Abstract
Background
Propensity for alcohol misuse may be linked to an individuals’ response to alcohol. This study examined the role of alcohol response phenotypes to future drinking problems.
Methods
One hundred four young heavy social drinkers participated in a within-subject, double-blind, placebo-controlled laboratory alcohol challenge study with 6-year follow-up. Participants were examined for subjective responses before and after receiving an intoxicating dose of alcohol (.8 g/kg) or a placebo beverage, given in random order. Follow-up was conducted in 5 waves over 6 years after the sessions to assess drinking behaviors and alcohol use disorder (AUD) symptoms. Retention was high with 98% (509 of 520) of possible follow-ups completed.
Results
Greater sensitivity to alcohol, in terms of stimulation and rewarding effects (like, want more) and lower sensitivity to alcohol sedation predicted greater number of AUD symptoms through 6 years of follow-up. Cluster analyses revealed that for half the sample, increasing levels of stimulation and liking were predictors of more AUD symptoms with the other half divided between those showing like and want more and want more alone as significant predictors.
Conclusions
The findings extend previous findings and offer new empirical insights into the propensity for excessive drinking and alcohol problems. Heightened alcohol stimulation and reward sensitivity robustly predicted more alcohol use disorder symptoms over time associated with greater binge-drinking frequency. These drinking problems were maintained and progressed as these participants were entering their third decade of life, a developmental interval when continued alcohol misuse becomes more deviant.
Keywords: Alcohol response, binge drinking, differentiator model, reward sensitivity, stimulation, subjective effects, trajectory
Heavy alcohol consumption elevates the risk for many adverse consequences, including disease, injury, and premature death (1–3). Although most individuals who drink can do so without incurring problems, others experience significant difficulties. Understanding the etiology of harmful drinking and its relation to inherent brain reward pathways and processes (4) is important for early identification, prevention, and treatment. One potentially important aspect of these processes consists of individual responses to alcohol. Research has attempted to elucidate these responses and how they may play a critical role in the development and exacerbation of loss of control over alcohol consumption, problems, and consequences (5–11). Several theoretical models have been proposed to explain how an individual’s response to alcohol may influence propensity to hazardous drinking. These all involve aspects of potentially rewarding and/or aversive responses to alcohol.
The low-level response model (12), the earliest of these models, posits that less sensitivity to alcohol increases risk for alcohol use disorders (AUDs). The model was developed primarily from a large longitudinal study of Schuckit and colleagues examining response to alcohol challenge. In that study, less intense alcohol responses, including subjective fatigue, stress hormone levels, and body sway increased the likelihood of subsequent alcohol abuse (AA) or alcohol dependence (AD) (9). These lower responses to alcohol were likened to a lack of inherent “brakes” that limit ethanol intake. However, this study lacked measurement of hedonic responses to alcohol.
Subsequent human data failed to support low-level responses in at-risk persons (13,14), and animal studies supported psychomotor stimulant mechanisms of drug reinforcement (15). Thus, a competing theory, the differentiator model (7), was introduced by Newlin and colleagues specifying that greater pleasurable and excitatory effects of alcohol during the ascending limb of the breath alcohol concentration (BrAC) curve, combined with lower sedative responses during the declining limb, increase risk for future AUD. The differentiator model differs from the low-level response model in that it likens alcohol response in persons at risk to having “an accelerator pedal without working brakes.” We have shown that although differential limb-specific effects are evident, responses simply measured at peak BrAC were predictive of future drinking at 2 years in at-risk heavy drinkers (16) leading to our proposed modified differentiator model. Finally, the incentive sensitization model (17,18) specifies the independence of neural system changes underlying development of addiction. The model posits that repeated heavy exposure to drugs (and alcohol) over time increases the salience of drug cues and sensitizes the neural systems of alcohol reward underlying motivation (wanting) but not hedonic (liking) effects.
Support for these models has come from animal studies (19), retrospective recall of alcohol responses in humans, or cross-sectional alcohol challenge designs (6). Although important, animal models have limitations in translation to the complexity of human behaviors. In humans, measuring alcohol responses by retrospective memory of early effects may incur recall bias and attenuate the likelihood of producing sufficiently precise information (20,21). Cross-sectional alcohol challenge designs overcome this problem but may not elucidate the full scope of subjective alcohol responses (8,11) or how alcohol responses relate to future behavior. Therefore, combining alcohol challenge measures with longitudinal follow-up is crucial to uncovering the relationship of both positively and negatively valenced alcohol responses to future drinking behaviors and AUD symptoms over time.
We therefore established the Chicago Social Drinking Project to conduct alcohol challenge and longitudinal research. We previously showed (16) that heavy social drinkers exhibited higher alcohol stimulation and reward (liking, wanting) and lower sedation compared with light drinker controls, and that these alcohol responses predicted future binge-drinking behaviors through intensive 2-year follow up (16). This 2-year period provided the first prospective information on drinking relative to baseline stimulation and sedation and formed the basis for the modified differentiator model. However, because most participants were then in their 20s, we were not able to examine the important transitional period between this decade of life and ages 30 and older, when the prevalence of binge drinking, drinking problems, and AUD declines sharply for many, but not all, individuals (22,23). This transitional period is a crucial developmental life-stage interval to fully test the modified differentiator model because those who do not reduce binge drinking by this phase may be at risk for chronic drinking problems and AUD symptoms, from which much physical morbidity and psychosocial impairment occurs.
Accordingly, an extensive, repeated-measure longitudinal follow-up of this sample was extended through 6 years after the original baseline placebo-controlled alcohol challenge. In this unique long-term follow-up, the main questions examined were as follows: 1) Do alcohol responses (stimulating, rewarding, and sedative) measured in the well-controlled laboratory predict the likelihood of meeting symptoms of AUD and frequency of binge drinking over a 6-year interval after the challenge? 2) Are there individual differences or subgroups evident in the predictive relationship of alcohol response to future drinking?
Methods and Materials
The study was approved by the University of Chicago Institutional Review Board. The design was a double-blinded, placebo-controlled, within-subjects study of responses to alcohol challenge, with longitudinal follow-up of alcohol drinking behaviors and problems in 190 non-alcohol-dependent social drinkers. The laboratory phase (March 2004 to July 2006) included three sessions. After laboratory testing, each participant entered the longitudinal follow-up phase, with no participants lost to follow-up. Follow-ups were conducted from March 2005 to October 2012, at the end of years 1, 2, 4, 5, and 6 after laboratory challenge. In this report, we focus on the 104 nonalcoholic, heavy social drinkers in the sample who met inclusion criteria (consume five or more drinks for men [four for women] on an occasion one to five times per week as their predominant adult pattern [i.e., at least the past 2 years], with at least 10 but no more than 40 standard drinks weekly). The binge criteria were consistent with Substance Abuse & Mental Health Services Administration and National Institute on Alcohol Abuse and Alcoholism guidelines (24,25); the weekly consumption criteria assured overall regular alcohol exposure (26).
