used to identify individual family groups (Linnell et al. 2007). For families with multiple observations, we used the centre of their utilized area as the position of observation. Estimates of the abundance of the breeding population of wolverines are based on repeated visits to previously known dens, plus extensive searches for new dens (Landa et al. 1998). Dens are normally revisited 3–6 times to affirm reproductive status (Brøseth, Tovmo & Andersen 2011). We hereafter refer to these observations as lynx and wolverine population sizes. These estimates contain no estimates of measurement error. Reconstructions of population size of lynx (Nilsen et al. 2012) and genetic capture–mark–recapture of wolverine (Brøseth et al. 2010), however, reveal that the national monitoring programme of lynx family groups and wolverine reproductions reflect population sizes well. All information regarding population size of large carnivores in Norway is obtained from the Norwegian Large Predator Monitoring Program at Rovdata (www.rovdata.no). A team of researchers work continuously to evaluate and improve the information pertaining to the large carnivore abundance. Temporal trends in the size of the lynx and wolverine populations are given in Fig. S2.
Climate data
We used remotely sensed vegetation green‐up to index climatic conditions and plant productivity as this predictor appears to be the one with the highest spatial resolution and best predictive power in terms of recruitment and body mass of semi‐domesticated reindeer (Bårdsen & Tveraa 2012; Tveraa et al. 2013). Based on 16‐day and 250‐m resolution MODIS EVI data, we estimated spring onset and plant productivity within the calving and summer pastures. Details regarding these methods can be found in Tveraa et al. (2013). Temporal trends in onset of spring greenness and plant productivity are given in Fig. S3.
Statistical analyses
Due to the large home ranges of lynx and wolverine (Herfindal et al. 2005; Persson, Wedholm & Segerstrom 2010), we aggregated the data into ten well‐defined management regions to reduce small‐scale stochastic variation in the position of lynx and wolverine observations on their abundance estimates (Fig. 1). In addition, this aggregation removed problems associated with the complex pasture use in the northern parts, where several reindeer herds utilize the same pastures but at different times of the year.
Our objective was to estimate the annual variation in number of reindeer (calves and adults) claimed lost, early recruitment and population growth rates as a function of food limitation and predation. Accounting for the impact of harvest on population dynamics may be difficult using discrete time series data both in seasonal environments and when harvest correlates positively to population size (Boyce, Sinclair & White 1999; Patterson & Power 2002). Accordingly, some authors have ignored harvest, calculating population growth rates, R t, as log(N t+1) – log(N t) where N t is population size in year t and N t+1 is population size in year t+1 (e.g. Patterson & Power 2002). Others calculated population growth rate, R t, as log(N t+1 + H t+1) – log(N t) where H t+1 is the number of animals harvested (e.g. Hobbs et al. 2012). We focus on the latter measure to facilitate comparison with the recent study of Hobbs et al. (2012). However, estimates of the former are given in Appendix S1. We used the previous year average calf slaughter mass as a measure of herd body condition. We entered reindeer population size, herd body condition in the previous year, plus date of onset of spring and plant productivity as predictors of food limitation, and population size of lynx and wolverine as predictors of predation risk. We entered region as a random factor with random intercept and estimated the effects of temporal variation in food limitation and predation in a generalized linear mixed modelling framework. Models were run using the ‘lme4’ package (Bates, Maechler & Bolker 2012) in r (R Core Team 2013). We used the log link function and assumed a Poisson distribution in the analyses of claimed losses and assumed a Gaussian distribution for early recruitment and population growth rates (cf. Tveraa et al. 2013). Details pertaining to choice of model structure and the robustness of the modelling approach are found in Appendix S1.
Results
Claims for losses
The number of reindeer calves and adults claimed lost increased with increasing population size, decreasing herd body condition the previous autumn, late spring onset and decreasing plant productivity (Fig. 2). Increasing lynx and wolverine populations also led to increased claims for compensation (Fig. 2). On average, the estimated claims for losses increased with 87 calves (95% CI = 80·3, 94·0) and 14 adult reindeer (95% CI = 10·2, 18·5) per lynx family group (Appendix S1, Table A1 and A2). For wolverine, the corresponding figures were 66 calves (95% CI = 59·5, 73·6) and five adults (95% CI = 0·6, 9·2). Comparisons of standardized regression coefficients showed that the effect of population size was 4–6 and 9–29 times stronger than the effects of lynx and wolverine predation on claims for losses of calves and adults, respectively (Fig. 3). The effect of herd body condition the previous autumn was 3–4 and 5–16 times stronger than the effects of predation on claims for losses of calves and adults, respectively (Fig. 3). The effect of onset of spring was of similar magnitude as the estimated effects of predation on claims for losses of calves, and 3–10 times stronger than the effects of predation on claims for losses of adults (Fig. 3). The effect of plant productivity was of similar magnitude as the effects of predation on claims for losses of calves, and 1–5 times stronger than the effects of predation on claims for losses of adults (Fig. 3).
