Abstract
Purpose
Downstaging after chemoradiotherapy (CRT) for rectal cancer usually occurs. The present study aimed to evaluate pathologic y-stage (yp-stage) and its influence on local recurrence and systemic recurrence in rectal cancer patients treated with CRT followed by surgical resection.
Methods
We retrospectively analyzed 261 patients underwent preoperative CRT and radical resection for rectal cancer between August 2004 and December 2010. Patients received preoperative CRT consisting of 5-fluorouracil and leucovorin delivered with concurrent pelvic radiation of 45.0-50.4 Gy, followed by radical surgery at 6-8 weeks after CRT.
Results
Of the 261 patients, 24 (9.2%) had yp-stage 0, 83 (31.8%) had yp-stage I, 86 (32.9%) had yp-stage II, and 68 (26.1%) had yp-stage III. Patients with yp-stage III had a greater prevalence of preoperative CEA, poorly differentiated tumor, lymphovascular invasion (LVI) and perineural invasion (PNI) than patients with lower yp-stages. We found that yp-stage, preoperative CEA, LVI, PNI and tumor regression grade were significant prognostic factors for both local and systemic recurrence. In multivariate analysis, yp-stage, LVI and PNI were significant factors for local and systemic recurrence. During the median follow-up of 37.5 months, the five-year local recurrence-free survival rate was 100.0%, 95.0%, 89.3%, and 80.6% of yp-stage 0-III, respectively. The five-year systemic recurrence-free survival was 95.8%, 75.3%, 71.4%, and 48.8% of yp-stages 0-III, respectively.
Conclusion
The yp-stage after preoperative CRT for rectal cancer is closely correlated with local and systemic recurrence-free survival. Therefore, yp-stage should be considered as a prognostic factor for rectal cancer patients having a course of preoperative CRT.
Keywords: Rectal neoplasms, Chemoradiotherapy, Pathologic y-staging
INTRODUCTION
Treatment strategies for patients with locally advanced rectal cancer have evolved over the past two decades. Preoperative chemoradiotherapy (CRT) has been considered one of the standard therapies for rectal cancer based on evidence of improved local control and survival [1]. Preoperative CRT downstages the neoplasm (Fig. 1), which leads to a substantial decrease in tumor size and invasion depth, and possibly lymph node sterilization [2].
Fig. 1.
(A) MRI imaging of patient before receiving preoperative chemoradiotherapy. (B) MRI imaging of patient after receiving preoperative chemoradiotherapy.
Several factors are considered in determining the prognosis of rectal cancer patients who receive a curative resection after preoperative CRT, including pathologic response, tumor regression grade (TRG), downstaging, CEA level, and circumferential resection margin (CRM) [3,4,5,6]. However, a study of the prognostic significance of pathologic y-stage (yp-stage) after preoperative CRT for rectal cancer is insufficient [7].
The present study aimed to evaluate the yp-stage and its influence on local recurrence and systemic recurrence in rectal cancer patients treated with CRT followed by surgical resection.
METHODS
Patients
We retrospectively reviewed 261 patients with rectal cancer without distant metastases who underwent radical surgery after preoperative CRT between August 2004 and December 2010. This study included all patients with rectal cancer and clinical stages II and III. Another 13 patients were added who received pretreatment because of cT2N0M0 tumors that were located within 5 cm from the anal verge. Preoperative clinical staging was based on a digital rectal examination using the Mason classification [8], transrectal ultrasound, colonoscopy, chest x-ray, blood test, serum CEA level, abdomen-pelvis CT and MRI. Tumors were staged according to the 7th American Joint Committee on Cancer TNM staging system [9].
Treatment
The whole pelvic field received 25-28 fractions of 180 cGy/day five times per week over five weeks, for a total of 4,500-5,040 cGy. Chemotherapy was administered intravenously and consisted of 5-fluorouracil (5-FU; 425 mg/m2/day) and leucovorin (20 mg/m2/day) for four days during the first and fifth weeks of radiotherapy.
The five-scale tumor regression was used for pathologic evidence of tumor response [10]. Restaging and radical surgery was performed at six to eight weeks after the completion of CRT. Total mesorectal excision and high ligation of inferior mesenteric artery was routinely performed. All patients had a postoperative chemotherapy course with 5-FU and leucovorin for an additional four cycles.
