Abstract
Moderate levels of aerobic exercise broadly enhance cognition throughout the lifespan. One hypothesized contributing mechanism is increased adult hippocampal neurogenesis. Recently, we measured the effects of voluntary wheel running on adult hippocampal neurogenesis in 12 different mouse strains, and found increased neurogenesis in all strains, ranging from 2 to 5 fold depending on the strain. The purpose of this study was to determine the extent to which increased neurogenesis from wheel running is associated with enhanced performance on the water maze for 5 of the 12 strains, chosen based on their levels of neurogenesis observed in the previous study (C57BL/6J, 129S1/SvImJ, B6129SF1/J, DBA/2J, and B6D2F1/J). Mice were housed with or without a running wheels for 30 days then tested for learning and memory on the plus water maze, adapted for multiple strains, and rotarod test of motor performance. The first 10 days, animals were injected with BrdU to label dividing cells. After behavioral testing animals were euthanized to measure adult hippocampal neurogenesis using standard methods. Levels of neurogenesis depended on strain but all mice had a similar increase in neurogenesis in response to exercise. All mice acquired the water maze but performance depended on strain. Exercise improved water maze performance in all strains to a similar degree. Rotarod performance depended on strain. Exercise improved rotarod performance only in DBA/2J and B6D2F1/J mice. Taken together, results demonstrate that despite different levels of neurogenesis, memory performance and motor coordination in these mouse strains, all strains have the capacity to increase neurogenesis and improve learning on the water maze through voluntary wheel running.
Keywords: exercise, adult neurogenesis, hippocampus, spatial learning, strain differences, rotarod
1. Introduction
Evidence has established that incorporating regular exercise into the life routine is critical for maintaining cognitive health throughout the lifespan [1]. Uncovering the neurological mechanisms is currently an active area of research. One consistent finding is enhanced volume of the hippocampus in association with physical exercise in humans [2]. Rodent models have also found certain regions of the hippocampus to enlarge as a result of wheel running exercise [3]. Large ensembles of neurons in the hippocampus become rhythmically active, with the amplitude and frequency of the rhythms closely related to the intensity of the physical activity [4, 5]. Expression of immediate early genes including Zif268, Arc, and c-Fos increases in the dentate gyrus in direct proportion to amount of running [6, 7]. Given the prominent role of the hippocampus in learning and memory, exercise-induced increases in neuronal activation, immediate early gene induction and associated morphological changes in the hippocampus likely contribute to the broad enhancement in cognitive performance observed from exercise in both humans and rodent animal models.
In 1999, Dr. Henriette van Praag and colleagues reported the seminal discovery that wheel running massively increases adult hippocampal neurogenesis in association with enhanced learning and memory performance on the Morris water maze task in C57BL/6J (B6) mice [8]. It is likely that the addition of new neurons contributes to the increased volume of the granule layer of the dentate gyrus in association with exercise in rodents and humans [2, 3]. Many studies have since confirmed that increased neurogenesis from exercise occurs in parallel with enhanced performance on hippocampal-dependent tasks in the B6 genotype [3, 9–15]. Whether or not the increased neurogenesis from running causally contributes to enhanced performance by adding large numbers of highly plastic units to the circuit has proven much more difficult to establish, and remains a hot topic of research and debate [9, 16–20].
One piece of information that would be useful for interpreting the significance of the association between running-induced neurogenesis and enhanced learning in B6 is to know the generality of the result for other genotypes besides B6. To the best of our knowledge, few studies have explored whether increased neurogenesis occurs in parallel with enhanced learning and memory in other strains of mice besides B6 in response to wheel running exercise. In at least some of the other strains that have been tested, the association was absent, prompting the need to evaluate the generality of the finding further [21]. For example, in one study, mice from four different lines that were selectively bred for increased voluntary wheel running displayed large increases in adult hippocampal neurogenesis from running but did not show an improvement in learning on the Morris water maze [21]. In fact, if anything, running worsened performance in these mice. These mice are not “normal” in the sense that they are highly physically active and display a number of interesting features including altered dopamine function not typically observed in other mouse strains [22]. Therefore, it would be useful to evaluate the association between exercise-induced neurogenesis and behavioral performance in other standard inbred strains in addition to B6 to resolve the generality of the finding.
Recently, we measured adult hippocampal neurogenesis in 10 different standard inbred strains and 2 F1 hybrids, housed either with or without a running wheel and found that neurogenesis was massively increased from wheel running in all 12 strains (the increase ranged from 2 to 5 fold, depending on the strain) [23]. A significant percentage of the strain variation in exercise-induced neurogenesis could be accounted for by the distance the animals ran. After removing variation related to distance run, strain still accounted for a significant percentage of the variation in levels of neurogenesis. Taken together, these results imply that the quantitative increase in total number of new neurons resulting from housing the animals with a running wheel differs between strains with some strains showing relatively more new neurons for the same amount of running as compared to others [23]. It would be useful to know whether strains that show relatively larger increases in neurogenesis from running would display relatively greater cognitive enhancement from running. If exercise-induced neurogenesis functionally contributes broadly to the pro-cognitive effects of exercise, one would expect to see a positive correlation between degree of exercise-induced neurogenesis and degree of cognitive enhancement.
The goal of this study was to first determine the generality of the finding that exercise enhances performance on the water maze in parallel with increased neurogenesis in 2 other standard inbred strains besides B6, DBA/2J (D2) and 129S1/SvImJ (129S1), and their F1 hybrids, B6D2F1/J (B6D2F1), and B6129SF1/J (B6129F1). We included the F1 hybrids along with the parental strains to for two reasons. First, we wanted to determine the pattern of inheritance of the alleles from each strain (e.g., dominant, recessive, additive, or over-dominant) on the running, neurogenesis and learning phenotypes. Second, we aimed to choose strains with a range in levels of exercise-induced neurogenesis and B6D2F1 and B6129F1 displayed among the greatest increases in neurogenesis from running in our previous study [23]. 129S1 and D2 displayed the lowest levels of neurogenesis under baseline sedentary or running conditions. B6 displayed the highest levels of neurogenesis under sedentary conditions, but the smallest increase in neurogenesis from wheel running. Because we hypothesized that exercise-induced neurogenesis is functionally related to enhanced performance on the water maze, we expected the hybrids to display the greatest enhancement in performance, and B6 the least enhancement, with the other strains in the middle.
The reason we used the plus water maze instead of the standard version of the water maze is because it is known that the different strains react to the standard version in ways that make differential behavior (in path length, latency to the platform, or time in target quadrant during the probe trial) difficult to interpret as differences in learning or memory. For example, some strains float rather than swim, or swim around the edge of the maze. The version of the plus water maze used in this study is the version that worked the best for a majority of strains without resulting in interference from idiosyncratic differences related to test reactivity [24].
In addition to the plus maze, we also wanted to measure performance on a behavioral task that has shown improvements from wheel running but not thought to be related to adult hippocampal neurogenesis. For this purpose we chose the rotarod, a motor performance task, thought to rely more on function of the cerebellum than hippocampus [9]. Our expectations for the rotarod were general improvements from wheel running across the strains following a pattern unrelated to levels of adult hippocampal neurogenesis.
2. Materials and methods
2.1 Animals
Male mice from the following strains were purchased from The Jackson Laboratory (Bar Harbor, ME), and arrived at the Beckman Institute animal facility at five weeks of age: (n=20 or 21 per strain: B6, 129S1, B6D2F1, D2, and B6129F1; n=102 mice total). Upon arrival, the mice were group housed mice (3 or 4 / cage) for one week in standard polycarbonate shoebox cages (dimensions 29×19×13 cm; L×W×H) with corncob bedding, Teklad 7012 (Harlan Teklad, Madison, WI, USA). Rooms were controlled for temperature (21 °C ± 1 °C) and photo-period (12 h L:D; lights on at 10:00 am and off at 10:00 pm). Food and water was provided ad libitum.
