Abstract
Background
Increased amounts of soluble E-cadherin (E-cad) have been found in the serum in various cancers, but the role of serum soluble E-cad in the prognosis of breast cancer patients has not been explored in Asian populations.
Material/Method
Blood samples from 111 consecutive patients diagnosed with breast cancer and 55 healthy controls were investigated. Serum soluble E-cad expression levels were measured by enzyme-linked immunosorbent assay (ELISA) with an immunoassay kit according to the manufacturer’s directions. Kaplan-Meier analyses were used to evaluate the association between serum soluble E-cad expression level and survival. All statistical tests were 2-sided.
Results
The serum levels of soluble E-cad in breast cancer patients were significantly higher than those of the control group (2218.9±319.6 ng/ml vs. 742.8±91.7 ng/ml, p<0.001). Serum levels of soluble E-cad correlated significantly with TNM stage (P=0.007), tumor grade (P=0.03), and lymph node metastasis (P<0.001). Kaplan-Meier analysis with the log-rank test indicated that high serum levels of soluble E-cad had a significant impact on overall survival (55.4% vs. 81.4%; P=0.032) and disease-free survival (36.8% vs. 67.8%; P=0.002) in breast cancer. Multivariate analysis revealed that serum levels of soluble E-cad were independently associated with overall survival and disease-free survival in breast cancer patients.
Conclusions
Serum soluble E-cad level is an independent prognostic factor in Asian breast cancer patients.
MeSH Keywords: Biological Markers, Breast Neoplasms, Cadherins, Prognosis
Background
Breast cancer is the most frequently diagnosed cancer in women and is the leading cause of cancer death in females worldwide, accounting for 23% of all cancer cases and 14% of cancer deaths [1]. Thus, early diagnosis and effective therapies for breast cancer are imperative. Many risk factors related to breast cancer tumorigenesis have been identified, such as female sex, increasing patient age, family history of breast cancer, early menarche, late menopause, older age at first live child-birth, prolonged hormone replacement therapy, previous exposure to therapeutic chest wall irradiation, benign proliferative breast disease, increased mammographic breast density, and genetic mutations such as those of the BRCA1/2 genes [2,3]. However, finding new molecular markers that can predict the effects of therapy and patient survival is still a difficult task.
Epithelial mesenchymal transition (EMT) has been shown to be an important mechanism contributing to cancer invasion and metastasis in epithelial-derived cancers [4,5]. During the process of EMT, epithelial cells lose their polarity and acquire a mesenchymal phenotype, becoming migratory and invasive [6]. E-cadherin (E-cad) belongs to a family of transmembrane glycoproteins, responsible for calcium-dependent cell-to-cell adhesion [7,8]. Soluble E-cad is generated by cleavage of the extracellular domain of the anchored protein (120 kDa), resulting in the release of fragments of 80 kDa [9]. Increased levels of its soluble form in the serum may reflect serious dysfunction of the cell surface molecule. Soluble cadherin fragments may retain specific biologic activities in the tumor environment favoring aggressiveness and metastasis by antagonizing the intact molecule or serving as an anchor for migration when bound to the extracellular matrix [9]. A hallmark of EMT is loss of E-cad, which is considered an epithelial differentiation marker in EMT studies [10]. Downregulation of E-cad is associated with tumor grade and stage and contributes to the transition of adenoma to carcinoma in animal models [11]. Gogali et al. found that serum levels of E-cad were significantly elevated in patients with non-small cell lung cancer (NSCLC) and small cell lung cancer (SCLC) compared with healthy controls, and serum soluble E-cad levels were correlated with presence of distant metastasis in patients with SCLC and NSCLC, suggesting that E-cad may have prognostic value [12]. However, the role of serum soluble E-cad in the prognosis of breast cancer patients has not been explored among Asian population. The aim of this study was to investigate the expression of serum soluble E-cad in breast cancer and its potential as a prognostic marker in Asian populations.