Follow-up examination after surgery was done every three months in the first two years and every six months after that. Routine follow-up examinations consisted of a digital rectal examination, laboratory test, serum CEA level, chest x-ray, and abdomen-pelvis CT. Chest CTs, pelvic MRIs, and PET scans were executed based on need. Recurrence was identified based on clinical examination and diagnostic imaging. Time to recurrence and time to the last follow-up were measured from the date of surgery.
Statistical analysis
The differences between groups were tested by the chi-square test and analysis of variance, as appropriate. Local and systemic recurrence free survival curves were calculated using the Kaplan-Meier method. The differences between the curves were evaluated using the log-rank test. Variables with a statistical P-value of <0.05 were entered into a Cox model multivariate analysis. Data were analyzed with IBM SPSS Statistics ver. 21.0 (IBM Co., Armonk, NY, USA).
RESULTS
The median age was 65.2 years (range, 31-92 years) and 202 patients (77.4%) were men. 179 of the patients (68.6%) were American Society of Anesthesiologists (ASA) grade 2, and 17 (6.5%) were ASA grade 3. The median body mass index (BMI) was 23.07 kg/m2 (range, 14.4-33.1 kg/m2). The median preoperative serum CEA level was 6.4 ng/mL (range, 0.2-149.5 ng/mL). The median duration between termination of preoperative CRT and surgery was 57 days (range, 35-96 days). The rate of CRM involvement (CRM < 1 mm) was 7.28% after preoperative CRT. The median number of lymph node in resected specimen was 14 (range, 0-77).
The clinical characteristics of the patients according to the yp-stage in rectal cancer patients treated with preoperative CRT followed by surgical resection are shown in Table 1. Preoperative CEA, poorly differentiated tumors, lymphovascular invasion (LVI), and perineural invasion (PNI) were higher in yp-stage III than in the order of stages (P < 0.05).
Table 1.
Patient and tumor characteristics
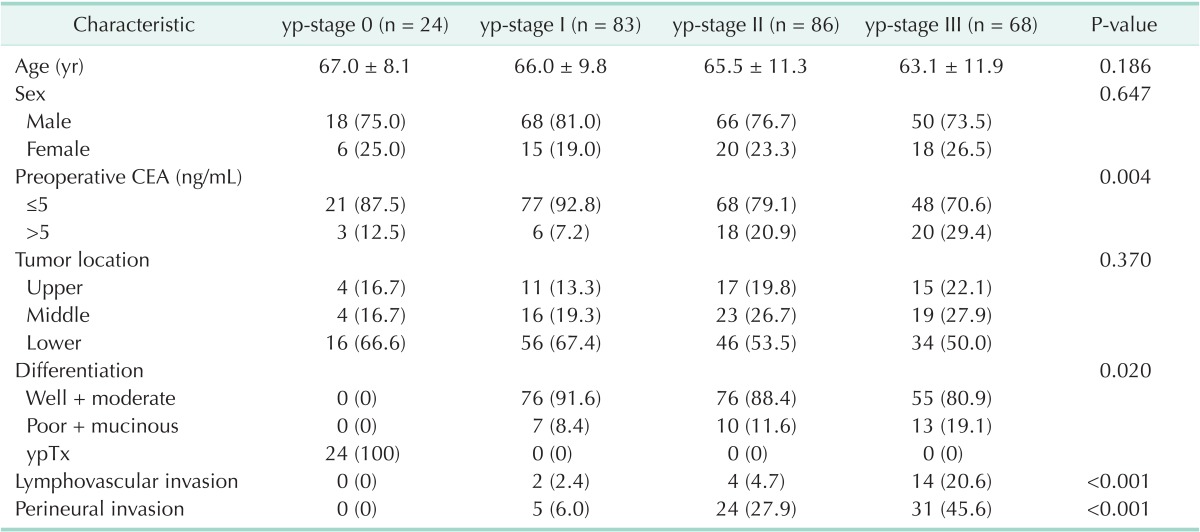
yp-stage, pathologic y-stage; ypTx, in microscopic findings, no viable tumor cells found in the bowel wall.