2.2 Experimental Design
After one week of habituation, the mice were divided into two groups by strain designated as Sedentary or Runner (n=10 or 11 per group). Sedentary mice were individually housed in standard shoebox cages whereas Runners were individually housed in cages (36×20×14 cm; L×W×H) with a 23-cm diameter wheel mounted to the cage top (Respironics, Bend, OR, USA). Wheel rotations were monitored continuously in 1 minute increments via magnetic switches interfaced to a computer throughout the experiment. The Sedentary mice were not housed in cages with locked wheels because mice climb in wheels and physical activity was intended to be minimal [25].
During the first 10 days of the Runner or Sedentary conditions, all the mice received 10 daily intraperitoneal (i.p.) injections of 5-bromo-2’-deoxyuridine (BrdU; 50 mg / kg) to label dividing cells. The mice remained undisturbed in their Runner or Sedentary conditions for an additional 20 days. On days 31–39, mice were tested on the plus (version 3) of the water maze [24] followed by the rotarod test of motor performance. After behavioral testing each day, animals were returned to their cages with or without running wheels so the Runner/Sedentary conditions continued throughout behavioral testing.
On day 42, all mice were deeply anesthetized with 200 mg/kg sodium pentobarbital injection (i.p.) and transcardially perfused with 4% paraformaldehyde in a phosphate buffer solution (PBS). Brains were removed, postfixed overnight in 4% paraformaldehyde in PBS, then transferred to 30% sucrose. Brains were sectioned into 40 µm coronal sections using a cryostat and stored in tissue cryoprotectant at −20 °C for immunohistochemistry.
2.3 Behavioral testing
2.3.1. 4-Arm Plus Water Maze
The dimensions and construction of the 4-arm plus maze is described in detail in Wahlsten et al. (2005) as version 3 [24]. In brief, the maze was constructed of white polypropylene (70 cm diameter, 20 cm high) and filled with 58 L of tap water to a depth of 15 cm. Water temperature was maintained at 25–26 °C throughout testing. White non-toxic tempera paint made the water opaque. The escape platform comprised white plastic mesh, 10 cm in diameter and was mounted on a clear plastic post submerged 0.5 cm below the water surface. A 8×10 cm mesh lid was fixed over the all arm ends to prevent the mice from jumping off the platform. Latency, path length, and swim speed were recorded by video tracking software (TopScan, CleverSystems, Reston, VA, USA).
Following Wahlsten et al. [24], on day 1, each mouse was allowed three 60 s trials of free swimming with a platform at the end of each arm. The mouse was placed in the middle of the maze and turned to face every position except the target platform. Mice that did not escape in 60 s were led to the closest platform. All mice remained on the platform for 30 s and then were returned to their cage for approximately 5 min before receiving another trial. Results of the day 1 trials indicated that the mice had a slight preference for the south and east platforms regardless of starting direction possibly because they were following the experimenter leaving the testing area. Therefore on the following days, all mice were trained to a non-preferred platform location, i.e., north or west.
Training was completed over five days using a single non-preferred platform. Each day animals received 3 trials separated by 5 min inter-trial intervals. A trial consisted of placing the animals in the center of the maze, facing one of three arms determined at random, never facing the target platform. If a mouse did not reach the platform in 60 s, it was gently led to the platform by the experimenter’s hand. All mice remained on the platform for 30 s and then were returned to their cages. Mice were trained on the maze in a random order with respect to the experimental groups, but the same order was used on each day of training.
2.3.2. Rotarod
Following plus water maze, mice were tested on a rotarod with a 63 cm height and 30 mm diameter dowel (AccuRotor, Rota Rod Tall Unit, Accuscan, Columbus, OH, USA). Mice were placed on the dowel starting at 0 rpm, accelerating at 60 rpm / minute [9]. A photobeam at the base stopped the timer automatically when the mouse fell off the dowel. Testing included three days with 4 consecutive trials each day.
2.4. Immunohistochemistry
A subset of each group (n=5–7 per group) was used to measure adult hippocampal neurogenesis using the BrdU method as described previously [23]. In brief, a 1-in-6 series of free floating sections were washed in tissue-buffering solution (TBS; 1.3% Trizma hydrochloride, 0.19% Trizma base, 0.9% sodium chloride) and then treated with 0.6% hydrogen peroxide in TBS for 30 min. To denature DNA, sections were treated for 120 min with a solution of 50% de-ionized formamide and 2×SSC buffer, rinsed for 15 min in 2×SSC buffer, then treated with 2 M hydrochloric acid for 30 min at 37 °C, then 0.1 M boric acid in TBS (pH 8.5) for 10 min at room temperature. Sections were then blocked with a solution of 0.3% Triton-X and 3% goat serum in TBS (TBS-X plus) for 30 min, and then incubated in primary antibody against BrdU made in rat (AbD Serotec, Raleigh, NC, USA, Catalog No. OBT0030) at a dilution of 1:100 in TBS-X plus for 72 h at 4 °C. Sections were then washed in TBS, blocked with TBS-X plus for 30 min, and then incubated in biotinylated secondary antibody against rat made in goat (Vector, Burlingame, CA, USA, Catalog No. BA-9400) at 1:250 in TBS-X plus for 100 min at room temperature. Sections were then treated for 60 min using the ABC system (Vector, Burlingame, CA, USA, Catalog No. PK-6100) and stained for 5 min using a DAB kit (Sigma, St. Louis, MO, USA, Catalog No.D4418).
2.5. Image Analysis
As described previously [23], the entire granule layer (bilateral), represented in the 1-in-6 series, was photographed by systematically advancing the field of view of the Zeiss brightfield light microscope and taking multiple photographs, via camera interfaced to computer, under 10 × (total 100 ×) magnification. These photographs were then analyzed using ImageJ software (NIH, Bethesda, MD, USA). In each image, the granule layer was traced to obtain the area and number of BrdU-positive nuclei were counted within the traced region automatically by setting a fixed threshold to remove the background. Number of BrdU-positive cells was also counted by hand in at least 20 sections from each group and values were regressed against the automated numbers. The R2 value for each batch of tissue was at least 0.94 and there were no significant difference between batches. To obtain unbiased estimates of total BrdU cell numbers, total counts were multiplied by 6 to account for the 1-in-6 series, and by 0.85, under the assumption that 15% of the nuclei counted would intersect with the plane of the section. This was estimated based on the observation that the average size of BrdU nuclei was 6 µm, which is 15% of 40 µm, the thickness of the section. Total number of BrdU+ neurons is defined as the total number of BrdU-positive cells multiplied by the fraction of BrdU-positive cells differentiated into neurons. This fraction was previously collected by our group for the strains used in this study using a double fluorescent labeling technique that revealed the proportion of BrdU+ cells co-labeled with the mature neuronal marker, NeuN [23]. The proportions used were as follows for sedentary and runners respectively taken from [23]: 0.77 and 0.89 for 129S1, 0.77 and 0.90 for B6129F1, 0.81 and 0.91 for B6, 0.81 and 0.91 for B6D2F1, and 0.79 and 0.89 for D2.
