Abstract
Background
Neuronal cells are vulnerable to many stresses that can cause apoptosis. Reprogramming of fibroblasts into induced neural stem cells (iNSCs) is a potentially unlimited source of neurons. Discovering agents that can provide neuronal protection against these apoptotic stimuli is important for developing therapeutic strategies for various brain diseases.
Material/Methods
We investigated the therapeutic effects of iNSCs against apoptosis activator II (AAII)-induced apoptosis of cortical neuronal cells. Apoptosis was confirmed by double immunocytochemistry with NeuN and 4′,6-diamidino-2-phenylindole using terminal deoxynucleotidyl transferase-mediated digoxigenin-dUTP-biotin nick-end labeling. We performed Western blot analyses for activated caspase-3, Bcl-2, phosphorylated Akt, and phosphorylated extracellular signal-regulated protein kinase (ERK). The level of vascular endothelial growth factor (VEGF) was analyzed using enzyme-linked immunosorbent assays (P<0.05).
Results
Cortical neuronal cells cultured with iNSCs had fewer apoptotic cells than those cultured without iNSCs. We found that cells cultured with iNSCs had a significantly lower caspase-3 level and a significantly higher Bcl-2 level than cells cultured without iNSCs. Cells cultured with iNSCs had higher VEGF levels than cells cultured without iNSCs. The levels of phosphorylated Akt and phosphorylated ERK were significantly higher in cells cultured with iNSCs than in cells cultured without iNSCs.
Conclusions
Our findings suggest that iNSCs activate Akt and ERK, which are associated with the inhibition of neuronal apoptosis. Thus, treatment with iNSCs may help reduce neuronal loss in brain disease. Further studies aimed at proving this hypothesis might help establish therapeutic agents that can prevent neuronal cell death and help cure neurodegenerative diseases.
MeSH Keywords: Apoptosis, Cerebral Cortex, Stem Cell Research
Background
Neural stem cells (NSCs) are well-characterized somatic stem cells and have extensive proliferation and self-renewal capacities; they also show an extensive capacity for differentiating into daughter cell types [1]. The reprogramming of fibroblasts into induced NSCs (iNSCs) is a potentially unlimited source of neurons. Furthermore, iNSCs have characteristics that are similar to brain tissue-derived NSCs [2]. A number of studies have reported that cell transplantation can help repair such damaged tissue in preclinical neurodegenerative disease models; these studies include the transplantation of bone marrow stromal cells [3] and embryonic stem cells [4].
Apoptosis is a type of programmed cell death that occurs during developmental conditions such as cerebral injury [5]. Hallmarks of apoptosis include cell body shrinkage, nuclear condensation, DNA fragmentation, mitochondrial depolarization, activation of apoptotic genes such as those encoding caspases, and mitochondrial release of apoptotic factors. Decreased cell volume is a typical morphological phenotype of apoptotic cells [6–13].
The phosphatidylinositol 3-kinase (PI3K) signaling pathway is implicated in cell survival and apoptosis [14]. Akt is a main effector in the PI3K signaling pathway and plays a key role in multiple cellular processes such as glucose metabolism, apoptosis, and cell proliferation [15,16]. The PI3K/Akt signaling pathway mediates mitogen-dependent growth and survival, and inhibition of this pathway results in apoptosis [17]. Phosphorylation of Akt inactivates pro-apoptotic factors and inhibits apoptosis; conversely, inhibition of the phosphorylation of Akt induces apoptosis [18,19].
Apoptosis activator II (AAII) is a known apoptosis activator [20–23], and transference of this compound to the cell induces apoptosis. In this study, we investigated whether iNSCs could effectively protect cortical cell growth after AAII treatment, and sought to identify the potential underlying mechanisms. We used a characterized cortical cell culture model of neuronal apoptosis and investigated the therapeutic effects of iNSCs.
We found that AAII-induced apoptosis was associated with the activation of caspase-3, but treatment with iNSCs blocked caspase-3 activation and inhibited apoptosis. We also found that iNSCs induced the phosphorylation of Akt and ERK, which was necessary for preventing apoptosis. Thus, iNSCs may have an anti-apoptotic role when cultured with neurons. The molecular mechanisms underlying this role are still unknown, but will be investigated further in future studies.