The yp-stage, histologic differentiation, LVI, PNI, TRG, and CRM were significant factors for local recurrence (Table 2).
Table 2.
Univariate analysis of prognostic factors associated with 5-year LRFS
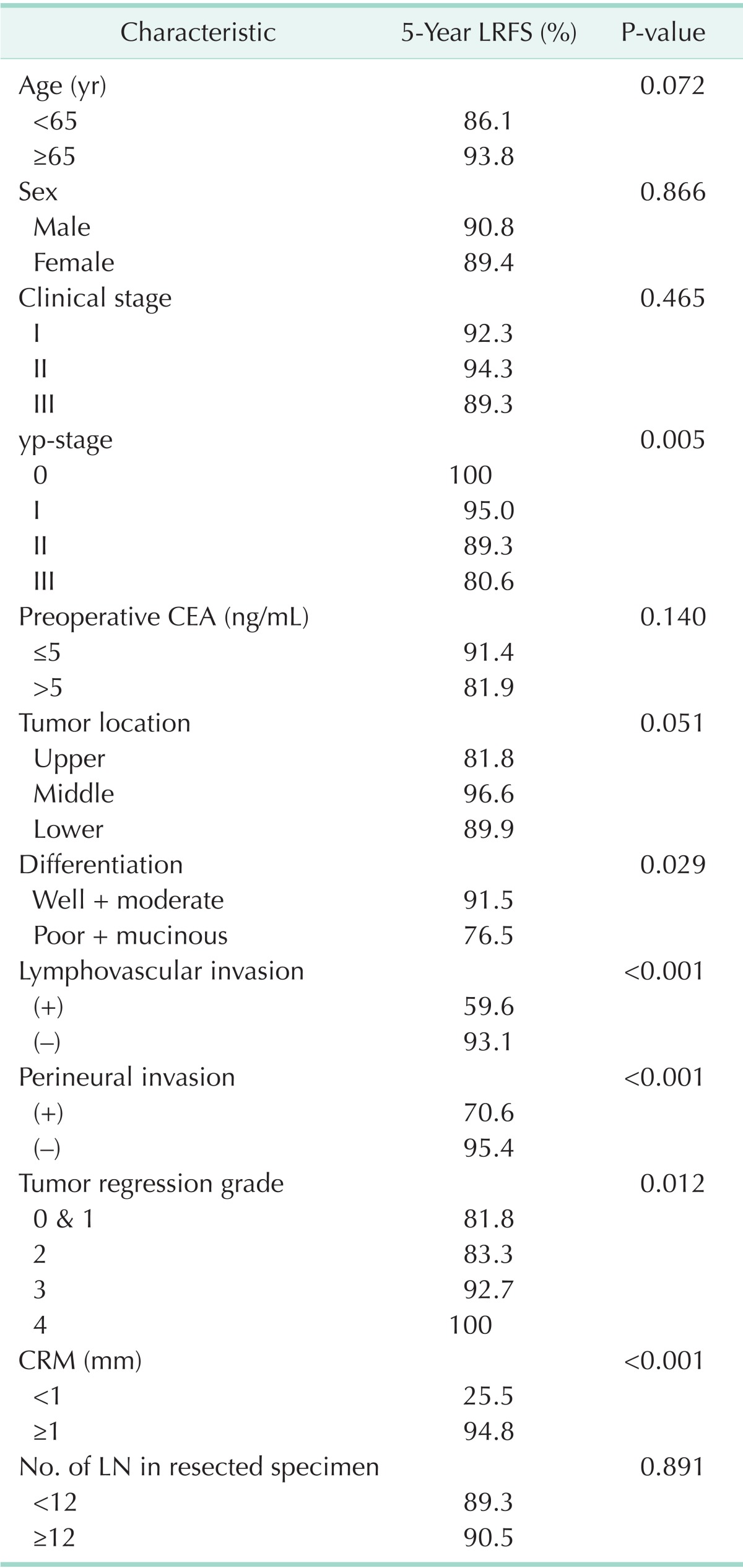
LRFS, local recurrence-free survival; yp-stage, pathologic y-stage; CRM, circumferential resection margin; LN, lymph node.
Additionally, the yp-stage, preoperative CEA, LVI, PNI and TRG were significant factors for systemic recurrence (Table 3).