2.6. Statistical Analysis
Data were analyzed using SAS version 9.3 (Cary, NC, USA). P < 0.05 was considered statistically significant. Average distance traveled on running wheels (km/day) over the first 30 days of wheel access (before behavioral testing) was analyzed using a 2-way repeated measures ANOVA with day as the within-subjects factor and strain as the between subjects factor. Average distance traveled (km/day) collapsed across all 30 days was also analyzed using a one-way analysis of variance (ANOVA) with strain as the factor. Total number of new (BrdU+) neurons and volume of the granule layer of the dentate gyrus was analyzed using a 2-way ANOVA with wheel access (Runner vs. Sedentary), strain, and the interaction between wheel access and strain factors. Total number of new neurons in Runners was also analyzed by analysis of covariance, with total running distance entered as the continuous covariate, and strain as the categorical variable. The correlation between running distance and number of new neurons was calculated using Pearson’s r for each strain separately. In addition, the neurogenic effect of exercise was calculated for each runner as the total number of BrdU+ neurons observed in the runner minus the average observed in sedentary animals from the same strain divided by the total distance traveled by that runner. The neurogenic effect of exercise was analyzed using a 1-way ANOVA with strain as the factor. For the plus water maze data, total incorrect arm entries was analyzed using two-way ANOVA with strain, wheel access and the interaction of strain and wheel access as factors. In addition, path length, latency to reach the platform, swim speed, and total number of incorrect arm entries were analyzed using a 3-way repeated measures ANOVA with day as the within subjects factor (1–5), and strain (5 levels) and wheel access (2 levels) as between subjects factors. Path length and swim speed were square root transformed to improve homogeneity of variance across groups. Average and maximum latency to fall from the rotarod (collapsed across the 4 trials per day over the 3 days) were analyzed by 2-way AVOVA with strain, wheel access, and the interaction between strain and wheel access as factors. Tukey posthoc tests were used to evaluate pairwise differences between means within the respective ANOVAs.
3. Results
3.1. Wheel Running
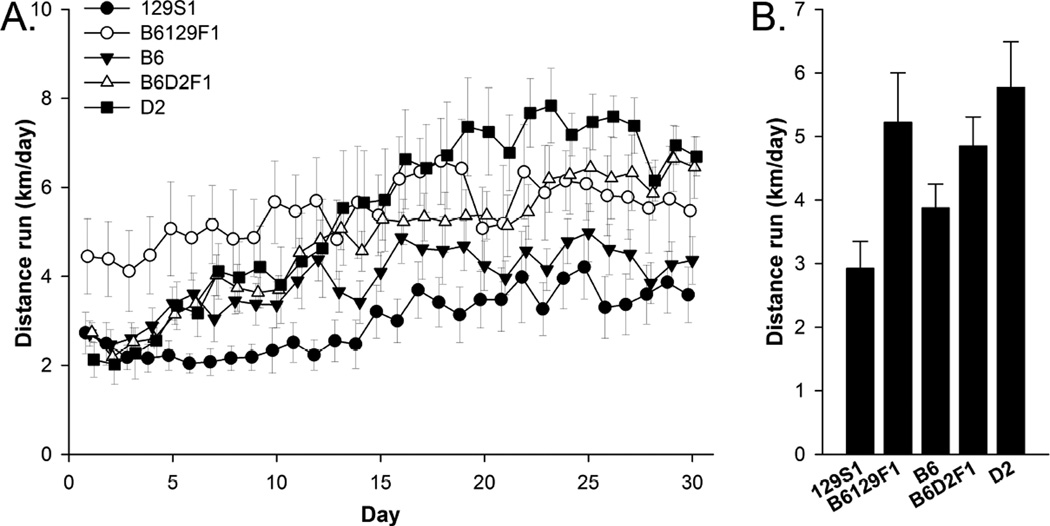
Running increased over the first 20 days and thereafter maintained a plateau in all the strains as indicated by a significant effect of day in the repeated measures analysis (Fig. 1A; F29, 1203 =26.29, P < 0.0001). The average level and rate of increase in running across the 30 days depended on strain as indicated by a significant main effect of strain (Fig. 1A; F4, 45 = 3.50, P = 0.01) and interaction between strain and day (Fig. 1A; F116, 1203 = 2.93, P < 0.0001). Average distance traveled on running wheels collapsed over the 30 days of uninterrupted wheel access also differed depended on mouse strain (Fig. 1B; F4, 45 = 2.79, P = 0.01). The 129S1 strain ran the least, B6 next, and then B6D2F1, B6129F1, and D2, the most. Posthoc tests revealed the following significant pairwise comparisons: D2 versus B6, D2 versus 129S1, B6129F1 versus 129S1, B6D2F1 versus 129S1.
Figure 1.
Wheel running. A) Average daily distance traveled (km/day) during the first 30 days of uninterrupted wheel access shown separately for the 5 strains. B) Average distance traveled (km/day) collapsed across the 30 days of wheel access. Level of running escalated by day and varied by strain. Standard error bars shown, n=10 or 11 animals per group.
3.2. Adult Hippocampal Neurogenesis
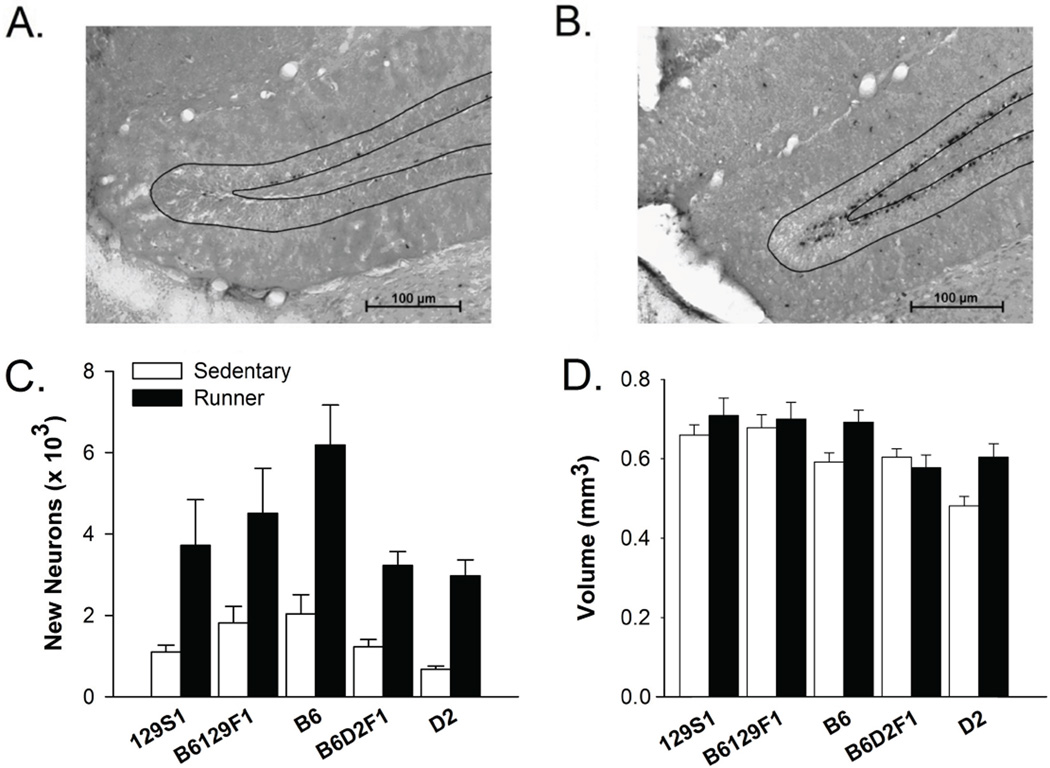
Total number of BrdU+/NeuN+ cells (BrdU+ neurons) in the granule layer differed depended on strain (F4,49 = 2.97, P = 0.03). B6 had the most new neurons, followed by B6129F1, 129S1, B6D2F1 and D2. Pair-wise posthoc tests revealed B6 was significantly different from all the other strains except B6129F1. No other pair-wise differences were significant. Access to a running wheel significantly increased the total number of new neurons in all the strains to a similar degree (F1, 49 = 41.03, P < 0.0001) (Fig. 2 A-C). Collapsing across strains, running mice had approximately 2900 more BrdU+ neurons than sedentary mice in the granule layer. The interaction between strain and access to a running wheel was not significant.
