Abstract
PACT (Protein kinase, interferon-inducible double stranded RNA dependent activator) and its murine ortholog RAX (PKR-associated protein X) were originally identified as a protein activator for the dsRNA-dependent, interferon-inducible protein kinase (PKR). Endogenous PACT/RAX activates PKR in response to diverse stress signals such as serum starvation, and peroxide or arsenite treatment. PACT/RAX heterodimerized with PKR and activated it with its third motif in the absence of dsRNA. The activation of PKR leads to enhanced eIF2α phosphorylation followed by apoptosis or inhibition of growth. Besides the role of activating PKR, PACT is associated with a ~500 kDa complex that contains Dicer, hAgo2, and TRBP (TAR RNA binding protein) and it associates with Dicer to facilitate the production of small interfering RNA. PACT/RAX plays an important role in diverse physiological and pathological processes. Pact−/− mice exhibit notable developmental abnormalities including microtia, with craniofacial ear, and hearing defects. Pact−/− mice had smaller body sizes and fertility defects, both of which were caused by defective pituitary functions. It was found that dRAX disrupted fly embryos homozygous, displayed highly abnormal commissural axon structure of the central nervous system, and 70% of the flies homozygous for the mutant allele died prior to adulthood. Using high density SNP genotyping arrays, it was found that a mutation in PRKRA (the PACT/RAX gene) is the causative genetic mutation in DYT16, a novel autosomal recessive dystonia-parkinsonism syndrome in Brazilian patients.
Keywords: PACT/RAX, PKR, TRBP, Dicer, DYT16
Introduction
A variety of eukaryotic, prokaryotic, viral and plant proteins have now been demonstrated to associate with dsRNA species, to regulate cellular signaling events and gene expression (St Johnston et al., 1992; Fierro-Monti and Mathews, 2000; Saunders and Barber, 2003). These dsRNA binding proteins, which we refer to as DRBPs, were first identified in the early 1990s and contain an evolutionarily conserved dsRNA binding motif (DRBM) of ~65–68 amino acids (Seeman et al., 1976; Ryter and Schultz, 1998).
More than 100 DRBPs have been identified and play diverse roles in cellular functions. An example is the interferon (IFN)-induced, double-stranded (ds) RNA activated protein kinase (PKR); it functions in dsRNA signaling and host defense against virus infection and Dicer, which is implicated in RNA interference (RNAi)-mediated gene silencing. Other DRBPs, such as Staufen, adenosine deaminase acting on RNA (ADAR), and spermatid perinuclear RNA binding protein (SPNR), are known to play essential roles in development, translation, RNA editing, and stability (Saunders and Barber, 2003). In many cases, homozygous or even heterozygous disruption of DRBPs in animal models results in embryonic lethality (Wang et al., 2000; Pires-daSilva et al., 2001). These results indicate the importance of DRBPs in cell physiology. Here, we provide an overview on the progress of research on a DRBP family member, PKR activator (PACT) or PKR-associated protein X (RAX).
PACT/RAX and PKR
PACT/RAX was first identified as the protein activator of PKR
PKR is a key mediator of the anti-viral and anti-proliferative effects of IFN (Hovanessian, 1989). PKR is expressed at low constitutive levels in cells, and is induced by treatment with IFN (Clemens and Elia, 1997). PKR kinase activity requires binding to an activator. The most well-known activator of PKR is dsRNA, and other polyanionic agents, such as heparin, have also been known to activate PKR in vitro (Galabru and Hovanessian, 1987). In interferon-treated cells, virus infection leads to activation of PKR by autophosphorylation, followed by eIF2α phosphorylation and inhibition of viral and cellular protein synthesis (Samuel et al., 1984; Samuel, 1993).
In uninfected cells, PKR is involved in diverse cellular functions such as growth regulation, apoptosis, and differentiation. In several experimental systems, overexpression of PKR can induce proliferation inhibition, apoptosis and differentiation promotion; however, overexpression of dominant negative PKR (K296R) results in tumorigenicity (Proud, 1995; Williams, 1997). Mutant PKR without dsRNA binding activity can still be activated by heparin in vitro and functional in yeast. These findings indicate that PKR may have other cellular activators which can activate PKR and regulate multiple physiological processes in the absence of virus infection.
