Abstract
The pathophysiology underlying spinal cord injury is complex. Mechanistic understanding of the adaptive responses to injury is critical for targeted therapy aimed at reestablishing lost connections between proximal and distal neurons. After injury, cell-type specific gene transcription programs govern distinct cellular behaviors, and chromatin regulators play a central role in shaping the chromatin landscape to adjust transcriptional profiles in a context-dependent manner. In this review, we summarize recent progress on the pleiotropic roles of chromatin regulators in mediating the diverse adaptive behaviors of neurons and glial cells after spinal cord injury, and wherever possible, discuss the underlying mechanisms and genomic targets. We specifically draw attention to the perspective that takes into consideration the impact of epigenetic modulation on axon growth potential, together with its effect on wound-healing properties of glial cells. Epigenetic modulation of chromatin state represents an emerging therapeutic direction to promote neural repair and axon regeneration after spinal cord injury.
Keywords: epigenetics, chromatin, spinal cord injury, axon regeneration, neural repair
Introduction
Following spinal cord injury (SCI), the connections between proximal and distal neurons are severed, resulting in neurological deficits that seldom recover. Several factors contribute to the failure of axon regeneration: astroglial scars at the injury site form an impenetrable barrier for axon regrowth, myelin debris constitutes an inhibitory environment hostile to growth cone navigation, and neurons in the central nervous system (CNS) exhibit an age-dependent decline in intrinsic axon growth potential. Additionally, the influx of immune cells and activation of resident microglia result in an inflammatory milieu that is toxic to local cells, including oligodendrocytes, which undergo demyelination, leading to denudation of remaining axons. CNS injury therefore triggers a complex, multicellular response that results in secondary tissue damage that further impedes axon regeneration. Certain aspects of the injury response, nevertheless, can promote wound healing and set the stage for neural repair. However, the diverse behaviors of different cell types, often exerting opposing functions, are rarely considered together in a particular experimental paradigm. It is clear that an integrated therapeutic approach is imperative to foster beneficial responses while attenuating detrimental events after SCI.
The pathophysiological events that occur after SCI are governed by cell-type specific gene transcription programs. A network of transcription factors works in close collaboration with histone-modifying enzymes and chromatin remodelers to reshape the chromatin landscape to regulate injury-induced gene changes unique to a particular cell type. The functions of individual chromatin regulators are just beginning to be unraveled. In this review, we summarize the emerging roles of chromatin regulators in contributing to the functional recovery after SCI, in particular, we discuss their roles in axon regeneration, astrogliosis, inflammation, demyelination, and progenitor cell differentiation. Notably, many recent studies have adopted a pharmacological approach to study the roles of epigenetic modulation in SCI, but this approach alone is inadequate for elucidating the mechanisms underlying functional improvement after SCI, partly because of the limited specificity of pharmacological agents. Future studies are required to tease out the contribution of individual chromatin regulators to the pathophysiological events after SCI in order to optimize treatment paradigms.
Chromatin modifications in the CNS
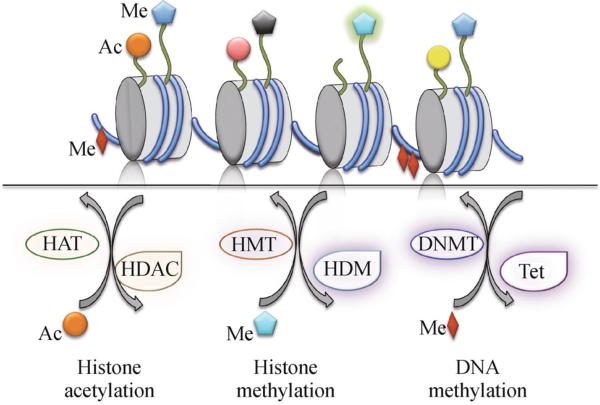
DNA is packaged into chromatin by wrapping around an octamer of four core histones (H2A, H2B, H3 and H4). The N-terminal tails of histones are subject to extensive post-translational modifications, which, together with direct DNA modifications, can impact local chromatin structure and thereby affect recruitment of transcriptional complexes and expression of target genes. Epigenetic regulation encompasses at least eight distinct types of chromatin modifications that influence gene expression without changing underlying DNA sequence (Kouzarides, 2007). For instance, covalent modifications of histone residues include acetylation, methylation (on lysines or arginines), phosphorylation, ubiquitination, sumoylation, and ribosylation, among others (Fig. 1). Modified histone residues are recognized by chromatin regulators with special domains that bind to modified histone residues. The recruited chromatin regulators may have additional enzymatic activities, such as ATPase activity, to further modify chromatin structure. Chromatin remodeling plays an essential role in neural development, plasticity, degeneration, and regeneration through modulation of the transcriptional responses that govern the developmental as well as adaptive behaviors of neurons and glial cells (Peleg, 2010; Konsoula and Barile, 2012; Trakhtenberg and Goldberg, 2012). Of the vast number of chromatin regulators, little is known of their biological functions, genomic targets, and mechanisms of action in the setting of CNS injury (refer to recent reviews on this topic) (Lindner et al., 2013; York et al., 2013). Gaining a deeper understanding will enable appropriate manipulation of epigenetic mechanisms to promote axon regeneration and neural repair after injury.
Figure 1.
Histone and DNA modifications. The covalent addition or removal of acetyl groups to the lysine residues on histone N-terminal tails is catalyzed by histone acetyltransferases (HATs) and histone deacetylases (HDACs), respectively. The covalent addition or removal of methyl groups to the lysine residues on histone tails is catalyzed by histone methyltransferases (HMTs) and histone demethylases (HDMs), respectively. The covalent addition of methyl groups to cytosine residues on DNA is catalyzed by DNA methytransferases (DNMTs), whereas the initial step of active DNA demethylation is catalyzed by the TET family of enzymes that convert the 5-methylcytosine (5-mC) to 5-hydroxymethylcytosine (5-hmC). Different colors of histone acetylation (depicted by round shapes) or histone methylation (depicted by star shapes) denote modifications on different lysine residues.
Histone-modifying enzymes in spinal cord injury
Among the many distinct epigenetic modifications, one of the best studied is reversible lysine acetylation of histone tails that is controlled by the activity of two groups of enzymes (Kouzarides, 2007). Histone acetyl transferases (HATs) add acetyl groups to lysine residues on the N-terminal tails of histones. This leads to neutralization of positive charges on histone tails, thus allowing unfolding of chromatin and better access of transcription factors for gene regulation. Histone deacetylases (HDACs), on the other hand, remove acetyl groups to compact chromatin, which generally exerts a repressive transcriptional effect (Fig. 1).
The HDAC family is classified into four classes based on structural differences and mechanisms of action (Kouzarides, 2007). The catalytic activity of the class I, II and IV HDACs is zinc-dependent, whereas the deacetylase activity of class III HDACs (Sirtuins 1-7) depends on the co-factor NAD+. Class I HDACs (HDAC1, 2, 3 and 8) are predominantly localized in the nucleus. In contrast, class IIa (HDAC4, 5, 7 and 9) and class IIb (HDAC6 and 10) HDACs shuttle between cytoplasm and nucleus. Class IV consists of only one member, HDAC11, which is widely expressed, but its function remains obscure (Broide et al., 2007). HDACs generally act as epigenetic co-repressors in transcriptional complexes that are recruited to specific genomic loci. The substrates of HDACs, however, are not restricted to histones, as many isoforms are localized in the cytoplasm and can catalyze deacetylation of non-histone proteins (see discussion below).
