Abstract
Purpose.
Allopregnanolone is a neurosteroid and powerful modulator of neuronal excitability. The neuroprotective effects of allopregnanolone involve potentiation of γ-aminobutyric acid (GABA) inhibitory responses. Although glutamate excitotoxicity contributes to ganglion cell death in glaucoma, the role of GABA in glaucoma remains uncertain. The aim of this study was to determine whether allopregnanolone synthesis is induced by high pressure in the retina and whether allopregnanolone modulates pressure-mediated toxicity.
Methods.
Ex vivo rat retinas were exposed to hydrostatic pressure (10, 35, and 75 mm Hg) for 24 hours. Endogenous allopregnanolone production was determined by liquid chromatography and tandem mass spectrometry (LC-MS/MS) and immunochemistry. We also examined the effects of allopregnanolone, finasteride, and dutasteride (inhibitors of 5α-reductase), picrotoxin (a GABAA receptor antagonist), and D-2-amino-5-phosphonovalerate (APV, a broad-spectrum N-methyl-D-aspartate receptor [NMDAR] antagonist).
Results.
Pressure loading at 75 mm Hg significantly increased allopregnanolone levels as measured by LC-MS/MS. Elevated hydrostatic pressure also increased neurosteroid immunofluorescence, especially in the ganglion cell layer and inner nuclear layers. Staining was negligible at lower pressures. Enhanced allopregnanolone levels and immunostaining were substantially blocked by finasteride, but more effectively inhibited by dutasteride and APV. Administration of exogenous allopregnanolone suppressed pressure-induced axonal swelling in a concentration-dependent manner, while picrotoxin overcame these neuroprotective effects.
Conclusions.
These results indicate that the synthesis of allopregnanolone is enhanced mainly via NMDARs in the pressure-loaded retina, and that allopregnanolone diminishes pressure-mediated retinal degeneration via GABAA receptors. Allopregnanolone and other related neurosteroids may serve as potential novel therapeutic targets for the prevention of pressure-induced retinal damage in glaucoma.
Keywords: transporter–knockout, Neurosteroid, allopregnanolone, glaucoma, GABA, neuroprotection
In ex vivo rat retinas exposed to hydrostatic pressure, we found that the synthesis of allopregnanolone is enhanced mainly via NMDA receptor activation, and that allopregnanolone diminishes pressure-mediated retinal degeneration via GABAA receptor activation.These observations suggest that neurosteroids such as allopregnanolone may serve as potential norvel therapeutic targets for the prevention of pressure-induced retinal damage in acute glaucoma.
Introduction
Although primary angle-closure glaucoma (PACG) is less common than primary open-angle glaucoma (POAG), PACG may cause blindness in a higher proportion of individuals, and be responsible for as much blindness as POAG.1–3 The prevalence of PACG is 0.5% worldwide, but highest in Asia (1.09%).4 It is estimated that 50% of global blindness by glaucoma results from PACG,5,6 particularly in Asia.4–8 Among all types of glaucoma, acute angle closure attacks (AAC) have a much greater likelihood of causing blindness, and at least 20% of patients with PACG present with AAC.2,9 The AAC is characterized by sudden increase in IOP that can reach 80 mm Hg.10 Without treatment, sustained IOP elevation damages retinal ganglion cells (RGCs), and leads to permanent visual disturbance in a significant number of those suffering an AAC.9,11,12 The mechanisms underlying pressure-induced RGC injury in AAC remain unclear.
Increased IOP may cause ocular ischemia, because the vascular perfusion pressure, defined as the difference between local arterial pressure and IOP, is decreased by elevated IOP. In this context, excitotoxicity, which has a key role in ischemic damage, is thought to contribute to glaucomatous retinal degeneration.13,14 Excitotoxic cell death does not always result from excess of glutamate, and extracellular levels of glutamate achieved during retinal ischemia13–15 may not be sufficient to induce neuronal damage under normal conditions.16 This suggests that clearance of glutamate or other factors are important in preventing glutamate-mediated retinal excitotoxicity. Glutamate is transported into Müller glia via glutamate transporters (mainly the glutamate aspartate transporter [GLAST]) and converted to the nontoxic amino acid glutamine by glutamine synthetase (GS). Our prior studies revealed that elevated hydrostatic pressure decreased glutamate clearance by suppressing GLAST and GS, and induced damage of RGC axons.17,18 Similarly, glutamate transporter–knockout mice show glaucomatous-type damage of the optic nerve.19
Glutamate and gamma-aminobutyric acid (GABA) are major excitatory and inhibitory retinal neurotransmitters, respectively. Glutamate is the neurotransmitter between photoreceptor cells and bipolar cells, and between bipolar cells and RGCs, whereas GABA is used by horizontal and amacrine cells in the lateral pathway, modulating neural transmission in outer and inner synaptic layers.20,21 Although there is evidence indicating a role for glutamate in glaucoma,18,19,22–25 GABAergic involvement has not been thoroughly investigated to our knowledge. It is hypothesized that the balance between glutamate and GABA is important to maintain retinal function and sensory information encoding. Loss of this balance could induce retinal degeneration and dysfunction. In fact, a significant dysfunction of the GABAergic system has been reported in rat retinas when IOP was experimentally increased by hyaluronic acid.26
Allopregnanolone is a neurosteroid that can be locally synthesized in the central nervous system (CNS) and a powerful modulator of neuronal excitability and neurotoxicity.27 Allopregnanolone potentiates the activity of GABAA receptors,28 and has neuroprotective properties in vitro29–32 and in vivo.33–35 Potential clinical targets for allopregnanolone36,37 include mood and anxiety disorders,38 alcoholism,39 sleep disorders,40 traumatic brain injury,41,42 and neurodegenerative disorders.43,44
Synthesis of allopregnanolone is increased locally in the CNS following acute behavioral45 or metabolic stress46,47 Thus, allopregnanolone may be a key mediator of neural stress responses and may serve to restore homeostatic mechanisms disrupted by acute stress.48,49 Whether allopregnanolone is produced in the retina in response to the acute stress of pressure elevation has not been studied to date to our knowledge. In the studies described here, we tested the hypothesis that retinal allopregnanolone synthesis is enhanced locally during pressure loading and that allopregnanolone helps to protect the retina from neurodegeneration via effects on GABAA receptors using a rat ex vivo model with hydrostatic pressure loading.
Materials and Methods
Protocols for animal use were approved by the Akita Graduate University Animal Studies Committee in accordance with the guidelines of the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Rat Ex Vivo Eyecup Preparation
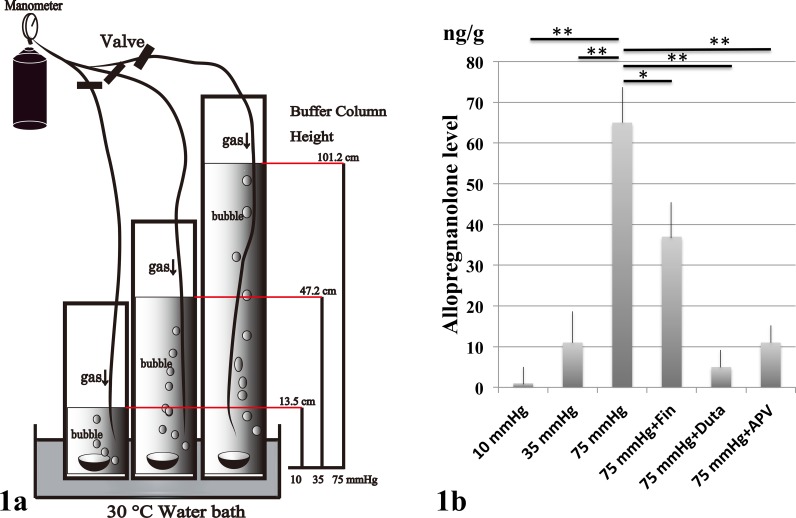
As described previously, rat ex vivo eyecups were prepared from approximately 30-day-old male Sprague-Dawley rats (Charles River Laboratories International, Inc., Wilmington, MA, USA) as reported previously.17,18 The anterior halves the enucleated eyes were carefully removed to make eyecup preparations. Eyecups were placed at the bottom of an acrylic cylinder filled with artificial cerebrospinal fluid (aCSF) containing (in mM): 124 NaCl, 5 KCl, 2 MgSO4, 2 CaCl2, 1.25 NaH2PO4, 22 NaHCO3, and 10 glucose, and incubated at 30°C for 24 hours (Fig. 1a). To simulate an IOP of 75 mm Hg, a pressure that can occur during a severe acute glaucoma attack, the CSF column height was adjusted to 101.2 cm. The depth of aCSF in the control column was adjusted to 13.5 cm to make a pressure of 10 mm Hg, and to 47.2 cm to create an intermediate pressure of 35 mm Hg. The 95% O2–5% CO2 gas mixture was delivered through disposable plastic tubing for infusion (200CP; Nipro Co., Osaka, Japan) that terminated 3 cm above the bottom of the cylinder. The pH was maintained at 7.35 to 7.40. In some experiments, allopregnanolone (10 nM, 100 nM, 1 μM), finasteride (100 nM, 1 μM), dutasteride (1 μM), picrotoxin (1 μM), and D-2-amino-5-phosphonovalerate (APV; 50 μM, 100 μM) were administered.