Figure 2.
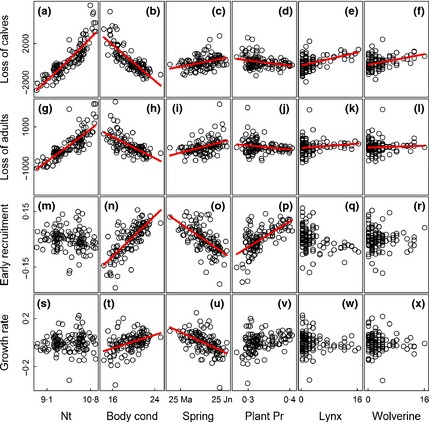
Regression lines (red lines) and partial residuals for the relationship between log population size (Nt), herd body condition in the previous fall (Body cond), onset of spring (Spring), plant productivity (Plant Pr), population size of lynx and wolverine and (a–f) claimed loss of calves (<1 year); (g–l) claimed loss of adults (>1 year); (m–r) early recruitment and (s–x) population growth rate.
Figure 3.
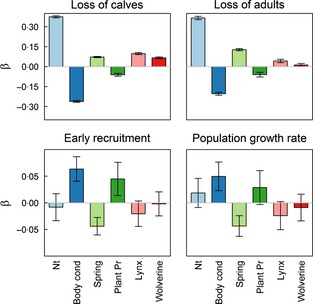
Standardized estimates of the impact of log population size (Nt), herd body condition in the previous fall (Body cond), onset of spring (Spring), plant productivity (Plant Pr) and population size of lynx and wolverine on claimed loss of calves (<1 year); claimed loss of adults (>1 year); early recruitment and population growth rate.
Early recruitment
Early recruitment, that is the number of calves marked in summer divided by the number of adult females in the population the previous winter, was unaffected by population size but increased with increasing herd body condition the previous autumn, early spring onset and increasing plant productivity (Fig. 2). We found no strong evidence for that the population sizes of lynx and wolverine impacted recruitment (Fig. 2). Comparisons of standardized regression coefficients showed that the effect of herd body condition was about three and 32 times more important than the effects of lynx and wolverine predation, respectively (Fig. 3). The corresponding figures for onset of spring and plant productivity were about two and 22 for lynx and wolverine predation, respectively (Fig. 3).
Population growth rate
Population growth rate was negatively affected by poor herd body condition and late onset of spring (Fig. 2). We found no strong evidence supporting that other predictors impacted population growth rate (Fig. 2). Comparisons of standardized regression coefficients showed that the effect of body condition was about two and six times stronger than the effects of lynx and wolverine population densities, respectively (Fig. 3) while the effect of onset of spring was 2–5 times stronger than the effects of lynx and wolverine predation (Fig. 3). For plant productivity, the corresponding figures were in the range 1–3 (Fig. 3).
Discussion
As expected, the number of reindeer claimed to have been lost increased with increasing lynx and wolverine population sizes. Density‐dependent and stochastic variations in food limitation were, nevertheless, the most important predictors of the reported losses to predators. Neither early recruitment nor population growth rates were significantly related to lynx and wolverine population sizes, but strongly related to density‐dependent and density‐independent food limitation. The strong impact of density‐dependent food limitation on early recruitment and population growth rates may suggest that losses to predators to some degree are compensatory.
The role of predation
We estimated an average increase in claimed losses of 87 calves and 14 adult reindeer per family group of lynx, and 66 calves and five adults per wolverine reproduction. These figures are similar to estimates of long‐term reduction in harvest due to lynx and wolverine across Swedish reindeer management units (Hobbs et al. 2012). In contrast, the estimates of lynx predation impact are only about one‐fourth of the estimates obtained in kill rate studies of lynx using radiotelemetry (Mattisson et al. 2011) assuming four adult lynx per family group (Andren et al. 2002). This difference is likely to be because the kill rate studies include both additive and compensatory mortality, while compensatory predation mortality to a large degree will be accounted for as being caused by reindeer population size, herd body condition and climatic conditions in our regression approach.
