Abstract
The response of bacteria, yeast, and mammalian and insects cells to oxidative stress is a topic that has been studied for many years. However, in most the reported studies, the oxidative stress was caused by challenging the organisms with H2O2 and redox-cycling drugs, but not by subjecting the cells to high concentrations of molecular oxygen. In this review we summarize available information about the effect of elevated oxygen concentrations on the physiology of microorganisms and cells at various culture conditions. In general, increased oxygen concentrations promote higher leakage of reactive oxygen species (superoxide and H2O2) from the respiratory chain affecting metalloenzymes and DNA that in turn cause impaired growth and elevated mutagenesis. To prevent the potential damage, the microorganisms and cells respond by activating antioxidant defenses and repair systems. This review described the factors that affect growth properties and metabolism at elevated oxygen concentrations that cells may be exposed to, in bioreactor sparged with oxygen enriched air which could affect the yield and quality of the recombinant proteins produced by high cell density schemes.
Keywords: Hyperoxia, Oxidative stress, ROS, Protein oxidation, SOD activity, Hyperoxygenation
Introduction
With the routine utilization of high density growth of microorganisms and mammalian cells for production of various biologicals, the use of oxygen-enriched air has become a common strategy. Elevated oxygen concentrations in the inlet gas may stress the cells in certain location in the bioreactor [1,2]. Although oxygen is essential to growth and production, it is also known to be toxic at elevated concentrations for a variety of cell types [3]. Most aerobic and microaerophilic organisms have developed protective responses to tolerate environmental oxygen concentrations. But when oxygen concentration surpasses the air saturation level, reactive oxygen species (ROS), such as superoxide (O2-) and hydrogen peroxide (H2O2), accumulate as byproducts of aerobic metabolism [4-6]. These molecules are toxic to the organisms since they are more reactive than molecular oxygen [7,8]. Most of the published research has focused on how these compounds are produced inside the cells; on how they damage the cellular components and on the defense mechanisms the cell activates to prevent the effect of these reactive species [8]. Yet, the damaging effect of molecular oxygen on the cell behavior has not been fully studied. The purpose of this review is to summarize the known information on the physiological response of organisms exposed to high molecular oxygen concentrations, especially the effect on growth, metabolism, enzyme activity, protein oxidation, and gene expression.
Effects of high molecular oxygen concentration on growth and metabolism of prokaryotes
In the late 1960s, it was reported that oxygen concentrations above 100% air saturation cause inhibition of metabolism and respiration in microorganisms [9]. A few years later, the labs of O.R. Brown and I. Fridovich investigated the toxic effect of hyperbaric oxygen on Escherichia coli B and K-12 strains, Streptococcus (Enterococcus) faecalis, Bacillus subtilis, and Saccharomyces cerevisiae by exposing the cultures to 1, 4.2 and 20 atm pressure of O2 [10-13]. They showed that growth and respiration of E. coli grown in minimal salts medium was rapidly but reversibly inhibited by oxygen at partial pressures above 1 atm. The growth-inhibitory effect of hyperbaric oxygen was partially reduced by the addition of yeast extract to the culture medium and it was later established that 10 specific amino acids, niacin, and thiamin were necessary to maintain the cells growth capability at the elevated oxygen concentrations [14]. It was found that biosynthesis of branched-chain amino acids is inhibited by the high oxygen concentration [11,15]. But even with amino acid supplementation, the growth of E. coli AB1157 in glucose minimal medium was impaired when the cells were exposed to 1 atm of O2, and the growth was more affected when the tricarboxylic acid (TCA) cycle-dependent carbon source succinate was used [16]. Based on their observations, the authors concluded that aconitase is one of the enzymes that is affected by molecular oxygen. The inhibition of the tricarboxylic acid cycle is expected to cause a decrease in respiration as a result of limiting electron flow from the substrates to oxygen. The oxygen toxicity effect was more severe at the exponential growth phase than at the stationary phase [17].
The addition of 0.1 mM thiamine to E. coli K-12 culture was found to protect the cells from the inhibitory effect of hyperoxygenation (4.2 atm of O2) that caused 3-fold decrease in the intracellular thiamin concentration [14]. Thiamin diphosphate is a cofactor involved in the pentose phosphate pathway and hence may affect NADPH biosynthesis. The synthesis and intracellular concentration of the pyridine nucleotide coenzymes were studied and found to be lower in oxygen-poisoned cells [18-21]. These findings contributed to the use of hyperoxygenation for medical applications. Hyperbaric oxygen has been used for the treatment of serious infections of Staphylococcus and Vibrio vulnificus; 90 min exposure to 2 atm of O2 significantly inhibited growth of Staphylococcus resistant strains in patients [22,23].