The 86 light drinkers in the original sample (i.e., those who consumed <6 drinks weekly with no/rare bingeing) are not described in this report because they continued with largely low-risk drinking through follow-up, averaging 1.7 ± .07 SEM drinking days per week, 1.8 ± .07 drinks per drinking day, and 3.1 ± .6 binge occasions per year. They showed primarily sedative alcohol responses (16), and no alcohol response factor predicted their future drinking behaviors or problems because there was little signal or variability in target behavior to detect.
Eligibility and Screening
Participants were recruited from advertisement and screened for these eligibility criteria: aged 21 to 35 years, weight 110 to 210 pounds, meeting heavy drinking criteria, and good general health with no current or past major medical or Axis I psychiatric disorders, including alcohol and drug dependence (excluding nicotine). Screening measures included Alcohol Quantity—Frequency (27), Timeline Follow-Back (28), the Alcohol Use Disorders Identification Test (AUDIT) (29), and a modified Structured Diagnostic Interview for DSM-IV (SCID) (30) with the screening modules for major psychiatric disorders and the full lifetime modules for AA and AD. Standard cutoff thresholds were used to rule out persons with psychiatric comorbidities, and candidates could not be taking centrally acting medications. Screening also included a brief physical examination, urine toxicology, and a blood sample for complete blood count and liver function panels. Candidates with a positive breathalyzer or urine toxicology screen (cocaine, opiates, benzodiazepines, amphetamines, barbiturates, and phencyclidine), a positive pregnancy test (women), or an abnormal blood chemistry or hepatic panel result (≥2 SD above mean) were excluded. Biological family history of AUD, a risk factor in development of AUD, was not an inclusion criterion but ascertained after enrollment through a family history tree and Research Diagnostic Criteria for alcohol consequences (31) to confirm likelihood of AUD in identified relatives. Positive family history (FH) was defined as having at least one primary relative or two or more secondary relatives with AUD, and negative FH was defined as having no AUD in the previous two generations. Twenty-three participants (22%) either did not meet these criteria or were unsure about family members, so they were not classified.
Laboratory Sessions
In the individual laboratory sessions separated by at least 48 hours, participants ingested a beverage given in random order that contained a high alcohol dose (.8 g/kg alcohol) or a placebo (.0 g/kg; 1% volume of ethanol as taste mask). Another session was conducted with .4 g/kg alcohol, but this dose was subthreshold to produce subjective changes (16) and not included in this study. Doses for women were 85% of those of men to adjust for sex differences in total body water (32). To reduce alcohol expectancy, the Alternative Substance Paradigm (33) was used, with instructions that the beverage might include alcohol, a stimulant, a sedative, a placebo, or a combination of these substances. All beverages contained water, flavored drink mix, a sucralose-based sugar substitute, and the applicable dose of 190-proof ethanol. The beverage was divided into two equal portions in clear-lidded cups. Each portion was consumed within 5 minutes with a 5-minute rest interval between portions. The sessions commenced between 3:00 and 5:00 PM and proceeded for 4.5 to 5 hours in comfortable, living room–like laboratory testing rooms. Each session began with self-report assessments of abstinence compliance of 48 hours for alcohol and drugs, and 3 hours for food, caffeine, and smoking. A urine sample was collected for a random drug toxicology screen before at least one session. For women, a urine sample was collected before every session to test for HcG to verify nonpregnancy. After these compliance measures, the participant then consumed a snack at 20% of daily kilocalorie needs consisting of 55% carbohydrate, 10% protein, and 35% fat. Prebeverage subjective and objective measures were conducted after the snack, followed by the beverage consumption interval accompanied by light conversation with the research assistant. Postbeverage measures were repeated at 30, 60, 120, and 180 minutes after beverage initiation. Between time points, the participant could view movies or read magazines from a standardized list provided by the study. At the end of each session when BrAC was ≤.04 mg/dL (.04%), a car service transported participants home. At the end of the third session, the participant was debriefed and given instructions for follow-up.
Objective Measures
Breathalyzer readings were obtained via the Alco-Sensor IV (Intoximeter, St. Louis, Missouri), which displays .000 mg/dL at each reading with actual values downloaded to a computer. Average BrAC peaked at 60 minutes following the initiation of drinking was .091 (.02 SD) mg/dL [see King et al. (16) for detailed BrAC curve data].
Subjective Measures
The main dependent measures for alcohol response in this report were the brief stimulation (B-STIM) and sedation (B-SED) subscales from the Brief Biphasic Alcohol Effects Scale (B-BAES) (34,35) and items from the Drug Effects Questionnaire (36) for like (“I LIKE the effects I am feeling right now”), with the midpoint indicating neutral, and want more (“I would like MORE of what I consumed right now”). On the basis of our previous work (16), net change scores were computed for stimulation and sedation as the rating at peak BrAC (60 minutes) from baseline, minus the same change score in the placebo session; for liking and wanting, net change scores were the 60-minute rating for alcohol minus placebo because there was no baseline. Secondary measures included similar net change scores at rising (30 minutes) and declining (120) BrAC limb as a direct test of the differentiator model. Participants were asked to respond on the basis of their current feeling state; the beverage content was not divulged.
Follow-up
Each follow-up took approximately 30 to 40 minutes to complete, including 20 to 25 minutes for self-report measures and 10 to 15 minutes for an interview. Self-report measures were completed by a Web-based program or by a mailed packet and included items for demographic updates and estimates of drinking quantity, frequency, and maximum quantity, the AUDIT, and the Drinker Inventory of Consequences—Recent (37). The interview was completed by telephone or online calling with a trained staff member and included a Timeline Follow-Back for the previous four Monday-to-Sunday weeks and SCID modules for past year incidence of the 11 items comprising DSM-IV AUDs (abuse and dependence) for years 1, 2, 5, and 6 (there was no follow-up at year 3, and SCID at year 4 to minimize participant burden). Compensation for each follow-up was a $40 gift card with a $20 bonus for timely completion.
