Abstract
Purpose.
Conjunctival epithelial T cells are dominated by CD3+CD56-TCRαβ+CD8αβ+ lymphocytes. In this study we explored the antigen experience status, mucosal homing phenotype, cytokine expression, and viral antigen recognition of conjunctival epithelial CD8+ T cells from healthy individuals.
Methods.
Following ocular surface impression cytology, conjunctival cells were recovered by gentle agitation and analyzed by flow cytometry for cell surface markers, cytokine production (stimulated by phorbol 12-myristate 13-acetate [PMA]/ionomycin), and Epstein-Barr virus (EBV)/cytomegalovirus (CMV) immunodominant epitope recognition using major histocompatibility complex (MHC) class I peptide tetramers.
Results.
In contrast to peripheral blood, conjunctival epithelial CD8+ T cells were dominantly CD45RA−CCR7− effector memory cells, and the vast majority expressed the mucosal homing integrin αEβ7. Conjunctival memory CD8+ T cells maintained effector functions with the ability to secrete IFN-γ and expression of Granzyme B, although they expressed significantly reduced amounts per cell compared to peripheral blood T cells. Interestingly, herpetic virus-specific CD8+ T cells recognizing epitopes derived from EBV and CMV could be detected in the conjunctival cells of healthy virus carriers, although they were generally at lower frequencies than in the peripheral blood of the same donor. Virus-specific conjunctival CD8+ T cells were dominated by CD45RA−CCR7− effector memory cells that expressed αEβ7.
Conclusions.
These data demonstrate that the majority of conjunctival epithelial CD8+ T cells are mucosal homing αEβ7+ effector memory T cells, which can recognize viral epitopes and are capable of secreting Granzyme B and IFN-γ.
Keywords: CD8+ T cell, conjunctiva, impression cytology, infection
Conjunctival epithelial T cells are dominated by CD3+CD56-TCRαβ+CD8αβ+ lymphocytes. The majority of these cells are mucosal homing αEβ7+ effector memory T cells, which can recognize viral epitopes and are capable of secreting Granzyme B and IFNγ.
Introduction
The ocular mucosa (conjunctiva and cornea) together with the lacrimal gland, eyelids, and sensory innervations form the lacrimal functional unit that regulates ocular surface immunity.1,2 Mucosal surfaces, including the conjunctiva, have adapted their “first line of defense” in order to undertake specialized roles such as gas exchange, absorption of nutrients, and secretion of mucin, but in the absence of protection afforded by keratinization, as in the skin.3 Keratinization is detrimental to the ocular mucosa, compromising both optical clarity and rendering hydrophobic properties that prohibit hydrophilic scaffolding for the retention of the tear film, a vital biofluid with immunoprotective mediators and nutritive molecules. Conversely, nonkeratinization of the ocular mucosa increases its susceptibility to infection and trauma.
Mucosal surfaces contain an abundance of intraepithelial lymphocytes (IEL).4–7 In the small bowel, CD8+ T cells dominate with one T cell for every 10 epithelial cells6,8; in the lung, CD8+ T cells are prevalent,9 while normal human conjunctiva populations include CD3+ cells, with a dominance of CD3+CD56−TCRαβCD8αβ T cells10 in the epithelium and CD4+ in the lamina propria.10–13 The effector function of TCRαβ+CD8αβ+ cells in the healthy conjunctival epithelium is not known. Repeated exposure to antigen may account for an altered role for epithelial CD8+ lymphocytes, some of which may become regulatory in nature, partly mediated by IL-10. These cells express CD103 (αE), which is associated with homing to mucosal tissues, but in the lung these cells are specific for respiratory viruses such as influenza and have a local cytotoxic function.14–17
Although the majority of the conjunctival CD8+ T cell populations are antigen experienced (CD45RO+ and therefore CD45RA−), their exact memory status remains poorly defined. The majority of CD8+CD103+ mucosal T cells are effector memory in phenotype.18 As well as central and effector memory CD8+ T cells, some cells “revert” to a CCR7−CD45RA+ (EM RA) phenotype and are capable of producing IFN-γ. This is of considerable interest, as a significant proportion of EM RA CD8+ T cells recognize persistent herpes viruses including cytomegalovirus (CMV) and Epstein-Barr virus (EBV), and this may have direct relevance to the ocular mucosa host immune response.19 These viruses have been implicated in a number of ocular surface disorders in both the immunocompromised and immunocompetent.20–23
It is currently not known whether the antigen specificity of the dominant CD8+ T cell population on the conjunctival epithelial surface reflects circulating peripheral blood levels or whether this represents a role in local immunity. The aim of this study was to characterize the conjunctival intraepithelial CD8+ T cell population in more detail by determining their antigen experience, expression patterns of mucosal homing markers, cytokine expression, and antigen specificity.
Materials and Methods
Study Subjects
Clinical data collection and patient sampling were undertaken following informed consent and ethical approval in accordance with the Declaration of Helsinki. Healthy volunteers were defined as individuals with no history or current clinical evidence of ocular, systemic inflammatory or autoimmune disease (including dry eye),24,25 contact lens wear, previous ocular surgery, cataract surgery within 3 months, or use of topical ophthalmic medication.
Two separate cohorts were evaluated: one cohort, n = 17 (median age 32 years [range, 21–81]; 10 male), for homing, memory, and cytokine expression and a second cohort, n = 10 (median age 33 years [range, 25–52]; 6 male), of known human leukocyte antigen (HLA) class I type and CMV/EBV status for virus-specific CD8+ T-cell responses with well-characterized tetramer responses. Conjunctival samples from the left and right eye of each donor were pooled in order to maximize the yield of cells for cytokine staining.