Material and Methods
Co-culture with a cortical primary culture
Cortical neurons obtained from the cerebral cortices of 17-day-old rat embryos were cultured as described previously [24]. The brains of embryonic rats (E16–E18) were removed from the skull, and both cortices were dissected from the brain stem and transferred into Dulbecco’s modified Eagle’s medium (DMEM) with 10% fetal bovine serum (FBS; both, GIBCO-BRL, Gaithersburg, MD, USA). The cortical tissue was mechanically minced and centrifuged (320 g for 2 min). Tissue pellets were resuspended in Hanks buffer free of Ca2+ and Mg2+ and centrifuged again. After resuspension, 1 mL of ethylenediaminetetraacetic acid (EDTA)/phosphate-buffered saline (PBS) solution (GIBCO-BRL) was added before additional centrifugation. Trypsinization was performed by adding 2 mL Trypsin/EDTA solution (0.25%/0.02%, GIBCO-BRL) to the 6-mL culture suspension, and the mixture was incubated for 2 min at room temperature under gentle agitation. After resuspension in DMEM with 10% FBS, samples were squirted twice through a syringe equipped with a 23-gauge needle. After resuspending the cells in the same medium and counting them using a hemocytometer, 1 000 000 cells were seeded per well into a 12-well plate (1 mL/well) coated with glass coverslips. Cells were grown under standard conditions at 37°C and 5% CO2 in Neurobasal medium with B27 (Invitrogen) and glutamine (Invitrogen). Immediately afterwards, NSCs were added. Wild-type control cells (cortical neurons without AAII and iNSC) were used in the control groups. The cortical primary culture was fixed after 3 days of co-culturing with 4% paraformaldehyde for 15 min at room temperature.
Generation of iNSCs
iNSCs were cultured as described previously [2]. Briefly, fibroblasts (5×104 cells) were infected with a pMX retrovirus expressing the reprogramming factors in different combinations for 48 h. Cells were cultured in standard NSC medium: DMEM/F-12 supplemented with N2 or B27 (GIBCO-BRL), 10 ng/mL epidermal growth factor, 10 ng/mL basic fibroblast growth factor (both from Invitrogen), 50 μg/mL bovine serum albumin (BSA; Fraction V; GIBCO-BRL), and 1× penicillin/streptomycin/glutamine (GIBCO-BRL). After the first mature iNSC clusters were observed, we either manually picked a mature iNSC clump or passaged and seeded whole dishes of cells onto either gelatin- or laminin-coated dishes and changed the medium every 24 h. Animal handling was in accordance with the animal protection guidelines of the Ministry for Primary Industries and the German animal protection laws.
Cell proliferation (WST-1) assays
The number of viable cells was determined by the cell proliferation assay kit, Cell Counting Kit-1 (Roche, Penzberg, Germany), following the standard protocol. The absorbance value at 450 nm was considered to be the relative number of viable cells. Bars represent the mean (±standard deviation [SD]) of 3 different cultures, with n=3 dishes/culture for each concentration.
Immunocytochemistry and terminal deoxynucleotidyl transferase-mediated digoxigenin-dUTP-biotin nick-end labeling (TUNEL) assay
The TUNEL assay using the In Situ Cell Death Detection Kit (Roche) was used to confirm apoptosis by demonstrating apoptotic bodies in primary cortical neurons. Briefly, primary cortical neurons were fixed with 4% formaldehyde and incubated at room temperature for 40 min. This was followed by several rinses in PBS and permeabilization in 0.2% Triton X-100 solution on ice for 5 min. Then 50 μL of TUNEL reaction mixture was added on coverslips before being incubated for 60 min at 37°C in a dark humidified chamber. Finally, the coverslips were incubated with 4′,6-diamidino-2-phenylindole (DAPI, Sigma-Aldrich, St. Louis, MO, USA) for 20 min at room temperature and examined with a model LSM 510 Confocal microscope (Carl Zeiss, Jen, Germany). Data are expressed as the ratio of TUNEL to total nuclei and the ratio of TUNEL to total neurons.
For immunostaining of cortical neuronal cells, cells were plated onto fibronectin-coated glass coverslips in 10% FBS for 4 h. Cells were then fixed with 4% paraformaldehyde. Fixed cells were washed in PBS containing 0.1% BSA and permeabilized with PBS-blocking buffer (PBS with 0.1% BSA and 0.3% Triton X-100) for 40 min at room temperature. Fixed cells were incubated with anti-NeuN primary antibody (Abcam, Newcastle, UK). The primary antibodies were detected with Alexa 596-conjugated goat anti-rabbit immunoglobulin (IgG; Molecular Probes, Eugene, OR, USA), and cyanine-conjugated goat anti-mouse IgG. The cells were rinsed with Tris-HCl buffer, mounted in glycerin, examined, and then photographed using a model LSM 310 microscope (Carl Zeiss). Data are expressed as the ratio of apoptotic neurons to total neurons.
Western blot analysis
Cells were resuspended in ice-cold cell lysis buffer (Cell Signaling Technology, Beverly, MA, USA) with the Protease Inhibitor Cocktail (complete mini tablet, Roche). Samples were incubated on ice for 30 min, and supernatants were recovered by centrifuging at 14 000 rpm at 4°C for 30 min. Protein concentrations were determined with a Bradford assay kit (Bio-Rad protein assay, Bio-Rad, Segrate, Italy) according to the manufacturer’s instructions. Samples of 50 μg of protein per lane were separated on sodium dodecyl sulfate-polyacrylamide gel electrophoresis (PAGE) 10% polyacrylamide NuPAGE gels (Invitrogen, San Diego, CA, USA) and transferred to polyvinylidene difluoride membranes (Millipore, Rome, Italy). The membranes were blocked in Tris-buffered saline with 0.1% Tween-20 and 3% milk, incubated with anti-cleaved caspase-3 (Cell signaling systems), anti-Bcl-2 (Cell signaling systems), and actin (Sigma) antibodies. Next, the membranes were incubated with horseradish peroxidase-conjugated anti-secondary IgG (Invitrogen) antibody and visualized using Super Signal West Pico Chemiluminescent Substrate (Pierce, IL, USA). Densitometric quantification of the bands was performed using ImageJ software (version 1.29x: NIH, Bethesda, MD, USA). Bars represent the mean (±SD) of three replicates.
