Abstract
Background
Complex fractionated atrial electrograms (CFAE) are morphologically more uniform in persistent longstanding as compared with paroxysmal atrial fibrillation (AF). It was hypothesized that this may result from a greater degree of repetitiveness in CFAE patterns at disparate left atrial (LA) sites in longstanding AF.
Methods and Results
CFAEs were obtained from recording sites outside the 4 pulmonary vein (PV) ostia and at a posterior and an anterior LA site during paroxysmal and longstanding persistent AF (10 patients each, 120 sequences total). To quantify repetitiveness in CFAE, the dominant frequency was measured from ensemble spectra using 8.4-second sequences, and repetitiveness was calculated by 2 novel techniques: linear prediction and Fourier reconstruction methods. Lower prediction and reconstruction errors were considered indicative of increasing repetitiveness and decreasing randomness. In patients with paroxysmal AF, CFAE pattern repetitiveness was significantly lower (randomness higher) at antral sites outside PV ostia as compared with LA free wall sites (P<0.001). In longstanding AF, repetitiveness increased outside the PV ostia, especially outside the left superior PV ostium, and diminished at the LA free wall sites. The result was that in persistent AF, there were no significant site-specific differences in CFAE repetitiveness at the selected LA locations used in this study. Average dominant frequency magnitude was 5.32±0.29 Hz in paroxysmal AF and higher in longstanding AF, at 6.27±0.13 Hz (P<0.001), with the frequency of local activation approaching a common upper bound for all sites.
Conclusions
In paroxysmal AF, CFAE repetitiveness is low and randomness high outside the PVs, particularly the left superior PV. As evolution to persistent longstanding AF occurs, CFAE repetitiveness becomes more uniformly distributed at disparate sites, possibly signifying an increasing number of drivers from remote PVs.
Keywords: atrial fibrillation, CFAE, dominant frequency, ensemble average, linear prediction
Complex fractionated atrial electrograms (CFAE) are frequently recorded at multiple left atrial (LA) sites during atrial fibrillation (AF).1 CFAEs are often characterized by their frequency content using the dominant frequency (DF), defined as the peak of greatest magnitude in the power spectrum, representing the largest fundamental periodicity.2–4 To further assess periodicities, the regularity index (RI), defined as the power in the DF divided by the spectral power in the range of interest (2 to 20 Hz),5 and the organizational index (OI), defined as the power in the DF and its harmonics divided by spectral power in the range of interest,6 were devised. Examples of these indices are shown and described in Figure 1. None of these indices, however, provides any information regarding the relative importance of repeating but nonperiodic components of the signal. If there are multiple fundamental frequencies in the signal, arising, for example, from independently firing sources driving AF, the DF only indicates the largest spectral peak and will thus detect only 1 of the sources, and the RI and OI will only include the spectral power related to the fundamental frequency and therefore are unable to detect repeating but nonperiodic patterns of CFAE deflections. Furthermore, when the standard Fourier transform method is used for analysis, CFAE signals are filtered; thus RI and OI may not accurately represent the original electrogram periodicity.7,8 Accurately quantifying both the periodic and the nonperiodic, repeating patterns in CFAEs, resulting possibly from multiple sources without distortion, might be useful in identifying the locations of key drivers and perpetuators of AF.
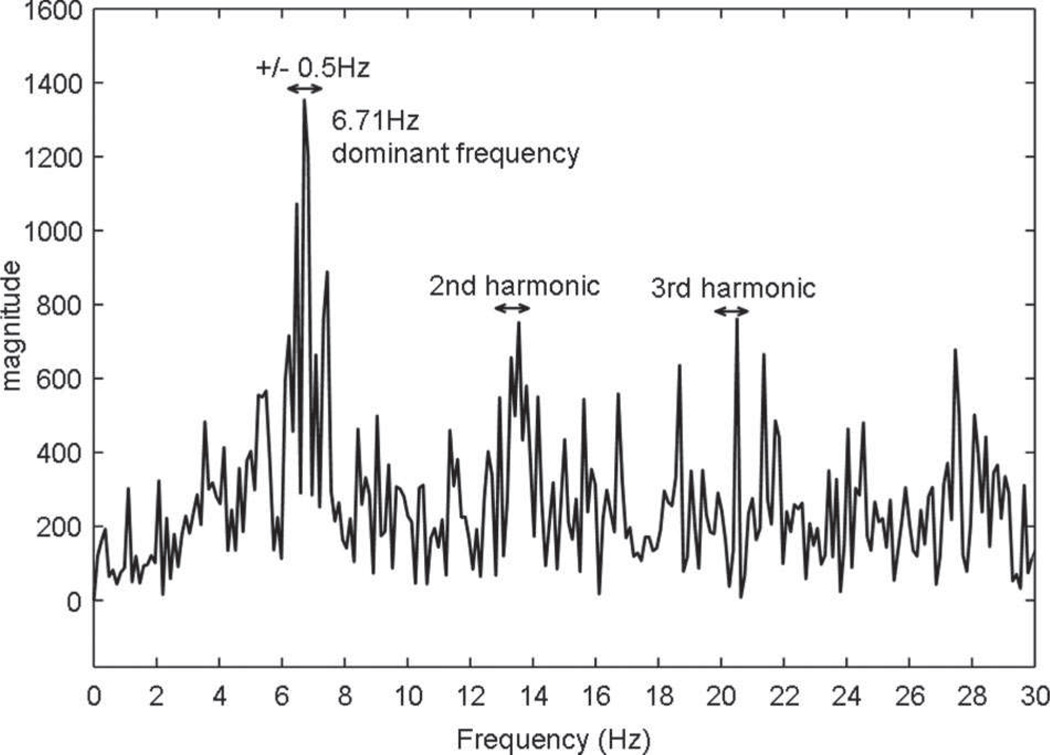
Figure 1.
Fourier power spectrum from 0 to 30 Hz is shown for a CFAE recording obtained from a site outside the LSPV of a patient with persistent AF. The DF is defined as the tallest peak in the power spectrum from 2 to 20 Hz and is located at 6.71 Hz. The RI is defined as the spectral power at the DF ±0.5 Hz (denoted by arrow) divided by the total spectral power in the region of interest (2 to 20 Hz). The OI is defined as the spectral power of the DF and its harmonics ±0.5 Hz combined (denoted by arrows) divided by the total spectral power in the region of interest (2 to 20 Hz).
