Abstract
Background and Purpose
The causes of tumor response variation to radiation remain obscure, thus hampering the development of predictive assays and strategies to decrease resistance. The present study evaluates the impact of host tumor stromal elements and the in vivo environment on tumor cell kill, and relationship between tumor cell radiosensitivity and the tumor control dose.
Material and Methods
Five endpoints were evaluated and compared in a radiosensitive DNA double-strand break repair-defective (DNA-Pkcs−/−) tumor line, and its DNA-PKcs repair competent transfected counterpart. In vitro colony formation assays were performed on in vitro cultured cells; on cells obtained directly from tumors, and on cells irradiated in situ. Permanent local control was assessed by the TCD50 assay. Vascular effects were evaluated by functional vascular density assays.
Results
The fraction of repair competent and repair deficient tumor cells surviving radiation did not substantially differ whether irradiated in vitro, i.e., in the absence of host stromal elements and factors, from the fraction of cells killed following in vivo irradiation. Additionally, the altered tumor cell sensitivity resulted in a proportional change in the dose required to achieve permanent local control. The estimated number of tumor cells per tumor, their cloning efficiency and radiosensitivity, all assessed by in vitro assays, were used to predict successfully, the measured tumor control doses.
Conclusion
The number of clonogens per tumor and their radiosensitivity govern the permanent local control dose.
Keywords: radiation, tumor control dose, intrinsic radiosensitivity, tumor vasculature, predictive assays
Introduction
Although employed as a principal method for the treatment of cancer for over 60 years, the biological causes of local treatment failure following radiation therapy remain obscure. The absence of such knowledge hampers the development of assays for identifying likely resistant vs. sensitive tumors as well as strategies to target and reduce resistance. Especially puzzling is the range of responses obtained in tumors matched for histology, and grade [1, 2]. Local failure not only directly causes or contributes to life shortening, but importantly, increases the probability of distant metastases [3–6].
Relatively recent studies have reported that the dose to achieve permanent local tumor control is determined by radiation damage of the tumor stroma, and more specifically, the tumor vasculature [7, 8]. This contrasts with studies indicating that the radiation sensitivity of the tumor cells is the primary determinant of response [9–12].
Garcia-Barros et al [7, 8] reported that permanent local control was achieved in tumors exposed to 15 Gy, when transplanted and treated in acid sphingomyelinase (asmase+/+) proficient mice, but was without effect on the growth of the same tumors in their asmase−/− littermates. The difference in tumor response was attributed to a more pronounced vascular endothelial apopotic response for tumors in asmase+/+ mice, than was observed in tumors grown and treated in their Niemann-Picks analogue asmase−/− littermates.
In apparent conflict with these results, Budach et al measured the dose of radiation required to achieve permanent local control of 50% of treated spontaneous FSaII tumors (TCD50) isotransplanted into C3H mice, as well as into Nude and SCID mice [9]. SCID mice are hypersensitive to radiation due to the absence of a competent DNA double-strand break repair gene, DNA-PKcs [13–15]. These investigators found that the TCD50 values did not differ for tumors in C3H, Nude and SCID mice. The role of tumor cell sensitivity vs. vascular endothelial sensitivity was further augmented by studies showing that as pertained to other cells and tissues derived from SCID mice, SCID vascular endothelium was hypersensitive to radiation compared to the endothelium and vasculature of DNA-PKcs proficient mice [10, 16].
The present study determines if and the extent to which the radiation killing of tumor cells in vitro is altered by the tumor host stroma and in vivo environment. The study utilizes an isogenic tumor pair, i.e., a sensitive parental DNA-PKcs defective tumor line from SCID mice, and the same cell clone transfected with a competent DNA-PKcs+/+ repair gene. The isogenic tumors were grown and treated in wild type, asmase+/+ Nude mice. The radiosensitivity of the two tumor lines following irradiation in vitro, i.e., in the absence of stromal elements, was compared to the sensitivity of the tumor cells when irradiated in vivo. Concurrently, the clonogenic surviving fraction and the functional vascular density of the irradiated tumors was assessed at various times following in vivo irradiation. To further assess the role of the clonogens per tumor and their intrinsic radiosensitivity as determinants of the tumor control dose, we calculated and compared the predicted tumor control doses based on the in vitro estimated number of clonogens per tumor and their radiosensitivity, to the measured 50% control doses.