Enzyme-linked immunosorbent assay (ELISA)
Quantification of vascular endothelial growth factor (VEGF) in conditioned medium was performed using ELISA (R&D systems) [25] according to the manufacturer’s instructions. VEGF expression was analyzed. The optimal density of the color reaction was detected at a wavelength of 450 nm using a chemiluminescence reader. The background signal detected at 450 nm was subtracted from the values. Delta values were normalized to the extinction obtained from standard curves, and protein contents were calculated for each sample.
Statistical analyses
Data are shown as the average and SD. Statistical analyses were performed using the t-test. The level of statistical significance was set at P < 0.05.
Results
Induction of NSC fate on fibroblasts
Stem cell factors with neural-specific transcription factors were used for the direct reprogramming of fibroblasts into iNSCs. The results were obtained within 4–5 weeks of infection using the following 5-factor (5F) combination: Brn4, Sox2, Klf4, c-Myc, and E47 (BSKME). After 5 weeks in neural differentiation medium, NSC cultures included Nestin-positive cells, indicating that fibroblasts were successfully reprogrammed to an NSC-like state with defined factors. Immunofluorescent analyses demonstrated the expression of neural lineage markers (Figure 1).
Figure 1.
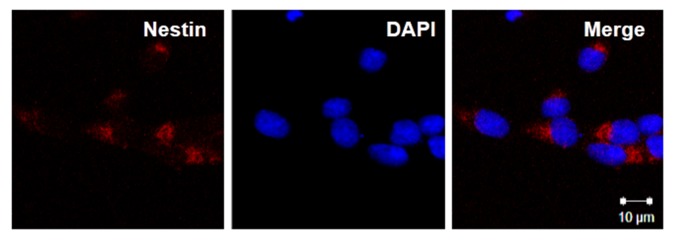
The differentiation potential of induced neural stem cells (iNSCs) in vitro. The differentiation potential of iNSCs was determined by immunocytochemistry with the antibodies neuronal marker (Nestin) and 4′,6-diamidino-2-phenylindole (DAPI), iNSC cultures contained NeuN-positive cells, indicating that fibroblasts were reprogrammed into an NSC-like state.
AAII induced neuronal apoptosis
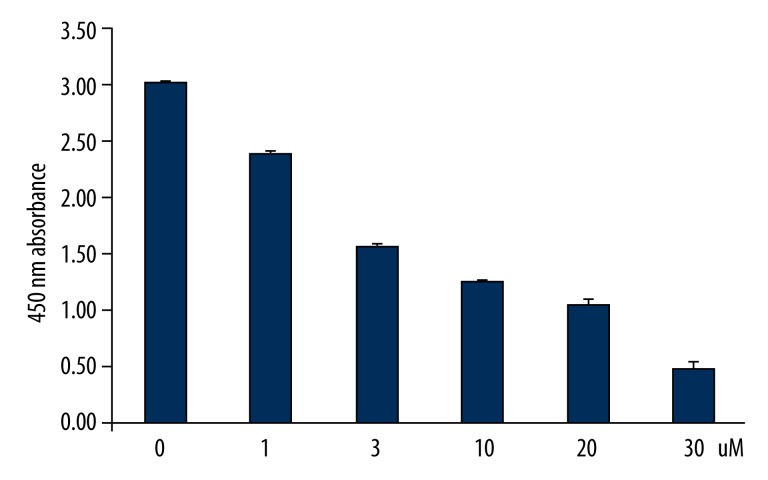
We first determined whether AAII-induced cell death was attributable to apoptosis. Cortical cells were cultured in a neural medium and exposed to AAII (0–30 μM) for 24 h. AAII inhibited the growth of cortical neuronal cells. The effect decreased in a dose-dependent fashion (Figure 2). Thus, the concentration of AAII was 3 μM for all further experiments.
Figure 2.
Dose-dependent neurotoxicity of apoptosis activator II (AAII) in cortical neuronal cells. Increasing concentrations of AAII were added to the culture media of cells, and the toxicity was estimated after 24 h using the WST-1 proliferation assay.
iNSCs were seeded and co-cultured with cortical cells for 48 h. To assess the potential effects of iNSCs, a transwell culture system was used. Cortical cells were exposed to AAII for 24 h. The rates of cortical cell proliferation were used to compare the cells cultured with and without iNSCs. Compared to the cortical neuronal cell cultures with iNSCs, cortical neuronal cells cultured without iNSCs exhibited decreased proliferation, suggesting that cortical neuronal cells benefit from being cultured in the presence of iNSCs (Figure 3).
