Abstract
Background
Pulmonary valve replacement (PVR) in repaired tetralogy of Fallot (TOF) reduces pulmonary regurgitation and decreases right ventricular (RV) dilation, but its long-term impact on ventricular tachycardia (VT) and mortality is unknown. This study aimed to determine the incidence of death and VT in TOF following PVR and test the hypothesis that PVR leads to improvement in these outcomes.
Methods and Results
A total of 98 patients with TOF and late PVR for RV dilation were identified. Matched controls were identified for 77 of these; controls had TOF with RV dilation but no PVR. Matching was done by age (±2y) and baseline QRS duration (±30msec). There were no significant differences in age, QRS duration, type or decade of initial repair, age at TOF repair, or presence of pre-PVR VT between the two groups; limited echocardiographic and MRI data showed no difference in left ventricular function but more RV dilation among PVR patients than controls. In the PVR group, there were 13 events over 272 patient-years. There was no significant change in QRS duration for any group. Overall 5- and 10-year freedom from death and/or VT was 80% and 41%. In the matched comparison, there were no significant differences seen in VT, death, or combined VT and/or death; p = 0.32, 0.06 (nearly favoring controls), 0.21.
Conclusions
This cohort experienced either VT or death every 20 patient-years. In a matched comparison with a similar TOF group, late PVR for symptomatic PR/RV dilation did not reduce the incidence of VT or death.
Keywords: Tetralogy of Fallot, Survival, Arrhythmia, Pulmonary Valve Replacement
Introduction
Surgical relief of right ventricular outflow tract obstruction in tetralogy of Fallot (TOF) often includes a transannular incision extending from the muscular infundibulum to the main pulmonary artery. While the afterload to the right ventricle (RV) is reduced, the tradeoff is pulmonary valvar incompetence, long-term pulmonary regurgitation (PR), and progressive RV dilation.1, 2 Placement of a valved homograft conduit from the right ventricle to the pulmonary artery, an alternative solution, typically has the same long-term complications due to progressive degradation in bioprosthetic leaflet tissue. RV dilatation is associated with vulnerability to arrhythmia; repaired TOF patients are known to be at increased long-term risk of mortality.3-5 A potential solution to this problem is pulmonary valve replacement (PVR), with the goal of reversing the process of RV dilation.
As the population of patients with repaired TOF ages, a growing number of patients can be expected to have PR and RV dilation. The question of when to perform a pulmonary valve replacement, and in whom, is becoming increasingly pressing.6-10 Clear guidelines to help inform this decision have proven difficult to identify.
The present study has two goals. The first is to characterize the incidence of death and ventricular tachycardia among all repaired TOF patients with RV dilation and late pulmonary valve replacement performed at our institution. The second is to determine if PVR in these patients is protective from these outcomes by retrospectively comparing this cohort to matched controls.
Methods
Statement of Responsibility
The authors had full access to and take full responsibility for the integrity of the data. All authors have read and agree to the manuscript as written.
Inclusion Criteria
Patients were identified in the database maintained within the cardiovascular program at our institution. At the time of database review (early 2007), 112 TOF patients had undergone PVR for RV dilation. Of these, only those with PVR greater than five years from their TOF repair were included, to focus on the impact of long-term volume load on the RV (leaving 101 patients in the study group). As well, those with significant confounding structural heart disease were excluded (3 patients with complete atrioventricular canal defect or Ebstein disease). The 98 remaining patients represented the final study cohort.
Descriptive and Baseline Data
Demographic, surgical, and electrophysiologic data included: age at review; type of initial TOF repair (transannular patch vs. conduit); era of and age at TOF repair; time between TOF repair and PVR; presence of pre-PVR ventricular tachycardia; decade of and age at PVR; and QRS duration at PVR (on the most recent ECG available occurring ≤1 year prior to valve replacement). The QRS duration was provided by the automated analysis performed by the General Electric Marquette Electronics mac5000 platform (Fairfield, CT) and was confirmed by manual inspection.
Baseline echocardiographic data included qualitative assessments of left and right ventricular function and RV dilation, on the latest evaluation prior to PVR (at most 3 years removed). Dysfunction or dilation was characterized as: none, mild, moderate, or severe. Baseline MRI data were LV and RV ejection fraction (EF), PR fraction, and right ventricular end diastolic volume (RVEDVi, indexed to body surface area, in ml/m2), again at most 3 years prior to PVR.
Outcome Data
The primary outcome variables were death and sustained ventricular tachycardia (VT). The former was identified within the hospital database system followed by search of the Social Security Death index. The latter was signaled by (1) a documented episode of spontaneous sustained VT (e.g. cardiac arrest); (2) concerning symptoms or Holter findings leading to electrophysiology study with inducible VT; (3) ICD implantation for VT; or (4) sustained VT documented by appropriate ICD discharge. For many of the analyses, a composite measure comprised of death and/or VT was used.