Figure 2.
Adult hippocampal neurogenesis. A) A representative section of the granule layer of the dentate gyrus (outlined) from a sedentary D2 mouse immunohistochemically stained to visualize BrdU. B) Same as A except from a D2 runner. C) Mean total number of BrdU+ neurons in mice housed with or without running wheels shown separately for the 5 strains. Levels of neurogenesis depended on strain. All strains displayed increased neurogenesis from running. D) Mean volume of the granule cell layer of the dentate gyrus across groups. Standard error bars shown, n=5–7 animals per group. Open bars represent the sedentary condition and closed bars represent the runner condition.
Volume of the dentate gyrus varied depending on strain (F4,51 = 7.54, P <0.0001; Fig. 2D). 129S1 displayed the largest volume followed by B6129F1, B6, B6D2F1, and D2. Posthoc tests revealed the following significant pairwise comparisons: 129S1 versus B6D2F1, 129D1 versus D2, B6 versus D2, B6129F1 versus B6D2F1, B6129F1 versus D2. Running increased volume across strains as indicated by a significant effect of running (F1,51 = 7.14, P = 0.01; Fig. 2D) but no interaction between running and strain was observed.
3.3. The Neurogenic Effect of Exercise
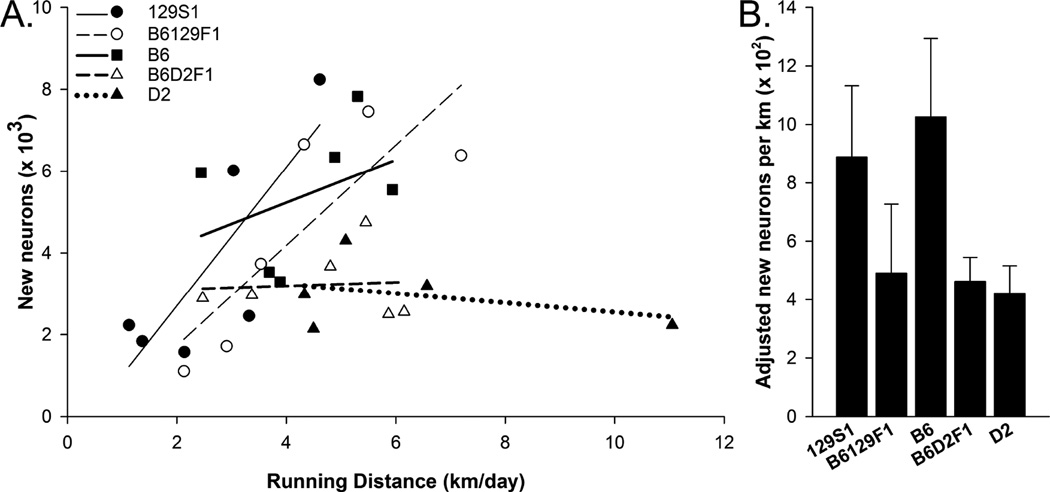
The relationship between distance traveled and number of new neurons depended on strain as indicated by a significant effect of strain (F4,19 = 3.71, P = 0.02) and interaction between strain and distance traveled (F4,19 = 3.93, P = 0.02) by analysis of covariance (Fig. 3A). The covariate, distance traveled was marginally not significant (F1,19 = 3.01, P = 0.10). 129-related strains (129S1, B6129F1) displayed a strong relationship between number of new neurons and level of wheel running (Pearsons r = 0.81, 0.83; P=0.049, P=0.039, respectively). B6 displayed a weaker positive relationship (Pearsons r = 0.38; NS). D2 and B6D2F1 displayed near zero or negative correlations (0.07, –0.37, NS), indicating no positive relationship between wheel running and number of new neurons. The neurogenic effect of exercise (number of new neurons per km from running) was similar in all strains as indicated by non-significant effect of strain (Fig. 3B; F4,24 = 1.19, P = 0.34). Hence, all strains showed increased neurogenesis with running, but this effect did not depend on running distance for the D2 related strains.
Figure 3.
The neurogenic effect of wheel running. A) Numbers of BrdU+ neurons plotted against average distance traveled in km/day across all the runners in the study. Each strain is shown separately along with linear trend lines. Analysis of covariance revealed significantly different linear trends depending on strain. B) Average neurogenic effect of wheel running (numbers of BrdU+ neurons/km) shown separately for each strain. The neurogenic effect of running was defined for each runner in the study as follows: total number of BrdU+ neurons observed in the runner minus average number in the sedentary group from the same strain divided by the total distance that runner traveled over the course of the study. No significant strain differences in the neurogenic effect of running were observed. Standard error bars shown, n=5–7 animals per group.
3.4. Behavioral Performance
3.4.1. Plus water maze (Fig. 4A)
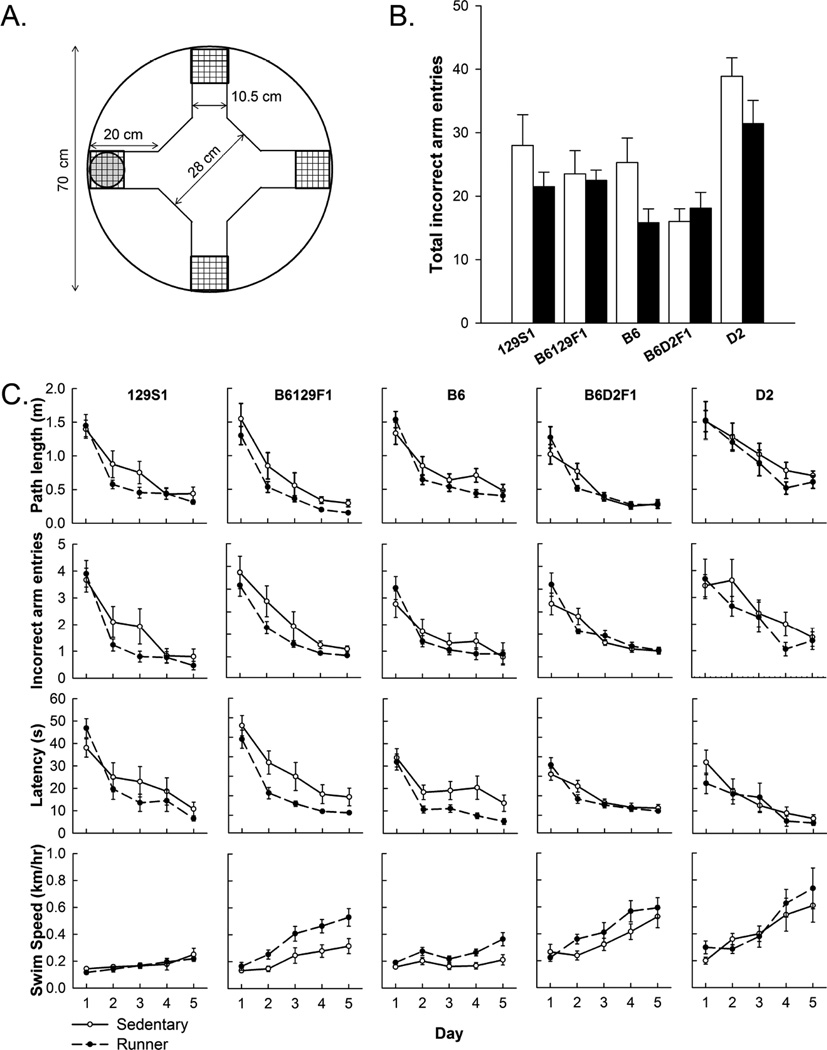
Figure 4.