In previous work, it was shown that the morphology of CFAE deflections depends on location and that it differs at the pulmonary vein (PV) ostia as compared with the LA free wall and whether AF is paroxysmal or longstanding.9 The morphologically most uniform CFAE deflections were recorded in longstanding persistent AF cases. In the present study, we developed novel techniques to quantify the degree of reproducibility or repeatability in CFAE patterns recorded from disparate LA sites. Using these techniques, we tested the hypothesis that repeatability of CFAE patterns is also site-specific during AF and that paroxysmal AF differs from longstanding persistent AF in these patterns and their distribution in the LA. Repetitiveness in CFAE signals was quantified using 2 novel indices of regularity, based on linear prediction and signal reconstruction techniques. With these 2 independent measurements described in the Methods section, both periodic as well as nonperiodic but repeating CFAE patterns could be detected.
Methods
Clinical Data and the Electrophysiology Procedure
Atrial electrograms recorded in a series of 20 patients referred to the Columbia University Medical Center cardiac electrophysiology laboratory for catheter ablation of AF were retrospectively analyzed. Ten patients had documented clinical paroxysmal AF, and all 10 had normal sinus rhythm as their baseline cardiac rhythm in the cardiac electrophysiology laboratory. Acute AF was induced, repeatedly if necessary, by burst atrial pacing from the coronary sinus or right atrial lateral wall, and the induced AF was allowed to persist for at least 10 minutes before any data collection. Ten other patients had longstanding persistent AF and had been in AF without interruption for 1 to 5 years before their catheter mapping and ablation procedure. LA electroanatomic mapping (CARTO, Biosense-Webster Inc, Diamond Bar, CA) was performed using a 3.5-mm irrigated-tip radiofrequency ablation catheter. The standard protocol involved placing multipolar electrode catheters in the right atrium and in the coronary sinus and a steerable catheter and circular “lasso” catheter in the LA through transseptal puncture. Using standard settings, electrograms recorded from the distal ablation electrode were filtered in hardware at acquisition to remove baseline drift and high-frequency noise. The filtered signals were then sampled at 977 Hz and stored. The digitized signals were analyzed with computer programs developed by the authors. Retrospective analysis of electrograms was approved by the Institutional Review Board at Columbia University Medical Center.
Only signals that were identified to be CFAEs by 2 cardiac electrophysiologists, using criteria similar to previously published ones,1 were included in this analysis. The local electrograms were considered to meet CFAE criteria if each component had multiple positive or negative deflections and if they were not separated by an isoelectric line >120 ms. This definition allowed continuous electric activity (lack of an isoelectric line segment) also to be classified as CFAE. One cardiac electrophysiologist recorded the data in the cardiac electrophysiology laboratory. The second electrophysiologist, who validated the CFAEs and selected the recording segments to be analyzed, was blinded to the patient identity, type of AF, and the anatomic recording site. CFAE recordings of >10 seconds in duration were obtained in each patient at antral locations outside the ostia of each of the PVs: the left superior pulmonary vein (LSPV), the left inferior pulmonary vein (LIPV), the right superior pulmonary vein (RSPV), and the right inferior pulmonary vein (RIPV). Similar recordings were obtained from 2 additional LA free wall sites, 1 in the mid posterior wall, and 1 on the anterior ridge at the base of the LA appendage (ANT). These recording sites were selected for their relative ease of reproducibility from one patient to the next. The PV ostial sites were in the antrum regions, about 1 cm outside the ostia as determined by CARTO and ultrasonic measurements. These sites were chosen on the basis of local electrogram morphology manifesting CFAE criteria, with no particular attention to the previously published anatomic sites for the ganglionic plexi. It has been shown that the assessment of fractionated electrograms requires a recording duration of >5 seconds at each site to obtain a consistent result.10 From each of the recordings at each recording site mentioned above, an 8.4-second CFAE sequence (8192 sample points) during AF was extracted and analyzed. Thus, a total of 120 CFAE sequences (6 anatomic locations ×20 patients), each of 8.4-second duration, were included in this analysis. All electrogram recordings were obtained at the same gain during sustained AF before any ablation.
Regularity and DF Measurements
Two independent methods were used to quantify repeating patterns in CFAE. The first one, linear prediction (LP), is a method that estimates, without filtering or distortion, future signal values from adaptively weighted past values. Using this method, periodic components are readily predicted. Also, nonperiodic repeatable components can be identified, as long as the predictive coefficients retain memory of the pattern at its next instance. Supposing this to be the case, then an increased level of regularity in CFAE signals may be defined as a decrease in prediction error. In contrast to RI and OI calculations that are used as measures of regularity in traditional DF analysis,5,6 with linear prediction, the signal is not distorted before measurement because no filtering is applied. The linear predictive weights (coefficients) are adapted using the method of finite differences, which was previously tested and validated in a canine postinfarction model,11 and later used to remove common mode artifact for improvement of cardiovascular signal quality12 and for motion artifact cancellation in tonometric recordings.13 Linear prediction was implemented using the following algorithm:
| (1a) |
| (1b) |
where v̲ is a vector of estimates of n future values of CFAE from discrete time epoch j to j−n+1, C is a matrix (linear transformation) of prior CFAE values, the elements of w̲ are the prediction coefficients (weights), and
| (2a) |
| (2b) |
| (2c) |
…
| (2d) |
where j−1, j−2, …, j−2n+1 are prior discrete time epochs, and the row vectors c̲1, c̲2, …, c̲n are sequences of prior CFAE values. Thus, the estimate vector v is composed of weighted prior values of the CFAE signal that are summed. The mean squared error (MSE) between the estimated and actual CFAE values can be approximated as
| (3) |
with column vector:
| (4) |
The MSE approximation becomes exact when the expectation operator is used rather than averaging a finite number of values. To update the weight vector, finite differences are taken:
| (5a) |
where
| (6a) |
| (6b) |
| (6c) |
where ± ω̲ are vectors having all elements set to zero except for the ith element, which has finite difference values of ±ωi. Thus
| (7a) |
| (7b) |
The weight vector is updated accordingly (ie, in the direction that has lower MSE):
| (8) |
which is repeated for all weights wi, i=1 to n, contained in weight vector w̲. Larger predictive order (larger n) is useful to detect repetitive patterns in CFAE deflections that are relatively disparate in time; however, the response time to adjust to new patterns will increase because the length of the weight vector requiring adjustment increases. Conversely, prediction using lesser order n will result in rapid response to new events but decreases the capability to accurately predict patterns that recur with longer time intervals in between. Therefore, a range of orders n was selected to account for regularly occurring patterns of both lesser and greater time intervals between occurrence. We selected orders from 10 to 60 incremented by 1 unit (10, 11, …, 60) which was done so that correspondence to both high- and low-frequency patterns could be obtained. For simplicity, the same finite difference, ±0.001 mV, or ≈0.1% of peak deflections, was used for adaptive update for all orders n. Decreased prediction error (MSE in Equation 3) is indicative of both greater periodicity of CFAE deflection patterns and greater repeatability of nonperiodic patterns in the CFAE deflections. Hence, this method detects any repeating patterns whether periodic or not. The root MSE was averaged at each anatomic location for each predictive order n and compared for paroxysmal versus longstanding persistent AF data.