Secondary outcome measures were (1) change in QRS duration and (2) change in echocardiographic and MRI indices listed above. For the former, the post-PVR QRS duration was taken from the last available ECG, occurring at least 1 month after valve replacement. Patients with a paced rhythm on either pre- or post-ECG were excluded from this portion of the analysis. For the latter, data from the last available MRI or echocardiogram were used, again at least one month following PVR; only patients with both baseline and follow-up exams were included in this analysis.
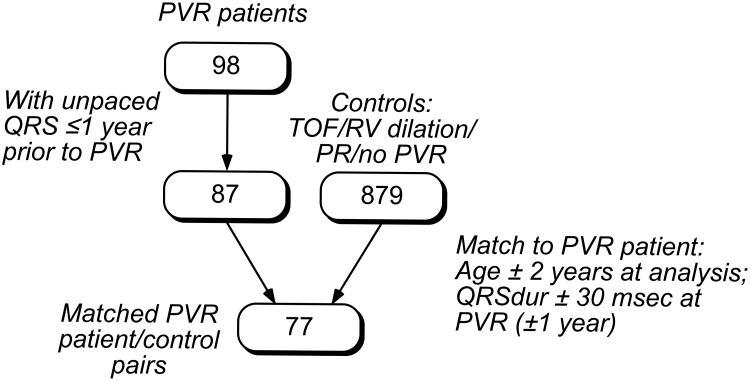
Control Subjects
Controls were selected from the pool of patients in our database with repaired TOF, PR and RV dilation, but no pulmonary valve replacement (879 patients). For each PVR patient, a paired control was identified in a two-step fashion (Figure 1). First, potential controls were identified that were matched by age ±2 years; they were ordered according to age similarity to the PVR patients. Then, within this group of prospects, the first patient satisfying the following criterion was selected: on an ECG occurring within one year of the PVR, the QRS duration (QRSdur) was within 30 msec of the PVR patient ECG (occurring at most one year prior to PVR).
Figure 1.
Identification of controls, resulting in 77 age- and QRS-duration matched pairs.
PVR patients with no available unpaced ECGs for measurement of QRS duration ≤1 year prior to PVR were excluded from this portion of the analysis (12 patients). Of the remaining 86 PVR patients, 77 controls were identified that matched the above criteria.
Baseline echocardiographic and MRI characteristics were recorded for the 77 pairs from the evaluation closest to the PVR date, no more than three years prior to it.
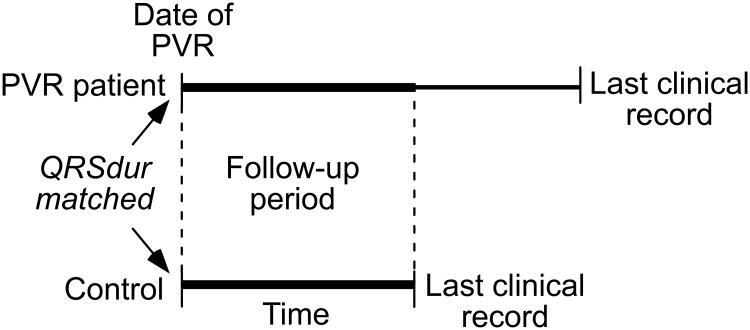
Follow-up Period
For the PVR patient/control comparison, primary outcome events were registered only if they occurred during a defined follow-up period (Figure 2), during which both patients were actively followed at CHB. For both groups, this period extends from the date of PVR until:
both patients alive: earlier of last clinical record for the PVR patient or control;
one patient deceased: last clinical record of the other patient.
Figure 2.
Illustration of the follow-up period during which clinical events are recorded, for a single PVR patient/control pair. The follow-up period begins at the date of PVR and continues to the earlier of the two last clinical records.
Six of the control patients themselves went on to PVR during the data analysis phase. For these patients, the follow-up period ended at the time of PVR.
Statistical Analysis
For the entire group of patients with repaired TOF and pulmonary valve replacement, times to the outcomes VT, death, and the composite measure (death and/or VT) were estimated using the Kaplan-Meier method; 95% confidence bands were calculated using the Greenwood formula. Changes in QRS duration and continuous MRI indices pre- to post-PVR were assessed using the paired t test. P values for changes in ordinal echocardiographic measures were calculated using the Wilcoxon signed-rank test. Comparison of QRS duration within groups (e.g. PVR or control) used all outcome data and was not limited to the ‘follow-up period’.
Baseline characteristics for a subgroup of PVR patients and their matched controls were compared using Fisher's exact test for categorical variables and either the two-sample t test or Wilcoxon rank sum test for continuous variables. Survival distributions for each of the outcomes were compared using the log-rank test. Cox proportional hazards models were used to adjust for any minor residual differences in age and baseline QRS duration between the groups. Unless noted otherwise, numbers in parentheses following mean and median values represent standard deviation and range, respectively.