In situ hybridization showed overlapping but distinct regional and cell layer-specific expression patterns of the 11 HDAC isoforms examined in the CNS. In the brain, HDACs are primarily expressed in neurons, whereas HDACs2–5 and 11 are also found in oligodendrocytes (Broide et al., 2007). In the spinal cord, HDAC11 exhibits the highest expression on the transcript level, while HDACs1–5 are expressed at an intermediate level and HDACs6–10 exhibit low expression. For a discussion on the roles of HDAC signaling in neuronal development and axon regeneration, refer to a recent review (Cho and Cavalli, 2014). Following SCI, little is known about the levels of expression, subcellular localization, transcriptional targets, or biological functions of the various HDAC isoforms in different cell types. What has been shown, however, is that global acetylation levels in the spinal cord are significantly reduced, and that administration of valproic acid (VPA), a class I HDAC inhibitor, can reverse hypoacetylation and improve functional recovery in rat SCI models (Table 1) (Lv et al., 2011; Lee et al., 2012; Lv et al., 2012). It is important to note that the mechanistic bases of VPA's effects might not necessarily be linked to chromatin modifications, as VPA is well known to affect multiple signaling pathways resulting in generally neurotrophic and neuroprotective effects (Monti et al., 2009). Furthermore, as discussed below, VPA lacks specificity on HDAC isoforms, and may also affect non-histone substrates and multiple cell types. Hence, understanding the function of individual HDAC isoforms in different cell types and in the context of SCI will be key for interpretation of the effects of HDAC inhibitors.
Table 1.
Summary of studies employing epigenetic modulators in spinal cord or optic nerve injury models
| Agents | Injury model | Species | Target | Treatment | In vivo effects |
|---|---|---|---|---|---|
| Valproic acid | T8 moderate contusion | Rat | Class I HDACs | 300 mg/kg i.p., twice daily immediately after injury for 2 wks | - ↑ AcH3, AcH4, Bcl-2, HSP-70 - Decreased apoptosis - Improved functional recovery (Lv et al., 2011) |
| Valproic acid | T9 moderate contusion | Rat | Class I HDACs | 300 mg/kg i.p., twice daily, starting 8 h post injury for 1wk | - ↑AcH3, BDNF, GDNF - Reduced apoptosis - Smaller lesion volume - Improved functional recovery (Lv et al., 2012) |
| Valproic acid | T9-T10 moderate contusion | Rat | Class I HDACs | 150 or 300 mg/kg s.c., immediately after injury then every 12 h for 5 d | - ↑ H3K9Ac - ↓ MMP-9, blood-spinal cord barrier breakdown, inflammation, apoptosis, lesion volume - Improved functional recovery (Lee et al., 2012) |
| Valproic acid | T9 clip compression | Rat | Class I HDACs | 200 mg/kg i.p., twice daily for 1 wk | - ↑ AcH3K, AcH3K18 - ↓ ED-1+ macrophages - Smaller lesion volume - Improved functional recovery (Yu et al., 2012) |
| Valproic acid | T9-T10 very severe contusion | Rat | Class I HDACs | 500 ng/d, osmotic pump for 3 d | - ↑ SOD - ↓ macrophages/microglia, astrocytes, P2X4R - Preserved tissue and nerve fibers - Improved functional recovery (Lu et al., 2013) |
| MS-275 | C5 dorsal column transection | Mouse | HDAC1 & 3 | 12.5 mg/kg s.c., immediately after injury, then daily for 4 d | - ↑ AcH4 - ↑ RAG transcription - Enhanced sensory axon regeneration (Finelli et al., 2013) |
| AAVP/CAF | T9-T10 dorsal hemi-crush | Mouse | PCAF | Sciatic nerve injection, 2 wks prior to injury | - ↑ H3K9Ac - ↑ RAG expression - Enhanced sensory axon regeneration (Puttagunta et al., 2014) |
| AVp300 | Optic nerve crush | Rat | p300 | Intraocular injection at time of injury | - ↑ Ac-p53, -C/EBP, H3K18Ac - ↑ RAG expression - Enhanced optic nerve regeneration - No effect on RGC survival (Gaub et al., 2011) |
| TSA | Optic nerve crush | Rat | Class I/II HDACs | 1, 10 or 100 ng/mL, injected into the vitreous at the time of injury. | - Improved RGC survival - No effect on axon regeneration (Gaub et al., 2011) |
Epigenetic reprogramming of axon regeneration
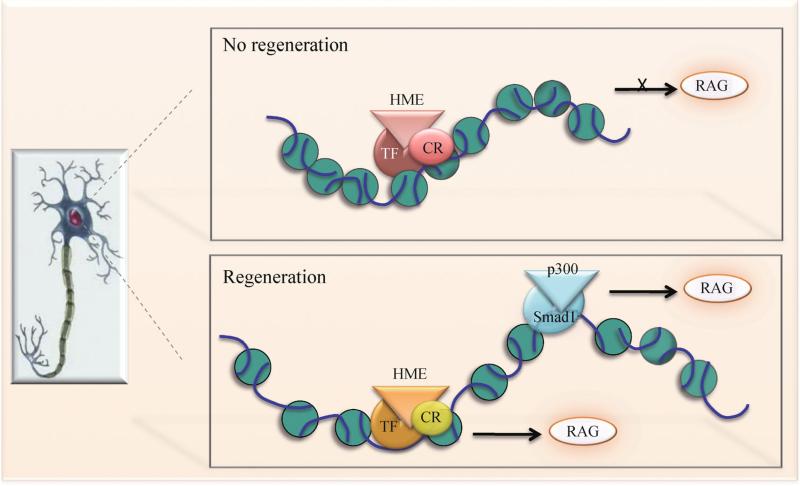
During CNS development, embryonic neurons are equipped with a high axon growth potential, but as neurons mature, pro-growth genes subsequently undergo long-lasting transcriptional alterations, and these changes are not readily reversed after injury, thus constituting a roadblock for axon regeneration (Liu et al., 2011) (Fig. 2). By contrast, in the peripheral nervous system (PNS), injured neurons are capable of reverting to a growth state by way of a transcription-dependent process that involves induction of a large repertoire of regeneration-associated genes (RAGs) (Richardson and Issa, 1984). Sensory neurons in the dorsal root ganglia (DRG) are particularly well suited for comparing the differential regenerative responses of the PNS versus the CNS. DRG neurons project a central branch into the spinal cord that is refractory to regeneration upon axotomy, but injury to their peripheral branch can trigger a retrograde propagation of injury signals, leading to a nuclear response that enhances axon growth potential of not only the peripheral but also the central branch (Neumann and Woolf, 1999; Richardson and Issa, 1984). A series of recent studies have revealed a critical role of histone-modifying enzymes in the epigenetic reprogramming of axon regeneration in DRG neurons (Figs. 2 and 3). Studies from our laboratory have found that both global and RAG-specific histone 4 acetylation (AcH4) levels are low in mature DRG neurons (Finelli et al., 2013). While peripheral axotomy of DRG neurons leads to a global elevation of AcH4 and gene specific enrichment of AcH4 at the promoters of multiple RAGs, central axotomy after SCI does not trigger such histone acetylation changes. Importantly, raising histone acetylation levels pharmacologically by administration of HDAC inhibitors such as trichostatin A (TSA), a broad inhibitor of class I and II HDACs, or MS-275, a more selective HDAC inhibitor, can induce multiple RAGs and enhance sensory axon regeneration after a dorsal column transection (Finelli et al., 2013) (Table 1). Meanwhile, Cho and colleagues elegantly showed that peripheral axotomy of DRG neurons triggers a retrograde calcium wave propagated to the nucleus to activate protein kinase C (PKCμ), which in turn induces nuclear export of HDAC5. The translocation in HDAC5 is linked to an increase in acetylation levels of histone 3 (AcH3) and axon regeneration after sciatic nerve transection (Cho et al., 2013). Furthermore, we have demonstrated a close collaboration of histone-modifying enzymes and Smad1, a proregenerative transcription factor that is induced and activated by peripheral, but not central axotomy (Zou et al., 2009; Parikh et al., 2011; Zhong and Zou, 2014). Promoter occupancy of Smad1 helps to recruit p300 (a HAT) and displace HDAC1, leading to an enrichment of AcH4 at promoters of Smad1 target genes such as Sprr1a, Npy, Galanin, and Vip, while increased histone acetylation, in turn, facilitates access of Smad1 to promoters (Finelli et al., 2013) (Fig. 3).
