Summary
Local recurrence (LR) rates in chordoma patients following surgery ± radiation therapy (RT) or definitive RT are high. Tumor hypoxia is associated with radioresistance and LR. In this prospective study, [18F]-FMISO-PET/CT detected hypoxic tumor sub-volumes in 60% of patients with chordoma of the mobile and sacrococcygeal spine, the majority of which were sufficiently large to allow an RT boost. Further study of hypoxia-directed, dose-escalated RT, particularly in patients at high risk for LR, is warranted.
Purpose/Objectives
Local recurrence (LR) rates in chordoma patients following surgery ± radiation therapy (RT) or definitive RT are high. Tumor hypoxia is associated with radioresistance and LR. [18F] fluoromisonidazole positron emission tomography/computed tomography (FMISO-PET/CT) can visualize skull base chordoma hypoxic sub-volumes (HSV) and feasibility of hypoxia-directed RT dose-escalation has been demonstrated in head and neck cancer. This study investigates FMISO-PET/CT detection of targetable HSVs in chordoma of the mobile or sacrococcygeal spine.
Methods and Materials
A prospective, pilot study of 20 patients with primary or locally recurrent chordoma of the mobile or sacrococcygeal spine treated with proton or combined proton/photon RT ± surgery was completed. FMISO-PET/CT was performed prior to RT and after 19.8-34.2 GyRBE (relative biologic effectiveness). Gross tumor volumes (GTV) were delineated and HSVs defined including voxels with standardized uptake values ≥ 1.4 times the muscle mean. Clinical characteristics and treatments received were compared between patients with and without HSVs.
Results
FMISO-PET/CT detected HSVs in 12 (60%; 12/20) patients. Baseline and interval HSV spatial concordance varied (0-94%). Eight HSVs were sufficiently large (≥ 5cc) to potentially allow an intensity modulated proton therapy boost. Patients with HSVs had significantly larger GTVs (median =410.0 cc vs. 63.4 cc; p=0.02) and were significantly more likely to have stage T2 tumors (5/12 vs. 0/8; p=0.04). After a median follow-up of 1.8 years (range: 0.2-4.4), a LR has yet to be observed. Three patients developed metastatic disease, 2 with HSVs.
Conclusions
FMISO-PET/CT detection of targetable HSVs within patients undergoing RT ± surgery for treatment of chordoma of the mobile and sacrococcygeal spine is feasible. The study's inability to attribute interval HSV changes to treatment, rapidly changing hypoxic physiology, or imaging inconsistencies is a limitation. Further study of double-baseline FMISO-PET/CT and hypoxia-directed RT dose-escalation, particularly in patients at high risk for LR, is warranted.
Introduction
Chordomas are tumors arising from notochordal remnants within the skull base, spine, and sacrum with an incidence of approximately 1 per 1,000,000, accounting for 1-4% of all primary bone tumors.[1-3] Despite its rarity, chordoma represents the most common primary bone tumor of the sacrum and the most common primary malignant tumor of the mobile spine.[4,5]
Wide surgical excision has been the cornerstone of management of chordomas of the mobile spine and sacrum.[6] While surgery can improve overall survival and has been associated with improved disease-free and local recurrence-free survival, it can result in significant morbidity and functional consequences, particularly when high sacrectomy is required.[4,7-9] Moreover, local recurrence rates in some surgical series are as high as 50-100% with subtotal resection compared to 0-53% with en bloc resection with clear margins.[4,9-18] Strategies combining high dose adjuvant radiation therapy (RT) and maximal resection have resulted in higher local control rates.[19,20]
In the past, the use of definitive RT at modest doses in the medically inoperable or those with unacceptable anticipated surgical morbidity resulted in unsatisfactory local control.[21] Investigators utilizing high-dose definitive particle therapy have recently reported encouraging outcomes with acceptable toxicity in this patient population.[19,22,23]
Tumor hypoxia has been associated with radioresistance as well as elevated risks of local recurrence and distant metastasis in gynecologic malignancies.[24,25] [18F] fluoromisonidazole positron emission tomography (FMISO-PET) can delineate areas of tumor hypoxia prior to and during RT and has been shown to be of prognostic value in patients with lung and head and neck cancers undergoing RT.[26,27] The feasibility of FMISO-PET/computed tomography (CT)-guided RT dose escalation to hypoxic tumor sub-volumes while respecting normal tissue tolerance has been demonstrated in head and neck cancer.[28] A recent series utilized FMISO-PET/CT to detect hypoxic areas of residual base of skull chordomas following incomplete surgical excision.[29] Whether the results of the aforementioned study are applicable to the typically much larger chordomas of the mobile and sacrococcygeal spine without prior surgical intervention is unknown.
