Abstract
BACKGROUND
Although obesity is an independent risk factor for heart failure (HF), once HF is established, obesity is associated with lower mortality. It is unclear if the weight loss due to advanced HF leads to this paradoxical finding.
OBJECTIVES
We sought to evaluate the prognostic impact of pre-morbid obesity in patients with HF.
METHODS
In the Atherosclerosis Risk in Communities (ARIC) study, we used body mass index (BMI) measured ≥6 months before incident HF (pre-morbid BMI) to evaluate the association of overweight (BMI 25 to <30 kg/m2) and obesity (≥30 kg/m2) compared to normal BMI (18.5 to <25 kg/m2) with mortality after incident HF.
RESULTS
Among 1,487 patients with incident HF, 35% were overweight and 47% were obese by pre-morbid BMI measured 4.3 ± 3.1 years before HF diagnosis. Over 10-year follow-up after incident HF, 43% of patients died. After adjustment for demographics and comorbidities, being premorbidly overweight (hazard ratio [HR]: 0.72; 95% confidence interval [CI[]: 0.58 to 0.90; p = 0.004) or obese (HR: 0.70; 95% CI: 0.56 to 0.87; p = 0.001) had a protective association with survival compared to normal BMI. The protective effect of overweight and obesity was consistent across subgroups based on a history of cancer, smoking, and diabetes.
CONCLUSIONS
Our results, for the first time, demonstrate that individuals who were overweight or obese before HF development have lower mortality once they have HF compared with normal BMI individuals. Thus, weight loss due to advanced HF may not completely explain the protective effect of higher BMI in HF patients.
Keywords: Obesity paradox, outcomes, overweight, premorbid
INTRODUCTION
Obesity has reached epidemic proportions in the United States, with more than two-thirds of adults being either overweight or obese (1). Obesity is linked to the development of cardiovascular diseases including atherosclerosis and hypertension (2). Although independently associated with the development of heart failure (HF) (3,4), obesity also has been shown to be associated with better survival once HF is established (5,6–9), often referred to as the “obesity paradox” (10).
One plausible explanation for this paradox: HF patients who gain or preserve their weight may represent a noncatabolic subgroup of HF patients with different neurohormonal, inflammatory, and metabolic profiles compared with HF patients who lose weight. The known protective effects of the ability to maintain or gain weight in other chronic diseases or catabolic states such as the acquired immunodeficiency syndrome, renal disease, and cancer lend support to this concept (11). Therefore, spontaneous weight loss (cachexia in extreme cases) after the development of HF may characterize a sicker group of patients with HF and, thus, may be associated with greater mortality (12).
However, it is not clear if weight loss after development of HF is the sole contributor to the obesity paradox or whether additional mechanisms, such as pre-existing obesity with possibly greater metabolic reserve prior to HF onset, contribute to the better survival of HF patients. Therefore, we examined the relationship of premorbid obesity (i.e., prior to incident HF) with mortality following incident HF. To our knowledge, this is the first study to investigate the prognostic implications of premorbid obesity with outcomes after the development of HF.
METHODS
STUDY COHORT
The ARIC (Atherosclerosis Risk in Communities) study is an ongoing community-based cohort study of 15,792 patients, comprised mostly of Caucasian and African-American men and women, aged 45 to 64 years at baseline (1987 to 1989) and sampled from 4 U.S. communities: Forsyth County, North Carolina; suburbs of Minneapolis, Minnesota; Washington County, Maryland; and Jackson, Mississippi (13). The institutional review boards from each site approved the ARIC study and all participants provided written informed consent. Standardized physical examinations and interviewer-administered questionnaires were conducted at baseline (visit 1) and at approximately 3-year follow-up intervals (visit 4: 1996 to 1998). Participant follow-up through annual telephone interviews, hospitalization, and vital status is ongoing. Individuals with missing anthropometry (n = 33), prevalent HF at the first study visit (n = 751), and those with missing data to determine prevalent HF at baseline (n = 289) were excluded (14). Participants with race not classified as white or black (n = 48), and blacks not from Jackson or Forsyth County (n = 120) were excluded due to their limited numbers.
ASCERTAINMENT OF HF CASES AND FOLLOW-UP
To determine HF cases, the following methods were used: 1) annual interviews of participants regarding interim hospitalizations (response rate: 93% to 96%); 2) review of discharge lists from local hospitals; and 3) survey of health department death certificate files and the national death index. Incident HF was defined as the first episode of either a hospitalization that included an International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) discharge diagnosis code for HF beginning with “428” (i.e., 428.0 to 428.9) in any position or a death certificate ICD-9 code beginning with “428” or ICD-10 code “I50” (HF or I50.0 to I50.9) in any position. For this study, incident HF was determined until December 31, 2004, date of last contact, or death (14).