Conjunctival Epithelium Collection and Recovery
Collection of conjunctival cells was undertaken with autoclaved synthetic membranes divided into two semicircles (measuring 13 × 6.5 mm2 each).26 Supor 200 polyethersulfone filters (0.2-μm Supor; Pall Corporation, Port Washington, NY, USA) were applied following instillation of 0.4% oxybuprocaine (as a topical anesthetic). Conjunctival ocular surface impression cytology (OSIC) was performed with four semicircle membranes per eye (equivalent to two full impressions) from the superior unexposed bulbar conjunctiva for 5 to 10 seconds using a sterile technique26,27 and before the application of topical fluorescein drops for clinical examination.
Membranes were removed and placed in 1.5 mL RPMI 1640 (Sigma-Aldrich, Dorset, UK) supplemented with 1% GPS (1.64 mM L-glutamine, 40 U/mL benzylpenicillin, 0.4 mg/mL streptomycin) (Sigma-Aldrich), 1% HEPES buffer (Sigma-Aldrich), and 10% heat inactivated fetal calf serum (HIFCS; Biosera Ltd., Ringmer, UK) in a sterile 5-mL universal container and processed within 6 hours after OSIC. In order to expedite cellular recovery, cells were recovered by gentle agitation with a pipette tip for 1 minute. Cell suspensions were transferred to a 1.5-mL Eppendorf tube and centrifuged (400g for 5 minutes).
The majority of the supernatant was discarded, and the cells were resuspended in RPMI, 10% HIFCS to a total volume of 100 μL. Cells were placed into each well of a 96-well plate for flow cytometric analysis as previously described.10,28
Preparation of Leukocytes
Peripheral blood was collected in EDTA tubes, centrifuged, and resuspended in 1:10 dilution of filter-sterilized red cell lysis buffer (8.29 g NH4Cl, 1 g KHCO3, and 37.2 mg EDTA per liter dH20). After 5 minutes at room temperature, the suspension was diluted with up to 15 mL RPMI to block further lysis. Following centrifugation, the pellet was resuspended in PBS at a concentration between 2 × 106 and 1 × 107 cells/mL.
Flow Cytometry
Flow cytometry was undertaken with a Dako Cyan ADP High Performance flow cytometer (Beckman Coulter, High Wycombe, UK). Multicolor cytometry compensation was performed using cells or compensation beads individually stained with each fluorochrome conjugated antibody in order to circumvent spectral overlap by adjusting for false positives from other fluorochromes. Analysis was undertaken with Kaluza for Windows (Beckman Coulter, Brea, CA, USA).
To characterize the cellular profile of the conjunctival ocular surface, nine color flow cytometry panels were developed. Commercially available antibodies to cell surface markers were employed, including mouse anti-human leukocyte markers CD45 (allophycocyanin or phycoerythrin), CD3 (AlexaFluor 780) (Ebioscience, Hatfield, UK), CD8α (Pacific Orange) (Invitrogen, Paisley, UK) or CD8β (PE Texas Red) (Beckman Coulter, High Wycombe, UK), CD56 (PE Cy7) (Biolegend, Cambridge, UK); memory markers including CD45RO (FITC), CD45RA (PE Texas Red) (Beckman Coulter, High Wycombe, UK), CCR7 (FITC) (R&D Systems, Abingdon, UK), and the homing markers αE (CD103) (FITC) (Dako, Ely, UK) and β7 (PE Cy5) (BD, Oxford, UK). All antibodies were titrated to predetermine optimal concentrations for multicolor staining. Appropriate panels were applied to cells recovered from conjunctival OSIC or peripheral blood.
Cells (100 μL, with a cell count per well ranging from 2 × 105 to 1 × 106 for leukocytes) or 20 μL positive and negative compensation beads were placed in a 96-well plate. Cells were centrifuged for 4 minutes at 400g at 4°C; the supernatant was removed and the 96-well plate gently vortexed. Cells were stained with surface marker antibodies (made up in 50 μL at appropriate dilutions) and incubated on ice in the dark for 20 minutes. One hundred microliters PBS/0.5% BSA was added to each well prior to further centrifugation and removal of supernatant. Cells were resuspended in 295 μL fluorescence-activated cell sorting (FACS) buffer and 5 μL counting beads prior to analysis. For dead cell exclusion, 30 μL Sytox blue (Invitrogen) was added to the stained cells at a concentration of 1/800 and incubated for 5 minutes immediately prior to running on the flow cytometer.
Intracellular Cytokine and Cytotoxic Protein Staining
For cytokine assays, conjunctival and peripheral blood leukocytes were stimulated for 3 hours at 37°C (5% CO2) with 250 ng/mL phorbol 12-myristate 13-acetate (PMA; Sigma-Aldrich) and 250 ng/mL ionomycin (Sigma-Aldrich), with the addition of 2 μg/mL Brefeldin A (Sigma-Aldrich) to prevent secretion of cytokine from the cells.
To discriminate dead cells, a Live Dead fixable yellow dye (Invitrogen) was used, with cells resuspended in 100 μL 1:1000 dilution for 30 minutes on ice in the dark. Surface marker antibodies in this panel were suspended in Fixation medium A (Fix & Perm; Invitrogen) under the same conditions as described for flow cytometry. Intracellular antibodies, mouse anti-human IFN-γ (eFluor 450; Ebioscience), IL-17a (FITC; Ebioscience), IL-22 (phycoerythrin; Ebioscience), and rat anti-human IL-10 (phycoerythrin; Biolegend), were suspended in Permeabilization medium B (Fix & Perm; Invitrogen) on ice in the dark and cell stained with these for 20 minutes before centrifugation and resuspension as described.
Anti-Granzyme B (PE) (BD, UK) was added after cells were fixed and permeabilized and prior to cell surface staining as described above.
