Abstract
The impact of syphilis reverse sequence screening has not been evaluated in community outreach. Using reverse sequence screening in neighborhoods identified with geographic information systems, we found that among 239 participants, 45 (19%) were seropositive. Of these, 3 (7%) had untreated syphilis, 33 (73%) had previously treated syphilis infection, and 9 (20%) had negative nontreponemal test results.
Highly effective curative strategies for syphilis have been available for more than 50 years, but the disease is far from eradication in the United States. In 2008, there were an estimated 117,000 prevalent syphilis infections in the United States and more than 50,000 incident infections.1 Despite a decrease in the national rates of congenital syphilis in 2010, there continues to be excess hospitalization costs and morbidity associated with maternal-to-child syphilis transmission.2
Persistence of the epidemic has been attributed to “high-transmitters” concentrated in specific populations (e.g., men who have sex with men) and/or geographic core areas.3,4 Current public health efforts focus on targeted syphilis testing for high-risk persons in both clinical and community settings.
The mainstay of syphilis diagnosis is serology, traditionally performed by screening with a nontreponemal antibody test, such as rapid plasma reagin or toluidine red unheated serum test (TRUST), followed by confirmation with a treponemal antibody assay (e.g., Treponema pallidum particle agglutination and syphilis-specific IgG enzyme-linked immunosorbent assay [EIA]). Many US laboratories have adopted a reverse algorithm for syphilis screening, primarily motivated by efforts to increase efficiency and reduce costs with high throughput screening.5 With this reverse syphilis screening (RSS) algorithm, samples are screened with an automated treponemal assay such as the EIA followed by nontreponemal testing on samples with positive treponemal results.6 If the nontreponemal test result is negative, a second treponemal specific test is recommended for further evaluation.7
To our knowledge, the application of the RSS algorithm in community screening programs targeting high-risk individuals has not been studied. Implementation of this algorithm faces operational barriers in health departments, which have limited resources to conduct patient follow-up if a second treponemal test is needed. Although public health programs may have disease intervention specialists (DIS) who can trace patients with syphilis to ensure treatment and partner notification, their priorities are focused on probable infectious cases to prevent disease transmission.8 Furthermore, current US budget reductions have made even these resources tenuous.9
The aim of this study was to assess the feasibility and impact of applying the RSS algorithm in a high-risk community population identified by geographic information systems incidence mapping. Specifically, we sought to quantify the proportion of clinically significant (untreated and/or newly diagnosed) syphilis infections identified by the treponemal EIA as part of a public health program.
Data for this study were collected with previously described geographic information systems methods.10 Briefly, residential addresses of incident tuberculosis, HIV, and syphilis cases in Wake County, North Carolina, between 2005 and 2007 were transformed into a kernel density map. Community-based syphilis screenings were conducted with Wake County Health and Human Services staff from February 6, 2009, to March 11, 2011, in areas with the highest densities of all three diseases. Screening sites were chosen based on availability of an area within the site to administer confidential surveys and acceptability by the site owner. Small incentives (snacks and a US $5 grocery gift card) were used to attract participants. Persons were excluded if they were 18 years or younger, did not speak English/Spanish, or previously participated in the study. Eligible participants were asked to complete a verbally administered questionnaire including comorbidities, perceived risk of infection and health care use, which have been previously described.11 Participants were screened for syphilis symptoms and asked about recent contacts to syphilis cases.
Screening was performed with the T. pallidum–specific IgG EIA (Trep-Sure; Trinity Biotech, Jamestown, NY). Specimens with positive EIA results had reflex TRUST testing performed. Health department records were reviewed for all cases with positive EIA results for evidence of prior infection and/or therapy and previous nontreponemal antibody titers.
Participants were instructed to come to the health department or return to the screening site for results. If the results from both the EIA and the TRUST were positive with no documentation of prior therapy, standard treatment of syphilis was offered. Participants who did not return for test results were actively pursued by DIS according to standard protocols based on patient’s age or titers.8 All participants with positive test results were followed up for 6 months to determine whether they received appropriate treatment.
The primary outcomes of the study were the proportion of positive treponemal EIAs and the proportion of untreated and/or newly diagnosed syphilis infections in our study population. Significance tests for comparisons between seropositive and seronegative participants and between patients with and without prior documented treatment included the 2-sample t test and Mann-Whitney test for continuous patient characteristics, and Pearson χ2 or Fisher exact test for categorical patient characteristics. SAS software, version 9.2 (SAS Institute, Cary, NC) was used for all statistical analysis.