Neither lynx nor wolverine abundance had any statistically significant impact on early recruitment. The estimated effects were, however, negative, and the importance of lynx was an order of magnitude larger than for wolverine. Lynx has been shown to select for calves of above average size (Nieminen 2010), reinforcing the view that lynx is a capable predator that might have an impact on the demography of reindeer. Reindeer have, however, evolved a suite of antipredator strategies, including migration and the use of alpine areas for calving and summer pastures which make them less vulnerable to lynx predation (Skogland 1991). This may explain the statistically insignificant relationship between lynx population size and reindeer recruitment. Overall, the small estimated effects of lynx and wolverine predation on early recruitment suggest that reproduction in semi‐domestic reindeer populations in Norway is mainly determined by food limitation. This is consistent with previous work that finds female body mass to be a main predictor of calf survival around birth and the first weeks thereafter. Females with high body mass appear more likely to conceive (Cameron & Smith 1993) and reproduce successfully (Fauchald et al. 2004) and less likely to lose their calves over the first weeks after calving (Tveraa et al. 2003).
No statistically significant negative impacts of lynx or wolverine population sizes on population growth rates were evident. This contrasts with results from the Swedish reindeer industry where both lynx and wolverine negatively affected population growth rates (Hobbs et al. 2012). More intensive predator control in Norway than in Sweden, leading to lower densities of lynx and wolverines in Norway than in Sweden, might be one reason for the difference between the neighbouring countries. More use of forested areas where reindeer are more vulnerable to lynx predation might be another reason for a stronger negative impact of lynx in Sweden than in Norway. Alternatively, differences in reindeer densities between the two countries might play a role. At present, we cannot disentangle these alternative hypotheses.
The role of food limitation
The strong relationship between the number of reindeer claimed lost due to predation and both population size and herd body condition suggested that the main driver of claims of losses was density‐dependent food limitation. This view was reinforced by the finding that early recruitment was positively related to plant productivity and early spring onset. Furthermore, population growth rates increased with early onset of spring. We found no evidence for a negative relationship between population size and population growth rate. However, herd body condition, which are negatively related to population size (Tveraa et al. 2013), positively affected population growth rates. Following Hobbs et al. (2012), we assessed whether the estimate of the effect of population size became negative when herd body condition was removed from the model and found no evidence for such a pattern (β = −0·003, 95% CI = −0·061, 0·056). In this context, we question whether our approach of adding harvest to population growth rates is a valid methodological approach in seasonal environments where the numeric effect of density on population growth rates is generally strongest in late winter (Boyce, Sinclair & White 1999), that is after the period when reindeer are harvested. In line with this, we found a strong negative relationship between population size and population growth rates when we did not add harvest to population growth rates (Appendix S1). Further research seems needed to evaluate these two alternative measures of population growth rates in harvested populations.
Strong bottom‐up climate impacts in ungulates have been suggested in systems that lack top‐down predator control (Wilmers et al. 2006). The reindeer populations in Fennoscandia have fluctuated in concert over the last decades. They increased during the seventies, peaked in the late eighties–early nineties and collapsed at the beginning of this millennium, before a new increase began (Tveraa et al. 2007; Moen 2008; Hausner et al. 2011). This large‐scale covariation supports the view that correlated large‐scale climate variability is a main driver of population dynamics. Tveraa et al. (2007) also found that Norwegian reindeer populations, except those subjected to intensive harvesting, fluctuated in concert with climate. This led to the conclusion that in the absence of a functional large carnivore guild, harvest was a prerequisite to ensure stable population sizes and resilience against poor climate. The role of predators in increasing resilience against poor climate is further supported by Wilmers et al. (2006) who reported that the collapse in the wolf population at Isle Royale led to increased moose population growth and vulnerability to climate.
A path to conflict management?