Figure 3.
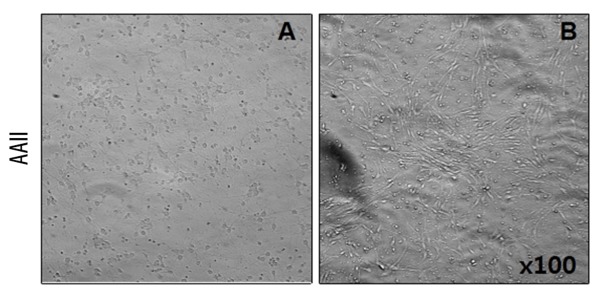
Effect of coculture with iNSCs on cortical neuronal cell proliferation. Cortical neuronal cells were cocultured with induced neuronal stem cells (iNSC). To assess potential effects of the iNSCs, a transwell culture system was used. The rates of proliferation of cortical neuronal cells cultured without iNSCs (A) or cocultured with iNSCs (B) was compared (×100).
Apoptotic cortical cells after culturing with iNSCs
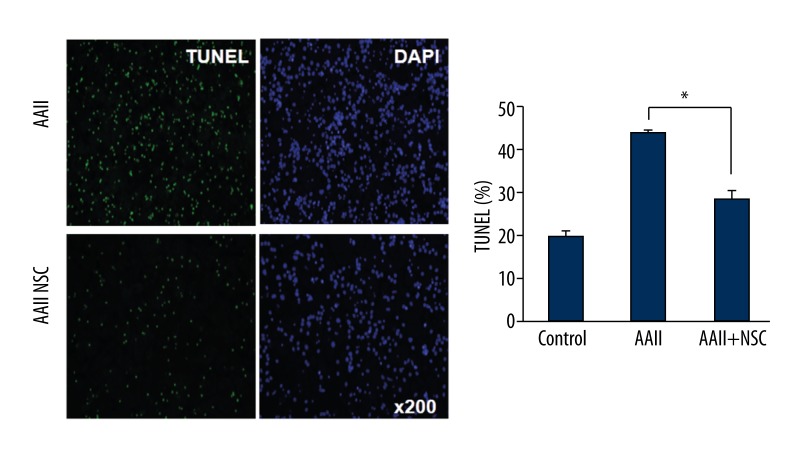
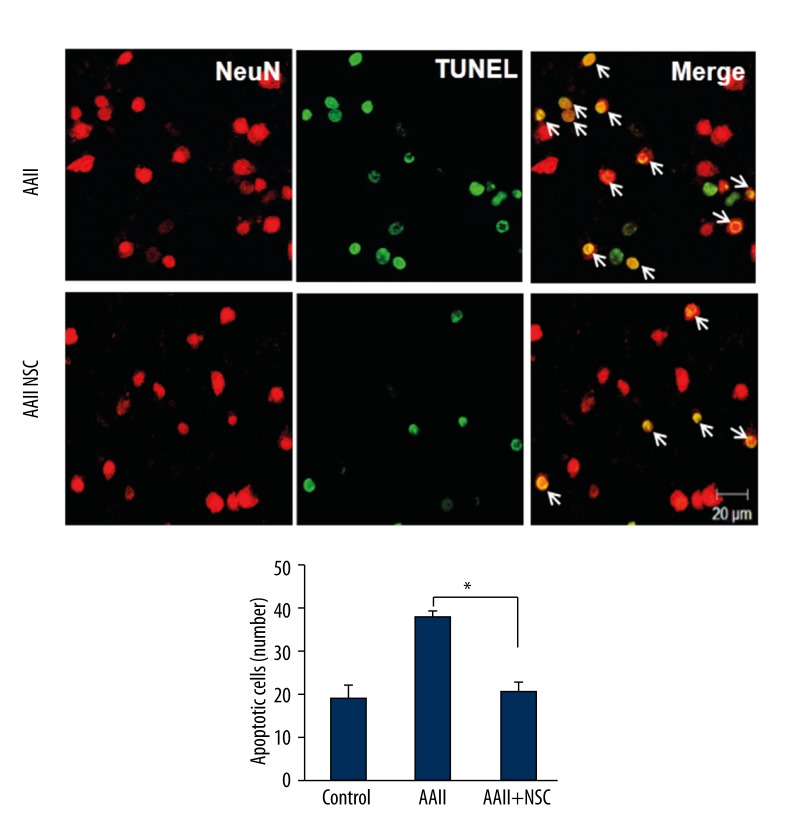
TUNEL is a sensitive assay for visualizing DNA damage in single cells. To assess potential apoptosis, apoptotic cells were detected using TUNEL assays after co-culturing with iNSCs. We assessed the cultures for apoptotic cells (TUNEL) and the specific neuronal marker (NeuN). After incubation, cultured cells were fixed and stained for NeuN and DAPI with TUNEL. Confocal microscopy was used to compare the cells cultured with and without iNSCs. The group of cells cultured with iNSCs had fewer apoptotic cells than the group cultured without iNSCs (P<0.05) (Figure 4). The number of neuronal apoptotic cells was lower in the group cultured with iNSCs than in the group cultured without iNSCs (P<0.05) (Figure 5). A secondary antibody was used to rule out non-specific staining (data not shown).