The study protocol was approved by the Duke University institutional review board and the Wake County Human Rights–Consumer Protection Committee. Written informed consent was obtained from each participant.
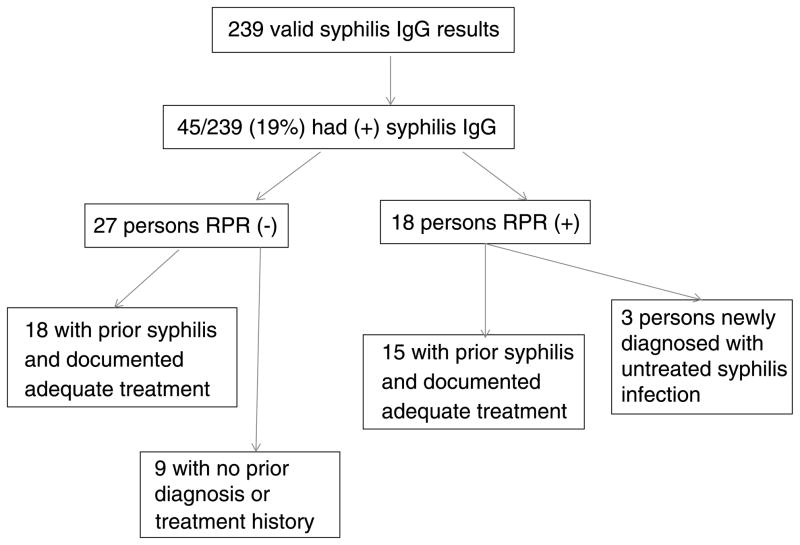
Of 247 participants recruited, 240 had blood drawn (2 persons left before blood draw and 5 persons had phlebotomy failures). One participant had an indeterminate treponemal EIA result. Among 239 participants with valid EIA results, 45 (19% [95% confidence interval, 14%–24%]) were seropositive (Fig. 1). Seropositive participants were older (mean age, 49.5 ± 10.5 years) than seronegative participants (mean age, 40.9 ± 12.9 years; P < 0.01). There was also a higher proportion of non-Hispanic African Americans (93% vs. 73%, P < 0.01) and US-born persons (93% vs. 79%, P = 0.03) in seropositive participants than in seronegative participants (Table 1).
Figure 1.
Follow-up of syphilis IgG-seropositive participants, Wake County, NC (2009–2011). Of 45 treponemal IgG-seropositive participants, 18 (40%) had positive nontreponemal test results. Of these 18 participants, 15 (83%) had a documented history of syphilis with adequate therapy. Only 3 participants, all asymptomatic, were newly diagnosed as having syphilis and had no documented history of previous treatment. None of the participants with positive IgG and negative TRUST results underwent treatment.
TABLE 1.
Characteristics of Eligible Study Participants by Syphilis Serostatus in Wake County, NC (2009–2012)
| Characteristic* | All Participants (n = 239) | Syphilis IgG Positive (n = 45) | Syphilis IgG Negative (n = 194) | P† |
|---|---|---|---|---|
| Mean age (SD), y | 42.5 (12.9) | 49.5 (10.5) | 40.9 (12.9) | G0.01 |
| Race‡ | 0.02 | |||
| Black, non-Hispanic | 178 (77) | 42 (93) | 136 (73) | G0.01 |
| White, non-Hispanic | 11 (5) | 0 | 11 (6) | 0.13 |
| Hispanic | 42 (18) | 3 (7) | 39 (21) | 0.03 |
| Native American | 3 (1) | 0 | 3 (2) | 1.0 |
| Sex | 0.50 | |||
| Male | 140 (59) | 24 (53) | 116 (60) | |
| Female | 99 (41) | 21 (47) | 78 (40) | |
| Foreign-born | 43 (18) | 3 (7) | 40 (21) | 0.03 |
| Prior incarceration | 158 (66) | 34 (76) | 124 (64) | 0.16 |
| Homeless (ever) | 90 (38) | 21 (47) | 69 (36) | 0.18 |
| Median (IQR) no. sexual partners in previous year | 1 (1–3) | 1 (1–3) | 1 (1–2) | 0.54 |
Values are presented as n (%) or mean (SD) or median (IQR).