Material and Methods
Patients and controls
The Ethics Committee at Yantaishan Hospital approved the study, in accordance with the Declaration of Helsinki. All individuals gave written informed consent for participation in the study. Blood samples from 111 consecutive patients diagnosed with breast cancer who underwent surgery at the Department of Breast Surgery, Yantaishan Hospital from May 2007 to August 2013 were investigated. The median follow-up time of the patients was 37 months. The patients had not received preoperative treatment such as radiation or chemotherapy. Forty-one patients underwent axillary lymph node resection, 37 patients underwent postoperative chemotherapy, and 29 patients underwent postoperative radiotherapy. Details of clinical and pathological characteristics of the patients are summarized in Table 1. The control group consisted of 55 healthy women whose ages were matched to the patient cohort. Blood samples were collected from the patients at the time of diagnosis, before surgery. The demographic and pathological data, including age, tumor size, axillary lymph node status, cancers stage according to the tumor-node-metastasis classification, histological type, status of ER and progesterone receptor (PR), and human epithelial receptor (HER)-2 protein status were obtained by a review of the patients’ medical records. A structured questionnaire was administered by well-trained interviewers to collect information on demographic and anthropometric characteristics of the enrolled subjects. Disease-free survival (DFS) was defined as the time between surgery and the date of first local recurrence, distant metastasis, second primary cancer of another organ, or death from any cause, whichever appeared first during follow-up. Patients known to be alive with no evidence of disease were censored at the last follow-up date. Overall survival (OS) was defined from surgery to death for any cause, and patients who were alive were censored at date of last follow-up visit.
Table 1.
Clinicopathological implication of soluble E-cad levels in patients with breast cancer.
| Variables | Cases (n) | Soluble E-cad (ng/ml) | |
|---|---|---|---|
| Mean ±SD | p-value | ||
| Age | |||
| <50 years old | 41 | 2189.3±288.4 | 0.54 |
| ≥50 years old | 70 | 2347.1±311.3 | |
| Menopausal status | |||
| Premenopausal | 36 | 2411.4±246.1 | 0.67 |
| Postmenopausal | 75 | 2011.9±328.2 | |
| TNM stage | |||
| I | 41 | 1873.2±128.3 | 0.007 |
| II | 33 | 2319.9±234.5 | |
| III | 37 | 2690.7±310.8 | |
| Grade | |||
| G1 (well differentiated) | 29 | 1933.5±198.9 | 0.03 |
| G2 (moderately differentiated) | 37 | 2355.9±320.8 | |
| G3 (poorly differentiated) | 45 | 2509.5±190.6 | |
| Histological type | |||
| Ductal invasive carcinoma | 47 | 2183.3±109.2 | 0.06 |
| Lobular invasive carcinoma | 33 | 2297.4±289.3 | |
| Others | 31 | 2210.9±299.8 | |
| Lymphonodus status | |||
| Positive | 49 | 2691.9±311.9 | <0.001 |
| Negative | 62 | 1689.4±208.3 | |
| ER status | |||
| Negative | 58 | 2191.5±100.4 | 0.11 |
| Positive | 53 | 2295.1±399.1 | |
| PR status | |||
| Negative | 67 | 2298.3±298.2 | 0.13 |
| Positive | 44 | 2197.1±100.2 | |
| HER2 status | |||
| Negative | 55 | 2107.6±188.3 | 0.09 |
| Positive | 56 | 2322.4±311.1 | |
Assay for serum levels of soluble E-cad
The samples were centrifuged and serum was kept at −80°C until use. Serum soluble E-cad expression levels were measured by enzyme-linked immunosorbent assay (ELISA) with an immunoassay kit (Miltenyi, Germany) according to the manufacturer’s directions. This test is based on the capture of E-cad using a solid-phase absorbed primary monoclonal antibody, followed by subsequent detection using a labeled secondary monoclonal antibody to E-cad. All samples were analyzed in duplicates. The optical density (OD) at 450 nm was determined. The standard curves were established with OD450 as the Y axis and the concentration of standard substance as the X axis. The level of protein was obtained through a standard curve.
Statistical analysis
The relationships between clinicopathologic variables and serum soluble E-cad expression were examined by χ2 analyses. The Receiver Operating Characteristic (ROC) curve was used for the prediction of cut-off values of the markers. Survival curves were calculated using the Kaplan-Meier method and compared with the log-rank test. Factors shown to be of prognostic significance in the univariate Cox regression model were evaluated in a multivariate Cox regression model. For all analyses, a P value less than 0.05 was considered statistically significant. All analyses were performed using SPSS18.0 (SPSS, Chicago, IL) and GraphPad5 software. All statistical tests were 2-sided.