Figure 4.
Detection of apoptosis in cortical neuronal cells by terminal deoxynucleotidyl transferase mediated digoxigenin-dUTP-biotin nick-end labeling (TUNEL). To examine the apoptotic cells in the cortical neuronal cells, we performed TUNEL staining. The cultured group with induced neuronal stem cells (iNSC) had fewer apoptotic cells compared to the cultured group without iNSCs. Histograms show the average number of TUNEL-positive cells as percentage for each group. * p<0.05.
Figure 5.
Detection of apoptosis in cortical neuronal cells by terminal deoxynucleotidyl transferase mediated digoxigenin-dUTP-biotin nick-end labeling (TUNEL) and neuronal nuclei (NeuN). To confirm the presence of neuronal apoptotic cells in the cortical neuronal cells, we performed double immunocytochemistry with TUNEL. Scale bar 20 um. Histograms show the average number of apoptotic cells as percentage for each group. * p<0.05.
Anti-apoptotic effects are mediated via activation of iNSCs
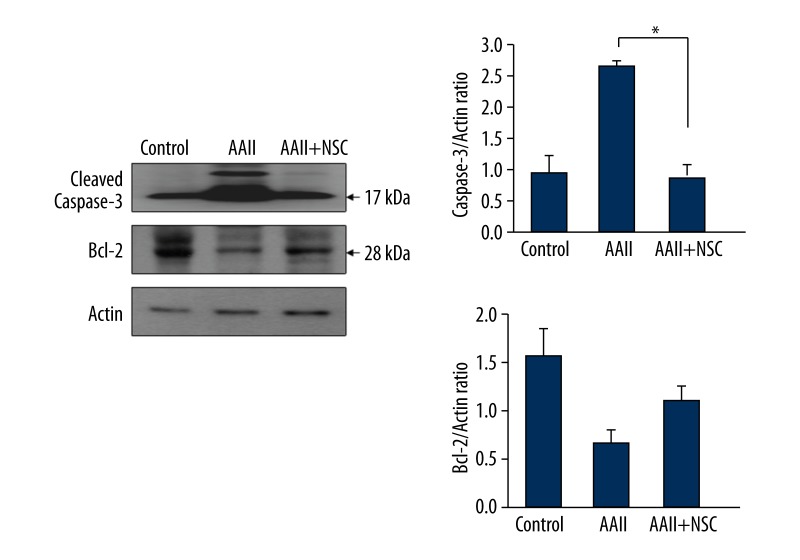
Caspase-3 activation is the key event in apoptotic cell death. To evaluate the apoptotic effect of iNSC co-culturing, we identified the levels of caspase-3 and Bcl-2 by Western blot analyses. The level of caspase-3 was significantly higher in cells cultured without iNSCs than in cells cultured with iNSCs. We also measured the level of Bcl-2, which is considered an important anti-apoptotic protein. The level of Bcl-2 was significantly higher in cells cultured with iNSCs than in cells cultured without iNSCs (Figure 6).
Figure 6.
Anti-apoptotic effects are mediated via activation of induced neural stem cells (iNSCs). To evaluate the anti-apoptotic effect, we performed Western blot analysis of cleaved caspase-3 and B-cell lymphoma 2 (Bcl-2). The cultured group with iNSCs induced a decrease in the level of caspase-3 and an increase in the level of Bcl-2. * p<0.05.
Anti-apoptotic proteins are elevated in the supernatants of co-cultured cells
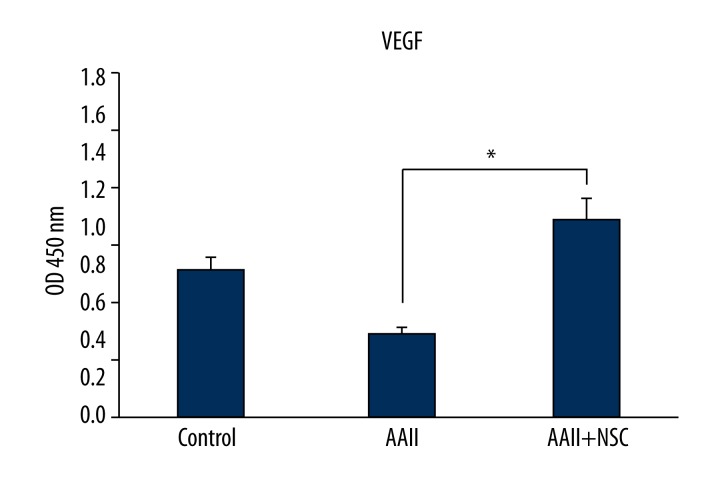
Stem cells release a large number of growth factors and cytokines that are important for the repair of injured tissues [26,27]. To examine whether iNSC co-culturing results in the secretion of essential repair-related growth factors, we performed ELISAs. ELISA confirmed that the levels of VEGF were increased in the cells cultured with iNSCs compared to in the cells cultured without iNSCs (P<0.05) (Figure 7).