High losses and associated low productivity in reindeer herds are characteristics of our study system that fuel the human–carnivore conflict. With respect to this, an important point that needs to be clearly articulated to managers and stakeholders is that losses of reindeer to carnivores have to be evaluated in a multiple competing hypotheses framework (Hebblewhite 2011). As expected, the number of reindeer claimed lost to predators increased with increasing lynx and wolverine abundances. However, density‐dependent food limitation and climatic driven variability in plant availability and productivity appeared as the most important predictors of variation in herd productivity and associated claims for compensation. Predator control and eradication, although being the oldest and most widespread action to protect livestock (Berger 2006), is therefore unlikely to reduce losses substantially, while actions that improve the nutritional status of reindeer are likely to succeed. Similar conclusions have been reached in other ungulate–carnivore systems. Female and fawn survival during winter and spring increased substantially when mule deer Odocoileus hemionus R. were fed ad libitum (Bishop et al. 2009) and predation rates by coyotes Canis latrans S. and mountain lions Puma concolor L. also decreased. Accordingly, predator removal had only marginal impact on vital rates of mule deer (Hurley et al. 2011). For free‐ranging reindeer, supplementary feeding is possible through the winter when snow scooters allow transport of food to remote pastures. Studies suggest that enhanced nutrition in late winter and spring increases calf survival shortly after parturition, but seems to have little effect on calf growth and survival over the summer (Fauchald et al. 2004; Ballesteros et al. 2013). High vulnerability to climatic perturbation and predation is expected to remain unless actions are taken to increase gain in body mass during summer. A solution to this is to reduce reindeer densities (Bårdsen & Tveraa 2012) through increased harvesting (Tveraa et al. 2007), that is, the human–carnivore conflict can be reduced through a reduction in reindeer over‐abundance. However, this is not necessarily regarded as an attractive solution by reindeer herders. While empirical data and models suggest that a reduction in reindeer densities will result in a substantial increase in economic income for individual herders from meat production (Kumpula, Colpaert & Nieminen 1998; Tahvonen, Kumpula & Pekkarinen 2014), herders may be sceptical to this conclusion and view a reduction in herd size as more likely to cause a reduction in capital and income. Clearly, there are many obstacles along this path to reconciliation. In the following section, we outline some of these problems and a possible way forward.
Disagreement over ecological knowledge
Although ecological knowledge is not the only element in conservation conflict management (Redpath et al. 2013), agreement around the relative importance of the ecological processes operating is likely to be crucial for reconcilement. Numerous scientific papers have shown that density‐dependent processes and climate are important for the productivity of the reindeer industry in Fennoscandia (Kumpula, Colpaert & Nieminen 1998; Tveraa et al. 2007; Hobbs et al. 2012). However, these findings are not necessarily supported by the herder's own experience. The proximate cause of death in free‐range ungulates will typically be predation (Linnell, Aanes & Andersen 1995) even when starvation is the ultimate cause (Tveraa et al. 2003; Griffin et al. 2011). The claim that food limitation is the main process causing high losses is therefore not consistent with the herder's local knowledge, as they predominantly find predator‐killed animals in the field. Furthermore, density as an important factor in herd productivity can only be detected through analyses, or at least a graphical representation, of data on productivity and densities from many years or areas. Such analyses are rarely available at scales relevant to the individual herder and therefore may be regarded as irrelevant.
We believe openness and easy access to continuously updated time series data on reindeer herd productivities and densities, predator densities and climate may improve this situation. More specifically, we plan to develop an adaptive monitoring programme (Lindenmayer & Likens 2010). An open‐access Web‐based data base system will be one pillar in this monitoring programme where interested parties can extract both predictor and response variables at the herd level and obtain figures of trends in statistics and relative effects of food limitation and predation. The idea is that making information easily available will make analyses like the ones presented in this article more acceptable, as anyone will be able to plot the trend in herd population sizes, calf slaughter weights, predator densities and climate variables and thereby look at the interrelationships for themselves. Furthermore, such a data base should make it attractive and easy for other researchers to evaluate the conclusions presented in this paper as new data appear, new analysis methods are developed and new questions arise. The second pillar involves individual‐based studies of reproductive success and losses of reindeer to carnivores carried out in close collaboration with herders. The concept is that this will elucidate important factors underlying losses to carnivores and promote awareness and discussions among herders related to these issues.
The challenge of common pool resource management
In Norway, economic incentives to reduce reindeer densities and thereby improve industry wide production have been tried several times in the last decades and failed (Ulvevadet & Hausner 2011). Conflict over common pool resources is one explanation for the lack of willingness to reduce herd size, as the cost of habitat degradation is shared among pastoralists while the benefits of adding animals to the herd are gained by the individual herders (Næss & Bårdsen 2010). Correspondingly, those with large herds tend to retain larger herds also after environmental crises resulting in herd collapses. Thus, herd accumulation may increase long‐term individual herder level viability (Næss & Bårdsen 2010) at the cost of a common, across herder increase in vulnerability to climatic perturbations (Wilmers et al. 2006; Tveraa et al. 2007). Clearly, to obtain a viable reduction in reindeer densities, this needs to become a common goal among herders that share pastures, and management structures will be needed that allow herders to have some control over each other's herd sizes.