Statistical Analyses
Baseline and 6-year follow-up background and drinking characteristics were compared by dependent sample Student t tests. Generalized equation estimation (GEE) models, which are similar to generalized linear mixed models for analyzing longitudinal data but are more robust in specifying variance-covariance structures using a sandwich algorithm, were used to examine alcohol responses and future drinking outcomes. The primary alcohol response variables were B-BAES derived B-STIM and B-SED to test directly low-level response and differentiator models. Secondary measures were DEQ derived “like” and “want more” to test the incentive sensitization theory. The primary outcome was the mean number of AUD symptoms met during follow-up, analyzed using GEE with a log link function for count data. The GEE models included standardized alcohol response (stimulation, sedation, wanting, liking), follow-up time, and their interaction. Whereas age, sex, race, education, and disinhibited personality (38) were not associated with AUD symptom count, FH was significantly associated (positive vs. negative: β [SE] = .327 [.125], p = .009); therefore, GEE analyses controlled for FH by including two dummy variables (FH positive vs. negative, FH unsure vs. negative). The analyses also controlled for BrAC given the variability in levels from oral alcohol administration. The false discovery rate method was used to correct p values for multiple comparisons (39).
As a complementary analysis for GEE to illustrate the AUD variation over follow-up, subgroups were formed on the basis of trajectory analysis of AUD symptom count derived from zero-inflated Poisson mixture model (40), with a cubic trajectory for each group and the number of groups determined by model Bayesian information criterion (BIC). Finally, to discern individual differences in the prospective relationship between acute alcohol responses and future drinking problems, exploratory k-means cluster analyses, which partitioned observations into k clusters with each observation belonging to a cluster with the nearest mean, were conducted (41) to create subgroups that exhibited different relationships between alcohol responses (stimulation, sedation, liking, wanting) and AUD symptoms. The optimal number of clusters was determined by the Variance Ratio Criterion (42). Statistical packages used were Stata 12.0 (Stata-Corp, College Station, Texas) for GEE and trajectory analyses and SPSS 17.0 (SPSS, Chicago, Illinois) for cluster analyses.
Results
Including missing follow-ups of two participants who withdrew (2 men in Year 4) and one who died (a woman in Year 3), 98% (509 of 520) of follow-ups were successfully conducted at 1, 2, 4, 5, and 6 years. Table 1 presents demographic and drinking characteristics at baseline when the average age was 25.3 years (range 21–34) years and at year 6 when the average age was 31.4 years (range 27–40). From baseline to Year 6, alcohol drinking decreased for any drinking frequency (from 51% to 44% of days), binge-drinking frequency (from 28% to 23% of days), and maximum number of drinks consumed on one occasion (from 9.7 to 8.4). The AUDIT scores decreased but remained above cutoff threshold levels for heavy drinking (43), and drinks per drinking day and alcohol consequences did not decrease over time (Table 1).
Table 1.
Heavy Drinker Background Characteristics at Baseline and at 6-year Follow-up
| Baseline | Six-year Follow-up | Significance Testing (Time) | |
|---|---|---|---|
| General Characteristics | |||
| Age (years) | 25.28 (.30) | 31.16 (.31) | t = −89.20, p < .001 |
| Education (years) | 15.70 (.14) | 16.26 (.20) | t = −4.29, p < .001 |
| Sex (male) | 61 (58.7%) | — | — |
| Race (Caucasian)a | 87 (83.7%) | — | — |
| FH Positiveb | 43 (52%) | — | — |
| Alcohol-Related Measuresc | |||
| AUDIT total score | 11.60 (.36) | 10.53 (.56) | t = 2.02, p = .046 |
| DrInC-2R total score | 14.24 (.86) | 14.29 (1.16) | t = −.04, p = .966 |
| AUD symptom countd | 1.67 (.18) | 1.26 (.17) | t = 2.12, p = .036 |
| Average Past Month Drinking Behaviore | |||
| Drinking frequency (days/month) | 14.32 (.51) | 12.33 (.72) | t = 2.63, p = .010 |
| Drinks per drinking day | 5.16 (.18) | 4.77 (.28) | t = 1.45, p = .150 |
| Max no. drinks per occasion | 9.69 (.40) | 8.43 (.45) | t = 2.60, p = .011 |
| Binge drinking frequency (days/month)f | 7.90 (.33) | 6.43 (.54) | t = 2.56, p = .012 |
| Liver Function Tests | |||
| AST (units/L)g | 23.0 (1.2) | 22.2 (1.2) | t = −1.05, p = .298 |
| ALT (units/L)g | 22.1 (2.1) | 21.6 (2.0) | t = −.49, p = .625 |
Data are mean (SEM) or n (%).
ALT, alanine aminotransferase; AST, aspirate aminotransferase; FH, family history.
Race was self-reported by the participant from a list consistent with National Institutes of Health classifications.
FH data are based on the 81 participants with whom FH positive or negative could be determined.
Alcohol-related measures are total scores for the Alcohol Use Disorder Identification Test (AUDIT) and the Drinker Inventory of Consequences-Recent (DrInC-2R) at baseline and at 6-year follow-up.
Alcohol use disorder symptom count was based on lifetime at baseline and past year AUD symptom count at 6-year follow-up.
Drinking variables were taken from Timeline Follow-Back Interview for the month preceding study enrollment at baseline and the month preceding 6-year follow-up.
Binge was defined as ≥5 drinks per occasion for men and ≥4 drinks for women.
AST and ALT were taken from blood samples in 85 participants at baseline and repeated in the laboratory at 5 years.
Table 2 depicts the GEE results for the main effects of each alcohol response variable and their interaction with follow-up time. Results showed that heightened sensitivity to alcohol stimulation, liking, and wanting and lower sensitivity to sedation were significant predictors of AUD symptoms. Consistent with the modified differentiator model, at peak BrAC (60-minute net change score), higher alcohol stimulation and lower sedation predicted increases in AUD symptoms over time, but they were not significant predictors on either the rising and declining BrAC limbs after correction for multiple comparisons. In terms of incentive sensitization theory, both alcohol liking and wanting predicted more AUD symptoms but did not predict additional increases in symptoms through the 6 years (no significant interactions with follow-up time).
Table 2.