Figure 1.
(a) Rat ex vivo eye cup preparations were placed at the bottom of an acrylic cylinder filled with aCSF, and incubated at 30°C for 24 hours. Hydrostatic pressure was calculated to be 10, 35, and 75 mm Hg when the height of aCSF liquid column was 13.5, 47.3, and 101.2 cm, respectively. Allopregnanolone, finasteride, and picrotoxin were added to the aCSF buffer during some experiments. (b) Measurement of allopregnanolone in the retinal extracts using LC-MS/MS. Allopregnanolone levels significantly increased at 75 mm Hg compared to lower pressure (10 or 35 mm Hg) or incubation with 1 μM finasteride (Fin), 1 μM dutasteride (Duta), and 50 μM APV. At 75 mm Hg, administration of 1 μM finasteride significantly depressed the increase of allopregnanolone. Administration of 1 μM dutasteride inhibited allopregnanolone levels more effectively than finasteride. Administration of 50 μM APV also significantly inhibited allopregnanolone levels at 75 mm Hg. P values are calculated by unpaired Student's t-test compared to 75 mm Hg (*P < 0.001, **P < 0.0001).
Liquid Chromatography and Tandem Mass Spectrometry (LC-MS/MS)
For sample preparation, a rat retina was homogenized in 1 mL 0.1 M potassium dihydrogen phosphate solution with Ultra-Turrax homogenizer (IKA, Staufen, Germany). As an internal standard, 2H4-allopregnanolone was added to the rat retina suspension. Allopregnanolone was extracted by 4 mL methyl tert-butyl ether (MTBE) from the remaining rat retina suspension. After the organic layer was evaporated to dryness, the extract was dissolved in 0.5 mL methanol and diluted with 1 mL distilled water, and then applied to an adande:I PAX cartridge (SHISEIDO, Tokyo, Japan) which had been successively conditioned with 3 mL methanol and 3 mL distilled water. After the cartridge was washed with 1 mL distilled water, 1 mL methanol/distilled water/acetic acid (45:55:1, vol/vol/vol), and 1 mL 1% pyridine solution, allopregnanolone was eluted with 1 mL methanol/pyridine (100:1, vol/vol). After evaporation, the residue was subjected to derivatization described below.
The sample was reacted with 50 μL mixed solution (80 mg of 2-methyl-6-nitrobenzoic anhydride, 20 mg 4-dimethylaminopyridine, and 40 mg picolinic acid in 1 mL acetonitrile) and 10 μL triethylamine for 30 minutes at room temperature. After the reaction, the sample was dissolved in 0.5 mL ethyl acetate/hexane/acetic acid (15:35:1, vol/vol) and the mixture was applied to an InertSep SI cartridge, which had been successively conditioned with 3 mL acetone and 3 mL hexane. The cartridge was washed with 1 mL hexane, 2 mL ethyl acetate/hexane (3:7, vol/vol), and 2.5 mL MTBE and then the derivatized allopregnanolone was eluted with 2.5 mL acetone/hexane (7:3, vol/vol). After evaporation, the residue was dissolved in 0.1 mL acetonitrile/distilled water (2:3, vol/vol) and 20 μL the solution was subjected to LC-MS/MS.50
An API-5000 triple stage quadruple mass spectrometer (AB SCIEX, Framingham, MA, USA) equipped with a positive ESI source and an HPLC system (SCL-10Avp system controller, LC-20AD pump, SIL-HTc column oven, CTO-20A auto-sampler; Shimadzu Corp., Kyoto, Japan) was used. A Capcellcore ADME column (SHISEIDO) was used at 50°C. The mobile phase consisting of 0.1% formic aid (Solvent A) and acetonitrile/methanol (9:1, vol/vol; Solvent B) was used with a gradient elution. For quantification of the steroids, the transitions m/z 424.4 → 283.3 and 428.4 → 287.3, were selected for allopregnanolone and 2H4-allopregnanolone, respectively.
Immunocytochemistry
For immunocytochemistry, eyecup preparations were fixed with 4% paraformaldehyde-0.1 M phosphate buffer for 30 minutes at 4°C (7 animals per experimental group) at the end of each experiment. Samples were washed with PBS and then incubated in blocking solution (1% donkey serum/PBS) for 2 hours at 25°C. Samples then were embedded in OCT compound (Sakura Global Holdings, Tokyo, Japan), and frozen with liquid nitrogen. Then, 20-μm cryosections were incubated with a primary antibody51,52 raised in sheep against 5α-reduced neurosteroids diluted 1:2500 in blocking solution for 48 hours at 4°C. This polyclonal antibody primarily recognizes allopregnanolone and has minimal cross-reactivity with other neurosteroids in rats53 (purchased from Robert Purdy, PhD, University of California-San Diego, San Diego, CA, USA).
After incubation with primary antibody, slices were rinsed with PBS and incubated with a secondary antibody, biotinylated rabbit anti-sheep IgG (diluted 1:500, Cat#BA-6000, AB_2336217; Vector Laboratories, Burlingame, CA, USA) and streptavidin conjugated with Alexa Fluor 488 (diluted 1:1000, Cat#S32354, AB_2315383; Molecular Probes, Carlsbad, CA, USA), for 2 hours at 25°C. The IgG binding sites were detected by confocal laser scanning microscopy (LSM510 Axiovert200M; Carl Zeiss Meditec, Göttingen, Germany); 4′6-diamidino-2-phenylindole (DAPI) was used for nuclear staining.
For double immunofluorescence, cryosections of fixed specimens were incubated at room temperature with a mixture of two primary antibodies: primary antibody raised in sheep against 5α-reduced neurosteroids (1:2500) and mouse anti-vimentin monoclonal antibody (1:100; Cat#MAB3400, AB_94843; Millipore, Billerica, MA, USA). Subsequent antibody detection was performed with a mixture of two secondary antibodies, biotinylated rabbit anti-sheep IgG (Cat#BA-6000, AB_2336217; Vector Laboratories) and rhodamine-conjugated goat anti-mouse IgG (1:200, Cat#ab5928, AB_955560; Abcam, Cambridge, MA, USA). After several washes with PBS, colocalization of 5α-reduced neurosteroids (allopregnanolone) and vimentin was observed under a confocal microscope.
For quantification of immunohistochemical data, images of each section (five sections per animal) were captured. Digital images were analyzed, and the average intensity of the tissue was measured using Image-Pro Plus software (Media Cybernetics, Rockville, MD, USA). All data were expressed as mean ± SEM. Student's t-test was used for comparisons between two groups.
Light Microscopy
At the end of each experiment, eyecup preparations were fixed in 2.5% glutaraldehyde in 0.1 M phosphate buffer overnight at 4°C. The fixed retinas were rinsed in 0.1 M phosphate buffer and placed in 1% buffered osmium tetroxide for 60 minutes. The retinas were dehydrated with an ethanol dilution series, embedded in epoxy resin (Epon 812; TAAB Laboratories, Aldermaston, UK) and cut into 1-μm thick semi-thin sections. The tissue then was stained with toluidine blue and evaluated by light microscopy.
Chemicals
Allopregnanolone was purchased from Wako Pure Chemical Industries, Ltd. (Cat#596-30841, CAS.NO 516-54-1; Osaka, Japan). Finasteride was purchased from Sigma-Aldrich Corp. (Cat#F1293-100MG, CAS.NO 98319-26-7; St. Louis, MO, USA). Picrotoxin and APV were purchased from Sigma-Aldrich Corp. (Cat#P1675, CAS.NO 124-87-8). Dutasteride was obtained from Adooq Bioscience LLC (Cat#A10338, CAS.NO 164656-23-9, Irvine, CA, USA). All other chemicals were purchased from Sigma-Aldrich Corp. or Nacalai Tesque (Kyoto, Japan). Allopregnanolone and dutasteride was dissolved in dimethyl sulfoxide (DMSO) as a 10-mM stock solution. Finasteride and picrotoxin were dissolved in ethanol as a 10-mM stock solution.
Data Analysis
We examined the middle portion of the retina, greater than 1200 μm away from the center of the optic disc along the inner limiting membrane (ILM) according to previously described methods.18 The nerve fiber layer thickness (NFLT) was measured by light microscopy along five lines perpendicular to the pigment epithelium at a distance of 15 μm from each other approximately 1200 μm away from the center of the optic disc. The average NFLT was determined in 10 different light micrographs taken from five to seven eyecup samples in each condition, divided by total retinal thickness, and mean ± SD was analyzed and compared to control.
