Abstract
Objectives
The aim of this study was to verify the clinical effectiveness of decompression in decreasing the size of a cyst. In addition to the different types of cysts, we tried to reveal what effect host factors, such as the initial size of the lesion and the age of the patient, have on the velocity of cyst shrinkage.
Materials and Methods
With the aid of a panoramic view, we measured the size of the cysts before and after decompression in 13 dentigerous cysts (DCs), 14 keratocystic odontogenic tumors (KTOCs), and 5 unicystic ameloblastoma (UA) cases. The velocity of shrinkage in the three cystic groups was calculated. Relationships between the age of the patient, the initial size of the cyst, and the shrinkage velocity were investigated.
Results
The three types of cysts showed no inter-type differences in their velocity of shrinkage. However, there was a statistically meaningful relationship between the initial size of the lesion and the absolute velocity of shrinkage in the DC group (P=0.02, R=0.65) and the KTOC group (P=0.02, R=0.56). There was also a significant relationship between the age of the patient and the absolute velocity of shrinkage in the KTOC group (P=0.04, R=0.45) and the UA group (P=0.04, R=0.46).
Conclusion
There was no difference in the decrease in size due to decompression among the different types of cysts. However, the age of the patient and the initial size of the lesion showed a significant relationship with the velocity of shrinkage.
Keywords: Decompression, Odontogenic cysts, Unicystic ameloblastoma
I. Introduction
A cyst is a pathological epithelial cell-lined cavity that is filled with fluid or soft material. They are frequently found as intra-bony lesions of the jaw, which can expand so drastically that sometimes the facial appearance can change. Although removal of the cyst is necessary in these cases, enucleation of these lesions can bring forth a risk of infection, fracture of the jaw, or nerve injury if the cyst is large enough to push on important anatomic structures. To avoid those complications, marsupialization or decompression is recommended to reduce the size of the cyst, hence making it safe to enucleate the lesion. The benefit of marsupialization or decompression includes maintenance of pulp vitality, preservation of the inferior alveolar nerve or maxillary sinus, prevention of fracture of the jaw, and low risk of recurrence1,2. Many previous studies have also suggested that marsupialization or decompression can be considered a good treatment option in large odontogenic cyst cases3,4,5. In particular, some studies have reported good post-op results in radicular cysts, dentigerous cysts (DCs), and keratocystic odontogenic tumors (KTOCs)6,7,8.
Ameloblastoma is the most frequent odontogenic cyst in the jaw, and shows high recurrence and invasiveness9. Among the four subtypes of ameloblastoma, unicystic ameloblastoma (UA) has rather low recurrence and invasiveness10. UA, with its cyst-like character, is considered to be a good indication for marsupialization or decompression5,11. However, there have been few studies that have focused on postoperative success based on the ameloblastoma's size, or other contributing factors affecting the velocity of its shrinkage.
This study is devised to evaluate the effectiveness of the decompression of cystic lesions of the mandible, especially, DC, KCOTs and UA using a panoramic view. Furthermore, to extract more meaningful clinical insights, we investigated the correlation between decompression and several host factors, such as age and the initial size of the cyst.
II. Materials and Methods
1. Subjects
Thirty-two patients with cystic lesions were chosen. Among them, 13 were DCs (12 males and 1 female; mean age 40.8 years), 14 were KTOC cases (10 males and 4 females; mean age 27.9 years), and 5 were UA cases (1 male and 4 females; mean age 18.6 years). The subjects were selected from patients who visited the Seoul National University Dental Hospital from 2000 to 2012. Among the mandibular cyst cases, only the cases in which the center of the lesion was localized to the posterior part of the premolar were selected. Decompression was performed under local anesthesia, making a hole through the mucosa and the submucosal bony surface in the vicinity of the lesion. After setting a tube through the hole, a biopsy was done in all cases to confirm that the lesion was indeed an odontogenic cyst. The patients were followed-up monthly.