Conflicting outcome of governmental instruments
The compensation scheme for losses to protected predators is managed by the Ministry of Climate and Environment. Recent research has suggested that overcompensation for losses due to predation may also be instrumental to herd accumulation, as it may ensure sufficient income at low slaughter intensities (Næss et al. 2011). Furthermore, in the Norwegian compensation system, large herds pave the way for larger payouts and therefore may hamper the willingness to reduce herd size (Næss et al. 2011). In contrast, the Ministry of Agriculture and Food tries to increase harvesting and thereby reduce reindeer densities through financial reward of slaughtering. In addition, an upper reindeer number is set for spatially defined management units through negotiations between government and herders. If effective incentives that increase harvest and reduce herd sizes are to be developed, the conflict between the different governmental instruments needs to be addressed. A predator compensation scheme based on predation risk as in Sweden (Zabel & Holm‐Müller 2008), rather than reported and documented losses, has been suggested as an approach to improve this situation as it is more likely to provide an incentive to minimize losses (Bulte & Rondeau 2005).
Future prospects
Given the discrepancy between livestock numbers claimed lost to predation and the numbers estimated lost, the carnivore–livestock conflict is likely to remain unless the current low‐harvest, high‐loss situation is turned into a high‐harvest, low‐loss situation that is perceived profitable by the herders (Redpath et al. 2013). Development of harvest models that explore the outcome of alternative harvest strategies may be one key to such a transition, as they may demonstrate the gain for the herders of more intensive harvesting and aid decision‐making. Indeed, such models are a prerequisite for operating within an adaptive management framework (Williams, Nichols & Conroy 2002). Furthermore, we believe an adaptive monitoring programme should include replicated experimental manipulations that involve both predator and reindeer densities, and long‐term detailed radiotelemetry studies that allow cause‐specific mortality estimates and the relative role of food limitation and predation to be disentangled (Griffin et al. 2011). Such studies will efficiently give reliable knowledge of predator–prey interactions needed to guide management and reduce today's human–carnivore conflict.
A major challenge in the process of achieving a sustainable reindeer herding industry with high productivity and low losses of animals is linked to the level at which reindeer herding strategies are decided. To a large degree, these decisions are made by individual households, with decision‐making at herd (which includes several households) and higher levels of aggregation occurring in diminishing degrees. This results in slaughter strategy decisions that do not necessarily attempt to maximize profit based on the foraging resources available, but rather aim at maximizing household utility. Predicting the outcome of management strategies has therefore proven difficult (Ulvevadet & Hausner 2011). The framework of management strategy evaluation (MSE) may be a promising tool to overcome these challenges because these models are designed to account for complex situations including multiple stakeholders and individual harvester decision‐making (Bunnefeld et al. 2011; Milner‐Gulland 2011). Sound ecological knowledge is only one building block in such a framework which elucidates the need for a better understanding of household‐level socio‐economic decision rules. In particular, such a modelling framework may allow the effects of changes in governmental instruments on household utilities, the economy of households and industry, and governmental biodiversity targets, to be evaluated.
Supporting information
Fig. S1. Temporal trends in reindeer data.
Fig. S2. Temporal trends in predator population densities.
Fig. S3. Temporal trends in onset of spring and plant productivity.
Appendix S1. Modelling approach, structure and robustness.
Acknowledgements
This research project was funded by the Norwegian Environment Agency, The Ministry of Food and Agriculture and the Norwegian Institute for Nature Research. The comments of Steve M. Redpath, Jenny Stien and two reviewers substantially improved the manuscript.
Data accessibility
Data are available from the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.jm7k1 (Tveraa et al. 2014).
References
- Andren, H. , Linnell, J.D.C. , Liberg, O. , Ahlqvist, P. , Andersen, R. , Danell, A. et al (2002) Estimating total lynx Lynx lynx population size from censuses of family groups. Wildlife Biology, 8, 299–306. [Google Scholar]
- Ballesteros, M. , Bårdsen, B.J. , Fauchald, P. , Langeland, K. , Stien, A. & Tveraa, T. (2013) Combined effects of long‐term feeding, population density and vegetation green‐up on reindeer demography. Ecosphere, 4, art45. [Google Scholar]
- Bårdsen, B.‐J. & Tveraa, T. (2012) Density‐dependence vs. density‐independence – linking reproductive allocation to population abundance and vegetation greenness. Journal of Animal Ecology, 81, 364–376. [DOI] [PubMed] [Google Scholar]
- Bates, D.M. , Maechler, M. & Bolker, B.M. (2012) lme4: Linear mixed‐effects models using Eigen and S4. url: http://cran.r-project.org/web/packages/lme4/index.html.