As a second independent measure of repeatability, or pattern reproducibility over time, CFAE signal reconstruction was also implemented using the Fourier reconstruction (FR) method. First, the Fourier transform of each CFAE was obtained (8192 sample points, sampling rate of 977 Hz); thus, the Fourier components had a frequency resolution of 0.12 Hz (sampling rate/sample points). These Fourier components were reordered from greatest to least power. The original CFAE signal was then reconstructed using a limited set of Fourier components in descending order of magnitude coefficient (ie, starting with the component having highest magnitude coefficient, where the magnitude is given as the square root of the real Fourier component squared plus the imaginary Fourier component squared). Therefore, independent peaks in the power spectrum, possibly corresponding to different generators, would be recruited for reconstruction, provided that the amplitude of the peaks, and thus their power, was sufficiently high. We selected 30 to 300 components for reconstruction at decade intervals (30, 40, …, 300). The lower end (30) resulted in an only rudimentary resemblance of the reconstruction to the original signal, whereas at the high end (300) the reconstruction was nearly perfect. The MSE was tabulated to determine the similarity of the reconstructed signal to the actual CFAE signal.
The DF was computed for all CFAE by use of the ensemble average method.14,15 This analysis was entirely automated, with the restriction that the DF was selected from the spectral range from 3 to 12 Hz.
When these techniques of quantifying repetitiveness are applied to relatively organized or obviously repetitive activities such as atrial tachycardia or simulated atrial signals, both LP and FR errors are expected to be low. Such examples are shown in Figure 2. As expected, the errors are low, and the actual values are affected by the order or the duration of the signal data used to make the prediction.
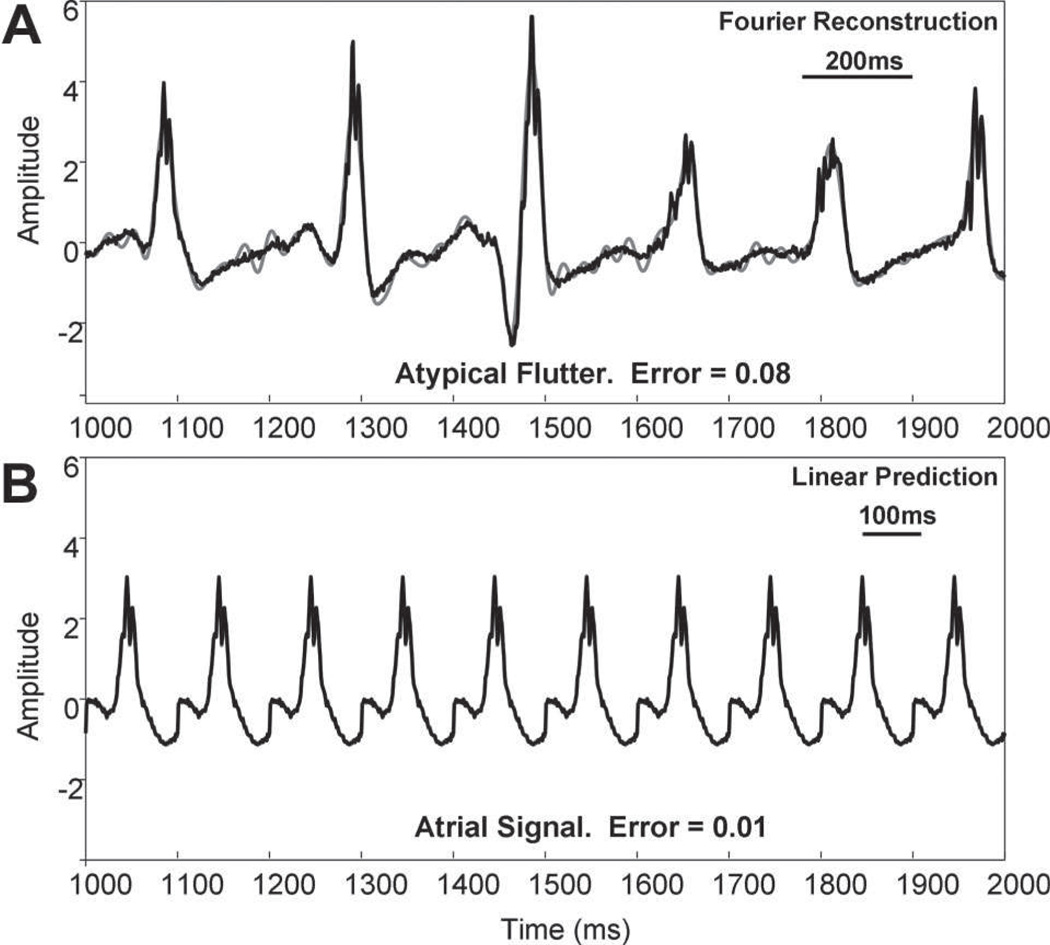
Figure 2.