PACT was identified as the protein activator of PKR. Using a yeast two-hybrid screening, it was found that PACT interacted with PKR. PACT was expressed in all kinds of tissues independent of IFN. It has DRBM, but it can activate PKR in the absence of dsRNA. In mammalian cells, overexpression of PACT can activate PKR, and then induce eIF2α phosphorylation and subsequent inhibition of protein synthesis. Co-expression of PKR and PACT leads to an anti-growth phenotype in Saccharomyces cerevisiae (Patel and Sen, 1998). PACT’s murine ortholog RAX (encoded by prkra gene) was independently discovered as a PKR activator (Ito et al., 1999). PACT and RAX are almost identical in their amino acid sequences; only 6 out of 313 residues are different with 4 similar substitutions.
PACT/RAX associates with PKR in regulating multiple physiological processes
Cellular PACT/RAX is a stress-activated, physiological activator of PKR that couples transmembrane stress signals and protein synthesis. IL-3 deprivation as well as diverse cell stress treatments including arsenite, thapsigargin, and H2O2, which are known to inhibit protein synthesis, induce the rapid phosphorylation of RAX followed by RAX-PKR association and activation of PKR (Ito et al., 1999; Patel et al., 2000). Knockdown of RAX expression by siRNA prevents TNFα induced PKR activation and eIF2α phosphorylation, IκB degradation, IRF-1 expression, and STAT1 phosphorylation, resulting in enhanced MEF cell survival. In contrast, expression of exogenous RAX, but not of the nonphosphorylatable, dominant-negative RAX (S18A) mutant, sensitizes cells to IFN/TNFα, mitomycin C (MMC), or serum deprivation in association with increased PKR activity and apoptosis. These responses indicate that RAX is required to activate PKR in response to a broad range of apoptosis-inducing cellular stresses (Bennett et al., 2006). The ER-stress-mediated eIF2α/ATF4/CHOP cell death pathway is, to some degree, dependent on PACT-mediated PKR activation apart from the PERK pathway. ER stress activates PKR, inducing the phosphorylation of eIF2α. ER-stress-mediated eIF2α/ATF4/CHOP signaling and associated cell death can be markedly reduced by PKR knockdown. This PKR activation is mediated by PACT, the expression of which is elevated by ER-stress (Lee et al., 2007).
We previously reported that the interactions between PKR and PACT/RAX modulate the effect of ethanol on protein synthesis and cell survival in the central nervous system. Exposure to ethanol increases the phosphorylation of PKR and eIF2α and enhances the association of RAX and PKR in the developing cerebellum. Overexpression of a wild-type RAX dramatically enhances sensitivity to ethanol-induced PKR/eIF2α phosphorylation. In contrast, overexpression of a mutant (S18A) RAX inhibited ethanol-mediated PKR/eIF2α activation (Chen et al., 2006).
A recent finding indicates that the RAX/PACT-PKR stress response pathway promotes p53 sumoylation and activation, leading to G1 cell cycle arrest. RAX/PACT interacts with the SUMO E2 ligase Ubc9 to stimulate p53-Ubc9 association and reversible p53 sumoylation on lysine 386. Expression of RAX/PACT in a variety of cell lines promotes p53 stability and increases PKR dependent p53-targeted gene expression (Bennett et al., 2012).
Molecular basis for PACT/RAX activating PKR
PACT/RAX contains three independent motifs. The first two resemble DRBM; the third one consists of 66 residues and appears to be an activator for interacting proteins (Peters et al., 2001). Among the three domains of PACT, the presence of either motif 1 or 2 is sufficient for high-affinity binding of PACT to PKR, while motif 3 is required for PKR activation in vitro and in vivo. In addition, domain 3 and eIF2α both interact with PKR through the same region within PKR, which is mapped to lie between amino acid residues 318 and 551 (Huang et al., 2002).
There is a PACT binding motif (PBM) located in the kinase domain of PKR. An intramolecular interaction between PBM and dsRBMs of PKR is responsible for keeping PKR in an inactive conformation. Disruption of this interaction by point mutations of appropriate residues or expression of a short decoy peptide representing PBM can both produce constitutively active PKR. Motif 3 of PACT can directly bind to PBM and disrupt the intramolecular interaction of PKR which leads to conformational change and autophosphorylation of PKR (Li et al., 2006).