ANTHROPOMETRY
Participants presented for each study visit after an overnight fast and measurements were taken in standard scrub attire. Weight was measured using a scale that was zeroed daily and calibrated quarterly. Premorbid body mass index (BMI) was defined as a BMI measurement from a study visit that occurred 6 months or more prior to the incident date of HF. Patients with HF were categorized by the premorbid BMI into normal (18.5 to <25 kg/m2), overweight (25 to <30 kg/m2), and obese (≥30 kg/m2) groups (15). Patients in the underweight category (BMI <18.5 kg/m2; n = 32) were excluded from this analysis because of small numbers and possible other pre-existing comorbidities that may have led to a cachectic state.
BASELINE COVARIATES
Ascertainment of demographics and comorbidities at each study visit has been described in detail previously (13). Age was assessed at the time of incident HF and sex, race, and education level were obtained from the baseline ARIC visit with interviewer-administered questionnaires. Comorbidities, including hypertension, history of myocardial infarction (MI), coronary heart disease (CHD), diabetes mellitus (DM) and stroke were assessed as present if these conditions were documented at any of the pre-HF study visits. History of MI was defined as self-report of physician-diagnosed MI or electrocardiographic diagnosis of silent MI. CHD was defined as history of MI, coronary revascularization, or coronary artery bypass surgery. Hypertension was defined by either a systolic blood pressure (SBP) ≥140 mm Hg or diastolic blood pressure ≥90 mm Hg measured with random-zero mercury manometers or recent anti-hypertensive medication use. Presence of DM was defined as either self-reported physician-diagnosed diabetes, recent diabetes medication use, or a blood glucose ≥126 mg/dl fasting or ≥200 mg/dl nonfasting (13).
Alcohol use, SBP, serum creatinine, total serum cholesterol, and insurance status also were collected from the pre-HF ARIC study visit. Patients were defined as smokers if the participants reported a history of current smoking at the pre-HF ARIC study visit. A history of cancer reported at any study visit prior to or including the pre-HF visit was used to define a positive history of cancer. Data for any variables with missing values at the pre-HF visit utilized available data from prior study visits. Renal function was quantified by the estimated glomerular filtration rate (eGFR) using the Modification of Diet in Renal Disease equation (16).
STATISTICAL ANALYSIS
Data are shown as mean ± standard deviation and percentages. Missing values were found to be less than 0.8%. Univariate differences among the 3 BMI groups were examined using the chi-square test for categorical variables and the analysis of variance (ANOVA) test for continuous variables. To assess the prognostic significance of premorbid BMI in HF patients, the endpoint was time to all-cause mortality after incident HF. We performed Kaplan-Meier survival analysis and used the log-rank test to compare time to death after incident HF among the 3 BMI groups. Because the number of patients after 10-year follow-up from the incident HF episode was small, we censored the follow-up after incident HF at 10 years. Cox proportional hazard models were used to examine the relationship between the BMI group and survival. Due to violation of the proportionality assumption, a time-dependent term, the product of BMI group and log time was added to the model to represent the nonhomogeneity of the hazard (17). The adjusted model was fitted by adding age, sex, race, history of MI, hypertension, CHD, DM, stroke, cancer, alcohol use, smoking status, insurance, education level, SBP, and eGFR as covariates. Due to concerns relating to smoking and history of cancer and their association with BMI status and mortality, as well as the fact that statistical adjustments may not be sufficient to control for duration, intensity, or timing of smoking exposure, we also conducted subgroup analyses by the presence or absence of a history of smoking and cancer. Similarly, due to prior studies demonstrating a possible differential effect of obesity and overweight on mortality in HF patients based on diabetic status, we also performed subgroup analysis by the presence or absence of DM (5,7,18–22). P values < 0.05 were considered to be significant. All analyses were performed using SAS version 9.3 (SAS Institute Inc., Cary, North Carolina).