2. Measurement of cystic lesion shrinkage
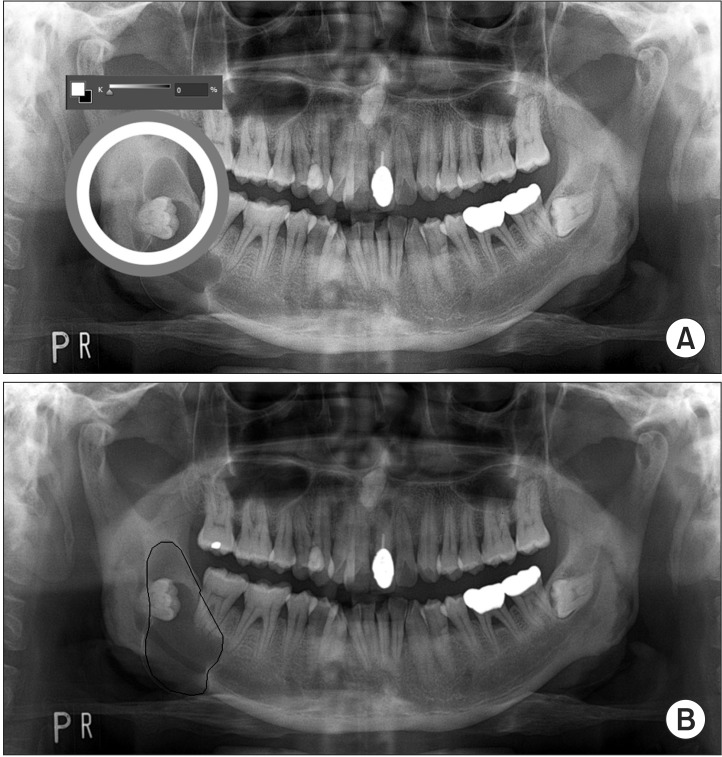
Because cystic lesions are relatively radiopaque, these looked darker than the jawbone in the panoramic view. A change of light and darkness in the panoramic view was remarkable around the boundary of the cystic lesion. By investigating the contrast of light and darkness in the panoramic view, the boundary of the cystic lesion was defined. Tracing of the defined boundary was performed using a computer program (SketchUp version 8.0 for Windows; Trimble Navigation Ltd., Sunnyvale, CA, USA). Then, the size of the marked area was calculated.(Fig. 1) Before and after decompression, the size of the cystic lesion was measured. Panoramic radiography was performed using panoramic units (Orthopantomograph OpR 100; Instrumentarium Co., Forchheim, Finland) operated at 70 kVp and 10 mA using photostimulable phosphor plates.
Fig. 1.
Measurement of the cystic lesion size in panoramic view. A. Investigation of the light and darkness contrast level around the boundary of the cystic lesion (circle). B. Traced boundary of the cystic lesion using a computer program in panoramic view (lined).
The absolute and relative velocities of shrinkage were calculated by measuring the change of size before, during, and after decompression. Here, the absolute velocity of shrinkage was defined as follows:
Absolute velocity of shrinkage (mm2/mo)=(size of decreasing area after decompression [mm2])/(duration of decompression [mo])
The relative velocity of shrinkage was defined as follows: Relative velocity of shrinkage (%/mo)=(percentage of decreasing area after decompression [%])/(duration of decompression [mo])
With the aid of statistics, we studied whether the absolute or relative velocity of shrinkage had a meaningful correlation with the host factors, such as the age of the patient and the initial size of the lesion.
3. Statistical analysis
Data were analyzed with PASW Statistics version 18.0 software (IBM Co., Armonk, NY, USA). A Kolmogorov- Smirnov test, Shapiro-Wilk test, Kruskal-Wallis test, Spearman's rank correlation analysis, Pearson's correlation analysis, and simple regression analysis were used to evaluate the change of the cystic lesion within each parameter. A P-value less than 0.05 was determined to be significant.