Fourier reconstruction and linear predictive results (gray traces) for the case of less complex signals (black traces). For ease of comparison, the same scales are used in both panels. A, Fourier reconstruction is shown for an electrogram recorded within the coronary sinus during atypical flutter. The error is only 0.08. B, The first deflection in A (duration, 100 ms) was repeated by simulation to show that linear prediction can model it almost exactly (error=0.01).
For illustrative purposes, the linear predictive values (orders 10 to 60) at the 6 anatomic locations were separately averaged for the 10 paroxysmal and for the 10 patients with persistent AF (n=10 for each). For statistical comparison, the linear predictive values were averaged together (n=51). The mean and standard deviation of these averages were then computed for each location and patient type (n=10 for each). The Student unpaired t test was used to determine statistical significance of the means (P<0.05; Sigma Plot version 9.01, 2004, Systat Software). The procedure was repeated with the 28 Fourier reconstruction calculations for each location and patient type.
Results
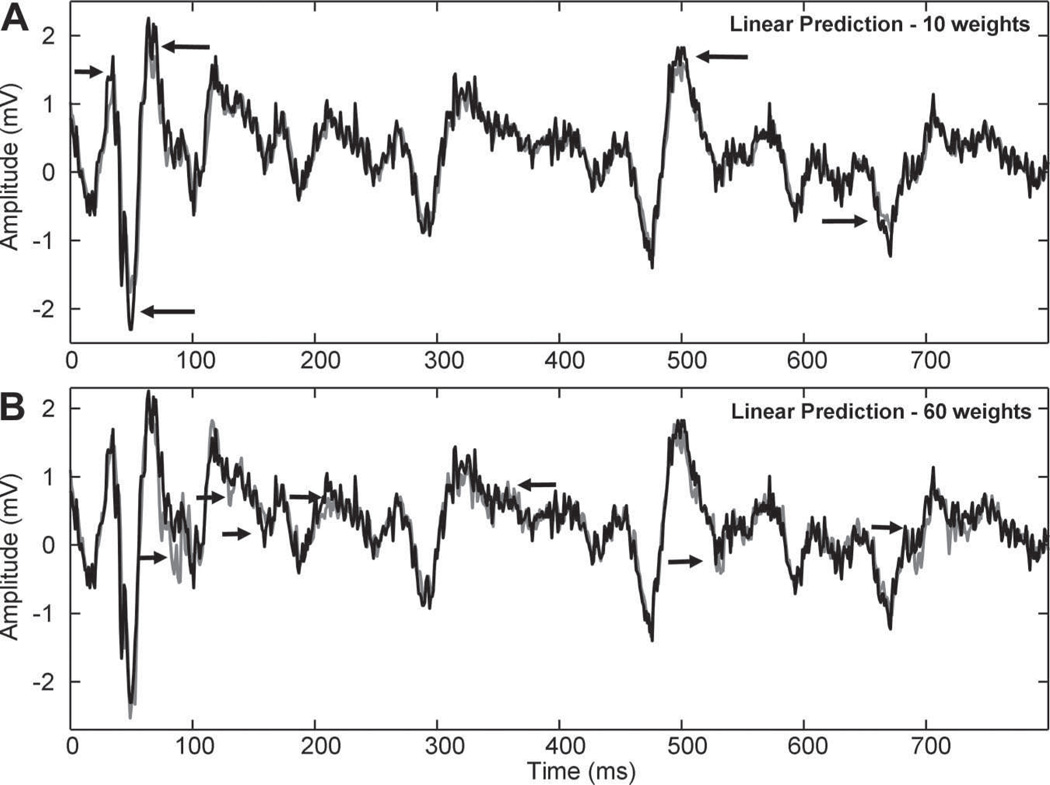
Examples of CFAE prediction are shown in Figure 3A for order n=10 and in Figure 3B for n=60. As noted by the arrows, discrepancies between predicted (gray) and actual signal (black) occur in the large-scale features when n=10 and in the small-scale detail when n=60. Narrower electrogram deflections are very accurately represented when n=10 in part because of the fast response time for this order of n. Longer-duration electrogram deflections are more accurately represented when n=60 in part because the large number of weights is sufficient to include in the weighting details occurring throughout the feature’s duration.
Figure 3.
Traces show CFAE signals (black) and linear predictive function (gray) using n=10 and n=60 coefficients. The same scales are used in both panels. The lower-order model (n=10) is more accurate at estimating detail and less accurate at estimating tall peaks (arrows in A). The higher-order model (n=60) is less accurate at estimating fine detail (arrows in B).
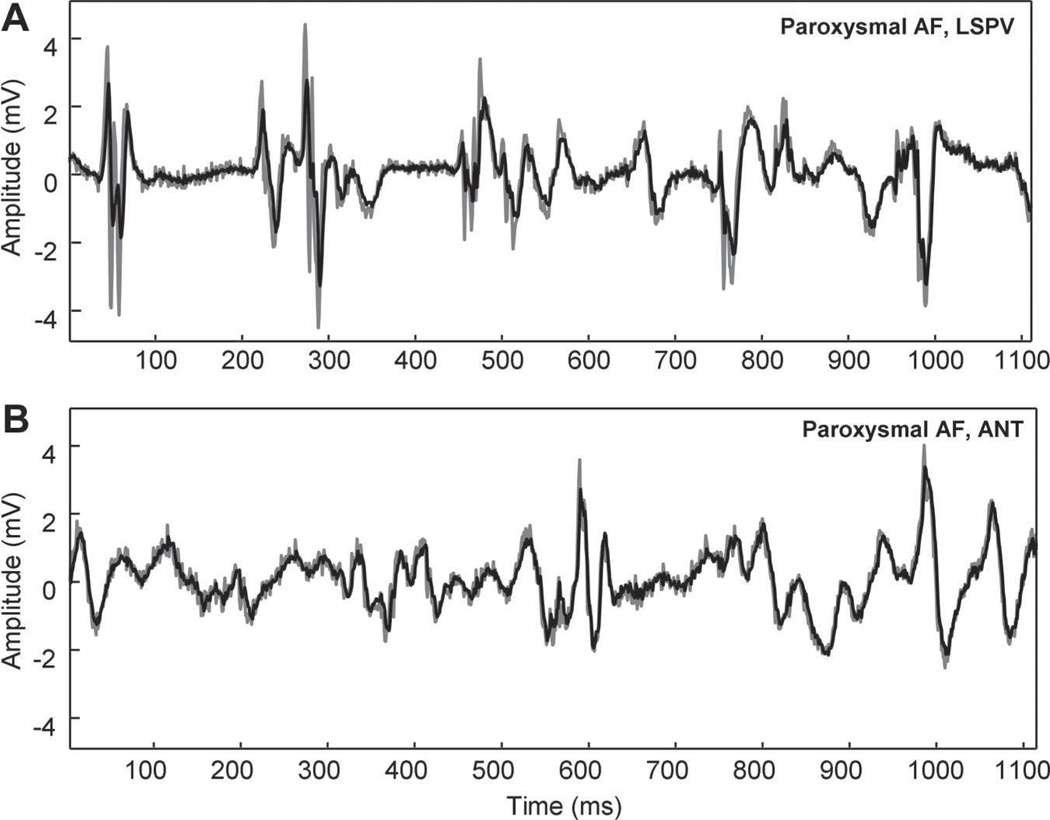
The measured error was partly dependent on location. In Figure 4, the original CFAE (black) and linear predictive estimate (gray, order n=10) are shown for recordings acquired outside the ostium of the LSPV and ANT free wall in the same paroxysmal AF patient. The shapes of the deflections in the LSPV recording (Figure 4A) are very different from one another and are not well predicted. Although there are differences in electrogram shape at the ANT (Figure 4B), similarly-shaped deflections such as the biphasic component at 0 to 50 ms recur, and the amplitude of all deflections are similar.
