Abstract
The present study established a model of RyR2 knockdown cardiomyocytes and elucidated the role of RyR2 in aconitine-induced arrhythmia. Cardiomyocytes were obtained from hearts of neonatal Sprague–Dawlay rats. siRNAs were used to down-regulate RyR2 expression. Reduction of RyR2 expression was documented by RT-PCR, western blot, and immunofluorescence. Ca2+ signals were investigated by measuring the relative intracellular Ca2+ concentration, spontaneous Ca2+ oscillations, caffeine-induced Ca2+ release, and L-type Ca2+ currents. In normal cardiomyocytes, steady and periodic spontaneous Ca2+ oscillations were observed, and the baseline [Ca2+]i remained at the low level. Exposure to 3 μM aconitine increased the frequency and decreased the amplitude of Ca2+ oscillations; the baseline [Ca2+]i and the level of caffeine-induced Ca2+ release were increased but the L-type Ca2+ currents were inhibited after application of 3 μM aconitine for 5 min. In RyR2 knockdown cardiomyocytes, the steady and periodic spontaneous Ca2+ oscillations almost disappeared, but were re-induced by aconitine without affecting the baseline [Ca2+]i level; the level of caffeine-induced Ca2+ release was increased but L-type Ca2+ currents were inhibited. Alterations of RyR2 are important consequences of aconitine-stimulation and activation of RyR2 appear to have a direct relationship with aconitine-induced arrhythmias. The present study demonstrates a potential method for preventing aconitine-induced arrhythmias by inhibiting Ca2+ leakage through the sarcoplasmic reticulum RyR2 channel.
Keywords: RyR2, Knockdown, Aconitine, Arrhythmia, Excitation–contraction coupling
1. Introduction
Aconite tuber, roots of aconite (Aconitum carmichaeli or A. kusnezofii), is an important oriental herbal medicine used for centuries in China and other countries to therapeutically increase the peripheral temperature, relieve rheumatic pain and treat neurological disorders [1]. The pharmacological effects of Aconitum alkaloids include positive inotropic effects, analgesic, anti-inflammatory, and antirheumatic activity as well as neurotransmission actions [2–4]. Aconitine and its structurally related analogs are known to injure both the central nervous system and the heart [5]. Its cardiotoxicity consists primarily of arrhythmic effects and it has been used as an experimental tool to induce tachyarrhythmias in animal models [6]. In spite of its potential toxic effects, aconitine remains a popular home remedy for several ailments in China. Although most mechanisms postulated for aconitine toxicity focus on voltage-dependent Na+ channels, little is known about its interaction with intracellular Ca2+ signals and Ca2+ handling proteins that regulate the cardiac excitation–contraction coupling (e–c coupling) system. We have shown previously that aconitine impairs the contractile function by disrupting the intracellular Ca2+ homeostasis in the cardiac e–c coupling of cultured cardiomyocytes and stimulating up-regulation of the type 2-ryanodine receptor (RyR2) level significantly [7]. The present study was therefore designed to establish a model of RyR2 knockdown (KD) cardiomyocytes and elucidate the role of RyR2 in aconitine-induced arrhythmia.
In cardiac muscle, the intracellular Ca2+ stores and the sarcoplasmic reticulum (SR) Ca2+ release play prominent roles in cardiac contractile activation and relaxation [8–10]. Ca2+ release from SR through the cardiac RyR2 channel is a fundamental event in cardiac muscle contraction, which is triggered by calcium-induced calcium release (CICR) [11]. Alterations in the sensitivity of RyR2 to Ca2+ release activation have been implicated in diseases including malignant hyperthermia and heart failure [12–14]. Mutations in RyR2 that are suspected to cause defective Ca2+ channel function and aberrant intracellular Ca2+ signals have recently been identified in catecholaminergic polymorphic ventricular tachycardia (CPVT) and arrhythmogenic right ventricular dysplasia (ARVD) patients [15]. Cardiomyocytes in RyR2 gene knock-out (KO) mice show a decrease in the rate of spontaneous diastolic depolarization and an absence of calcium sparks [16].
We report here that RyR2 gene expression was strongly reduced in cultured neonatal rat cardiomyocytes by exogenous transcription of small interfering RNAs (siRNAs). Moreover, we examined the effects of aconitine on the relative intracellular Ca2+ concentration, spontaneous Ca2+ oscillations, caffeine-induced Ca2+ release and L-type Ca2+ channel currents in control and RyR2 KD cardiomyocytes.
2. Materials and methods
2.1. Animals and chemicals
Cardiomyocytes were obtained by dissociating hearts of neonatal Sprague–Dawlay rats (1–3 days old). The experimental protocol for animals was approved by the Medical Ethical Committee of Tsinghua University. Aconitine (content ≥98%) was purchased from the National Institute for the Control of Pharmaceutical & Biological Products (China).
2.2. RyR2 gene knockdown techniques
2.2.1. Preparation of siRNAs for targeting on RyR2 gene
siRNAs were designed by Ambion (Ambion Inc., USA) according to the guidelines for effective knockdown. Three double-stranded siRNAs for targeting rat RyR2 gene were as follows: target 1 for RyR2 (siRNA1), 5′-CCUUGAACAGAAAUCUAAGtt-3′ (sense) and 5′-CUUAGAUUUCUGUUCAAGGtg-3′ (antisense); target 2 for RyR2 (siRNA2), 5′-CCCUCAGAGAUCAAAGAAAtt-3′ (sense) and 5′-UUUCUUUGAUCUCUGAGGGtg-3′ (antisense); target 3 for RyR2 (siRNA3), 5′-GCCAUGAAAAGAGUUGAUCtt-3′ (sense) and 5′-GAUCAACUCUUUUCAUGGCtt-3′ (antisense). The positive control siRNAs (anti-GAPDH siRNA) were purchased from Ambion. All siRNAs were provided in a freeze-dried, preannealed, HPLC-purified form (>80%). 20 nmol of annealed siRNA was resuspended into 200 μl RNA-free ddH2O as a stock solution at the concentration of 100 μM, and stored at −70 °C until required.