Table 3.
Univariate analysis of prognostic factors associated with 5-year SRFS
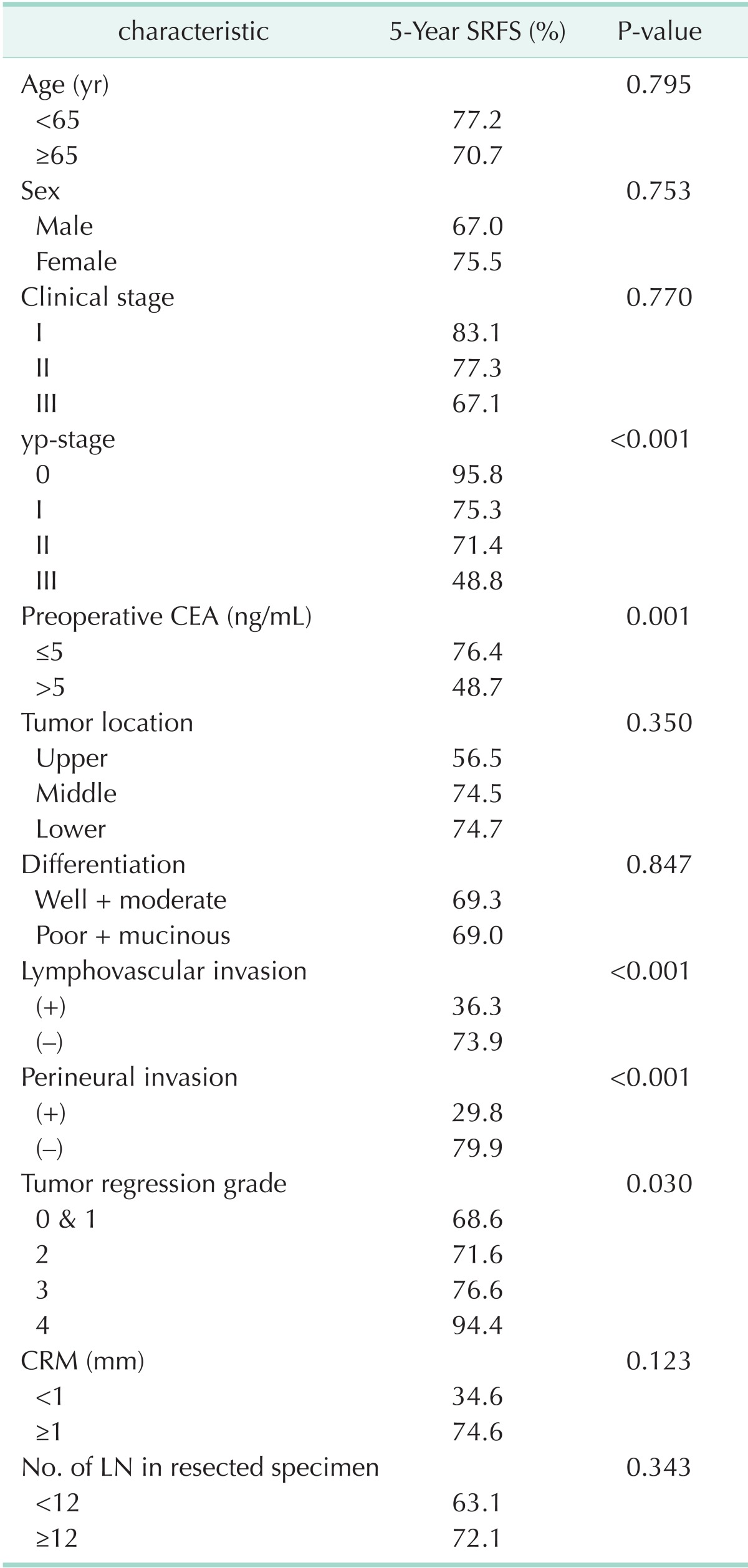
SRFS, systemic recurrence-free survival; yp-stage, pathologic y-stage; CRM, circumferential resection margin; LN, lymph node.
The local and systemic recurrences for all significant factors from the univariate analysis were included in the multivariate analysis. The yp-stage, LVI, and PNI were shown to be significant factors in the multivariate analysis for local and systemic recurrence. Notably, CRM was a significant factor for local recurrence only (Tables 4, 5).