Figure 2.
Nuclear epigenetic events for RAGs induction. In mature neurons in the CNS, pro-growth genes are generally downregulated by a repressive transcriptional complex, which may encompass transcription factor (TF), histone-modifying enzyme (HME) and chromatin remodeler (CR), to compact local chromatin. Upon stimulation, activated proregenerative transcription factors, such as Smad1, are recruited to the respective regulatory elements on specific genomic loci, and orchestrate recruitment of regeneration-associated HMEs and CRs to relax local chromatin, which further facilitates promoter occupancy of TFs, leading to a chromatin landscape favorable for expression of regeneration-associated genes (RAGs).
Figure 3.
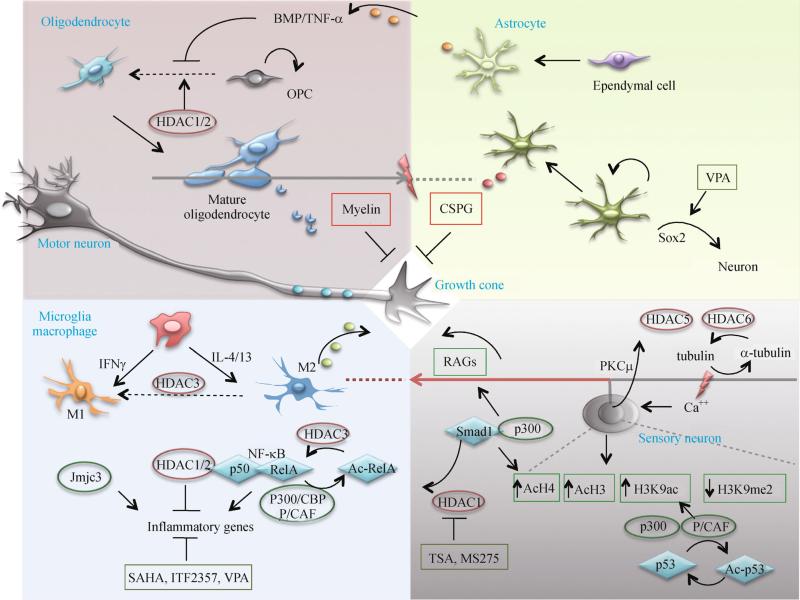
Injury responses of neurons and glia after SCI. Axon regeneration is controlled by intrinsic proregenerative transcriptional programs and influenced by environmental signals. Myelin debris from demyelinating oligodendrocytes and chondroitin sulfate proteoglycans (CSPG) secreted from reactive astrocytes constitute a roadblock for axonal regeneration. M2 macrophages can promote axonal regrowth. A nuclear program is activated by peripheral axotomy of DRG neurons, leading to increased histone acetylation and induction of RAGs. Histone-modifying enzymes participate in various aspects of injury responses in neurons and glial cells. Note that the epigenetic regulators controlling macrophage polarization have only been demonstrated in non-spinal cord injury settings.
Puttagunta and colleagues further analyzed residue specific histone acetylation in DRG neurons after a peripheral axotomy, and demonstrated an increase in acetylation of histone 3 lysine 9 (H3K9ac), a marker of actively transcribed genes, and a decrease in H3K9 methylation (H3K9me2), a repressive marker, at the promoters of GAP43, Galanin, and brain-derived neurotrophic factor (BDNF). p300/CBP-associated factor (P/CAF), a H3K9ac-specific acetyltransferase, is enriched at the promoters of these RAGs, and this process is dependent upon retrograde signaling mediated by the activation of the extracellular signal-regulated kinase (ERK) pathway (Puttagunta et al., 2014). P/CAF overexpression enhances regeneration of ascending sensory neurons after SCI (Puttagunta et al., 2014). In the same study, Puttagunta et al. also investigated DNA methylation patterns and found that a number of genes displayed differential promoter and CGI DNA methylation following peripheral versus central axotomy, however, these changes do not appear to correlate with gene expression. It is worth mentioning that in DRGs, sensory neurons are vastly out-numbered by satellite and glial cells, thus DNA lysates from whole DRGs consist mostly of glial chromatin. Hence, neuronal enriched chromatin would be better suited for studying the role of DNA methylation in axon regeneration. Earlier studies have shown that the folate pathway can mediate CNS axon regeneration through global and gene-specific DNA methylation, suggesting that DNA methylation represents yet another epigenetic mechanism mediating CNS recovery (Iskandar et al., 2010).
Histone acetylation also plays a role in axon regeneration in other subtypes of neurons. In a rat optic nerve crush model, overexpression of p300 by adenovirus can enrich AcH3 at the promoters of GAP43, coronin1b and Sprr1a and induce their expression, as well as enhance optic nerve regeneration, but not retinal ganglionic cell (RGC) survival (Gaub et al., 2011). In contrast, the HDAC inhibitor TSA increases RGC survival but not axonal regeneration after optic nerve crush. Therefore, HAT activation and HDAC inhibition appear to work on different substrates in RGCs.
It is worth noting that the substrates of the histone-modifying enzymes are not restricted to histones. p53, a transcription factor classically known as a tumor suppressor, is subject to the acetyltransferase activity of CBP/p300. Acetylated p53 has been shown to regulate GAP43 and promote axon outgrowth (Tedeschi et al., 2009) (Fig. 3). In addition, TSA treatment prevents growth cone collapse and enhances neurite outgrowth of cerebellar granule cells on both permissive and inhibitory substrates, and this effect is mediated partly through CBP/p300 and P/CAF-dependent p53 acetylation, leading to histone H3K9/14 acetylation and upregulation of GAP43 (Gaub et al., 2010). Notably, many isoforms of HDACs are also found in axons. For instance, during demyelination or other neurotoxic conditions, HDAC1 is shuttled out of the nucleus to form complexes with kinesin motor proteins, thereby hindering mitochondrial transport (Kim et al., 2010). HDAC6 is mainly a cytoplasmic protein and its inhibition by TSA enhances neurite outgrowth on nonpermissive substrates via a transcription-independent mechanism that is likely through acetylation of α-tubulin (Rivieccio et al., 2009; Tang, 2014). Moreover, upon peripheral axotomy of DRG neurons, HDAC5 in the axon is activated by PKC, and functions as an α-tubulin deacetylase to contribute to growth cone dynamics and axon regeneration (Cho and Cavalli, 2012). In this context, HDAC5 inhibition blocks axon regeneration in a sciatic nerve injury model. It is therefore important to be cognizant of different subcellular locations of HDACs and non-histone substrates.
In summary, a full regenerative program may engage a diverse set of proregenerative transcription factors, each orchestrating recruitment of specific histone-modifying enzymes or chromatin remodelers to the regulatory elements of target RAGs to reshape local chromatin structure in favor of regeneration (Fig. 2). The abundance and spatial proximity of various modifications on histone residues make it likely for crosstalk to take place between different modifications. The next challenge is to reveal the biological functions of individual chromatin regulators and their genomic targets in SCI. It is worth mentioning that ChIP-string and bioinformatics analyses suggest that genes with similar functions may be regulated by the same transcription module, thus ensuring orchestrated timing for transcriptional induction (Ernst et al., 2011; Ram et al., 2011). Genome wide mapping of DNA methylation and histone modifications will therefore provide a blueprint of the chromatin changes underlying the differential regenerative capacities of PNS versus CNS neurons, young versus old, and motor versus sensory neurons. Bioinformatic analyses may lead to identification of novel proregenerative transcription modules besides the aforementioned Smad1/p300/HDAC1 and p300/CBP/PCAF complexes that could be targeted therapeutically. One advantage of modulating epigenetic factors is that, if done correctly, an ensemble of RAGs could be induced, as opposed to induction of single genes. Hence, a new therapeutic direction is to create a proregenerative chromatin landscape to facilitate recovery following injury.