2.2.2. Cell cultures and siRNA transfection
Primary cardiomyocyte cultures were obtained as previously described [17]. Cells were seeded into six-well plates at a final density of 1.5–3 × 105 cells per well in DMEM/F12 media (Invitrogen Corporation, USA) supplemented with 10% fetal bovine serum (FBS) (Hyclone Laboratories Inc., USA) for 3 days under normal growth conditions (37 °C, 91% humidity and 5% CO2). All siRNAs were transfected into cardiomyocytes monolayers by using the siPORT™ NeoFX™ lipid-based reagent for reverse transfection (Ambion Inc., USA) according to the transfection protocol. After 6 h of transfection, the complexes were removed from the six-well plates. 2 ml fresh media containing 10% FBS was added to the plates. After 6 and 72 h, cardiomyocytes were used for subsequent experiments, each of which was performed at least three times. The controls were analyzed in parallel.
To optimize the contribution of the siRNA amount and the siPORT™ NeoFX™ volume to transfection efficiency in six-well plates, total volume of siRNA (2 μM) was varied between 1.25 and 37.5 μl, and siPORT™ NeoFX™ volume was varied between 2 and 6 μl per well. The cytotoxicity was evaluated by MTT assays. Down-regulation of RyR2 expression was identified by RT-PCR, western blot, and immunofluorescence analyses.
2.2.3. RT-PCR analyses
Total cellular RNA was extracted by PUREGENE™ RNA isolation kit (Gentra Systems, USA). Spectrophotometry at 260 and 280 nm was performed to measure the amount and purity of RNA. The specific primers used for RT-PCR are described in Ref. [7]. The GADPH primer was as follows: sense, 5′-TTGGCCGTATTGGCCGC-3′; antisence, 5′-GTGCCATTGAAC-TTGCCGTG-3′. The PCR condition was 95 °C for 1 min 30 s, 94 °C for 30 s, 52–60 °C for 40 s, and 72 °C for 30 s, 35 cycles, with a final extension for 5–7 min at 72 °C. In all experiments, the threshold count values were normalized to the internal control of GAPDH before calculating the changes for all of the genes. The products were separated by 1.5% agarose gel stained with ethidium bromide (EB). Intensity of the DNA bands was analyzed using the Totallab TL100 software (Nonlinear Inc., USA).
2.2.4. Western blot analyses
Protocols described in reference 18 were followed [18]. Protein samples (120 μg of total protein per lane) were separated by 5% SDS-PAGE gels for RyR and transferred to PVDF membranes. The membranes were probed with the monoclonal anti-RyR (1:2500, MA3-925) (Affinity Bioreagents, USA). A peroxidase-conjugated goat anti-mouse IgG was used as a secondary antibody (1:5000) (Affinity Bioreagents, USA). β-Actin was used as the internal standard for analysis of the protein. Signals were detected using the super signal ECL substrate (Pierce Biotechnology Inc., USA). The Totallab TL100 software was used to analyze the protein bands (Nonlinear Inc., USA).
2.2.5. Immunofluorescence assays
For immunofluorescence assays, cardiomyocytes were fixed with the mixture of methanol and acetone (v/v, 1:1) and blocked in 10% horse serum for 30 min at 37 °C. They were then incubated with the primary monoclonal anti-RyR (1:1000, MA3-925) (Affinity Bioreagents, USA) for 1 h at 37 °C, followed by goat anti-mouse IgG–FITC (1:100) secondary antibody (Santa Cruz Biotechnology Inc., USA) in dark chamber for 30 min at 37 °C. After that, cells were subsequently stained with PI (5 μg/ml, PBS) for 5 min and detected using a ZEISS LSM510 META laser-scanning confocal microscope (MIC Bergen, USA). Fluorescence recordings were done with either 488 nm laser excitation (520LP emission) or 543 nm laser excitation (580LP emission).
2.3. Assays of Ca2+ signals
2.3.1. Assays of the spontaneous Ca2+ oscillations and the relative intracellular Ca2+ concentration by Ca2+ imaging
Ca2+ imaging experiments were performed to measure the spontaneous Ca2+ oscillations and the relative intracellular Ca2+ concentration in control and RyR2 KD cardiomyocytes with aconitine-stimulation [19]. Cardiomyocytes were inoculated in 35 mm dishes (MatTeK Corporation, USA) and loaded with 5 μM fluo-3 AM (Sigma Chemical Co., USA). Intracellular fluo-3 AM was excited by a DG-4 quartz lamp filtered at 488 nm, and emission wavelengths were monitored with a 520 nm long-pass filter in 37 °C perfusion solution (in mM: 145 NaCl, 5.4 KCl, 0.5 MgCl2, 1.2 CaCl2, 5 HEPES, 5.5 Glucose, 0.3 NaH2PO4, pH 7.4) [20]. Rapid scanning of the observation field (5 s/scan) was used to minimize photo-oxidation artifacts. The images were recorded by a CoolSNAP fx CCD camera (Roper Scientific, USA). Ca2+ oscillations were recorded and analyzed using MetaFluor software (Universal Imaging Corporation, USA).
2.3.2. Assessments content of SR Ca2+ release stimulated by caffeine
To evaluate the SR Ca2+ loading state, caffeine (20 mM) was applied to stimulate Ca2+ release from SR. After the relative intracellular free Ca2+ concentration ([Ca2+]i) transients of the fluo-3 AM loaded cardiomyocytes became stable, a rapid pulse of 20 mM caffeine (200 ms) was applied to the cardiomyocytes via a micropipette (~4 μm at tip). The micropipette was placed downstream with respect to the superfusate flow, which prevented caffeine leaking out of the pipette from diffusing to the cardiomyocytes. Baseline values for [Ca2+]i were measured in individual cells. Resting and peak [Ca2+]i during caffeine application were each converted to total cytosolic Ca2+ taking into account estimated Ca2+ binding at high and low affinity sites. The difference (Δ[Ca2+]i) was taken as the total level of caffeine-induced Ca2+ release. Cells without treatment were initiated by a puff of caffeine to induce SR Ca2+ release. After washed out the caffeine with perfusion solution, cells were treated with 3 μM aconitine for 5 min, and then initiated with the puff of caffeine to induce SR Ca2+ release.