Table 4.
Multivariate analysis of 5-year LRFS
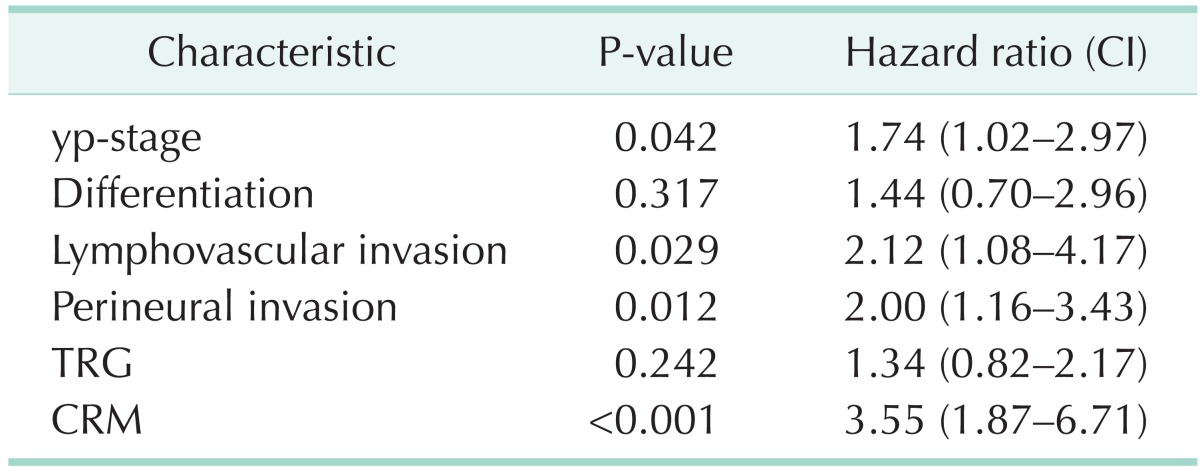
LRFS, local recurrence-free survival; CI, confidence interval; yp-stage, pathologic y-stage; TRG, tumor regression grade; CRM, circumferential resection margin.
Table 5.
Multivariate analysis of 5-year SRFS
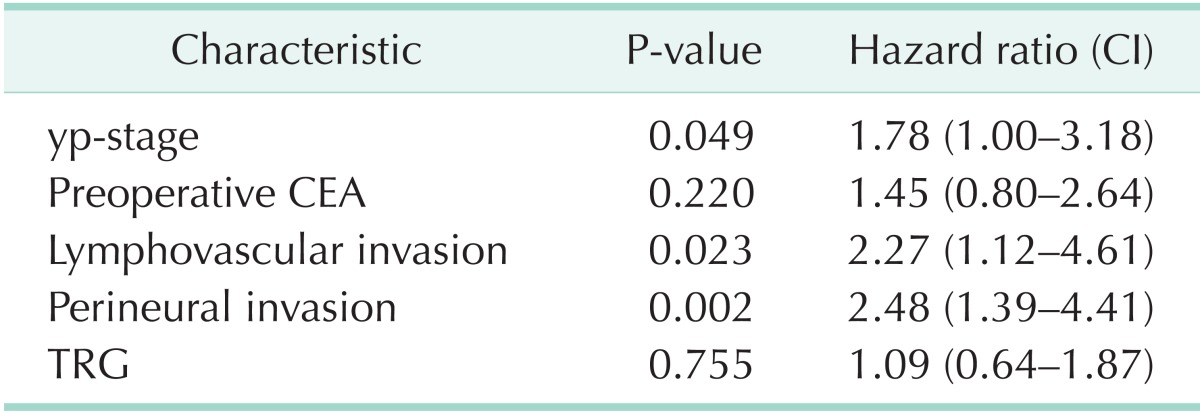
SRFS, systemic recurrence-free survival; CI, confidence interval; yp-stage, pathologic y-stage; TRG, tumor regression grade.