Alcohol Response Variable Predictors of DSM-IV AUD Symptoms Through Follow-up
| Six-year Follow-up Outcome: AUD Symptom Count
| |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Alcohol Responses | Peak BrAC (T2)
|
Rising Limb (T1)
|
Declining Limb (T3)
|
||||||
| β | SE | p | β | SE | p | β | SE | p | |
| Stimulation (B-STIM) | −.044 | .066 | .506 | .054 | .063 | .396 | .059 | .063 | .351 |
| Stimulation × Follow-up Time | .029 | .013 | .025a | .022 | .013 | .082 | .012 | .013 | .343 |
| Sedation (B-SED) | .111 | .062 | .074 | −.018 | .064 | .773 | −.023 | .062 | .707 |
| Sedation × Follow-up Time | −.042 | .013 | .001a | −.028 | .013 | .033 | −.029 | .013 | .026 |
| Like | .156 | .061 | .011a | .183 | .065 | .005a | .111 | .060 | .064 |
| Like × Follow-up Time | .023 | .013 | .072 | .021 | .014 | .117 | .019 | .012 | .113 |
| Want More | .158 | .063 | .012a | .192 | .064 | .003a | .243 | .063 | <.001a |
| Want More × Follow-up Time | .011 | .013 | .417 | .023 | .014 | .089 | .006 | .013 | .631 |
Results from generalized equation estimation analyses (coefficient [β], SE, and p value) for B-BAES and DEQ derived alcohol response measures and their interaction with follow-up time. Follow-up time refers to Structured Clinical Interview for DSM interview intervals 1, 2, 5, and 6 years after alcohol challenge. Alcohol responses were net change score from alcohol session minus placebo at peak breath alcohol content (Time 2 [T2], 60 minutes), rising limb (Time 1 [T1], 30 minutes), and declining limb (Time [T3], 120 minutes), relative to baseline (T0). Note follow-up time was included in each model and significant (ps < .003), indicating a decrease in AUD symptoms for the whole sample over follow-up but not shown for ease of presentation. All analyses controlled for family history of alcohol use disorder and BrAC level at that interval.
AUD, alcohol use disorder; BrAC, breath alcohol content.
Significant p values after false discovery rate correction for multiple comparisons.
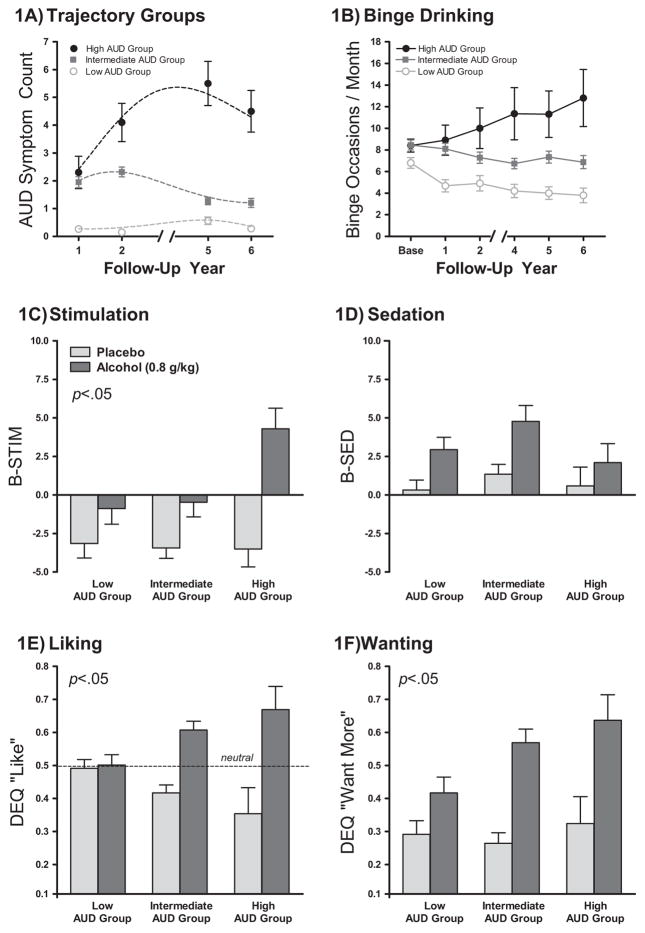
Trajectory analysis of AUD symptom counts over follow-up showed the model BIC for two, three, or four trajectory groups of −674, −667, and −672, respectively. That is, 2ΔBIC increased by 15 comparing the two- and three-trajectory models but decreased by 10 when comparing three- and four-trajectory models. Using 2ΔBIC ≥10 as strong evidence for rejecting the null model (40), the three-group model best described the data. This yielded low (n = 34), intermediate (n = 60), and high (n = 10) AUD symptom groups. The validity of these groups as showing meaningful behavioral differences was supported by other measures including drinking quantity, drinking frequency, binge-drinking frequency, AUDIT, and Drinker Inventory of Consequences scores, which showed a linear progression among the AUD groups (Table 3). Notably, all participants in the high AUD group met AD criteria at least once during follow-up, compared with 26% in the intermediate group and none in the low group. The progression of AUD symptoms and binge-drinking frequency are shown in Figure 1A and B. Alcohol responses, relative to placebo, for stimulation, sedation, like, and want more for the AUD groups are shown in Figure 1C and F. There was a linear progression in responses with the high AUD symptom group showing pronounced stimulation, like, and want more ratings compared with the other groups. Sedation did not differ across groups.
Table 3.
General and Drinking Characteristics in AUD Symptom Count Groups from Trajectory Analysis
| Low AUD Symptom Count (n = 34) | Intermediate AUD Symptom Count (n = 60) | High AUD Symptom Count (n = 10) | p Value | |
|---|---|---|---|---|
| General Characteristics | ||||
| Age at study enrollment (years) | 25.82 (.53) | 25.08 (.38) | 24.60 (1.09) | .179 |
| Sex (male) | 17 (50%) | 38 (63%) | 6 (60%) | .318 |
| FH positivea | 10 (43%) | 27 (54%) | 6 (75%) | .053 |
| DSM-IV Diagnostic Criteria | ||||
| AUD symptom countb | .88 (.13) | 3.17 (.16) | 6.60 (.52) | <.001 |
| Alcohol dependence (DSM-IV)b | 0% | 23% | 100% | <.001 |
| Alcohol abuse without dependence (DSM-IV)b | 32% | 72% | 0% | <.001 |
| Average for 6 Years Drinking Behavior and Related Measuresc | ||||
| Drinking frequency (days/month) | 14.86 (1.16) | 17.35 (.71) | 20.10 (2.43) | .008 |
| Drinks per drinking day | 5.88 (.39) | 6.92 (.31) | 9.82 (1.03) | <.001 |
| Maximum no. drinks per occasion | 10.56 (.81) | 12.45 (.53) | 17.60 (2.83) | <.001 |
| Binge drinking frequency (days/month) | 7.82 (.63) | 11.10 (.52) | 17.30 (2.24) | <.001 |
| Average Alcohol Drinking Measuresd | ||||
| AUDIT total score | 10.18 (.59) | 15.60 (.67) | 23.20 (1.80) | <.001 |
| DrInC-2R total score | 14.97 (1.43) | 25.30 (1.39) | 46.00 (3.07) | <.001 |
Data are mean (SEM) or %. AUD symptom count groups based on Bayesian information criterion models and trajectory analysis. Analyses included χ2, Fisher’s exact, and linear trend analysis as appropriate. Significance testing results reflect the comparison of AUD subgroups, as high > intermediate > low for all comparisons with the exception of alcohol abuse without dependence, which was significant for intermediate > low > high AUD symptom count group.