RESULTS
The study cohort consisted of 1.487 HF patients categorized as normal, overweight, or obese based on BMI obtained at least 6 months prior to incident HF. Overall, 54% of the HF patients were male and 66% were white; they averaged 67 years of age at the time of diagnosis. Premorbidly, the majority of patients were either overweight (35%) or obese (47%). The premorbid BMI was measured 4.3 ± 3.1 years before the HF diagnosis at the last ARIC study visit that occurred at least 6 months prior to incident HF. Baseline characteristics of patients with incident HF by the 3 premorbid BMI groups are shown in Table 1. Compared to HF patients with normal BMI, obese HF patients were younger, more often African American, and less likely to have health insurance, and attained a lower education level. Overweight and obese HF patients had higher prevalence of comorbidities such as DM and hypertension, as well as higher SBP. Smoking was associated with lower BMI. There was no significant difference in the time period of measurement of BMI prior to incident HF among the 3 BMI categories.
TABLE 1.
Baseline Characteristics in Patients with Incident HF
| Normal Weight n = 274 | Over-weight n = 518 | Obese n = 695 | p Value | ||
|---|---|---|---|---|---|
| Age at incident HF, yrs | 67 ± 6 | 67 ± 7 | 66 ± 7 | 0.02 | |
| Male | 149 (54) | 338 (65) | 316 (45) | <0.0001 | |
| Black | 73 (27) | 155 (30) | 279 (40) | 0.0001 | |
| Education level | Did not graduate HS | 101 (37) | 198 (38) | 303 (44) | 0.02 |
| HS graduate | 105 (38) | 173 (33) | 248 (36) | ||
| Higher than HS | 67 (25) | 146 (28) | 143 (21) | ||
| Health insurance | 232 (85) | 423 (82) | 541 (78) | 0.03 | |
| Diabetes mellitus | 58 (21) | 185 (36) | 359 (52) | <0.0001 | |
| Hypertension | 168 (61) | 358 (69) | 537 (77) | <0.0001 | |
| History of MI (by history or ECG) | 56 (20) | 123 (24) | 159 (23) | 0.57 | |
| History of CAD | 55 (20) | 127 (25) | 143 (21) | 0.19 | |
| History of stroke | 17 (6) | 44 (8) | 47 (7) | 0.39 | |
| History of cancer | 41 (15) | 68 (13) | 79 (11) | 0.29 | |
| Current smoker | 129 (47) | 162 (32) | 149 (22) | <0.0001 | |
| History of alcohol use | 117 (43) | 225 (44) | 227 (33) | 0.0004 | |
| Total serum cholesterol, mg/dl | 204 ± 42 | 207 ± 46 | 210 ± 46 | 0.27 | |
| Blood pressure | |||||
| Systolic, mm Hg | 137 ± 26 | 138 ± 25 | 141 ± 24 | 0.01 | |
| Diastolic, mm Hg | 71 ± 13 | 72 ± 13 | 73 ± 13 | 0.08 | |
| Estimated GFR, ml/min/1.73 m2 | 85 ± 26 | 82 ± 23 | 83 ± 25 | 0.14 | |
| Time to HF, yrs* | 4.2 ± 3.2 | 4.2 ± 3.1 | 4.4 ± 3.2 | 0.49 | |
Time from HF = time between the pre-HF ARIC study visit and incident HF hospitalization.
Values are n (%) or mean ± standard deviation.
ARIC = Atherosclerosis Risk in Communities study; CAD = coronary artery disease; ECG = electrocardiogram; GFR = glomerular filtration rate; HF = heart failure; HS = high school; MI = myocardial infarction.
Over a follow-up period of 10 years, a total of 43% of all HF patients died. The Central Illustration shows the Kaplan-Meier survival curves by BMI group over 10-year follow-up after incident HF. The overweight and obese groups had better survival compared with the normal-weight group. Over time, the survival curve for the obese patients appeared to converge towards the other groups, and crossed over the overweight group at ~7.5 years follow-up. On the other hand, the curves for overweight appeared to remain parallel to the normal-weight group. Compared to normal BMI, overweight (hazard ratio [HR]: 0.77; 95% confidence interval [CI]: 0.62 to 0.96) and obesity (HR: 0.75; 95% CI: 0.61 to 0.91) were associated with improved survival. As expected from the Kaplan-Meier curves, the interaction between BMI group and log time was not significant for the overweight group (p = 0.74), but was significant for the obese group (p = 0.02). The interaction terms were therefore included in the models. As shown in Table 2, even after adjusting for covariates, premorbid overweight and obesity remained independent predictors of improved survival compared with normal BMI.
CENTRAL ILLUSTRATION. Kaplan-Meier Survival Curves in HF Patients by Pre-HF BMI Categories.