2.3.3. L-type Ca2+ currents assay by patch clamp
Intracellular Ca2+ currents were recorded using the whole-cell patch clamp technique. The culture media was replaced with the external solution (in mM: 120 NaCl, 5 KCl, 1 MgCl2, 3.6 CaCl2, 10 HEPES, 20 TEA, 0.001 TTX, pH 7.4 (TEAOH)) before recording. The patch pipettes (4–6 M resistance) were filled with the internal solution (in mM: 120 CsCl, 3 MgCl2, 5 HEPES, 10 EGTA, 5 MgATP, pH 7.4 (CsOH)) [21]. Whole-cell path clamp recordings were made at room temperature (21–23 °C) with the Axopatch-200B amplifier (Axon Instruments, USA) in conjunction with pClamp9 software (Axon Instruments, USA). Cardiomyocytes were clamped at −40 mV and step depolarized to +50 mV in 10 mV increments. Ca2+ current recordings were filtered with a current filter at 5 kHz.
2.4. Statistical analyses
Data were presented as mean ± S.E. Differences between means were determined using the Student’s t-test for paired observations. Differences were considered statistically significant when p-value <0.05.
3. Results
3.1. Maximal reduction of RyR2 mRNA level was achieved by exogenous transcription of siRNA3 48 h after transfection
In order to examine the silencing specificity of the three siRNAs in primary cultured cardiomyocytes from hearts of neonatal SD rats (1–3 days), mRNA levels of RyR2 were investigated by RT - PCR. Transfection of cardiomyocytes with the siRNA3 template led to significant reduction of mRNA level of RyR2.
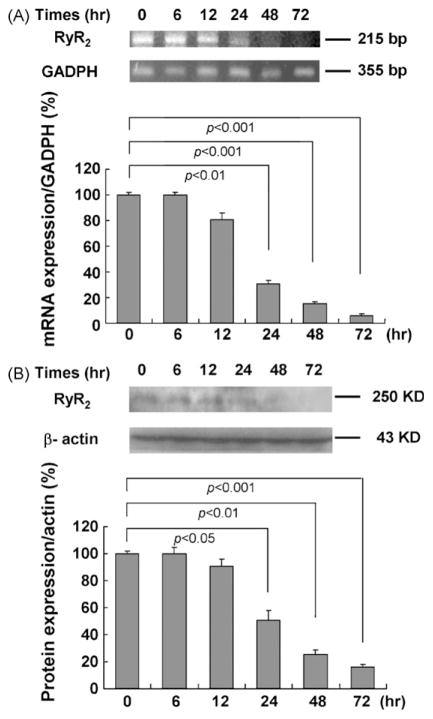
To further examine siRNA specificity, reduction of RyR2 expression in cardiomyocytes after delivery of siRNA3 for 6–72 h was documented by mRNA level (semiquantitative RT-PCR) paralleled with the corresponding protein expression (western blot) (Fig. 1A and B). Changes in mRNA and protein levels of RyR2 were noted in cardiomyocytes with siRNA transfection after 24 h. We can also see that changes in mRNA and protein levels after 48–72 h were more significant than other time point. But it was shown in MTT assays that cytotoxicity of the cardiomyocytes with transfection after 72 h was more obvious than that in cardiomyocytes with transfection after 48 h, so in the subsequent experiments, we use the KD cadiomyocytes with transfection after 48 h.
Fig. 1.
Effects of siRNA3 target on mRNA and protein levels of RyR2 in rat primary cardiomyocytes with transfection after 6–72 h tested by RT-PCR and western blot. (A) Changes in mRNA levels of RyR2 in cardiomyocytes with siRNA3 transfection after 6–72 h. GAPDH served as an internal control for analysis of the PCR productions. (B) Changes in protein expressions of RyR2 in cardiomyocytes with siRNA3 transfection after 6–72 h. β-Actin was used as a standard for analysis of the protein samples. Data are presented as the mean ± S.E. Differences were considered statistically significant when p-value <0.05, t-test, n = 4.
Of the three siRNAs, the maximal silencing effect of RyR2 was achieved with the siRNA3 targeting rat RyR2 gene from 2137 to 2155 bp. The silencing effects of siRNA1 and siRNA2 targeting 1850–1868 bp and 1902–1920 bp, respectively, were less obvious than that of siRNA3 (XM_341548). The optimal siRNA amount and siPORT™ NeoFX™ volume that yielded the best transfection efficiency in six-well plates were under the following conditions: total volume of media is 1 ml, total volume of siRNA (2 μM) is 5 μl, and the siPORT™ NeoFX™ volume is 4 μl per well.
3.2. The mRNA and protein levels of RyR2 were increased by aconitine
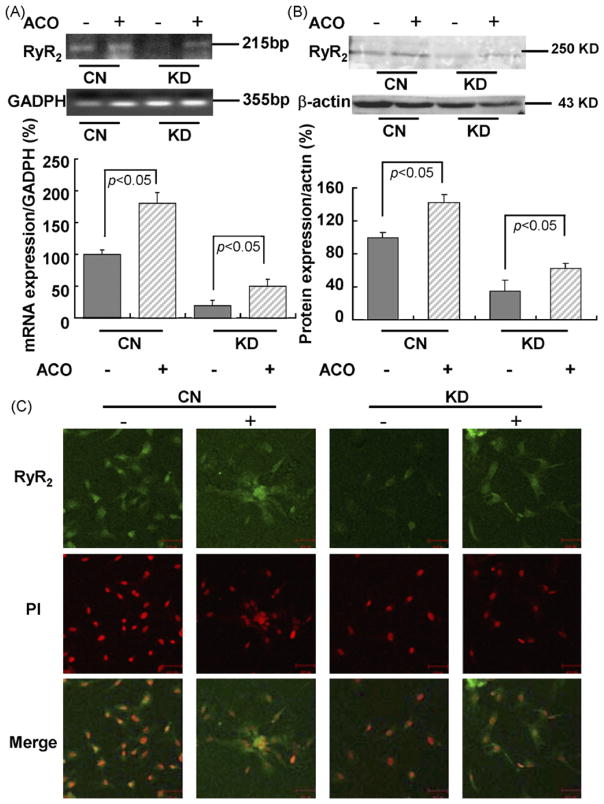
To examine the effect of aconitine on RyR2, RT-PCR, western blot, and immunofluorescence were used to examine RyR2 expression. As illustrated in Fig. 2A and B, mRNA and protein levels were increased both in control and KD cardiomyocytes induced by 3 μM aconitine for 0.5 h. We can also see that alteration of RyR2 expression in KD cardiomyocytes is more obvious rather than that in control cardiomyocytes. (mRNA level: from 100.05 ± 6.31 to 182.51 ± 17.85% in CN cardiomyocytes and from 22.76 ± 8.42 to 51.89 ± 12.33% in KD cardiomyocytes, respectively; protein level: from 100.08 ± 6.04 to 142.39 ± 9.61% in CN cardiomyocytes and from 35.12 ± 11.3 to 62.38 ± 6.74% in KD cardiomyocytes, respectively.)