Viral Peptide MHC-I Tetramer Staining
Conjunctival and matched peripheral blood CD8+ T cells were incubated with pooled CMV or EBV peptide–major histocompatibility complex (MHC) class I tetramers conjugated to APC as previously described by Khan et al.29 Serostatus for CMV was determined by detection of anti-CMV IgG antibodies using ELISA (BioCheck, Inc., Foster City, CA, USA) according to manufacturer's instructions and for EBV as previously described.30 The HLA status was also determined as previously described.29 For donors of known HLA class I type and CMV/EBV status, MHC class I tetramers of the following HLA allele/epitope peptide combinations were employed: CMV—HLA-A1 VTEHDTLLY (pp50 derived), HLA-A1 YSEHPTFTSQY (pp65), HLA-A2 NLVPMVATV (pp65), HLA-B7 RPHERNGFTVL (pp65), HLA-B8 ELKRKMIYM (IE1), HLA-B8 QIKVRVDMV (IE-1); EBV—HLA-A2 GLCTLVAML (BMFL1 derived), HLA-A2 YVLDHLIVV (BMRF1), HLA-B8 RAKFKQLL (BZLF1; all lytic cycle), and HLA-B8 FLRGRAYGL (EBNA3A; latent cycle). For details of peptide sequences see Khan et al.29 Briefly, cells were incubated at room temperature for 20 minutes with pooled CMV or EBV tetramers prior to cell surface staining as described above.
Statistical Analysis
Nonparametric comparisons were undertaken with the Wilcoxon matched-pairs signed rank test and correlations by Spearman's correlation using Prism version 5.0 for Macintosh (GraphPad Software, La Jolla, CA, USA).
Results
Memory and Homing: Human Conjunctival CD8+ T Cells Are Mucosal Homing Effector Memory Cells
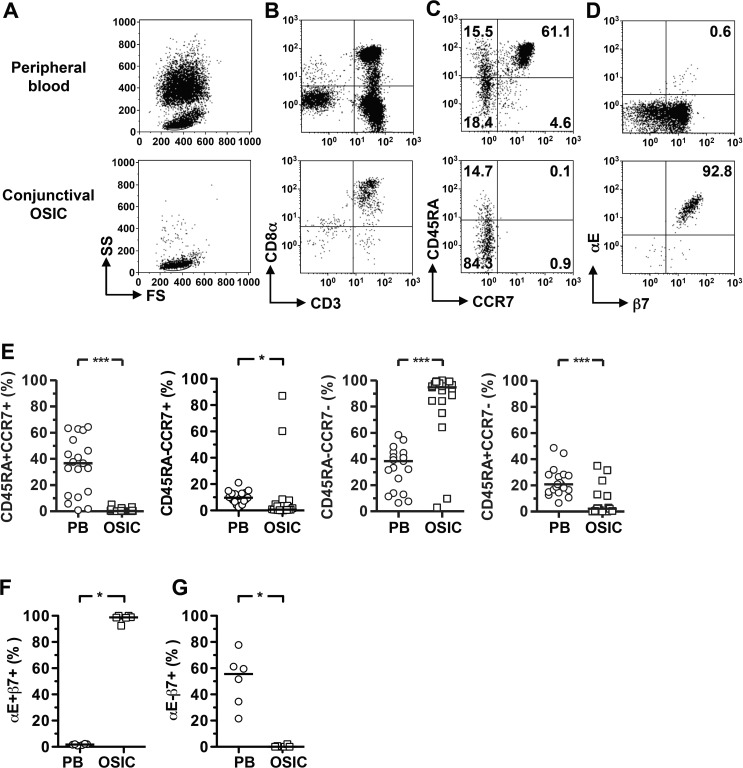
In order to characterize the memory status of conjunctival CD8+ T cells, we determined the expression of an isoform of CD45, CD45RA, and lymph node homing receptor CCR7. For the majority of donors, conjunctival epithelial CD8+ T cells were predominantly CD45RA−CCR7− effector memory (EM) cells as compared to peripheral blood, with a consequent reduction in the naïve population (Figs. 1A–C, 1E; Table). In addition, there were significantly fewer EM RA+ (CCR7−CD45RA+) CD8+ T cells and central memory CD8+ T cells in most donors, with the exception of two subjects who showed a very high frequency of central memory cells and corresponding reduction in EM frequency.
Figure 1.
Human conjunctival CD8+ lymphocytes are mucosal homing, effector memory T cells. Flow cytometry plots were gated as previously described.10 (A) Data are shown for live CD45+ cells to define the scatter gates. In (B), cells were additionally gated on CD56− cells; (C, D) show the gated CD56−CD3+CD8αβ+ lymphocyte population. Representative plots showing mucosal homing integrin expression and memory phenotype identified by CD45RA and CCR7 coexpression pattern on CD3−CD8+CD56− live lymphocytes (C, D). Significant differences in expression of homing markers (F, G) and memory phenotype (E) between conjunctival epithelial leukocyte and peripheral blood populations were observed. Statistical analysis was undertaken by the Wilcoxon matched-pairs signed rank test; P > 0.05; ***P < 0.001; **P < 0.01; *P < 0.05. FS, forward scatter; SS, side scatter; PB, peripheral blood; OSIC, conjunctival ocular surface impression cytology.
Table.