- Berger, K.M. (2006) Carnivore‐livestock conflicts: effects of subsidized predator control and economic correlates on the sheep industry. Conservation Biology, 20, 751–761. [DOI] [PubMed] [Google Scholar]
- Bishop, C.J. , White, G.C. , Freddy, D.J. , Watkins, B.E. & Stephenson, T.R. (2009) Effect of enhanced nutrition on mule deer population rate of change. Wildlife Monographs, 172, 1–28. [Google Scholar]
- Boyce, M.S. , Sinclair, A.R.E. & White, G.C. (1999) Seasonal compensation of predation and harvesting. Oikos, 87, 419–426. [Google Scholar]
- Bråthen, K.A. , Ims, R.A. , Yoccoz, N.G. , Fauchald, P. , Tveraa, T. & Hausner, V.A. (2007) Induced shift in ecosystem productivity? Extensive scale effects of abundant large herbivores. Ecosystems, 10, 773–789. [Google Scholar]
- Brøseth, H. & Tovmo, M. (2012) Number of family groups, population estimate and population development of lynx in Norway (in Norwegian). pp. 28. NINA Report 859.
- Brøseth, H. , Tovmo, M. & Andersen, R. (2011) Yngleregistreringer av jerv i Norge i 2011 (in Norwegian). pp. 26. NINA Report 757.
- Brøseth, H. , Flagstad, Ø. , Wärdig, C. , Johansson, M. & Ellegren, H. (2010) Large‐scale noninvasive genetic monitoring of wolverines using scats reveals density dependent adult survival. Biological Conservation, 143, 113–120. [Google Scholar]
- Bulte, E.H. & Rondeau, D. (2005) Why compensating wildlife damages may be bad for conservation. Journal of Wildlife Management, 69, 14–19. [Google Scholar]
- Bunnefeld, N. , Börger, L. , van Moorter, B. , Rolandsen, C.M. , Dettki, H. , Solberg, E.J. & Ericsson, G. (2011) A model‐driven approach to quantify migration patterns: individual, regional and yearly differences. Journal of Animal Ecology, 80, 466–476. [DOI] [PubMed] [Google Scholar]
- Cameron, R.D. & Smith, W.T. (1993) Calving success of female caribou in relation to body weight. Canadian Journal of Zoology, 71, 480–486. [Google Scholar]
- Dickman, A.J. , Macdonald, E.A. & Macdonald, D.W. (2011) A review of financial instruments to pay for predator conservation and encourage human‐carnivore coexistence. Proceedings of the National Academy of Sciences, 108, 13937–13944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dijk, J. , Gustavsen, L. , Mysterud, A. , May, R. , Flagstad, O. , Brøseth, H. et al (2008) Diet shift of a facultative scavenger, the wolverine, following recolonization of wolves. Journal of Animal Ecology, 77, 1183–1190. [DOI] [PubMed] [Google Scholar]
- Estes, J.A. , Terborgh, J. , Brashares, J.S. , Power, M.E. , Berger, J. , Bond, W.J. et al (2011) Trophic downgrading of planet earth. Science, 333, 301–306. [DOI] [PubMed] [Google Scholar]
- Fauchald, P. , Tveraa, T. , Henaug, C. & Yoccoz, N. (2004) Adaptive regulation of body reserves in reindeer, Rangifer tarandus: a feeding experiment. Oikos, 107, 583–591. [Google Scholar]
- Griffin, K.A. , Hebblewhite, M. , Robinson, H.S. , Zager, P. , Barber‐Meyer, S.M. , Christianson, D. et al (2011) Neonatal mortality of elk driven by climate, predator phenology and predator community composition. Journal of Animal Ecology, 80, 1246–1257. [DOI] [PubMed] [Google Scholar]
- Hausner, V.H. , Fauchald, P. , Tveraa, T. , Pedersen, E. , Jernsletten, J. , Ulvevadet, B. , Ims, R.A. , Yoccoz, N.G. & Bråthen, K.A. (2011) The ghost of development past: the impact of economic security policies on Saami pastoral ecosystems. Ecology and Society, 16, 4. [Google Scholar]
- Hebblewhite, M. (2011) Unreliable knowledge about economic impacts of large carnivores on bovine calves. Journal of Wildlife Management, 75, 1724–1730. [Google Scholar]