Fig. 2.
Effects of aconitine on mRNA and protein levels of RyR2 in control cardiomyocytes and knockdown cardiomyocytes (with siRNA3 transfection after 48 h) tested by RT-PCR, western blot, and immunostaining. (A and B) Changes in mRNA and protein levels of RyR2 in CN and KD cardiomyocytes before and after aconitine application for 0.5 h. GAPDH and β-actin were used as standards for analyses of the protein and total RNA samples, respectively. Data are presented as the mean ± S.E. Differences were considered statistically significant when p-value <0.05, t-test, n = 4. (C) Immunostaining of RyR2 fluorescence in CN and KD cardiomyocytes before and after aconitine application, respectively, using the anti-RyR primary antibody (scale bar: 50 μm). Immunofluorescence staining on cardiomyocytes RyR positive signals is better observed in the cytosol (green signals). All nuclei were stained with PI (CN, control; KD, knockdown; ACO, aconitine).
Immunostaining was used to localize RyR2 in cardiomyocytes (Fig. 2C). Fluorescence in cardiomyocytes stained with primary anti-RyR and FITC-labeled secondary antibodies was noted in control cardiomyocytes with cytosolic prominence likely caused by active RyR2 expression. We observed the reduction of RyR2 expression in all cardiomyocytes visible in the field, which indicates a very efficient siRNA delivery 48 h after transfection. We can also see in Fig. 2C that, consistent with RT-PCR and western blot results, RyR2 fluorescence was significantly increased after exposure to 3 μM aconitine for 0.5 h in RyR2 KD cardiomyocytes.
3.3. Aconitine-induced recovery of spontaneous Ca2+ oscillations without affecting the baseline [Ca2+]i in RyR2 KD cardiomyocytes
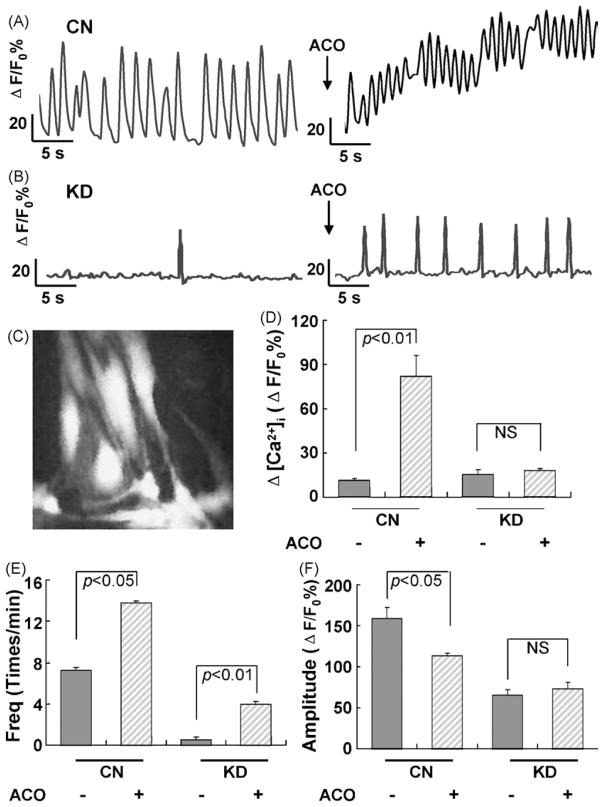
We used digital imaging technique to explore the effects of aconitine on spontaneous Ca2+ oscillations and the relative intracellular Ca2+ concentration (baseline [Ca2+]i) in control and RyR2 KD cardiomyocytes (Fig. 3A and B). In control cardiomyocytes, steady and periodic spontaneous Ca2+ oscillations were observed, and the baseline [Ca2+]i remained at low level. Application of 3 μM aconitine resulted in a significant increase in the frequency (nearly 2-fold higher, from 7.25 ± 0.25 to 13.75 ± 0.35, p < 0.05) and a decrease in the amplitude of spontaneous Ca2+ oscillations (from 159.41 ± 12.72 to 113.34 ± 3.34 ΔF/F0%, p < 0.05). A significant elevation of the baseline [Ca2+]i (from 11.38 ± 1.07 to 82.14 ± 13.66 ΔF/F0%, p < 0.01) was also observed.
Fig. 3.
Effects of aconitine on the relative intracellular Ca2+ concentration and spontaneous Ca2+ oscillations in control cardiomyocytes and knockdown cardiomyocytes (with siRNA3 transfection after 48 h) examined by Ca2+ imaging. (A and B) Representative recordings of the spontaneous Ca2+ oscillations in a given CN and KD cardiomyocyte before and after aconitine application, respectively. Traces indicate relative changes of fluo-3 intensity (ΔF/F0%) over time in cells randomly selected from the culture dish. Each trace represents ΔF/F0% of an individual cell acquired every 5 s. (C) The photograph shows fluorescent image of cardiomyocytes loaded with fluo-3 AM (scale bar: 50 μm). (D–F) Effects of aconitine on the baseline [Ca2+]i, the frequency and amplitude of spontaneous Ca2+ oscillations in CN and KD cardiomyocyes before and after aconitine application. Data are presented as the mean ± S.E. Differences were considered statistically significant when p-value <0.05, t-test, n = 4 plates (CN, control; KD, knockdown; ACO, aconitine).
In contrast, in RyR2 KD cardiomyocytes, steady and periodic spontaneous Ca2+ oscillations disappeared completely. Interestingly, application of 3 μM aconitine re-induced spontaneous Ca2+ oscillations (frequency: from 0.5 ± 0.29 to 4.4 ± 0.2, p < 0.01; amplitude: from 65.52 ± 6.66 to 73.45 ± 7.42 ΔF/F0%) without affecting the baseline [Ca2+]i level (15.23 ± 3.38 vs. 17.93 ± 1.36 ΔF/F0%) (Fig. 3D–F).