Summary of Percentage Frequencies of CD8+ T-Cell Populations and Median Fluorescent Intensity (MFI) From Ocular Surface Impression Cytology of the Conjunctival Epithelium and Matched Peripheral Blood
|
CD8+ T-Cell Populations |
OSIC |
Blood |
Significance,P< 0.05 |
| Homing | |||
| αEβ7, % | 99 (92–100) | 1.8 (0.9–2.3) | P= 0.03 |
| α4β7, % | 0.1 (0–2) | 56 (21–78) | P= 0.03 |
| Memory | |||
| Naïve, % | 0.2 (0–5) | 37 (1–64) | P= 0.0002 |
| CM, % | 0.7 (0–87) | 10 (3–21) | P= 0.046 |
| EM, % | 95 (3–100) | 38 (6–58) | P= 0.0004 |
| EMRA, % | 2 (0–35) | 21 (7–49) | P= 0.0004 |
| Cytotoxicity | |||
| Granzyme | 55 (34–68) | 38 (29–49) | P= 0.03 |
| Granzyme MFI | 3.9 (2.8–4.7) | 10.0 (7.6–14.9) | P= 0.03 |
| Cytokines | |||
| IFN-γ, % | 41 (25–83) | 33 (19–54) | P = 0.22 |
| IFN-γ MFI, % | 78 (58–111) | 139 (50–185) | P = 0.16 |
| IL-17, % | 1 (0–4.5) | 0.3 (1–1.3) | P = 0.44 |
| IL-22, % | 0.53 (0–1) | 0.39 (0.1–1) | P = 0.84 |
| IL-10, % | 0 (0–0.6) | 0.1 (0–0.2) | P = 1.00 |
| CMV/EBV-specific CD8+ T cells | |||
| EM, % | 87 (73–98) | 47 (9–80) | P= 0.03 |
| EMRA, % | 7 (0–27) | 47 (7–67) | P= 0.03 |
Numbers referred to are medians with ranges in parentheses. n = 6 for OSIC and blood for all cohorts except the memory population, where n = 17. Statistical analysis was undertaken by the Wilcoxon matched-pairs signed rank test; significance was taken at P < 0.05 (bold). CM, central memory; EM, effector memory; EMRA, effector memory RA.
Almost all (median 99%) conjunctival CD8+ T cells expressed integrins related to mucosal homing (αEβ7 [CD103]), while only a very small population was observed in the peripheral blood (Figs. 1B, 1D; Table). By contrast, <1% of conjunctival CD8+ T cells were αE−β7+ assumed to be α4+β7+, compared to >50% in peripheral blood (Fig. 1G). These data demonstrate an antigen-experienced and mucosal homing phenotype of conjunctival CD8+ T cells.
Function of Conjunctival Epithelial CD8+ T Cells: Conjunctival CD8+ T Cells Demonstrate Cytotoxicity, Albeit With Low Expression Levels of Granzyme B and IFN-γ
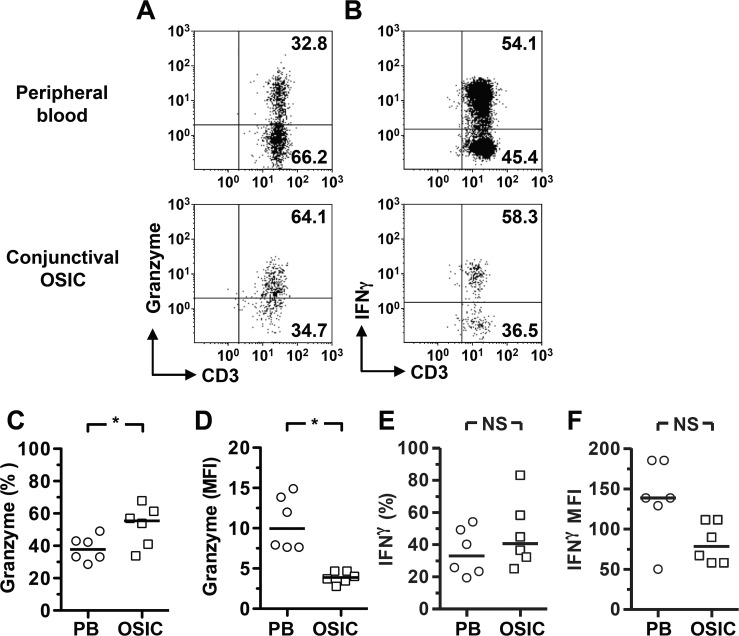
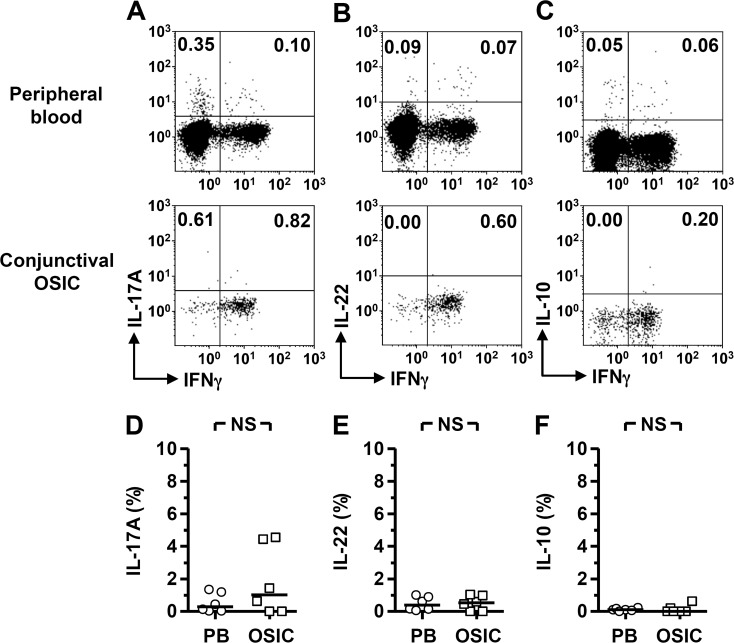
The cytotoxic potential of conjunctival CD8+ T cells was examined using Granzyme B expression as a marker. The population of CD8+CD45RO+ memory T cells within the conjunctiva was found to constitutively express Granzyme B, higher than the frequency of positive cells as compared to peripheral blood (Figs. 2A, 2C; Table). This contrasted with a significantly lower quantity of Granzyme B per cell in the conjunctival CD8+ T cells versus peripheral blood (Fig. 2D; Table). When stimulated with PMA/ionomycin, antigen-experienced conjunctival CD8+ T cells were capable of producing IFN-γ. Although there was no significant difference in the quantity produced per cell, there was a clear trend toward lower expression in the conjunctival cells (Figs. 2B, 2E, 2F). Analysis of additional inflammatory cytokines, IL-17 and IL-22, did not reveal any significant differences between conjunctival and peripheral blood CD8+ T cells; only small frequencies of CD8+ T cells secreted these cytokines (Figs. 3A, 3B, 3D, 3E). There was also little evidence for a regulatory CD8+ T-cell population, with few cells secreting IL-10 (Figs. 3C, 3F) and no detectable FoxP3 expression (data not shown).