The impact of dissolved-oxygen (dO2) concentration on E. coli processes for production of recombinant proteins is uncertain [24,25]. Increasing dO2 concentration from 50% to 100% air saturation (0.105 to 0.21 atm of O2) did not affect the specific activity and the volumetric productivity of the expressed recombinant β-galactosidase, nor the growth rate, biomass concentration, or plasmid content of E. coli TB-1/pUC19 and TB-1/p1034 [24]. However, the usage of oxygen enriched air (0.40 atm of O2) to control dO2 concentration above 20% air-saturation in a glucose defined medium contributed to higher biomass and higher acetate concentrations but decreased the specific production of rhGH produced by E. coli K-12 strain compared with air supply control experiment [25]. In contrast, the productivity of rhGH in E. coli BL21 growing in a complex medium and using 0.93 atm of O2 in inlet gas to control dO2 at the same levels (20% air saturation), was 2-fold higher than in air-control culture [26]. In addition to the difference in the E. coli strain and induction time, the availability of amino acids in the complex medium might explain the contrasting results. By using a higher oxygen concentration (0.68 atm of O2) in the inlet gas to maintain a dO2 of 30% air-saturation in a glucose defined medium, Lara et al. [27] did not observe any acetate or other organic acid accumulation. The authors concluded that any potential oxidative stress due to the oxygen content in the gas anywhere in the bioreactor was negligible since no changes on overflow metabolism such as acetate excretion was observed.
Longer exposure time to elevated oxygen concentrations and stronger oxidative stress might occur in large bioreactors when gas blending or pure oxygen is used for scaling-up E. coli cultivations [1,27]. Potential physiological effects of the use of oxygen enriched air were studied by Baez and Shiloach [28]. No significant effects on the growth rate, biomass yield, and maximum acetate accumulation was found when E. coli batch cultures were exposed to dO2 of 300% air-saturation (~0.63 atm of O2 inlet gas). However, oxygen uptake and acetate production rates decreased when oxygen concentration was increased in chemostat cultures [28]. An increasing maintenance coefficient was suggested by Castan et al. [25] as a result of the respiration changes caused by high oxygen; this observation is in line with the maintenance coefficient of E. coli MG1655 growing in glucose mineral medium that was found to increase from 0.66 to 0.85 mmol/g-h following increase in oxygen concentration from 30% to 300% air saturation (Baez, unpublished data).
Effects of elevated molecular oxygen concentration on growth and metabolism of eukaryotes
Unlike prokaryotes, eukaryotes are more sensitive to atmospheric oxygen concentration above 40% [29,30]. Cultures of human myeloid leukemia U-937 cells exposed to 50% of O2 (~240% air saturation) showed alteration of the mitochondrial respiratory chain, lactate and alanine accumulation, and strong growth inhibition compared with cells exposed to 21% O2 [31]. Exposing Chinese hamster ovary (CHO) cells to 80% O2 (~380% air saturation) inhibited cell growth together with 65% higher glutathione accumulation compared with cultures grown at 20% O2 [30]. Specific growth rate, production productivity of erythropoietin (EPO), and final cell yield of CHO cell transfected with the human EPO gene was significantly lower at 200% air saturation, but the cells were unaffected by oxygen concentrations between 10% and 100% air saturation [32]. Hyperoxic conditions (200% air saturation) also caused lower fucosylation pattern in the produced EPO. On the other hand, moderate oxygenation of CHO cell culture (80% air saturation) did not affect viability, productivity, and cell-specific glutathione production rate compared with a control culture exposed to 30% air saturation [33,34]. When mouse-mouse hybridoma cells were exposed to 200%, 300% and 476% air saturation, the cells were not able to grow and an association between DNA strand breakage and hyperoxia was observed compared with a control culture growing at 10% air saturation [35]. Viral infection of mammalian and insect cells is associated with increased oxidative stress [36,37]. The increase in dissolved oxygen (dO2) concentrations decreased the viability and increased the protein carbonyl content and lipid oxidation of infected Tn-5B1-4 cells, but did not affect the viability, protein and lipid oxidation of Sf-9 cells [38,39]. When, mouse lung epithelial type II cells (MLE-12) were exposed to hyperoxia (95% O2) they became larger and showed significant decrease in basal oxygen consumption rate, glycolytic capacity and growth rate compared to cultures under normoxia (room air) [40]. Since elevated O2 concentrations inhibit complex I and II activities and decrease the oxygen consumption rate via complex I and II, but not complex IV, in isolated mitochondria, it was concluded that complexes I and II are specific targets of hyperoxia [40,41]. S. cerevisiae mutants devoid of mitochondrial superoxide dismutase activity were more vulnerable to hyperoxia (100% O2) than S. cerevisiae lacking cytosolic superoxide dismutase activity, suggesting that the mitochondrial respiratory chain is the target of hyperoxia toxicity [42].