PACT/RAX itself is regulated by phosphorylation on specific residues, such as serine 18. The non-phosphoryla-table form of RAX, RAX (S18A), although is still able to bind dsRNA and associate with PKR, fails to activate PKR in response to cellular stress. Furthermore, stable expression of RAX(S18A) results in a dominant-negative effect characterized by a deficiency of eIF2α phosphorylation, delay of translation inhibition, and failure to undergo rapid apoptosis following removal of interleukin-3 (Bennett et al., 2004). There are another two serine residues, whose phosphorylation was essential for the cellular actions of PACT. Constitutive phosphorylation of one of the two residues, Ser246, was required for stress-induced phosphorylation of the other, Ser287. Substitution of either of them by threonine or aspartic acid, but not alanine, was tolerated. Substitution of both residues with the phosphoserine mimetic, aspartic acid, produces a mutant PACT which will induce PKR activation and apoptosis, even in unstressed cells (Peters et al., 2006). While in stressed cells, mutant PACT activates PKR more effectively and has a tighter association with PKR (Peters et al., 2009). Phosphorylation at serine 246 and 287 is essential for PACT-PACT and PACT-PKR interaction. Point mutation at these sites of PACT abolish PACT–PACT interaction and PKR activation (Singh and Patel, 2012).
Other dsRNA binding proteins regulate PACT/RAX-PKR association by interacting with PACT/RAX
Proteins with conserved dsRBMs can usually interact with each other through these structures. The TAR RNA binding Protein (TRBP) is an example. TRBP and PACT bind to each other through their dsRBMs and their Medipal domains. TRBP binds to PACT to inhibit PACT’s activity on PKR. PACT-induced PKR phosphorylation is restored in Tarbp2 −/− murine tail fibroblasts and in HEK293T or HeLa cells when TRBP expression is reduced by RNA interference. In HEK293T and HeLa cells, arsenite, peroxide, and serum starvation-mediated stresses dissociate the TRBP-PACT interaction and increase PACT-induced PKR activation, demonstrating the relevance of this control in a physiologic context. TRBP negatively regulates PACT-mediated activation of PKR, and this regulation is disrupted by cellular stresses (Daher et al., 2009).
PACT/RAX and RNA silencing pathway
Basics in RNA interference
RNA interference (RNAi) is an evolutionarily conserved mechanism for gene silencing mediated through small RNAs of 22nt (Hannon, 2002). At least two classes of small RNAs have been described in mammals. micro-RNAs (miRNA) produced from hairpin precursors and small interfering RNAs (siRNAs) derived from long double-stranded RNAs (dsRNAs) (Tomari and Zamore, 2005). Both miRNAs and siRNAs are generated by RNase III-type nuclease Dicer, and are assembled into an effector complex termed RNA-induced silencing complex (RISC) (Bernstein et al., 2001; Provost et al., 2002). Although two Dicer enzymes (Dcr1 and Dcr2) have been found in fruit flies and are responsible for the generation of miRNAs and siRNAs, respectively, there exists only one single Dicer in humans, which produces both miRNAs and siRNAs. Besides the processing of miRNA, human Dicer has also been proposed to have a role in RISC assembly, because a depletion of Dicer results in a defect of RNA interference (RNAi) process mediated by siRNA duplex in human cell lines (Doi et al., 2003). Argonaute proteins are the core components of the RISC. Argonaute proteins are capable of interacting directly with small RNAs through their PAZ domains (to the 3′ end) and through the PIWI and the middle domains (to the 5′ end). A small RNA guides the RISC to its target RNA, which leads to mRNA degradation and/or to translational repression (Carmell et al., 2002; Liu et al., 2004; Meister et al., 2005).
PACT is highly involved in Small RNA-mediated gene silencing pathway
Recently, TRBP was reported to interact with Dicer and human Ago2 (hAgo2). The depletion of TRBP by RNAi causes defects of siRNA- or miRNA-mediated RNA silencing processes in human cell lines (Chendrimada et al., 2005). PACT is similar to TRBP in the domain structure. Lee et al. first identified that PACT is associated with a ~500 kDa complex that contains Dicer, hAgo2, and TRBP. The interaction with Dicer involves the third DRBM of PACT and the N-terminal region of Dicer containing the helicase motif. Like TRBP, PACT is not required for the pre-microRNA cleavage reaction step. However, the depletion of PACT strongly affects the accumulation of mature miRNA in vivo and moderately reduces the efficiency of small interfering RNA-induced RNAi (Lee et al., 2006). It was