Astrogliosis and functional heterogeneity of astrocytes
The glial scar is traditionally considered to be detrimental to functional recovery after CNS injury as it presents a physical barrier for axon regeneration (Silver and Miller, 2004) (Fig. 3). However, the glial scar is also beneficial in restricting inflammation and preserving tissue integrity (Faulkner et al., 2004). After SCI, newly proliferated astroglia extend elongated processes at the scar borders, via a Stat3-dependent mechanism, to corral inflammatory and fibrotic cells, thus enabling the separation of viable neurons from the injury milieu (Wanner et al., 2013). Recent advances have revealed a vast degree of functional heterogeneity among reactive astrocytes, and genomic analysis has revealed distinct transcriptomes of different subtypes of astrocytes reacting to different types of CNS injury (Zamanian et al., 2012). Many previously unappreciated features of reactive astrocytes also came to light with improved genetic tools and in vivo imaging techniques. For instance, in vivo tracking of GFP+ protoplasmic astrocytes unexpectedly showed minimal cell migration toward a punctate wound, but instead, the increased astrocytes at the lesion site stem from proliferation of local astrocytes at juxtavascular locations. Moreover, a given astrocyte undergoes no more than a single division, generating two daughter cells that remain spatially close for up to a month post-injury (Bardehle et al., 2013). Fate mapping studies have also revealed an unexpected source of reactive astrocytes after SCI. In the spinal cord, ependymal cells lining the central canal have stem cell properties that enable them to differentiate into either astrocytes or oligodendrocytes (Barnabé-Heider et al., 2010). Interestingly, ependymal-derived astrocytes are mainly confined to the lesion center, whereas local reactive astrocytes mostly form the outer boundaries of the glial scar. In a mouse mutant model with genetic deletion of all Ras genes in ependymal cells (FoxJ1-rasless), which blocks their proliferation and progeny generation, lesions were larger and neuronal loss greater when compared to controls after SCI, thus demonstrating the importance of the ependymal-derived cellular component of the glial scar in providing scaffolding to restrict expansion of tissue damage (Sabelström et al., 2013). Defining the epigenetic mechanisms that regulate reactive phenotypes of astrocytes and progenitor cells after SCI would help to design strategies to promote wound healing and preserve tissue integrity after injury.
Furthermore, certain mature astrocytes exposed to CNS injury resume properties of earlier developmental stages, including expression of nestin and vimentin, along with acquisition of stem cell properties. These reactive astrocytes exhibit multipotency and spherogenic potential in vitro, but remain within the astroglial lineage in vivo (Buffo et al., 2008). Recent studies have reported the possibility of in vivo reprogramming of parenchymal astrocytes in the striatum directly into neurons by viral delivery of three neural conversion factors achaete-scute complex-like 1 (Ascl1), brain-2 (Brn2a), and myelin transcription factor-like 1(Myt1l) (Torper et al., 2013). The transcription factor Sox2 alone reportedly is capable of reprogramming resident astrocytes in the striatum into proliferating neuroblasts in both young adult and aged mice, and treatment with BDNF and noggin or VPA enabled the maturation of these neuroblasts into functional neurons (Niu et al., 2013). Reactive astrocytes can also be reprogrammed into functional neurons following either a cortical stab wound or in an Alzheimer's disease mouse model by forced expression of neural transcription factor, NeuroD1 (Guo et al., 2014). Similarly, in the injured spinal cord, local astrocytes can be reprogrammed to neurons by Sox2, and neuronal maturation and survival of Sox2-reprogrammed local astrocytes can be further enhanced by VPA (Su et al., 2014). Pericytes are another major source of proliferating scar-forming cells after CNS injury (Göritz et al., 2011). Pericyte-derived cells can be reprogrammed into neuronal cells by forced coexpression of Sox2 and Mash1 (Karow et al., 2012). Reprogramming of astrocytes and pericytes into functional neurons may thus represent an important repair strategy after CNS injury.
Transplanting exogenous NSCs has also been shown to enhance neural repair after SCI. Grafted NSCs embedded in fibrin matrices with growth factors can differentiate into neurons with extensive axonal elongation and form functional relays (Lu et al., 2012). Given the important role of epigenetic regulation during neural development, epigenetic factors may be exploited to optimize cellular reprogramming and stem cell-based replacement therapies. Indeed, during differentiation of multipotent neural progenitor cells (NPCs), VPA promotes neuronal differentiation and inhibits astrocyte and oligodendrocyte differentiation (Hsieh et al., 2004). Transplanted NPCs in the injured spinal cord undergo differentiation that is generally restricted to the glial lineage, but VPA treatment enhances neuronal differentiation and improves hindlimb functional recovery (Abematsu et al., 2010). Notably, the promoting effect of VPA on neuronal differentiation may in fact not be related to HDAC inhibition, as HDAC1 and 2 are required for the progression of NPCs to neurons during development as double deletion of HDAC1 and 2 severely disrupts cortical development and causes excessive cell death (Montgomery et al., 2009).
Epigenetic regulation of the inflammatory response after SCI
SCI triggers a multiphasic immune response. During the early phase, recruitment of neutrophils occurs within a few hours, followed by peak infiltration of blood monocytes at 7 days, and peak T cell lymphocyte infiltration at 9 days post-injury (Beck et al., 2010). Blood-born monocytes differentiate into phagocytic macrophages, which together with activated resident microglia, clear up cell debris (Popovich and Jones, 2003). Immune cells also produce reactive oxygen and nitrogen species (ROS/RNS) that partake in oxidative damage to spinal cord tissue (Carlson et al., 1998). The late phase of inflammation is detected at 14 days post-injury and can last as long as 6 months. Interestingly, blocking the late phase of inflammation impairs locomotor recovery, suggesting a reparative role for late inflammation after SCI (Beck et al., 2010). Hence, selecting an appropriate time frame for immunomodulatory therapy after SCI is imperative.
Remarkably, macrophages can simultaneously inflict injury and facilitate repair after SCI. These divergent effects are attributed to functional polarization of macrophages dictated by a delicate imbalance of pro- and anti-inflamma-tory signals in the injured spinal cord (Aguzzi et al., 2013). For instance, interferon-γ (IFNγ), in conjunction with activated toll-like receptors (TLRs), induces pro-inflamma-tory, classically activated M1 macrophages. In contrast, alternatively activated, anti-inflammatory M2 macrophages are regulated by interleukin 4 (Il-4) and IL-13, and function to promote wound healing (Gordon and Martinez, 2010). In uninjured CNS, microglia and macrophages exhibit a M2 phenotype (Ponomarev et al., 2007). After SCI, both M1 and M2 macrophages are present at the injury site, however, M2 cells are incapable of maintaining their phenotype, resulting in a predominance of the M1 phenotype over time (David and Kroner, 2011). When isolated M2 macrophages are injected intraspinally after SCI, they transform into the M1 phenotype along with repression of M2-associated genes, thus demonstrating that lesion-associated factors indeed suppress the M2 phenotype. Unlike cutaneous wound healing where a shift from M1 to M2 macrophages occurs to ensure a transition from an early pro-inflammatory phase to a later wound healing phase, this M1-to-M2 macrophage shift does not occur after SCI. Consistently, after SCI, the pro-inflammatory cytokine IFNγ is upregulated, whereas intraspinal IL-4 and-13 remain low (Kigerl et al., 2009).
Epigenetic regulation may play a role in maintaining the M2 phenotype (Fig. 3). In this regard, HDAC3 has been shown to function as a brake to prevent M2 induction by deacetylating histone tails at regulatory regions of many IL-4 regulated genes. HDAC3 deletion, on the other hand, releases this epigenomic brake and enhances anti-inflammatory properties of macrophages (Mullican et al., 2011). One likely scenario is that SCI induces an upregulation or increased activity of HDAC3 in macrophages, thereby promoting a shift from a M2 to M1 phenotype at the injury site. If proven to be true, selective inhibition of HDAC3 activity in macrophages may represent a promising therapeutic direction.