Results
All PVR patients
Patient Characteristics And Baseline Data
For the 98 PVR patients in the study cohort, Table 1 contains demographic, surgical, and baseline electrophysiologic data. The earliest TOF repair for this patient cohort was 1958. 18 patients (19.1%) had QRS duration >180 msec. During the period between 1997-2003, six selected cases included surgical cryoablation in the right ventricular outflow tract as VT treatment or prophylaxis based on pre-operative electrophysiological mapping (this intervention was not adopted as a routine part of PVR at our institution). The total time during which these patients were followed at CHB is 1,879 years, 272 following PVR (mean 2.8±4.3y; median 1.1y, 1 mo–25 y).
Table 1.
Demographic, Surgical, and Electrophysiologic Patient Characteristics. TAP = Transannular patch; other abbreviations defined in the text.
| Variable | Value | |
|---|---|---|
|
|
|
|
| mean | median | |
| Age at Review (y) | 30.1 (13.7) | 26.6 (7.2-61.5) |
| Age at TOF Repair (y) | 4.9 (6.5) | 2.0 (0-29.1) |
| TOF repair to PVR (y) | 19.7 (9.4) | 18.8 (5.5-47.7) |
| Age at PVR (y) | 24.6 (13.0) | 20.7 (5.7-57.3) |
| QRS duration at PVR (msec) | 158 (30) | 158 (78-236) |
| TOF Repair type | # | % |
| TAP | 72 | 73.5 |
| Conduit | 26 | 26.5 |
| Pre-PVR VT? | 6 | 6.1 |
| Decade of PVR | ||
| 1980s | 5 | 5.1 |
| 1990s | 15 | 15.3 |
| 2000-2006 | 78 | 79.6 |
| RV Cryoablation at PVR | 7 | 7.1 |
Outcomes
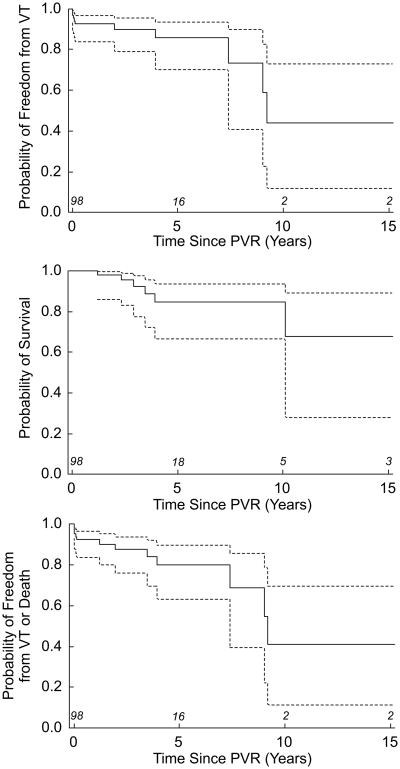
Table 2 shows the prevalence of the outcome variables death and VT and the composite measure (death and/or VT) in the PVR population. The event incidence was 4.8 per 100 patient-years. There was no perioperative mortality (the earliest patient death occurred 1.2y following PVR). Figure 3 shows freedom from the primary outcome variables. Five and 10-year measures of freedom from the composite outcome were 80% and 41%, respectively. Five of the 7 patients who had undergone intraoperative cryoablation experienced VT during follow-up.
Table 2.
PVR Patient Outcomes.
| Outcome | Number | % |
|---|---|---|
|
|
|
|
| Death | 6 | 6.1 |
| VT | 11 | 11.2 |
| Death and/or VT | 13 | 13.3 |
| Event-free survival | 85 | 86.7 |
Figure 3.
Survival functions for 98 PVR patients. Outcome measures are (a) VT; (b) death; and (c) death and/or VT.
For the 55 patients in whom non-paced pre- and post-PVR ECG data were available, there was no significant change in pre- vs. post-PVR QRS duration: 157.7 ± 29.6 msec vs. 154.3 ± 30.5 msec, p = 0.09 (time from PVR to ECG: mean = 3.0±2.6y; median 2.4y, 1mo-13.2y).
Table 3 summarizes baseline and follow-up echocardiographic and MRI data. The qualitative echo data shows a decrease in the degree of RV dysfunction and dilation. The MRI data shows a substantial decrease in the PR% and RVEDVi, with a suggestion of a trivial decrease in LV function but no change in RV function. The median time between PVR and evaluation are 6.5y (0.1-60.9y) and 1.1y (0.5-7.3y) for echo and MRI, respectively. Overall, LV function was well preserved for the study group both before and after surgery.
Table 3.