Molecular responses to elevated oxygen concentrations
Accumulation of reactive oxygen species (ROS), and effect of oxygen on protein and lipid oxidation
When cells are exposed to high extracellular oxygen concentration, oxygen diffuses through the membranes and abstract electrons from reduced flavoenzymes to produce partially reduced oxygen species such as superoxide (O2-) and hydrogen peroxide (H2O2) [5,8,43,44]. Since ROS production-rate is proportional to collision frequency of oxygen and redox enzymes, the rate of O2- and H2O2 formation inside the cells depends directly on the oxygen concentration in the extracellular environment [4,5,44,45]. The association between hyperoxia and accumulation of ROS was shown in 1982 by Crapo and colleagues [46,47]. In later publications, it was shown that cultures of CD14+ monocyte and HeLa-20 cells exposed to 40% O2 and 80% O2 respectively produced 2-fold higher ROS amounts than cultures under normal atmospheric oxygen concentration [41,48]. Similarly, Entamoeba histolytica and Drosophila melanogaster flies exposed to high-oxygen environment (90-95% O2) showed 2-fold increases in ROS accumulation compared with flies exposed to normal oxygen conditions [49,50]. Using isolated mitochondria, it was shown that the majority of ROS detected in the cells were derived from the mitochondrial electron transport chain [41]. This was also established in bacteria, where the main source of endogenous superoxide (O2-) was found to be the respiratory chain [4,51]. It was also demonstrated that the formation rate of O2- increase in proportion to the oxygen concentration [4]. Hence, it was proposed that at hyperoxia conditions, the main ROS accumulated in the mitochondrial matrix is H2O2. The proposed steps for its accumulation are the following: when cells are exposed to an increasing oxygen concentration, there is higher leakage of electrons from complex I and III of the respiratory chain leading to an increase in superoxide production as described in Figure 1. This superoxide is immediately converted to H2O2 by the mitochondrial superoxide dismutase [42]. At lower oxygen concentrations, catalases and glutathione peroxidase systems minimize the accumulation of H2O2 [44], but at higher oxygen concentrations, these antioxidant defenses are overwhelmed resulting in accumulation of H2O2 which can diffuse freely from the mitochondria reaching targets that can be damaged such as dehydratases and DNA [5,8].
Figure 1.
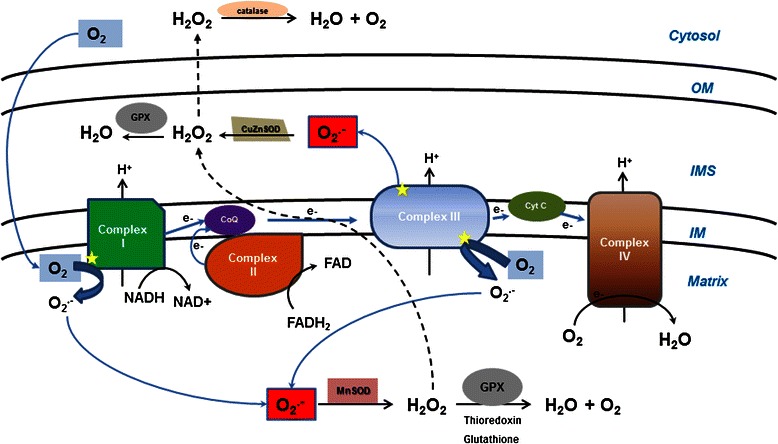
Elevated oxygen triggers intracellular ROS accumulation. The ubisemiquinone intermediates present in complexes I and III of the respiratory chain are the primary source of univalent reduction of oxygen into superoxide (O2 .-) (indicated by stars). At over-oxygenation conditions, electrons leak from complexes I and III generating superoxide increases. Superoxide is converted immediately to hydrogen peroxide (H2O2) by manganese superoxide dismutase (MnSOD) or copper/zinc superoxide dismutase (Cu/ZnSOD). At normoxic conditions, the catalases and peroxidase systems minimize the accumulation of H2O2 but at higher oxygen concentrations; these antioxidant defenses can be overwhelmed and the accumulating H2O2 diffuse freely from the mitochondria (dashed lines) reaching targets that can be damaged such as dehydratases and DNA. Abbreviations: CoQ, coenzyme Q10; Cyt C, cytochrome C; GPX, glutathione peroxidase; IM, inner membrane; IMS, intermembrane space; OM, outer membrane.
Similarly two transcription activators (Yap1 and Skn7) involved in the oxidative stress response and redox homeostasis that are normally induced by H2O2, were also activated at elevated oxygen conditions. These suggested that elevated oxygen promotes H2O2 accumulation which in turn activated the transcription of Yap1 and Skn7 factors [42,52].
The increased generation of ROS during exposure to high-oxygen concentrations can overwhelm the antioxidant defense mechanisms and eventually cause cell death [53]. It is interesting to note here that hyperoxia did not increase the ROS content in oxygen-tolerant HeLa-80 cells or in oxygen-resistant Drosophila melanogaster flies as it did in the non-resistant controls [41,50]. Compared with the wild Type HeLa 20, the oxygen-tolerant HeLa-80 cell did not show significant differences in antioxidant enzymes or compounds nor differential susceptibility to apoptotic death; the only difference was the lower intracellular ROS accumulation by the oxygen-tolerant cell line [41]. The oxygen-tolerant flies showed similar antioxidant enzyme activity as the control flies but demonstrated lower H2O2 accumulation, lipid peroxidation and protein oxidation [50]. Based on the above, it was concluded that oxygen-tolerant cells do not rely on increased activity of antioxidant defenses but on an increased efficiency of mitochondrial metabolism which decreased ROS leakage from the electron-rich intermediates of the mitochondrial electron transport chain.