We report the results of a prospective study exploring the feasibility of FMISO-PET/CT for visualization of hypoxic sub-volumes in chordoma patients undergoing RT ± surgery. The relationship between hypoxic sub-volumes and clinical characteristics are delineated. Treatment outcomes are reported.
Methods
Patient Population, Treatment and Follow-up
The study cohort consisted of adult patients with primary or locally recurrent chordoma of the mobile or sacrococcygeal spine treated with proton or combined proton/photon RT ± surgery at a single institution between January 2009 and November 2012 as part of a prospective study (institutional protocol # 07-162). Patients with recurrent chordoma after RT, tumors < 1cm, FMISO allergies, or Karnofsky performance status <60 were excluded. RT and surgical treatments were similar to those described in a phase II trial of high dose proton/photon RT for spinal sarcomas.[19] Patients with locally recurrent disease after prior surgery, referred after intralesional/incomplete tumor surgery, or undergoing definitive RT without surgery could receive nilotinib in conjunction with RT as part of a phase I trial of concurrent RT and nilotinib for high risk chordoma (institutional protocol # 11-072). Following treatment, history and physical examinations and imaging studies were performed according to the National Comprehensive Cancer Network guidelines.[6] The date of last follow-up was December 11, 2013. This study was approved by the institutional review board.
FMISO-PET/CT and Determination of Hypoxic Tumor Volume
Gross tumor volumes (GTV) were manually delineated on each slice of the treatment planning CT with the assistance of magnetic resonance imaging (MRI) fusions (T1+ gadolinium, T2). Combined FMISO-PET/CT was performed prior to RT and again after 19.8-34.2 GyRBE (relative biologic effectiveness) on a 64-slice Siemens Biograph 64 PET/CT scanner (Siemens Medical Solutions, Knoxville, TN) 2 hours following intravenous administration of 350-400 MBq of the investigational hypoxia tracer, [18F] FMISO (PETNET Solutions, Knoxville, TN). FMISO-PET data sets were co-registered with their concurrently acquired CT scans, which were then co-registered with their respective treatment planning CT scans. The co-registered treatment planning CT data were then fused to the FMISO-PET images onto which the GTV was automatically transferred. Standardized uptake values (SUV) based on the total body weight were calculated (HybridViewer, Hermes Medical Solutions, Stockholm, Sweden). The mean SUV for normal muscle, using gluteal or paraspinal muscle as the reference site, was calculated by drawing a circular, 2D region of interest. Hypoxic tumor tissue was determined to be present if any voxels had an SUV ≥ 1.4 times the mean SUV of muscle.[27] The total hypoxic sub-volume was calculated by summing the volume of these voxels. The stability of hypoxic sub-volumes was assessed in patients with hypoxia at both time-points by fusing the treatment planning CT, baseline FMISO-PET, and interval FMISO-PET (MIMvista, MIM Software, Inc., Cleveland, OH). The volume of spatially stable hypoxic tumor tissue present at both time points was measured and reported as a percentage of the visualized hypoxic sub-volume at interval scanning. FMISO-PET/CT images were not used to plan delivered treatments.
The pre-specified threshold for determination of FMISO-PET/CT feasibility in detection of macroscopic hypoxic chordoma sub-volumes was positive tracer uptake in 4 of 20 patients.
Statistical Methods
Distributions of Clinical Characteristics at Baseline and Treatments Received Stratified by the Presence or Absence of Hypoxia
The distributions of categorical and continuous clinical characteristics and treatments received were enumerated and compared across the cohort in patients with and without hypoxic sub-volumes at either time-point. A chi-square metric was used to compare the distributions of categorical factors including tumor stage, gender, tumor location, treatments received, and surgical margin status.[30] A non-parametric Mann-Whitney U test was used to compare the distributions of continuous factors including age, maximum tumor diameter, gross tumor volume, and RT dose.[31] A two-sided p-value < 0.05 was considered significant. R version 3.0.2 was used for all statistical calculations (R Foundation for Statistical Computing, Vienna, Austria).[32]
Results
Patient Population, FMISO-PET/CT and Treatment
Twenty patients meeting entry criteria were enrolled and treated during the study period. All patients successfully underwent FMISO-PET/CT prior to RT and again after a mean RT dose of 26.7 GyRBE (SD= 3.7). Hypoxic sub-volumes were detected in 9 patients prior to RT (median volume: 7.5 cc; range: 0.04-272.4) and in 10 patients at interval FMISO-PET/CT during RT (median volume: 8.5 cc; range: 0.3-418.8). Visualized FMISO-PET/CT hypoxic sub-volumes were contained entirely within the contoured GTV in all but one evaluated patient. In this individual, 6.5% and 3.1% of the hypoxic sub-volumes on baseline and interval FMISO-PET/CT, respectively, occurred in the tissue immediately adjacent to the contoured GTV.