Baseline and follow-up (a) qualitative echocardiographic measures and (b) quantitative MRI indices.
| Baseline | Last | p | |
|---|---|---|---|
|
|
|
|
|
| a. Echo | #, % | #, % | |
| RV Dysfunction (n = 40) | 0.03 | ||
| None | 11 (27.5) | 15 (37.5) | |
| Mild | 16 (39.0) | 19 (47.5) | |
| Moderate | 9 (22.5) | 2 (5.0) | |
| Severe | 4 (10.0) | 4 (10.0) | |
| Median | mild | mild | |
| LV Dysfunction (n = 57) | 0.37 | ||
| None | 43 (75.4) | 41 (71.9) | |
| Mild | 13 (22.8) | 11 (19.3) | |
| Moderate | 1 (1.8) | 3 (5.3) | |
| Severe | 0 (0.0) | 2 (3.5) | |
| Median | none | none | |
| RV Dilation (n = 27) | <0.001 | ||
| None | 0 (0.0) | 6 (22.2) | |
| Mild | 3 (11.1) | 11 (40.7) | |
| Moderate | 11 (40.7) | 6 (22.2) | |
| Severe | 13 (48.2) | 4 (14.8) | |
| Median | moderate | mild | |
| b. MRI | |||
| RV EF % (n = 36) | |||
| Mean | 46 (9) | 45 (12) | 0.34 |
| Median | 46 (20-67) | 46 (15-68) | |
| LV EF % (n = 34) | |||
| Mean | 60 (8) | 57 (9) | 0.04 |
| Median | 61 (44-76) | 57 (18-70) | |
| PR % (n = 20) | |||
| Mean | 51 (13) | 7 (9) | <0.001 |
| Median | 50 (25-75) | 4 (0-37) | |
| RVEDVi (n = 35) | |||
| Mean | 194 (71) | 131 (45) | <0.001 |
| Median | 177 (77-424) | 124 (56-308) |
PVR patient/Control pairs
Patient characteristics and baseline data
Table 4 shows select demographic, surgical, electrophysiologic, echocardiographic, and MRI characteristics of the 77 PVR/control pairs. Comparing the two populations, there were no significant differences in age at review; age, repair type, or decade at TOF repair; presence of pre-PVR VT; or baseline QRS duration. The qualitative echocardiographic data suggest no differences in LV function but an increased prevalence of RV dysfunction and dilation in the PVR group. By MRI, there is no difference in LV EF; a slightly decreased RV EF (borderline p value) and significantly higher RVEDVi are present in the PVR group. Significant (≥moderate) qualitative echocardiographic right ventricular outflow tract obstruction was uncommon, occurring in 13% and 10% of PVR patients and controls, respectively (p = 0.37).
Table 4.
Comparison of Demographic, Surgical, Electrophysiologic, Echocardiographic, and MRI Baseline Characteristics of PVR and Control Groups.
| PVR | Control | p | |
|---|---|---|---|
|
|
|
|
|
| Median Age at Review (y) | 25.5 (7.3-59.9) | 25.8 (7.2-60.8) | 0.98 |
| TOF Repair type (#, %) | 0.86 | ||
| TAP | 56 (72.7) | 54 (70.1) | |
| Conduit | 21 (27.3) | 23 (29.9) | |
| Decade of TOF repair (#, %) | 0.84 | ||
| 1950-1969 | 7 (9.1) | 5 (6.5) | |
| 1970-1979 | 23 (29.9) | 18 (23.4) | |
| 1980-1989 | 26 (33.8) | 31 (40.3) | |
| 1990-1999 | 21 (27.3) | 23 (29.9) | |
| Median Age at TOF repair (y) | 1.3 (0-27.1) | 2.9 (0-28.4) | 0.17 |
| Pre-PVR VT | 6 (7.8) | 7 (9.1) | 1.00 |
| Median QRSdur at PVR (msec) | 158 (86-224) | 148 (82-200) | 0.11 |
| Echo parameters (median) | |||
| LV dysfunction (n=68) | none | none | 0.13 |
| RV dysfunction (n=43) | mild | none | <0.001 |
| RV dilation (n=21) | moderate | moderate | 0.03 |
| MRI Parameters | |||
| RV EF % (n=33) | |||
| Mean | 46 (10) | 50 (8) | 0.04 |
| Median | 44 (20-67) | 52 (33-63) | |
| LV EF % (n=31) | |||
| Mean | 61 (9) | 59 (6) | 0.39 |
| Median | 62 (41-76) | 58 (44-71) | |
| RVEDVi (n=33) | |||
| Mean | 196 (76) | 132 (38) | <0.001 |
| Median | 194 (65-424) | 122 (75-232) |
The aggregate durations over which PVR patients/controls were followed, including pre-PVR time, was 1418 and 1492 years, respectively, with median value = 17.3y (0.0-50.0y) and 15.9y (0.6-53.9y). For each group, the total post PVR duration was 171.6 y, with median 1.4y (0.01-13.2y) and mean 2.2y (2.7y).