AUD, alcohol use disorder; AUDIT, Alcohol Use Disorder Identification Test; DrInC-2R, Drinker Inventory of Consequences—Recent; FH, family history.
FH based on 81 participants who could be classified as either FH+ or FH−.
Variables from Structured Clinical Interview for DSM interview indicate maximum number of AUD symptoms from 11 DSM-IV criteria met during any follow-up at 1, 2, 5, or 6 years, and alcohol abuse and alcohol dependent diagnoses consistent with DSM-IV criteria for alcohol abuse and dependence.
Drinking variables were average maxima of 1-, 2-, 4-, 5-, and 6-year follow-up.
AUDIT and DrInC-2R average maximum total scores at 1-, 2-, 4-, 5-, and 6-year follow-up.
Figure 1.
Baseline alcohol response factors in alcohol use disorder (AUD) trajectory subgroups. (A) Three trajectory groups were identified based on AUD symptoms (total alcohol abuse and alcohol dependence symptoms; 0–11 possible) over 6 years of follow-up occurring at Years 1, 2, 5, and 6. The symbols represent the mean AUD symptoms at each follow-up interval for each group with the dotted lines representing model fits. (B) Binge drinking frequency over follow-up in the AUD trajectory groups. (C–F). Stimulation, sedation, liking and wanting for alcohol, and placebo at 60 minutes (peak breath alcohol content) for the AUD symptom subgroups derived from trajectory analyses. Results on figures are mainly for visual purposes and were derived from linear trend analyses comparing groups on the net change score of alcohol response relative to placebo to be consistent with other analyses in this study, with p < .05 indicating a significant difference across groups. BAES, Biphasic Alcohol Effects Scale; DEQ, Drug Effects Questionnaire.
Additional exploratory examination of individual differences by cluster analysis revealed three clusters that best described the relationship of baseline alcohol responses to future AUD symptoms (Figure 2). Among half the participants (n = 51), increasing levels of stimulation and liking were predictors of more AUD symptoms; the remaining half included clusters with increasing levels of liking and wanting more (n = 25) or wanting more alone (n = 28) as significant predictors. The cluster groups did not differ on age, sex, race, education, FH, or absolute number of AUD symptoms.
Figure 2.
Cluster analysis results of the prospective relationship of B-BAES and DEQ derived alcohol response to alcohol use disorder (AUD) symptom count over time. Raw data for both alcohol response measures (x axes) and mean AUD symptoms (y axes) are displayed for ease of presentation. Cluster analyses were conducted on standardized scores for alcohol responses (to allow direct comparisons across measures), log-transformed mean AUD symptoms (to correct for skewness), and the increase in AUD symptoms during Years 5 and 6 relative to Year 0, 1, and 2 (to account for AUD changes over follow-up). Cluster analysis revealed three subgroups on the relationship of stimulation, sedation, liking, and wanting to AUD symptoms over time. Regression analyses were used to assess the relationship between these variables.
Discussion
Our results provide important evidence for the role of alcohol stimulation and reward sensitivity, in addition to lower sedation, in the development of future AUD symptoms and persistence and exacerbation of bingeing and other drinking behaviors. The study extends our previous findings (16) to a critical longer 6-year follow-up interval when most participants were entering their third decade of life and provides crucial and broader longitudinal evidence to reconcile theoretical debate about models of alcohol response and risk for future drinking problems. Young adult heavy drinkers who exhibited an alcohol response phenotype characterized by greater sensitivity to stimulant and rewarding effects progressed in their drinking and reported more AUD symptoms over a 6-year interval approaching their early 30s compared with those without this response profile. The high and intermediate AUD trajectory groups showed clinically relevant signs of departure from drinking norms over follow-up: average binge-drinking frequency throughout follow-up was 62% and 40% of days, respectively, compared with 28% of days in the low AUD group. Furthermore, these groups averaged 6.6 and 3.2 AUD symptoms, respectively, during their heaviest follow-up. Extrapolating to the new DSM-5, these would correspond to mild to severe AUD (44). The 6-year follow-up interval therefore provided a more comprehensive picture than in our original report of 2 years of follow-up (16) and support the link between alcohol response and progression of excessive drinking, particularly during the developmental transition to entering early middle adulthood.
In terms of theoretical models, the data provide the most support for the modified differentiator model (16), because higher stimulation and lower sedation during peak BrAC at an intoxicating dose of alcohol were predictive of the increase over time in AUD symptoms. These effects were not predictive when measured during rising and declining BrAC limbs, which may be due to greater variability in responses at those temporal phases as compared with the peak BrAC interval at 60 minutes. Historically, the low-level response model has been widely accepted, with less intense alcohol effects assumed to be the mechanism for risk of development of AUD above and beyond known risk factors such as FH (9). However, as we previously suggested (5), a paradigm shift to greater emphasis on reward sensitivity in the development of AUD is now of paramount importance in the alcohol field. Although not explicitly stated, the lower-level response model does not apply across the spectrum of subjective alcohol responses but appears rather to focus on alcohol’s impairing and sluggish effects. At peak BrAC, the only low-level response that predicted future AUD was sedation, with high level of response, in terms of stimulation and reward, also predictors. Therefore, future alcohol challenge research should examine both stimulating and sedating effects of alcohol within the same sample for a complete picture of the differential effects of alcohol.
Investigation of individual differences showed that half of participants comprised a cluster group showing an association of hedonic effects (i.e., stimulation and liking) and AUD symptoms. The other half of the sample was equally divided among those showing liking and wanting, or wanting alone, as predictors. Therefore, incentive sensitization (wanting but not liking) was evident only among a quarter of the sample. If the theory is correct, with continued excessive drinking over a longer interval, more participants would likely be included in this cluster, and longer-term follow-up is underway. The cluster analysis approach revealed that sedation was not as highly associated with AUD as stimulating and rewarding effects. This individual difference approach complemented the GEE and trajectory group approach by elucidating individual differences in the relationships among the alcohol responses and AUD symptoms and underscore that the relationships of different alcohol response factors to future drinking problems is multifaceted.
The neurobiological underpinnings of the pleasurable effects of alcohol are complex and not well understood but likely involve inherent brain reward pathways including opioidergic and dopaminergic circuits (45,46). In addition, neuroadaptations in gamma-aminobutyric acid–ergic, glutaminergic, and corticotrophin systems are also likely involved in critical changes in the reward circuitry due to repeated alcohol exposures (4). On a behavioral level, animal research indicates that alcohol, and other addictive substances, are reinforcing through approach behavior processes and psychomotor activation (15). Although translation to human research has been challenging (47,48), the observed sensitivity to hedonic (liking) and motivational (wanting) effects of alcohol, as well as stimulatory effects, may provide important clues for targeting research on neurobiological substrates. Heightened striatal and mesolimbic system activity likely underlie these rewarding and euphoric alcohol effects (49). Indeed, work by Volkow and colleagues (50) suggests that faster increases in dopamine correspond to more intense reinforcing effects of drugs, including alcohol. Whether long-term use depletes dopamine function and reduces alcohol sensitivity remains an empirical question.