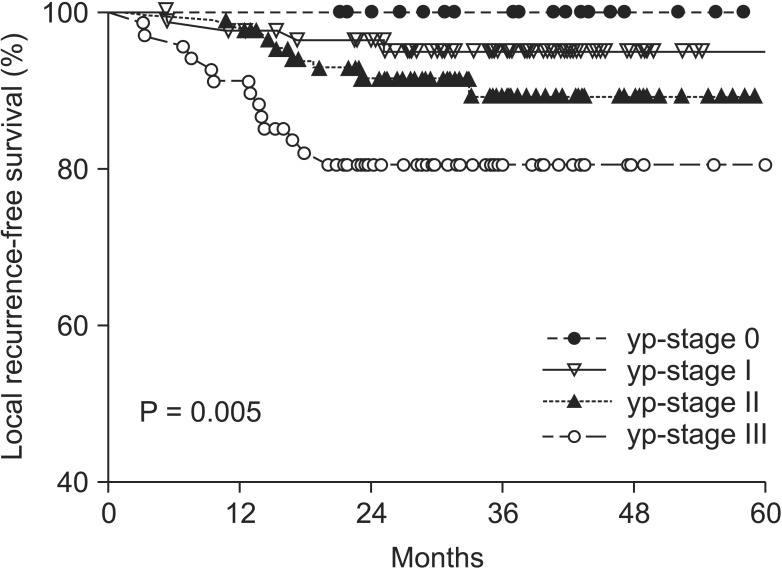
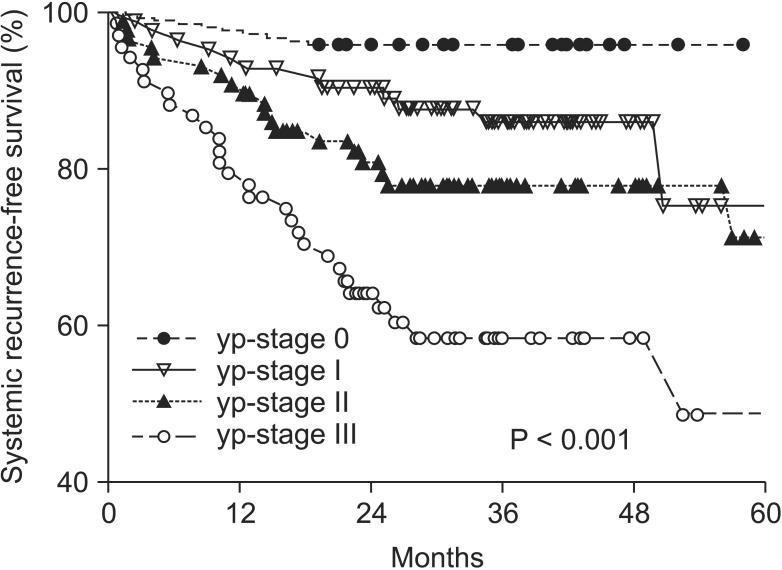
The median follow-up period was 37.5 months. Local and systemic recurrence occurred in 25 (9.58%) and 60 patients (22.99%). There was a significant correlation between yp-stage and five-year LRFS and SRFS. The five-year LRFS was 100.0% for yp-stage 0, 95.0% for yp-stage I, 89.3% for yp-stage II, and 80.6% for yp-stage III (Fig. 2) (P = 0.005). The five-year SRFS was 95.8% for yp-stage 0, 75.3% for yp-stage I, 71.4% for yp-stage II, and 48.8% for yp-stage III (Fig. 3) (P < 0.001).
Fig. 2.
Proportion of five-year local recurrence-free survival. yp-stage, pathologic y-stage.
Fig. 3.
Proportion of five-year systemic recurrence-free survival. yp-stage, pathologic y-stage.
DISCUSSION
Preoperative CRT is now considered one of the standard therapies for locally advanced rectal cancer [1,11]. Preoperative CRT downstages the neoplasm resulting in more sphincter-saving operations and improved local control compared to surgery alone [2].
Suarez et al. [12] reported that the group with a higher TRG showed a better disease-free survival (DFS) rate than those with a lower TRG rate, and that the TRG was a more significant prognostic factor than pathologic downstaging. Park et al. [13] insisted that the TRG, which reflects the effect of CRT, is thought to be an additional prognostic factor that, along with the TNM stage, affects the survival and the recurrence rates. However, Abdul-Jalil et al. [14] reported that the TRG was not a significant factor after preoperative CRT in rectal cancer. Our study showed that TRG was one of several effective factors of local and systemic recurrence in univariate analysis. However, the multivariate analysis showed that the TRG was not significant factor. In TRG 4, local recurrence did occur; however, two patients (5.6%) showed systemic recurrence during the follow-up period.