The density of degenerated ganglion cells (GCs) was determined by counting 10 fields of 500 μm length at 10 different locations in light micrographs taken from the block of the middle retinal part 950 to 1450 μm away from the center of the optic disc.
The severity of neuronal damage was assessed by light microscopy in 10 fields from each experiment using a neuronal damage score (NDS) as described previously.54 The NDS was determined in 10 different light micrographs taken from five to seven eyecup samples in each condition. The NDS rates neuronal damage in the inner nuclear layer (INL) and the inner plexiform layer (IPL) on a 0 to 4 scale, with 0 signifying no neuronal damage and 4 indicating very severe damage. Criteria used in establishing the degree of neuronal damage included the extent of cytoplasmic swelling in the IPL and the number of neurons in the INL showing signs of severe cytoplasmic swelling and coarse clumping of nuclear chromatin. The highest NDS rating (4) is given when the IPL shows apparent spongiform appearance due to dendritic swelling, and when most cell bodies in the INL show severe cytoplasmic swelling and coarse clumping of nuclear chromatin. If the damage is of a lesser degree, a rating of 3 is given. Score NDS 2 is assigned when cell bodies in the INL are sporadically swollen. In NDS 1, damage does not fulfill higher criteria, but the retinas differ from controls (NDS 0). Fine dendritic swelling in a limited area of the IPL without damage in the INL is described by NDS 1.
These morphometric parameters were assessed by three raters who remained unaware of the experimental condition. Upon completion of data assessment, significance of individual differences among raters was evaluated using five randomly selected samples in each morphometric parameter by 1-way ANOVA followed by a post hoc test. There were no significant differences among the raters in any of the morphometric measurements.
Data were double-checked and analyzed using the Statistical Package for Bioscience V9.53 (SPBS; Nankodo Publisher, Tokyo, Japan) on a personal computer. Each parameter was compared to control group or pressure-loaded group by Student's unpaired t-test. For all analyses, P values were considered statistically significant, when the values were less than 0.05 (2-tailed).
Results
LC-MS/MS Data
We initially examined the effects of elevated hydrostatic pressure on neurosteroid levels in rat ex vivo eyecups using LC-MS/MS. Five eyes were examined by LC-MS/MS in each condition. The LC-MS/MS analysis revealed a significant increase of allopregnanolone at 75 mm Hg compared to 10 or 35 mm Hg (Fig. 1b). The increase in allopregnanolone synthesis induced by high pressure was significantly diminished, but not completely eliminated by 1 μM finasteride, an agent that primarily inhibits type II 5α-reductase, a key enzyme in allopregnanolone synthesis (Fig. 1b). In contrast, 1 μM dutasteride, a broader spectrum 5α-reductase inhibitor, almost completely inhibited allopregnanolone synthesis induced by high pressure (Fig. 1b). Administration of 50 μM APV, an N-methyl-D-aspartate receptor (NMDAR) antagonist, also significantly decreased allopregnanolone synthesis.
Immunocytochemistry
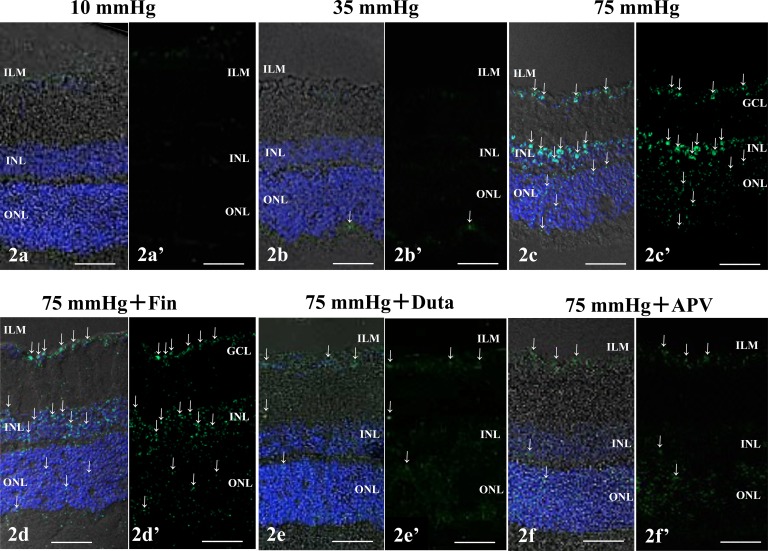
To determine regional and cellular changes in allopregnanolone levels, we examined the effects of elevated hydrostatic pressure on neurosteroid immunostaining using an antibody against 5α-reduced steroids that primarily recognizes allopregnanolone. Immunostaining was negligible at 10 (Figs. 2a, 2a') and 35 (Figs. 2b, 2b') mm Hg. At 75 mm Hg, positive allopregnanolone immunofluorescence was detected in the ganglion cell layer (GCL), INL, and the outer nuclear layer (Figs. 2c, 2c'). The enhanced staining induced by elevated hydrostatic pressure was significantly dampened, but not eliminated by 1 μM finasteride (Figs. 2d, 2d'). Administration of 1 μM dutasteride (Fig. 2e, 2e') and 50 μM APV (Figs. 2f, 2f') even more markedly inhibited pressure-induced changes in immunofluorescence.
Figure 2.
Immunofluorescent localization of allopregnanolone by confocal microscopy. (a–f) Merge of differential interference contrast images and fluorescence images using DAPI and an antibody against 5α-reduced neurosteroids antibody. (a'–f') Fluorescence images using anti-5α-reduced neurosteroids antibody without DAPI nuclear staining. (a, a') Allopregnanolone immunostaining was minimal in a retina incubated at 10 mm Hg. (b, b') At 35 mm Hg, the retina showed marginal changes in fluorescence. Arrows indicate the weak fluorescence in the corresponding lesions in (b) and (b'). (c, c') Positive immunofluorescence of allopregnanolone was observed in the GCL, the INL, and the ONL at 75 mm Hg. Arrows indicate the fluorescence in the corresponding panels in (c) and (c'). (d, d') Compared to the fluorescence induced at 75 mm Hg, less prominent fluorescence was detected especially in the ILM after 1 μM finasteride (Fin) treatment at 75 mm Hg. Positive fluorescence also was observed in the GCL and the ONL. Arrows indicate the fluorescence in the corresponding panels in (d) and (d'). (e, e') Administration of 1 μM dutasteride (Duta) almost completely reduced the fluorescence induced at 75 mm Hg. Arrows indicate the fluorescence in the corresponding panels in (e) and (e'). (f, f') Administration of 50 μM APV markedly inhibited the immunofluorescence of allopregnanolone at 75 mm Hg. Arrows indicate the fluorescence in the corresponding panels in (f) and (f'). Scale bars: 20 μm.
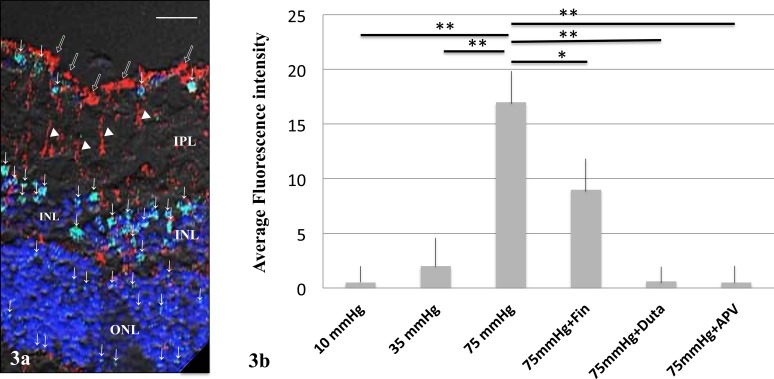
To further characterize the cellular localization of allopregnanolone, we used double immunofluorescence labeling with the neurosteroids antibody and antivimentin antibody at 75 mm Hg (Fig. 3a). Vimentin was specifically expressed in Müller cell end feet and cell bodies (Fig, 3a). Double-labeled cells were not detected (Fig. 3a). Fluorescence intensities in each condition are summarized in Figure 3b.
Figure 3.
(a) Immunofluorescent colocalization of allopregnanolone and vimentin by laser scanning microscopy. Allopregnanolone staining (green; FITC) was observed in the GCL, INL, and ONL at 75 mm Hg, and indicated by arrows. Antivimentin antibody (red; rhodamine) was localized to the Müller cell end feet (open arrows) and Müller cell bodies (white triangles). Double-labeled structures were not detected. Scale bars: 8 μm. (b) Summary of immunostaining studies shows fluorescence intensity by antiallopregnanolone antibody (arbitrary units) as mean ± SEM. Fluorescent intensity significantly increased at 75 mm Hg compared to 10 or 35 mm Hg. The increase in fluorescence induced by high pressure was significantly decreased by 1 μM finasteride (Fin). Administration of 1 μM dutasteride (Duta), almost completely inhibited the fluorescence increase. Administration of 50 μM APV also significantly decreased fluorescence. P values are calculated by unpaired Student's t-test compared to 75 mm Hg (*P < 0.001, **P < 0.0001).