High oxygen concentrations also induced ROS accumulation in bacteria; it was found that the production of periplasmic superoxide increased proportionally to the oxygen concentration [4]. Superoxide released by the respiratory chain in the periplasm provoked glutathione efflux from the cells. In the absence of SodC activity, glutathione can catalyze the dismutation of superoxide into H2O2, protecting the cell from oxidative stress [54]. E. coli grown at 300% dO2 air saturation (~0.63 atm of O2) accumulated 22% higher intracellular ROS compared to cultures grown at 30% dO2 [28]. However, this ROS increase did not cause any detectable change in the oxidation pattern of proteins after 3 hours of induction at 300% dO2 [55]. In another example the carbonyl content of the total proteins of Lactobacillus sake increased 2.2 and 11.2 fold in insensitive and sensitive strains respectively after 72 hours of exposure to 0.9 atm of O2 [56]. The increased protein damage reported may have resulted from longer exposure to oxidative stress imposed by elevated O2. It is important to emphasize that when oxygen concentration in bacterial cultures is increased 10-fold, the intracellular ROS concentration is increased only 1.2 fold, while smaller oxygen increase (2 to 5 fold) in eukaryotes promote 2-fold higher intracellular ROS accumulation [28,41,48-50].
Inducible responses and enzyme inactivation
The physiological response of microorganisms to oxygen has been studied by comparing normoxic (room air) with anaerobic conditions. However, physiological response to increasing oxygen concentrations from normoxia to hyperoxia is also important since a set of protecting genes involved in redox homeostasis and oxidative stress are also activated. An example is the 36 fold activation of sulfiredoxin (Srx1) transcription and 12 fold activation of NADPH dehydrogenase (Oye3) transcription in S. cerevisiae exposed to hyperoxic conditions [42]. E. coli MG1655 growing in glucose minimal medium or E. coli AB1157 growing in complex medium activated the transcription of SoxRS-controlled genes in response to oxygen increase unlike genes under OxyR control [28,55]. Using sodA-lacZ and sodB-lacZ fusions, it was shown that oxygen activates sodA expression while it has an opposite effect on sodB expression [57]. sodA expression at 0.21 atm and 0.6 atm of O2 was 35 and 45 fold higher compared with anaerobic conditions. At the same time, 0.21 atm of O2 repressed sodB expression by two fold compared with the expression at anaerobic conditions. When exposed to increased oxygen, the double mutant E. coli strain sodA- sodB- activated the transcription of the genes under control of both the OxyR and SoxRS regulons [28]. In addition to the 32-fold up-regulation of soxS gene, two oxidant-resistant isozymes, aconitase A (acnA) and fumarase C (fumC), were up-regulated in the sodA- sodB- mutant, an indication that the unstable aconitase B and fumarases A and B were inactivated by the oxygen-shift [28]. The inactivation of these enzymes could be attributed to the accumulation of intracellular superoxide [58]. This is probable since most dehydratases containing the [4Fe-4S]2+ cluster and metalloenzymes in general, can be easily inactivated by superoxide generated through hyperoxygenation and redox-cycling agents [21,58-61]. In Pseudomonas putida KT2440, elevated oxygen was found to activate gene expression of the iron-sulfur cluster system while decreasing intracellular levels of free iron in the cells [62].
E. coli contains at least three distinct superoxide dismutases (SODs): Mn-containing SOD (gene product of sodA) and Fe-containing SOD (gene product of sodB), both localized in the cytoplasm, and the hybrid CuZn-containing SOD (gene product of sodC) which is located in the periplasmic space [63-65]. It has been known that these SODs enzymes play an essential role in protecting the bacteria from oxygen poisoning. Exposing E. coli or S. cerevisiae cells to 1 atm of O2 after growing at air atmosphere induce an increase in the SOD activity which allows the cells to resist the lethal effect of 20 atm of O2 [12,66]. However, microorganisms that were unable to respond to increase O2 concentration by increasing SOD activity, like B. subtilis, were sensitive to hyperbaric dissolved oxygen even when the catalase activity was increased [66]. Construction of mutant strains lacking these scavenging enzymes has confirmed the important role of SODs at elevated oxygen concentrations. Hence, S. cerevisiae mutants with compromised SOD activity were extremely sensitive to hyperoxia [42,67,68]. Similarly, E. coli strains lacking Mn-SOD and Fe-SOD were sensitive to elevated oxygen concentrations and showed higher rate of spontaneous mutagenesis than the parental strains [28,69]. This double mutant E. coli strain, as a result of being auxotrophic for branched-chain amino acids, was unable to grow in aerobic minimal-medium [70,71]. Additional example of the important role of SOD at elevated oxygen concentration is the behavior of Lactobacillus sake, the lower SOD activity in the sensitive strain causes higher superoxide accumulation and higher damage of the the [Fe-S]-cluster-containing enzymes [56]. All the above demonstrates that one of the critical roles of SOD is maintaining the stability of [4Fe-4S] cluster of dihydroxyacid dehydratase, fumarase A and B, promoting the aerobic growth [72,73].
Conclusions
The use of oxygen enriched air has become an accepted practice currently utilized to support the aerobic growth of both prokaryotic and eukaryotic cells in bioreactors in an effort to increase the cell density and to improve process productivity. However, exposing cells to high oxygen concentrations is known to enhance the accumulation of intracellular ROS which can reach harmful levels that can overwhelm the antioxidant defenses and repair systems of the cells. The observations summarized here indicate that bacteria are more resistant to hyperoxygenation than mammalian cells. The ability of bacteria to control tightly the ROS homeostasis at elevated oxygen concentrations allows them to survive better in hyperoxia. The addition of agents that reduce the leak of electrons from respiratory chain hence reduce ROS accumulation, may prevent the damage of elevated oxygen concentration.