Materials and Methods
Mice, isogenic tumor cells and tumors
The DNA double-strand break repair deficient and proficient tumor lines used in this study, have been previously described [10, 11] and Supplementary Methods. For selection purposes the DNA-PKcs expression vector was co-transfected with the pSV2 neo plasmid. Source tumors were initiated from in vitro cultured cells. Experimental tumors were initiated by subcutaneous chunk transfer of source tumors to the hind leg of 8–9 week old male NCr/Sed nu/nu mice provided by the Cox-7 defined flora animal facility in MGH. The tumor volume doubling-times of both isogenic FSC and T53 tumors was two days over the caliper measured size range of 5 to 8 mm average diameter. All animal care and procedures were carried out and performed following the guidelines of Public Health Service Policy on Humane Care of Laboratory Animals and approved by the Institution Animal Care and Use Committee at the MGH.
Irradiation
Radiation was administered with 320 kVp X-rays (half value layer = 1.5 mm copper), at 1.66 Gray (Gy) per minute for cells and 3.76 Gy per minute for tumors. For irradiation of tumors under conditions of uniform hypoxia, a spring loaded brass clamp was applied to the root of the thigh for 3 minutes prior to and during radiation [17]. All cells and tumors were processed identically, including control tumors which were clamped and sham irradiated [18].
Colony Formation Assay
Regardless of the source of the cells, i.e., from in vitro cell cultures or from tumors, single cell suspensions were prepared and irradiated 18–24 hours later. Lethally irradiated feeder cells (20 Gy) were used to maintain a constant number of cells per flask [19]. All surviving fraction data was multiplicity corrected. Tumors were excised, weighted, cut into approximately 1mm pieces and single cell suspensions were prepared enzymatically, (Supplementary Methods). Five to six mm average diameter FSC and T53 tumors (approximately 25 mg tumor tissue) yielded approximately 5 X 106 trypan excluding cells. Cells obtained from FSC initiated tumors failed to form colonies in G418 containing selection medium, whereas the vivo to in vitro cloning efficiency of cells from T53 initiated tumors, did not significantly differ ± G418.
Tumor control dose (TCD50) assay
Following a pilot assay, 56 mice bearing 5 to 6 mm diameter FSC tumors and 56 bearing T53 tumors were entered into the TCD50 assay. Prior studies with the FSC and T53 tumor have shown that they contain a similar hypoxic fraction [11]. However, to reduce any tumor-to-tumor variability in tumor oxygenation, a spring-loaded clamp was placed around the upper thigh for 3 minutes prior to and during irradiation. Following irradiation, the mice were examined twice weekly for the presence of tumor and progressive tumor growth. Tumor bearing mice were sacrificed when tumors reached an average diameter of 7–8 mm. In the absence of palpable tumor and progressive growth, mice were followed for 120 days. Eight mice were irradiated at each of seven dose levels. The TCD50 values and their 95% confidence intervals were calculated as previously described [20].
Functional vascular density
Functional tumor vessels were labeled via tail vein injection of 10 μg of biotinylated lectin (Vector Laboratories, Burlingame CA) as previously described [10, 21, 22]. Vessel density was assessed in control and irradiated mice at 24, 96 and 288 hours following irradiation. Additional details are provided in Supplementary Methods.
Statistics
Survival curve terminal slopes were calculated by least square fitting of log transformed surviving fraction data. Tests of significance were calculated with unpaired, two tailed T tests. Confidence intervals are 95% standard error of the mean, unless otherwise indicated.