Light Microscopic Findings
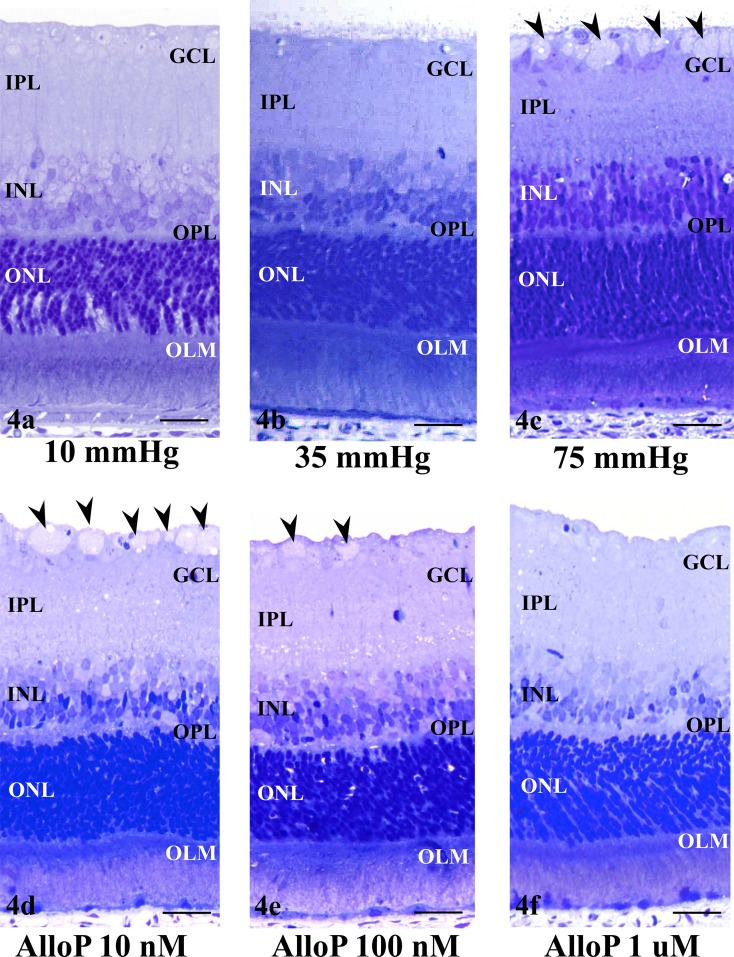
We next examined the effects of exogenously administered allopregnanolone on retinal morphology under pressure loading. Consistent with our previous reports,17,18 retinas incubated at 10 (Fig. 4a) or 35 (Fig. 4b) mm Hg exhibited a normal appearance. Retinas incubated at 75 mm Hg showed axonal swelling in the nerve fiber layer (NFL) at 75 mm Hg (Fig. 4c). A few GC nuclei became pyknotic and shrunken at 75 mm Hg.
Figure 4.
(a, b) Light micrographs of rat ex vivo eye cup preparations incubated at 10 (a) and 35 (b) mm Hg. At 10 and 35 mm Hg, the retina showed normal structure. (c) Pressure loading at 75 mm Hg for 24 hours induced axonal swelling (arrowheads) in the NFL. (d–f) Light micrographs of retinas incubated with 10 nM (d), 100 nM (e), and 1 μM allopregnanolone (AlloP, [f]) at 75 mm Hg. (d) At 75 mm Hg, the extent of swollen axons (arrowheads) were reduced in the NFL of the retina incubated with 10 nM allopregnanolone. (e) A retina incubated with 100 nM allopregnanolone at 75 mm Hg exhibited sparse distribution of the swollen axons (arrowheads). (f) Administration of 1 μM allopregnanolone exhibited no remarkable changes in the NFL. (a–f) Scale bars: 15 μm.
Administration of allopregnanolone inhibited pressure-induced axonal swelling in a concentration-dependent manner. The extent of swollen axons in the retina at 75 mm Hg was partially inhibited by 10 nM allopregnanolone (Fig. 4d). In the presence of 100 nM allopregnanolone, elevated hydrostatic pressure failed to induce significant morphological alterations except for a small number of swollen axons and vacuole formation in the IPL and INL (Fig. 4e). Administration of 1 μM allopregnanolone substantially inhibited axonal swelling (Fig. 4f).
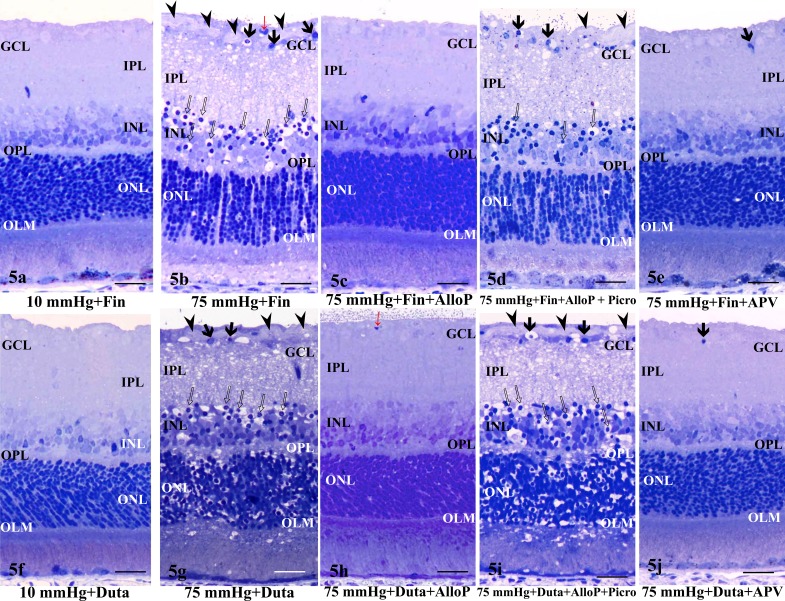
To determine whether the increase in endogenous allopregnanolone levels observed by LC-MS/MS and immunohistochemistry also is neuroprotective, we examined the effects of finasteride. At 10 mm Hg, the retinas did not exhibit specific morphological changes in the presence of 1 μM finasteride (Fig. 5a). At 75 mm Hg in the presence of 1 μM finasteride, however, retinas developed severe retinal damage characterized by edematous changes in the IPL and bull's eye formation in the INL along with axonal swelling (Fig. 5b). In this condition, NFL thickness ratings were increased relative to controls and similar to retinas incubated without finasteride at 75 mm Hg. Administration of 1 μM allopregnanolone was neuroprotective against the retinal degeneration observed in the presence of 1 μM finasteride and high pressure (Fig. 5c). To determine whether the neuroprotective effects of allopregnanolone involve GABAA receptors, 1 μM picrotoxin (a GABAA receptor antagonist) was administered in combination with 1 μM finasteride and 1 μM allopregnanolone. We found that picrotoxin overcame the neuroprotective effects of allopregnanolone under hyperbaric conditions (Fig. 5d). Retinas treated with 100 μM APV in the presence of high pressure plus finasteride showed substantial neuroprotection, but still exhibited changes in RGC nuclei (Fig. 5e).
Figure 5.
(a, b) Light micrographs of retinas incubated with 1 μM finasteride (Fin) at 10 (a) and 75 (b) mm Hg. (a) At 10 mm Hg, the retina incubated with finasteride showed no remarkable changes. (b) Administration with 1 μM finasteride induced excitotoxic changes characterized by bull's eye formation in the INL (open arrows) and edematous IPL along with axonal swelling in the NFL (arrowheads) in the retina at 75 mm Hg. Red arrow indicates blood capillary. Arrows indicate the degenerated ganglion cells. (c) Combination of 1 μM finasteride (Fin) and 1 μM allopregnanolone (AlloP) blocked the retinal excitotoxic degeneration at 75 mm Hg. (d) At 1 μM, picrotoxin (Picro) overcame the protective effect of 1 μM allopregnanolone in a pressure-loaded retina incubated with 1 μM finasteride at 75 mm Hg. Note the edematous IPL. Open arrows: Bull's eye formation in the INL. Arrowhead: Axonal swelling in the NFL. Arrows: Degenerated ganglion cells. (e) At 75 mm Hg, the excitotoxic changes induced by finasteride were inhibited in the retina incubated with 100 μM APV, but pyknotic ganglion cell nuclei remained (arrow). No remarkable changes were observed in other layers of the retina.