3.4. The level of caffeine-induced Ca2+ release in the cardiomyocytes was increased by aconitine
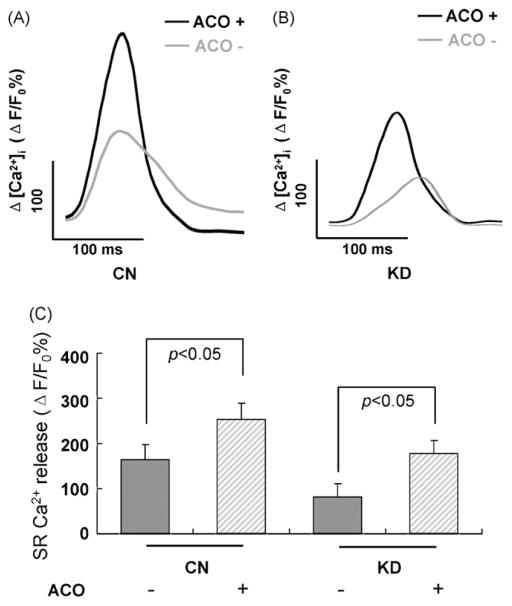
The SR Ca2+ release from the SR was estimated by the traditional caffeine puff. The Ca2+ content released from SR during the caffeine contracture was measured as ΔF/F0%. Superimposed Ca2+ transients in control and RyR2 KD cardiomyocytes were performed (Fig. 4A and B). It was shown that Ca2+ transients were relatively smaller in RyR2 KD cardiomyocytes than those in control cardiomyocytes (164.25 ± 33.29 vs. 83.89 ± 28.36 ΔF/F0%). The alterations of transient amplitudes, however, were amplified significantly in both of these two kinds of cardiomyocytes when the caffeine contracture was done in 3 μM aconitine-containing perfusion solution for 5 min (CN cardiomyocytes: from 164.25 ± 33.29 to 254.17 ± 35.41 ΔF/F0%, p < 0.05; KD cardiomyocytes: from 83.89 ± 28.36 to 178.26 ± 29.32 ΔF/F0%, p < 0.05, respectively) (Fig. 4C).
Fig. 4.
Effects of aconitine on sensitivity to caffeine contracture in control cardiomyocytes and knockdown cardiomyocytes (with siRNA3 transfection after 48 h) examined by Ca2+ imaging. (A and B) Caffeine-induced Ca2+ transients in CN and KD cardiomyocytes before (gray trace) and after (black trace) aconitine application for 5 min. Each trace represents ΔF/F0% of an individual cell acquired every 200 ms. (C) Average Δ[Ca2+]i for caffeine-induced Ca2+ transients. Data are presented as the mean ± S.E. Differences were considered statistically significant when p-value <0.05, t-test, n = 5 cells per plate, four plates (CN, control; KD, knockdown; ACO, aconitine).
3.5. L-type Ca2+ currents in cardiomyocytes were inhibited by aconitine
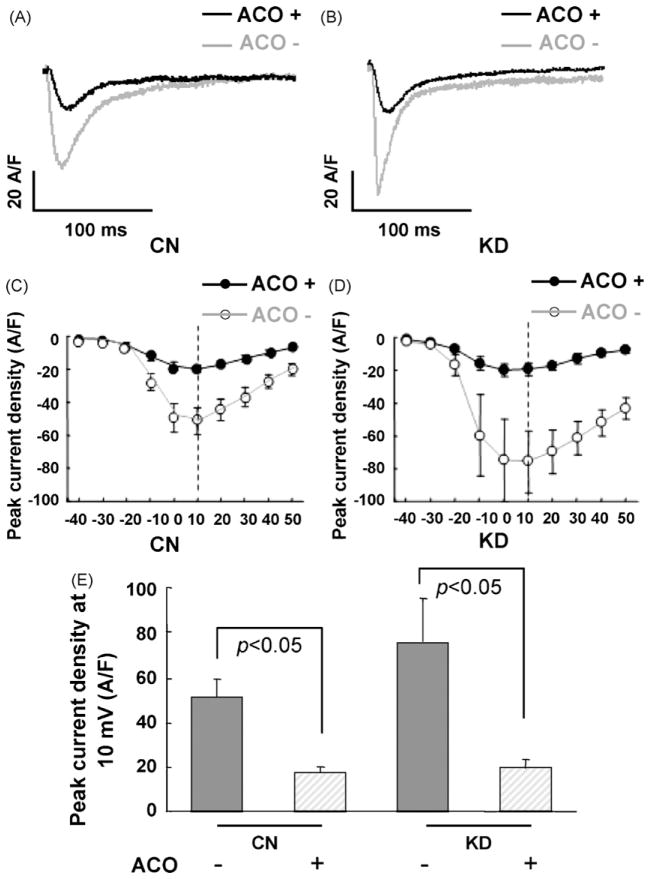
Current responses to a voltage step from −40 to 50 mV were recorded in control and RyR2 KD cardiomyocytes and estimated as current density (A/F) (Fig. 5A and B). Both in control and KD cardiomyocytes, I–V curves showed that activation of L-type Ca2+ current began at −30 mV and peak currents occurred between 0 and +10 mV in the absence and presence of 3 μM aconitine (Fig. 5C and D).
Fig. 5.
Effects of aconitine on the L-type Ca2+ currents in control cardiomyocytes and knockdown cardiomyocytes (with siRNA3 transfection after 48 h) examined by patch clamp. (A and B) L-type Ca2+ currents recorded at 10 mV command potential in CN and KD cardiomyocyes before (gray trace) and after (black trace) aconitine application for 5 min. (C and D) ICa–V relationships in CN (hollow circle) and KD cardiomyocytes (solid circle) before (gray trace) and after (black trace) aconitine application. (E) Peak current density at 10 mV command potential in CN and KD cardiomyocyes. Data are presented as the mean ± S.E. Differences were considered statistically significant when p-value <0.05, t-test, n = 5 cells per plate, five plates (CN, control; KD, knockdown; ACO, aconitine).