The hypoxic sub-volume identified prior to RT resolved on interval scanning in two patients. Three patients without hypoxic sub-volumes prior to RT developed hypoxic sub-volumes at interval scanning. Hypoxic sub-volumes were cumulatively detected by FMISO-PET/CT in a total of 12 unique patients (60%; 12/20). Hypoxic sub-volumes were visualized at both time-points in seven patients. The percentage of the hypoxic sub-volume visualized on interval FMISO-PET/CT that was also hypoxic on baseline imaging in each of these individuals is reported in Table 1. Of the identified hypoxic sub-volumes, 66.7% (8/12) were of sufficient size (≥5 cc) to potentially allow delivery of an intensity modulated proton therapy (IMPT) boost.
Table 1.
Hypoxic sub-volume characteristics for patients with visualized hypoxic sub-volumes on both baseline (scan 1) and interval (scan 2) FMISO-PET/CT.
| Patient Number | FMISO-PET/CT Scan | HSV (cm3) | HSV Overlap (cm3) [%]* |
|---|---|---|---|
| 1 | 1 | 11.7 | -- |
| 2 | 6.4 | 3.4 [53.5] | |
| 2 | 1 | 7.2 | -- |
| 2 | 0.2 | 0.2 [94.4] | |
| 3 | 1 | 23.8 | -- |
| 2 | 12.6 | 0.2 [1.9] | |
| 4 | 1 | 0.6 | -- |
| 2 | 0.7 | 0 [0] | |
| 5 | 1 | 12.4 | -- |
| 2 | 14.8 | 2.3 [15.5] | |
| 6 | 1 | 1147.8 | -- |
| 2 | 868.2 | 793 [91.3] | |
| 7 | 1 | 94.5 | -- |
| 2 | 18.9 | 8 [42.3] |
Interval hypoxic sub-volume overlapping with baseline hypoxic sub-volume expressed as a percentage of the interval hypoxic sub-volume.
Abbreviations: FMISO-PET/CT: [18F] fluoromisonidazole positron emission tomography/computed tomography; HSV: hypoxic sub-volume
Seventeen (85%; 17/20) patients received pre-operative RT ± post-operative RT to a median total dose of 70.2 GyRBE (range: 50.4-77.4). Negative margins were achieved in 14 (82.4%; 14/17) of these patients. Because of unacceptable anticipated surgical morbidity the remaining three (15%; 3/20) patients underwent definitive RT to a median dose of 77.4 GyRBE (range: 73.8-77.4). As shown in Table 2, patients with hypoxic tumor sub-volumes at either time-point had significantly larger gross tumor volumes (median = 410.0 cc vs. 63.4 cc; p= 0.02) and were significantly more likely to have stage T2 (≥8 cm) tumors (5/12 vs. 0/8; p= 0.04) compared to those without hypoxic sub-volumes. A trend towards larger maximum tumor diameter was likewise observed in patients with hypoxic sub-volumes compared to those without (median = 8.7 cm vs. 5.8 cm; p= 0.11). There were no significant differences in age, gender, tumor location, surgical margin status, RT dose, or nilotinib use between patients with and without hypoxic tumor sub-volumes.
Table 2. Clinical characteristics and treatments received stratified by the presence or absence of hypoxia.