Results
Stability and radiation sensitivity of cells obtained from tumors
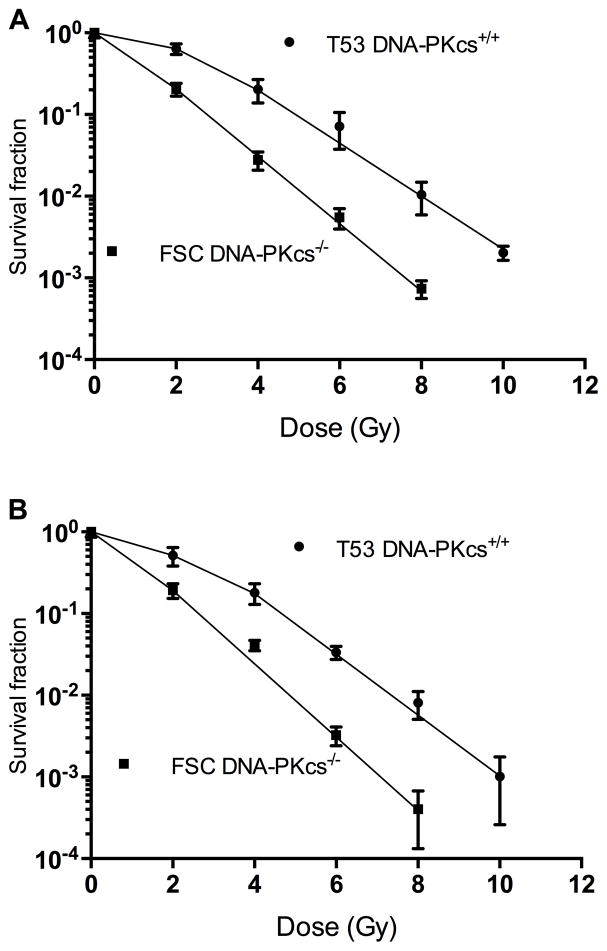
The clonogenic survival of the irradiated DNA-PKcs−/− cell line, FSC, and its DNA-PKcs+/+counterpart, T53, is shown in Figure 1. Panel A shows the response of cells that were cultured and irradiated in the absence of stromal elements and factors. The clonogenic response of cells obtained directly from tumors is shown in panel B. For both sets of data, FSC cells exhibit minimal resistance to low doses of radiation, whereas DNA-PKcs+/+ T53 cells are characterized by a relatively resistant shoulder region between the dose range of 0 to 4 Gy.
Figure 1.
DNA-PKcs −/− FSC cells (closed circle) and the same cells transfected with functional DNA-PKcs+/+ (T53), (closed square) were cultured for more than 20 passages in vitro and irradiated. The fraction of cells surviving irradiation is shown in Panel A. Panel B; same as in panel A, except FSC and T53 cells were obtained from F2–F3 generation tumors, from which a single cell suspension was prepared, plated and irradiated 18–24 hours later. Confidence intervals are one standard deviation.
To further characterize and assess the stability of the cells when grown in vivo, the data in Figure 1 were fit to the equation: ln Sf = −D/Do + ln (n), over the dose range of 2–8 Gy for FSC cells and 4–10 Gy for T53 cells, where Sf = surviving fraction, Do = the dose of radiation that reduces the surviving fraction on the exponential portion of the survival curve by a factor of “e”, D = the administered dose, and “n” equals the constant (survival curve extrapolation number). The coefficients of determination of the survival curve slopes, R2, ranged from 0.992 to 0.998 for the 4 curves shown in Figure 1. For both cell lines, neither the survival curve slopes or constants significantly differed, regardless of whether the cells were from in vitro cultures or directly from tumors (p value range = 0.15 to 0.80), whereas the slopes and constants significantly differed between the FSC and T53 lines (p < 0.05). The values of the parameter “n” and 95% confidence intervals of the mean are: 1.56 (1.49 – 1.64) and 6.36 (2.91 – 13.87) for FSC and T53 respectively, and “Do” = 1.00 (0.92 – 1.08) and 1.21 (1.01–1.38) Gy for FSC and T53 cells respectively.