Topova et al. [15] analyzed 174 patients treated with preoperative CRT. The study identified pathologic response as a significant factor after preoperative CRT in rectal cancer. Garcia-Aguilar et al. [16] analyzed 168 patients and concluded that pathologic complete response (pCR) was associated with improved local control and survival after preoperative CRT followed by radical resection with mesorectal excision. The patients were all staged by ultrasound as stages II and III. Kuo et al. [7] insisted that rectal cancer in yp-stage 0 showed good DFS. However, they found no difference between local control rates and overall survival. Similarly, the present study showed that about 9.2% of patients had pCR after preoperative CRT. The five-year DFS was significantly improved in the pCR group as compared with non-pCR group (95.8% in pCR group vs. 75.1% in non-pCR group, P = 0.03).
Lee et al. [17] analyzed 328 patients treated with preoperative CRT. They identified LVI was a significant factor of DFS and overall survival rates after preoperative CRT in rectal cancer. Similarly, our study found a significant correlation between LVI and local and systemic recurrences. PNI was also recognized as an important prognostic factor in the study by Liebig et al. [18]. The authors reported significantly improved rates of cancer-specific overall survival and DFS rates in patients with PNI-negative tumors. In our study, there was a significant correlation between PNI and local and systemic recurrences.
Kuo et al. [7] enrolled 248 patients treated with preoperative CRT. They determined that the pathologic stage is correlated with DFS and the tumor recurrence rate in locally advanced rectal cancer after preoperative CRT. Rodel et al. [19] analyzed 385 patients treated with preoperative CRT. Their study identified yp-stage as a good prognostic factor for patients treated with preoperative CRT. Our study found that yp-stages 0, I, and II showed better five-year LRFS and five-year SRFS than yp-stage III.
Preoperative CRT is important in the treatment algorithm of rectal cancer. The yp-stage after preoperative CRT for rectal cancer is closely correlated with LRFS and SRFS, regardless of initial clinical staging. Therefore, yp-stage should be considered as a prognostic factor for rectal cancer patients having a course of preoperative CRT.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Kapiteijn E, Marijnen CA, Nagtegaal ID, Putter H, Steup WH, Wiggers T, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med. 2001;345:638–646. doi: 10.1056/NEJMoa010580. [DOI] [PubMed] [Google Scholar]
- 2.Sauer R, Becker H, Hohenberger W, Rodel C, Wittekind C, Fietkau R, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731–1740. doi: 10.1056/NEJMoa040694. [DOI] [PubMed] [Google Scholar]
- 3.Huh JW, Kim HR, Kim YJ. Clinical prediction of pathological complete response after preoperative chemoradiotherapy for rectal cancer. Dis Colon Rectum. 2013;56:698–703. doi: 10.1097/DCR.0b013e3182837e5b. [DOI] [PubMed] [Google Scholar]
- 4.Lee YC, Hsieh CC, Chuang JP. Prognostic significance of partial tumor regression after preoperative chemoradiotherapy for rectal cancer: a meta-analysis. Dis Colon Rectum. 2013;56:1093–1101. doi: 10.1097/DCR.0b013e318298e36b. [DOI] [PubMed] [Google Scholar]
- 5.Roy P, Serra S, Kennedy E, Chetty R. The prognostic value of grade of regression and oncocytic change in rectal adenocarcinoma treated with neo-adjuvant chemoradiotherapy. J Surg Oncol. 2012;105:130–134. doi: 10.1002/jso.22073. [DOI] [PubMed] [Google Scholar]