Plus water maze. A) A diagram of the plus water maze used drawn to scale (see also [24]). Overlaying grids represent mesh lids covering the arm end. The platform is shown in the west arm. B) Average total number of incorrect arm entries across all 15 trials (3 trials per day for 5 days) shown separately by strain and running group. Open bars represent the sedentary condition and closed bars represent the runner condition. C) Path length (m), incorrect arm entries, latency (sec), and swim speed (km/hr), averaged across three trials per day over the 5 days of acquisition shown for each strain as separate graphs organized into columns. Runners are shown as filled circles and sedentary as open circles. All strains learned the plus water maze. Performance depended on strain. All strains displayed enhanced performance from wheel running. Standard error bars shown, n=10 or 11 animals per group.
All mice learned the water maze, as indicated by significantly decreased path length (F4, 356 = 145.94, P < 0.0001), shorter latency (F4,356 = 137.76, P < 0.0001) and significantly fewer total incorrect arm entries (F4, 356 = 81.29, P < 0.0001) across days.
Strain significantly influenced overall performance on the plus maze as measured by total incorrect arm entries across all trials (F4, 89 = 10.26, P < 0.0001; Fig. 4B). Running improved overall performance (F1, 89 = 4.58, P = 0.035). The interaction between strain and wheel access was not significant. However, the effect of running tended to be stronger in B6129F1, 129S1 and B6 as compared to D2 and B6D2F1. Overall, B6D2F1 performed best as indicated by fewest total incorrect arm entries, followed by B6129F1, then B6, 129S1, and D2. Posthoc pair-wise comparisons indicated that D2 was different from all the other strains. B6D2F1 also differed from all the others. No other pairwise differences were significant.
Results of the repeated measures analysis of the 5 days of acquisition indicated significant main effects of strain (F4, 87 = 18.44, P < 0.0001) and access to a running wheel (F1, 87 = 8.65, P = 0.004) for path length (Fig. 4C). The steepness of the learning curves depending on strain, as indicated by a significant interaction between strain and day for path length (F16, 356 = 1.70, P = 0.045). No other interactions were significant.
Results of the repeated measures analysis of incorrect arm entries were similar to path length. Strain was significant (F4, 87 = 9.89, P < 0.0001) and access to running reduced incorrect arm entries (F1, 87 = 4.69, P = 0.03). The interaction between day and access to running wheels was significant (F4, 356 = 2.79, P = 0.03) indicating running accelerated the rate of learning across strains. No other interactions were significant.
Latency to the platform followed similar pattern as path length. Strain was significant (F4,87 = 7.14, P < 0.0001) and access to a running wheel reduced latency (F1,87 = 9.48, P = 0.003). The interaction between day and strain was significant (F16,356 = 3.18, P < 0.0001). No other factors or interactions were significant.
Swim speed significantly increased with day (F4, 356 = 46.50, P < 0.0001), starting from an average of 0.19 (± 0.010 SE) km/hr on day 1 and ending with 0.43 (± 0.029 SE) km/hr on day 5, collapsed across strains. Some strains swam faster than others (F4,87 = 28.08, P < 0.0001). D2 swam the fastest at 0.44 (± 0.031 SE) km/hr whereas 129S1 swam the slowest, 0.17 (± 0.009 SE) km/hr. Some strains showed steeper increases in swim speed across days than others as indicated by a significant interaction between day and strain (F16,356 = 4.14, P < 0.0001). Access to a running wheel significantly increased swim speed on the water maze (F1,87 = 13.68, P = 0.0002) across strains. The interaction between day and access to running wheel was marginally not significant (F4,356 = 2.39, P = 0.0506). The interaction between strain and access to a running wheel was marginally not significant (F4,87 = 2.19, P = 0.0762).
3.4.2. Rotarod
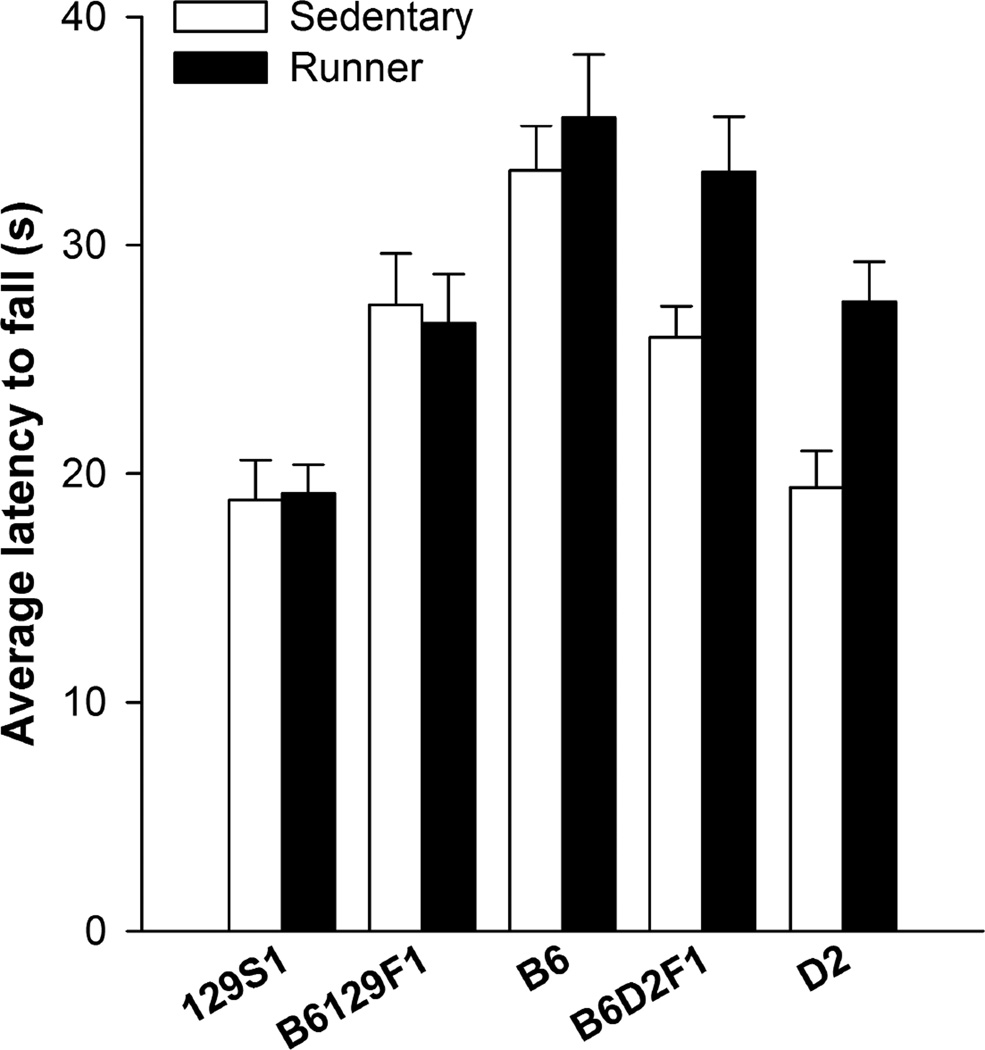
Average latency to fall from the accelerating rotarod remained stable across days and significantly differed between strains (F4, 267 = 31.12, P < 0.0001). All pair-wise differences between strains were significant (P < 0.05) except the comparison of the two F1 hybrids. B6 mice had the longest latency to fall, then B6D2F1, B6129F1, D2, and 129S1 (Fig. 5). Access to a running wheel significantly improved performance in strains with D2 genetic background (D2, and B6D2F1) but not in the others. This was indicated by a significant main effect of access to a running wheel (F1, 267 = 13.28, P = 0.0003) and significant interaction between strain and wheel access (F4, 267 = 3.62, P = 0.0069). Pair-wise comparisons revealed that wheel running only improved rotarod performance in D2 and B6D2F1 strains (both P < 0.02; all other strains NS). No other effects or interactions were significant. Results for maximum latency were similar. An effect of strain (F4, 267 = 27.61, P < 0.0001), running (F1, 267 = 8.71, P = 0.003), and running-by-strain interaction (F4, 267 = 4.13, P = 0.003) was observed. The D2 and B6D2F1 displayed improved performance from running whereas the other strains did not. Average latency collapsed across all 12 trials over the 3 days, and maximum latency across all 12 trials showed similar results. Main effects of strain (average latency, F4, 87 = 21.32, P < 0.0001; maximum latency, F4, 87 = 18.68, P < 0.0001), running (average latency, F1, 87 = 9.31, P = 0.003; maximum latency, F1, 87 = 6.13, P = 0.015), and marginally non-significant interaction between strain and running were observed (average latency, F4, 87 = 2.17, P = 0.08; maximum latency, F4, 87 = 2.45, P = 0.05).