Results
Serum levels of soluble E-cad and its association with clinicopathological characteristics
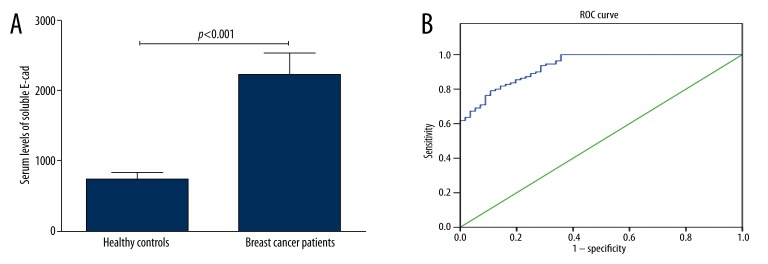
The serum levels of soluble E-cad in breast cancer patients were significantly higher than those of the control group (2218.9±319.6 ng/ml vs. 742.8±91.7ng/ml, p<0.001, Figure 1A). Since soluble E-cad level was significantly elevated in the serum of breast cancer patients when compared with healthy controls, ROC curve analysis was used to explore the potential of using serum soluble E-cad as a biomarker for breast cancer. The analysis revealed that serum level of soluble E-cad was a potential biomarker for differentiating breast cancer patients from controls and was under the ROC curve (AUC) of 0.935 [95% confidence interval (CI)=0.900–0.969] (Figure 1B). At a cut-off value of 1239.3 ng/ml, the sensitivity was 79.1% and the specificity was 89.3%. The 95th percentile of soluble E-cad values for the healthy control group, 1246.7 ng/ml, was used as the cutoff value for the following analyses. We then investigated the association between serum levels of soluble E-cad and clinicopathological characteristics. The relationships between serum levels of soluble E-cad and clinicopathological characteristics of patients with breast cancer are shown in Table 1. Serum levels of soluble E-cad correlated significantly with TNM stage (P=0.007), tumor grade (P=0.03), and lymph node metastasis (P<0.001). There was no significant correlation of serum soluble E-cad levels with age (p=0.54), menopausal status (p=0.67), histological type (p=0.06), expression of ER (p=0.11), expression of PR (p=0.13), and expression of HER2 (P=0.09).
Figure 1.
The serum soluble E-cad levels. (A) The serum levels of soluble E-cad in breast cancer patients were significantly higher than those of the control group (2218.9±319.6 ng/ml vs. 742.8±91.7 ng/ml, p<0.001). (B) ROC curve for the serum soluble E-cad differentiating breast cancer and control group. The analysis revealed that serum level of soluble E-cad was a potential biomarker for differentiating breast cancer patients from controls and was under the ROC curve of 0.935 [95% confidence interval (CI)=0.900–0.969].