Figure 7.
Anti-apoptotic proteins are elevated in supernatants of cocultured cells. To examine whether induced neural stem cells (iNSCs) coculture secrete VEGF for the repair, we performed enzyme-linked immunosorbent assays (ELISA). * p<0.05.
Anti-apoptotic effects are mediated by Akt and ERK phosphorylation
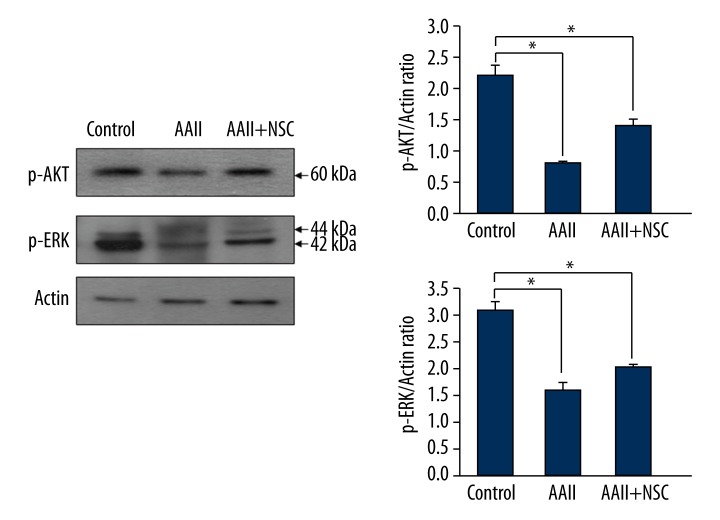
Phosphorylation of Akt inactivates apoptotic factors and phosphorylation of ERK is activated by growth signals. Therefore, Akt and ERK are known to inhibit apoptosis. To examine the underlying mechanisms of these processes, we analyzed a variety of signaling cascades involved in cell proliferation and survival. We performed Western blot analyses for phosphorylated Akt (p-Akt) and phosphorylated ERK (p-ERK) and found that the levels of both p-Akt and p-ERK were significantly higher in cells cultured with iNSCs than in cells cultured without iNSCs (P<0.05 for both) (Figure 8).
Figure 8.
Anti-apoptotic effects are mediated via phosphorylated Akt (p-Akt) and phosphorylated extracellular signal-related kinase (p-ERK). We performed Western blot analyses for p-Akt and p-ERK. The p-Akt and p-ERK were significantly higher in the cultured cells with induced neural stem cells (iNSCs). * p<0.05.
Discussion
Apoptosis of neurons in the central nervous system has been observed in a wide range of animals [28]. Here, we showed that most cortical cell neurons undergoing apoptosis were protected by co-culturing them with iNSCs. We successfully generated iNSCs that had the ability to self-renew and were nearly identical to brain tissue-derived NSCs in terms of their morphology, gene expression profile, and epigenetics [2]. The iNSCs can be engrafted in the stem cell niches of the adult mouse brain, where they can continue to proliferate. Therefore, these cells have potential therapeutic applications. We hypothesized that iNSCs would protect cortical cells against AAII-induced apoptosis. Our findings support our hypothesis and show that iNSCs can prevent cortical neuronal death via mechanisms that are dependent on the activation of Akt and ERK.
Apoptosis is controlled by many apoptosis-related proteins. Caspases are a highly conserved family of cysteine proteases and have been found to play a role in cell apoptosis [29]. Caspase-3 is suggested to be the principal inducer of apoptosis [30]. Bcl-2, an anti-apoptotic member of the protein family, determines the mitochondrial response to apoptotic stimuli and is crucial for determining whether cells survive or undergo apoptosis [31,32]. The present study extends these observations to cortical cells by demonstrating that caspase-3 also plays an important role in the activation of apoptosis. Under the experimental conditions of the present study, cells cultured with iNSCs showed decreased cleaved caspase-3 levels and increased Bcl-2 levels, suggesting that iNSCs exert an anti-apoptotic effect on cortical cells.
Akt and ERK are known to inhibit apoptosis by inhibiting caspase activation. In the present study, we found that iNSCs enhanced the activation of Akt and ERK in cells cultured with iNSCs, suggesting that iNSC-induced activation of Akt and ERK might be correlated with the anti-apoptotic effect.
We also found that iNSCs promote the recovery of cortical cells by suppressing apoptosis. The anti-apoptotic effect may result from the iNSC-mediated increase in the expression of growth factors via activation of the Akt and ERK pathways. Based on these results, iNSCs may exert a protective effect and improve neuronal recovery following apoptosis.