Tumor cell survival following in vivo irradiation
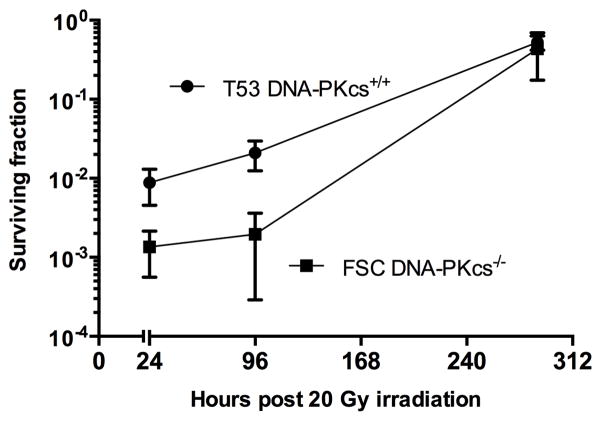
To determine if the in vivo environment altered the fraction of tumor cells sterilized by radiation, tumors were treated with 20 Gy upon reaching an average diameter of 5.5–6.0 mm. The results are shown in Figure 2. As was observed following the irradiation of in vitro cultured cells, the fraction of DNA-PKcs+/+ T53 cells surviving in vivo irradiation, substantially exceeded the fraction of FSC cells surviving in vivo irradiation. It is to be noted that 20 Gy under hypoxic conditions is approximately equivalent to 7.5–8 Gy administered under fully oxygenated conditions. The results provide no evidence of substantial or significant enhancement of radiation cell killing due to in vivo micro-environmental factors at 24 hr following irradiation, nor of post irradiation enhancement of radiation killing between 24 hr and 96 hr following irradiation. At 288 hr following irradiation, the fraction of tumor cells capable of clonal growth approximates that observed in control (unirradiated) FSC and T53 tumors. It is to be noted, the data plotted in Figure 2 is the fraction of tumor cells that formed colonies. At 288 hr post irradiation, the size of T53 tumors and number of cells per tumor substantially exceeded the size of FSC tumors.
Figure 2.
Clonogenic survival of FSC (close circles) and T53 cells (closed squares) following in vivo irradiation. F2–F3 tumors were exposed to 20 Gy irradiation under uniform (clamp) hypoxic conditions. At 24, 96 and 288 hours following irradiation, the tumors were excised; a single cell suspension was prepared, counted, and plated for colony formation. The surviving fraction data is normalized against control (unirradiated) tumors. Confidence intervals are one standard deviation.
Functional vascular density following tumor irradiation
Photomicrographs of functional vessels in control and irradiated tumors are shown in Supplement Figure 1. Neither the number of functional vessels per high-power field in control tumors or irradiated tumors at 24, 96 and 288 hours following 20 Gy irradiation, nor the number of vessels in FSC vs. T53 tumors significantly differed. Vessels per field ranged from 14.9 ± 5.7 to 16.4 ± 7.6 in FSC tumors and 14.6 ± 7.1 to 16.5 ± 5.5 in T53 tumors (n = > 3 X 8 fields per time point). The vascular density of control FSC and T53 tumors did not significantly differ, P = 0.7; control vs irradiated tumors (all time points) P> 0.8, and > 0.2, for FSC and T53 respectively. For irradiated FSC vs T53 tumors, differences in vascular density were not significant (P > 0.3 for any point).
Dose of radiation leading to permanent local control
To determine if and the extent to which tumor response evaluated for the end-point of permanent local control was impacted by the difference in the intrinsic radiosensitivity of the tumor cells, we performed 50% tumor control dose assays. Of the 112 mice entered into the experiment, five mice died without tumor or progressive tumor growth prior to 50% recurrence of tumors within the same dose cohort, and were not included in the analysis. Most prematurely dyeing mice bore lung metastases, which were judged to be the cause of death.
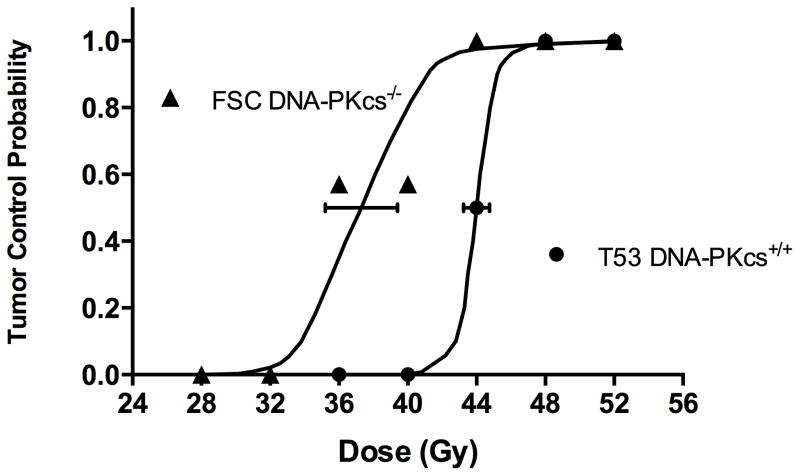
The results of the TCD50 assays are shown in Figure 3. The dose of radiation yielding permanent local control of 50% of T53 DNA-PKcs+/+ tumors is significantly greater than the dose to control 50% of repair defective DNA-PKcs−/− tumors, i.e., 44.0 Gy (43.3 Gy – 44.8, 95% CI) vs. 37.3 Gy (35.2 Gy – 39.4 Gy, 95% CI), P<0.01.