Acknowledgements
Funding was provided by the intramural program of the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health. The authors would like to thank Mrs. D. Livnat for critical editorial assistance.
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
AB collected all the relevant publications, wrote the manuscript and produced figure. JS revised and formatted the manuscript. Both authors read and approved the final manuscript.
Contributor Information
Antonino Baez, Email: abaezro@gmail.com.
Joseph Shiloach, Email: yossi@nih.gov.
References
- 1.Oosterhuis NMG, Kossen NWF. Dissolved oxygen concentration profiles in a production-scale bioreactor. Biotechnol Bioeng. 1984;26:546–550. doi: 10.1002/bit.260260522. [DOI] [PubMed] [Google Scholar]
- 2.Konz JO, King J, Cooney CL. Effects of oxygen on recombinant protein expression. Biotechnol Prog. 1998;14:393–409. doi: 10.1021/bp980021l. [DOI] [PubMed] [Google Scholar]
- 3.Kazzaz JA, Xu J, Palaia TA, Mantell L, Fein AM, Horowitz S. Cellular oxygen toxicity: oxidant injury without apoptosis. J Biol Chem. 1996;271:15182–15186. doi: 10.1074/jbc.271.25.15182. [DOI] [PubMed] [Google Scholar]
- 4.Korshunov S, Imlay JA. Detection and quantification of superoxide formed within the periplasm of Escherichia coli. J Bacteriol. 2006;188:6326–6334. doi: 10.1128/JB.00554-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Imlay JA. Cellular defenses against superoxide and hydrogen peroxide. Annu Rev Biochem. 2008;77:755–776. doi: 10.1146/annurev.biochem.77.061606.161055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Messner KR, Imlay JA. The identification of primary sites of superoxide and hydrogen peroxide formation in the aerobic respiratory chain and sulfite reductase complex of Escherichia coli. J Biol Chem. 1999;274:10119–10128. doi: 10.1074/jbc.274.15.10119. [DOI] [PubMed] [Google Scholar]
- 7.Korshunov S, Imlay JA. Two sources of endogenous hydrogen peroxide in Escherichia coli. Mol Microbiol. 2010;75:1389–1401. doi: 10.1111/j.1365-2958.2010.07059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Imlay JA. The molecular mechanisms and physiological consequences of oxidative stress: lessons from a model bacterium. Nat Rev Microbiol. 2013;11:443–454. doi: 10.1038/nrmicro3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haugaard N. Cellular mechanisms of oxygen toxicity. Physiol Rev. 1968;48:311–373. doi: 10.1152/physrev.1968.48.2.311. [DOI] [PubMed] [Google Scholar]
- 10.Brown OR. Reversible inhibition of respiration of Escherichia coli by hyperoxia. Microbios. 1972;5:7–16. [PubMed] [Google Scholar]
- 11.Boehme DE, Vincent K, Brown OR. Oxygen and toxicity inhibition of amino acid biosynthesis. Nature. 1976;262:418–420. doi: 10.1038/262418a0. [DOI] [PubMed] [Google Scholar]
- 12.Gregory EM, Goscin SA, Fridovich I. Superoxide dismutase and oxygen toxicity in a eukaryote. J Bacteriol. 1974;117:456–460. doi: 10.1128/jb.117.2.456-460.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gregory EM, Fridovich I. Induction of superoxide dismutase by molecular oxygen. J Bacteriol. 1973;114:543–548. doi: 10.1128/jb.114.2.543-548.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown OR, Seither RL. Oxygen and redox-active drugs: shared toxicity sites. Fundam Appl Toxicol. 1983;3:209–214. doi: 10.1016/S0272-0590(83)80127-4. [DOI] [PubMed] [Google Scholar]
- 15.Brown OR, Yein F. Dihydroxyacid dehydratase: the site of hyperbaric oxygen poisoning in branch-chain amino acids biosynthesis. Biochem Biophys Res Commun. 1978;85:1219–1224. doi: 10.1016/0006-291X(78)90672-1. [DOI] [PubMed] [Google Scholar]
- 16.Gardner PR, Fridovich I. Superoxide sensitivity of the Escherichia coli aconitase. J Biol Chem. 1991;266:19328–19333. [PubMed] [Google Scholar]
- 17.Harley JB, Santangelo GM, Rasmussen H, Goldfine H. Dependence of Escherichia coli hyperbaric oxygen toxicity on the lipid acyl chain composition. J Bacteriol. 1978;134:808–820. doi: 10.1128/jb.134.3.808-820.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brown OR, Yein F, Boehme D, Foudin L, Song CS. Oxygen poisoning of NAD biosynthesis: a proposed site of cellular oxygen toxicity. Biochem Biophys Res Commun. 1979;91:982–990. doi: 10.1016/0006-291X(79)91976-4. [DOI] [PubMed] [Google Scholar]