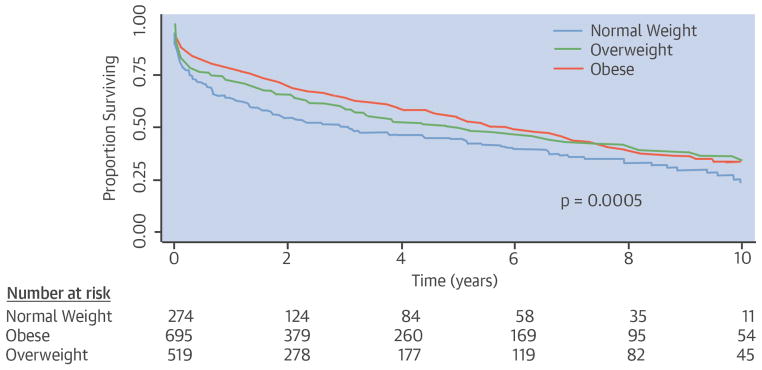
Survival after the development of heart failure (HF) differed significantly among body mass index (BMI) groups defined by pre-HF BMI. The overweight and obese patients had better survival compared with the normal-weight group.
TABLE 2.
Hazard Ratios for All-cause Mortality after Incident HF
| BMI Category | Incident HF | Deaths over 10 Years | Adjusted Hazard Ratio (95% CI)† | p Value†† |
|---|---|---|---|---|
| Normal | 274 | 141 (51) | 1 | |
| Overweight | 519 | 232 (45) | 0.72 (0.58 – 0.90) | 0.99 |
| Obese | 695 | 265 (38) | 0.70 (0.56 – 0.87) | 0.02 |
The models included the following covariates in addition to BMI and time-dependent term of group*log (time in years): age; sex; race; education level; health insurance; diabetes; hypertension; history of MI, CAD, or stroke; cancer; smoking; alcohol use; systolic blood pressure; total cholesterol; and estimated GFR.
For BMI group*log time interaction
Values are n or n (%).
BMI = body mass index; CI = confidence interval; other abbreviations as in Table 1.
A sensitivity analysis was performed after excluding patients with a presentation of fatal incident HF. Again, the overweight (adjusted HR: 0.72; 95% CI: 0.57 to 0.90; interaction with log time p = 0.37) and obese (adjusted HR: 0.69; 95% CI: 0.56 to 0.86; interaction with log time p < 0.001) HF patients had significantly better survival compared with normal BMI HF patients.
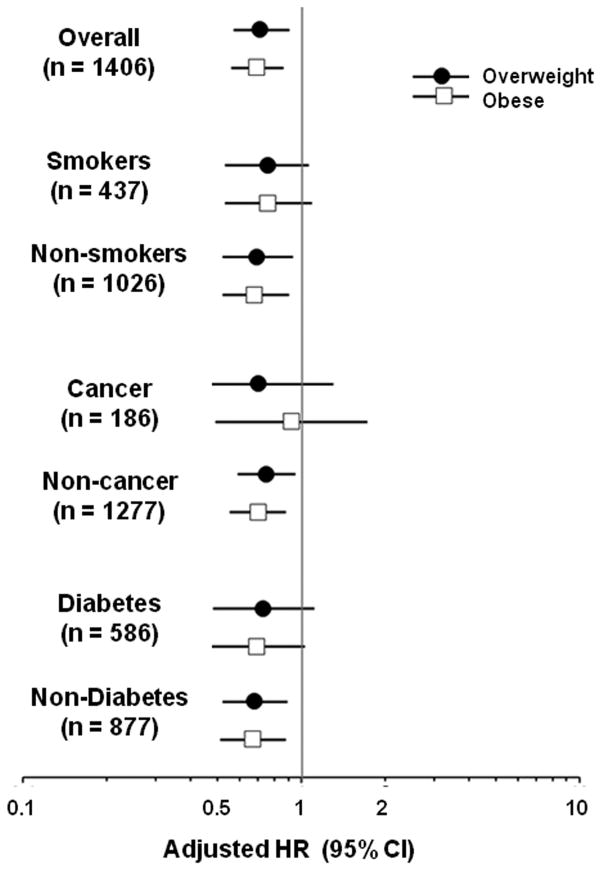
Subgroup analyses were conducted in HF patients based on smoking, history of cancer, and the presence or absence of diabetes (Figure 1). The beneficial trends associated with premorbid overweight and obesity compared with normal weight, similar to those observed in the overall cohort, were noted in all subgroups, although not all differences reached statistical significance.