| Clinical Characteristic | Hypoxia Present (n=12) | Hypoxia Absent (n=8) | p-value |
|---|---|---|---|
| Age (years) (%) | p = 0.60 | ||
| Median [range] | 60.5 [35-73] | 56.5 [37-67] | |
| <60 | 6 (50.0) | 5 (62.5) | |
| >60 | 6 (50.0) | 3 (37.5) | |
| Median Maximum Tumor Diameter (cm) [range] | 8.7 [3.0-22.8] | 5.75 [3.6-6.8] | p = 0.11 |
| Tumor Stage (%) | p = 0.04 | ||
| T1 (<8 cm) | 7 (58.3) | 8 (100) | |
| T2 (≥8 cm) | 5 (41.6) | 0 (0) | |
| Median Gross Tumor Volume (cc) [range] | 410 [51.0-2398.0] | 63.4 [17.0-463.2] | p = 0.02 |
| Gender (%) | p = 0.58 | ||
| Female | 6 (50.0) | 5 (62.5) | |
| Male | 6 (50.0) | 3 (37.5) | |
| Tumor Location (%) | p = 0.45 | ||
| Cervical | 0 (0) | 1 (12.5) | |
| Lumbar | 2 (16.7) | 1 (12.5) | |
| Sacrococcygeal | 10 (83.3) | 6 (75.0) | |
| Radiation Therapy (%) | p = 0.80 | ||
| Definitive RT | 2 (16.7) | 1 (12.5) | |
| Pre-op RT +/- Post-op Boost | 10 (83.3) | 7 (87.5) | |
| Median RT Dose (GyRBE) [range] | |||
| Definitive | 75.6 [73.8-77.4] | 77.4 | NA |
| Pre-Op | 50.4 [45-50.4] | 50.4 [50.4-50.4] | p = 0.48 |
| Post-Op | 19.8 [19.8-27.0] | 19.8 [19.8-22.0] | p = 0.42 |
| Total Pre-op + Post-op | 70.2 [50.4-77.4] | 70.2 [70.2-72.4] | p = 0.74 |
| Surgical Margin Status (%) | p = 0.32 | ||
| Positive | 1 (10.0) | 2 (28.6) | |
| Negative | 9 (90.0) | 5 (71.4) | |
| Nilotinib (%) | p = 0.80 | ||
| Yes | 2 (16.7) | 0 (0) | |
| No | 10 (83.3) | 8 (100) |
Abbreviations: RT: radiation therapy; RBE: relative biological effectiveness; NA: not applicable
Treatment Outcomes
After a median follow-up of 1.8 years (range: 0.2-4.6), no local recurrences were observed. Three patients developed recurrent/progressive disease after a median of 10.7 months (range: 9.7-25.5). Two of these patients developed distant metastases and died of chordoma at 1.6 and 1.8 years post-treatment. Both had hypoxic sub-volumes at initial evaluation. The third patient, who was without a hypoxic sub-volume, progressed regionally in the iliac bone 2.1 years after definitive RT and was managed with salvage RT. He subsequently developed a metastatic lesion in the L5 vertebral body 1.8 years thereafter for which he was undergoing RT at last follow-up.
A third death occurred in a patient with a 10 cm, 751 cc sacral chordoma with a hypoxic sub-volume who required steroids for management of persistent pain following definitive RT. She died of diabetic ketoacidosis, likely a steroid complication, which was not initially recognized by her local physicians 3.5 months after completion of RT without evidence of progressive disease.
Discussion
In this prospective study of 20 patients treated with RT ± surgery for chordoma of the mobile and sacrococcygeal spine, we found that the use of FMISO-PET/CT for detection of hypoxic tumor sub-volumes is feasible. Specifically, hypoxic sub-volumes were cumulatively identified in 60% (12/20) of patients, 66.7% (8/12) of which were of sufficient size (≥5 cc) to potentially allow delivery of an IMPT boost. The presence of hypoxic sub-volumes is significantly associated with increasing gross tumor volume and stage T2 tumors (≥8 cm). While the use of FMISO-PET/CT has recently been reported in incompletely resected skull base chordomas, to our knowledge, the current study represents the first and largest investigation of FMISO-PET/CT in primary or locally recurrent chordomas of the mobile and sacrococcygeal spine.[29]
After a median follow-up of 1.8 years, no local recurrences were observed, consistent with the favorable local control outcomes observed in a prior series of patients treated with high-dose RT and surgery.[19] While the majority of patients in our study were treated with a combination of RT and surgery, three patients underwent definitive RT. One is without evidence of progressive disease at 1.8 years post-RT with concurrent nilotinib, one developed bone-only metastases for which he has undergone further RT, and one died of diabetic ketoacidosis 3.5 months after RT completion. A recently published series of 24 chordoma patients treated with definitive proton/photon RT to a median dose of 77.4 GyRBE with longer follow-up reports an encouraging 5-year local progression-free survival of ∼80%.[22] A second series using definitive carbon ion RT to 73.6 GyRBE reported a somewhat more favorable local-control rate of 89% with a median follow-up of 6.7 years.[23] Nevertheless, these data suggest that further efforts to improve local-control among patients treated with definitive RT are necessary. Dose-escalated definitive RT is associated with improved local control; however dose escalation to entire tumor volumes is unlikely to be possible due to unacceptable long-term toxicity.[20,22,23,33]
Tumor hypoxia has been associated with radioresistance and an elevated risk of local recurrence in gynecologic malignancies.[24,25] Feasibility of the use of FMISO-PET/CT for planning hypoxia-guided boost volumes in head and neck cancer has been demonstrated.[28] Our study found that the use of FMISO-PET/CT is feasible for visualization of hypoxic chordoma sub-volumes. A future goal of the currently reported study is to determine whether hypoxia-directed IMPT dose escalation, respecting normal tissue RT tolerance, is technically possible in chordoma. Such a treatment approach may result in improved local control in patients undergoing definitive RT or with residual disease after surgery without increased long-term toxicity. For the purpose of illustration, Figure 1 demonstrates FMISO-PET/CT visualization of hypoxic sub-volumes prior to RT (panel B) and following 25.2 GyRBE (panel C) in a 49-year-old man who underwent definitive combined proton/photon RT to a total dose of 73.8 GyRBE (panel D) with concurrent nilotinib for treatment of a 22.8 cm sacral chordoma. Panel E illustrates a theoretical IMPT plan designed to deliver 77.4 GyRBE to the entire gross tumor volume with an integrated boost to the combined pre-treatment and interval hypoxic sub-volumes plus a 3 mm expansion to a total dose of 84.9 GyRBE. Further study is needed to determine whether such dose-escalated treatments are feasible and result in improved outcomes.