Figure 2.
Conjunctival epithelial CD8+ lymphocytes display a cytotoxic phenotype, expressing Granzyme B and IFN-γ. Flow cytometry plots were gated on live CD45+CD56−CD8+CD45RO+ cells. The inclusion of the CD3 marker provides confirmation that the gated cells are indeed T cells. Representative plots show constitutive expression of Granzyme B (A) and IFN-γ following stimulation (B), with percentages (C, E) and median fluorescence intensities (MFI) (D, F) for the cohort. Statistical analysis was undertaken by the Wilcoxon matched-pairs signed rank test; NS, not significant, P > 0.05; *P < 0.05. PB, peripheral blood; OSIC, conjunctival ocular surface impression cytology.
Figure 3.
Conjunctival epithelial CD8+ lymphocytes do not display a proinflammatory or regulatory phenotype. Flow cytometry plots were gated on live CD45+CD56−CD8+CD45RO+ cells. Representative plots showing production of IL-17, -22, and -10 among conjunctival and matched peripheral blood (PB) cells (A–C). Comparative analyses are shown in (D–F). Statistical analysis was undertaken by the Wilcoxon matched-pairs signed rank test; NS, not significant, P > 0.05. OSIC, conjunctival ocular surface impression cytology.
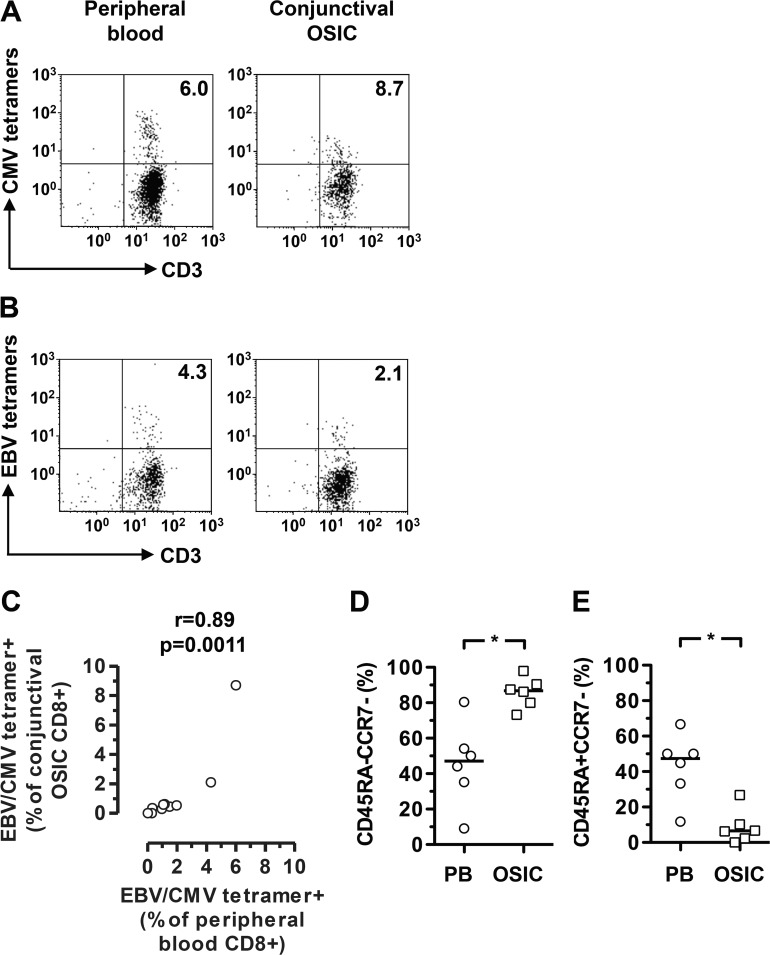
Detection of EBV- and CMV-Specific T Cells Within the Conjunctival Epithelial CD8+ T-Cell Population
To identify antigen-specific T cells from the conjunctival impression samples, we used pools of HLA class I tetramers containing peptides derived from either EBV or CMV herpetic viruses, with donors preselected for EBV and CMV seropositive status and tetramer staining in the peripheral blood. As these are detected in significant numbers in the peripheral blood of healthy donors, this might allow detection from the very low numbers of cell yields from OSIC. We observed a positive correlation between the frequencies of EBV/CMV-specific CD8+ T cells from the conjunctiva and those in the peripheral blood (Figs. 4A–C; Table). Overall, however, there was an underrepresentation of CMV- and EBV-specific CD8+ T cells in the conjunctiva compared to the peripheral blood, apart from one donor who demonstrated a high frequency of CMV-specific CD8+ T cells (Fig. 4C). The memory status of the CMV- and EBV-specific conjunctival CD8+ T cells reflected those of the total CD8+ T cell population, with significant elevation of EM cells and a reduction in EM RA+ cells, as compared to peripheral blood (Figs. 4D, 4E).
Figure 4.