Figure 5.
Rotarod. A) Average latency (s) to fall from the accelerating rotarod across the 12 trials (4 trials per day over the 3 days) shown separately for each strain and running condition. B) Maximum latency (s) of all the 12 trials by group. Performance on the rotarod depended on strain. Running improved performance only in strains with D2 alleles (D2 and B6D2F1). Standard error bars shown, n=10 or 11 animals per group. Open bars represent the sedentary condition and closed bars represent the runner condition.
4. DISCUSSION
Our study is the first to compare effects of wheel running on acquisition of the plus water maze across multiple standard inbred strains and their F1 hybrids. Results support a small improvement in performance from running in all the strains. The effect size for learning improvement is similar to what has been observed on the standard version of the water maze in B6 mice [8–10, 21]. The multi-strain adapted version of the plus maze served well in the sense that all the strains reacted similarly to the task (e.g., floating was not a problem, the animals appeared to be equally motivated) [24]. The similar reactivity to the test across strains likely made it possible to reveal the subtle effects of exercise on improving learning across the genetically divergent strains. Each strain that showed improved performance on the water maze also showed increased adult hippocampal neurogenesis from wheel running (see Fig. 2C). Hence, the positive association between increased adult hippocampal neurogenesis and enhanced performance on the plus water maze generalizes across the five genetically divergent mouse strains used in our study, B6, D2, 129S1, B6D2F1, and B6129F1.
Consistent with previous reports, significant strain differences in all the outcome measures including levels of wheel running, adult hippocampal neurogenesis and performance on the plus maze and rotarod could be detected indicating genetic influences on these traits [23, 24, 26–29]. Heritability estimates are given in Table 1. In general, D2 alleles appeared to decrease levels of neurogenesis (Fig 2C) and decrease performance on the plus maze relative to B6 alleles (Fig. 4B,C), which increased neurogenesis and enhanced performance. This pattern is consistent with the literature [27]. 129S1 alleles decreased levels of neurogenesis relative to B6 (Fig 2C), but maintained similar performance on the plus maze and similar enhancements from running as B6 (Fig. 4B,C).
Table 1.
Heritability estimates
| Trait | Sedentary | Runner | ||
|---|---|---|---|---|
| R2 | P-value | R2 | P-value | |
| Wheel running | ||||
| Total dist. Traveled | 0.25 | 0.01 | ||
| Neurogenesis | ||||
| Total # BrdU+ neurons | 0.35 | 0.02 | 0.26 | 0.10 |
| Volume granule layer | 0.58 | <0.0001 | 0.30 | 0.05 |
| Plus water maze | ||||
| Incorrect arm entries | 0.32 | < 0.01 | 0.38 | < 0.01 |
| Path length | 0.36 | < 0.01 | 0.51 | < 0.0001 |
| Latency | 0.19 | 0.05 | 0.30 | < 0.01 |
| Swim speed | 0.46 | < 0.0001 | 0.58 | < 0.0001 |
| Rotarod | ||||
| Average | 0.50 | <0.0001 | 0.45 | < 0.01 |
| Maximum | 0.46 | < 0.0001 | 0.43 | < 0.0001 |
Although more strains are needed to provide a reasonable estimate of the genetic correlation between levels of adult neurogenesis and performance on the plus maze, contrary to our predictions, results from the present study suggest that the correlation may be weak if present at all. A previous study using 10 different recombinant inbred lines derived from B6 and D2 (BXD RI lines) found a positive genetic correlation between levels of adult neurogenesis under sedentary conditions and acquisition of the standard water maze [27]. Our results are consistent with this report in that B6 displayed increased neurogenesis and enhanced plus water maze performance relative to D2. However, a population derived from only B6 and D2 may not be sufficient to establish the generality of the correlation between levels of neurogenesis and water maze performance. 129S1 showed low numbers of new neurons similar to D2 (Fig. 2C) but average performance on the plus maze similar to B6 (Fig. 4C). Therefore, collectively, the data including 129S1 suggest performance on the water maze is unrelated to levels of adult hippocampal neurogenesis across strains. More genotypes different from B6, D2, and 129S1 are needed to evaluate the generality of the correlation hypothesis. It is possible that knowing levels of neurogenesis is not enough information to predict strain variation in learning, as many features of the brain vary between strains and change as a result of exercise (e.g., vasculature, numbers of glial cells, microglia activation, changes in synapses, concentrations of trophic factors and growth factors) that could contribute to differential performance [3, 30–33].
4.1. Descriptive synthesis of results for each strain
The D2 strain displayed the lowest level of adult neurogenesis under sedentary conditions, and the greatest proportional increase in neurogenesis from running, approximately a 5 fold increase relative to sedentary levels (Fig. 2C). Despite the 5-fold increase, average numbers of new neurons in runners were still the lowest of all the strains measured. These results are very similar to our previous report [23]. In the present study, D2 ran the most of all 5 strains (Fig. 1), with substantial within strain variation. Despite the large variation, the quantitative amount of running had little influence on total numbers of neurons in this genotype (Fig. 3A). The neurogenic effect of exercise as defined by the numbers of new neurons that can be attributed to running divided by the total distance traveled was the smallest in D2 of all the strains (Fig. 3B). Of all 5 strains, D2 displayed the flattest learning curve on the plus maze, as measured by path length, or incorrect arm entries (Fig. 4C). The D2 strain also showed the smallest effect of exercise on improving acquisition of the plus maze (Fig. 4B,C). However, D2 displayed the greatest improvement in rotarod performance from wheel running of all the strains (Fig 5).
In stark contrast to D2, the B6 strain displayed the highest level of adult neurogenesis under sedentary conditions, and a 3-fold increase in neurogenesis from running, ending with the highest level of adult neurogenesis of all the strains under runner conditions (Fig. 2C). A positive relationship was observed between distance traveled and numbers of new neurons in this genotype, unlike D2, despite substantially less within-strain variation in running than D2 (Fig. 3A). The B6 genotype displayed the highest neurogenic response to running of all the strains (Fig. 3B). B6 mice displayed a steeper learning curve than D2, but similar to 129S1 (Fig. 4C). The effect of exercise on improving acquisition was more obvious in B6 than D2 but not as obvious as 129S1 (Fig. 4B,C). No effect of exercise on rotarod was observed, though B6 performance under baseline sedentary conditions was better than the performance of any of the other strains even under runner conditions, therefore the lack of effect of running in improving rotarod performance in B6 could be a ceiling effect (Fig. 5).