Comparing the peak currents density at 10 mV, I–V relationships showed that L-type Ca2+ currents in RyR2 KD cardiomyocytes were higher than those in control cardiomyocytes (52.84 ± 13.66 A/F vs. 75.77 ± 18.80 A/F). We can also see that both in control and RyR2 KD cardiomyocytes, the L-type Ca2+ current density at peak current was significantly lower in the presence of 3 μM aconitine as compared to that in the absence of aconitine (Fig. 5C and D). L-type Ca2+ currents density in control cardiomyocytes was decreased by application of 3 μM aconitine (52.84 ± 13.66 A/F vs. 19.51 ± 4.01 A/F, p < 0.05). And that in RyR2 KD cardiomyocytes was decreased from 75.77 ± 18.80 to 21.98 ± 2.44 A/F by application of 3 μM aconitine (p < 0.05) (Fig. 5E).
4. Discussion
The present study established a model of RyR2 KD cardiomyocytes and found calcium homeostasis in these myocytes were seriously affected. In addition, we studied the possible role of RyR2 in aconitine-induced arrhythmias by comparing the effects of the presence and absence of aconitine in control cardiomyocytes and in cardiomyocytes with reduced RyR2 mRNA and protein levels. We concluded that alterations of RyR2 are important consequences of aconitine-stimulation and activation of RyR2 expression appear to have a direct relationship with aconitine-induced arrhythmias. This conclusion was mainly based on the observations that (1) aconitine induced up-regulation of RyR2 mRNA and protein levels both in control and RyR2 KD cardiomyocytes; (2) aconitine-induced recovery of spontaneous Ca2+ oscillations without affecting the baseline [Ca2+]i in RyR2 KD cardiomyocytes; (3) the level of caffeine-induced Ca2+ release in the cardiomyocytes was increased by aconitine; (4) L-type Ca2+ channel currents in cardiomyocytes were inhibited by aconitine. The present study therefore provides an insight into a useful method for preventing aconitine-induced arrhythmias by reducing Ca2+ leakage through the SR RyR2 channel.
Mutations in the human RyR2 gene have been shown to cause different forms of cardiac arrhythmias characterized by stress-, emotion-, and physical exercise-induced ventricular tachycardia (VT). However, the molecular and cellular mechanisms underlying these forms of ventricular arrhythmias are not clear [22]. It has been hypothesized that disease-associated RyR2 mutations are likely to cause the channel to open spontaneously or to increase its sensitivity to activation by luminal Ca2+ [23,24]. In an earlier study we found that aconitine could enhance mRNA and protein levels of RyR2 gene [7]. We conjectured, on these grounds, that alterations in RyR2 channel function induced by aconitine would influence the properties of SR Ca2+ release and spontaneous Ca2+ oscillations, and thus cause the occurrence of triggered arrhythmias. In order to elucidate the crucial role of RyR2 in aconitine-induced arrhythmia, we use the RyR2 gene KD cardiomyocytes model to further study the role of RyR2 in aconitine-induced arrhythmic toxicity.
The siRNA method for reducing gene expression is a rapidly evolving tool in molecular biology. There are several methods for preparing siRNA [25]. Here, we report that reduction of RyR2 gene expression can be obtained by the chemical synthesis siRNAs with the aid of lipid-based reagent for reverse transfection (Fig. 1). Moreover, RT-PCR shows a more pronounced reduction of RyR2 mRNA level compared to protein expression by western blot. This finding is likely due to drastic interference and specific degradation of mRNA by siRNA, indicating that our procedure of RNA silencing is effective in neonatal rat cardiomyocytes.
It is known that [Ca2+]i occupies a key role in cardiac e–c coupling. During an action potential (AP), the influx of extracellular Ca2+ via L-type Ca2+ channels triggers SR Ca2+ release, resulting in an increase in [Ca2+]i and myofilament activation [26]. When SR Ca2+ content reaches a critical level, spontaneous SR Ca2+ release in the form of Ca2+ waves or Ca2+ oscillations occurs in cardiac cells [27]. The systolic dysfunction observed after transient Ca2+ overload is the result of both decreased systolic Ca2+ and reduced myofilament sensitivity to Ca2+ [28].
In our experiments, we tested the spontaneous Ca2+ oscillations and [Ca2+]i in control and RyR2 KD cardiomyocytes with aconitine-stimulation (Fig. 3). We found that aconitine induced a sharp increase in the relative [Ca2+]i and aberrant spontaneous Ca2+ oscillations in control cardiomyocytes indicating that the malfunctions of spontaneous Ca2+ oscillations and the ability to contract and relax in aconitine-induced cardiomyocytes are due to Ca2+ overload of the cytosolic Ca2+ concentration. In RyR2 KD cardiomyocytes with aconitine-stimulation, the spontaneous Ca2+ oscillations were recovered partly, also reflects the properties of RyR2 in aconitine-induced cardiomyocytes. It has been suggested that the occurrence of Ca2+ oscillations in RyR2 (wild type) cells depends completely on the expression of RyR2 [27], which is consistent with our results that modulating RyR2 function has a close relationship with the intracellular Ca2+ concentration and spontaneous Ca2+ oscillations in cardiomyocytes with aconitine-stimulation.
In order to verify whether the overload of the cytosolic Ca2+ concentration and the alteration of spontaneous Ca2+ oscillations were triggered by excess enhancement of the SR Ca2+ release in aconitine-induced cardiomyocytes, we investigated the level of caffeine-induced Ca2+ release in control and RyR2 KD cardiomyocytes.
RyR2 regulates SR Ca2+ release and thus, the Ca2+ content of SR luminal storage. We investigated the SR Ca2+ release by caffeine stimulation. Rapid application of caffeine solution induces a [Ca2+]i transient and contracture in cardiomyocytes, and the amplitude can be used as an index of SR Ca2+ releasable content [29]. Fig. 4 demonstrates that higher level of caffeine-induced Ca2+ release was observed in both control and RyR2 KD cardiomyocytes with aconitine-stimulation. These data suggest that aconitine caused enhancing SR Ca2+ release by RyR2 luminal Ca2+ activation and an increase in RyR2 gene expression due to aconitine results in an increase in SR Ca2+ leak and intracellular Ca2+ concentration. It has been shown that an increase in RyR2 activity lowered the amplitude of propagating Ca2+ waves, but increased the frequency of propagating Ca2+ waves and this increase escalates the propensity for triggered arrhythmia [23,24]. An increased propensity for spontaneous Ca2+ oscillations and decreased propagating Ca2+ waves under the condition of intracellular Ca2+ overload triggered by excess enhancement of the SR Ca2+ release may underlie a mechanism of aconitine-induced cardiac arrhythmia. It has now become clear that alterations in RyR2 channel function as a result of genetic defects can cause ventricular arrhythmias [30]. Our findings also provide evidence that acquired dysfunction of RyR2 could lead to arrhythmia susceptibility.