III. Results
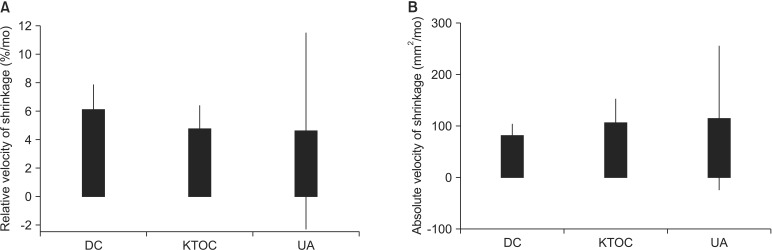
In the DC group, the initial mean size of the lesion and the standard deviation (SD) in the panoramic view were 1,474.3 mm2 and 930.7 mm2. The decompression lasted for 6.9 months and a 39.4% reduction was obtained during the procedure. The mean decrement of the lesions was 580.9 mm2. The relative velocity of shrinkage (mean±SD) was 6.1±2.9%/mo and the absolute velocity of shrinkage was 81.1±40.0 mm2/mo. In the KTOC group, the initial mean size of the lesion (mean± SD) was 2,356.3±1,135.7 mm2 and the decompression was performed for 7.4 months. After decompression, we found a 33.6% reduction in the size of the lesion. The mean decrement of the lesions was 791.7 mm2. The relative velocity of shrinkage (mean±SD) was 4.7±2.9%/mo and the absolute velocity of shrinkage was 106.0±81.9 mm2/mo. In the UA group, the initial mean size of the lesion (mean±SD) was 2,115.4±888.9 mm2 in the panoramic view. After 13.4 months of decompression, a 36.7% reduction was found. The mean decrement of the lesions was 776.4 mm2. The relative velocity of shrinkage (mean±SD) was 4.6±5.6%/mo and the absolute velocity of shrinkage (mean±SD) was 115.8±112.9 mm2/mo.(Table 1) No statistically significant inter-group difference was found in the absolute or relative velocities of shrinkage.(Fig. 2)
Table 1.
Mean values of the initial size of the cysts, duration of decompression, shrinkage of the cystic lesion after decompression, and velocity of shrinkage after decompression
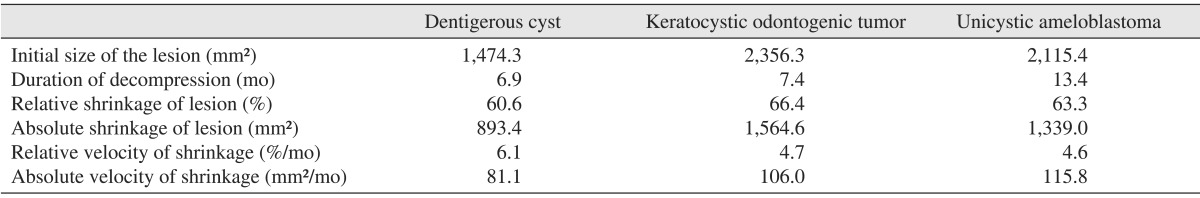
Fig. 2.
No statistically significant difference in relative (A) and absolute velocity of shrinkage among the three groups (B) (P>0.05). (DC: dentigerous cyst, KTOC: keratocystic odontogenic tumor, UA: unicystic ameloblastoma)
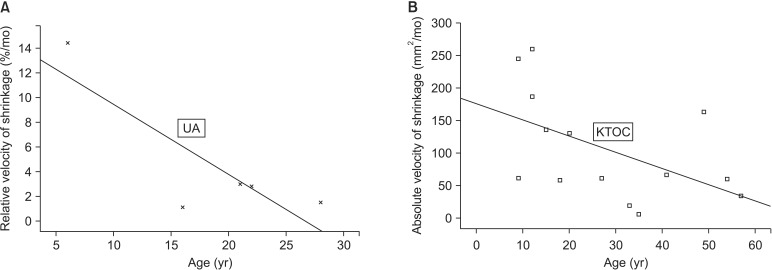
Some of the host factors seemed to contribute to the process of healing after decompression. The age of the patient showed a statistically significant correlation with the relative velocity of shrinkage in the UA group only (P=0.04, R=0.46).(Fig. 3. A) In the KTOC group, the absolute velocity of shrinkage was significantly associated with the age of the patient (P=0.04, R=0.45).(Fig. 3. B)
Fig. 3.