Stem cells secret a number of cytokines and growth factors. Several growth factors and cytokines have demonstrated beneficial effects in the wound-healing process [33]. Mesenchymal stem cells produce growth factors and cytokines, all of which are upregulated during the injury phase of wound healing [34,35]. In this study, iNSC co-culturing resulted in the secretion of essential repair-related growth factors. In addition, VEGF expression was regulated in the group of cells cultured with iNSCs.
Conclusions
We found that iNSCs inhibited AAII-induced cellular apoptosis in an apoptotic model of rat cortical neuronal cells. Further, iNSCs activated Akt and ERK, which are associated with the inhibition of neuronal apoptosis. Thus, treatment with iNSCs may be one way of reducing neuronal loss in brain diseases. Further studies aimed at proving this hypothesis might help in the development of therapeutic agents that can prevent neuronal cell death, and hence cure neurodegenerative diseases.
Acknowledgements
We thank Dong Wook Han PhD. for technical assistance.
Footnotes
Source of support: This work was supported by a grant of the Korean Health Technology R&D Project, Ministry of Health & Welfare (HI12C0337), Republic of Korea
References
- 1.Reynoids BA, Welss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255:1707–10. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- 2.Han DW, Tapia N, Hermann A, et al. Direct reprogramming of fibroblasts into neural stem cells by defined factors. Cell Stem Cell. 2012;6:465–72. doi: 10.1016/j.stem.2012.02.021. [DOI] [PubMed] [Google Scholar]
- 3.Guo F, Lv S, Lou Y, et al. Bone marrow stromal cells enhance the angiogenesis in ischaemic cortex after stroke: involvement of notch signalling. Cell Biol Int. 2012;36:997–1004. doi: 10.1042/CBI20110596. [DOI] [PubMed] [Google Scholar]
- 4.Hicks AU, Lappalainen RS, Narkilahti S, et al. Transplantation of human embryonic stem cellderived neural precursor cells and enriched environment after cortical stroke in rats: cell survival and functional recovery. Eur J Neurosci. 2009;29:562–74. doi: 10.1111/j.1460-9568.2008.06599.x. [DOI] [PubMed] [Google Scholar]
- 5.Burg ED, Remillard CV, Yuan JX. K+ channels in apoptosis. J Membr Biol. 2006;209(1):3–20. doi: 10.1007/s00232-005-0838-4. [DOI] [PubMed] [Google Scholar]
- 6.Taatjes DJ, Sobel BE, Budd RC. Morphological and cytochemical determination of cell death by apoptosis. Histochem Cell Biol. 2008;129(1):33–43. doi: 10.1007/s00418-007-0356-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nagat S. Apoptosis by death factor. Cell. 1997;88:355–65. doi: 10.1016/s0092-8674(00)81874-7. [DOI] [PubMed] [Google Scholar]
- 8.Jacobson MD, Weil M, Raff MC. Programmed cell death in animal development. Cell. 1997;88:347–54. doi: 10.1016/s0092-8674(00)81873-5. [DOI] [PubMed] [Google Scholar]
- 9.Ohyama H, Yamada T, Watanabe I. Cell volume reduction associated with interphase death in rat thymocytes. Radiat Res. 1981;85:333–39. [PubMed] [Google Scholar]
- 10.Thomas N, Bell PA. Glucocorticoid-induced cell size changes and nuclear fragility in rat thymocytes. Mol Cell Endocrinol. 1981;22:71–84. doi: 10.1016/0303-7207(81)90103-9. [DOI] [PubMed] [Google Scholar]
- 11.Sanderson CJ. Morphological aspects of lymphocyte mediated cytotoxicity. In: Clark WR, Golstein P, editors. Mechanisms of Cell Mediate Cytotoxity. Plenum; New York: 1980. pp. 3–21. [Google Scholar]
- 12.Trump BF, Beresesky IK, Osornio-Vargas AR. Cell death and the disease process. The role of calcium. In: Bowen ID, Lockshin RA, editors. Cell Death in Biology and Pathology. Chapman Hall; New York: 1980. pp. 209–42. [Google Scholar]
- 13.Wyllie AH. Clucocorticoid-induced thymocyte apoptosis is associated with endogenous nuclease activation. Nature. 1980;284:555–56. doi: 10.1038/284555a0. [DOI] [PubMed] [Google Scholar]
- 14.Schorey JS, Cooper AM. Macrophage signaling upon mycobacterial infection: the MAP kinases lead the way. Cell Microbiol. 2003;5:133–42. doi: 10.1046/j.1462-5822.2003.00263.x. [DOI] [PubMed] [Google Scholar]
- 15.Cardone MH, Roy N, Stennicke HR, et al. Regulation of cell death protease caspase-9 by phosphorylation. Science. 1998;282:1318–21. doi: 10.1126/science.282.5392.1318. [DOI] [PubMed] [Google Scholar]