(f, g) Light micrographs of retinas incubated with 1 μM dutasteride at 10 (f) and 75 (g) mm Hg. (f) At 10 mm Hg, the retina incubated with dutasteride (Duta) showed no remarkable changes. (g) Administration with 1 μM dutasteride induced excitotoxic changes characterized by bull's eye formation in the INL (open arrows) and edematous IPL along with axonal swelling in the NFL (arrowheads) in the retina at 75 mm Hg. Arrows indicate the degenerated ganglion cells. (h) Combination with 1 μM dutasteride (Duta) and 1 μM allopregnanolone (AlloP) blocked the retinal excitotoxic degeneration at 75 mm Hg. Red arrow indicates blood capillary. (i) At 1 μM, picrotoxin (Picro) overcame the protection effect of 1 μM allopregnanolone in a pressure-loaded retina incubated with 1 μM dutasteride at 75 mm Hg. Open arrows: Bull's eye formation in the INL (red arrows). Arrowhead: Axonal swelling in the NFL. Arrow: Degenerated ganglion cells. (j) At 75 mm Hg, excitotoxic changes induced by dutasteride were inhibited in a retina incubated with 100 μM APV, but pyknotic ganglion cell nuclei remained (arrow). No remarkable changes were observed in other layers of the retina. (a–j) Scale bars: 15 μm.
The LC-MS/MS and immunohistochemistry revealed that dutasteride was more effective than finasteride at inhibiting steroidogenesis. Therefore, we also examined the effects of dutasteride on retinal histology. At 10 mm Hg, the retinas did not show any remarkable changes in the presence of 1 μM dutasteride (Fig. 5f). At 75 mm Hg, administration of 1 μM dutasteride, however, resulted in severe changes characterized by an edematous appearance in the IPL and bull's eye formation in the INL along with axonal swelling (Fig. 5g). In addition, destruction of the ONL was found. Administration of 1 μM allopregnanolone was neuroprotective against the retinal degeneration observed in the presence of 1 μM dutasteride and high pressure (Fig. 5h). However, administration of 1 μM picrotoxin overcame the neuroprotective effects of allopregnanolone in the retina treated with 1 μM dutasteride under hyperbaric conditions (Fig. 5i). At 75 mm Hg, 100 μM APV-treated retinas showed substantial neuroprotection against dutasteride-induced toxicity (Fig. 5j), consistent with results from APV administration to finasteride-treated retinas. However, APV failed to protect RGC nuclei.
A quantitative assessment of structural changes induced by high pressure (75 mm Hg) and/or administration of allopregnanolone, finasteride, dutasteride, picrotoxin, and APV is summarized in Table 1. The NFLT in retinas incubated at 75 mm Hg was significantly increased compared to that in control retinas incubated at 10 mm Hg. Administration of allopregnanolone at 75 mm Hg prevented the NFLT increase in a concentration-dependent fashion. The NFLT also was significantly increased after exposure to high pressure in the presence of finasteride or dutasteride. Allopregnanolone significantly decreased the NFLT at 75 mm Hg in the presence of finasteride or dutasteride. Administration of APV also decreased the NFLT at 75 mm Hg in the presence of finasteride or dutasteride. In contrast, a combination of finasteride, allopregnanolone, and picrotoxin or a combination of dutasteride, allopregnanolone, and picrotoxin significantly increased the NFLT at 75 mm Hg.
Table 1.
Effects of Pressure Elevation, Allopregnanolone, Finasteride, and Picrotoxin on NFLT and NDS
|
Condition (n) |
NFLT vs. RT, % (P) |
NDS (P) |
| 10 mm Hg (5) | 2.4 ± 1.5 (–) | 0.1 ± 0.1 (–) |
| 10 mm Hg + 1 μM AlloP (5) | 2.3 ± 1.5 (1.000) | 0.2 ± 0.1 (1.000) |
| 10 mm Hg + 1 μM Fin (6) | 2.2 ± 2.2 (0.815) | 0.4 ± 0.5 (0.079) |
| 75 mm Hg (7) | 12.8 ± 3.5 (0.0001)* | 0.9 ± 0.4 (<0.0001)* |
| 75 mm Hg + 10 nM AlloP (6) | 8.0 ± 3.0 (0.0044)* | 0.8 ± 0.4 (<0.0001)* |
| 75 mm Hg + 100 nM AlloP (6) | 3.0 ± 2.0 (0.458) | 0.3 ± 0.3 (0.061) |
| 75 mm Hg + 1 μM AlloP (5) | 2.4 ± 1.3 (0.676) | 0.3 ± 0.2 (0.510) |
| 75 mm Hg + 1 μM Fin (5) | 11.5 ± 3.4 (0.0006)* | 3.4 ± 0.1 (<0.0001)* |
| 75 mm Hg + 1 μM Fin + 1 μM AlloP (6) | 2.7 ± 2.1 (0.718) | 0.2 ± 0.2 (0.174) |
| 75 mm Hg + 1 μM Fin + 1 μM AlloP + 1 μM Picro (5) | 13.8 ± 4.3 (0.0005)* | 3.7 ± 1.2 (<0.0001)* |
| 75 mm Hg + 1 μM Fin + 100 μM APV (5) | 2.0 ± 1.0 (0.633) | 0.2 ± 0.1 (0.153) |
| 75 mm Hg + 1 μM Duta (5) | 10.5 ± 2.50(0.0003)* | 4.0 ± 0.5 (<0.0001)* |
| 75 mm Hg + 1 μM Duta + 1 μM AlloP (6) | 2.2 ± 1.0 (0.858) | 0.2 ± 0.2 (0.338) |
| 75 mm Hg + 1 μM Duta + 1 μM AlloP + 1 μM Picro (5) | 10.6 ± 2.3 (0.0002)* | 3.9 ± 0.7 (<0.0001)* |
| 75 mm Hg + 1 μM Duta + 100 μM APV (5) | 1.7 ± 0.8 (0.384) | 0.2 ± 0.1 (0.153) |
Data are the mean ± SD; NFLT vs. RT (%) refers to the NFLT percentage of total RT. P values were calculated versus control (10 mm Hg) by Student's t-test. AlloP, allopregnanolone; Fin, finasteride; Picro, picrotoxin.
P < 0.001.
The NDS were significantly increased after exposure to high pressure and worsened by a combination of finasteride and high pressure (0.9 ± 0.4 with high pressure alone versus 3.4 ± 0.1 with finasteride plus high pressure, P < 0.0001) or by a combination of dutasteride and high pressure (1.0 ± 0.5 with high pressure alone versus 4.0 ± 0.5 with dutasteride plus high pressure, P < 0.0001). Allopregnanolone significantly decreased the NDS at 75 mm Hg in the presence of finasteride or dutasteride. Administration of APV also decreased the NDS at 75 mm Hg in the presence of finasteride or dutasteride. In contrast, a combination of finasteride, allopregnanolone, and picrotoxin or a combination of dutasteride, allopregnanolone, and picrotoxin significantly increased the NDS at 75 mm Hg.
A quantitative assessment of GC damage in the presence of the 5α-reductase inhibitors (finasteride and dutasteride) and the NMDAR antagonist APV is summarized in Table 2. At 75 mm Hg, the density of damaged GCs was greater in retinas treated with finasteride alone or dutasteride alone compared to control retinas incubated at 10 mm Hg. The density of damaged GCs was significantly increased after incubation with finasteride, and decreased by a combination of finasteride and APV at 75 mm Hg (38.7 ± 6.1 with finasteride versus 2.3 ± 1.2 with finasteride plus APV, P < 0.0001). The density of damaged GCs also was significantly increased after incubation with dutasteride, and decreased by a combination of dutasteride and APV at 75 mm Hg (33.2 ± 4.0 with dutasteride versus 2.5 ± 1.6 with dutasteride plus APV, P < 0.0001).
Table 2.
Effects of Pressure Elevation, Finasteride, Dutasteride, and the NMDAR Antagonist (APV) on the Density of Damaged GCs in the Middle Retina
|
Condition (n) |
GC ± SD (P) |
| 10 mm Hg (5) | 0.6 ± 0.4 (–) |
| 75 mm Hg (7) | 8.8 ± 3.6 (0.0005)* |
| 75 mm Hg + 1 μM Fin (5) | 38.7 ± 6.1 (<0.0001)* |
| 75 mm Hg + 1 μM Fin + 100 μM APV (5) | 2.3 ± 1.2 (0.0169)† |
| 75 mm Hg + 1 μM Duta (5) | 33.2 ± 4.0 (<0.0001)* |
| 75 mm Hg + 1 μM Duta + 100 μM APV (5) | 2.5 ± 1.6 (0.0328)† |
Data are the mean ± SD. The density of damaged GCs was counted per 500 μm of retina. P values were calculated versus control (10 mm Hg) by Student's t-test. P values are calculated by Student's t-test compared to 10 mm Hg. Duta, dutasteride.
P < 0.001.
P < 0.05.