Figure 4.
Comparison of linear prediction outside the LSPV (A) versus ANT LA free wall (B) in a patient with paroxysmal AF for order n=10 (error=0.63 and 0.35, respectively). Shown in each panel, which use the same scale, are the original signal (black) and linear prediction (gray). There is poorer representation of peak deflections at the LSPV location (A).
Repeatability indices from averaged paroxysmal and persistent AF data were calculated for LP and FR error. As described in the Methods section, the values were averaged for all orders n and are plotted in Figure 5A and 5B. The averaged mean LP and FR errors decrease by comparably small magnitudes from paroxysmal to longstanding persistent AF as recorded outside the ostia of the LIPV, RSPV, and RIPV but by a larger magnitude at the LSPV ostium (Figure 5A and 5B). At the posterior and anterior LA recording sites, there are small increases in error from paroxysmal to persistent except at the posterior left atrium, where the FR error is the same for paroxysmal and longstanding AF. The lowest error (highest repeatability) in absolute value is observed at the anterior base of the LA appendage for both techniques of measurement.
Figure 5.
Mean linear prediction error (A) and reconstruction error (B) for modeling CFAE. A and B show the errors for each location when the values calculated are averaged. These average values show that overall error for representation of CFAE decreases from paroxysmal (black) to persistent AF (gray dashed), particularly at the LSPV. At the anterior and posterior LA, there is mostly an increase in error from paroxysmal to persistent AF.
The mean values for LP and FR errors as indices of repeatability or reproducibility are summarized in Table 1. The error decreases from paroxysmal to persistent AF patients by both measures at the PV ostial sites (the first row) and increases by both measures at the LA free wall sites (the second row). It is also evident that the mean error for the antral regions is substantially higher compared with mean LA free wall recording sites in paroxysmal AF, whereas the errors are more similar in value for longstanding AF. The significance in the error differences for the 4 columns of Table 1 are shown in Table 2. Both techniques show a trend of increasing repeatability from the antral sites to the posterior wall to the anterior recording site in paroxysmal AF (Figure 5). The difference is significant (Table 2, first two rows). For longstanding persistent AF, however, there were no significant differences between the mean errors at the LA free wall versus the PV ostia (Figure 5 and Table 2, rows 3 and 4). Thus, the repeatable CFAE patterns were spatially more uniformly distributed in the patients with persistent AF. Comparing one type of AF versus the other, the LP technique detected a significantly higher repeatability at the anterior wall recording site in paroxysmal AF compared with longstanding AF, whereas the FR method detected a significantly higher error outside the LSPV ostium in paroxysmal AF compared with longstanding AF (Figure 5 and Table 2, rows 5 and 6).
Table 1.
Mean Error
| Location | PAR-LP | PER-LP | PAR-FR | PER-FR |
|---|---|---|---|---|
| PV | 0.48±0.15 | 0.44±0.11 | 0.36±0.19 | 0.30±0.14 |
| LA | 0.34±0.08 | 0.40±0.08 | 0.25±0.10 | 0.27±0.09 |
PAR indicates paroxysmal; PER, persistent.
Table 2.
Significance of Error Differences
| Row | Comparison of | For | Model | Significance |
|---|---|---|---|---|
| 1 | PV vs LA | Paroxysmal | LP | P<0.001 |
| 2 | PV vs LA | Paroxysmal | FR | P=0.032 |
| 3 | PV vs LA | Persistent | LP | P=0.169 |
| 4 | PV vs LA | Persistent | FR | P=0.283 |
| 5 | PAR vs PER | LSPV | FR | P=0.036 |
| 6 | PAR vs PER | LA | LP | P=0.024 |
PV indicates pulmonary vein ostia; LA, left atrial free wall; PAR, paroxysmal; and PER, persistent.