Figure 1.
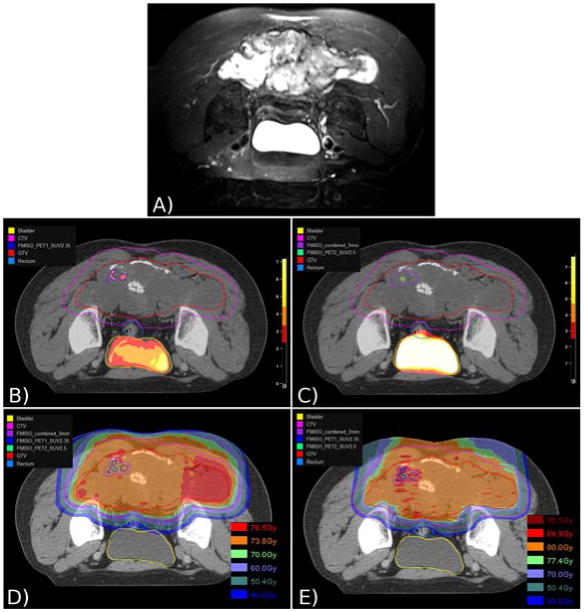
[18F] fluoromisonidazole positron emission tomography/computed tomography (FMISO-PET/CT) visualization of hypoxic chordoma sub-volumes (HSV) and theoretical use for dose-escalated intensity modulated proton therapy (IMPT). (A) Pre-treatment T2-weighted MRI demonstrating a 22.8 cm sacral chordoma. (B) Pre-treatment FMISO-PET/CT fused with radiation planning CT showing contoured HSV, gross tumor volume (GTV), clinical target volume (CTV), bladder, and rectum. (C) FMISO-PET/CT fused with radiation planning CT after 25.2 GyRBE (relative biologic effectiveness) with additionally contoured interval HSV and 3 mm expansion of combined HSVs. (D) Actual delivered proton/photon treatment plan to 73.8 GyRBE to the GTV. (E) Theoretical IMPT plan to 77.4 GyRBE to the GTV with integrated boost to the combined pre-treatment and interval HSVs to 84.9 GyRBE.
Several points require further clarification. First, recent studies employing short-interval, double-baseline FMISO-PET/CTs in head and neck cancer suggest that there may be significant variability in visualized hypoxic sub-volumes independent of treatment.[34,35] Among patients with hypoxia on both baseline and interval imaging in the current study, we observed varied spatial concordance of hypoxic sub-volumes ranging between 0 and 94%. Given that all patients received radiotherapy between baseline and interval FMISO-PET/CT, we are unable to determine whether these changes are related to treatment vs. intrinsic variation due to transient, acute tumor hypoxia or other physiologic causes. Future studies in chordoma patients employing double-baseline FMISO-PET/CT in addition to interval scans may allow greater understanding of the spatial and temporal stability of visualized hypoxic sub-volumes. Such information would be of value for defining appropriate hypoxia-directed IMPT boost volumes. Second, there is insufficient spatial information available in the surgical specimens and too great an interval between FMISO-PET/CT and surgery to allow pathologic confirmation of hypoxia within FMISO-avid tumor sub-volumes in the current study. Completion of FMISO-PET/CTs immediately prior to surgery with use of implanted fiducials, if technically possible, may allow for such investigation in the future. In addition, emerging evidence suggests that poor perfusion on dynamic contrast enhanced MRI (DCE-MRI) may be correlated with hypoxia as visualized by FMISO-PET.[36] Future study of simultaneous FMISO-PET and DCE-MRI in chordoma patients using a combined PET-MRI scanner may be of value should direct pathologic confirmation prove technically challenging. Third, while the reported early local control outcomes are encouraging, a median follow-up of 1.8 years is insufficient to document chordoma cures. Fourth, the small sample size and paucity of events precludes meaningful study of the influence of hypoxic sub-volumes, or sub-volume characteristics on treatment outcomes. Longer follow-up and a future, larger prospective study could address these concerns. Fifth, the majority of patients enrolled in this study underwent a combination of preoperative RT, en bloc surgical resection with negative margins, and an additional postoperative RT boost to the tumor bed. Given the excellent local control results achieved in such patients, FMISO-PET/CT-guided RT dose escalation may be more likely to benefit patients undergoing definitive RT, those with high risk disease such as locally recurrent tumor after prior surgery, or those with large-volume residual disease. Further study of FMISO-PET/CT in this subgroup would be of value.