The relationship between the age of patients and the velocity of shrinkage in the two cystic lesion groups. A. Relationship between the age and the relative velocity of shrinkage in the UA group (P=0.04, R=0.46). B. Relationship between the age and the absolute velocity of shrinkage in the KTOC group (P=0.04, R=0.45). (UA: unicystic ameloblastoma, KTOC: keratocystic odontogenic tumor)
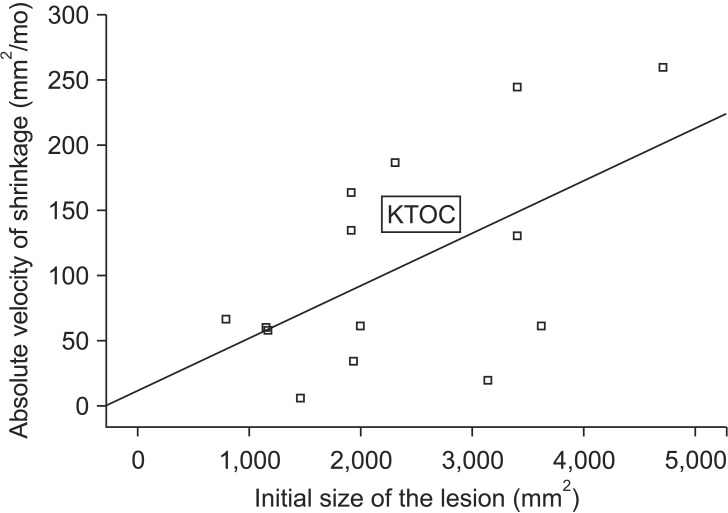
When analyzing the initial size of the lesion, all three kinds of cystic lesions showed no correlation between the initial size of the lesion and the relative velocity of shrinkage. However, in the DC and KTOC groups, absolute velocity of shrinkage was larger when the initial size of the lesion was larger (DC: P=0.02, R=0.65; KTOC: P=0.02, R=0.56).(Fig. 4)
Fig. 4.
The relationship between the initial size of the lesion and the absolute velocity of shrinkage in the KTOC group (P=0.02, R=0.56). (KTOC: keratocystic odontogenic tumor)
IV. Discussion
Computed tomography (CT) is one of the best tools to evaluate the size of an intra-bony lesion of the jaw. Unlike the panoramic image, which reproduces the actual anatomy on a two-dimensional plane, CT data provides three-dimensional images with precision. In some previous studies, researchers have tried to use CT as a tool to measure the size of the cyst but the procedure had various limitations such as high cost and the large amount of radiation associated with CT scans.
Resorting to panoramic view instead of CT, we referred to Yoshiura and Kubota's report12,13 that says that the area of the cyst measured on the panoramic image has a linear relationship with the volume of the lesion on CT when the lesion exists in the posterior part of the mandible. They argue that this is because odontogenic cysts rarely expand bucco-lingually in the posterior part of the premolar region12,13. With the aid of this report, we used a panoramic view to study the size of the cysts located in the posterior part of the mandible.