Other chromatin regulators also participate in the regulation of inflammatory genes, but their contribution to the functional polarization of macrophages remains to be clarified (Fig. 3). In the inflammatory response to lipopolysaccharide (LPS), a potent TLR ligand, Jmjd3, a histone demethylase, acts to remove repressive histone modifications to control gene expression (De Santa et al., 2009). NF-κB plays a dual role as both a pro- and an anti-inflammatory transcriptional complex. HDAC1 forms a repressive transcriptional complex with p50, a subunit of NF-κB to suppress inflammatory genes, such as TNF-α, CXCL10, and MMP-13, thereby limiting the inflammatory response to liver injury (Oakley et al., 2005; Elsharkawy et al., 2010). HDAC2, via its association with HDAC1, can indirectly interact with NF- κB, and act as a co-repressor (Ashburner et al., 2001). The NF-κB complex also recruits the p300/CBP and P/CAF coactivators to induce target genes by either altering chromatin structure and/or acetylation of transcription factors (Chen et al., 2001). Additionally, RelA, another subunit of NF-κB, can itself be subject to reversible acetylation mediated by p300/CBP and HDAC3. In fact, HDAC3 can deacetylate RelA, leading to nuclear export of the NF-κB complex (Chen et al., 2001). Given that TNF-α and NF-κB, as well as many other inflammatory genes are activated within hours after SCI, it is likely that various isoforms of HDACs and HATs participate in their transcriptional regulation after SCI (Bartholdi and Schwab, 1997; Bethea et al., 1998; Xu et al., 1998; Carmel et al., 2001). Two potent HDAC inhibitors, suberoylanilide hydroxamic acid (SAHA) and its structural analog ITF2357, can suppress pro-inflammatory gene expression in LPS-stimulated glial cells by affecting access of transcription factors c-Fos, c-Jun and FRA-2 to DNA (Faraco et al., 2009). Moreover, intraspinal infusion of VPA significantly attenuates microglia or macrophage accumulation in the lesion core, along with reduced tissue loss and improved hindlimb locomotor function in a rat spinal cord contusion model (Lu et al., 2013). However, the effect of VPA is reportedly not through inhibition of HDACs, but rather by way of p38 MAPK activation to downregulate P2X4R, a P2X receptor subtype that binds to extracellular ATP released from injured neurons and activated glia as an excitotoxin in SCI (Lu et al., 2013). In fact, treatment with two other HDAC inhibitors, TSA and sodium butyrate, actually upregulates P2X4R in LPS-stimulated microglia (Popovich and Long-brake, 2008). Therefore, interpretation of the effects of nonspecific pharmacological agents warrants caution.
Aside from their effects on wound healing, microglia/ macrophages can directly affect the regenerative capacity of injured neurons (Fig. 3). In optic nerve injury models, lens injury can promote axon regeneration, and zymosan, a yeast wall component, has been identified as a potent macrophage stimulant. Zymosan-activated macrophages are capable of promoting axon regeneration of RGC and DRG neurons, albeit with some degree of neurotoxicity (Gensel et al., 2009). Combinatorial treatment of intraocular injection of zymosan with PTEN deletion or elevation of cAMP results in long-distance axon regeneration (de Lima et al., 2012). It is unclear whether macrophage polarization plays a role in axon regeneration after SCI. Interestingly, both M1 and M2 macrophages can promote axon growth but the former stimulates a short arborizing growth pattern and are neurotoxic, while the latter induce axonal elongation with less branching, even on non-permissive substances (Kigerl et al., 2009). In summary, understanding the role of specific chromatin regulators in polarizing macrophages and the mechanisms by which microglia/macrophages interact with injured neurons to promote axon regeneration will help tailor immunomodulatory therapies for SCI.
Demyelination and oligodendrocyte differentiation
After SCI, demyelination is initiated during primary injury and continues to take place well into the chronic stages of injury. During primary demyelination, the impact of the trauma results in the death of oligodendrocytes and denudation of remaining intact axons. In contrast, secondary demyelination is a chronic process involving axonal injury that subsequently triggers oligodendrocyte necrosis and apoptosis, which in turn leads to demyelination (Wisniewski and Bloom, 1975). In rats, demyelination has been shown to persist as long as 450 days post injury (Totoiu and Keirstead, 2005).
After SCI, oligodendrocyte progenitor cells (OPCs) proliferate and increase in number within days (Ishii et al., 2001; McTigue et al., 2001), however, their differentiation into mature oligodendrocytes is inhibited (Suyama et al., 2007). Various transcription factors are involved in regulating OPC differentiation, including Nkx2.2, Olig1, Olig2, and Sox10 (Stolt et al., 2002). After SCI, reactive astrocytes inhibit the differentiation of OPCs into mature oligodendrocytes by releasing bone morphogenetic proteins (BMPs), which promotes OPC differentiation into astrocytes at the expense of oligodendrocytes (Wang et al., 2011)(Fig. 3). This effect can be reversed by noggin, a BMP receptor antagonist. Similarly, TNF-α secreted by reactive astrocytes can also inhibit the differentiation of OPCs into oligodendrocytes, and this effect can be ameliorated by neutralizing antibodies against either TNF-α or its receptor (Su et al., 2011).
During development, selective ablation of both HDAC1 and 2 in the oligodendrocyte lineage show that both are required for oligodendrocyte specification and differentiation, partly by inhibiting β-catenin/TCF7L2 complex formation in the canonical Wnt pathway (Ye et al., 2009). Additionally, HDAC11 regulates morphological maturation of OL-1 oligodendroglia cells (Liu et al., 2009). It has been reported that remyelination capability declines with age, and epigenetic regulation plays a role in this decline. Specifically, HDAC1 and 2 recruitment to the promoter regions of transcriptional repressors Sox2 and Hes5 is important for remyelination in young mice, but this recruitment becomes impaired with age (Shen et al., 2008). It is possible that similar epigenetic mechanisms might regulate remyelination efficiency after SCI. Gaining further knowledge about the epigenetic regulation of OPC differentiation into remyelinating oligodendrocytes following SCI is necessary before HDAC inhibitors or other drugs can be employed to modulate this response in a beneficial manner.
Conclusions
Epigenetic mechanisms are involved in regulating the diverse adaptive behaviors of neurons and glial cells after SCI. Systematic studies will be required to characterize the complex epigenetic changes that are induced by SCI. Achieving a fine balance between negating detrimental and fostering beneficial injury responses requires taking an integrated approach to understand the functions and genomic targets of histone-modifying enzymes and chromatin remodelers in mediating cell-type-specific and context-dependent adaptive behaviors. Insights into the mechanisms by which the chromatin landscape can be reshaped in favor of axon regeneration and neural tissue repair would enlighten us on how epigenetic modulation can be harnessed to enhance tissue repair and axon regeneration after SCI.
Acknowledgements
We apologize to colleagues whose work could not be cited owing to space limitations. H. Z. is supported by NIH (NS073596) and IrmaT. Hirschl/Monique Weill-Caulier Foundation.
Footnotes
Compliance with ethics guidlines
Jamie K. Wong and Hongyan Zou declare that they have no conflict of interest. This manuscript is a review article and does not involve a research protocol requiring approval by the relevant institutional review board or ethics committee.