then verified that human TRBP and PACT directly interact with each other and associate with Dicer to stimulate the cleavage of double-stranded or short hairpin RNA to siRNA (Kok et al., 2007). The analysis of the Dicer cleavage products generated in vitro revealed the presence of a cleavage intermediate when pre-miRNA was processed by recombinant Dicer alone. This intermediate was not observed during pre-miRNA cleavage by endogenous Dicer. It demonstrates that one of the roles of AGO2, PACT and TRBP proteins is to assure better synchronization of cleavages triggered by two RNase III domains of Dicer (Koscianska et al., 2011). The ATP-independent dsRNA-specific diffusion activity of TRBP and PACT contributes to enhancing siRNA and miRNA processing by Dicer (Koh et al., 2013). Further research revealed that PACT in complex with Dicer inhibits the processing of pre-siRNA substrates when compared with Dicer and a Dicer–TRBP complex. In addition, PACT and TRBP show non-redundant effects on the production of different-sized miRNAs (isomiRs), which in turn alter target binding specificities (Lee et al., 2013). Monomeric TRBP binds to siRNA at the higher affinity compared to the affinity for its own homodimerization. In contrast, the affinity between PACT and siRNA is lower than that of the homodimerization or that between TRBP and siRNA. Thus, siRNA may be more readily incorporated into a RISC loading complex, interacting with TRBP (instead of PACT) in vivo (Takahashi et al., 2013). The cytoplasmic RISC proteins PACT, TRBP, and Dicer are steroid receptor RNA activators (SRA) binding nuclear receptor (NR) co-regulators that target steroid-responsive promoters and regulate NR activity and downstream gene expression (Redfern et al., 2013). Recently, the rough endoplasmic reticulum was found to have a central nucleation site of siRNA-mediated RNA silencing. Also, TRBP and PACT are key factors anchoring RISC to ER membranes in an RNA-independent manner (Stalder et al., 2013). Increasing evidence shows that PACT/RAX is highly involved in several critical steps of the Small RNA-mediated gene silencing pathway, indicating that PACT/RAX may play an important role in diverse physiologic and pathological processes.
PACT/RAX and development
To determine the physiological functions of PACT/RAX, Pact−/− mice are generated. The single-copy mouse prkra gene is disrupted and expression of the protein is completely ablated. The most notable phenotypes of the Pact−/− mouse are reduced size and severe microtia. As a result of the congenital abnormality of both outer and middle ears, these mice are hearing impaired (Rowe et al., 2006). In addition, Pact−/− mice have smaller body size and fertility defects caused by defective pituitary functions. Pact−/− mice exhibit anterior pituitary lobe (AL) hypoplasia, which develops postnatally, when the second phase of pituitary expansion occurs (Peters et al., 2009). In another knockout mouse model, deletion of the entire Rax gene results in no mice homozygous for the mutant allele. Also, there are no embryos obtained by mating heterozygous mice at either E3.5, 7, or 14 that was nullizygous for the Rax gene, which means the Rax gene is embryonic lethal in mice at a preimplantation stage of development. In Drosophila, dRax (loqs/R3D1, homologous gene of PACT/RAX) is expressed at high levels in the developing nerve cord. Fly embryos homozygous with dRax disruption display highly abnormal commissural axon structure of the CNS; 70% of the flies homozygous for the mutant allele die prior to adulthood. Surviving male flies have reduced fertility and female flies are sterile (Bennett et al., 2008). Notably, these defects are not present in 2 models of PKR-deficient mice (Yang et al., 1995; Abraham et al., 1999), supporting additional roles of PACT beyond activating PKR at the physiological level. PACT/RAX’s role in the gene silencing pathway may be partially responsible for these defects.
PACT and diseases
Dystonia and parkinsonism may present as part of the same genetic disorder. By high-density genome-wide SNP genotyping of two unrelated families with novel autosomal recessive dystonia-parkinsonism syndrome, Camargos et al. identify a mutation within the PACT gene prkra in a novel disease locus DYT16. These patients have progressive, generalized, early-onset dystonia with axial muscle involvement, oromandibular (sardonic smile), laryngeal dystonia and, in some cases, parkinsonian features. These patients do not respond to levodopa therapy, which may be partially correlated with its interplay with PKR or the gene silencing pathway (Bando et al., 2005; Camargos et al., 2008).