Serum levels of soluble E-cad and clinicopathological factors associated with disease-free survival and overall survival in patients with breast cancer
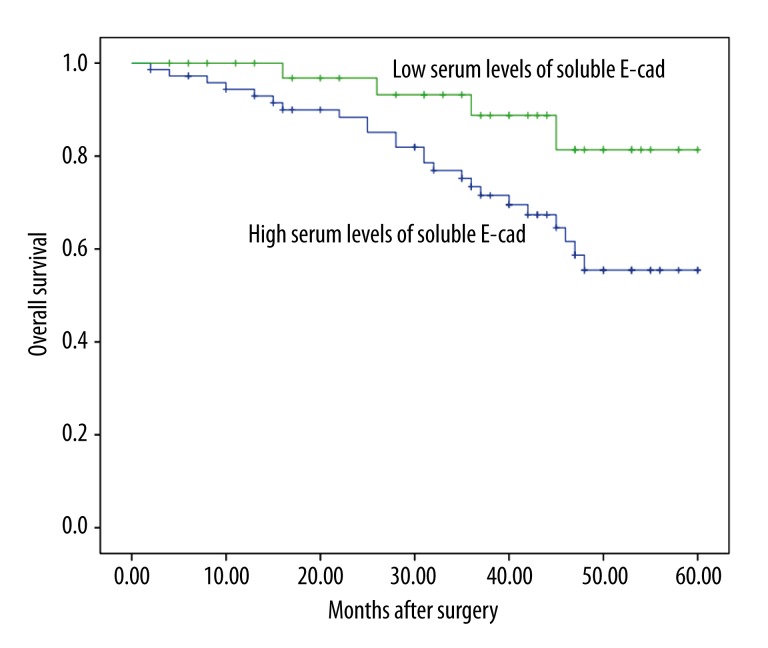
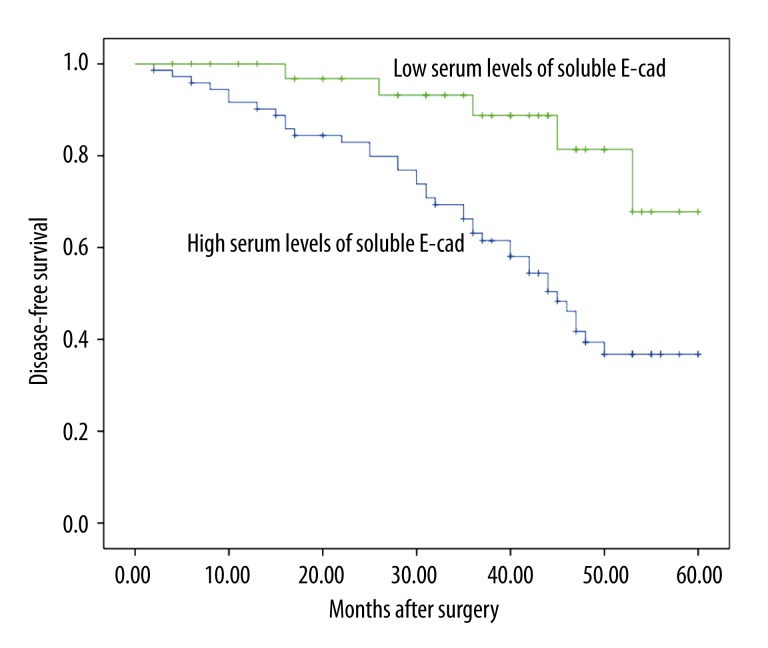
Using a cutoff value of 1246.7 ng/ml for soluble E-cad, patients with breast cancer (n=111) were divided into a high expression group (n=77) and a low expression group (n=34). Kaplan-Meier analysis with the log-rank test indicated that high serum levels of soluble E-cad had a significant impact on overall survival (55.4% vs. 81.4%; P=0.032; Figure 2) and disease-free survival (36.8% vs. 67.8%; P=0.002; Figure 3) in breast cancer. Univariate and multivariate analyses were utilized to evaluate whether the serum levels of soluble E-cad and various clinicopathological features were independent prognostic parameters of breast cancer patients’ outcomes. Multivariate analysis revealed that serum levels of soluble E-cad (HR=2.97, 95% CI: 1.28–9.11; P=0.017), TMN stage (HR=3.31, 95% CI: 1.38–7.29; P=0.02), and lymph node status (HR=2.98, 95% CI: 1.86–10.02; P=0.012) were independently associated with the overall survival (Table 2), and that serum levels of soluble E-cad (HR=3.38, 95% CI: 2.47–11.89; P=0.009), tumor grade (HR=3.19, 95% CI: 2.13–10.02; P=0.003), and lymph node status (HR=4.12, 95% CI: 1.98–15.21; P=0.007) were independent prognostic factors for disease-free survival in breast cancer patients (Table 2).
Figure 2.
Kaplan-Meier analysis with the log-rank test indicated that high serum levels of soluble E-cad had a significant impact on overall survival (55.4% vs. 81.4%; P=0.032) in breast cancer.
Figure 3.
Kaplan-Meier analysis with the log-rank test indicated that high serum levels of soluble E-cad had a significant impact on disease-free survival (36.8% vs. 67.8%; P=0.002) in breast cancer.
Table 2.