References
- Abematsu M, Tsujimura K, Yamano M, Saito M, Kohno K, Kohyama J, Namihira M, Komiya S, Nakashima K. Neurons derived from transplanted neural stem cells restore disrupted neuronal circuitry in a mouse model of spinal cord injury. J Clin Invest. 2010;120(9):3255–3266. doi: 10.1172/JCI42957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguzzi A, Barres BA, Bennett ML. Microglia: scapegoat, saboteur, or something else? Science. 2013;339(6116):156–161. doi: 10.1126/science.1227901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner BP, Westerheide SD, Baldwin AS., Jr The p65 (RelA) subunit of NF-κB interacts with the histone deacetylase (HDAC) corepressors HDAC1 and HDAC2 to negatively regulate gene expression. Mol Cell Biol. 2001;21(20):7065–7077. doi: 10.1128/MCB.21.20.7065-7077.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardehle S, Krüger M, Buggenthin F, Schwausch J, Ninkovic J, Clevers H, Snippert HJ, Theis FJ, Meyer-Luehmann M, Bechmann I, Dimou L, Götz M. Live imaging of astrocyte responses to acute injury reveals selective juxtavascular proliferation. Nat Neurosci. 2013;16(5):580–586. doi: 10.1038/nn.3371. [DOI] [PubMed] [Google Scholar]
- Barnabé-Heider F, Göritz C, Sabelström H, Takebayashi H, Pfrieger FW, Meletis K, Frisén J. Origin of new glial cells in intact and injured adult spinal cord. Cell Stem Cell. 2010;7(4):470–482. doi: 10.1016/j.stem.2010.07.014. [DOI] [PubMed] [Google Scholar]
- Bartholdi D, Schwab ME. Expression of pro-inflammatory cytokine and chemokine mRNA upon experimental spinal cord injury in mouse: an in situ hybridization study. Eur J Neurosci. 1997;9(7):1422–1438. doi: 10.1111/j.1460-9568.1997.tb01497.x. [DOI] [PubMed] [Google Scholar]
- Beck KD, Nguyen HX, Galvan MD, Salazar DL, Woodruff TM, Anderson AJ. Quantitative analysis of cellular inflammation after traumatic spinal cord injury: evidence for a multiphasic inflammatory response in the acute to chronic environment. Brain. 2010;133(Pt 2):433–447. doi: 10.1093/brain/awp322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bethea JR, Castro M, Keane RW, Lee TT, Dietrich WD, Yezierski RP. Traumatic spinal cord injury induces nuclear factor-κB activation. J Neurosci. 1998;18(9):3251–3260. doi: 10.1523/JNEUROSCI.18-09-03251.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broide RS, Redwine JM, Aftahi N, Young W, Bloom FE, Winrow CJ. Distribution of histone deacetylases 1-11 in the rat brain. J Mol Neurosci. 2007;31(1):47–58. doi: 10.1007/BF02686117. [DOI] [PubMed] [Google Scholar]
- Buffo A, Rite I, Tripathi P, Lepier A, Colak D, Horn AP, Mori T, Götz M. Origin and progeny of reactive gliosis: A source of multipotent cells in the injured brain. Proc Natl Acad Sci USA. 2008;105(9):3581–3586. doi: 10.1073/pnas.0709002105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson SL, Parrish ME, Springer JE, Doty K, Dossett L. Acute inflammatory response in spinal cord following impact injury. Exp Neurol. 1998;151(1):77–88. doi: 10.1006/exnr.1998.6785. [DOI] [PubMed] [Google Scholar]
- Carmel JB, Galante A, Soteropoulos P, Tolias P, Recce M, Young W, Hart RP. Gene expression profiling of acute spinal cord injury reveals spreading inflammatory signals and neuron loss. Physiol Genomics. 2001;7(2):201–213. doi: 10.1152/physiolgenomics.00074.2001. [DOI] [PubMed] [Google Scholar]
- Chen LF, Fischle W, Verdin E, Greene WC. Duration of nuclear NF-kappaB action regulated by reversible acetylation. Science. 2001;293(5535):1653–1657. doi: 10.1126/science.1062374. [DOI] [PubMed] [Google Scholar]
- Cho Y, Cavalli V. HDAC5 is a novel injury-regulated tubulin deacetylase controlling axon regeneration. EMBO J. 2012;31(14):3063–3078. doi: 10.1038/emboj.2012.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho Y, Cavalli V. HDAC signaling in neuronal development and axon regeneration. Curr Opin Neurobiol. 2014;27C:118–126. doi: 10.1016/j.conb.2014.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho Y, Sloutsky R, Naegle KM, Cavalli V. Injury-induced HDAC5 nuclear export is essential for axon regeneration. Cell. 2013;155(4):894–908. doi: 10.1016/j.cell.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David S, Kroner A. Repertoire of microglial and macrophage responses after spinal cord injury. Nat Rev Neurosci. 2011;12(7):388–399. doi: 10.1038/nrn3053. [DOI] [PubMed] [Google Scholar]
- de Lima S, Koriyama Y, Kurimoto T, Oliveira JT, Yin Y, Li Y, Gilbert HY, Fagiolini M, Martinez AM, Benowitz L. Full-length axon regeneration in the adult mouse optic nerve and partial recovery of simple visual behaviors. Proc Natl Acad Sci USA. 2012;109(23):9149–9154. doi: 10.1073/pnas.1119449109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Santa F, Narang V, Yap ZH, Tusi BK, Burgold T, Austenaa L, Bucci G, Caganova M, Notarbartolo S, Casola S, Testa G, Sung WK, Wei CL, Natoli G. Jmjd3 contributes to the control of gene expression in LPS-activated macrophages. EMBO J. 2009;28(21):3341–3352. doi: 10.1038/emboj.2009.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsharkawy AM, Oakley F, Lin F, Packham G, Mann DA, Mann J. The NF-κB p50:p50:HDAC-1 repressor complex orchestrates transcriptional inhibition of multiple pro-inflammatory genes. J Hepatol. 2010;53(3):519–527. doi: 10.1016/j.jhep.2010.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst J, Kheradpour P, Mikkelsen TS, Shoresh N, Ward LD, Epstein CB, Zhang X, Wang L, Issner R, Coyne M, Ku M, Durham T, Kellis M, Bernstein BE. Mapping and analysis of chromatin state dynamics in nine human cell types. Nature. 2011;473(7345):43–49. doi: 10.1038/nature09906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraco G, Pittelli M, Cavone L, Fossati S, Porcu M, Mascagni P, Fossati G, Moroni F, Chiarugi A. Histone deacetylase (HDAC) inhibitors reduce the glial inflammatory response in vitro and in vivo. Neurobiol Dis. 2009;36(2):269–279. doi: 10.1016/j.nbd.2009.07.019. [DOI] [PubMed] [Google Scholar]
- Faulkner JR, Herrmann JE, Woo MJ, Tansey KE, Doan NB, Sofroniew MV. Reactive astrocytes protect tissue and preserve function after spinal cord injury. J Neurosci. 2004;24(9):2143–2155. doi: 10.1523/JNEUROSCI.3547-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finelli MJ, Wong JK, Zou H. Epigenetic regulation of sensory axon regeneration after spinal cord injury. J Neurosci. 2013;33(50):19664–19676. doi: 10.1523/JNEUROSCI.0589-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaub P, Joshi Y, Wuttke A, Naumann U, Schnichels S, Heiduschka P, Di Giovanni S. The histone acetyltransferase p300 promotes intrinsic axonal regeneration. Brain. 2011;134(Pt 7):2134–2148. doi: 10.1093/brain/awr142. [DOI] [PubMed] [Google Scholar]
- Gaub P, Tedeschi A, Puttagunta R, Nguyen T, Schmandke A, Di Giovanni S. HDAC inhibition promotes neuronal outgrowth and counteracts growth cone collapse through CBP/p300 and P/CAF-dependent p53 acetylation. Cell Death Differ. 2010;17(9):1392–1408. doi: 10.1038/cdd.2009.216. [DOI] [PubMed] [Google Scholar]
- Gensel JC, Nakamura S, Guan Z, van Rooijen N, Ankeny DP, Popovich PG. Macrophages promote axon regeneration with concurrent neurotoxicity. J Neurosci. 2009;29(12):3956–3968. doi: 10.1523/JNEUROSCI.3992-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon S, Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity. 2010;32(5):593–604. doi: 10.1016/j.immuni.2010.05.007. [DOI] [PubMed] [Google Scholar]