The 129S1 genotype displayed the second lowest levels of neurogenesis under sedentary conditions of the 5 strains, and approximately 4-fold increase from running (Fig. 2C). This genotype displayed a positive relationship between distance traveled and numbers of new neurons, and the second highest neurogenic response to running among the 5 strains (Fig. 3A). 129S1 displayed average performance on the plus maze relative to the other strains, and strong improvements from running (Fig. 4B,C). No effect of exercise on rotarod was observed, though this genotype displayed the worst performance under sedentary and runner conditions (Fig. 5).
4.2. Patterns of inheritance
Wheel running was greater in B6129F1 relative to their parental strains indicating over-dominance, or non-additive effects of B6 combined with 129S1 alleles. In contrast, wheel running in B6D2F1 was intermediate between the parental strains indicating additive effects of B6 combined with D2 alleles (Fig. 2B). Adult neurogenesis measured under both sedentary and runner conditions in B6129F1 mice was intermediate between B6 and 129S1 indicating additive genetic effects under both environmental conditions. Neurogenesis levels under sedentary conditions in B6D2F1 were intermediate between the parental strains, also indicating additive genetic effects. However, levels of neurogenesis under runner conditions in B6D2F1 were similar to D2, indicating D2 alleles were dominant over B6 (Fig. 2C). Total number of incorrect arm entries on the plus maze was lower in the F1 hybrids then their parental strains, except for sedentary B6129F1 which showed intermediate performance relative to the parental strains. These results suggest that the effects of D2 and 129S1 alleles on plus maze learning display over-dominance when combined with B6, or in one case, additive effects when 129S1 alleles are combined with B6 and animals are housed under sedentary conditions (Fig. 4B). Latency to fall from the rotarod under both sedentary and runner conditions in the F1 hybrids was intermediate between the parental strains suggesting additive effects of D2 and 129S1 alleles when combined with B6 alleles on motor performance (Fig. 5).
4.3. Methodological limitations
One problem we encountered in analyzing the relationship between exercise-induced neurogenesis and performance on the plus maze was how to represent the neurogenic influence of running. The issue is exceedingly important, as results and interpretations are different depending on which measure is used. For example, exercise-induced neurogenesis could be represented as a difference or as a proportion of levels observed in animals housed under sedentary conditions? Alternatively, level of exercise-induced neurogenesis could be represented as the Preason’s correlation between distance traveled and number of new neurons. If represented as a difference, then the effect of exercise was the same in all genotypes (Fig. 2C). Because, we detected a significant improvement of exercise and no interaction between exercise and strain on the plus water maze (Fig. 4C), results are consistent with the hypothesis that running-induced neurogenesis is associated with improved performance on the maze. If on the other hand, exercise-induced neurogenesis is represented as a proportion of sedentary levels, then D2 displayed the greatest increases in neurogenesis and B6 and B6129F1 the smallest increases. Combined with the water maze data, these results would suggest exercise-induced neurogenesis is dissociated from improved learning on the water maze, otherwise D2 should have displayed greater enhancements in learning from running than B6 and B6129F1, but D2 displayed the least enhancement from running of all the strains (Fig. 4C). Finally, if the correlation between distance run and neurogenesis is used as the metric for representing exercise-induced neurogenesis, then 129S1 and B6129F1 were the high-responders, B6 was in the middle, and D2 and B6D2F1 near zero. In this case, exercise-induced neurogenesis would be strongly correlated with degree of exercise-induced learning enhancement, since the B6129F1 and 129S1 displayed the greatest enhancement on learning from running, B6 was in the middle, and the D2 strains were the least responsive (Fig. 4C).
It is important to note here that some of the variation in neurogenesis between the strains could reflect differences in rates of cell death, with greater levels of neurogenesis occurring to compensate for greater cell death [34, 35]. Cell death was not directly measured in our study. However, volume of the dentate gyrus significantly increased in response to running in most strains (Fig. 2D) suggesting that whatever level of cell death may have been happening as a result of wheel running, neurogenesis more than compensated for the loss. To more precisely address the issue of cell death and total numbers of functional neurons in the dentate gyrus, it would be useful to quantify the total number of granule neurons directly using stereological methods applied to thinner sections. To the extent that increased neurogenesis reflects increased cell death, the observation of increased neurogenesis within a particular strain relative to another or in response to exercise is not necessarily expected to enhance performance.
Another important feature of our study to note is that the two F1 strains that were included in the study were generated from crossing a B6 dam with either a D2 or 129S1 sire to produce the F1 progeny. Hence, the maternal environment was held constant for comparisons of the F1 strains with B6. However, comparisons of the F1 strains with 129S1 or D2 are confounded by maternal influences (i.e., maternal environmental effects from the 129S1 and D2 dams could contribute to the differences between these strains and the F1 hybrids).
4.4. Conclusions
Given the complexities in interpreting correlations between behavioral performance and neurogenesis across strains as discussed above, rather than use strain comparisons to study the functional significance of exercise-induced neurogenesis, it may be more fruitful to directly manipulate neurogenesis within a single strain. Recent advances in optogenetics [36, 37] and DREADDs [38, 39], provide the tools, at least in theory, necessary to temporarily inactivate cohorts of new neurons to evaluate their influence on learning or other behavioral measures. The functional significance of new neurons in the hippocampus, specifically the massive increases in hippocampal neurogenesis from wheel running that occurs across strains, is still open for debate. Because of the importance of the hippocampus in learning and memory [40–42], it seems likely that changes in numbers of new neurons in this region will be related to how exercise protects and enhances cognition throughout the lifespan [1].
Highlights.