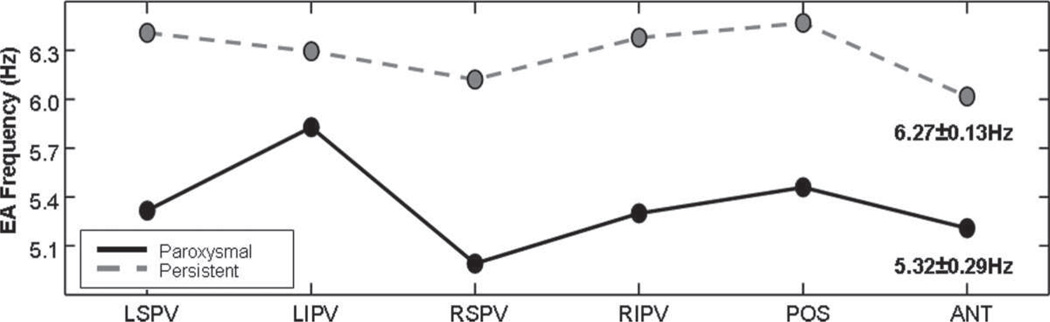
The results of DF analysis are shown in Figure 6. We used the ensemble analysis method to calculate the frequency of activation. The mean DF value for all locations is 5.32±0.29 Hz for paroxysmal and 6.27±0.13 Hz for longstanding persistent AF, values significantly different in both mean and standard deviation (P<0.001). The significant difference in standard deviations reflects the differences in frequencies of activation among the disparate recording sites. Note that the DF magnitude is higher at all recording sites during longstanding AF, compared with the paroxysmal AF, a finding that suggests that frequency of local atrial activation becomes faster as well as being more spatially homogeneous in persistent AF. Indeed, the DF appears to approach an upper bound value for all the LA sites used in this study, somewhere between 6 and 6.5 Hz, as paroxysmal AF evolves into longstanding persistent AF.
Figure 6.
Mean dominant frequency, based on ensemble spectral analysis. DFs are higher-valued and more similar at all locations in persistent AF (gray dashed) compared with paroxysmal AF (black). DFs are more variable in paroxysmal AF.
Discussion
In paroxysmal AF, there is a low degree of repeatability and more randomness in CFAE patterns recorded at the antral sites outside the PV ostia. The degree of repeatability increases (LP and FR errors decrease) and the randomness decreases as the recording sites move away from outside the PV ostia to the middle of posterior wall, and repeatability increases even more, further away from the PVs at the anterior base of the LA appendage (Figure 5, Table 1). In longstanding persistent AF, compared with paroxysmal AF, CFAE recordings manifest a higher degree of repeatable patterns at the antral sites outside the PV ostia, especially at the LSPV, and less repeatability at the posterior and anterior LA (Figure 5, Table 1). The overall effect is that repeatability of CFAE deflections becomes more spatially uniform when the antral sites outside the PV ostia are compared with selected sites in the LA free wall as paroxysmal AF evolves into longstanding persistent AF. Furthermore, the frequency of local atrial activation, as reflected by DF, becomes higher and more uniform at all recording locations in longstanding as compared with paroxysmal AF, and appears to approach a common upper bound value (Figure 6).
Underlying Mechanisms of CFAEs
The CFAE recordings manifest little or no obvious recurrent pattern on cursory inspection. Many but not necessarily all such signals may represent arrhythmogenic substrate for AF.1,16 Elimination of CFAEs by catheter ablation may result in changes in AF patterns, in transformation into “mappable” atrial flutter, and even in termination of longstanding AF acutely with emergence of sinus rhythm.16 –18 These phenomena, however, are not universal and predictable and certainly do not occur in all patients with AF. Therefore, all CFAE sites should not be equated with AF sources, and the first reasonable step for their further investigation is an objective characterization of these complex recordings. Previously, CFAE recordings have been categorized objectively by their frequency content15 and by their morphological characteristics.9 Quantifying CFAEs by the techniques described in this study may further be used for this aim.
The CFAE recordings at sites of wave break at the periphery of the sources have been demonstrated in experimental models of AF.5 If the sources and the drivers of paroxysmal AF, for example, rotors, predominate in the antral regions, then one would also expect a higher incidence of wave break sites in these areas. Because wave breaks may occur unpredictably, more random and less repeatable CFAE patterns would result. This conjecture is also compatible with our recently published observations of CFAE morphology characteristics observed outside the PV ostia.9 As the recording sites move away from the sources, the CFAE patterns may be preferentially determined by local geometry of the atrial tissue, which may dictate a preferred direction of activation. Repetitive patterns may begin to emerge, lowering the prediction error in CFAE patterns at sites such as the isthmus between the anterior mitral annulus and the LA appendage. As AF becomes more persistent and eventually longstanding, as the result of changes at the tissue level, other generators and drivers, such as microreentry, may form in regions away from the PVs and the antral regions. Consequently, the arrhythmogenic medium may become more anatomically homogenous and accordingly the CFAE patterns may reflect other phenomenon such as local reentry rather than random wave break. The LP and FR errors would then be expected to become more evenly distributed at disparate LA recording sites, including those far away from the PVs, as was observed in this study. Finally, our results also show that our analysis, which identifies the degree of repeatability, provides independent information additional to the frequency content of the CFAE recordings. For example, a comparison of Figure 5 and Figure 6 reveals that the sites of lowest error are not necessarily the sites manifesting highest rate of activation and that the indices LP and FR errors are not simply functions of DF.
Clinical Correlates and Implications
PV isolation alone may be an adequate strategy for many patients with paroxysmal AF without significant structural heart disease. By contrast, the impact of PV isolation alone is less efficacious in longstanding AF, and more than just PV isolation is needed for optimal results.19–21 What other sites should be the targets for catheter ablation after PV disconnection in longstanding AF is still being debated. In an attempt to identify arrhythmogenic sites more efficaciously, CFAE analysis has become a new area of investigation.22 However, targeting all CFAE sites is limited by the need for extensive ablation, increased power delivery, increased fluoroscopy and procedural times, and therefore greater risk for collateral injury and potential for proarrhythmia.20–24 Thus, there is a need for quantitative markers in characterizing certain site-specific CFAE patterns, particularly those that are indicative of underlying arrhythmia mechanisms, which could lead to appropriate ablation targets. The results of the present investigation show that repeatability of CFAE patterns becomes less site-specific and therefore less discriminatory in longstanding AF, in which the need for ablation at sites distant from the PVs is most important. Our results also suggest that during the evolution from paroxysmal to longstanding AF, frequency of local LA activation, as measured by DF, becomes higher and more uniform at disparate locations of the LA. Thus, DF also becomes less discriminatory and therefore a less useful guide for identifying ablation targets in longstanding AF.