Frequency of cytomegalovirus (CMV)- and Epstein-Barr virus (EBV)-specific T cells among conjunctival epithelial CD8+ T cells reflects that of peripheral blood. Flow cytometry plots were gated on live CD45+CD56−CD8+ cells. Conjunctival CD8+ T cells were stained with pooled CMV or EBV peptide MHC class I tetramers. The inclusion of the CD3 marker provides confirmation that the gated cells are indeed T cells. Representative plots identifying CMV- and EBV-specific T cells are shown in (A, B), respectively. Correlation between frequencies of CMV- or EBV-specific CD8+ T cells in peripheral blood and conjunctiva is shown in (C). Differences between effector memory and effector memory RA subsets within the virus-specific T cell populations in peripheral blood and conjunctiva are shown in (D, E). Statistical analysis was undertaken by a Spearman correlation or Wilcoxon matched-pairs signed rank test. EBV, Epstein-Barr virus; CMV, cytomegalovirus; PB, peripheral blood; OSIC, conjunctival ocular surface impression cytology; *P < 0.05.
Discussion
The superficial layers of the conjunctival epithelium have previously been shown to be dominated by CD8+αβ+ T cells. In this study we have demonstrated that the majority of these cells express the mucosal homing integrin αEβ7 and are of an EM (CD45RA−CCR7−) phenotype. There is a clear functional ability; these cells express Granzyme B and are capable of expressing IFN-γ. Their ability to recognize a number of peptide/HLA complexes from DNA viruses suggests that these cells provide immune surveillance functions at the ocular surface.
Circulating T cells are able to be recruited to mucosal tissues such as the gut by expression of the integrin pairing of α4 and β7, which allows binding to MadCAM-1 on the lamina propria vascular endothelium.31 It is suggested that a switch to αEβ7 (CD103) on these mucosal homing T cells directs lymphocytes within the lamina propria to bind to E-cadherin on epithelial cells.32,33 It is has been proposed that E-cadherin adhesion (which has been identified on normal human conjunctival epithelial cells34) is dependent on CCR9–CCL25 interaction.35 The mechanism by which α4 is switched to αE is not fully understood; but there is evidence from the gut in diseases such as graft-vs-host disease (GVHD) that CD103 expression is mediated by TGFβ on epithelial cells.36 There is also evidence in the skin that following exposure to herpes simplex virus (HSV) antigen, CD103 is required for formation and/or survival of CD8+ resident memory cells, and entry into the epithelium is dependent on TGFβ and IL-15.37 It is not known whether the recruitment to the conjunctival epithelium is dependent on CD103 expression, but its presence on virtually all CD8+ T cells in this study strongly suggests that this is the case.
The priming of naïve T cells by antigen when taking place in draining lymph nodes, including mucosa-associated lymphoid tissues (MALT), results in an alteration in cell surface marker expression including a switch from a high (CD45RA) to a low molecular weight isoform (CD45RO) of the CD45 common leukocyte antigen marker.19 Education in the local environment of mucosal lymphoid tissues has been shown to induce the expression of αEβ7 on T cell subsets, although alternative routes of immunization, such as subcutaneous yellow fever vaccination in humans, may also induce mucosal homing.35,38 The dominant expression of αEβ7 on the conjunctival CD8+ T cells in this study suggests that the rules governing the recruitment to the conjunctival epithelium are the same as those previously defined for other mucosal tissues.
Effector memory cells typically secrete IFN-γ upon stimulation,18 yet there is evidence of a regulatory/suppressive phenotype in CD103+ (αEβ7) cells, including the suppression of pathogenic Th17 cells implicated in corneal barrier dysregulation in an experimental murine dry eye model.15,39 In the lung, the dominant CD103+CD8+ T cells demonstrate a lower cytotoxic potential as compared to the CD103−CD8+ T cells.14 Our data suggest that conjunctival epithelial CD8+ T cells are overwhelmingly CD103+, retaining their cytotoxic function and capacity to secrete IFN-γ, although the levels produced, at least within the healthy human conjunctiva, might be partially attenuated.
We found no evidence that significant numbers of conjunctival epithelial CD8+ T cells had a regulatory phenotype, although our studies were restricted to IL-10 and FoxP3 expression. This is an interesting observation given the recent finding of CD8+ Tregs playing a role in the regulation of the afferent arm of Th17-mediated ocular surface damage in a desiccating stress murine model of Sjögren's syndrome.39 It is possible that these cells are recruited in response to ocular surface inflammatory disorders including dry eye disease. The composition of conjunctival epithelial leukocytes alters in response to severe inflammatory diseases such as Stevens-Johnson syndrome, including a reduction in the dominant CD8+ T cell population and the recruitment of neutrophils, which persist.28 It would be interesting to determine how the function of CD8+ T cells is influenced by pathological states, in particular, viral infections relevant to the ocular surface (e.g., HSV). In addition, any potential regulatory function would ideally need to be addressed with assays that allow cell–cell contact, although this is clearly very challenging with such small cell numbers.
Herpetic viruses such as CMV are known to cause devastating retinitis in immunocompromised individuals, and CMV DNA has been found to be elevated in the conjunctiva of patients with human immunodeficiency virus (HIV).20 Furthermore, EBV is detected in the conjunctiva of nearly half of patients who are HIV positive or negative (healthy controls), but up to 83% of HIV-positive patients who developed ocular surface neoplasia had the EBV virus within malignant tissue.20,21 Susceptibility to these viruses is not confined to the immunocompromised alone, however, with CMV corneal endotheliitis being well documented in immunocompetent populations where CMV seropositivity is frequent.22,23 Our findings that EBV- and CMV-specific CD8+ T cells are detectable in the conjunctival epithelium suggests that a broad range of virus-specific T cells may be able to home to this tissue and provide immune protection. It will be important in future studies not only to determine the range of antigens recognized by these CD8+ T cells, but also to confirm their function in response to native antigen.