Outcomes
Table 5 shows the incidence of the outcome variables for the PVR patient/control pairs during follow-up. Nine patients (12%) in the PVR group experienced an event during this time, compared with 5 patients in the control group (7%). Per 100 patient-years, the incidence of an event was 5.3 and 2.9 for PVR patient and control, respectively. For the PVR group, the median QRS duration among patients with death and/or VT, vs. those with event-free survival, was 177 msec (134-216 msec), and 156 (86-224 msec), respectively (p = 0.046); For the control group, these values were 174 msec (88-200 msec) and 146 msec (82 – 200 msec); p = 0.01.
Table 5.
PVR and Control Group Outcomes, # (%).
| Outcome | PVR | Control |
|---|---|---|
|
|
|
|
| Death | 4 (5.2) | 0 (0.0) |
| VT | 8 (10.4) | 5 (6.5) |
| Death and/or VT | 9 (11.7) | 5 (6.5) |
| Event-free Survival | 68 (88.3) | 72 (93.5) |
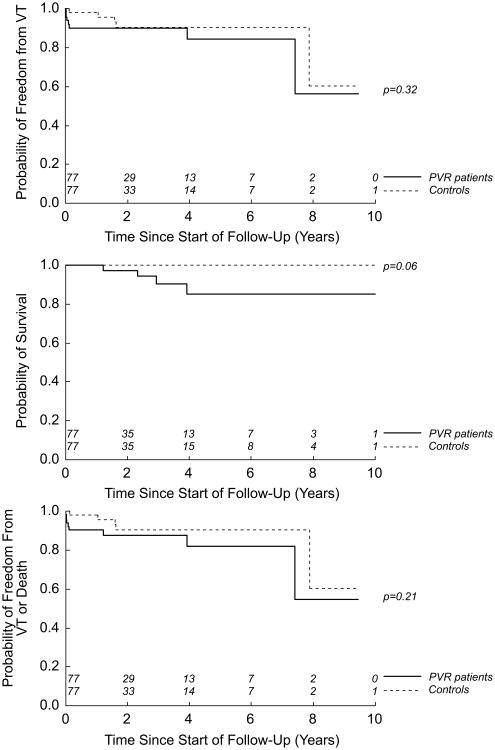
Figure 4 compares these two groups. The panels address freedom from VT; probability of survival; and freedom from the composite measure (death and/or VT), respectively. There are no significant differences between the groups for any of these measures. Adjustment for age and baseline QRS duration does not affect these comparisons. With regard to survival, a benefit to non-intervention approaches significance.
Figure 4.
Survival functions for 77 PVR patient/control pairs. Outcome measures are (a) VT; (b) death; and (c) death and/or VT.
Pre- and post-PVR non-paced ECGs were available for 46 patient pairs. For the PVR group, the QRS duration mean values were 160.8 msec (±25.0) and 156.9 msec (±26.7), respectively; for the controls, they were 153.3 msec (±26.4) and 151.9 msec (±26.2). Comparing PVR patients and controls, there was no difference seen in: baseline QRS duration (p=0.16); time between ECGs (p=0.27); and change in QRS duration (p=0.38).
Discussion
Most patients with repaired tetralogy of Fallot are subject to right ventricular volume overload as a consequence of pulmonary valvar incompetence. This chronic load leads to progressive right ventricular dilation which can be associated with substantial morbidity and even mortality.3 Pulmonary valve replacement is increasingly being used in these patients as a treatment for pulmonary insufficiency.
It has been suggested that PVR is protective against the development of future ventricular arrhythmia.11 The degree to which RV remodeling following PVR may reduce the arrhythmogenic substrate, however, is unknown. The QRS duration has been viewed as a proxy for this process, with some reports showing a reduction in duration after PVR.12 There remain no published data demonstrating a clear survival benefit from PVR, however, with only one matched trial examining this outcome.13 As well, the optimal timing for PVR remains undetermined.
The current series catalogues the entire experience of our center with PVR in TOF, specifically looking at long-term survival and freedom from VT. These results are analyzed by comparing PVR patients to a similar cohort of TOF patients with pulmonary insufficiency in whom PVR had not been performed. The primary outcome variables of death and ventricular tachycardia were chosen both for their clinical importance and for their readily identifiable nature in a retrospective fashion, minimizing missed instances of either.