Summary
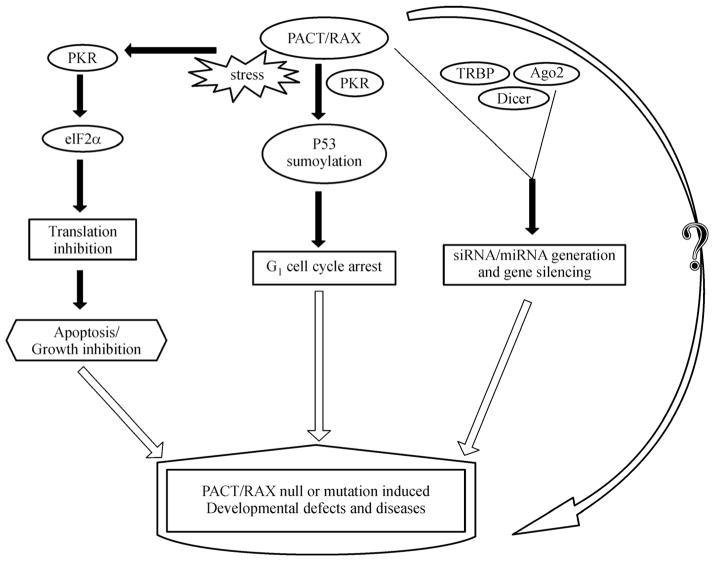
PACT/RAX is a stress responsive protein. Various cellular stresses can promote PACT/RAX and PKR interaction, resulting in the inhibition of protein synthesis and induction of growth arrest or apoptosis. Some of RAX/PACT-PKR signals are mediated by p53. PACT/RAX can also regulate gene silencing by modulating siRNA/miRNA generation. PACT/RAX knockout or mutation leads to some diseases and serious development defects, indicating its important regulatory role in cell physiology and development (Fig. 1). Our understanding of PACT/RAX’s function is very limited. Therefore, research on cell biology of PACT/RAX is important and will provide potential new approaches for disease prevention and intervention.
Figure 1.
Role of PACT/RAX in the development and diseases. PACT/RAX is activated by various cellular stresses and interacts with PKR, resulting in eIF2α phosphorylation and subsequent inhibition of protein synthesis and induction of apoptosis or growth inhibition. RAX/PACT-PKR interaction promotes p53 sumoylation, leading to G1 cell cycle arrest. PACT/RAX associates with TRBP, Dicer and Ago2 and regulates siRNA/miRNA generation and gene silencing. PACT/RAX knockout or mutation leads to diseases and serious development defects through unknown mechanisms. PACT/RAX is an important stress responsive protein and plays important in cell physiology and development.
Acknowledgments
We would like to thank Jacqueline A. Frank for reading this manuscript. This research was supported by grants from the Ministry of Science and Technology of China (2010CB912000), the National Natural Science Foundation of China (31271142), Program of Clinical Research Center, Institute for Nutritional Sciences and Xuhui Central Hospital (CRC20100010). Dr J. Luo was also supported by a grant from NIH/NIAAA (AA015407).
Footnotes
Compliance with ethics guidelines
Yue Yong, Jia Luo, and Zunji Ke declare that they have no conflict of interest. This paper does not involve human and animal studies, so it is unnecessary to include Human and animal right and informed concent.
Contributor Information
Jia LUO, Email: jialuo888@uky.edu.
Zun-Ji KE, Email: zunjike@gmail.com.
References
- Abraham N, Stojdl DF, Duncan PI, Méthot N, Ishii T, Dubé M, Vanderhyden BC, Atkins HL, Gray DA, McBurney MW, Koromilas AE, Brown EG, Sonenberg N, Bell JC. Characterization of transgenic mice with targeted disruption of the catalytic domain of the double-stranded RNA-dependent protein kinase, PKR. J Biol Chem. 1999;274(9):5953–5962. doi: 10.1074/jbc.274.9.5953. [DOI] [PubMed] [Google Scholar]
- Bando Y, Onuki R, Katayama T, Manabe T, Kudo T, Taira K, Tohyama M. Double-strand RNA dependent protein kinase (PKR) is involved in the extrastriatal degeneration in Parkinson’s disease and Huntington’s disease. Neurochem Int. 2005;46(1):11–18. doi: 10.1016/j.neuint.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Bennett RL, Blalock WL, Abtahi DM, Pan Y, Moyer SA, May WS. RAX, the PKR activator, sensitizes cells to inflammatory cytokines, serum withdrawal, chemotherapy, and viral infection. Blood. 2006;108(3):821–829. doi: 10.1182/blood-2005-11-006817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett RL, Blalock WL, Choi EJ, Lee YJ, Zhang Y, Zhou L, Oh SP, May WS. RAX is required for fly neuronal development and mouse embryogenesis. Mech Dev. 2008;125(9–10):777–785. doi: 10.1016/j.mod.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett RL, Blalock WL, May WS. Serine 18 phosphorylation of RAX, the PKR activator, is required for PKR activation and consequent translation inhibition. J Biol Chem. 2004;279(41):42687–42693. doi: 10.1074/jbc.M403321200. [DOI] [PubMed] [Google Scholar]