Figure 3.
Permanent local tumor control at 120 days following irradiation. Tumors initiated from radiosensitive DNA-PKcs deficient FSC cells are indicated by closed triangles; transfected repair competent cells are indicated by closed circles. Ninety-five percent confidence intervals at the TCD50 are shown.
Discussion
The major finding of the present study are that transfect ion of a DNA-PKcs defective tumor line with a competent DNA-PKcs double-strand break repair gene increased the cells’ intrinsic radiation resistance in vitro and in vivo, and resulted in a proportional increase in the dose required to achieve permanent local tumor control. In spite of the difference in the in vitro and in vivo killing of FSC vs T53 tumor cells, and the tumors’ control doses, the functional vessel density FSC and T53 tumors did not differ.
The results of the present study are thus inconsistent with a significant contribution of radiation induced tumor vascular damage as a determinant of response for the endpoint of permanent local control. The results suggests that with knowledge of the number of stem cells per tumor and their intrinsic radiosensitivity, the dose required for permanent local control can be estimated. Based on studies by Hill and Milas [23] the in vivo to in vitro plating efficiency of cells obtained directly from tumors generally underestimates but nevertheless inversely correlates with the number of tumor cells required for successful tumor transplantation (P < 0.01). This suggests that the in vivo to in vitro plating efficiencies can be used to approximate the fraction or rank-order of tumor cells that are cancer stem cells. Assuming the survival of 1 or more clonogen per tumor results in local treatment failure, and tumor cells which forms colonies of greater than 50 cells in vitro will sustain tumor growth in vivo, with knowledge of the number of clonogens per tumor and their intrinsic radiosensitivity, the predicted 50% tumor control dose (TCD50p) is:
| 1 |
where N = the number of tumor cells per tumor; PE = fraction of explanted tumor cells that form colonies in vitro, and n and Do are as defined above [18]. Substitution of the cell survival parameters Do and n, and the number of trypan blue excluding tumor cells per tumor (N), and PE (PE = 0.22 ± 0.03 and 0.23 ± 0.07 for FSC and T53 respectively) into equation 1, yields an estimate of the tumors’ relative TCD50 values if the number of cancer stem cells per tumor is not accurately assessed by the colony formation assay, and the actual TCD50 values if it is. For the FSC and T53 tumors in the present study, the predicted TCD50 values are 14.6 Gy and 19.1 Gy for FSC and T53 tumors respectively, for irradiation under fully oxygenated conditions. Assuming an OER of 2.5 pertains to cells irradiated under oxygenated conditions in vitro vs. hypoxic conditions in vivo [24, 25], the predicted 50% tumor control doses of 36.5 Gy for FSC and 48 Gy T53 tumors, closely approximate the measured TCD50 values of 37 Gy and 44 Gy.
The results presented here pertain to single fraction irradiation. For conventional multifraction radiotherapy, re-oxygenation and differences in the tumor cell proliferation rate between treatments may be expected to impact the local control dose. Nevertheless, compelling evidence suggests the number of stem cells at the start of treatment and their radiosensitivity, impacts the tumor control dose regardless whether radiation is administered in single or multiple fractions. Yaromina et al [26] assessed the relationship between the control doses of ten human squamous cell tumor lines treated by single doses under conditions of clamp hypoxia, vs. 30 fraction treatment over 6 weeks, under normal blood flow conditions. A highly significant correlation was observed; R2 = 0.82, P = 0.002.