The relative decrease in EM RA+ CD8+ T cells in the conjunctiva, which is the dominant phenotype for peripheral CMV-specific CD8+ T cells in healthy subjects, may explain the slight underrepresentation of these cells in the conjunctival epithelium of the majority of donors analyzed. The absence of EM RA+ CD8+ T cells in the conjunctiva may reflect either an inability of these cells to migrate through the conjunctival epithelium and/or a reduction of αEβ7+ EBV/CMV-specific CD8+ T cells. This latter point is supported by the data from Piet et al.14 showing that although influenza-specific CD8+ T cells expressed αEβ7, this molecule was largely absent from the EBV- and CMV-specific cells. A more detailed analysis of the antigen specificity and phenotype of conjunctival epithelial CD8+ T cells would be required to determine the relative contribution of these pathways, as well as a study of T cells specific for other viruses more commonly associated with the ocular surface, for example, HSV.
In this study OSIC was confined to the superior bulbar conjunctival population of cells, which has previously been demonstrated to have a higher proportion of CD3+ T cells than in the inferior tarsal and bulbar conjunctiva.13 It would be interesting to determine CD8+ T-cell memory and phenotype in other areas of the ocular surface. These data for the first time suggest that functional EM conventional CD8αβ+ T cells are recruited to the conjunctival epithelium and are capable of providing immune protection against a range of pathogens that can access the ocular surface
Acknowledgments
Presented in part at the annual meeting of the Association for Research in Vision and Ophthalmology, Fort Lauderdale, Florida, United States, May 2010, and the International Congress of Mucosal Immunology, Paris, France, July 2011.
Supported by the Wellcome Trust, United Kingdom (Clinical Research Fellowship to GPW), the Birmingham Eye Foundation (Registered Charity United Kingdom Charity 257549), and a Fight for Sight New Lecturers Grant (GPW).
Disclosure: G.P. Williams, None; A. Pachnio, None; H.M. Long, None; S. Rauz, None; S.J. Curnow, None
References
- 1. Zierhut M, Dana MR, Stern ME, Sullivan DA. Immunology of the lacrimal gland and ocular tear film. Trends Immunol. 2002; 23: 333–335. [DOI] [PubMed] [Google Scholar]
- 2. Stern ME, Schaumburg CS, Dana R, Calonge M, Niederkorn JY, Pflugfelder SC. Autoimmunity at the ocular surface: pathogenesis and regulation. Mucosal Immunol. 2010; 3: 425–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lawson LB, Norton EB, Clements JD. Defending the mucosa: adjuvant and carrier formulations for mucosal immunity. Curr Opin Immunol. 2011; 23: 414–420. [DOI] [PubMed] [Google Scholar]
- 4. Allansmith MR, Greiner JV, Baird RS. Number of inflammatory cells in the normal conjunctiva. Am J Ophthalmol. 1978; 86: 250–259. [DOI] [PubMed] [Google Scholar]
- 5. Knop E, Knop N. Anatomy and immunology of the ocular surface. Chem Immunol Allergy. 2007; 92: 36–49. [DOI] [PubMed] [Google Scholar]
- 6. Hayday A, Theodoridis E, Ramsburg E, Shires J. Intraepithelial lymphocytes: exploring the Third Way in immunology. Nat Immunol. 2001; 2: 997–1003. [DOI] [PubMed] [Google Scholar]
- 7. Knop E, Knop N. The role of eye-associated lymphoid tissue in corneal immune protection. J Anat. 2005; 206: 271–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. MacDonald TT, Monteleone I, Fantini MC, Monteleone G. Regulation of homeostasis and inflammation in the intestine. Gastroenterology. 2011; 140: 1768–1775. [DOI] [PubMed] [Google Scholar]
- 9. Power CK, Burke CM, Sreenan S, Hurson B, Poulter LW. T-cell and macrophage subsets in the bronchial wall of clinically healthy subjects. Eur Respir J. 1994; 7: 437–441. [DOI] [PubMed] [Google Scholar]
- 10. Williams GP, Denniston AK, Oswal KS, et al. The dominant human conjunctival epithelial CD8αβ+ T cell population is maintained with age but the number of CD4+ T cells increases. Age (Dordr). 2012; 34: 1517–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sacks EH, Wieczorek R, Jakobiec FA, Knowles DM II. Lymphocytic subpopulations in the normal human conjunctiva. A monoclonal antibody study. Ophthalmology. 1986; 93: 1276–1283. [DOI] [PubMed] [Google Scholar]
- 12. Hingorani M, Metz D, Lightman SL. Characterisation of the normal conjunctival leukocyte population. Exp Eye Res. 1997; 64: 905–912. [DOI] [PubMed] [Google Scholar]
- 13. Reinoso R, Martin-Sanz R, Martino M, et al. Topographical distribution and characterization of epithelial cells and intraepithelial lymphocytes in the human ocular mucosa. Mucosal Immunol. 2012; 5: 455–467. [DOI] [PubMed] [Google Scholar]
- 14. Piet B, de Bree GJ, Smids-Dierdorp BS, et al. CD8(+) T cells with an intraepithelial phenotype upregulate cytotoxic function upon influenza infection in human lung. J Clin Invest. 2011; 121: 2254–2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Koch SD, Uss E, van Lier RAW, ten Berge IJM. Alloantigen-induced regulatory CD8+CD103+ T cells. Hum Immunol. 2008; 69: 737–744. [DOI] [PubMed] [Google Scholar]