Limitations
Our patient population was small, and the fact that these patients were referred for a catheter ablation procedure could have introduced a selection bias. The duration of longstanding AF in 10 patients was also not uniform and varied from 1 year to 5, from one patient to the next. It is quite likely that the arrhythmia substrate after 5 years of uninterrupted AF is different than the underlying substrate after 1 year of uninterrupted AF. Thus, our results should not be extrapolated to all patients with paroxysmal and longstanding AF before they can be confirmed in a prospective study with larger number of patients. Another limitation of our study is the fact that CFAE mapping was not performed globally in the entire LA but was limited to selected sites for practicality and ease of reproducibility from one patient to the next. Consequently, many LA regions, such as the interatrial septum and the LA roof, are not represented. Other CFAE patterns might emerge if these other regions are investigated as well. Without these additional data, the scope of our conclusions must remain limited. Finally, our observations remain hypothesis-generating; their validity requires further testing, as mentioned above, before they may become clinically useful and provide practical information to guide catheter ablation.
Conclusions
The results of this investigation extend the previous findings in CFAE morphology9 and show the feasibility of objectively quantifying repeating patterns in CFAE recordings even when they are nonperiodic. The degree of repeatability is site-specific and different in paroxysmal compared with longstanding AF. Finally, these indices of repeatability in CFAE patterns do not appear to be simply a function of the frequency content of the local electrograms and provide additional independent information.
CLINICAL PERSPECTIVE.
Catheter ablation has become a frequently performed procedure for treating atrial fibrillation (AF). Even though electric disconnection of the regions harboring the pulmonary vein ostia remains the first step in AF ablation, a substantial minority of the patients with paroxysmal AF and the vast majority of the patients with longstanding AF require more than pulmonary vein isolation for optimal results. Recently, complex fractionated electrograms (CFAE) have been a focus of investigation as targets for AF ablation. Our understanding of AF pathophysiology has advanced substantially over the past decade through experimental data revealing the role of atrial remodeling. Signaling pathways leading to fibrosis and connexin-40 alterations result in structural remodeling in longstanding AF. Consequently, CFAEs may represent different underlying phenomena in paroxysmal compared with longstanding AF, with measurable differences in CFAE morphology and amplitude as well as in atrial distribution of CFAEs between paroxysmal and longstanding AF. We used a novel technique to detect repeating morphological patterns not necessarily occurring at harmonics of the dominant frequency; our study shows that in paroxysmal AF, CFAE repetitiveness is low and randomness high at the antral regions, and repetitiveness increases significantly in the left atrium farther away from these regions. In longstanding AF, however, this gradient in repetitiveness is not evident, and repetitive CFAE patterns become more evenly distributed. Such investigations in CFAE characteristics that may be quantifiable will help generate testable hypotheses as to which types of CFAE are appropriate and effective targets for catheter ablation.
Acknowledgments
Sources of Funding
Dr Einstein was supported by institutional career development award KL2 RR024157 from the National Institutes of Health, by a Victoria and Esther Aboodi Assistant Professorship, by the Louis V. Gerstner Jr Scholars program, and by the Lewis Katz Cardiovascular Research Prize for a Young Investigator.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
None.
References
- 1.Nademanee K, McKenzie J, Kosar E, Schwab M, Sunsaneewitayakul B, Vasavakul T, Khunnawat C, Ngarmukos T. A new approach for catheter ablation of atrial fibrillation: mapping of the electrophysiologic substrate. J Am Coll Cardiol. 2004;43:2044–2053. doi: 10.1016/j.jacc.2003.12.054. [DOI] [PubMed] [Google Scholar]
- 2.Botteron GW, Smith JM. A technique for measurement of the extent of spatial organization of atrial activation during atrial fibrillation in the intact human heart. IEEE Trans Biomed Eng. 1995;42:579–586. doi: 10.1109/10.387197. [DOI] [PubMed] [Google Scholar]
- 3.Botteron GW, Smith JM. Quantitative assessment of the spatial organization of atrial fibrillation in the intact human heart. Circulation. 1996;93:513–518. doi: 10.1161/01.cir.93.3.513. [DOI] [PubMed] [Google Scholar]
- 4.Sanders P, Berenfeld O, Hocini M, Jais P, Vaidyanathan R, Hsu LF, Garrigue S, Takahashi Y, Rotter M, Sacher F, Scavee C, Ploutz-Snyder R, Jalife J, Haissaguerre M. Spectral analysis identifies sites of high-frequency activity maintaining atrial fibrillation in humans. Circulation. 2005;112:789–797. doi: 10.1161/CIRCULATIONAHA.104.517011. [DOI] [PubMed] [Google Scholar]
- 5.Kalifa J, Tanaka K, Zaitsev AV, Warren M, Vaidyanathan R, Auerbach D, Pandit S, Vikstrom KL, Ploutz-Snyder R, Talkachou A, Atienza F, Guiraudon G, Jalife J, Berenfeld O. Mechanisms of wave fractionation at boundaries of high-frequency excitation in the posterior left atriumof the isolated sheep heart during atrial fibrillation. Circulation. 2006;113:626–633. doi: 10.1161/CIRCULATIONAHA.105.575340. [DOI] [PubMed] [Google Scholar]
- 6.Everett THIV, Moorman JR, Akar JG, Haines DE. Assessment of global atrial fibrillation organization to optimize timing of atrial defibrillation. Circulation. 2001;103:2857–2861. doi: 10.1161/01.cir.103.23.2857. [DOI] [PubMed] [Google Scholar]