FIGURE 1. Adjusted Risk of Mortality Associated with BMI Category.
The adjusted hazard ratios (HR) and 95% confidence intervals (CI) for mortality risk for the obese and overweight groups compared to the normal-weight group (reference group; HR = 1) are shown on a logarithmic scale for the overall cohort, and for the subgroups stratified by smoking, cancer, and diabetes. The results are consistent across all subgroups.
DISCUSSION
This study demonstrates that among individuals who develop HF in the community, the majority are premorbidly overweight or obese. Additionally, for the first time, we have shown that patients who are overweight or obese before incident HF have better survival after they develop HF compared with patients with normal BMI. This association is independent of the patients’ demographic profile and comorbidities. Furthermore, this trend occurred irrespective of smoking status, history of cancer, or diabetes.
Our findings of an association between a higher premorbid BMI and improved survival following incident HF could suggest that obese patients have a higher metabolic reserve compared with normal-weight patients, providing them with a survival advantage when cardiac cachexia ensues after HF development. Several studies have demonstrated an obesity paradox in patients with chronic and acute decompensated HF, i.e., a survival advantage of higher BMI measured in patients with established HF (5,7–9, 18–22). It must be noted that none of the previous studies demonstrating the obesity paradox in HF used the BMI (or BMI-equivalent variable) prior to development of HF. Moreover, very few studies had a follow-up >5 years (23,24). Because prior studies used the BMI of patients with established HF, they were unable to distinguish between the effect of weight loss between the time of development of HF and the BMI measurement as a marker of more advanced HF versus the possible survival advantage of pre-existing obesity or overweight. Our study goes a major step further than prior studies by demonstrating that higher premorbid BMI is independently associated with a long-term survival advantage over a long (10-year) follow-up period.
Several proposed mechanisms could contribute to this apparent obesity paradox, including the fact that HF is a catabolic state leading to cachexia, and obese and overweight patients may have better outcomes as they have higher metabolic reserves (12,25). Another hypothesis is that obesity alters the natural history of HF through neurohumoral pathways. Higher levels of serum lipoproteins may neutralize bacterial lipopolysaccharides and thus attenuate the detrimental cytokine response in HF (26–29). Adipose tissue may produce higher levels of soluble tumor necrosis factor (TNF) receptors that serve as a reservoir for harmful circulating TNF (30). Levels of circulating stem cells are also higher in obese individuals (31). Furthermore, obese patients have decreased adiponectin levels and an attenuated renin-angiotensin system and catecholamine response, both of which are associated with improved HF survival (26,32).
Another possible explanation is that obese or overweight individuals may present with and be diagnosed with HF at an earlier stage due to symptoms exacerbated by excess body weight, such as dyspnea and edema (that is, the obesity paradox may represent a lead-time bias). Furthermore, obese patients have a higher prevalence of comorbidities such as hypertension and DM, as supported by our study, and may represent a higher-risk population for HF. Another possibility: the higher prevalence of hypertension, as well as higher blood pressures, in the overweight and obese patients may allow greater up-titration of disease-modifying HF therapies. It is interesting to note that the protective effect of obesity was greatest during the initial years (Central Illustration) with significant interaction between BMI group and time, suggesting perhaps that during the later years, the complications of obesity-associated comorbidities catch up and lead to a greater decline in survival. This finding would also be expected if a lead-time bias is contributing with an earlier presentation with HF due to obesity-enhanced symptoms. Unlike our analysis, most studies that have examined the obesity paradox in patients with established HF have had shorter follow-up periods, usually <5 years. In contrast, the protective effect of being overweight did not appear to decrease over time. Although a history of cancer and smoking are associated with lower BMI and higher mortality, perhaps confounding the analyses of BMI and survival, our subgroup analyses did suggest that the observed results were independent of smoking or cancer status.
Previous studies that have evaluated the association between obesity and cardiovascular outcomes have used various indices of obesity, including BMI, waist circumference and waist-hip ratio, and percent body fat (22,23). Whereas, waist-hip ratio and waist circumference are better predictors of central obesity, BMI reflects generalized obesity (34,35). Based on previous analyses conducted on the ARIC cohort (14), which had confirmed that obesity and overweight are independent risk factors for developing HF, the degree and pattern of relationships for the development of HF were comparable for all 3 indices of obesity. Also, because most previous studies that have evaluated the association between obesity and cardiovascular outcomes have used BMI, we used BMI as the index of obesity in our study.