- 19.Brown OR, Song CS. Pyridine nucleotide coenzyme biosynthesis: a cellular site of oxygen toxicity. Biochem Biophys Res Commun. 1980;93:172–178. doi: 10.1016/S0006-291X(80)80262-2. [DOI] [PubMed] [Google Scholar]
- 20.Draczynska-Lusiak B, Brown OR. Protein A of quinolinate synthetase is the site of oxygen poisoning of pyridine nucleotide coenzyme synthesis in Escherichia coli. Free Radic Biol Med. 1992;13:689–693. doi: 10.1016/0891-5849(92)90042-F. [DOI] [PubMed] [Google Scholar]
- 21.Gardner PR, Fridovich I. Quinolinate synthetase: the oxygen-sensitive site of the novo NAD (P) + biosynthesis. Arch Biochem Biophys. 1991;284:106–111. doi: 10.1016/0003-9861(91)90270-S. [DOI] [PubMed] [Google Scholar]
- 22.Tamura T, Iida K, Saito M, Shiota S, Nakayama H, Yoshida S. Effect of hyperbaric oxygen on Vibrio vulnificus and murine infection caused by it. Microbiol Immunol. 2012;56:673–679. doi: 10.1111/j.1348-0421.2012.00491.x. [DOI] [PubMed] [Google Scholar]
- 23.Tsuneyoshi I, Boyle WA, Kanmura Y, Fujimoto T. Hyperbaric hyperoxia suppresses growth of Staphylococcus aureus, including methicillin-resistant strains. J Anesth. 2001;15:29–32. doi: 10.1007/s005400170048. [DOI] [PubMed] [Google Scholar]
- 24.Li X, Robbins JW, Taylor KB. Effect of the levels of dissolved oxygen on the expression of recombinant proteins in four recombinant Escherichia coli strains. J Ind Microbiol. 1992;9:1–10. doi: 10.1007/BF01576362. [DOI] [PubMed] [Google Scholar]
- 25.Castan A, Nasman A, Enfors SO. Oxygen enriched air supply in Escherichia coli processes: production of biomass and recombinant human growth hormone. Enzyme Microb Technol. 2002;30:847–854. doi: 10.1016/S0141-0229(01)00490-2. [DOI] [Google Scholar]
- 26.Shang L, Tian PY, Kim NJ, Chang HN, Hahm MS. Effects of oxygen supply modes on the production of human growth hormone in different scale bioreactors. Chem Eng Technol. 2009;32:600–605. doi: 10.1002/ceat.200800481. [DOI] [Google Scholar]
- 27.Lara AR, Knabben I, Regestein L, Sassi J, Caspeta L, Ramirez OT, Buchs J. Comparison of oxygen enriched air vs. pressure cultivations to increase oxygen transfer and to scale-up plasmid DNA production fermentations. Eng Life Sci. 2011;11:1–5. doi: 10.1002/elsc.201000104. [DOI] [Google Scholar]
- 28.Baez A, Shiloach J: Escherichia coliavoids high dissolved oxygen stress by activation of SoxRS and manganese-superoxide dismutase.Microb Cell Fact 2013, 12:23. [DOI] [PMC free article] [PubMed]
- 29.Gille JJ, Wortelboer HM, Joenje H. Effect of normobaric hyperoxia on antioxidant defenses of HeLa and CHO cells. Free Radic Biol Med. 1988;4:85–91. doi: 10.1016/0891-5849(88)90068-8. [DOI] [PubMed] [Google Scholar]
- 30.Lin AA, Miller WM. Modulation of glutathione level in CHO cells: effects of oxygen concentration and prior exposure to hypoxia. Ann N Y Acad Sci. 1992;665:117–126. doi: 10.1111/j.1749-6632.1992.tb42579.x. [DOI] [PubMed] [Google Scholar]
- 31.Scatena R, Messana I, Martorana GE, Gozzo ML, Lippa S, Maccaglia A, Bottoni P, Vincenzoni F, Nocca G, Castagnola M, Giardina B. Mitochondrial damage and metabolic compensatory mechanisms induced by hyperoxia in the U-937 cell line. J Biochem Mol Biol. 2004;37:454–459. doi: 10.5483/BMBRep.2004.37.4.454. [DOI] [PubMed] [Google Scholar]
- 32.Restelli V, Wang MD, Huzel N, Ethier M, Perreault H, Butler M. The effect of dissolved oxygen on the production and the glycosylation profile of recombinant human erythropoietin produced from CHO cells. Biotechnol Bioeng. 2006;94:481–494. doi: 10.1002/bit.20875. [DOI] [PubMed] [Google Scholar]
- 33.Gomez N, Ouyang J, Nguyen MD, Vinson AR, Lin AA, Yuk IH. Effect of temperature, pH, dissolved oxygen, and hydrolysate on the formation of triple light chain antibodies in cell culture. Biotechnol Prog. 2010;26:1438–1445. doi: 10.1002/btpr.465. [DOI] [PubMed] [Google Scholar]
- 34.Nienow AW, Langheinrich C, Stevenson NC, Emery AN, Clayton TM, Slater NK. Homogenisation and oxygen transfer rates in large agitated and sparged animal cell bioreactors: Some implications for growth and production. Cytotechnology. 1996;22:87–94. doi: 10.1007/BF00353927. [DOI] [PubMed] [Google Scholar]
- 35.Cacciuttolo MA, Trinh L, Lumpkin JA, Rao G. Hyperoxia induces DNA damage in mammalian cells. Free Radic Biol Med. 1993;14:267–276. doi: 10.1016/0891-5849(93)90023-N. [DOI] [PubMed] [Google Scholar]
- 36.Schweizer M, Peterhans E. Oxidation stress in cells infected with bovine viral diarrhoea virus: a crucial step in the induction of apoptosis. J Gen Virol. 1999;80:1147–1155. doi: 10.1099/0022-1317-80-5-1147. [DOI] [PubMed] [Google Scholar]