- 6.Rullier A, Gourgou-Bourgade S, Jarlier M, Bibeau F, Chassagne-Clement C, Hennequin C, et al. Predictive factors of positive circumferential resection margin after radiochemotherapy for rectal cancer: the French randomised trial ACCORD12/0405 PRODIGE 2. Eur J Cancer. 2013;49:82–89. doi: 10.1016/j.ejca.2012.06.028. [DOI] [PubMed] [Google Scholar]
- 7.Kuo LJ, Liu MC, Jian JJ, Horng CF, Cheng TI, Chen CM, et al. Is final TNM staging a predictor for survival in locally advanced rectal cancer after preoperative chemoradiation therapy. Ann Surg Oncol. 2007;14:2766–2772. doi: 10.1245/s10434-007-9471-z. [DOI] [PubMed] [Google Scholar]
- 8.Mason AY. President's address. Rectal cancer: the spectrum of selective surgery. Proc R Soc Med. 1976;69:237–244. doi: 10.1177/003591577606900401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, editors. AJCC cancer staging manual. 7th ed. New York: Springer; 2010. [Google Scholar]
- 10.Dworak O, Keilholz L, Hoffmann A. Pathological features of rectal cancer after preoperative radiochemotherapy. Int J Colorectal Dis. 1997;12:19–23. doi: 10.1007/s003840050072. [DOI] [PubMed] [Google Scholar]
- 11.Improved survival with preoperative radiotherapy in resectable rectal cancer. Swedish Rectal Cancer Trial. N Engl J Med. 1997;336:980–987. doi: 10.1056/NEJM199704033361402. [DOI] [PubMed] [Google Scholar]
- 12.Suarez J, Vera R, Balen E, Gomez M, Arias F, Lera JM, et al. Pathologic response assessed by Mandard grade is a better prognostic factor than down staging for disease-free survival after preoperative radiochemotherapy for advanced rectal cancer. Colorectal Dis. 2008;10:563–568. doi: 10.1111/j.1463-1318.2007.01424.x. [DOI] [PubMed] [Google Scholar]
- 13.Park YJ, Oh BR, Lim SW, Huh JW, Joo JK, Kim YJ, et al. Clinical significance of tumor regression grade in rectal cancer with preoperative chemoradiotherapy. J Korean Soc Coloproctol. 2010;26:279–286. doi: 10.3393/jksc.2010.26.4.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abdul-Jalil KI, Sheehan KM, Kehoe J, Cummins R, O'Grady A, McNamara DA, et al. The prognostic value of tumour regression grade following neoadjuvant chemoradiation therapy for rectal cancer. Colorectal Dis. 2014;16:O16–O25. doi: 10.1111/codi.12439. [DOI] [PubMed] [Google Scholar]
- 15.Topova L, Hellmich G, Puffer E, Schubert C, Christen N, Boldt T, et al. Prognostic value of tumor response to neoadjuvant therapy in rectal carcinoma. Dis Colon Rectum. 2011;54:401–411. doi: 10.1007/DCR.0b013e3182070efb. [DOI] [PubMed] [Google Scholar]
- 16.Garcia-Aguilar J, Hernandez de Anda E, Sirivongs P, Lee SH, Madoff RD, Rothenberger DA. A pathologic complete response to preoperative chemoradiation is associated with lower local recurrence and improved survival in rectal cancer patients treated by mesorectal excision. Dis Colon Rectum. 2003;46:298–304. doi: 10.1007/s10350-004-6545-x. [DOI] [PubMed] [Google Scholar]
- 17.Lee JH, Jang HS, Kim JG, Cho HM, Shim BY, Oh ST, et al. Lymphovascular invasion is a significant prognosticator in rectal cancer patients who receive preoperative chemoradiotherapy followed by total mesorectal excision. Ann Surg Oncol. 2012;19:1213–1221. doi: 10.1245/s10434-011-2062-z. [DOI] [PubMed] [Google Scholar]
- 18.Liebig C, Ayala G, Wilks J, Verstovsek G, Liu H, Agarwal N, et al. Perineural invasion is an independent predictor of outcome in colorectal cancer. J Clin Oncol. 2009;27:5131–5137. doi: 10.1200/JCO.2009.22.4949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rodel C, Martus P, Papadoupolos T, Fuzesi L, Klimpfinger M, Fietkau R, et al. Prognostic significance of tumor regression after preoperative chemoradiotherapy for rectal cancer. J Clin Oncol. 2005;23:8688–8696. doi: 10.1200/JCO.2005.02.1329. [DOI] [PubMed] [Google Scholar]