The recent HF guidelines from the American College of Cardiology and American Heart Association do not specifically recommend weight reduction in obese patients with HF based on the lack of data demonstrating a beneficial effect in this population (36). Although our study suggests that patients who are overweight/obese before the development of HF have better survival compared with patients of normal weight, it does not answer whether targeted weight reduction in obese patients with HF is beneficial or not. Only a randomized controlled trial of targeted weight reduction in obese patients with HF could help resolve that question.
STUDY LIMITATIONS
Our study has inherent limitations associated with an observational cohort study, including those of possible residual confounding from unmeasured covariates. In addition, identification of the cases relied on ICD–9 codes; only hospitalized HF and incident fatal HF were included because we lacked consistent data on outpatient HF. However, validation of HF hospitalizations in an ARIC community surveillance study in 2005 has shown that the sensitivity and positive predictive value of ICD code 428.x in any position for HF classified by subsequent medical record review by ARIC criteria were 0.95 and 0.77, respectively, for combined acute decompensated HF and chronic HF (in comparison to 0.83 and 0.78, respectively, by Framingham criteria) (37). Also, the fact that there was a long time period between measurement of BMI and incident HF (average 4.2 years) makes it unlikely that our cohort included HF cases in whom weight loss as a result of HF would have occurred.
Furthermore, community surveillance reports have indicated that 74% of outpatient HF cases are hospitalized within 1.7 years (38). Since a diagnosis of HF in obese individuals may be less specific than that in normal-weight individuals, there is a possibility of differential misclassification bias. Also, we were unable to adjust for level of fitness, which has been shown to modify the association of BMI with prognosis in HF (39,40). Fatal initial HF episodes were determined from death certificates, which may overestimate or underestimate the true number of cases. We did not have a record of medical therapies instituted following incident HF and were unable to adjust for potential differences in therapy by BMI status. Additionally, the type of HF (HF with preserved or reduced ejection fraction) was not known.
CONCLUSIONS
The majority of patients with incident HF in the community have pre-existing overweight or obesity. Once the overweight/obese patients develop HF, they have lower mortality compared with HF patients with prior normal BMI. These results suggest that a significant component of the obesity paradox is driven by premorbid obesity and it is, therefore, unlikely that cardiac cachexia due to advanced HF is the only mechanism contributing to the observed obesity paradox in established HF. Future studies are needed to confirm our observations in other cohorts.
PERSPECTIVES.
COMPETENCY IN MEDICAL KNOWLEDGE
Although higher body mass index is associated with an increased risk of developing clinical heart failure (HF), patients with HF who are overweight or obese have better survival rates than those with normal weight. This obesity paradox also applies to patients who were overweight or obese before incident HF hospitalization, suggesting that the paradox is not entirely accounted for by weight loss or cardiac cachexia due to HF.
TRANSLATIONAL OUTLOOK
Further research is needed to understand the mechanisms responsible for the protective effect of premorbid overweight and obesity and the effect of intentional weight loss on clinical outcomes in patients with HF.
Acknowledgments
The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C). The authors thank the staff and participants of the ARIC study for their important contributions.