- 37.Wang Y, Oberley LW, Murhammer DW. Evidence of oxidative stress following the viral infection of two lepidopteran insect cell lines. Free Radic Biol Med. 2001;31:1448–1455. doi: 10.1016/S0891-5849(01)00728-6. [DOI] [PubMed] [Google Scholar]
- 38.Saarinen MA, Murhammer DW. The response of virally infected insect cells to dissolved oxygen concentration: recombinant protein production and oxidative damage. Biotechnol Bioeng. 2002;81:106–114. doi: 10.1002/bit.10460. [DOI] [PubMed] [Google Scholar]
- 39.Zhang F, Saarinen MA, Itle LJ, Lang SC, Murhammer DW, Linhardt RJ. The effect of dissolved oxygen (DO) concentration on the glycosylation of recombinant protein produced by the insect cell-baculovirus expression system. Biotechnol Bioeng. 2002;77:219–224. doi: 10.1002/bit.10131. [DOI] [PubMed] [Google Scholar]
- 40.Das KC: Hyperoxia decreases glycolytic capacity, glycolytic reserve and oxidative phosphorylation in MLE-12 cells and inhibits complex I and II function, but not complex IV in isolated mouse lung mitochondria.PLoS One 2013, 8:e73358. [DOI] [PMC free article] [PubMed]
- 41.Campian JL, Qian M, Gao X, Eaton JW. Oxygen tolerance and coupling of mitochondrial electron transport. J Biol Chem. 2004;279:46580–46587. doi: 10.1074/jbc.M406685200. [DOI] [PubMed] [Google Scholar]
- 42.Fujiwara H, Kawai S, Murata K. Significance of sulfiredoxin/peroxiredoxin and mitochondrial respiratory chain in response to and protection from 100% O2 in Saccharomyces cerevisiae. Mitochondrion. 2013;13:52–58. doi: 10.1016/j.mito.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 43.McDonald CA, Fagan RL, Collard F, Monnier VM, Palfey BA. Oxygen reactivity in flavoenzymes: context matters. J Am Chem Soc. 2011;133:16809–16811. doi: 10.1021/ja2081873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Turrens JF. Mitochondrial formation of reactive oxygen species. J Physiol. 2003;552:335–344. doi: 10.1113/jphysiol.2003.049478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Imlay JA. Pathways of oxidative damage. Annu Rev Microbiol. 2003;57:395–418. doi: 10.1146/annurev.micro.57.030502.090938. [DOI] [PubMed] [Google Scholar]
- 46.Freeman BA, Crapo JD. Hyperoxia increases oxygen radical production in rat lungs and lung mitochondria. J Biol Chem. 1981;256:10986–10992. [PubMed] [Google Scholar]
- 47.Turrens JF, Freeman BA, Levitt JG, Crapo JD. The effect of hyperoxia on superoxide production by lung submitochondrial particles. Arch Biochem Biophys. 1982;217:401–410. doi: 10.1016/0003-9861(82)90518-5. [DOI] [PubMed] [Google Scholar]
- 48.Yamasaki N, Tsuboi H, Hirao M, Nampei A, Yoshikawa H, Hashimoto J. High oxygen tension prolongs the survival of osteoclast precursors via macrophage colony-stimulating factor. Bone. 2009;44:71–79. doi: 10.1016/j.bone.2008.09.015. [DOI] [PubMed] [Google Scholar]
- 49.Akbar MA, Chatterjee NS, Sen P, Debnath A, Pal A, Bera T, Das P. Genes induced by a high-oxygen environment in Entamoeba histolytica. Mol Biochem Parasitol. 2004;133:187–196. doi: 10.1016/j.molbiopara.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 50.Zhao HW, Ali SS, Haddad GG. Does hyperoxia selection cause adaptive alterations of mitochondrial electron transport chain activity leading to a reduction of superoxide production? Antioxid Redox Signal. 2012;16:1071–1076. doi: 10.1089/ars.2011.4504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Imlay JA, Fridovich I. Assay of metabolic superoxide production in Escherichia coli. J Biol Chem. 1991;266:6957–6965. [PubMed] [Google Scholar]
- 52.Molin M, Yang J, Hanzén S, Toledano MB, Labarre J, Nyström T. Life span extension and H2O2 resistance elicited by caloric restriction require the peroxiredoxin Tsa1 in Saccharomyces cerevisiae. Mol Cell. 2011;43:823–833. doi: 10.1016/j.molcel.2011.07.027. [DOI] [PubMed] [Google Scholar]
- 53.Buccellato LJ, Tso M, Akinci OI, Chandel NS, Budinger GR. Reactive oxygen species are required for hyperoxia-induced Bax activation and cell death in alveolar epithelial cells. J Biol Chem. 2004;279:6753–6760. doi: 10.1074/jbc.M310145200. [DOI] [PubMed] [Google Scholar]
- 54.Oktyabrsky ON, Muzyka NG, Ushakov VY, Tyulenev AV, Smirnova GV: Endogeneous and exogenous superoxide provokes glutathione efflux fromEscherichia colicells.Res Microbiol, In press. [DOI] [PubMed]