Discussion
In the present study, we used an ex vivo rat model that incubated dissected eyecups under hydrostatic pressure for 24 hours. The hydrostatic pressure was adjusted to simulate conditions in the normal retina (10 mm Hg) and conditions that can occur during an AAC attack (75 mm Hg). The advantages of this model include preservation of retinal morphology without baseline ischemic degeneration17,18 making it possible to investigate direct effects of pressure-loading on histology and neurosteroidogenesis. Furthermore, the ex vivo model avoids the influence of circulating steroids.
We were interested in determining whether isolated retinas have the ability to produce neurosteroids and whether neurosteroid production is altered by the high pressure of an AAC glaucoma attack. The LC-MS/MS analysis and immunohistochemistry revealed that allopregnanolone levels are minimal under low pressure, but significantly increased under high pressure. Thus, it is likely that neurosteroidogenesis is enhanced during an AAC attack. It is known that stressful events, like acute swim stress45 or metabolic stressors, including ammonia48 and acetaldehyde,47 elevate allopregnanolone levels in the brain to concentrations that modulate GABAA receptors. This is consistent with the present findings that pressure loading, a form of acute stress, induced allopregnanolone production in the retina.
Allopregnanolone is synthesized from pregnenolone by the sequential action of three enzymes, 3β-hydroxysteroid dehydrogenase/isomerase, 5α-reductase, and 3α-hydroxysteroid deoxygenase (3α-HSD).44,55–57 The conversion from progesterone to 5α-dihydroprogesterone (5α-DHP) is catalyzed by 5α-reductase, and 3α-HSD either converts 5α-DHP into allopregnanolone or converts allopregnanolone into 5α-DHP.57 The present study revealed that finasteride, an agent that primarily inhibits type II 5α-reductase with less effect on type I 5α-reductase,58 and dutasteride, an effective inhibitor of Types I and II 5α-reductase,59 markedly dampened allopregnanolone synthesis with dutasteride being more effective than finasteride. Taken together, it appears that Types I and II 5α-reductase contribute to allopregnanolone production in the pressure-loaded retina.
Although it is unknown how acute stress promotes neurosteroidogenesis, several signaling pathways could be involved.47,60 These signaling pathways include endogenous ligands that act on key steps in neurosteroid synthesis, such as acyl-Coenzyme A (CoA) binding domain protein-1,51,61 triakontatetraneuro-peptide (DBI-17-50),51,61 adrenocorticotropic hormone (ACTH),55,60 and corticosterone.55,60 Increases in intracellular calcium also can trigger neurosteroid synthesis.56,61 In the retina, calcium influx via NMDARs enhances pregnenolone formation and the synthesis of neurosteroids.56,61 As previously reported,17,18 pressure loading depresses the expression of two key proteins involved in glutamate homeostasis, GLAST, and glutamine synthetase. Subsequent elevation of extracellular glutamate likely triggers calcium influx via NMDAR activation and allopregnanolone formation. Consistent with this, we found that a broad-spectrum NMDAR antagonist blocks allopregnanolone production in the hyperbaric condition. We17 previously found that full protection of the retina from hyperbaric injury requires block of NMDARs and ionotropic non-NMDARs; thus, the mechanisms underlying hyperbaric neurosteroid production may be more complex.
Using our rat ex vivo glaucoma model, we previously demonstrated high pressure induced down-regulation of GLAST and glutamine synthetase, suggesting that abnormalities in glutamate metabolism are involved in the pathogenesis of glaucoma. Although glutamate excitotoxicty could mediate RGC death in glaucoma, few studies have examined the relevance of GABA to glaucoma.26,62 Moreno et al.26 reported a significant dysfunction of the retinal GABAergic system in rats exposed experimentally to elevated IOP induced by hyaluronic acid. Additionally, unilateral elevation of IOP affects the expressed levels of GABAA receptor proteins measured immunohistochemically in the primary visual cortex of adult monkeys,62 suggesting that GABA may have a role in glaucomatous neuropathy. Allopregnanolone acts at GABAA receptors to positively modulate the effects of GABA. In part through its effects on GABAA receptors, allopregnanolone is neuroprotective against apoptosis and glutamate-mediated excitotoxicity.63 In addition to its ability to modulate postsynaptic GABAA responses, recent evidence suggests that allopregnanolone modulates glutamate release via presynaptic GABAA receptors.64 The inhibition of neuronal excitability resulting from potentiation of GABAA inhibitory responses is considered largely responsible for the neuroprotective effects of allopregnanolone.35 These findings raise the possibility that allopregnanolone also may serve as a neuroprotectant against pressure-induced injury in the retina. Although neuroprotection by other neurosteroids has been reported in glaucomatous animals,65–67 to our knowledge, allopregnanolone has not yet been investigated in glaucoma. Thus, the present study described the first evidence for the involvement of allopregnanolone in glaucomatous conditions. The finding that exogenous administration of allopregnanolone prevents pressure-induced axonal injury indicates that allopregnanolone and related compounds may be useful in protecting glaucomatous eyes from neuronal degeneration. To determine whether the neuroprotective effects of allopregnanolone result from effects on GABAA receptors, we used picrotoxin, a GABAA receptor antagonist, and found that picrotoxin overcame allopregnanolone-mediated neuroprotection.
Previous studies have shown that administration of finasteride induced excitotoxic neuronal cell death in the hippocampus and cerebellum in fetal sheep brain.68 In the present study, we found that finasteride administration in the presence of high pressure resulted in retinal degeneration with edematous changes in the IPL and bull's eye formation in the INL, which are characteristic of excitotoxic retinal damage.69,70 To determine the contribution of excitotoxicity to this retinal degeneration, we examined APV, a broad-spectrum NMDAR antagonist, in finasteride-treated retinas under hyperbaric conditions, and found that APV substantially inhibited the finasteride-induced retinal degeneration. These findings indicated that finasteride induces excitotoxicity in the pressure-loaded retinas. Consistent with this, dutasteride-induced retinal degeneration also was inhibited by APV under hyperbaric conditions. Taken together, administration of finasteride or dutasteride may induce excitotoxicity during IOP elevation.
Finasteride is used clinically for the treatment of benign prostatic hyperplasia (BPH) and androgenetic alopecia (AGA), while dutasteride use is restricted to the treatment of BPH. Because both conditions (BPH and AGA) are common in males, it is plausible that significant numbers of men with glaucoma take 5α-reductase inhibitors. In this case, their eyes may be at risk of retinal damage as a result of inhibition of allopregnanolone synthesis during periods of elevated IOP. Although adverse ophthalmologic effects have not been reported with finasteride, psychiatric side effects, including depression, may develop after finasteride or dutasteride administration.58,71,72
Taken together, our findings indicates that enhanced neurosteroid synthesis and GABAA receptor activation have important roles in maintaining retinal integrity under hyperbaric conditions, helping to protect the retina from pressure-induced damage. Today, most treatments for glaucoma are directed at lowering IOP even if IOP lowering itself does not prevent optic nerve damage or visual field loss. Furthermore, loss of ganglion cells continues to progress even after IOP is lowered.73,74 Thus, interventions that only focus on lowering IOP may not be optimal for some glaucoma patients. The present study demonstrated that allopregnanolone exerts neuroprotective effects against glaucomatous pressure-induced injuries. In rodents and humans, however, allopregnanolone and its analogues appear to be well-tolerated and these agents can improve clinical symptoms (anxiety and anhedonia) at levels that do not cause significant adverse effects.43 Thus, allopregnanolone and related compounds may serve as potential therapeutics to protect glaucomatous eyes from pressure-induced injuries.
Acknowledgments
The authors thank Yoko Hayami and Sanae Takaseki for technical support.
Supported in part by JSPS KAKENHI Grant No. 24592666 (MI) and National Institutes of Health (NIH; Bethesda, MD, USA) Grants MH077791, MH101874, and AA017413 (CFZ) and the Bantly Foundation. The authors alone are responsible for the content and writing of the paper.