Totals for each variable may not add up to 100% secondary to observations with missing data.
Significance tests for comparisons between syphilis seropositive and seronegative results based on 2-sample t test for parametric, Mann-Whitney test for nonparametric continuous patient characteristics, and Pearson χ2 or Fisher exact test for categorical patient characteristics.
Because participants were allowed to report more than 1 race/ethnicity, total categories may exceed 100%.
Of the 18 participants with both positive treponemal EIA and TRUST results, 15 (83%) had a documented history of treated syphilis. Only three participants, all asymptomatic, were newly diagnosed as having syphilis (Fig. 1). Two of these patients had a TRUST of 1:2, and 1 had a TRUST of 1:1, which likely represented cases of late latent syphilis rather than early infection. Unfortunately, repeated attempts to contact these patients were not successful after 6 months of follow-up.
Among the 45 with positive treponemal EIA result, 27 individuals (60%) had negative TRUST results (Fig. 1). Eighteen of these 27 persons had documented previous syphilis infection with prior therapy. However, 9 had no prior syphilis diagnosis and were asymptomatic without known contact to an early syphilis case. DIS attempted to contact these 9 persons, but none presented with symptoms or for additional syphilis testing by the end of the study period.
The 9 of 27 participants with discordant results and no history of syphilis had similar characteristics to the 18 of 27 participants with a history of treated syphilis, with respect to age, proportion of black non-Hispanic race, proportion of men, history of incarceration, and homelessness. However, the 9 participants reported a lower number of sexual partners (median, 1; interquartile range [IQR], 0–1) compared with those with documented prior infection/therapy (median, 2; IQR, 1–3; P = 0.05).
In this study using the RSS algorithm among a high-risk community population, we found 19% seropositivity with the treponemal EIA. This seropositivity rate was higher than rates in other studies (2%–4%)7,12 in large part because we screened a targeted high-risk community population rather than a clinic population.
Although the RSS algorithm facilitated detection of newly diagnosed syphilis (3/45, or 7%) among those tested, we found a high prevalence of previously treated syphilis infections (33/45, or 73%). We identified 12 persons with positive EIA results and no syphilis history using health department records. However, despite multiple attempts by DIS to contact these persons, none presented for therapy. Implementing newer rapid syphilis tests that incorporate both treponemal and nontreponemal antibodies may improve engagement in care in these high-risk populations.13,14
This study highlights how the RSS algorithm may not be suitable for screening a population with a high prevalence of previously treated syphilis infections. Although our study targeted community participants, similar conclusions could be drawn for other high-prevalence populations, such as HIV or sexually transmitted disease clinic patients, where the RSS algorithm may be more costly than beneficial. RSS may also not be ideal in resource-limited public health programs whose priorities are to ensure treatment for individuals with early or infectious syphilis.
The study was limited by its small sample size and the nonrandom fashion in which participants elected to participate. It is difficult to know whether the high prevalence of prior syphilis infection in our study reflected the prevalence in the communities or the higher-risk individuals who attended the community-based voluntary screenings. Limited public health resources prevented repeat testing with a second treponemal test to distinguish false-positive treponemal EIA results,7 but given the high documented prevalence of disease in the population, most of the positive test results likely represented true positives. In addition, we did not have the resources to conduct nontreponemal tests in addition to the treponemal EIAs for screening, which would have facilitated direct comparisons between the RSS versus traditional algorithms with respect to the proportion of new or prior syphilis infections detected.
Our data suggest that using the RSS for a targeted community screening program among a high-prevalence population will primarily find the “footprint” of old syphilis, rather than detect cases of public health interest. Although treponemal screening followed by reflex nontreponemal titers allowed detection of a few newly diagnosed cases, the impact and cost-effectiveness of this strategy need further evaluation in public health community-based programs.
Acknowledgments
The authors thank Muhammad L. Mackalo and Darvlyn Mclean of the North Carolina Department of Health, Carter Vickery of Wake County Community Services; Yvonne Torres, Carla Piedrahita, and Debbie Turner of Wake County Human Services; and the following Duke faculty, staff, and students who assisted with the community screenings: David Holland, Susanna Naggie, Marshall Ahearn, Sujit Suchindran, and Paul Park. This work was supported by National Institute of Health (NIH) T32 AIDS Training Grant (5T32-AI007392-22, to N.D.G.) and a developmental grant from the Duke Center for AIDS Research, an NIH-funded program (P30A164518, to J.E.S.).