- 16.Datta SR, Dudek H, Tao X, et al. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91:231–41. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- 17.Shin DY, Kim GY, Lee JH, et al. Apoptosis induction of human prostate carcinoma DU145 cells by diallyl disulfide via modulation of JNK and PI3K/AKT signaling pathways. Int J Mol Sci. 2012;13:14158–71. doi: 10.3390/ijms131114158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li Y, Jiang Y, Wan Y, et al. Medroxyprogestogen enhances apoptosis of SKOV-3 cells via inhibition of the PI3K/Akt signaling pathway. Biomed Res. 2013;27:43–50. doi: 10.7555/JBR.27.20120051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lubinus M, Meier KE, Smith EA, et al. Independent effects of platelet derived growth factor isoforms on mitogen-activated protein kinase activation and mitogenesis in human dermal fibroblasts. J Biol Chem. 1994;269:9822–25. [PubMed] [Google Scholar]
- 20.Nguyen JT, Wells JA. Direct activation of the apoptosis machinery as a mechanism to target cancer cells. Proc Natl Acad Sci USA. 2003;100:7533–38. doi: 10.1073/pnas.1031631100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nguyen TV, Jayaraman A, Quaglino A, Pike CJ. Androgens selectively protect against apoptosis in hippocampal neurones. J Neuroendocrinol. 2010;22:1013–22. doi: 10.1111/j.1365-2826.2010.02044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alavian KN, Sgadò P, Alberi L, et al. Elevated P75NTR expression causes death of engrailed-deficient midbrain dopaminergic neurons by Erk1/2 suppression. Neural Dev. 2009;4:11. doi: 10.1186/1749-8104-4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jayaraman A, Pike CJ. Progesterone attenuates oestrogen neuroprotection via downregulation of oestrogen receptor expression in cultured neurones. J Neuroendocrinol. 2009;21:77–81. doi: 10.1111/j.1365-2826.2008.01801.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beaudoin GM, III, Lee SH, Singh D, et al. Culturing pyramidal neurons from the early postnatal mouse hippocampus and cortex. Nat Protoc. 2012;7(9):1741–54. doi: 10.1038/nprot.2012.099. [DOI] [PubMed] [Google Scholar]
- 25.Neuhoff S, Moers J, Rieks M, et al. Proliferation, differentiation, and cytokine secretion of human umbilical cord blood-derived mononuclear cells in vitro. Exp Hematol. 2007;35(7):1119–31. doi: 10.1016/j.exphem.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 26.Takai K, Hara J, Matsumoto K, et al. Hepatocyte growth factor is constitutively produced by human bone marrow stromal cells and indirectly promotes hematopoiesis. Blood. 1997;89(5):1560–65. [PubMed] [Google Scholar]
- 27.Labouyrie E, Dubus P, Groppi A, et al. Expression of neurotrophins and their receptors in human bone marrow. Am J Pathol. 1999;154(2):405–15. doi: 10.1016/S0002-9440(10)65287-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roth KA, D’Sa C. Apoptosis and brain development. Ment Retard Dev Disabil Res Rev. 2001;7(4):261–66. doi: 10.1002/mrdd.1036. [DOI] [PubMed] [Google Scholar]
- 29.Dai HY, Liu L, Qin SK, et al. Lobaplatin suppresses proliferation and induces apoptosis in the human colorectal carcinoma cell Line LOVO in vitro. Biomed Pharmacother. 2011;65(3):137–41. doi: 10.1016/j.biopha.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 30.Armstrond RC, Aja TJ, Hoand KD, et al. Activation of the CED3/ICE-related protease-CPP32 in cerebellar granule neurons undergoing apoptosis but not necrosis. Trends Neurosci. 1997;17:553–62. doi: 10.1523/JNEUROSCI.17-02-00553.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hwang L, Choi IY, Kim SE, et al. Dexmedetomidine ameliorates intracerebral hemorrhage-induced memory impairment by inhibiting apoptosis and enhancing brain-derived neurotrophic factor expression in the rat hippocampus. Int J Mol Med. 2013;31:1047–56. doi: 10.3892/ijmm.2013.1301. [DOI] [PubMed] [Google Scholar]
- 32.Cory S, Huang DC, Adams JM. The Bcl-2 family: roles in cell survival and oncogenesis. Oncogene. 2003;22:8590–607. doi: 10.1038/sj.onc.1207102. [DOI] [PubMed] [Google Scholar]
- 33.Werner S, Grose R. Regulation of wound healing by growth factors and cytokines. Physiol Rev. 2003;83:835–70. doi: 10.1152/physrev.2003.83.3.835. [DOI] [PubMed] [Google Scholar]
- 34.Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med. 1999;341:738–46. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- 35.Kane CJ, Hebda PA, Mansbridge JN, Hanawalt PC. Direct evidence for spatial and temporal regulation of transforming growth factor beta 1 expression during cutaneous wound healing. J Cell Physiol. 1999;148:157–73. doi: 10.1002/jcp.1041480119. [DOI] [PubMed] [Google Scholar]