Despite numerous strengths in this study, including placebo-controlled assessment of alcohol responses, repeated follow-ups, and successful retention (98%), there are several limitations worth noting. First, light social drinkers were enrolled originally as a control group, but they were not appropriate for testing of the models in this study. Their drinking over follow-up remained light (51), and analyses of the prospective role of alcohol response to future drinking and alcohol problems were not significant, likely due to limited target behavior to detect. Although some light drinkers showed positive-like alcohol responses, as a group, their predominantly high sedative alcohol responses appear to protect against alcohol problem development in adulthood in addition to other psychosocial factors that may have mitigated their risk for heavy drinking (52,53). Second, whether the observed alcohol responses were inherent or if they were acquired or sensitized over time in our sample with repeated binge-drinking exposures in early adulthood remains unknown. Unfortunately, for ethical reasons, alcohol challenge cannot be conducted in the United States in persons under age 21 to address this issue. Third, participants’ actual in vivo alcohol responses may differ from those measured in the laboratory and differ depending on the dose. Environmental and contextual effects are known to affect alcohol responses (54–59) but such effects were minimized in our well-controlled laboratory environment, and our dose was chosen as an alcohol challenge to reach the limit for impaired driving in the United States. Finally, consistent with DSM-5, a dimensional approach of AUD symptom count was the primary outcome. However, it was based on the 11 DSM-IV criteria for AA and AD; this differs slightly from DSM-5, which replaces alcohol-related legal problems with an item for craving, which was not ascertained in this study (60). Further, developmental diagnoses (i.e., conduct disorder or attention-deficit disorder) that may relate to drinking propensity (61,62) were not ascertained.
In sum, our results offer new empirical insights into the propensity for alcohol misuse. Persons who continue to binge drink in their 30s show increasing signs of departure from normative social behavior and incur consequences and risk for health harms from chronic excessive drinking (63–65). Study findings indicate that heightened rather than lower alcohol stimulation and reward sensitivity accounted for a large proportion of future drinking escalations and AUD symptoms. Alcohol challenge responses prospectively predicted alcohol problems through 6 years of follow-up and during the developmental phase when many young heavy drinkers mature and binge-drinking behaviors becomes less normative (22,23). Delineation of alcohol response phenotypes may add an important component to prevention and educational efforts to reduce alcohol harm in society. Additional neurobiological, genetic, and epigenetic research will provide important information on precise brain pathways and mechanisms underlying this phenotype.
Acknowledgments
This research was supported by Grant Nos. R01-AA013746 (to AK) and K05AA014223 (to DH) from the National Institute on Alcohol Abuse and Alcoholism, the Alcoholic Beverage Medical Research Foundation (DC), the University of Chicago Comprehensive Cancer Center (Grant No. P30-CA14599), the National Center for Research Resources, the National Institutes of Health Roadmap for Medical Research (Grant No. UL1 RR024999), and the New York State Psychiatric Institute.
We thank study consultant Sean O’Connor, M.D., and Lingjiao Zhang, M.S., and Michael Palmeri for technical assistance.
Footnotes
Dr. King has provided consultation to Lundbeck and the Respiratory Health Association. Drs. Cao and Hasin and Mr. McNamara have no conflicts of interest or disclosures.
ClinicalTrials.gov: Chicago Social Drinking Project; http://clinicaltrials.gov/ct2/show/NCT00961792; NCT00961792
References
- 1.Room R, Babor T, Rehm J. Alcohol and public health. Lancet. 2005;365:519–530. doi: 10.1016/S0140-6736(05)17870-2. [DOI] [PubMed] [Google Scholar]
- 2.Barbosa C, Taylor B, Godfrey C, Rehm J, Parrott S, Drummond C. Modelling lifetime QALYs and health care costs from different drinking patterns over time: A Markov model. Int J Methods Psychiatr Res. 2010;19:97–109. doi: 10.1002/mpr.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dawson DA, Grant BF. The “gray area” of consumption between moderate and risk drinking. J Stud Alcohol Drugs. 2011;72:453–458. doi: 10.15288/jsad.2011.72.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nestler EJ. Is there a common molecular pathway for addiction? Nat Neurosci. 2005;8:1445–1449. doi: 10.1038/nn1578. [DOI] [PubMed] [Google Scholar]
- 5.King AC, Roche DJ, Rueger SY. Subjective responses to alcohol: A paradigm shift may be brewing. Alcohol Clin Exp Res. 2011;35:1726–1728. doi: 10.1111/j.1530-0277.2011.01629.x. [DOI] [PubMed] [Google Scholar]
- 6.Morean ME, Corbin WR. Subjective response to alcohol: A critical review of the literature. Alcohol Clin Exp Res. 2010;34:385–395. doi: 10.1111/j.1530-0277.2009.01103.x. [DOI] [PubMed] [Google Scholar]
- 7.Newlin DB, Thomson JB. Alcohol challenge with sons of alcoholics: A critical review and analysis. Psychol Bull. 1990;108:383–402. doi: 10.1037/0033-2909.108.3.383. [DOI] [PubMed] [Google Scholar]
- 8.Newlin DB, Renton RM. High risk groups often have higher levels of alcohol response than low risk: The other side of the coin. Alcohol Clin Exp Res. 2010;34:199–202. doi: 10.1111/j.1530-0277.2009.01081.x. author reply 203–195. [DOI] [PubMed] [Google Scholar]
- 9.Schuckit MA. Low level of response to alcohol as a predictor of future alcoholism. Am J Psychiatry. 1994;151:184–189. doi: 10.1176/ajp.151.2.184. [DOI] [PubMed] [Google Scholar]
- 10.Schuckit MA, Smith TL. An 8-year follow-up of 450 sons of alcoholic and control subjects. Arch Gen Psychiatry. 1996;53:202–210. doi: 10.1001/archpsyc.1996.01830030020005. [DOI] [PubMed] [Google Scholar]