To summarize, the number of clonogens per tumor and their radiosensitivity is the principle determinant of tumor response for the endpoint of permanent local control. The results suggest both parameters may be estimated by in vitro assays.
Supplementary Material
Acknowledgments
Grant support: Supported by Federal Share/NCI Proton Beam Program Income, NIHC06 CA059267 (LE Gerweck), and in part by Federal Share/NCI Proton Beam Income (P Huang)
Drs. Herman Suit and Joao Seco for careful examination of the manuscript and suggestions; Yujiao Liu for technical assistance
Footnotes
Conflict of Interest: The authors disclose no potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Peters LJ, Fletcher GH. Causes of failure of radiotherapy in head and neck cancer. Radiother and Oncol. 1983;1:53–63. doi: 10.1016/s0167-8140(83)80007-3. [DOI] [PubMed] [Google Scholar]
- 2.Peters LJ, Withers HR, Thames HD, Jr, Fletcher GH. Tumor radioresistance in clinical radiotherapy. Int J Radiat Oncol Biol Phys. 1982;8:101–8. doi: 10.1016/0360-3016(82)90392-3. [DOI] [PubMed] [Google Scholar]
- 3.Fuks Z, Leibel SA, Wallner KE, et al. The effect of local control on metastatic dissemination in carcinoma of the prostate: long-term results in patients treated with 125I implantation. Int J Radiat Oncol Biol Phys. 1991;21:537–47. doi: 10.1016/0360-3016(91)90668-t. [DOI] [PubMed] [Google Scholar]
- 4.Leibel SA, Scott CB, Mohiuddin M, et al. The effect of local-regional control on distant metastatic dissemination in carcinoma of the head and neck: results of an analysis from the RTOG head and neck database. Int J Radiat Oncol Biol Phys. 1991;21:549–56. doi: 10.1016/0360-3016(91)90669-u. [DOI] [PubMed] [Google Scholar]
- 5.Suit HD. Local control and patient survival. Int J Radiat Oncol Biol Phys. 1992;23:653–60. doi: 10.1016/0360-3016(92)90025-d. [DOI] [PubMed] [Google Scholar]
- 6.Bernstein TE, Endreseth BH, Romundstad P, Wibe A Norwegian Colorectal Cancer R. Improved local control of rectal cancer reduces distant metastases. Colorectal Dis. 2012;14:e668–78. doi: 10.1111/j.1463-1318.2012.03089.x. [DOI] [PubMed] [Google Scholar]
- 7.Garcia-Barros M, Paris F, Cordon-Cardo C, et al. Tumor response to radiotherapy regulated by endothelial cell apoptosis. Science. 2003;300:1155–9. doi: 10.1126/science.1082504. [DOI] [PubMed] [Google Scholar]
- 8.Garcia-Barros M, Thin TH, Maj J, et al. Impact of stromal sensitivity on radiation response of tumors implanted in SCID hosts revisited. Cancer Res. 2010;70:8179–86. doi: 10.1158/0008-5472.CAN-10-1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Budach W, Taghian A, Freeman J, Gioioso D, Suit HD. Impact of stromal sensitivity on radiation response of tumors. J Natl Cancer Inst. 1993;85:988–93. doi: 10.1093/jnci/85.12.988. [DOI] [PubMed] [Google Scholar]
- 10.Ogawa K, Boucher Y, Kashiwagi S, Fukumura D, Chen D, Gerweck LE. Influence of tumor cell and stroma sensitivity on tumor response to radiation. Cancer Res. 2007;67:4016–21. doi: 10.1158/0008-5472.CAN-06-4498. [DOI] [PubMed] [Google Scholar]
- 11.Gerweck LE, Vijayappa S, Kurimasa A, Ogawa K, Chen DJ. Tumor cell radiosensitivity is a major determinant of tumor response to radiation. Cancer Res. 2006;66:8352–5. doi: 10.1158/0008-5472.CAN-06-0533. [DOI] [PubMed] [Google Scholar]
- 12.Fertil B, Malaise EP. Intrinsic radiosensitivity of human cell lines is correlated with radioresponsiveness of human tumors: analysis of 101 published survival curves. Int J Radiat Oncol Biol Phys. 1985;11:1699–707. doi: 10.1016/0360-3016(85)90223-8. [DOI] [PubMed] [Google Scholar]