- 7.Ng J, Kadish AH, Goldberger JJ. Effect of electrogram characteristics on the relationship of dominant frequency to atrial activation rate in atrial fibrillation. Heart Rhythm. 2006;3:1295–1305. doi: 10.1016/j.hrthm.2006.07.027. [DOI] [PubMed] [Google Scholar]
- 8.Ng J, Kadish AH, Goldberger JJ. Technical considerations for dominant frequency analysis. J Cardiovasc Electrophysiol. 2007;18:757–764. doi: 10.1111/j.1540-8167.2007.00810.x. [DOI] [PubMed] [Google Scholar]
- 9.Ciaccio EJ, Biviano AB, Whang W, Gambhir A, Garan H. Different characteristics of complex fractionated atrial electrograms in acute paroxysmal versus longstanding persistent atrial fibrillation. Heart Rhythm. 2010;9:1207–1215. doi: 10.1016/j.hrthm.2010.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin YJ, Tai CT, Kao T, Chang SL, Wongcharoen W, Lo LW, Tuan TC, Udyavar AR, Chen YJ, Higa S, Ueng KC, Chen SA. Consistency of complex fractionated atrial electrograms during atrial fibrillation. Heart Rhythm. 2008;5:406–412. doi: 10.1016/j.hrthm.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 11.Ciaccio EJ. Localization of the slow conduction zone during reentrant ventricular tachycardia. Circulation. 2000;102:464–469. doi: 10.1161/01.cir.102.4.464. [DOI] [PubMed] [Google Scholar]
- 12.Ciaccio EJ, Micheli-Tzanakou E. Development of gradient descent adaptive algorithms to remove common mode artifact for improvement of cardiovascular signal quality. Ann Biomed Eng. 2007;35:1146–1155. doi: 10.1007/s10439-007-9294-x. [DOI] [PubMed] [Google Scholar]
- 13.Ciaccio EJ, Drzewiecki GM. Tonometric arterial pulse sensor with noise cancellation. IEEE Trans Biomed Eng. 2008;55:2388–2396. doi: 10.1109/TBME.2008.925692. [DOI] [PubMed] [Google Scholar]
- 14.Ciaccio EJ, Biviano AB, Whang W, Wit AL, Garan H, Coromilas J. New methods for estimating local electrical activation rate during atrial fibrillation. Heart Rhythm. 2009;6:21–32. doi: 10.1016/j.hrthm.2008.10.016. [DOI] [PubMed] [Google Scholar]
- 15.Ciaccio EJ, Biviano AB, Whang W, Wit AL, Coromilas J, Garan H. Optimized measurement of activation rate at left atrial sites with complex fractionated electrograms during atrial fibrillation. J Cardiovasc Electrophysiol. 2010;21:133–143. doi: 10.1111/j.1540-8167.2009.01595.x. [DOI] [PubMed] [Google Scholar]
- 16.Verma A, Novak P, Macle L, Whaley B, Beardsall M, Wulffhart Z, Khaykin Y. A prospective, multicenter evaluation of ablating complex fractionated electrograms (CFEs) during atrial fibrillation (AF) identified by an automated mapping algorithm: acute effects on AF and efficacy as an adjuvant strategy. Heart Rhythm. 2008;5:198–205. doi: 10.1016/j.hrthm.2007.09.027. [DOI] [PubMed] [Google Scholar]
- 17.Haissaguerre M, Hocini M, Sanders P, Sacher F, Rotter M, Takahashi Y, Rostock T, Hsu LF, Bordachar P, Reuter S, Roudaut R, Clementy J, Jais P. Catheter ablation of long-lasting persistent atrial fibrillation: clinical outcome and mechanisms of subsequent arrhythmias. J Cardiovasc Electrophysiol. 2005;16:1138–1147. doi: 10.1111/j.1540-8167.2005.00308.x. [DOI] [PubMed] [Google Scholar]
- 18.Oral H, Chugh A, Good E, Wimmer A, Dey S, Gadeela N, Sankaran S, Crawford T, Sarrazin JF, Kuhne M, Chalfoun N, Wells D, Frederick M, Fortino J, Benloucif-Moore S, Jongnarangsin K, Pelosi F, Jr, Bogun F, Morady F. Radiofrequency catheter ablation of chronic atrial fibrillation guided by complex electrograms. Circulation. 2007;115:2606–2612. doi: 10.1161/CIRCULATIONAHA.107.691386. [DOI] [PubMed] [Google Scholar]
- 19.Knecht S, Hocini M, Wright M, Lellouche N, O’Neill MD, Matsuo S, Nault I, Chauhan VS, Makati KJ, Bevilacqua M, Lim KT, Sacher F, Deplagne A, Derval N, Bordachar P, Jais P, Clementy J, Haissaguerre M. Left atrial linear lesions are required for successful treatment of persistent atrial fibrillation. Eur Heart J. 2008;29:2359–2366. doi: 10.1093/eurheartj/ehn302. [DOI] [PubMed] [Google Scholar]
- 20.Willems S, Klemm H, Rostock T, Brandstrup B, Ventura R, Steven D, Risius T, Lutomsky B, Meinertz T. Substrate modification combined with pulmonary vein isolation improves outcome of catheter ablation in patients with persistent atrial fibrillation: a prospective randomized comparison. Eur Heart J. 2006;27:2871–2878. doi: 10.1093/eurheartj/ehl093. [DOI] [PubMed] [Google Scholar]
- 21.Fassini G, Riva S, Chiodelli R, Trevisi N, Berti M, Carbucicchio C, Maccabelli G, Giraldi F, Della Bella P. Left mitral isthmus ablation associated with PV isolation: long-term results of a prospective randomized study. J Cardiovasc Electrophysiol. 2005;16:1150– 1156. doi: 10.1111/j.1540-8167.2005.50192.x. [DOI] [PubMed] [Google Scholar]
- 22.Bencsik G, Martinek M, Hassanein S, Aichinger J, Nesser HJ, Purerfellner H. Acute effects of complex fractionated atrial electrogram ablation on dominant frequency and regulatory index for the fibrillatory process. Europace. 2009;11:1011–1017. doi: 10.1093/europace/eup113. [DOI] [PubMed] [Google Scholar]
- 23.Lin YJ, Tai CT, Chang SL, Lo LW, Tuan TC, Wongcharoen W, Udyavar AR, Hu YF, Chang CJ, Tsai WC, Kao T, Higa S, Chen SA. Efficacy of additional ablation of complex fractionated atrial electrograms for catheter ablation of nonparoxysmal atrial fibrillation. J Cardiovasc Electrophysiol. 2009;20:607– 615. doi: 10.1111/j.1540-8167.2008.01393.x. [DOI] [PubMed] [Google Scholar]
- 24.Jais P, Hocini M, Hsu LF, Sanders P, Scavee C, Weerasooriya R, Macle L, Raybaud F, Garrigue S, Shah DC, Le Metayer P, Clementy J, Haissaguerre M. Technique and results of linear ablation at the mitral isthmus. Circulation. 2004;110:2996–3002. doi: 10.1161/01.CIR.0000146917.75041.58. [DOI] [PubMed] [Google Scholar]