The subjects of this study underwent decompression before receiving enucleation surgery. Decompression with marsupialization is a non-invasive treatment option for odontogenic cysts. Marsupialization is a surgical procedure in which the supra-wall of the cyst is removed and the internal wall of the cyst is sutured with the oral mucosa so that part of the lining epithelium can be transitioned into the oral epithelium. With decompression, we expect to reduce the size of the lesion by inserting a rubber tube or a stent through a small hole into the cyst14. Both of these procedures disrupt the continuity of the cystic wall, and the suturing of the internal tissue of the cyst with the oral mucosa in marsupialization is challenging to the operator. Moreover, post-operative deformation or surface depression is less likely with decompression compared to with marsupialization; hence prosthetic reconstruction is easier in decompression cases15,16. However, decompression also has limitations, according to Bramley et al., some studies have reported that the tissue left after decompression could develop into a more aggressive lesion17,18,19,20. Because of this, periodic follow-ups and radiographic imaging should be conducted after decompression, and we followed this principle in this research.
Marker et al.21 recommended that the decompression procedure should be performed for over a year, and reported that the longer the decompression period lasted (to the extent that the drain be removed spontaneously), the better the prognosis was. Their alleged criterion of success was a 50% to 60% decrease in the cystic volume. According to the recommendation of Marker et al.21, the duration of decompression in our study was too short. The duration after decompression was 6.9 months for the DC group, 7.4 months for the KTOC group, and 13.4 months for the UA group. However, in spite of the short duration of decompression, we at least fulfilled the minimum and most crucial goal: the size of the lesion was reduced enough to be enucleated without damaging the adjacent tissue. Considering the fact that most of the shrinkage observed by Miyawaki et al.22 was accomplished during the first 3 months after the decompression, it is reasonable to conclude that a considerable amount of shrinkage had already been attained during the follow-up period in this study.
In terms of the effect of the cyst type on the velocity of shrinkage, Gao et al.23 reported that decompression was more effective with radicular cysts than with KTOCs or UA. Kubota et al.6 also obtained similar results: the velocity of shrinkage after decompression was faster in radicular cysts than in KTOCs or DCs. In contrast, Kim and Lee15 reported that DCs shrank more dramatically after decompression compared to KTOCs or radicular cysts. However, Anavi et al.1 reported that very few statistically significant inter-group differences were found in the velocity of shrinkage among radicular cysts, DCs, and KTOCs. In this study, there were also no intergroup differences in the velocity of shrinkage.
Previous studies have noted that shrinkage is faster when the initial size of the cyst is larger24,25. Although Anavi et al.1 reported that the relative velocity of shrinkage was faster in the initially small-sized cases, his result can be interpreted in different ways given that the absolute velocity of shrinkage could give opposite results.
In this study, however, UA showed little correlation between the initial size of the lesion and its shrinkage velocity. The reason for this is not clear but may involve the histologic diversity of UA cases. Among Ackermann's classification of UA (the unilocular luminal type, the intraluminal with solid growth inside the lumen of the cystic lesion type, and the intraluminal growth with mural invasion within adjacent tissues type)26, the third type shows the highest aggressiveness and recurrence. The inter-type difference in behavior and recurrence seems to impose different characteristics on its response to decompression. Further research on these classification differences is necessary.
There is little consensus about whether the velocity of shrinkage is affected by the age of the patient. Kubota et al.6 said that there seemed to be little correlation between the age of the patient and the relative velocity of shrinkage. A similar result was obtained for the UA group by Gao et al.23. However, Anavi et al.1 reported that the period that was required to decrease the size of the cyst to a certain level was shorter when the patient was under 18 years old. Kim and Lee15 also revealed that the relative velocity of shrinkage was faster when the patient was younger, especially in the DC cases.
In this study, the relative velocity of shrinkage was higher when the patients were younger in the UA group. In the KTOC cases, the absolute velocity of shrinkage showed similar results.
Although active osteogenic metabolism can partly explain these results, more focused research is required to verify the effect of decompression on cystic pathophysiology.
V. Conclusion
Among the DC, KTOC and UA groups, there was little inter-group difference in shrinkage velocity. In the DC and the KTOC groups, the absolute velocity of shrinkage was faster for a larger initial size of the lesion (DC: P=0.02, R=0.65; KCOT: P=0.02, R=0.56). Also, in the KTOC and UA groups, the absolute velocity of shrinkage was faster for younger patients (KCOT: P=0.04, R=0.45; UA: P=0.04, R=0.46).