- 11.Quinn PD, Fromme K. Subjective response to alcohol challenge: A quantitative review. Alcohol Clin Exp Res. 2011;35:1759–1770. doi: 10.1111/j.1530-0277.2011.01521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schuckit MA. Self-rating of alcohol intoxication by young men with and without family histories of alcoholism. J Stud Alcohol. 1980;41:242–249. doi: 10.15288/jsa.1980.41.242. [DOI] [PubMed] [Google Scholar]
- 13.Finn PR, Pihl RO. Men at high risk for alcoholism: The effect of alcohol on cardiovascular response to unavoidable shock. J Abnorm Psychol. 1987;96:230–236. doi: 10.1037//0021-843x.96.3.230. [DOI] [PubMed] [Google Scholar]
- 14.Gianoulakis C, Beliveau D, Angelogianni P, Meaney M, Thavundayil J, Tawar V, et al. Different pituitary beta-endorphin and adrenal cortisol response to ethanol in individuals with high and low risk for future development of alcoholism. Life Sci. 1989;45:1097–1109. doi: 10.1016/0024-3205(89)90167-7. [DOI] [PubMed] [Google Scholar]
- 15.Wise RA, Bozarth MA. A psychomotor stimulant theory of addiction. Psychol Rev. 1987;94:469–492. [PubMed] [Google Scholar]
- 16.King AC, de Wit H, McNamara PJ, Cao D. Rewarding, stimulant, and sedative alcohol responses and relationship to future binge drinking. Arch Gen Psychiatry. 2011;68:389–399. doi: 10.1001/archgenpsychiatry.2011.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robinson TE, Berridge KC. The neural basis of drug craving: An incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- 18.Robinson TE, Berridge KC. Incentive-sensitization and addiction. Addiction. 2001;96:103–114. doi: 10.1046/j.1360-0443.2001.9611038.x. [DOI] [PubMed] [Google Scholar]
- 19.Crabbe JC, Phillips TJ, Belknap JK. The complexity of alcohol drinking: Studies in rodent genetic models. Behav Genet. 2010;40:737–750. doi: 10.1007/s10519-010-9371-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sackett DL. Bias in analytic research. J Chronic Dis. 1979;32:51–63. doi: 10.1016/0021-9681(79)90012-2. [DOI] [PubMed] [Google Scholar]
- 21.Hassan E. Recall bias can be a threat to retrospective and prospective research designs. [Accessed August 13, 2013];Internet J Epideomiol. 2006 :3. Available at: http://archive.ispub.com/journal/the-internet-journal-of-epidemiology/volume3-number-2/recall-bias-can-be-a-threat-to-retrospective-and-prospective-research-designs.html#sthash.qJ1jAk3I.dpbs.
- 22.Hasin DS, Stinson FS, Ogburn E, Grant BF. Prevalence, correlates, disability, and comorbidity of DSM-IV alcohol abuse and dependence in the United States: Results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry. 2007;64:830–842. doi: 10.1001/archpsyc.64.7.830. [DOI] [PubMed] [Google Scholar]
- 23.Naimi TS, Brewer RD, Mokdad A, Denny C, Serdula MK, Marks JS. Binge drinking among US adults. JAMA. 2003;289:70–75. doi: 10.1001/jama.289.1.70. [DOI] [PubMed] [Google Scholar]
- 24.Substance Abuse & Mental Health Services Administration. National Survey on Drug Use and Health. Bethesda, MD: Office of Applied Studies, SAMHSA; 2005. [Google Scholar]
- 25.National Institute on Alcohol Abuse and Alcoholism. Helping Patients Who Drink Too Much: A Clinician’s Guide. Bethesda, MD: National Institutes of Health; 2005. NIH Publication No. 05-3769. [Google Scholar]
- 26.Gmel G, Kuntsche E, Rehm J. Risky single-occasion drinking: Bingeing is not bingeing. Addiction. 2011;106:1037–1045. doi: 10.1111/j.1360-0443.2010.03167.x. [DOI] [PubMed] [Google Scholar]
- 27.Cahalan D, Cisin IH, Crossley HM. American Drinking Practices: A National Study of Drinking Behavior and Patterns [monograph] New Brunswick, NJ: Rutgers Center of Alcohol Studies; 1969. [Google Scholar]
- 28.Sobell LC, Sobell MB. Alcohol Timeline Follow-Back Users’ Manual. Toronto, Canada: Addiction Research Foundation; 1995. [Google Scholar]
- 29.Babor TF, de la Fuente JR, Saunders J, Grant M. AUDIT: The Alcohol Use Disorders Identification Test: Guidelines for Use in Primary Health Care. Geneva: World Health Organization; 1992. [Google Scholar]
- 30.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders, Research version, Patient Edition (SCID-I/P) New York: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- 31.Andreasen NC, Endicott J, Spitzer RL, Winokur G. The family history method using diagnostic criteria. Reliability and validity. Arch Gen Psychiatry. 1977;34:1229–1235. doi: 10.1001/archpsyc.1977.01770220111013. [DOI] [PubMed] [Google Scholar]
- 32.Watson PE, Watson ID, Batt RD. Total body water volumes for adult males and females estimated from simple anthropometric measurements. Am J Clin Nutr. 1980;33:27–39. doi: 10.1093/ajcn/33.1.27. [DOI] [PubMed] [Google Scholar]
- 33.Conrad MC, McNamara PJ, King AC. The alternative substance paradigm: effectiveness of beverage blinding and effects on acute alcohol responses. Exp Clin Psychopharmacol. 2012;20:382–389. doi: 10.1037/a0029261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rueger SY, McNamara PJ, King AC. Expanding the utility of the Biphasic Alcohol Effects Scale (BAES) and initial psychometric support for the Brief-BAES (B-BAES) Alcohol Clin Exp Res. 2009;33:916–924. doi: 10.1111/j.1530-0277.2009.00914.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rueger SY, King AC. Validation of the Brief Biphasic Alcohol Effects Scale (B-BAES) Alcohol Clin Exp Res. 2013;37:470–476. doi: 10.1111/j.1530-0277.2012.01941.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morean ME, de Wit H, King A, Sofuoglu M, Rueger SY, O’ Malley S. The Drug Effects Questionnaire: psychometric support across multiple substances. Psychopharmacology (Berl) 2013;227:177–192. doi: 10.1007/s00213-012-2954-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miller WR, Tonigan JS, Longabaugh R. The Drinker Inventory of Consequences (DrInC): An Instrument for Assessing Adverse Consequences of Alcohol Abuse. Rockville, MD: National Institute on Alcohol Abuse and Alcoholism; 1995. [Google Scholar]
- 38.Zuckerman M, Eysenck S, Eysenck HJ. Sensation seeking in England and America: Cross-cultural, age, and sex comparisons. J Consult Clin Psychol. 1978;46:139–149. doi: 10.1037//0022-006x.46.1.139. [DOI] [PubMed] [Google Scholar]