Nevertheless, it appears that the use of FMISO-PET/CT for visualization of targetable hypoxic tumor sub-volumes within patients undergoing RT for chordomas of the mobile and sacrococcygeal spine is feasible. Further study of its application in hypoxia-directed, dose-escalated RT, particularly in patients at high risk for local recurrence, is warranted.
Acknowledgments
Financial Support: This project was supported in part by the Federal share of program income earned by Massachusetts General Hospital on C06 CA059267, Proton Therapy Research and Treatment Center. Additional support was provided by the Jennifer Hunter Yates Sarcoma Foundation, the Kenneth Stanton Fund, the Cassandra Moseley-Berry Fund and the Moseley Family.
Conflict of Interest: Dr. Shih reports grants from the Ira J. Spiro Award for Translational Research, the National Cancer Institute, the National Institutes of Health, and the Radiation Therapy Oncology Group; personal fees from Novartis, Merck, UpToDate, and the International Journal of Radiation Oncology*Biology*Physics (senior editor). Dr. Schwab reports personal fees from Stryker Spine and Biom'Up. Dr. Hornicek reports grants from the National Institutes of Health; board or panel membership at ISOLS, AATB, and the FDA; personal fees from Stryker Corporation and BioMed Valley Discoveries; patent NSC23925 related to drug resistance mechanisms. Dr. DeLaney reports grants from the National Cancer Institute; personal fees from Amgen, UpToDate, and royalties from Wolters Kluwer Heath.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Healey JH, Lane JM. Chordoma: A critical review of diagnosis and treatment. Orthop Clin North Am. 1989;20:417–426. [PubMed] [Google Scholar]
- 2.McMaster ML, Goldstein AM, Bromley CM, Ishibe N, Parry DM. Chordoma: Incidence and survival patterns in the united states, 1973-1995. Cancer Causes Control. 2001;12:1–11. doi: 10.1023/a:1008947301735. [DOI] [PubMed] [Google Scholar]
- 3.Salisbury JR. The pathology of the human notochord. J Pathol. 1993;171:253–255. doi: 10.1002/path.1711710404. [DOI] [PubMed] [Google Scholar]
- 4.Boriani S, Bandiera S, Biagini R, Bacchini P, Boriani L, Cappuccio M, Chevalley F, Gasbarrini A, Picci P, Weinstein JN. Chordoma of the mobile spine: Fifty years of experience. Spine (Phila Pa 1976) 2006;31:493–503. doi: 10.1097/01.brs.0000200038.30869.27. [DOI] [PubMed] [Google Scholar]
- 5.Cheng EY, Ozerdemoglu RA, Transfeldt EE, Thompson RC., Jr Lumbosacral chordoma. Prognostic factors and treatment. Spine (Phila Pa 1976) 1999;24:1639–1645. doi: 10.1097/00007632-199908150-00004. [DOI] [PubMed] [Google Scholar]
- 6.Biermann JS, Adkins DR, Agulnik M, Benjamin RS, Brigman B, Butrynski JE, Cheong D, Chow W, Curry WT, Frassica DA, Frassica FJ, Hande KR, Hornicek FJ, Jones RL, Mayerson J, McGarry SV, McGrath B, Morris CD, O'Donnell RJ, Randall RL, Santana VM, Satcher RL, Siegel HJ, von Mehren M, Bergman MA, Sundar H. Bone cancer. J Natl Compr Canc Netw. 2013;11:688–723. doi: 10.6004/jnccn.2013.0088. [DOI] [PubMed] [Google Scholar]
- 7.Ruggieri P, Angelini A, Ussia G, Montalti M, Mercuri M. Surgical margins and local control in resection of sacral chordomas. Clin Orthop Relat Res. 2010;468:2939–2947. doi: 10.1007/s11999-010-1472-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Samson IR, Springfield DS, Suit HD, Mankin HJ. Operative treatment of sacrococcygeal chordoma. A review of twenty-one cases. J Bone Joint Surg Am. 1993;75:1476–1484. doi: 10.2106/00004623-199310000-00008. [DOI] [PubMed] [Google Scholar]
- 9.Schwab JH, Healey JH, Rose P, Casas-Ganem J, Boland PJ. The surgical management of sacral chordomas. Spine (Phila Pa 1976) 2009;34:2700–2704. doi: 10.1097/BRS.0b013e3181bad11d. [DOI] [PubMed] [Google Scholar]