- Göritz C, Dias DO, Tomilin N, Barbacid M, Shupliakov O, Frisén J. A pericyte origin of spinal cord scar tissue. Science. 2011;333(6039):238–242. doi: 10.1126/science.1203165. [DOI] [PubMed] [Google Scholar]
- Guo Z, Zhang L, Wu Z, Chen Y, Wang F, Chen G. In Vivo direct reprogramming of reactive glial cells into functional neurons after brain injury and in an Alzheimer’s disease model. Cell Stem Cell. 2014;14(2):188–202. doi: 10.1016/j.stem.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh J, Nakashima K, Kuwabara T, Mejia E, Gage FH. Histone deacetylase inhibition-mediated neuronal differentiation of multi-potent adult neural progenitor cells. Proc Natl Acad Sci USA. 2004;101(47):16659–16664. doi: 10.1073/pnas.0407643101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii K, Toda M, Nakai Y, Asou H, Watanabe M, Nakamura M, Yato Y, Fujimura Y, Kawakami Y, Toyama Y, Uyemura K. Increase of oligodendrocyte progenitor cells after spinal cord injury. J Neurosci Res. 2001;65(6):500–507. doi: 10.1002/jnr.1180. [DOI] [PubMed] [Google Scholar]
- Iskandar BJ, Rizk E, Meier B, Hariharan N, Bottiglieri T, Finnell RH, Jarrard DF, Banerjee RV, Skene JH, Nelson A, Patel N, Gherasim C, Simon K, Cook TD, Hogan KJ. Folate regulation of axonal regeneration in the rodent central nervous system through DNA methylation. J Clin Invest. 2010;120(5):1603–1616. doi: 10.1172/JCI40000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karow M, Sánchez R, Schichor C, Masserdotti G, Ortega F, Heinrich C, Gascón S, Khan MA, Lie DC, Dellavalle A, Cossu G, Goldbrunner R, Götz M, Berninger B. Reprogramming of pericyte-derived cells of the adult human brain into induced neuronal cells. Cell Stem Cell. 2012;11(4):471–476. doi: 10.1016/j.stem.2012.07.007. [DOI] [PubMed] [Google Scholar]
- Kigerl KA, Gensel JC, Ankeny DP, Alexander JK, Donnelly DJ, Popovich PG. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J Neurosci. 2009;29(43):13435–13444. doi: 10.1523/JNEUROSCI.3257-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JY, Shen S, Dietz K, He Y, Howell O, Reynolds R, Casaccia P. HDAC1 nuclear export induced by pathological conditions is essential for the onset of axonal damage. Nat Neurosci. 2010;13(2):180–189. doi: 10.1038/nn.2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konsoula Z, Barile FA. Epigenetic histone acetylation and deacetylation mechanisms in experimental models of neurodegenerative disorders. J Pharmacol Toxicol Methods. 2012;66(3):215–220. doi: 10.1016/j.vascn.2012.08.001. [DOI] [PubMed] [Google Scholar]
- Kouzarides T. Chromatin modifications and their function. Cell. 2007;128(4):693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Lee JY, Kim HS, Choi HY, Oh TH, Ju BG, Yune TY. Valproic acid attenuates blood-spinal cord barrier disruption by inhibiting matrix metalloprotease-9 activity and improves functional recovery after spinal cord injury. J Neurochem. 2012;121(5):818–829. doi: 10.1111/j.1471-4159.2012.07731.x. [DOI] [PubMed] [Google Scholar]
- Lindner R, Puttagunta R, Di Giovanni S. Epigenetic regulation of axon outgrowth and regeneration in CNS injury: the first steps forward. Neurotherapeutics. 2013;10(4):771–781. doi: 10.1007/s13311-013-0203-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Hu Q, D'ercole AJ, Ye P. Histone deacetylase 11 regulates oligodendrocyte-specific gene expression and cell development in OL-1 oligodendroglia cells. Glia. 2009;57(1):1–12. doi: 10.1002/glia.20729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K, Tedeschi A, Park KK, He Z. Neuronal intrinsic mechanisms of axon regeneration. Annu Rev Neurosci. 2011;34(1):131–152. doi: 10.1146/annurev-neuro-061010-113723. [DOI] [PubMed] [Google Scholar]
- Lu P, Wang Y, Graham L, McHale K, Gao M, Wu D, Brock J, Blesch A, Rosenzweig ES, Havton LA, Zheng B, Conner JM, Marsala M, Tuszynski MH. Long-distance growth and connectivity of neural stem cells after severe spinal cord injury. Cell. 2012;150(6):1264–1273. doi: 10.1016/j.cell.2012.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu WH, Wang CY, Chen PS, Wang JW, Chuang DM, Yang CS, Tzeng SF. Valproic acid attenuates microgliosis in injured spinal cord and purinergic P2X4 receptor expression in activated microglia. J Neurosci Res. 2013;91(5):694–705. doi: 10.1002/jnr.23200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv L, Han X, Sun Y, Wang X, Dong Q. Valproic acid improves locomotion in vivo after SCI and axonal growth of neurons in vitro. Exp Neurol. 2012;233(2):783–790. doi: 10.1016/j.expneurol.2011.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv L, Sun Y, Han X, Xu CC, Tang YP, Dong Q. Valproic acid improves outcome after rodent spinal cord injury: potential roles of histone deacetylase inhibition. Brain Res. 2011;1396:60–68. doi: 10.1016/j.brainres.2011.03.040. [DOI] [PubMed] [Google Scholar]
- McTigue DM, Wei P, Stokes BT. Proliferation of NG2-positive cells and altered oligodendrocyte numbers in the contused rat spinal cord. J Neurosci. 2001;21(10):3392–3400. doi: 10.1523/JNEUROSCI.21-10-03392.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery RL, Hsieh J, Barbosa AC, Richardson JA, Olson EN. Histone deacetylases 1 and 2 control the progression of neural precursors to neurons during brain development. Proc Natl Acad Sci USA. 2009;106(19):7876–7881. doi: 10.1073/pnas.0902750106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monti B, Polazzi E, Contestabile A. Biochemical, molecular and epigenetic mechanisms of valproic acid neuroprotection. Curr Mol Pharmacol. 2009;2:95–109. doi: 10.2174/1874467210902010095. [DOI] [PubMed] [Google Scholar]
- Mullican SE, Gaddis CA, Alenghat T, Nair MG, Giacomin PR, Everett LJ, Feng D, Steger DJ, Schug J, Artis D, Lazar MA. Histone deacetylase 3 is an epigenomic brake in macrophage alternative activation. Genes Dev. 2011;25(23):2480–2488. doi: 10.1101/gad.175950.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann S, Woolf CJ. Regeneration of dorsal column fibers into and beyond the lesion site following adult spinal cord injury. Neuron. 1999;23(1):83–91. doi: 10.1016/s0896-6273(00)80755-2. [DOI] [PubMed] [Google Scholar]
- Niu W, Zang T, Zou Y, Fang S, Smith DK, Bachoo R, Zhang CL. In vivo reprogramming of astrocytes to neuroblasts in the adult brain. Nat Cell Biol. 2013;15(10):1164–1175. doi: 10.1038/ncb2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley F, Mann J, Nailard S, Smart DE, Mungalsingh N, Constandinou C, Ali S, Wilson SJ, Millward-Sadler H, Iredale JP, Mann DA. Nuclear factor-κB1 (p50) limits the inflammatory and fibrogenic responses to chronic injury. Am J Pathol. 2005;166(3):695–708. doi: 10.1016/s0002-9440(10)62291-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh P, Hao Y, Hosseinkhani M, Patil SB, Huntley GW, Tessier-Lavigne M, Zou H. Regeneration of axons in injured spinal cord by activation of bone morphogenetic protein/Smad1 signaling pathway in adult neurons. Proc Natl Acad Sci USA. 2011;108(19):E99–E107. doi: 10.1073/pnas.1100426108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peleg S. Memory impairment in mice altered histone acetylation is associated with age-dependent. Science. 2010;328:753–756. doi: 10.1126/science.1186088. [DOI] [PubMed] [Google Scholar]