On the other hand, recent investigations show that SR Ca2+ release appears to modulate the sarcolemmal L-type Ca2+ currents, suggesting a retrograde communication from the SR to the sarcolemmal L-type Ca2+ channels in cardiac e–c coupling [31]. In this regard, it is worthy to identify the effect of aconitine on L-type Ca2+ currents in control and RyR2 KD cardiomyocytes.
As reported, aconitine binds to Na+ channels and prolongs the voltage-dependent Na+ channel open state, favoring entry of a large quantity of Na+ into cytosol. This influx then induces AP and activates the L-type Ca2+ currents [32]. In the present study, tetrodotoxin (TTX, 1 μM) was used to inhibit the effect of the Na+ channel activation induced by aconitine.
As shown in Fig. 5, compared with the control cardiomyocytes, the L-type Ca2+ currents were increased in RyR2 KD cardiomyocytes. Moreover, application of aconitine resulted in a decrease in magnitude of the L-type Ca2+ currents in control and RyR2 KD cardiomyocytes. The physiological function of the L-type current in skeletal muscle fibers is unclear. Some proposal reported that a depletion of SR Ca2+ would result in decreased SR Ca2+ release on plasma membrane depolarization, resulting in a smaller rise in triadic Ca2+, which in turn would stimulate Ca2+ influx via the L-type Ca2+ channel [31]. Our results are consistent with the proposal that aconitine stimulation-induced SR Ca2+ leakage through RyR2 channel, which depressed L-type Ca2+ current. Our results support the hypothesis of a retrograde communication between the SR Ca2+ release channel and the L-type Ca2+ channel (DHPR). This communication may be mediated in some cases by a direct interaction between the two channel proteins, L-type Ca2+ channel and RyR2 [33].
Acknowledgments
The authors want to thank Ms. Carlyn Tan from Northwestern University (Chicago) for her help in revising this manuscript. The present work was supported by grants of the ‘Tenth-five’ National Key Technologies R&D Programme (No. 2004BA721A11) and the Ph.D. Programs Foundation of Ministry of Education of China (No. 20060003036).
Abbreviations
- RyR2
type 2-ryanodine receptor
- KD
knockdown
- e–c coupling
excitation–contraction coupling
- ACO
aconitine
- SR
sarcoplasmic reticulum
- siRNA
small interfering RNA
References
- 1.Sato H, Yamada C, Konno C, Ohizumi Y, Endo K, Hikino H. Pharmacological actions of aconitine alkaloids. Tohoku J Exp Med. 1979;128:175–87. doi: 10.1620/tjem.128.175. [DOI] [PubMed] [Google Scholar]
- 2.Honerjager P, Meissner A. The positive inotropic effect of aconitine. Naunyn Schmiedebergs Arch Pharmacol. 1983;322:49–58. doi: 10.1007/BF00649352. [DOI] [PubMed] [Google Scholar]
- 3.Hikino H, Konno C, Takata H, Yamada Y, Yamada C, Ohizumi Y. Anti-inflammatory principles of aconitum roots. J Pharm Dyn. 1980;3:514–25. doi: 10.1248/bpb1978.3.514. [DOI] [PubMed] [Google Scholar]
- 4.Herzog W, Feibel R, Bryant S. The effect of aconitine on the giant axon of the squid. J Gen Physiol. 1964;47:719–33. doi: 10.1085/jgp.47.4.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ameri A. The effects of Aconitum alkaloids on the central nervous system. Prog Neurobiol. 1998;56:211–35. doi: 10.1016/s0301-0082(98)00037-9. [DOI] [PubMed] [Google Scholar]
- 6.Levy S, Breithardt G, Campbell RW, Camm AJ, Daubert JC, Allessie M, et al. Working Group on Arrhythmias of the European Society of Cardiology. Atrial fibrillation: current knowledge and recommendations for management. Eur Heart J. 1998;19:1294–320. doi: 10.1053/euhj.1998.1050. [DOI] [PubMed] [Google Scholar]
- 7.Fu M, Wu M, Wang JF, Qiao YJ, Wang Z. Disruption of the intracellular Ca2+ homeostasis in the cardiac excitation-contraction coupling is a crucial mechanism of arrhythmic toxicity in aconitine-induced cardiomyocytes. Biochem Biophys Res Commun. 2007;354:929–36. doi: 10.1016/j.bbrc.2007.01.082. [DOI] [PubMed] [Google Scholar]
- 8.Eisner DA, Choi HS, Diaz ME, O’Neill SC, Trafford AW. Integrative analysis of calcium cycling in cardiac muscle. Circ Res. 2000;87:1087–94. doi: 10.1161/01.res.87.12.1087. [DOI] [PubMed] [Google Scholar]
- 9.Bers DM. Cardiac excitation-contraction coupling. Nature. 2002;415:198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- 10.MacLennan DH, Kranias EG. Phospholamban: a crucial regulator of cardiac contractility. Nat Rev Mol Cell Biol. 2003;4:566–77. doi: 10.1038/nrm1151. [DOI] [PubMed] [Google Scholar]
- 11.Fabiato A. Simulated calcium current can both cause calcium loading in and trigger calcium release from the sarcoplasmic reticulum of a skinned canine cardiac Purkinje cell. J Gen Physiol. 1985;85:291–320. doi: 10.1085/jgp.85.2.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mickelson JR, Louis CF. Malignant hyperthermia: excitation-contraction coupling, Ca2+ release channel, and cell Ca2+ regulation defects. Physiol Rev. 1996;76:537–92. doi: 10.1152/physrev.1996.76.2.537. [DOI] [PubMed] [Google Scholar]
- 13.Loke J, MacLennan DH. Malignant hyperthermia and central core disease: disorders of Ca2+ release channels. Am J Med. 1998;104:470–86. doi: 10.1016/s0002-9343(98)00108-9. [DOI] [PubMed] [Google Scholar]