- 39.Benjamini Y, Hochbert Y. Controlling the false discovery rate: A pratical and powerful approach to multiple testing. J R Statist Soc B. 1995;57:289–300. [Google Scholar]
- 40.Jones BL, Nagin DS, Roeder K. A SAS procedure based on mixture models for estimating developmental trajectories. Sociol Method Res. 2001;29:374–393. [Google Scholar]
- 41.Scott AJ, Knott M. A cluster analysis method for grouping means in the analysis of variance. Biometrics. 1974;30:507–512. [Google Scholar]
- 42.Caliński T, Harabasz J. A dendrite method for cluster analysis. Commun Stat Theory Meth. 1974;3:1–27. [Google Scholar]
- 43.Babor TF, De La Fuente JR, Saunders J, Grant M. The Alcohol Use Disorders Identification Test: Guidelines for Use in Primary Health Care. Geneva: World Health Organization; 1992. WHO Publication No. 924. [Google Scholar]
- 44.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-V. 5. Washington, DC: American Psychiatric Association; 2013. [Google Scholar]
- 45.Cowen MS, Lawrence AJ. The role of opioid-dopamine interactions in the induction and maintenance of ethanol consumption. Prog Neuropsychopharmacol Biol Psychiatry. 1999;23:1171–1212. doi: 10.1016/s0278-5846(99)00060-3. [DOI] [PubMed] [Google Scholar]
- 46.Gianoulakis C. Implications of endogenous opioids and dopamine in alcoholism: Human and basic science studies. Alcohol Alcohol Suppl. 1996;1:33–42. [PubMed] [Google Scholar]
- 47.Barkley-Levenson AM, Crabbe JC. Bridging animal and human models: Translating from (and to) animal genetics. Alcohol Res. 2012;34:325–335. [PMC free article] [PubMed] [Google Scholar]
- 48.Crabbe JC, Bell RL, Ehlers CL. Human and laboratory rodent low response to alcohol: Is better consilience possible? Addict Biol. 2010;15:125–144. doi: 10.1111/j.1369-1600.2009.00191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Volkow ND, Fowler JS, Wang GJ, Baler R, Telang F. Imaging dopamine’s role in drug abuse and addiction. Neuropharmacology. 2009;56(Suppl 1):3–8. doi: 10.1016/j.neuropharm.2008.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Volkow ND, Fowler JS, Wang GJ, Swanson JM, Telang F. Dopamine in drug abuse and addiction: Results of imaging studies and treatment implications. Arch Neurol. 2007;64:1575–1579. doi: 10.1001/archneur.64.11.1575. [DOI] [PubMed] [Google Scholar]
- 51.Dawson DA. US low-risk drinking guidelines: an examination of four alternatives. Alcohol Clin Exp Res. 2000;24:1820–1829. [PubMed] [Google Scholar]
- 52.Chassin L, Fora DB, King KM. Trajectories of alcohol and drug use and dependence from adolescence to adulthood: The effects of familial alcoholism and personality. J Abnorm Psychol. 2004;113:483–498. doi: 10.1037/0021-843X.113.4.483. [DOI] [PubMed] [Google Scholar]
- 53.Hill KG, White HR, Chung IJ, Hawkins JD, Catalano RF. Early adult outcomes of adolescent binge drinking: Person- and variable-centered analyses of binge drinking trajectories. Alcohol Clin Exp Res. 2000;24:892–901. [PMC free article] [PubMed] [Google Scholar]
- 54.Cooper ML, Frone MR, Russell M, Mudar P. Drinking to regulate positive and negative emotions: A motivational model of alcohol use. J Pers Soc Psychol. 1995;69:990–1005. doi: 10.1037//0022-3514.69.5.990. [DOI] [PubMed] [Google Scholar]
- 55.Doty P, de Wit H. Effect of setting on the reinforcing and subjective effects of ethanol in social drinkers. Psychopharmacology (Berl) 1995;118:19–27. doi: 10.1007/BF02245245. [DOI] [PubMed] [Google Scholar]
- 56.Labbe AK, Maisto SA. Alcohol expectancy challenges for college students: A narrative review. Clin Psychol Rev. 2011;31:673–683. doi: 10.1016/j.cpr.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 57.Sayette MA, Creswell KG, Dimoff JD, Fairbairn CE, Cohn JF, Heckman BW, et al. Alcohol and group formation: A multimodal investigation of the effects of alcohol on emotion and social bonding. Psychol Sci. 2012;23:869–878. doi: 10.1177/0956797611435134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sher KJ. Subjective effects of alcohol: The influence of setting and individual differences in alcohol expectancies. J Stud Alcohol. 1985;46:137–146. doi: 10.15288/jsa.1985.46.137. [DOI] [PubMed] [Google Scholar]
- 59.Goldman MS, Brown SA, Christiansen BA. Expectancy theory: Thinking about drinking. In: Blane H, Leonard K, editors. Psychological Theories of Drinking and Alcoholism. New York: Guilford Press; 1987. pp. 181–226. [Google Scholar]
- 60.Hasin D, O’Brien CP, Auriacombe M, Borges G, Bucholz K, Budney A, et al. DSM-5 criteria for substance use disorders: Recommendations and rationale. Am J Psychiatry. 2013;170:834–851. doi: 10.1176/appi.ajp.2013.12060782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Flory K, Lynam DR. The relation between attention deficit hyperactivity disorder and substance abuse: What role does conduct disorder play? Clin Child Fam Psychol Rev. 2003;6:1–16. doi: 10.1023/a:1022260221570. [DOI] [PubMed] [Google Scholar]
- 62.Knop J, Penick EC, Nickel EJ, Mortensen EL, Sullivan MA, Murtaza S, et al. Childhood ADHD and conduct disorder as independent predictors of male alcohol dependence at age 40. J Stud Alcohol Drugs. 2009;70:169–177. doi: 10.15288/jsad.2009.70.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dawson DA. Defining risk drinking. Alcohol Res Health. 2011;34:144–156. [PMC free article] [PubMed] [Google Scholar]
- 64.Murray RP, Connett JE, Tyas SL, Bond R, Ekuma O, Silversides CK, et al. Alcohol volume, drinking pattern, and cardiovascular disease morbidity and mortality: Is there a U-shaped function? Am J Epidemiol. 2002;155:242–248. doi: 10.1093/aje/155.3.242. [DOI] [PubMed] [Google Scholar]
- 65.Bobak M, Room R, Pikhart H, Kubinova R, Malyutina S, Pajak A, et al. Contribution of drinking patterns to differences in rates of alcohol related problems between three urban populations. J Epidemiol Commun Health. 2004;58:238–242. doi: 10.1136/jech.2003.011825. [DOI] [PMC free article] [PubMed] [Google Scholar]