- Henden, J.A. , Ims, R.A. , Yoccoz, N.G. & Killengreen, S.T. (2011) Declining willow ptarmigan populations: the role of habitat structure and community dynamics. Basic and Applied Ecology, 12, 413–422. [Google Scholar]
- Herfindal, I. , Linnell, J.D.C. , Odden, J. , Nilsen, E.B. & Andersen, R. (2005) Prey density, environmental productivity and home‐range size in the Eurasian lynx (Lynx lynx). Journal of Zoology, 265, 63–71. [Google Scholar]
- Hobbs, N.T. , Andren, H. , Persson, J. , Aronsson, M. & Chapron, G. (2012) Native predators reduce harvest of reindeer by Sámi pastoralists. Ecological Applications, 22, 1640–1654. [DOI] [PubMed] [Google Scholar]
- Hurley, M.A. , Unsworth, J.W. , Zager, P. , Hebblewhite, M. , Garton, E.O. , Montgomery, D.M. , Skalski, J.R. & Maycock, C.L. (2011) Demographic response of mule deer to experimental reduction of coyotes and mountain lions in southeastern Idaho. Wildlife Monographs, 178, 1–33. [Google Scholar]
- Ims, R.A. , Yoccoz, N.G. , Bråthen, K.A. , Fauchald, P. , Tveraa, T. & Hausner, V. (2007) Can reindeer overabundance cause a trophic cascade? Ecosystems, 10, 607–622. [Google Scholar]
- Killengreen, S.T. , Lecomte, N. , Ehrich, D. , Schott, T. , Yoccoz, N.G. & Ims, R.A. (2011) The importance of marine vs. human‐induced subsidies in the maintenance of an expanding mesocarnivore in the arctic tundra. Journal of Animal Ecology, 80, 1049–1060. [DOI] [PubMed] [Google Scholar]
- Kumpula, J. , Colpaert, A. & Nieminen, M. (1998) Reproduction and productivity of semidomesticated reindeer in northern Finland. Canadian Journal of Zoology, 76, 269–277. [Google Scholar]
- Landa, A. , Tufto, J. , Franzen, R. , Bø, T. , Lindén, M. & Swenson, J.E. (1998) Active wolverine Gulo gulo dens as a minimum population estimator in Scandinavia. Wildlife Biology, 4, 159–168. [Google Scholar]
- Lindenmayer, D.B. & Likens, G.E. (2010) Effective Ecological Monitoring. CSIRO, Collingwood, Australia. [Google Scholar]
- Linnell, J.D.C. , Aanes, R. & Andersen, R. (1995) Who killed Bambi? The role of predation on neonatal mortality of temperate ungulates. Wildlife Biology, 1, 209–223. [Google Scholar]
- Linnell, J.D.C. , Odden, J. , Andren, H. , Liberg, O. , Andersen, R. , Moa, P. et al (2007) Distance rules for minimum counts of Eurasian lynx Lynx lynx family groups under different ecological conditions. Wildlife Biology, 13, 447–455. [Google Scholar]
- Mattisson, J. , Odden, J. , Nilsen, E.B. , Linnell, J.D.C. , Persson, J. & Andren, H. (2011) Factors affecting Eurasian lynx kill rates on semi‐domestic reindeer in northern Scandinavia: can ecological research contribute to the development of a fair compensation system? Biological Conservation, 144, 3009–3017. [Google Scholar]
- Milner‐Gulland, E.J. (2011) Integrating fisheries approaches and household utility models for improved resource management. Proceedings of the National Academy of Sciences, 108, 1741–1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moen, J. (2008) Climate change: effects on the ecological basis for reindeer husbandry in Sweden. Ambio, 37, 304–311. [DOI] [PubMed] [Google Scholar]
- Næss, M.W. & Bårdsen, B.J. (2010) Environmental stochasticity and long‐term livestock viability‐herd‐accumulation as a risk reducing strategy. Human Ecology, 38, 3–17. [Google Scholar]
- Næss, M.V. , Bårdsen, B.‐J. , Pedersen, E. & Tveraa, T. (2011) Pastoral herding strategies and governmental management objectives: predation compensation as a risk buffering strategy in the Saami reindeer husbandry. Human Ecology, 39, 489–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieminen, M. (2010) The impact of large carnivores on the mortality of semi‐domesticated reindeer (Rangifer tarandus) calves in Kainuu, southeastern reindeer herding region in Finland. Rangifer, 30, 79–88. [Google Scholar]