- Ponomarev ED, Maresz K, Tan Y, Dittel BN. CNS-derived interleukin-4 is essential for the regulation of autoimmune inflam mation and induces a state of alternative activation in microglial cells. J Neurosci. 2007;27(40):10714–10721. doi: 10.1523/JNEUROSCI.1922-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popovich PG, Jones TB. Manipulating neuroinflammatory reactions in the injured spinal cord: back to basics. Trends Pharmacol Sci. 2003;24(1):13–17. doi: 10.1016/s0165-6147(02)00006-8. [DOI] [PubMed] [Google Scholar]
- Popovich PG, Longbrake EE. Can the immune system be harnessed to repair the CNS? Nat Rev Neurosci. 2008;9:481–493. doi: 10.1038/nrn2398. [DOI] [PubMed] [Google Scholar]
- Puttagunta R, Tedeschi A, Sória MG, Hervera A, Lindner R, Rathore KI, Gaub P, Joshi Y, Nguyen T, Schmandke A, Laskowski CJ, Boutillier AL, Bradke F, Di Giovanni S. PCAF-dependent epigenetic changes promote axonal regeneration in the central nervous system. Nat Commun. 2014;5:3527. doi: 10.1038/ncomms4527. [DOI] [PubMed] [Google Scholar]
- Ram O, Goren A, Amit I, Shoresh N, Yosef N, Ernst J, Kellis M, Gymrek M, Issner R, Coyne M, Durham T, Zhang X, Donaghey J, Epstein CB, Regev A, Bernstein BE. Combinatorial patterning of chromatin regulators uncovered by genome-wide location analysis in human cells. Cell. 2011;147(7):1628–1639. doi: 10.1016/j.cell.2011.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson PM, Issa VM. Peripheral injury enhances central regeneration of primary sensory neurones. Nature. 1984;309(5971):791–793. doi: 10.1038/309791a0. [DOI] [PubMed] [Google Scholar]
- Rivieccio MA, Brochier C, Willis DE, Walker BA, D'Annibale MA, McLaughlin K, Siddiq A, Kozikowski AP, Jaffrey SR, Twiss JL, Ratan RR, Langley B. HDAC6 is a target for protection and regeneration following injury in the nervous system. Proc Natl Acad Sci USA. 2009;106(46):19599–19604. doi: 10.1073/pnas.0907935106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabelström H, Stenudd M, Réu P, Dias DO, Elfineh M, Zdunek S, Damberg P, Göritz C, Frisén J. Resident neural stem cells restrict tissue damage and neuronal loss after spinal cord injury in mice. Science. 2013;342(6158):637–640. doi: 10.1126/science.1242576. [DOI] [PubMed] [Google Scholar]
- Shen S, Sandoval J, Swiss VA, Li J, Dupree J, Franklin RJ, Casaccia-Bonnefil P. Age-dependent epigenetic control of differentiation inhibitors is critical for remyelination efficiency. Nat Neurosci. 2008;11(9):1024–1034. doi: 10.1038/nn.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver J, Miller JH. Regeneration beyond the glial scar. Nat Rev Neurosci. 2004;5(2):146–156. doi: 10.1038/nrn1326. [DOI] [PubMed] [Google Scholar]
- Stolt CC, Rehberg S, Ader M, Lommes P, Riethmacher D, Schachner M, Bartsch U, Wegner M. Terminal differentiation of myelin-forming oligodendrocytes depends on the transcription factor Sox10. Genes Dev. 2002;16(2):165–170. doi: 10.1101/gad.215802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Z, Niu W, Liu ML, Zou Y, Zhang CL. In vivo conversion of astrocytes to neurons in the injured adult spinal cord. Nat Commun. 2014;5:3338. doi: 10.1038/ncomms4338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Z, Yuan Y, Chen J, Zhu Y, Qiu Y, Zhu F, Huang A, He C. Reactive astrocytes inhibit the survival and differentiation of oligodendrocyte precursor cells by secreted TNF-α. J Neurotrauma. 2011;28(6):1089–1100. doi: 10.1089/neu.2010.1597. [DOI] [PubMed] [Google Scholar]
- Suyama K, Watanabe M, Sakai D, Osada T, Imai M, Mochida J. Nkx2.2 expression in differentiation of oligodendrocyte precursor cells and inhibitory factors for differentiation of oligodendrocytes after traumatic spinal cord injury. J Neurotrauma. 2007;24(6):1013–1025. doi: 10.1089/neu.2006.0151. [DOI] [PubMed] [Google Scholar]
- Tang BL. Class II HDACs and neuronal regeneration. J Cell Biochem. 2014;115(7):1225–1233. doi: 10.1002/jcb.24802. [DOI] [PubMed] [Google Scholar]
- Tedeschi A, Nguyen T, Puttagunta R, Gaub P, Di Giovanni S. A p53-CBP/p300 transcription module is required for GAP-43 expression, axon outgrowth, and regeneration. Cell Death Differ. 2009;16(4):543–554. doi: 10.1038/cdd.2008.175. [DOI] [PubMed] [Google Scholar]
- Torper O, Pfisterer U, Wolf DA, Pereira M, Lau S, Jakobsson J, Björklund A, Grealish S, Parmar M. Generation of induced neurons via direct conversion in vivo. Proc Natl Acad Sci USA. 2013;110(17):7038–7043. doi: 10.1073/pnas.1303829110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Totoiu MO, Keirstead HS. Spinal cord injury is accompanied by chronic progressive demyelination. J Comp Neurol. 2005;486(4):373–383. doi: 10.1002/cne.20517. [DOI] [PubMed] [Google Scholar]
- Trakhtenberg EF, Goldberg JL. Epigenetic regulation of axon and dendrite growth. Front Mol Neurosci. 2012;5:24. doi: 10.3389/fnmol.2012.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Cheng X, He Q, Zheng Y, Kim DH, Whittemore SR, Cao QL. Astrocytes from the contused spinal cord inhibit oligodendrocyte differentiation of adult oligodendrocyte precursor cells by increasing the expression of bone morphogenetic proteins. J Neurosci. 2011;31(16):6053–6058. doi: 10.1523/JNEUROSCI.5524-09.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanner IB, Anderson MA, Song B, Levine J, Fernandez A, Gray-Thompson Z, Ao Y, Sofroniew MV. Glial scar borders are formed by newly proliferated, elongated astrocytes that interact to corral inflammatory and fibrotic cells via STAT3-dependent mechanisms after spinal cord injury. J Neurosci. 2013;33(31):12870–12886. doi: 10.1523/JNEUROSCI.2121-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisniewski HM, Bloom BR. Primary demyelination as a nonspecific consequence of a cell-mediated immune reaction. J Exp Med. 1975;141(2):346–359. doi: 10.1084/jem.141.2.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Fan G, Chen S, Wu Y, Xu XM, Hsu CY. Methylprednisolone inhibition of TNF-α expression and NF-κB activation after spinal cord injury in rats. Brain Res Mol Brain Res. 1998;59(2):135–142. doi: 10.1016/s0169-328x(98)00142-9. [DOI] [PubMed] [Google Scholar]
- Ye F, Chen Y, Hoang T, Montgomery RL, Zhao XH, Bu H, Hu T, Taketo MM, van Es JH, Clevers H, Hsieh J, Bassel-Duby R, Olson EN, Lu QR. HDAC1 and HDAC2 regulate oligodendrocyte differentiation by disrupting the β-catenin-TCF interaction. Nat Neurosci. 2009;12(7):829–838. doi: 10.1038/nn.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- York EM, Petit A, Roskams AJ. Epigenetics of neural repair following spinal cord injury. Neurotherapeutics. 2013;10(4):757–770. doi: 10.1007/s13311-013-0228-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamanian JL, Xu L, Foo LC, Nouri N, Zhou L, Giffard RG, Barres BA. Genomic analysis of reactive astrogliosis. J Neurosci. 2012;32(18):6391–6410. doi: 10.1523/JNEUROSCI.6221-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong J, Zou H. BMP signaling in axon regeneration. Curr Opin Neurobiol. 2014;27C:127–134. doi: 10.1016/j.conb.2014.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou H, Ho C, Wong K, Tessier-Lavigne M. Axotomy-induced Smad1 activation promotes axonal growth in adult sensory neurons. J Neurosci. 2009;29(22):7116–7123. doi: 10.1523/JNEUROSCI.5397-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]