Clinicopathological factors associated with disease-free survival and overall survival in patients with breast cancer.
| Variable | Disease-free survival | Overall survival | ||||||
|---|---|---|---|---|---|---|---|---|
| Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | |||||
| P-value | Hazard ratio | 95% CI | P-value | P-value | Hazard ratio | 95% CI | P-value | |
| Age | 0.76 | 0.93 | 0.29–2.24 | 0.71 | 0.62 | 1.21 | 0.31–2.36 | 0.66 |
| Menopausal status | 0.35 | 1.11 | 0.26–3.12 | 0.38 | 0.29 | 1.38 | 0.47–3.11 | 0.32 |
| TNM stage | 0.07 | 2.38 | 0.92–6.77 | 0.06 | 0.04 | 3.31 | 1.38–7.29 | 0.02 |
| Grade | 0.01 | 3.19 | 2.13–10.02 | 0.003 | 0.08 | 2.39 | 0.87–6.32 | 0.09 |
| Histological type | 0.12 | 0.79 | 0.23–3.19 | 0.24 | 0.33 | 1.28 | 0.38–3.03 | 0.67 |
| Lymphonodus status | 0.03 | 4.12 | 1.98–15.21 | 0.007 | 0.009 | 2.98 | 1.86–10.02 | 0.012 |
| ER status | 0.34 | 1.67 | 0.57–2.78 | 0.18 | 0.19 | 1.39 | 0.38–2.93 | 0.31 |
| PR status | 0.38 | 1.93 | 0.78–3.19 | 0.21 | 0.27 | 1.71 | 0.27–3.12 | 0.29 |
| HER2 status | 0.41 | 1.98 | 0.68–2.99 | 0.19 | 0.32 | 1.38 | 0.82–3.58 | 0.11 |
| Serum soluble E-cad level | 0.01 | 3.38 | 2.47–11.89 | 0.009 | 0.03 | 2.97 | 1.28–9.11 | 0.017 |
Discussion
In recent years, several biomarkers of breast cancer have been proposed, including Ki-67, Bcl-2, HER-2, ER, PR, P53, PAR1, and FGFR1. However, the biological potential of breast cancer is difficult to predict completely with the use of these biomarkers. Therefore, it is urgent to identify novel biomarkers of clinical stage, prognosis, and treatment to improve the survival rate of breast cancer patients [13–15].
EMT, defined by loss of epithelial characteristics and acquisition of mesenchymal phenotype, is a key developmental program that the cancer cells often activate, which leads to invasion and metastasis [16]. EMT can help cancer cells detach from a solid tumor and intravasate. In breast cancer, the phenomenon of EMT has been shown to take place in highly aggressive tumors with a basal-like phenotype, which frequently show overexpression of mesenchymal proteins, including vimentin and smooth-muscle-actin, and cadherin switching [17,18]. One of the key characteristics of EMT is loss of E-cad, which is a marker of epithelial morphology [16,19]. E-cad is a member of the large family of Ca++-dependent transmembrane glycoproteins that mediate cell-cell adhesion. It is a single-span transmembrane glycoprotein of 5 repeats and a cytoplasmic domain, which is expressed primarily in epithelial cells. Its extracellular domain has Ca++-dependent homophillic adhesion function, and its cytoplasmic domain contains a juxtamembrane domain and β-catenin-binding domain [20,21]. Epigenetic processes involving hypermethylation and/or transcriptional alternations such as deacetylation have been shown to be responsible for downregulation or loss of E-cad in tumors [20]. Several transcriptional repressors of E-cad have been identified, including the zinc-finger factors, snail, slug, ZEB1, ZEB2, and bHLH (basic helix-loop-helix) factors such as E12/E47 and twist [20,22,23]. Mechanisms for loss of E-cadherin-mediated adhesions include inactivating mutations, epigenetic silencing, proteolytic cleavage, endocytosis, proteasomal degradation, and increased expression of nonepithelial cadherins [24,25].
Increased amounts of soluble E-cad have been found in the serum or urine in various cancers, including gastric cancer, bladder cancer, prostate cancer, ovarian cancer, lung cancer, and colorectal cancer, as well as in melanoma [9,12,26–29]. Brouxhon et al. found that soluble E-cad was associated with and activated many of the oncogenic pathways that tumors utilized for growth and survival, as well as serum levels in patients correlated with clinical response, suggesting that targeted therapy against soluble E-cad in combination with other therapies might offer a novel therapeutic strategy for the treatment of breast cancers [30]. Hofmann et al. investigated whether serum soluble E-cad levels could assess prognosis after preoperative systemic chemotherapy (PST) in patients with locally advanced breast cancer. They finally found that serum soluble E-cad levels might be a marker predicting response to PST for patients with breast cancer [31]. However, the role of serum soluble E-cad in the prognosis of breast cancer patients has not been explored in Asian populations. To the best of our knowledge, this is the first study to report a correlation between soluble E-cad and the prognosis of breast cancer in Asians. This study has 3 findings. Firstly, the serum soluble E-cad levels in breast cancer patients were significantly higher than in healthy controls, and high serum soluble E-cad level was found to significantly correlate with TNM stage, tumor grade, and lymph node metastasis. Secondly, Kaplan-Meier analysis with the log-rank test indicated that high serum levels of soluble E-cad had a significant impact on overall survival and disease-free survival in breast cancer. Thirdly, multivariate analysis revealed that serum level of soluble E-cad was independently associated with the overall survival and disease-free survival in breast cancer. All these results suggest that serum level of soluble E-cad can predict prognosis of breast cancer patients after surgery.