Footnotes
Conflicts of interest and source of funding: The authors have no conflicts of interests to declare.
References
- 1.Satterwhite CL, Torrone E, Meites E, et al. Sexually transmitted infections among US women and men: Prevalence and incidence estimates, 2008. Sex Transm Dis. 2013;40:187–193. doi: 10.1097/OLQ.0b013e318286bb53. [DOI] [PubMed] [Google Scholar]
- 2.Owusu-Edusei K, Jr, Introcaso CE, Chesson HW. Hospitalization cost of congenital syphilis diagnosis from insurance claims data in the United States. Sex Transm Dis. 2013;40:226–229. doi: 10.1097/OLQ.0b013e31827c5b9f. [DOI] [PubMed] [Google Scholar]
- 3.Gesink DC, Sullivan AB, Miller WC, et al. Sexually transmitted disease core theory: Roles of person, place, and time. Am J Epidemiol. 2011;174:81–89. doi: 10.1093/aje/kwr035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosenberg D, Moseley K, Kahn R, et al. Networks of persons with syphilis and at risk for syphilis in Louisiana: Evidence of core transmitters. Sex Transm Dis. 1999;26:108–114. doi: 10.1097/00007435-199902000-00009. [DOI] [PubMed] [Google Scholar]
- 5.Loeffelholz MJ, Binnicker MJ. It is time to use treponema-specific antibody screening tests for diagnosis of syphilis. J Clin Microbiol. 2012;50:2–6. doi: 10.1128/JCM.06347-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Owusu-Edusei K, Jr, Peterman TA, Ballard RC. Serologic testing for syphilis in the United States: A cost-effectiveness analysis of two screening algorithms. Sex Transm Dis. 2011;38:1–7. doi: 10.1097/OLQ.0b013e3181ec51f1. [DOI] [PubMed] [Google Scholar]
- 7.Discordant results from reverse sequence syphilis screening—Five laboratories, United States, 2006–2010. MMWR Morb Mortal Wkly Rep. 2011;60:133–137. [PubMed] [Google Scholar]
- 8.Schaffzin JK, Koumans EH, Kahn RH, et al. Evaluation of syphilis reactor grids: Optimizing impact. Sex Transm Dis. 2003;30:700–706. doi: 10.1097/01.OLQ.0000079518.04451.9D. [DOI] [PubMed] [Google Scholar]
- 9.McDonough JE. Budget sequestration and the U.S. health sector. N Engl J Med. 2013;368:1269–1271. doi: 10.1056/NEJMp1303266. [DOI] [PubMed] [Google Scholar]
- 10.Goswami ND, Hecker EJ, Vickery C, et al. Geographic information system–based screening for TB, HIV, and syphilis (GIS-THIS): A cross-sectional study. PLoS One. 2012;7:e46029. doi: 10.1371/journal.pone.0046029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goswami ND, Hecker E, Holland DP, et al. Feasibility and willingness-to-pay for integrated community-based tuberculosis testing. BMC Infect Dis. 2011;11:305. doi: 10.1186/1471-2334-11-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park IU, Chow JM, Bolan G, et al. Screening for syphilis with the treponemal immunoassay: Analysis of discordant serology results and implications for clinical management. J Infect Dis. 2011;204:1297–1304. doi: 10.1093/infdis/jir524. [DOI] [PubMed] [Google Scholar]
- 13.Castro AR, Esfandiari J, Kumar S, et al. Novel point-of-care test for simultaneous detection of nontreponemal and treponemal antibodies in patients with syphilis. J Clin Microbiol. 2010;48:4615–4619. doi: 10.1128/JCM.00624-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yin YP, Chen XS, Wei WH, et al. A dual point-of-care test shows good performance in simultaneously detecting nontreponemal and treponemal antibodies in patients with syphilis: A multisite evaluation study in China. Clin Infect Dis. 2013;56:659–665. doi: 10.1093/cid/cis928. [DOI] [PMC free article] [PubMed] [Google Scholar]