ABBREVIATIONS
- ARIC
Atherosclerosis Risk in Communities
- BMI
body mass index
- CHD
coronary heart disease
- DM
diabetes mellitus
- eGFR
estimated glomerular filtration rate
- HF
heart failure
- MI
myocardial Infarction
Footnotes
Conflict(s) Of Interest/Disclosure(s): None of the authors have any financial or other relations that could lead to a conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Flegal KM, Carroll MD, Kit BK, et al. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA. 2012;307:491–7. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 2.Yusuf S, Hawken S, Ounpuu S, et al. Obesity and the risk of myocardial infarction in 27,000 participants from 52 countries: a case-control study. Lancet. 2005;366:1640–9. doi: 10.1016/S0140-6736(05)67663-5. [DOI] [PubMed] [Google Scholar]
- 3.Kenchaiah S, Evans JC, Levy D, et al. Obesity and the risk of heart failure. N Engl J Med. 2002;347:305–13. doi: 10.1056/NEJMoa020245. [DOI] [PubMed] [Google Scholar]
- 4.Kenchaiah S, Sesso HD, Gaziano JM. Body mass index and vigorous physical activity and the risk of heart failure among men. Circulation. 2009;119:44–52. doi: 10.1161/CIRCULATIONAHA.108.807289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Horwich TB, Fonarow GC, Hamilton MA, et al. The relationship between obesity and mortality in patients with heart failure. J Am Coll Cardiol. 2001;38:789–95. doi: 10.1016/s0735-1097(01)01448-6. [DOI] [PubMed] [Google Scholar]
- 6.Kenchaiah S, Pocock SJ, Wang D, et al. Body mass index and prognosis in patients with chronic heart failure: insights from the Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity (CHARM) program. Circulation. 2007;116:627–36. doi: 10.1161/CIRCULATIONAHA.106.679779. [DOI] [PubMed] [Google Scholar]
- 7.Fonarow GC, Srikanthan P, Costanzo MR, et al. An obesity paradox in acute heart failure: analysis of body mass index and inhospital mortality for 108,927 patients in the Acute Decompensated Heart Failure National Registry. Am Heart J. 2007;153:74–81. doi: 10.1016/j.ahj.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 8.Lavie CJ, Alpert MA, Arena R, et al. Impact of obesity and the obesity paradox on prevalence and prognosis in heart failure. JACC Heart Fail. 2013;1:93–102. doi: 10.1016/j.jchf.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 9.Shah R, Gayat E, Januzzi JL, Jr, et al. Body mass index and mortality in acutely decompensated heart failure across the world: a global obesity paradox. J Am Coll Cardiol. 2014;63:778–85. doi: 10.1016/j.jacc.2013.09.072. [DOI] [PubMed] [Google Scholar]
- 10.Lavie CJ, De SA, Alpert MA, et al. Obesity paradox, cachexia, frailty, and heart failure. Heart Fail Clin. 2014;10:319–26. doi: 10.1016/j.hfc.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 11.Boban M, Persic V, Jovanovic Z, et al. Obesity dilemma in the global burden of cardiovascular diseases. Int J Clin Pract. 2014;68:173–9. doi: 10.1111/ijcp.12254. [DOI] [PubMed] [Google Scholar]
- 12.Anker SD, Ponikowski P, Varney S, et al. Wasting as independent risk factor for mortality in chronic heart failure. Lancet. 1997;349:1050–3. doi: 10.1016/S0140-6736(96)07015-8. [DOI] [PubMed] [Google Scholar]
- 13.The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 14.Loehr LR, Rosamond WD, Poole C, et al. Association of multiple anthropometrics of overweight and obesity with incident heart failure: the Atherosclerosis Risk in Communities study. Circ Heart Fail. 2009;2:18–24. doi: 10.1161/CIRCHEARTFAILURE.108.813782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults--The Evidence Report. National Institutes of Health Obes Res. 1998;6 (Suppl 2):51S–209S. No authors listed. [PubMed] [Google Scholar]
- 16.Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–70. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 17.Ata N, Sozer MT. Cox regression models with nonproportional hazards applied to lung cancer survival data. Hacet J Math Stat. 2007;36:157–67. [Google Scholar]
- 18.Arena R, Myers J, Abella J, et al. Influence of etiology of heart failure on the obesity paradox. Am J Cardiol. 2009;104:1116–21. doi: 10.1016/j.amjcard.2009.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bozkurt B, Deswal A. Obesity as a prognostic factor in chronic symptomatic heart failure. Am Heart J. 2005;150:1233–9. doi: 10.1016/j.ahj.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 20.Curtis JP, Selter JG, Wang Y, et al. The obesity paradox: body mass index and outcomes in patients with heart failure. Arch Intern Med. 2005;165:55–61. doi: 10.1001/archinte.165.1.55. [DOI] [PubMed] [Google Scholar]