Conclusions
This study demonstrated that serum levels of soluble E-cad may be an independent prognostic factor for predicting poor prognosis in breast cancer patients. This finding could be used to stratify patients and help select appropriate therapeutic regimens for breast cancer patients.
Footnotes
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Source of support: Departmental sources
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Friebel TM, Domchek SM, Rebbeck TR. Modifiers of Cancer Risk in BRCA1 and BRCA2 Mutation Carriers: Systematic Review and Meta-Analysis. J Natl Cancer Inst. 2014;106(6):dju091. doi: 10.1093/jnci/dju091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fasching PA, Ekici AB, Wachter DL, et al. Breast Cancer Risk – From Genetics to Molecular Understanding of Pathogenesis. Geburtshilfe Frauenheilkd. 2013;73:1228–35. doi: 10.1055/s-0033-1360178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li LP, Lu CH, Chen ZP, et al. Subcellular proteomics revealed the epithelial-mesenchymal transition phenotype in lung cancer. Proteomics. 2011;11:429–39. doi: 10.1002/pmic.200900819. [DOI] [PubMed] [Google Scholar]
- 5.Soltermann A, Tischler V, Arbogast S, et al. Prognostic significance of epithelial-mesenchymal and mesenchymal-epithelial transition protein expression in non-small cell lung cancer. Clin Cancer Res. 2008;14:7430–37. doi: 10.1158/1078-0432.CCR-08-0935. [DOI] [PubMed] [Google Scholar]
- 6.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–28. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang G, Dong W, Shen H, et al. A comparison of Twist and E-cadherin protein expression in primary non-small-cell lung carcinoma and corresponding metastases. Eur J Cardiothorac Surg. 2011;39:1028–32. doi: 10.1016/j.ejcts.2011.01.023. [DOI] [PubMed] [Google Scholar]
- 8.Liu Y, Zhao J, Zhang PY, et al. MicroRNA-10b targets E-cadherin and modulates breast cancer metastasis. Med Sci Monit. 2012;18(8):BR299–308. doi: 10.12659/MSM.883262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Noe V, Fingleton B, Jacobs K, et al. Release of an invasion promoter E-cadherin fragment by matrilysin and stromelysin-1. J Cell Sci. 2001;114:111–18. doi: 10.1242/jcs.114.1.111. [DOI] [PubMed] [Google Scholar]
- 10.Zheng G, Lyons JG, Tan TK, et al. Disruption of E-cadherin by matrix metalloproteinase directly mediates epithelial-mesenchymal transition downstream of transforming growth factor-beta1 in renal tubular epithelial cells. Am J Pathol. 2009;175:580–91. doi: 10.2353/ajpath.2009.080983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2:442–54. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- 12.Gogali A, Charalabopoulos K, Zampira I, et al. Soluble adhesion molecules E-cadherin, intercellular adhesion molecule-1, and E-selectin as lung cancer biomarkers. Chest. 2010;138:1173–79. doi: 10.1378/chest.10-0157. [DOI] [PubMed] [Google Scholar]
- 13.Min W, Wang B, Li J, et al. The expression and significance of five types of miRNAs in breast cancer. Med Sci Monit. 2014;20:97–104. doi: 10.12659/MSMBR.891246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arana S, Vasquez-Del-Aguila J, Espinosa M, et al. Lymphatic mapping could not be impaired in the presence of breast carcinoma and coexisting small lymphocytic lymphoma. Am J Case Rep. 2013;14:322–25. doi: 10.12659/AJCR.884000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tvrdik D, Skalova H, Dundr P, et al. Apoptosis – associated genes and their role in predicting responses to neoadjuvant breast cancer treatment. Med Sci Monit. 2012;18(1):BR60–67. doi: 10.12659/MSM.882205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guarino M, Rubino B, Ballabio G. The role of epithelial-mesenchymal transition in cancer pathology. Pathology. 2007;39:305–18. doi: 10.1080/00313020701329914. [DOI] [PubMed] [Google Scholar]