- 14.Marx SO, Reiken S, Hisamatsu Y, Jayaraman T, Burkhoff D, Rosemblit N, et al. PKA phosphorylation dissociates FKBP12. 6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts. Cell. 2000;101:365–76. doi: 10.1016/s0092-8674(00)80847-8. [DOI] [PubMed] [Google Scholar]
- 15.George CH, Higgs GV, Lai FA. Ryanodine receptor mutations associated with stress-induced ventricular tachycardia mediate increased calcium release in stimulated cardiomyocytes. Circ Res. 2003;93:531–40. doi: 10.1161/01.RES.0000091335.07574.86. [DOI] [PubMed] [Google Scholar]
- 16.Yang HT, Tweedie D, Wang S, Guia A, Vinogradova T, Bogdanov K, et al. The ryanodine receptor modulates the spontaneous beating rate of cardiomyocytes during development. Proc Natl Acad Sci USA. 2002;99:9225–30. doi: 10.1073/pnas.142651999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zahabi A, Deschepper CF. Long-chain fatty acids modify hypertrophic responses of cultured primary neonatal cardiomyocytes. J Lipid Res. 2001;42:1325–30. [PubMed] [Google Scholar]
- 18.Temsah RM, Netticadan T, Chapman D, Takeda S, Mochizuki S, Dhalla NS. Alterations in sarcoplasmic reticulum function and gene expression in ischemic-reperfused rat heart. Am J Physiol. 1999;277:H584–94. doi: 10.1152/ajpheart.1999.277.2.H584. [DOI] [PubMed] [Google Scholar]
- 19.Zhou C, Wen ZX, Shi DM, Xie ZP. Muscarinic acetylcholine receptors involved in the regulation of neural stem cell proliferation and differentiation in vitro. Cell Biol Int. 2004;28:63–7. doi: 10.1016/j.cellbi.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 20.Knollmann BC, Chopra N, Hlaing T, Akin B, Yang T, Ettensohn K, et al. Casq2 deletion causes sarcoplasmic reticulum volume increase, premature Ca2+ release, and catecholaminergic polymorphic ventricular tachycardia. J Clin Invest. 2006;116:2510–20. doi: 10.1172/JCI29128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reppel M, Sasse P, Piekorz R, Tang M, Roell W, Duan YQ, et al. S100A1 enhances the L-type Ca2+ current in embryonic mouse and neonatal rat ventricular cardiomyocytes. J Biol Chem. 2005;280:36019–28. doi: 10.1074/jbc.M504750200. [DOI] [PubMed] [Google Scholar]
- 22.Bassani RA, Bassani JW, Bers DM. Mitochondrial and sarcolemmal Ca2+ transport reduce [Ca2+]i during caffeine contractures in rabbit cardiac myocytes. J Physiol. 1992;453:591–608. doi: 10.1113/jphysiol.1992.sp019246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiang D, Xiao B, Zhang L, Chen SR. Enhanced basal activity of a cardiac Ca2+ release channel (ryanodine receptor) mutant associated with ventricular tachycardia and sudden death. Circ Res. 2002;91:218–25. doi: 10.1161/01.res.0000028455.36940.5e. [DOI] [PubMed] [Google Scholar]
- 24.Tiso N, Stephan DA, Nava A, Bagattin A, Devaney JM, Stanchi F, et al. Identification of mutations in the cardiac ryanodine receptor gene in families affected with arrhythmogenic right ventricular cardiomyopathy type 2 (ARVD2) Hum Mol Genet. 2001;10:189–94. doi: 10.1093/hmg/10.3.189. [DOI] [PubMed] [Google Scholar]
- 25.Priori SG, Napolitano C, Tiso N, Memmi M, Vignati G, Bloise R, et al. Mutations in the cardiac ryanodine receptor gene (hRyR2) underlie catecholaminergic polymorphic ventricular tachycardia. Circulation. 2001;103:196–200. doi: 10.1161/01.cir.103.2.196. [DOI] [PubMed] [Google Scholar]
- 26.Seth M, Sumbilla C, Mullen SP, Lewis D, Klein MG, Hussain A, et al. Sarco(endo)plasmic reticulum Ca2+ ATPase (SERCA) gene silencing and remodeling of the Ca2+ signal mechanism in cardiac myocytes. Proc Natl Acad Sci USA. 2004;101:16683–8. doi: 10.1073/pnas.0407537101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jiang D, Xiao B, Yang D, Wang R, Choi P, Zhang L, et al. RyR2 mutations linked to ventricular tachycardia and sudden death reduce the threshold for store-overload-induced Ca2+ release (SOICR) Proc Natl Acad Sci USA. 2004;101:13062–7. doi: 10.1073/pnas.0402388101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Holt E, Christensen G. Transient Ca2+ overload alters Ca2+ handling in rat cardiomyocytes: effects on shortening and relaxation. Am J Physiol. 1997;273:H573–82. doi: 10.1152/ajpheart.1997.273.2.H573. [DOI] [PubMed] [Google Scholar]
- 29.Catterall WA, Trainer V, Baden DG. Molecular properties of the sodium channel: a receptor for multiple neurotoxins. Bull Soc Pathol Exot. 1992;85:481–5. [PubMed] [Google Scholar]
- 30.Zhang XQ, Song J, Rothblum L, Lun M, Wang X, Ding F, et al. Overexpression of Na+/Ca2+ exchanger alters contractility and SR Ca2+ content in adult rat myocytes. Am J Physiol Heart Circ Physiol. 2001;281:H2079–88. doi: 10.1152/ajpheart.2001.281.5.H2079. [DOI] [PubMed] [Google Scholar]
- 31.Balog EM, Gallant EM. Modulation of the sarcolemmal L-type current by alteration in SR Ca2+ release. Am J Physiol. 1999;276:C128–35. doi: 10.1152/ajpcell.1999.276.1.C128. [DOI] [PubMed] [Google Scholar]
- 32.Sterling NW. Comparison of aconitine-modified human heart (hH1) and rat skeletal (μ1) muscle Na+ channels: an important role for external Na+ ions. J Physiol. 2002;538:759–71. doi: 10.1113/jphysiol.2001.012915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakai J, Dirksen RT, Nguyen HT, Pessah IH, Beam KG, Allen PD. Enhanced dihydropyridine receptor channel activity in the presence of ryanodine receptor. Nature. 1996;6380:72–5. doi: 10.1038/380072a0. [DOI] [PubMed] [Google Scholar]