Ninety-eight patients were included in the descriptive portion of the study. Our institutional preference for neonatal repair is reflected in the median age at TOF repair of 2.0y (contrasting with recent reported European series).14, 15 In children born since 1990, 15 out of 19 had a full repair prior to 13 months of age. Nearly three quarters of the repairs used a transannular patch. The mean time from TOF repair to PVR was nearly 20 years. Six percent of the population undergoing PVR had documented pre-surgical VT. The number of PVRs performed during the decades at CHB has risen dramatically, with 80% of the total surgical volume occurring during the years 2000-2006. The median age at PVR was 21 years, with a somewhat prolonged mean QRS duration equal to 158 msec. Nineteen percent of patients had a QRS duration greater than 180 msec, a figure associated with increased risk of VT and sudden death.16
The incidence of death and/or VT was 4.8 events per 100 patient-years. The prevalence of all-cause mortality in this population, 6.1%, is similar to the rates of sudden cardiac death reported by other groups late after TOF repair without PVR.3, 13, 17 The 5- and 10-year measures of freedom from death and/or VT, 80% and 41%, vary from those in another recent large series of 158 post-PVR patients; however the two studies differ in both inclusion criteria and the definition of endpoint events.15
There was no significant change in QRS duration following PVR in our cohort. Post-operative changes in this measure may be complex, as published reports have had variable findings; from an overall reduction,12 to a lengthening,13 to a brief period of QRS shortening followed by lengthening.18 Both echocardiographic and MRI data showed a significant reduction in RV dilation, in line with multiple published series.14, 19-21 By MRI, the RV function remained unchanged while there was a very slight decrease in LV EF (although there was no change in qualitative echocardiographic LV function).
The second objective of this work was to provide a context for these outcome data by comparing to similar controls from a single institution. During the period of time contained in this report, there was no uniform practice policy governing the decision to proceed to PVR. The decision to refer to surgery was made by individual practitioners based on a combination of clinical factors, including symptoms and rhythm disturbances, and qualitative and quantitative measures such as exercise test, echocardiography, and MRI findings. Controls were matched to PVR patients by age and QRS duration. The former criterion was chosen given the progressive nature of RV dilation10; the latter was based on reports identifying the QRS duration as a marker of both right ventricular dilation and predisposition to ventricular arrhythmia.4, 5 Baseline analysis of the two groups revealed no significant differences in age at review; era and type of TOF of repair; baseline QRS duration; and the presence of pre-PVR VT. In the subpopulation in whom paired echo and MRI data were available, there was no difference in LV function (by echocardiogram or MRI). By both modalities, the PVR group had more RV dilation. As well, by echo, RV function was qualitatively mildly more impaired in the PVR group, compared with normal in the controls; by MRI, there was a slightly lower RV EF in the former than the latter (borderline p value).
The incidence of death and/or VT for the PVR and control populations, per 100 patient years, was 5.2 and 2.9, respectively. The median QRS duration was longer for patients with events, for both PVR and control groups. For none of the outcome variables did the PVR group have an improved outcome compared with matched controls; for the survival outcome, these patients approached a trajectory that was worse. As well, there was no difference in the change in QRS duration between PVR and control populations during the course of the study, similar to another recent report.13
These results suggest that in this population, PVR may not have lead to long-term benefits of arrhythmia reduction from RV remodeling in spite of a reduction in RV size. These findings are not inconsistent with a growing body of literature suggesting that there is a “window of opportunity” for intervention beyond which, for example, restoration of normal right ventricular dimensions becomes impossible.19, 21, 22
Study Limitations
Three limitations should be mentioned. First, this study was a non-randomized comparison of intervention vs. non-intervention, not a randomized controlled clinical trial; as such, PVR patients and their controls were imperfectly matched. The subpopulation of PVR patients with available MRI data, for example, had more RV dilation and slightly worse RV function than their paired controls (LV function, however, a primary predictor of adverse outcomes23, was well matched; also, for the entire patient cohort, there were no differences in many other predictors of future adverse outcomes). Second, as a consequence of the disproportionate number of PVRs performed recently and the conservative definition used for the follow-up period, the median follow-up time is quite limited. It may be that a protective effect will not be seen until these patients are followed further into the future. Third, it should be noted that these results make no comment on any beneficial impact that PVR might have on patients' subjective symptomatology or objective exercise capacity.
Conclusion
In a large cohort of patients with repaired TOF and RV dilation at our institution, the incidence of death or sustained VT following PVR is considerable, at one event per 20 patient years. QRS duration did not change significantly following PVR over the course of the study. Compared with a group of similar controls, PVR did not result in improved survival or decreased incidence of VT. These data support continued efforts to refine the proper clinical indications and timing for referral to pulmonary valve replacement in patients with repaired tetralogy of Fallot.
Clinical Perspective.