Disclosure: M. Ishikawa, None; T. Yoshitomi, None; C.F. Zorumski, None; Y. Izumi, None
References
- 1. Foster PJ, Oen FT, Machin D, et al. The prevalence of glaucoma in Chinese residents of Singapore: a cross-sectional population survey of the Tanjong Pagar district. Arch Ophthalmol. 2000; 118: 1105–1111. [DOI] [PubMed] [Google Scholar]
- 2. Foster PJ, Basanhu J, Alsbirk PH, Munkhbayar D, Uranchimeg D, Johnson GJ. Glaucoma in Mongolia: a population-based survey in Hövsgöl Province, Northern Mongolia. Arch Ophthalmol. 1996; 114: 1235–1241. [DOI] [PubMed] [Google Scholar]
- 3. Dandona L, Dandona R, Mandel P, et al. Angle-closure glaucoma in an urban population in southern India. The Andhra Padesh eye disease study. Ophthalmology. 2000; 107: 1710–1716. [DOI] [PubMed] [Google Scholar]
- 4. Quigley HA. Number of people with glaucoma worldwide. Br J Ophthalmol. 1996; 80: 389–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tham YC, Li X, Wong TY, Quigley HA, Aung T, Cheng CY. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014; 121: 2081–2090. [DOI] [PubMed] [Google Scholar]
- 6. Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006; 90: 262–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Resnikoff S, Pascolini D, Etya'ale D, et al. Global data on visual impairment in the year 2002. Bull World Health Organ. 2004; 82: 844–851. [PMC free article] [PubMed] [Google Scholar]
- 8. Cheng JW, Zong Y, Zeng YY, Wei RL. The prevalence of primary angle closure glaucoma in adult Asians: a systematic review and meta-analysis. PLoS One. 2014; 24: e103222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Aung T, Ang LP, Chan SP, Chew PT. Acute primary angle-closure: long-term intraocular pressure outcome in Asian eyes. Am J Ophthalmol. 2001; 131: 7–12. [DOI] [PubMed] [Google Scholar]
- 10. Ritch R. Chapter 4. Glaucoma. In: Silverstone B, Lang MA, Rosenthal BP, Faye EE. eds Vision Impairment and Vision Rehabilitation. New York, NY: Oxford University Press, Inc.; 2000: 53–81. [Google Scholar]
- 11. Lowe RF. Primary angle-closure glaucoma: a review 5 years after bilateral surgery. Br J Ophthalmol. 1973; 57: 457–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lowe RF. Acute angle-closure glaucoma the second eye: an analysis of 200 cases. Br J Ophthalmol. 1962; 46: 641–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Louzada-Junior P, Dias JJ. Santos, Lachat JJ, Bradford HF, Coutinho-Netto J. Glutamate release in experimental ischaemia of the retina: an approach using microdialysis. J Neurochem. 1992; 59: 358–363. [DOI] [PubMed] [Google Scholar]
- 14. Neal MJ, Cunningham JR, Hutson PH, Hogg J. Effects of ischaemia on neurotransmitter release from the isolated retina. J Neurochemi. 1994; 62: 1025–1033. [DOI] [PubMed] [Google Scholar]
- 15. Müller A, Villain M, Bonne C. The release of amino acids from ischemic retina. Exp Eye Res. 1997; 64: 291–293. [DOI] [PubMed] [Google Scholar]
- 16. Casper DS, Trelstad RL, Reif-Lehrer L. Glutamate-induced cellular injury in isolated chick embryo retina: Müller cell localization of initial effects. J Comp Neurol. 1982; 209: 79–90. [DOI] [PubMed] [Google Scholar]
- 17. Ishikawa M, Yoshitomi T, Zorumski CF, Izumi Y. Effects of acutely elevated hydrostatic pressure in the rat ex vivo retinal preparation. Invest Ophthalmol Vis Sci. 2010; 51: 6414–6423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ishikawa M, Yoshitomi T, Zorumski CF, Izumi Y. Down regulation of glutamine synthetase via GLAST suppression induces retinal axonal swelling in a rat ex vivo hydrostatic pressure model. Invest Ophthalmol Vis Sci. 2011; 52: 6604–6616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Harada T, Harada C, Nakamura K, et al. The potential role of glutamate transporters in the pathogenesis of normal tension glaucoma. J Clin Invest. 2007; 117: 1763–1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kalloniatis M, Tomisich G. Amino acid neurochemistry of the vertebrate retina. Prog Retin Eye Res. 1999; 18: 811–866. [DOI] [PubMed] [Google Scholar]
- 21. Yang XL. Characterization of receptors for glutamate and GABA in retinal neurons. Prog Neurobiol. 2004; 73: 127–150. [DOI] [PubMed] [Google Scholar]
- 22. Naskar R, Vorwerk CK, Dreyer EB. Concurrent downregulation of a glutamate transporter and receptor in glaucoma. Invest Ophthalmol Vis Sci. 2000; 41: 1940–1944. [PubMed] [Google Scholar]
- 23. Martin KR, Levkovitch-Verbin H, Valenta D, Baumrind L, Pease ME, Quigley HA. Retinal glutamate transporter changes in experimental glaucoma and after optic nerve transection in the rat. Invest Ophthalmol Vis Sci. 2002; 43: 2236–2243. [PubMed] [Google Scholar]
- 24. Moreno MC, Sande P, Marcos HA, de Zavalia N, Sarmiento MI, Rosenstein ME. Effect of glaucoma on the retinal glutamate/glutamine cycle activity. FASEB J. 2005; 19: 1161–1162. [DOI] [PubMed] [Google Scholar]
- 25. Nucci C, Tartaglione R, Rombola L, Morrone LA, Fazzi E, Bagetta G. Neurochemical evidence to implicate elevated glutamate in the mechanisms of high intraocular pressure (IOP)-induced retinal ganglion cell death in rat. Neurotoxicology. 2005; 26: 935–941. [DOI] [PubMed] [Google Scholar]
- 26. Moreno MC, de Zavalía N, Sande P, et al. Effect of ocular hypertension on retinal GABAergic activity. Neurochem Int. 2008; 52: 675–682. [DOI] [PubMed] [Google Scholar]
- 27. Reddy DS. Neurosteroids: endogenous role in the human brain and therapeutic potentials. Prog Brain Res. 2010; 186: 113–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Weir CJ, Ling AT, Belelli D, Wildsmith JA, Peters JA, Lambert JJ. The interactioof anaesthetic steroids with recombinant glycine and GABAA receptors. Br J Anaesth. 2004; 92: 704–711. [DOI] [PubMed] [Google Scholar]
- 29. Kajta M, Budziszewska B, Lason W. Allopregnanolone attenuates kainate-induced toxicity in primary cortical neurons and PC12 neuronal cells. Pol J Pharmaco. 1999; 51: 531–534. [PubMed] [Google Scholar]
- 30. Frank C, Sagratella S. Neuroprotective effects of allopregnenolone on hippocampal irreversible neurotoxicity in vitro. Prog Neuropsychopharmacol Biol Psychiatr. 2000; 24: 1117–1126. [DOI] [PubMed] [Google Scholar]
- 31. Ardeshiri A, Kelley MH, Korner IP, Hurn PD, Herson PS. Mechanism of progesterone neuroprotection of rat cerebellar Purkinje cells following oxygen-glucose deprivation. Eur J Neurosci. 2006; 24: 2567–2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ishihara Y, Kawami T, Ishida A, Yamazaki T. Allopregnanolone-mediated protective effects of progesterone on tributyltin-induced neuronal injury in rat hippocampal slices. J Steroid Biochem Mol Biol. 2013; 135: 1–6. [DOI] [PubMed] [Google Scholar]
- 33. Ciriza I, Azcoitia I, Garcia-Segura LM. Reduced progesterone metabolites protect rat hippocampal neurones from kainic acid excitotoxicity in vivo. J Neuroendocrinol. 2004; 16: 58–63. [DOI] [PubMed] [Google Scholar]
- 34. He J, Hoffman SW, Stein DG. Allopregnanolone, a progesterone metabolite, enhances behavioral recovery and decreases neuronal loss after traumatic brain injury. Restor Neurol Neurosci. 2004; 22: 19–31. [PubMed] [Google Scholar]
- 35. Chesnoy-Marchais D. Progesterone and allopregnanolone enhance the miniature synaptic release of glycine in the rat hypoglossal nucleus. Eur J Neurosci. 2009; 30: 2100–2111. [DOI] [PubMed] [Google Scholar]
- 36. Brambilla P, Perez J, Barale F, Schettini G, Soares JC. GABAergic dysfunction in mood disorders. Mol Psychiatry. 2003; 8: 721–737. [DOI] [PubMed] [Google Scholar]
- 37. Luscher B, Shen Q, Sahir N. The GABAergic deficit hypothesis of major depressive disorder. Mol Psychiatry. 2011; 16: 383–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Girdler SS, Klatzkin R. Neurosteroids in the context of stress: implications for depressive disorders. Pharmacol Ther. 2006; 116: 125–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Morrow AL, VanDoren MJ, Fleming R, Penland S. Ethanol and neurosteroid interactions in the brain. Int Rev Neurobiol. 2001; 46: 349–377. [DOI] [PubMed] [Google Scholar]