- Bennett RL, Pan Y, Christian J, Hui T, May WS., Jr The RAX/PACT-PKR stress response pathway promotes p53 sumoylation and activation, leading to G1 arrest. Cell Cycle. 2012;11(2):407–417. doi: 10.4161/cc.11.2.18999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein E, Caudy AA, Hammond SM, Hannon GJ. Role for a bidentate ribonuclease in the initiation step of RNA interference. Nature. 2001;409(6818):363–366. doi: 10.1038/35053110. [DOI] [PubMed] [Google Scholar]
- Camargos S, Scholz S, Simón-Sánchez J, Paisán-Ruiz C, Lewis P, Hernandez D, Ding J, Gibbs JR, Cookson MR, Bras J, Guerreiro R, Oliveira CR, Lees A, Hardy J, Cardoso F, Singleton AB. DYT16, a novel young-onset dystonia-parkinsonism disorder: identification of a segregating mutation in the stress-response protein PRKRA. Lancet Neurol. 2008;7(3):207–215. doi: 10.1016/S1474-4422(08)70022-X. [DOI] [PubMed] [Google Scholar]
- Carmell MA, Xuan Z, Zhang MQ, Hannon GJ. The Argonaute family: tentacles that reach into RNAi, developmental control, stem cell maintenance, and tumorigenesis. Genes Dev. 2002;16(21):2733–2742. doi: 10.1101/gad.1026102. [DOI] [PubMed] [Google Scholar]
- Chen G, Ma C, Bower KA, Ke Z, Luo J. Interaction between RAX and PKR modulates the effect of ethanol on protein synthesis and survival of neurons. J Biol Chem. 2006;281(23):15909–15915. doi: 10.1074/jbc.M600612200. [DOI] [PubMed] [Google Scholar]
- Chendrimada TP, Gregory RI, Kumaraswamy E, Norman J, Cooch N, Nishikura K, Shiekhattar R. TRBP recruits the Dicer complex to Ago2 for microRNA processing and gene silencing. Nature. 2005;436(7051):740–744. doi: 10.1038/nature03868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens MJ, Elia A. The double-stranded RNA-dependent protein kinase PKR: structure and function. J Interferon Cytokine Res. 1997;17(9):503–524. doi: 10.1089/jir.1997.17.503. [DOI] [PubMed] [Google Scholar]
- Daher A, Laraki G, Singh M, Melendez-Peña CE, Bannwarth S, Peters AH, Meurs EF, Braun RE, Patel RC, Gatignol A. TRBP control of PACT-induced phosphorylation of protein kinase R is reversed by stress. Mol Cell Biol. 2009;29(1):254–265. doi: 10.1128/MCB.01030-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi N, Zenno S, Ueda R, Ohki-Hamazaki H, Ui-Tei K, Saigo K. Short-interfering-RNA-mediated gene silencing in mammalian cells requires Dicer and eIF2C translation initiation factors. Curr Biol. 2003;13(1):41–46. doi: 10.1016/s0960-9822(02)01394-5. [DOI] [PubMed] [Google Scholar]
- Fierro-Monti I, Mathews MB. Proteins binding to duplexed RNA: one motif, multiple functions. Trends Biochem Sci. 2000;25(5):241–246. doi: 10.1016/s0968-0004(00)01580-2. [DOI] [PubMed] [Google Scholar]
- Galabru J, Hovanessian A. Autophosphorylation of the protein kinase dependent on double-stranded RNA. J Biol Chem. 1987;262(32):15538–15544. [PubMed] [Google Scholar]
- Hannon GJ. RNA interference. Nature. 2002;418(6894):244–251. doi: 10.1038/418244a. [DOI] [PubMed] [Google Scholar]
- Hovanessian AG. The double stranded RNA-activated protein kinase induced by interferon: dsRNA-PK. J Interferon Res. 1989;9(6):641–647. doi: 10.1089/jir.1989.9.641. [DOI] [PubMed] [Google Scholar]
- Huang X, Hutchins B, Patel RC. The C-terminal, third conserved motif of the protein activator PACT plays an essential role in the activation of double-stranded-RNA-dependent protein kinase (PKR) Biochem J. 2002;366(Pt 1):175–186. doi: 10.1042/BJ20020204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito T, Yang M, May WS. RAX, a cellular activator for double-stranded RNA-dependent protein kinase during stress signaling. J Biol Chem. 1999;274(22):15427–15432. doi: 10.1074/jbc.274.22.15427. [DOI] [PubMed] [Google Scholar]