Tetralogy of Fallot, the most common cyanotic congenital heart defect, combines a large ventricular septal defect with right ventricular outflow tract obstruction. In addition to closure of the ventricular septal defect, the traditional surgical repair for this lesion has included disruption of the pulmonary valve annulus with an outflow tract patch. The tradeoff for this very effective reduction in right ventricular pressure load is exposure of the right ventricular myocardium to a long-standing volume load, from pulmonary regurgitation. Aging patients with repaired tetralogy of Fallot have been shown to be predisposed to exercise intolerance, arrhythmia, and premature death. Accordingly, a growing amount of attention is being paid to the potential impact of the chronic increase in right ventricular work to which these patients are exposed. Pulmonary valve replacement (PVR) is an intuitively appealing means of addressing this problem. PVR has been shown to dramatically reduce the amount of pulmonary regurgitation and lead to a reduced size of the right ventricular cavity. The risk/benefit calculus for this procedure, however, has not been well established. The current report uses a pair of retrospective matched cohorts to investigate the impact of PVR on survival and ventricular tachycardia. In the cohorts studied, PVR failed to reduce ventricular tachycardia or prolong survival. These results serve as a cautionary tale; they may help to guide expectations following this surgery, for patient and physician alike. At a minimum, they support continued thoughtful and systematic investigations into the specific indications for, and timing of, pulmonary valve replacement.
Acknowledgments
The authors would like to thank Dr. James E. Lock for his helpful comments during the preparation of this manuscript.
Funding Sources: Boston Children's Heart Foundation. NIH Loan Repayment Program. NIH T32 (National Research Training Grant)
Footnotes
Disclosures: None
References
- 1.Bouzas B, Kilner PJ, Gatzoulis MA. Pulmonary regurgitation: not a benign lesion. European Heart Journal. 2005;26:433–439. doi: 10.1093/eurheartj/ehi091. [DOI] [PubMed] [Google Scholar]
- 2.Rebergen SA, Chin JG, Ottenkamp J, van der Wall EE, de Roos A. Pulmonary regurgitation in the late postoperative follow-up of tetralogy of Fallot. Volumetric quantitation by nuclear magnetic resonance velocity mapping. Circulation. 1993;88:2257–2266. doi: 10.1161/01.cir.88.5.2257. [DOI] [PubMed] [Google Scholar]
- 3.Murphy JG, Gersh BJ, Mair DD, Fuster V, McGoon MD, Ilstrup DM, McGoon DC, Kirklin JW, Danielson GK. Long-term outcome in patients undergoing surgical repair of tetralogy of Fallot. N Engl J Med. 1993;329:593–599. doi: 10.1056/NEJM199308263290901. [DOI] [PubMed] [Google Scholar]
- 4.Gatzoulis MA, Till JA, Somerville J, Redington AN. Mechanoelectrical interaction in tetralogy of Fallot. QRS prolongation relates to right ventricular size and predicts malignant ventricular arrhythmias and sudden death. Circulation. 1995;92:231–237. doi: 10.1161/01.cir.92.2.231. [DOI] [PubMed] [Google Scholar]
- 5.Abd El Rahman MY, Abdul-Khaliq H, Vogel M, Alexi-Meskishvili V, Gutberlet M, Lange PE. Relation between right ventricular enlargement, QRS duration, and right ventricular function in patients with tetralogy of Fallot and pulmonary regurgitation after surgical repair. Heart. 2000;84:416–420. doi: 10.1136/heart.84.4.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van der Wall EE, Mulder BJ. Pulmonary valve replacement in patients with tetralogy of Fallot and pulmonary regurgitation: early surgery similar to optimal timing of surgery? Eur Heart J. 2005;26:2614–2615. doi: 10.1093/eurheartj/ehi613. [DOI] [PubMed] [Google Scholar]
- 7.Quintessenza JA, Jacobs JP, Chai PJ, Morell VO, Giroud JM, Boucek RJ. Late replacement of the pulmonary valve: when and what type of valve? Cardiol Young. 2005;15(Suppl 1):58–63. doi: 10.1017/s1047951105001046. [DOI] [PubMed] [Google Scholar]
- 8.Davlouros PA, Karatza AA, Gatzoulis MA, Shore DF. Timing and type of surgery for severe pulmonary regurgitation after repair of tetralogy of Fallot. Int J Cardiol. 2004;97(Suppl 1):91–101. doi: 10.1016/j.ijcard.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 9.Lim C, Lee JY, Kim WH, Kim SC, Song JY, Kim SJ, Choh JH, Kim CW. Early replacement of pulmonary valve after repair of tetralogy: is it really beneficial? Eur J Cardiothorac Surg. 2004;25:728–734. doi: 10.1016/j.ejcts.2004.01.036. [DOI] [PubMed] [Google Scholar]