- 16. Xystrakis E, Dejean AS, Bernard I, et al. Identification of a novel natural regulatory CD8 T-cell subset and analysis of its mechanism of regulation. Blood. 2004; 104: 3294–3301. [DOI] [PubMed] [Google Scholar]
- 17. Uss E, Rowshani AT, Hooibrink B, Lardy NM, van Lier RAW, ten Berge IJM. CD103 is a marker for alloantigen-induced regulatory CD8+ T cells. J Immunol. 2006; 177: 2775–2783. [DOI] [PubMed] [Google Scholar]
- 18. Gupta S, Bi R, Su K, Yel L, Chiplunkar S, Gollapudi S. Characterization of naïve, memory and effector CD8+ T cells: effect of age. Exp Gerontol. 2004; 39: 545–550. [DOI] [PubMed] [Google Scholar]
- 19. Faint JM, Annels NE, Curnow SJ, et al. Memory T cells constitute a subset of the human CD8+CD45RA+ pool with distinct phenotypic and migratory characteristics. J Immunol. 2001; 167: 212–220. [DOI] [PubMed] [Google Scholar]
- 20. Lee-Wing MW, Hodge WG, Diaz-Mitoma F. The prevalence of herpes family virus DNA in the conjunctiva of patients positive and negative for human immunodeficiency virus using the polymerase chain reaction. Ophthalmology. 1999; 106: 350–354. [DOI] [PubMed] [Google Scholar]
- 21. Simbiri KO, Murakami M, Feldman M, et al. Multiple oncogenic viruses identified in ocular surface squamous neoplasia in HIV-1 patients. Infect Agents Cancer. 2010; 5: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chee SP, Bacsal K, Jap A, Se-Thoe SY, Cheng CL, Tan BH. Corneal endotheliitis associated with evidence of cytomegalovirus infection. Ophthalmology. 2007; 114: 798–803. [DOI] [PubMed] [Google Scholar]
- 23. Koizumi N, Suzuki T, Uno T, et al. Cytomegalovirus as an etiologic factor in corneal endotheliitis. Ophthalmology. 2008; 115: 292–297, e293. [DOI] [PubMed] [Google Scholar]
- 24. Behrens A, Doyle JJ, Stern L, et al. Dysfunctional tear syndrome: a Delphi approach to treatment recommendations. Cornea. 2006; 25: 900–907. [DOI] [PubMed] [Google Scholar]
- 25. The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf. 2007; 5: 75–92. [DOI] [PubMed] [Google Scholar]
- 26. Brignole-Baudouin F, Ott AC, Warnet JM, Baudouin C. Flow cytometry in conjunctival impression cytology: a new tool for exploring ocular surface pathologies. Exp Eye Res. 2004; 78: 473–481. [DOI] [PubMed] [Google Scholar]
- 27. Brignole F, Pisella PJ, Goldschild M. De Saint Jean M, Goguel A, Baudouin C. Flow cytometric analysis of inflammatory markers in conjunctival epithelial cells of patients with dry eyes. Invest Ophthalmol Vis Sci. 2000; 41: 1356–1363. [PubMed] [Google Scholar]
- 28. Williams GP, Tomlins PJ, Denniston AK, et al. Elevation of conjunctival epithelial CD45INTCD11b(+)CD16(+)CD14(−) neutrophils in ocular Stevens-Johnson syndrome and toxic epidermal necrolysis. Invest Ophthalmol Vis Sci. 2013; 54: 4578–4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Khan N, Hislop A, Gudgeon N, et al. Herpesvirus-specific CD8 T cell immunity in old age: cytomegalovirus impairs the response to a coresident EBV infection. J Immunol. 2004; 173: 7481–7489. [DOI] [PubMed] [Google Scholar]
- 30. Henle W, Henle GE, Horwitz CA. Epstein-Barr virus specific diagnostic tests in infectious mononucleosis. Hum Pathol. 1974; 5: 551–565. [DOI] [PubMed] [Google Scholar]
- 31. Svensson M, Marsal J, Ericsson A, et al. CCL25 mediates the localization of recently activated CD8alphabeta(+) lymphocytes to the small-intestinal mucosa. J Clin Invest. 2002; 110: 1113–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cepek KL, Parker CM, Madara JL, Brenner MB. Integrin alpha E beta 7 mediates adhesion of T lymphocytes to epithelial cells. J Immunol. 1993; 150: 3459–3470. [PubMed] [Google Scholar]
- 33. Cepek KL, Shaw SK, Parker CM, et al. Adhesion between epithelial cells and T lymphocytes mediated by E-cadherin and the alpha E beta 7 integrin. Nature. 1994; 372: 190–193. [DOI] [PubMed] [Google Scholar]
- 34. Scott RA, Lauweryns B, Snead DM, Haynes RJ, Mahida Y, Dua HS. E-cadherin distribution and epithelial basement membrane characteristics of the normal human conjunctiva and cornea. Eye (Lond). 1997; 11 (pt 5): 607–612. [DOI] [PubMed] [Google Scholar]
- 35. Agace WW. T-cell recruitment to the intestinal mucosa. Trends Immunol. 2008; 29: 514–522. [DOI] [PubMed] [Google Scholar]
- 36. El-Asady R, Yuan R, Liu K, et al. TGF-{beta}-dependent CD103 expression by CD8(+) T cells promotes selective destruction of the host intestinal epithelium during graft-versus-host disease. J Exp Med. 2005; 201: 1647–1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mackay LK, Rahimpour A, Ma JZ, et al. The developmental pathway for CD103(+)CD8+ tissue-resident memory T cells of skin. Nat Immunol. 2013; 14: 1294–1301. [DOI] [PubMed] [Google Scholar]
- 38. Masopust D, Choo D, Vezys V, et al. Dynamic T cell migration program provides resident memory within intestinal epithelium. J Exp Med. 2010; 207: 553–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zhang X, Schaumburg CS, Coursey TG, et al. CD8(+) cells regulate the T helper-17 response in an experimental murine model of Sjogren syndrome. Mucosal Immunol. 2014; 7: 417–427. [DOI] [PMC free article] [PubMed] [Google Scholar]