- 55.Baez A, Majdalani N, Shiloach J: Production of recombinant protein by a novel oxygen-induced system inEscherichia coli.Microb Cell Fact 2014, 13:50. [DOI] [PMC free article] [PubMed]
- 56.Amanatidou A, Bennik MH, Gorris LG, Smid EJ. Superoxide dismutase plays an important role in the survival of Lactobacillus sake upon exposure to elevated oxygen. Arch Microbiol. 2001;176:79–88. doi: 10.1007/s002030100297. [DOI] [PubMed] [Google Scholar]
- 57.Matsumura Y, Takagi M, Imanaka T. Regulation of Escherichia coli superoxide dismutase genes (sodA and sodB) by oxygen. Biotechnol Lett. 1993;15:229–234. doi: 10.1007/BF00128310. [DOI] [Google Scholar]
- 58.Gu M, Imlay JA. Superoxide poisons mononuclear iron enzymes by causing mismetallation. Mol Microbiol. 2013;89:123–134. doi: 10.1111/mmi.12263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Varghese S, Tang Y, Imlay JA. Contrasting sensitivities of Escherichia coli aconitases A and B to oxidation and iron depletion. J Bacteriol. 2003;185:221–230. doi: 10.1128/JB.185.1.221-230.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liochev SI, Fridovich I. Fumarase C, the stable fumarase of Escherichia coli, is controlled by the soxRS regulon. Proc Natl Acad Sci USA. 1992;89:5892–5896. doi: 10.1073/pnas.89.13.5892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cunningham L, Gruer MJ, Guest JR. Transcriptional regulation of the aconitase genes (acnA and acnB) of Escherichia coli. Microbiology. 1997;143:3795–3805. doi: 10.1099/00221287-143-12-3795. [DOI] [PubMed] [Google Scholar]
- 62.Follonier S, Escapa IF, Fonseca PM, Henes B, Panke S, Zinn M, Prieto MA: New insights on the reorganization of gene transcription in Pseudomonas putida KT2440 at elevated pressure.Microb Cell Fact 2013, 12:30. [DOI] [PMC free article] [PubMed]
- 63.Benov L, Chang LY, Day B, Fridovich I. Copper, zinc superoxide dismutase in Escherichia coli: periplasmic localization. Arch Biochem Biophys. 1995;319:508–511. doi: 10.1006/abbi.1995.1324. [DOI] [PubMed] [Google Scholar]
- 64.Gort AS, Ferber DM, Imlay JA. The regulation and role of the periplasmic copper, zinc superoxide dismutase of Escherichia coli. Mol Microbiol. 1999;32:179–191. doi: 10.1046/j.1365-2958.1999.01343.x. [DOI] [PubMed] [Google Scholar]
- 65.Yost FJ, Fridovich I. An iron-containing superoxide dismutase from Escherichia coli. J Biol Chem. 1973;248:4905–4908. [PubMed] [Google Scholar]
- 66.Gregory EM, Fridovich I. Oxygen toxicity and the superoxide Dismutase. J Bacteriol. 1973;114:1193–1197. doi: 10.1128/jb.114.3.1193-1197.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Outten CE, Falk RL, Culotta VC. Cellular factors required for protection from hyperoxia toxicity in Saccharomyces cerevisiae. Biochem J. 2005;388:93–101. doi: 10.1042/BJ20041914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.van Loon APGM, Pesold-Hurt B, Schatz G. A yeast mutant lacking mitochondrial manganese-superoxide dismutase is hypersensitive to oxygen. Proc Natl Acad Sci USA. 1986;83:3820–3824. doi: 10.1073/pnas.83.11.3820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Farr SB, Ari RD, Touati D. Oxygen-dependent mutagenesis in Escherichia coli lacking superoxide dismutase. Proc Natl Acad Sci USA. 1986;83:8268–8272. doi: 10.1073/pnas.83.21.8268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Carlioz A, Touati D. Isolation of superoxide dismutase mutants in Escherichia coli: is superoxide dismutase necessary for aerobic life? EMBO J. 1986;5:623–630. doi: 10.1002/j.1460-2075.1986.tb04256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Imlay JA, Fridovich I. Suppression of oxidative envelope damage by pseudoreversion of a superoxide dismutase-deficient mutant of Escherichia coli. J Bacteriol. 1992;174:953–961. doi: 10.1128/jb.174.3.953-961.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Brown OR, Smyk-Randall E, Draczynsca-Lusiak B, Fee JA. Dihydroxyacid dehydratase, a [4Fe-4S] cluster-containing enzyme in Escherichia coli: effects of intracellular superoxide dismutase on its inactivation by oxidant stress. Arch Biochem Biophys. 1995;319:10–22. doi: 10.1006/abbi.1995.1262. [DOI] [PubMed] [Google Scholar]
- 73.Flint DH, Tuminello JF, Emptage MH. The inactivation of Fe-S cluster containing hydro-lyases by superoxide. J Biol Chem. 1993;268:22369–22376. [PubMed] [Google Scholar]