- 10.Geva T. Indications and timing of pulmonary valve replacement after tetralogy of Fallot repair. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2006:11–22. doi: 10.1053/j.pcsu.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 11.Therrien J, Siu SC, Harris L, Dore A, Niwa K, Janousek J, Williams WG, Webb G, Gatzoulis MA. Impact of pulmonary valve replacement on arrhythmia propensity late after repair of tetralogy of Fallot. Circulation. 2001;103:2489–2494. doi: 10.1161/01.cir.103.20.2489. [DOI] [PubMed] [Google Scholar]
- 12.van Huysduynen BH, van Straten A, Swenne CA, Maan AC, van Eck HJ, Schalij MJ, van der Wall EE, de Roos A, Hazekamp MG, Vliegen HW. Reduction of QRS duration after pulmonary valve replacement in adult Fallot patients is related to reduction of right ventricular volume. Eur Heart J. 2005;26:928–932. doi: 10.1093/eurheartj/ehi140. [DOI] [PubMed] [Google Scholar]
- 13.Gengsakul A, Harris L, Bradley TJ, Webb GD, Williams WG, Siu SC, Merchant N, McCrindle BW. The impact of pulmonary valve replacement after tetralogy of Fallot repair: a matched comparison. Eur J Cardiothorac Surg. 2007;32:462–8. doi: 10.1016/j.ejcts.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 14.Ghez O, Tsang VT, Frigiola A, Coats L, Taylor A, Van Doorn C, Bonhoeffer P, De Leval M. Right ventricular outflow tract reconstruction for pulmonary regurgitation after repair of tetralogy of Fallot. Preliminary results. Eur J Cardiothorac Surg. 2007;31:654–658. doi: 10.1016/j.ejcts.2006.12.031. [DOI] [PubMed] [Google Scholar]
- 15.Oosterhof T, Meijboom FJ, Vliegen HW, Hazekamp MG, Zwinderman AH, Bouma BJ, van Dijk AP, Mulder BJ. Long-term follow-up of homograft function after pulmonary valve replacement in patients with tetralogy of Fallot. Eur Heart J. 2006;27:1478–1484. doi: 10.1093/eurheartj/ehl033. [DOI] [PubMed] [Google Scholar]
- 16.Gatzoulis MA, Balaji S, Webber SA, Siu SC, Hokanson JS, Poile C, Rosenthal M, Nakazawa M, Moller JH, Gillette PC, Webb GD, Redington AN. Risk factors for arrhythmia and sudden cardiac death late after repair of tetralogy of Fallot: a multicentre study. Lancet. 2000;356:975–981. doi: 10.1016/S0140-6736(00)02714-8. [DOI] [PubMed] [Google Scholar]
- 17.Silka MJ, Hardy BG, Menashe VD, Morris CD. A population-based prospective evaluation of risk of sudden cardiac death after operation for common congenital heart defects. J Am Coll Cardiol. 1998;32:245–251. doi: 10.1016/s0735-1097(98)00187-9. [DOI] [PubMed] [Google Scholar]
- 18.Oosterhof T, Vliegen HW, Meijboom FJ, Zwinderman AH, Bouma B, Mulder BJ. Long-term effect of pulmonary valve replacement on QRS duration in patients with corrected tetralogy of Fallot. Heart. 2007;93:506–509. doi: 10.1136/hrt.2006.094169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dave HH, Buechel ER, Dodge-Khatami A, Kadner A, Rousson V, Bauersfeld U, Pretre R. Early insertion of a pulmonary valve for chronic regurgitation helps restoration of ventricular dimensions. Ann Thorac Surg. 2005;80:1615–1620. doi: 10.1016/j.athoracsur.2005.04.058. discussion 1620-1611. [DOI] [PubMed] [Google Scholar]
- 20.Buechel ER, Dave HH, Kellenberger CJ, Dodge-Khatami A, Pretre R, Berger F, Bauersfeld U. Remodelling of the right ventricle after early pulmonary valve replacement in children with repaired tetralogy of Fallot: assessment by cardiovascular magnetic resonance. Eur Heart J. 2005;26:2721–2727. doi: 10.1093/eurheartj/ehi581. [DOI] [PubMed] [Google Scholar]
- 21.Therrien J, Provost Y, Merchant N, Williams W, Colman J, Webb G. Optimal timing for pulmonary valve replacement in adults after tetralogy of Fallot repair. Am J Cardiol. 2005;95:779–782. doi: 10.1016/j.amjcard.2004.11.037. [DOI] [PubMed] [Google Scholar]
- 22.Oosterhof T, van Straten A, Vliegen HW, Meijboom FJ, van Dijk AP, Spijkerboer AM, Bouma BJ, Zwinderman AH, Hazekamp MG, de Roos A, Mulder BJ. Preoperative thresholds for pulmonary valve replacement in patients with corrected tetralogy of Fallot using cardiovascular magnetic resonance. Circulation. 2007;116:545–551. doi: 10.1161/CIRCULATIONAHA.106.659664. [DOI] [PubMed] [Google Scholar]
- 23.Knauth AL, Gauvreau K, Powell AJ, Landzberg MJ, Walsh EP, Lock JE, Delnido PJ, Geva T. Ventricular Size and Function Assessed by Cardiac MRI Predict Major Adverse Clinical Outcomes Late After Tetralogy of Fallot Repair. Heart. 2008;94:211–216. doi: 10.1136/hrt.2006.104745. [DOI] [PubMed] [Google Scholar]