- 17.Blick T, Widodo E, Hugo H, et al. Epithelial mesenchymal transition traits in human breast cancer cell lines. Clin Exp Metastasis. 2008;25:629–42. doi: 10.1007/s10585-008-9170-6. [DOI] [PubMed] [Google Scholar]
- 18.Sarrio D, Rodriguez-Pinilla SM, Hardisson D, et al. Epithelial-mesenchymal transition in breast cancer relates to the basal-like phenotype. Cancer Res. 2008;68:989–97. doi: 10.1158/0008-5472.CAN-07-2017. [DOI] [PubMed] [Google Scholar]
- 19.Guarino M. Epithelial-mesenchymal transition and tumour invasion. Int J Biochem Cell Biol. 2007;39:2153–60. doi: 10.1016/j.biocel.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 20.Baranwal S, Alahari SK. Molecular mechanisms controlling E-cadherin expression in breast cancer. Biochem Biophys Res Commun. 2009;384:6–11. doi: 10.1016/j.bbrc.2009.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Roy F, Berx G. The cell-cell adhesion molecule E-cadherin. Cell Mol Life Sci. 2008;65:3756–88. doi: 10.1007/s00018-008-8281-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vesuna F, van Diest P, Chen JH, Raman V. Twist is a transcriptional repressor of E-cadherin gene expression in breast cancer. Biochem Biophys Res Commun. 2008;367:235–41. doi: 10.1016/j.bbrc.2007.11.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peinado H, Ballestar E, Esteller M, Cano A. Snail mediates E-cadherin repression by the recruitment of the Sin3A/histone deacetylase 1 (HDAC1)/HDAC2 complex. Mol Cell Biol. 2004;24:306–19. doi: 10.1128/MCB.24.1.306-319.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cavallaro U. N-cadherin as an invasion promoter: a novel target for antitumor therapy? Curr Opin Investig Drugs. 2004;5:1274–78. [PubMed] [Google Scholar]
- 25.Van Aken E, De Wever O, Correia da Rocha AS, Mareel M. Defective E-cadherin/catenin complexes in human cancer. Virchows Archiv. 2001;439:725–51. doi: 10.1007/s004280100516. [DOI] [PubMed] [Google Scholar]
- 26.Kuefer R, Hofer MD, Zorn CS, et al. Assessment of a fragment of e-cadherin as a serum biomarker with predictive value for prostate cancer. Br J Cancer. 2005;92:2018–23. doi: 10.1038/sj.bjc.6602599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilmanns C, Grossmann J, Steinhauer S, et al. Soluble serum E-cadherin as a marker of tumour progression in colorectal cancer patients. Clin Exp Metastasis. 2004;21:75–78. doi: 10.1023/b:clin.0000017204.38807.22. [DOI] [PubMed] [Google Scholar]
- 28.Gadducci A, Ferdeghini M, Cosio S, et al. Preoperative serum E-cadherin assay in patients with ovarian carcinoma. Anticancer Res. 1999;19:769–72. [PubMed] [Google Scholar]
- 29.Durkan GC, Brotherick I, Mellon JK. The impact of transurethral resection of bladder tumour on serum levels of soluble E-cadherin. BJU Int. 1999;83:424–28. doi: 10.1046/j.1464-410x.1999.00972.x. [DOI] [PubMed] [Google Scholar]
- 30.Brouxhon SM, Kyrkanides S, Teng X, et al. Soluble-E-cadherin activates HER and IAP family members in HER2+ and TNBC human breast cancers. Mol Carcinog. 2013 doi: 10.1002/mc.22048. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hofmann G, Balic M, Dandachi N, et al. The predictive value of serum soluble E-cadherin levels in breast cancer patients undergoing preoperative systemic chemotherapy. Clin Biochem. 2013;46:1585–89. doi: 10.1016/j.clinbiochem.2013.06.010. [DOI] [PubMed] [Google Scholar]