- Nilsen, E. , Brøseth, H. , Odden, J. & Linnell, J.C. (2012) Quota hunting of Eurasian lynx in Norway: patterns of hunter selection, hunter efficiency and monitoring accuracy. European Journal of Wildlife Research, 58, 325–333. [Google Scholar]
- Patterson, B.R. & Power, V.A. (2002) Contributions of forage competition, harvest, and climate fluctuation to changes in population growth of northern white‐tailed deer. Oecologia, 130, 62–71. [DOI] [PubMed] [Google Scholar]
- Persson, J. , Wedholm, P. & Segerstrom, P. (2010) Space use and territoriality of wolverines (Gulo gulo) in northern Scandinavia. European Journal of Wildlife Research, 56, 49–57. [Google Scholar]
- R Core Team (2013) R: A Language and Environment for Statistical Computing, Version 3.0.0. R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]
- Redpath, S.M. , Young, J. , Evely, A. , Adams, W.M. , Sutherland, W.J. , Whitehouse, A. et al (2013) Understanding and managing conservation conflicts. Trends in Ecology & Evolution, 28, 100–109. [DOI] [PubMed] [Google Scholar]
- Sæther, B.E. (1997) Environmental stochasticity and population dynamics of large herbivores: a search for mechanisms. Trends in Ecology & Evolution, 12, 143–149. [DOI] [PubMed] [Google Scholar]
- Skogland, T. (1991) What are the effects of predators on large ungulate populations. Oikos, 61, 401–411. [Google Scholar]
- Tahvonen, O. , Kumpula, J. & Pekkarinen, A.‐J. (2014) Optimal harvesting of an age‐structured, two‐sex herbivore–plant system. Ecological Modelling, 272, 348–361. [Google Scholar]
- Tovmo, M. & Brøseth, H. (2011) Lynx monitoring in selected areas 2011 (in Norwegian). pp. 28. NINA Report 750.
- Tveraa, T. , Fauchald, P. , Henaug, C. & Yoccoz, N.G. (2003) An examination of a compensatory relationship between food limitation and predation in semi‐domestic reindeer. Oecologia, 137, 370–376. [DOI] [PubMed] [Google Scholar]
- Tveraa, T. , Fauchald, P. , Yoccoz, N.G. , Ims, R.A. , Aanes, R. & Høgda, K.A. (2007) What regulate and limit reindeer populations in Norway? Oikos, 116, 706–715. [Google Scholar]
- Tveraa, T. , Stien, A. , Bårdsen, B.J. & Fauchald, P. (2013) Population densities, vegetation green‐up, and plant productivity: impacts on reproductive success and juvenile body mass in reindeer. PLoS One, 8, e56450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tveraa, T. , Stien, A. , Brøseth, H. & Yoccoz, N.G. (2014) Data from “The role of predation and food limitation on claims for compensation, reindeer demography and population dynamics”. Dryad Digital Repository, 10.5061/dryad.jm7k1. [DOI] [PMC free article] [PubMed]
- Ulvevadet, B. & Hausner, V.H. (2011) Incentives and regulations to reconcile conservation and development: thirty years of governance of the Sami pastoral ecosystem in Finnmark, Norway. Journal of Environmental Management, 92, 2794–2802. [DOI] [PubMed] [Google Scholar]
- Williams, B.K. , Nichols, J.D. & Conroy, M.D. (2002) Analysis and Management of Animal Population. Academic Press, San Diego, CA, USA. [Google Scholar]
- Wilmers, C.C. , Post, E. , Peterson, R.O. & Vucetich, J.A. (2006) Predator disease out‐break modulates top‐down, bottom‐up and climatic effects on herbivore population dynamics. Ecology Letters, 9, 383–389. [DOI] [PubMed] [Google Scholar]
- Zabel, A. & Holm‐Müller, K. (2008) Conservation performance payments for carnivore conservation in Sweden. Conservation Biology, 22, 247–251. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Temporal trends in reindeer data.
Fig. S2. Temporal trends in predator population densities.
Fig. S3. Temporal trends in onset of spring and plant productivity.
Appendix S1. Modelling approach, structure and robustness.