- Koh HR, Kidwell MA, Ragunathan K, Doudna JA, Myong S. ATP-independent diffusion of double-stranded RNA binding proteins. Proc Natl Acad Sci USA. 2013;110(1):151–156. doi: 10.1073/pnas.1212917110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kok KH, Ng MH, Ching YP, Jin DY. Human TRBP and PACT directly interact with each other and associate with dicer to facilitate the production of small interfering RNA. J Biol Chem. 2007;282(24):17649–17657. doi: 10.1074/jbc.M611768200. [DOI] [PubMed] [Google Scholar]
- Koscianska E, Starega-Roslan J, Krzyzosiak WJ. The role of Dicer protein partners in the processing of microRNA precursors. PLoS ONE. 2011;6(12):e28548. doi: 10.1371/journal.pone.0028548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee ES, Yoon CH, Kim YS, Bae YS. The double-strand RNA-dependent protein kinase PKR plays a significant role in a sustained ER stress-induced apoptosis. FEBS Lett. 2007;581(22):4325–4332. doi: 10.1016/j.febslet.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Lee HY, Zhou K, Smith AM, Noland CL, Doudna JA. Differential roles of human Dicer-binding proteins TRBP and PACT in small RNA processing. Nucleic Acids Res. 2013;41(13):6568–6576. doi: 10.1093/nar/gkt361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Hur I, Park SY, Kim YK, Suh MR, Kim VN. The role of PACT in the RNA silencing pathway. EMBO J. 2006;25(3):522–532. doi: 10.1038/sj.emboj.7600942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Peters GA, Ding K, Zhang X, Qin J, Sen GC. Molecular basis for PKR activation by PACT or dsRNA. Proc Natl Acad Sci USA. 2006;103(26):10005–10010. doi: 10.1073/pnas.0602317103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Carmell MA, Rivas FV, Marsden CG, Thomson JM, Song JJ, Hammond SM, Joshua-Tor L, Hannon GJ. Argonaute2 is the catalytic engine of mammalian RNAi. Science. 2004;305(5689):1437–1441. doi: 10.1126/science.1102513. [DOI] [PubMed] [Google Scholar]
- Meister G, Landthaler M, Peters L, Chen PY, Urlaub H, Lührmann R, Tuschl T. Identification of novel argonaute-associated proteins. Curr Biol. 2005;15(23):2149–2155. doi: 10.1016/j.cub.2005.10.048. [DOI] [PubMed] [Google Scholar]
- Patel CV, Handy I, Goldsmith T, Patel RC. PACT, a stress-modulated cellular activator of interferon-induced double-stranded RNA-activated protein kinase, PKR. J Biol Chem. 2000;275(48):37993–37998. doi: 10.1074/jbc.M004762200. [DOI] [PubMed] [Google Scholar]
- Patel RC, Sen GC. PACT, a protein activator of the interferon-induced protein kinase, PKR. EMBO J. 1998;17(15):4379–4390. doi: 10.1093/emboj/17.15.4379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters GA, Hartmann R, Qin J, Sen GC. Modular structure of PACT: distinct domains for binding and activating PKR. Mol Cell Biol. 2001;21(6):1908–1920. doi: 10.1128/MCB.21.6.1908-1920.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters GA, Li S, Sen GC. Phosphorylation of specific serine residues in the PKR activation domain of PACT is essential for its ability to mediate apoptosis. J Biol Chem. 2006;281(46):35129–35136. doi: 10.1074/jbc.M607714200. [DOI] [PubMed] [Google Scholar]
- Peters GA, Seachrist DD, Keri RA, Sen GC. The double-stranded RNA-binding protein, PACT, is required for postnatal anterior pituitary proliferation. Proc Natl Acad Sci USA. 2009;106(26):10696–10701. doi: 10.1073/pnas.0900735106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pires-daSilva A, Nayernia K, Engel W, Torres M, Stoykova A, Chowdhury K, Gruss P. Mice deficient for spermatid perinuclear RNA-binding protein show neurologic, spermatogenic, and sperm morphological abnormalities. Dev Biol. 2001;233(2):319–328. doi: 10.1006/dbio.2001.0169. [DOI] [PubMed] [Google Scholar]
- Proud CG. PKR: a new name and new roles. Trends Biochem Sci. 1995;20(6):241–246. doi: 10.1016/s0968-0004(00)89025-8. [DOI] [PubMed] [Google Scholar]