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Anavi Y, Gal G, Miron H, Calderon S, Allon DM. Decompression of odontogenic cystic lesions: clinical long-term study of 73 cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;112:164–169. doi: 10.1016/j.tripleo.2010.09.069. [DOI] [PubMed] [Google Scholar]
- 2.Thoma KH. Oral surgery. 3rd ed. St. Louis: Mosby; 1958. [Google Scholar]
- 3.Pogrel MA. Decompression and marsupialization as a treatment for the odontogenic keratocyst. Oral Maxillofac Surg Clin North Am. 2003;15:415–427. doi: 10.1016/S1042-3699(03)00038-4. [DOI] [PubMed] [Google Scholar]
- 4.Dolanmaz D, Etoz OA, Pampu A, Kalayci A, Gunhan O. Marsupialization of unicystic ameloblastoma: a conservative approach for aggressive odontogenic tumors. Indian J Dent Res. 2011;22:709–712. doi: 10.4103/0970-9290.93461. [DOI] [PubMed] [Google Scholar]
- 5.Yokobayashi Y, Yokobayashi T, Nakajima T, Oyama T, Fukushima M, Ishiki T. Marsupialization as a possible diagnostic aid in cystic ameloblastoma. Case report. J Maxillofac Surg. 1983;11:137–141. doi: 10.1016/s0301-0503(83)80033-2. [DOI] [PubMed] [Google Scholar]
- 6.Kubota Y, Imajo I, Itonaga R, Takenoshita Y. Effects of the patient's age and the size of the primary lesion on the speed of shrinkage after marsupialisation of keratocystic odontogenic tumours, dentigerous cysts, and radicular cysts. Br J Oral Maxillofac Surg. 2013;51:358–362. doi: 10.1016/j.bjoms.2012.07.017. [DOI] [PubMed] [Google Scholar]
- 7.Yahara Y, Kubota Y, Yamashiro T, Shirasuna K. Eruption prediction of mandibular premolars associated with dentigerous cysts. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;108:28–31. doi: 10.1016/j.tripleo.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 8.Oka S, Kubota Y, Yamashiro T, Ogata S, Ninomiya T, Ito S, et al. Effects of positive pressure in odontogenic keratocysts. J Dent Res. 2005;84:913–918. doi: 10.1177/154405910508401008. [DOI] [PubMed] [Google Scholar]
- 9.Sampson DE, Pogrel MA. Management of mandibular ameloblastoma: the clinical basis for a treatment algorithm. J Oral Maxillofac Surg. 1999;57:1074–1077. doi: 10.1016/s0278-2391(99)90328-2. [DOI] [PubMed] [Google Scholar]
- 10.Lau SL, Samman N. Recurrence related to treatment modalities of unicystic ameloblastoma: a systematic review. Int J Oral Maxillofac Surg. 2006;35:681–690. doi: 10.1016/j.ijom.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 11.Furuki Y, Fujita M, Mitsugi M, Tanimoto K, Yoshiga K, Wada T. A radiographic study of recurrent unicystic ameloblastoma following marsupialization. Report of three cases. Dentomaxillofac Radiol. 1997;26:214–218. doi: 10.1038/sj.dmfr.4600250. [DOI] [PubMed] [Google Scholar]
- 12.Yoshiura K, Higuchi Y, Araki K, Shinohara M, Kawazu T, Yuasa K, et al. Morphologic analysis of odontogenic cysts with computed tomography. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997;83:712–718. doi: 10.1016/s1079-2104(97)90325-5. [DOI] [PubMed] [Google Scholar]
- 13.Kubota Y, Yamashiro T, Oka S, Ninomiya T, Ogata S, Shirasuna K. Relation between size of odontogenic jaw cysts and the pressure of fluid within. Br J Oral Maxillofac Surg. 2004;42:391–395. doi: 10.1016/j.bjoms.2004.02.032. [DOI] [PubMed] [Google Scholar]