- 40. Matsumoto K1, Guidotti A, Costa E. Stress and sleep/emotional disorder: possible involvement of neurosteroid allopregnanolone [in Japanese]. Nihon Yakurigaku Zasshi. 2005; 126: 107–112. [DOI] [PubMed] [Google Scholar]
- 41. Guennoun R, Labombarda F, Gonzalez Deniselle MC, Liere P, De Nicola AF, Schumacher M. Progesterone and allopregnanolone in the central nervous system: response to injury and implication for neuroprotection [published online ahead of print September 6, 2014]. J Steroid Biochem Mol Biol. doi:10.1016/j.jsbmp.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 42. Pibiri F, Nelson M, Guidotti A, Costa E, Pinna G. Decreased corticolimbic allopregnanolone expression during social isolation enhances contextual fear: a model relevant for posttraumatic stress disorder. Proc Natl Acad Sci U S A. 2008; 105: 5567–5572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zorumski CF, Mennerick S, Isenberg KE, Covey DF. Potential clinical uses of neuroactive steroids. Curr Opin Investig Drugs. 2000; 1: 360–369. [PubMed] [Google Scholar]
- 44. Gunn BG, Brown AR, Lambert JJ, Belelli D. Neurosteroids and GABAA receptor interactions: a focus on stress. Front Neurosci. 2011; 5: 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Purdy RH, Morrow AL, Moore PH Jr, Paul SM. Stress-induced elevations of γ-aminobutyric acid type A receptor-active steroids in the rat brain. Proc Natl Acad Sci U S A. 1991; 88: 4553–4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tokuda K, Izumi Y, Zorumski CF. Locally-generated acetaldehyde contributes to the effects of ethanol on neurosteroids and LTP in the hippocampus. Neurol Clin Neurosci. 2013; 1: 138–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Izumi Y, Svrakic N, O'Dell K, Zorumski CF. Ammonia inhibits long-term potentiation via neurosteroid synthesis in hippocampal pyramidal neurons. Neuroscience. 2013; 233: 166–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Vallee M, Rivera JD, Koob GF, Purdy RH, Fitzgerald RL. Quantification of neurosteroids in rat plasma and brain following swim stress and allopregnanolone administration using negative chemical ionization gas chromatography/mass spectrometry. Anal Biochem. 2000; 287: 153–166. [DOI] [PubMed] [Google Scholar]
- 49. Crawley JN, Glowa JR, Majewska MD, Paul SM. Anxiolytic activity of an endogenous adrenal steroid. Brain Res. 1986; 398: 382–385. [DOI] [PubMed] [Google Scholar]
- 50. Pesaresi M, Maschi O, Giatti S, Garcia-Segura LM, Caruso D, Melcangi RC. Sex differences in neuroactive steroid levels in the nervous system of diabetic and non-diabetic rats. Horm Behav. 2010; 57: 46–55. [DOI] [PubMed] [Google Scholar]
- 51. Saalmann YB, Kirkcaldie MT, Waldron S, Calford MB. Cellular distribution of the GABAA receptor-modulating 3α-hydroxy, 5α-reduced pregnane steroids in the adult rat brain. J Neuroendocrinol. 2007; 19: 272–284. [DOI] [PubMed] [Google Scholar]
- 52. Tokuda K, O'Dell KA, Izumi Y, Zorumski CF. Midazolam inhibits hippocampal long-term potentiation and learning through dual central and peripheral benzodiazepine receptor activation and neurosteroidogenesis. J Neurosci. 2010; 30: 16788–16795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Bernardi F, Salvestroni C, Casarosa E, et al. Aging is associated with changes in allopregnanolone concentrations in brain, endocrine glands and serum in male rats. Eur J Endocrinol. 1998; 138: 316–321. [DOI] [PubMed] [Google Scholar]
- 54. Izumi Y, Kirby CO, Benz AM, Olney JW, Zorumski CF. Swelling of Müller cells induced by AP3 and glutamate transport substrates in rat retina. Glia. 1996; 17: 285–293. [DOI] [PubMed] [Google Scholar]
- 55. Rone MB, Fan J, Papadopoulos V. Cholesterol transport in steroid biosynthesis: role of protein-protein interactions and implications in disease states. Biochim Biophys Acta. 2009; 1791: 646–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Guarneri P, Russo D, Cascio C, De Leo G, Piccoli F, Guarneri R. Induction of neurosteroid synthesis by NMDA receptors in isolated rat retina: a potential early event in excitotoxicity. Eur J Neurosci. 1998; 10: 1752–1763. [DOI] [PubMed] [Google Scholar]
- 57. Belelli D, Lambert JJ. Neurosteroids: endogenous regulators of the GABA receptor. Nat Rev Neurosci. 2005; 6: 565–575. [DOI] [PubMed] [Google Scholar]
- 58. Tian G. 17β-(N-tert-butylcarbamoyl)-4-aza-5α-androstan-1-en-3-one is an active site-directed slow time-dependent inhibitor of human steroid 5α-reductase. Biochemistry. 1994; 33: 2291–2296. [DOI] [PubMed] [Google Scholar]
- 59. Clark RV, Hermann DJ, Cunningham GR, Wilson TH, Morrill BB, Hobbs S. Marked suppression of dihydrotestosterone in men with benign prostatic hyperplasia by dutasteride, a dual 5α-reductase inhibitor. J Clin Endocrinol Metab. 2004; 89: 2179–2184. [DOI] [PubMed] [Google Scholar]
- 60. Do Rego JL, Seong JY, Burel D, et al. Regulation of neurosteroid biosynthesis by neurotransmitters and neuropeptides. Front Endocrinol. 2012; 3: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ferrarese C, Mennini T, Pecora N, et al. Diazepam binding inhibitor (DBI) increases after acute stress in rat. Neuropharmacology. 1991; 30: 1445–1452. [DOI] [PubMed] [Google Scholar]
- 62. Lam DY, Kaufman PL, Gabelt BT, To EC, Matsubara JA. Neurochemical correlates of cortical plasticity after unilateral elevated intraocular pressure in a primate model of glaucoma. Invest Ophthalmol Vis Sci. 2003; 44: 2573–2581. [DOI] [PubMed] [Google Scholar]
- 63. Marx CE, Bradford DW, Hamer RM, et al. Pregnenolone as a novel therapeutic candidate in schizophrenia: emerging preclinical and clinical evidence. Neuroscience. 2011; 191: 78–90. [DOI] [PubMed] [Google Scholar]
- 64. Iwata S, Wakita M, Shin MC, Fukuda A, Akaike N. Modulation of allopregnanolone on excitatory transmitters release from single glutamatergic terminal. Brain Res Bull. 2013; 93: 39–46. [DOI] [PubMed] [Google Scholar]
- 65. Guarneri P, Cascio C, Russo D, et al. Neurosteroids in the retina: neurodegenerative and neuroprotective agents in retinal degeneration. Ann N Y Acad Sci. 2003; 1007: 117–128. [DOI] [PubMed] [Google Scholar]
- 66. Fourgeux C, Martine L, Pasquis B, et al. Steady-state levels of retinal 24S-hydroxycholesterol are maintained by glial cells intervention after elevation of intraocular pressure in the rat. Acta Ophthalmol. 2012; 90: e560–567. [DOI] [PubMed] [Google Scholar]
- 67. Fourgeux C, Martine L, Acar N, Bron AM, Creuzot-Garcher CP, Bretillon L. In vivo consequences of cholesterol-24S-hydroxylase (CYP46A1) inhibition by voriconazole on cholesterol homeostasis and function in the rat retina. Biochem Biophys Res Commun. 2014; 446: 775–781. [DOI] [PubMed] [Google Scholar]
- 68. Yawno T, Hirst JJ, Castillo-Melendez M, Walker DW. Role of neurosteroids in regulating cell death and proliferation in the late gestation fetal brain. Neuroscience. 2009; 163: 838–47. [DOI] [PubMed] [Google Scholar]
- 69. Izumi Y, Benz AM, Kurby CO, et al. An ex vivo rat retinal preparation for excitotoxicity studies. J Neurosci Methods. 1995; 60: 219–225. [DOI] [PubMed] [Google Scholar]
- 70. Izumi Y, Kirby CO, Benz AM, Olney JW, Zorumski CF. Müller cell swelling, glutamate uptake, and excitotoxic neurodegeneration in the isolated rat retina. Glia. 1999; 25: 379–389. [PubMed] [Google Scholar]
- 71. Irwig MS. Depressive symptoms and suicidal thoughts among former users of finasteride with persistent sexual side effects. J Clin Psychiatry. 2012; 73: 1220–1223. [DOI] [PubMed] [Google Scholar]
- 72. Traish AM, Hassani J, Guay AT, Zitzmann M, Hansen ML. Adverse side effects of 5α-reductase inhibitors therapy: persistent diminished libido and erectile dysfunction and depression in a subset of patients. J Sex Med. 2011; 8: 872–884. [DOI] [PubMed] [Google Scholar]
- 73. Shahsuvaryan ML. Glaucomatous optic neuropathy management: the role of neuroprotective agents. Med Hypothesis Discov Innov Ophthalmol. 2013; 2: 41–46. [PMC free article] [PubMed] [Google Scholar]
- 74. Tsai JC. Canadian Journal of Ophthalmology Lecture: translational research advances in glaucoma neuroprotection. Can J Ophthalmol. 2013; 48: 141–145. [DOI] [PubMed] [Google Scholar]