- 13.Peterson SR, Kurimasa A, Oshimura M, Dynan WS, Bradbury EM, Chen DJ. Loss of the catalytic subunit of the DNA-dependent protein kinase in DNA double-strand-break-repair mutant mammalian cells. Proc Natl Acad Sci U S A. 1995;92:3171–4. doi: 10.1073/pnas.92.8.3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kirchgessner CU, Patil CK, Evans JW, et al. DNA-dependent kinase (p350) as a candidate gene for the murine SCID defect. Science. 1995;267:1178–83. doi: 10.1126/science.7855601. [DOI] [PubMed] [Google Scholar]
- 15.Blunt T, Finnie NJ, Taccioli GE, et al. Defective DNA-dependent protein kinase activity is linked to V(D)J recombination and DNA repair defects associated with the murine scid mutation. Cell. 1995;80:813–23. doi: 10.1016/0092-8674(95)90360-7. [DOI] [PubMed] [Google Scholar]
- 16.Shinohara ET, Geng L, Tan J, et al. DNA-dependent protein kinase is a molecular target for the development of noncytotoxic radiation-sensitizing drugs. Cancer Res. 2005;65:4987–92. doi: 10.1158/0008-5472.CAN-04-4250. [DOI] [PubMed] [Google Scholar]
- 17.Suit HD, Maeda M. Hyperbaric oxygen and radiobiology of a C3H mouse mammary carcinoma. J Natl Cancer Inst. 1967;39:639–52. [PubMed] [Google Scholar]
- 18.Gerweck LE, Zaidi ST, Zietman A. Multivariate determinants of radiocurability. I: Prediction of single fraction tumor control doses. Int J Radiat Oncol Biol Phys. 1994;29:57–66. doi: 10.1016/0360-3016(94)90226-7. [DOI] [PubMed] [Google Scholar]
- 19.Gerweck LE, Kornblith PL, Burlett P, Wang J, Sweigert S. Radiation sensitivity of cultured human glioblastoma cells. Radiology. 1977;125:231–4. doi: 10.1148/125.1.231. [DOI] [PubMed] [Google Scholar]
- 20.Walker AM, Suit HD. Assessment of local tumor control using censored tumor response data. Int J Radiat Oncol Biol Phys. 1983;9:383–6. doi: 10.1016/0360-3016(83)90300-0. [DOI] [PubMed] [Google Scholar]
- 21.Chang YS, di Tomaso E, McDonald DM, Jones R, Jain RK, Munn LL. Mosaic blood vessels in tumors: frequency of cancer cells in contact with flowing blood. Proc Natl Acad Sci U S A. 2000;97:14608–13. doi: 10.1073/pnas.97.26.14608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hashizume H, Baluk P, Morikawa S, et al. Openings between defective endothelial cells explain tumor vessel leakiness. Am J Patho. 2000;156:1363–80. doi: 10.1016/S0002-9440(10)65006-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hill RP, Milas L. The proportion of stem cells in murine tumors. Int J Radiat Oncol Biol Phys. 1989;16:513–8. doi: 10.1016/0360-3016(89)90353-2. [DOI] [PubMed] [Google Scholar]
- 24.Hall Eric, Giaccia Amato J. Oxygen Effect and Reoxygenation. In: Hall Eric, Giaccia Amato J., editors. Radiobiology for the Radiologist. 7. Philadelphia, PA: Lippincott Williams & Wilkins; 2012. pp. 86–103. [Google Scholar]
- 25.Powers WE, Tolmach LJ. A multicomponent x-ray survival curve for mouse lymphosarcoma cells irradiated in vivo. Nature. 1963;197:710–1. doi: 10.1038/197710b0. [DOI] [PubMed] [Google Scholar]
- 26.Yaromina A, Krause M, Thames H, et al. Pre-treatment number of clonogenic cells and their radiosensitivity are major determinants of local tumour control after fractionated irradiation. Radiother and Oncol. 2007;83:304–10. doi: 10.1016/j.radonc.2007.04.020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.