- 14.Kim SM, Chung SW, Cha IH, Nam W. Normal eruption guidance of unerupted permanent teeth associated with dentigerous cyst by decompression: 5 cases report. J Korean Assoc Oral Maxillofac Surg. 2009;35:271–275. [Google Scholar]
- 15.Kim YH, Lee EW. Comparison of clinico-histopathologic findings before and after decompression of odontogenic cyst in the jaw. J Korean Assoc Oral Maxillofac Surg. 2005;31:150–160. [Google Scholar]
- 16.Bodner L, Bar-Ziv J. Characteristics of bone formation following marsupialization of jaw cysts. Dentomaxillofac Radiol. 1998;27:166–171. doi: 10.1038/sj/dmfr/4600344. [DOI] [PubMed] [Google Scholar]
- 17.Forssell K, Sainio P. Clinicopathological study of keratinized cysts of the jaws. Proc Finn Dent Soc. 1979;75:36–45. [PubMed] [Google Scholar]
- 18.Thomas M, Tackett JC, Desai P. The incredible odontogenic keratocyst. N Y State Dent J. 1992;58:31–33. [PubMed] [Google Scholar]
- 19.Peterson LJ. Contemporary oral and maxillofacial surgery. 3rd ed. St. Louis: Mosby; 1998. [Google Scholar]
- 20.Jackson RF, Kramer HS, Hyde GM, Eisenberg E, Topazian RG. Clinicopathologic conferences. Case 45, part II: dentigerous cyst of the mandible with ameloblastomatous changes. J Oral Maxillofac Surg. 1983;41:407–408. doi: 10.1016/s0278-2391(83)80013-5. [DOI] [PubMed] [Google Scholar]
- 21.Marker P, Brøndum N, Clausen PP, Bastian HL. Treatment of large odontogenic keratocysts by decompression and later cystectomy: a long-term follow-up and a histologic study of 23 cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996;82:122–131. doi: 10.1016/s1079-2104(96)80214-9. [DOI] [PubMed] [Google Scholar]
- 22.Miyawaki S, Hyomoto M, Tsubouchi J, Kirita T, Sugimura M. Eruption speed and rate of angulation change of a cyst-associated mandibular second premolar after marsupialization of a dentigerous cyst. Am J Orthod Dentofacial Orthop. 1999;116:578–584. doi: 10.1016/s0889-5406(99)70192-7. [DOI] [PubMed] [Google Scholar]
- 23.Gao L, Wang XL, Li SM, Liu CY, Chen C, Li JW, et al. Decompression as a treatment for odontogenic cystic lesions of the jaw. J Oral Maxillofac Surg. 2014;72:327–333. doi: 10.1016/j.joms.2013.07.035. [DOI] [PubMed] [Google Scholar]
- 24.Zhao Y, Liu B, Han QB, Wang SP, Wang YN. Changes in bone density and cyst volume after marsupialization of mandibular odontogenic keratocysts (keratocystic odontogenic tumors) J Oral Maxillofac Surg. 2011;69:1361–1366. doi: 10.1016/j.joms.2010.05.067. [DOI] [PubMed] [Google Scholar]
- 25.Shudou H, Sasaki M, Yamashiro T, Tsunomachi S, Takenoshita Y, Kubota Y, et al. Marsupialisation for keratocystic odontogenic tumours in the mandible: longitudinal image analysis of tumour size using 3D visualised CT scans. Int J Oral Maxillofac Surg. 2012;41:290–296. doi: 10.1016/j.ijom.2011.10.015. [DOI] [PubMed] [Google Scholar]
- 26.Ackermann GL, Altini M, Shear M. The unicystic ameloblastoma: a clinicopathological study of 57 cases. J Oral Pathol. 1988;17:541–546. doi: 10.1111/j.1600-0714.1988.tb01331.x. [DOI] [PubMed] [Google Scholar]