- 10.Aas M, Moe L, Gamlem H, Skretting A, Ottesen N, Bruland OS. Internal radionuclide therapy of primary osteosarcoma in dogs, using 153sm-ethylene-diamino-tetramethylene-phosphonate (edtmp) Clin Cancer Res. 1999;5:3148s–3152s. [PubMed] [Google Scholar]
- 11.Baratti D, Gronchi A, Pennacchioli E, Lozza L, Colecchia M, Fiore M, Santinami M. Chordoma: Natural history and results in 28 patients treated at a single institution. Ann Surg Oncol. 2003;10:291–296. doi: 10.1245/aso.2003.06.002. [DOI] [PubMed] [Google Scholar]
- 12.Bergh P, Kindblom LG, Gunterberg B, Remotti F, Ryd W, Meis-Kindblom JM. Prognostic factors in chordoma of the sacrum and mobile spine: A study of 39 patients. Cancer. 2000;88:2122–2134. doi: 10.1002/(sici)1097-0142(20000501)88:9<2122::aid-cncr19>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 13.Fuchs B, Dickey ID, Yaszemski MJ, Inwards CY, Sim FH. Operative management of sacral chordoma. J Bone Joint Surg Am. 2005;87:2211–2216. doi: 10.2106/JBJS.D.02693. [DOI] [PubMed] [Google Scholar]
- 14.Hanna SA, Aston WJ, Briggs TW, Cannon SR, Saifuddin A. Sacral chordoma: Can local recurrence after sacrectomy be predicted? Clin Orthop Relat Res. 2008;466:2217–2223. doi: 10.1007/s11999-008-0356-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hulen CA, Temple HT, Fox WP, Sama AA, Green BA, Eismont FJ. Oncologic and functional outcome following sacrectomy for sacral chordoma. J Bone Joint Surg Am. 2006;88:1532–1539. doi: 10.2106/JBJS.D.02533. [DOI] [PubMed] [Google Scholar]
- 16.Ozaki T, Hillmann A, Winkelmann W. Surgical treatment of sacrococcygeal chordoma. J Surg Oncol. 1997;64:274–279. doi: 10.1002/(sici)1096-9098(199704)64:4<274::aid-jso5>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 17.Yonemoto T, Tatezaki S, Takenouchi T, Ishii T, Satoh T, Moriya H. The surgical management of sacrococcygeal chordoma. Cancer. 1999;85:878–883. [PubMed] [Google Scholar]
- 18.York JE, Kaczaraj A, Abi-Said D, Fuller GN, Skibber JM, Janjan NA, Gokaslan ZL. Sacral chordoma: 40-year experience at a major cancer center. Neurosurgery. 1999;44:74–79. doi: 10.1097/00006123-199901000-00041. discussion 79-80. [DOI] [PubMed] [Google Scholar]
- 19.DeLaney TF, Liebsch NJ, Pedlow FX, Adams J, Dean S, Yeap BY, McManus P, Rosenberg AE, Nielsen GP, Harmon DC, Spiro IJ, Raskin KA, Suit HD, Yoon SS, Hornicek FJ. Phase ii study of high-dose photon/proton radiotherapy in the management of spine sarcomas. Int J Radiat Oncol Biol Phys. 2009;74:732–739. doi: 10.1016/j.ijrobp.2008.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park L, Delaney TF, Liebsch NJ, Hornicek FJ, Goldberg S, Mankin H, Rosenberg AE, Rosenthal DI, Suit HD. Sacral chordomas: Impact of high-dose proton/photon-beam radiation therapy combined with or without surgery for primary versus recurrent tumor. Int J Radiat Oncol Biol Phys. 2006;65:1514–1521. doi: 10.1016/j.ijrobp.2006.02.059. [DOI] [PubMed] [Google Scholar]
- 21.Fuller DB, Bloom JG. Radiotherapy for chordoma. Int J Radiat Oncol Biol Phys. 1988;15:331–339. doi: 10.1016/s0360-3016(98)90012-8. [DOI] [PubMed] [Google Scholar]
- 22.Chen YL, Liebsch N, Kobayashi W, Goldberg S, Kirsch D, Calkins G, Childs S, Schwab J, Hornicek F, DeLaney T. Definitive high-dose photon/proton radiotherapy for unresected mobile spine and sacral chordomas. Spine (Phila Pa 1976) 2013;38:E930–936. doi: 10.1097/BRS.0b013e318296e7d7. [DOI] [PubMed] [Google Scholar]
- 23.Imai R, Kamada T, Tsuji H, Sugawara S, Serizawa I, Tsujii H, Tatezaki S. Effect of carbon ion radiotherapy for sacral chordoma: Results of phase i-ii and phase ii clinical trials. Int J Radiat Oncol Biol Phys. 2010;77:1470–1476. doi: 10.1016/j.ijrobp.2009.06.048. [DOI] [PubMed] [Google Scholar]