- Provost P, Dishart D, Doucet J, Frendewey D, Samuelsson B, Rådmark O. Ribonuclease activity and RNA binding of recombinant human Dicer. EMBO J. 2002;21(21):5864–5874. doi: 10.1093/emboj/cdf578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redfern AD, Colley SM, Beveridge DJ, Ikeda N, Epis MR, Li X, Foulds CE, Stuart LM, Barker A, Russell VJ, Ramsay K, Kobelke SJ, Li X, Hatchell EC, Payne C, Giles KM, Messineo A, Gatignol A, Lanz RB, O’Malley BW, Leedman PJ. RNA-induced silencing complex (RISC) Proteins PACT, TRBP, and Dicer are SRA binding nuclear receptor coregulators. Proc Natl Acad Sci USA. 2013;110(16):6536–6541. doi: 10.1073/pnas.1301620110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe TM, Rizzi M, Hirose K, Peters GA, Sen GC. A role of the double-stranded RNA-binding protein PACT in mouse ear development and hearing. Proc Natl Acad Sci USA. 2006;103(15):5823–5828. doi: 10.1073/pnas.0601287103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryter JM, Schultz SC. Molecular basis of double-stranded RNA-protein interactions: structure of a dsRNA-binding domain complexed with dsRNA. EMBO J. 1998;17(24):7505–7513. doi: 10.1093/emboj/17.24.7505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel CE. The eIF-2 alpha protein kinases, regulators of translation in eukaryotes from yeasts to humans. J Biol Chem. 1993;268(11):7603–7606. [PubMed] [Google Scholar]
- Samuel CE, Duncan R, Knutson GS, Hershey JW. Mechanism of interferon action. Increased phosphorylation of protein synthesis initiation factor eIF-2 alpha in interferon-treated, reovirus-infected mouse L929 fibroblasts in vitro and in vivo. J Biol Chem. 1984;259(21):13451–13457. [PubMed] [Google Scholar]
- Saunders LR, Barber GN. The dsRNA binding protein family: critical roles, diverse cellular functions. FASEB J. 2003;17(9):961–983. doi: 10.1096/fj.02-0958rev. [DOI] [PubMed] [Google Scholar]
- Seeman NC, Rosenberg JM, Rich A. Sequence-specific recognition of double helical nucleic acids by proteins. Proc Natl Acad Sci USA. 1976;73(3):804–808. doi: 10.1073/pnas.73.3.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh M, Patel RC. Increased interaction between PACT molecules in response to stress signals is required for PKR activation. J Cell Biochem. 2012;113(8):2754–2764. doi: 10.1002/jcb.24152. [DOI] [PubMed] [Google Scholar]
- St Johnston D, Brown NH, Gall JG, Jantsch M. A conserved double-stranded RNA-binding domain. Proc Natl Acad Sci USA. 1992;89(22):10979–10983. doi: 10.1073/pnas.89.22.10979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stalder L, Heusermann W, Sokol L, Trojer D, Wirz J, Hean J, Fritzsche A, Aeschimann F, Pfanzagl V, Basselet P, Weiler J, Hintersteiner M, Morrissey DV, Meisner-Kober NC. The rough endoplasmatic reticulum is a central nucleation site of siRNA-mediated RNA silencing. EMBO J. 2013;32(8):1115–1127. doi: 10.1038/emboj.2013.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Miyakawa T, Zenno S, Nishi K, Tanokura M, Ui-Tei K. Distinguishable in vitro binding mode of monomeric TRBP and dimeric PACT with siRNA. PLoS ONE. 2013;8(5):e63434. doi: 10.1371/journal.pone.0063434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomari Y, Zamore PD. Perspective: machines for RNAi. Genes Dev. 2005;19(5):517–529. doi: 10.1101/gad.1284105. [DOI] [PubMed] [Google Scholar]
- Wang Q, Khillan J, Gadue P, Nishikura K. Requirement of the RNA editing deaminase ADAR1 gene for embryonic erythropoiesis. Science. 2000;290(5497):1765–1768. doi: 10.1126/science.290.5497.1765. [DOI] [PubMed] [Google Scholar]
- Williams BR. Role of the double-stranded RNA-activated protein kinase (PKR) in cell regulation. Biochem Soc Trans. 1997;25(2):509–513. doi: 10.1042/bst0250509. [DOI] [PubMed] [Google Scholar]
- Yang YL, Reis LF, Pavlovic J, Aguzzi A, Schäfer R, Kumar A, Williams BR, Aguet M, Weissmann C. Deficient signaling in mice devoid of double-stranded RNA-dependent protein kinase. EMBO J. 1995;14(24):6095–6106. doi: 10.1002/j.1460-2075.1995.tb00300.x. [DOI] [PMC free article] [PubMed] [Google Scholar]