- 21.Kapoor JR, Heidenreich PA. Obesity and survival in patients with heart failure and preserved systolic function: a U-shaped relationship. Am Heart J. 2010;159:75–80. doi: 10.1016/j.ahj.2009.10.026. [DOI] [PubMed] [Google Scholar]
- 22.Lavie CJ, Osman AF, Milani RV, et al. Body composition and prognosis in chronic systolic heart failure: the obesity paradox. Am J Cardiol. 2003;91:891–4. doi: 10.1016/s0002-9149(03)00031-6. [DOI] [PubMed] [Google Scholar]
- 23.Zamora E, Lupon J, Urrutia A, et al. Obesity and long-term prognosis in heart failure: the paradox persists. Rev Esp Cardiol. 2010;63:1210–2. doi: 10.1016/s1885-5857(10)70238-3. [DOI] [PubMed] [Google Scholar]
- 24.Zamora E, Lupon J, de AM, et al. The obesity paradox in heart failure: is etiology a key factor? Int J Cardiol. 2013;166:601–5. doi: 10.1016/j.ijcard.2011.11.022. [DOI] [PubMed] [Google Scholar]
- 25.Davos CH, Doehner W, Rauchhaus M, et al. Body mass and survival in patients with chronic heart failure without cachexia: the importance of obesity. J Card Fail. 2003;9:29–35. doi: 10.1054/jcaf.2003.4. [DOI] [PubMed] [Google Scholar]
- 26.Kistorp C, Faber J, Galatius S, et al. Plasma adiponectin, body mass index, and mortality in patients with chronic heart failure. Circulation. 2005;112:1756–62. doi: 10.1161/CIRCULATIONAHA.104.530972. [DOI] [PubMed] [Google Scholar]
- 27.Lavie CJ, Ventura HO. Weighing in on obesity and the obesity paradox in heart failure. J Card Fail. 2011;17:381–3. doi: 10.1016/j.cardfail.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 28.Rauchhaus M, Coats AJ, Anker SD. The endotoxin-lipoprotein hypothesis. Lancet. 2000;356:930–3. doi: 10.1016/S0140-6736(00)02690-8. [DOI] [PubMed] [Google Scholar]
- 29.Rauchhaus M, Clark AL, Doehner W, et al. The relationship between cholesterol and survival in patients with chronic heart failure. J Am Coll Cardiol. 2003;42:1933–40. doi: 10.1016/j.jacc.2003.07.016. [DOI] [PubMed] [Google Scholar]
- 30.Mohamed-Ali V, Goodrick S, Bulmer K, et al. Production of soluble tumor necrosis factor receptors by human subcutaneous adipose tissue in vivo. Am J Physiol. 1999;277:E971–E975. doi: 10.1152/ajpendo.1999.277.6.E971. [DOI] [PubMed] [Google Scholar]
- 31.Blogowski W, Serwin K, Budkowska M, et al. Clinical analysis of systemic and adipose tissue levels of selected hormones/adipokines and stromal-derived factor-1. J Biol Regul Homeost Agents. 2012;26:607–15. [PubMed] [Google Scholar]
- 32.Weber MA, Neutel JM, Smith DH. Contrasting clinical properties and exercise responses in obese and lean hypertensive patients. J Am Coll Cardiol. 2001;37:169–74. doi: 10.1016/s0735-1097(00)01103-7. [DOI] [PubMed] [Google Scholar]
- 33.Clark AL, Fonarow GC, Horwich TB. Waist circumference, body mass index, and survival in systolic heart failure: the obesity paradox revisited. J Card Fail. 2011;17:374–80. doi: 10.1016/j.cardfail.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 34.Folsom AR, Kushi LH, Anderson KE, et al. Associations of general and abdominal obesity with multiple health outcomes in older women: the Iowa Women’s Health Study. Arch Intern Med. 2000;160:2117–28. doi: 10.1001/archinte.160.14.2117. [DOI] [PubMed] [Google Scholar]
- 35.Nicklas BJ, Cesari M, Penninx BW, et al. Abdominal obesity is an independent risk factor for chronic heart failure in older people. J Am Geriatr Soc. 2006;54:413–20. doi: 10.1111/j.1532-5415.2005.00624.x. [DOI] [PubMed] [Google Scholar]
- 36.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62:e147–e239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 37.Rosamond WD, Chang PP, Baggett C, et al. Classification of heart failure in the atherosclerosis risk in communities (ARIC) study: a comparison of diagnostic criteria. Circ Heart Fail. 2012;5:152–9. doi: 10.1161/CIRCHEARTFAILURE.111.963199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roger VL, Weston SA, Redfield MM, et al. Trends in heart failure incidence and survival in a community-based population. JAMA. 2004;292:344–50. doi: 10.1001/jama.292.3.344. [DOI] [PubMed] [Google Scholar]
- 39.Lavie CJ, Cahalin LP, Chase P, et al. Impact of cardiorespiratory fitness on the obesity paradox in patients with heart failure. Mayo Clin Proc. 2013;88:251–8. doi: 10.1016/j.mayocp.2012.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lavie CJ, McAuley PA, Church TS, et al. Obesity and cardiovascular diseases: implications regarding fitness, fatness, and severity in the obesity paradox. J Am Coll Cardiol. 2014;63:1345–54. doi: 10.1016/j.jacc.2014.01.022. [DOI] [PubMed] [Google Scholar]