- 24.Bristow RG, Hill RP. Hypoxia and metabolism. Hypoxia, DNA repair and genetic instability. Nat Rev Cancer. 2008;8:180–192. doi: 10.1038/nrc2344. [DOI] [PubMed] [Google Scholar]
- 25.Hockel M, Schlenger K, Aral B, Mitze M, Schaffer U, Vaupel P. Association between tumor hypoxia and malignant progression in advanced cancer of the uterine cervix. Cancer Res. 1996;56:4509–4515. [PubMed] [Google Scholar]
- 26.Eschmann SM, Paulsen F, Reimold M, Dittmann H, Welz S, Reischl G, Machulla HJ, Bares R. Prognostic impact of hypoxia imaging with 18f-misonidazole pet in non-small cell lung cancer and head and neck cancer before radiotherapy. J Nucl Med. 2005;46:253–260. [PubMed] [Google Scholar]
- 27.Koh WJ, Bergman KS, Rasey JS, Peterson LM, Evans ML, Graham MM, Grierson JR, Lindsley KL, Lewellen TK, Krohn KA, et al. Evaluation of oxygenation status during fractionated radiotherapy in human nonsmall cell lung cancers using [f-18]fluoromisonidazole positron emission tomography. Int J Radiat Oncol Biol Phys. 1995;33:391–398. doi: 10.1016/0360-3016(95)00170-4. [DOI] [PubMed] [Google Scholar]
- 28.Lee NY, Mechalakos JG, Nehmeh S, Lin Z, Squire OD, Cai S, Chan K, Zanzonico PB, Greco C, Ling CC, Humm JL, Schoder H. Fluorine-18-labeled fluoromisonidazole positron emission and computed tomography-guided intensity-modulated radiotherapy for head and neck cancer: A feasibility study. Int J Radiat Oncol Biol Phys. 2008;70:2–13. doi: 10.1016/j.ijrobp.2007.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mammar H, Kerrou K, Nataf V, Pontvert D, Clemenceau S, Lot G, George B, Polivka M, Mokhtari K, Ferrand R, Feuvret L, Habrand JL, Pouyssegur J, Mazure N, Talbot JN. Positron emission tomography/computed tomography imaging of residual skull base chordoma before radiotherapy using fluoromisonidazole and fluorodeoxyglucose: Potential consequences for dose painting. Int J Radiat Oncol Biol Phys. 2012;84:681–687. doi: 10.1016/j.ijrobp.2011.12.047. [DOI] [PubMed] [Google Scholar]
- 30.Agresti A. Inference for contingency tables, categorical data analysis. 2. New York, NY: John Wiley & Sons; 2002. [Google Scholar]
- 31.Hollander M, Wolfe DA. Nonparametric statistical methods. 2. New York, NY: John Wiley and sons; 1999. [Google Scholar]
- 32.R Core Team. R: A language and environment for statistical computing. [Accessed on February 3, 2014]; Avaliable from: http://www.R-project.org/
- 33.Pieters RS, Niemierko A, Fullerton BC, Munzenrider JE. Cauda equina tolerance to high-dose fractionated irradiation. Int J Radiat Oncol Biol Phys. 2006;64:251–257. doi: 10.1016/j.ijrobp.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 34.Lin Z, Mechalakos J, Nehmeh S, Schoder H, Lee N, Humm J, Ling CC. The influence of changes in tumor hypoxia on dose-painting treatment plans based on 18f-fmiso positron emission tomography. Int J Radiat Oncol Biol Phys. 2008;70:1219–1228. doi: 10.1016/j.ijrobp.2007.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nehmeh SA, Lee NY, Schroder H, Squire O, Zanzonico PB, Erdi YE, Greco C, Mageras G, Pham HS, Larson SM, Ling CC, Humm JL. Reproducibility of intratumor distribution of (18)f-fluoromisonidazole in head and neck cancer. Int J Radiat Oncol Biol Phys. 2008;70:235–242. doi: 10.1016/j.ijrobp.2007.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jansen JF, Schoder H, Lee NY, Wang Y, Pfister DG, Fury MG, Stambuk HE, Humm JL, Koutcher JA, Shukla-Dave A. Noninvasive assessment of tumor microenvironment using dynamic contrast-enhanced magnetic resonance imaging and 18f-fluoromisonidazole positron emission tomography imaging in neck nodal metastases. Int J Radiat Oncol Biol Phys. 2010;77:1403–1410. doi: 10.1016/j.ijrobp.2009.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]