Running increased neurogenesis and enhanced plus water maze learning across 5 strains
Running, neurogenesis, and learning displayed significant heritability
Level of neurogenesis was a poor predictor of learning between strains
ACKNOWLEDGEMENTS
We wish to thank the Beckman Institute Animal Facility for help with animal care. This work was supported by grants from National Institutes of Health, MH083807 and DA027487. The authors also wish to thank Ashley Masnik for help with data collection.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Hillman CH, Erickson KI, Kramer AF. Be smart, exercise your heart: exercise effects on brain and cognition. Nat Rev Neurosci. 2008;9:58–65. doi: 10.1038/nrn2298. [DOI] [PubMed] [Google Scholar]
- 2.Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, et al. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci U S A. 2011;108:3017–3022. doi: 10.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clark PJ, Brzezinska WJ, Puchalski EK, Krone DA, Rhodes JS. Functional analysis of neurovascular adaptations to exercise in the dentate gyrus of young adult mice associated with cognitive gain. Hippocampus. 2009;19:937–950. doi: 10.1002/hipo.20543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kuo TB, Li JY, Chen CY, Yang CC. Changes in hippocampal theta activity during initiation and maintenance of running in the rat. Neuroscience. 2011;194:27–35. doi: 10.1016/j.neuroscience.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 5.McFarland WL, Teitelbaum H, Hedges EK. Relationship between hippocampal theta activity and running speed in the rat. Journal of comparative and physiological psychology. 1975;88:324–328. doi: 10.1037/h0076177. [DOI] [PubMed] [Google Scholar]
- 6.Clark PJ, Bhattacharya TK, Miller DS, Rhodes JS. Induction of c-Fos, Zif268, and Arc from acute bouts of voluntary wheel running in new and pre-existing adult mouse hippocampal granule neurons. Neuroscience. 2012;184:16–27. doi: 10.1016/j.neuroscience.2011.03.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clark PJ, Kohman RA, Miller DS, Bhattacharya TK, Haferkamp EH, Rhodes JS. Adult hippocampal neurogenesis and c-Fos induction during escalation of voluntary wheel running in C57BL/6J mice. Behav Brain Res. 2010;213:246–252. doi: 10.1016/j.bbr.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci U S A. 1999;96:13427–13431. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clark PJ, Brzezinska WJ, Thomas MW, Ryzhenko NA, Toshkov SA, Rhodes JS. Intact neurogenesis is required for benefits of exercise on spatial memory but not motor performance or contextual fear conditioning in C57BL/6J mice. Neuroscience. 2008;155:1048–1058. doi: 10.1016/j.neuroscience.2008.06.051. [DOI] [PubMed] [Google Scholar]
- 10.van Praag H, Shubert T, Zhao C, Gage FH. Exercise enhances learning and hippocampal neurogenesis in aged mice. J Neurosci. 2005;25:8680–8685. doi: 10.1523/JNEUROSCI.1731-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li H, Liang A, Guan F, Fan R, Chi L, Yang B. Regular treadmill running improves spatial learning and memory performance in young mice through increased hippocampal neurogenesis and decreased stress. Brain Res. 2013;1531:1–8. doi: 10.1016/j.brainres.2013.07.041. [DOI] [PubMed] [Google Scholar]
- 12.Mustroph ML, Chen S, Desai SC, Cay EB, DeYoung EK, Rhodes JS. Aerobic exercise is the critical variable in an enriched environment that increases hippocampal neurogenesis and water maze learning in male C57BL/6J mice. Neuroscience. 2012;219:62–71. doi: 10.1016/j.neuroscience.2012.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marlatt MW, Potter MC, Lucassen PJ, van Praag H. Running throughout middle-age improves memory function, hippocampal neurogenesis, and BDNF levels in female C57BL/6J mice. Developmental neurobiology. 2012;72:943–952. doi: 10.1002/dneu.22009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clark PJ, Bhattacharya TK, Miller DS, Kohman RA, DeYoung EK, Rhodes JS. New neurons generated from running are broadly recruited into neuronal activation associated with three different hippocampus-involved tasks. Hippocampus. 2012;22:1860–1867. doi: 10.1002/hipo.22020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mustroph ML, Stobaugh DJ, Miller DS, DeYoung EK, Rhodes JS. Wheel running can accelerate or delay extinction of conditioned place preference for cocaine in male C57BL/6J mice, depending on timing of wheel access. Eur J Neurosci. 2011;34:1161–1169. doi: 10.1111/j.1460-9568.2011.07828.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meshi D, Drew MR, Saxe M, Ansorge MS, David D, Santarelli L, et al. Hippocampal neurogenesis is not required for behavioral effects of environmental enrichment. Nat Neurosci. 2006;9:729–731. doi: 10.1038/nn1696. [DOI] [PubMed] [Google Scholar]
- 17.Groves JO, Leslie I, Huang GJ, McHugh SB, Taylor A, Mott R, et al. Ablating adult neurogenesis in the rat has no effect on spatial processing: evidence from a novel pharmacogenetic model. PLoS genetics. 2013;9:e1003718. doi: 10.1371/journal.pgen.1003718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Winocur G, Becker S, Luu P, Rosenzweig S, Wojtowicz JM. Adult hippocampal neurogenesis and memory interference. Behav Brain Res. 2012;227:464–469. doi: 10.1016/j.bbr.2011.05.032. [DOI] [PubMed] [Google Scholar]
- 19.Winocur G, Wojtowicz JM, Sekeres M, Snyder JS, Wang S. Inhibition of neurogenesis interferes with hippocampus-dependent memory function. Hippocampus. 2006;16:296–304. doi: 10.1002/hipo.20163. [DOI] [PubMed] [Google Scholar]
- 20.Wojtowicz JM, Askew ML, Winocur G. The effects of running and of inhibiting adult neurogenesis on learning and memory in rats. Eur J Neurosci. 2008;27:1494–1502. doi: 10.1111/j.1460-9568.2008.06128.x. [DOI] [PubMed] [Google Scholar]
- 21.Rhodes JS, van Praag H, Jeffrey S, Girard I, Mitchell GS, Garland T, Jr, et al. Exercise increases hippocampal neurogenesis to high levels but does not improve spatial learning in mice bred for increased voluntary wheel running. Behav Neurosci. 2003;117:1006–1016. doi: 10.1037/0735-7044.117.5.1006. [DOI] [PubMed] [Google Scholar]
- 22.Rhodes JS, Garland T. Differential sensitivity to acute administration of Ritalin, apomorphine, SCH 23390, but not raclopride in mice selectively bred for hyperactive wheel-running behavior. Psychopharmacology. 2003;167:242–250. doi: 10.1007/s00213-003-1399-9. [DOI] [PubMed] [Google Scholar]
- 23.Clark PJ, Kohman RA, Miller DS, Bhattacharya TK, Brzezinska WJ, Rhodes JS. Genetic influences on exercise-induced adult hippocampal neurogenesis across 12 divergent mouse strains. Genes Brain Behav. 2011;10:345–353. doi: 10.1111/j.1601-183X.2010.00674.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wahlsten D, Cooper SF, Crabbe JC. Different rankings of inbred mouse strains on the Morris maze and a refined 4-arm water escape task. Behav Brain Res. 2005;165:36–51. doi: 10.1016/j.bbr.2005.06.047. [DOI] [PubMed] [Google Scholar]
- 25.Rhodes JS, Koteja P, Swallow JG, Carter PA, Garland T. Body temperatures of house mice artificially selected for high voluntary wheel-running behavior: repeatability and effect of genetic selection. J Therm Biol. 2000;25:391–400. doi: 10.1016/s0306-4565(99)00112-6. [DOI] [PubMed] [Google Scholar]
- 26.Lightfoot JT, Turner MJ, Daves M, Vordermark A, Kleeberger SR. Genetic influence on daily wheel running activity level. Physiological genomics. 2004;19:270–276. doi: 10.1152/physiolgenomics.00125.2004. [DOI] [PubMed] [Google Scholar]
- 27.Kempermann G, Gage FH. Genetic determinants of adult hippocampal neurogenesis correlate with acquisition, but not probe trial performance, in the water maze task. Eur J Neurosci. 2002;16:129–136. doi: 10.1046/j.1460-9568.2002.02042.x. [DOI] [PubMed] [Google Scholar]
- 28.Kempermann G, Chesler EJ, Lu L, Williams RW, Gage FH. Natural variation and genetic covariance in adult hippocampal neurogenesis. Proc Natl Acad Sci U S A. 2006;103:780–785. doi: 10.1073/pnas.0510291103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rustay NR, Wahlsten D, Crabbe JC. Assessment of genetic susceptibility to ethanol intoxication in mice. Proc Natl Acad Sci U S A. 2003;100:2917–2922. doi: 10.1073/pnas.0437273100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kohman RA, Bhattacharya TK, Wojcik E, Rhodes JS. Exercise reduces activation of microglia isolated from hippocampus and brain of aged mice. Journal of neuroinflammation. 2013;10:114. doi: 10.1186/1742-2094-10-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Redila VA, Christie BR. Exercise-induced changes in dendritic structure and complexity in the adult hippocampal dentate gyrus. Neuroscience. 2006;137:1299–1307. doi: 10.1016/j.neuroscience.2005.10.050. [DOI] [PubMed] [Google Scholar]
- 32.Neeper SA, Gomez-Pinilla F, Choi J, Cotman C. Exercise and brain neurotrophins. Nature. 1995;373:109. doi: 10.1038/373109a0. [DOI] [PubMed] [Google Scholar]
- 33.Carro E, Nunez A, Busiguina S, Torres-Aleman I. Circulating insulin-like growth factor I mediates effects of exercise on the brain. J Neurosci. 2000;20:2926–2933. doi: 10.1523/JNEUROSCI.20-08-02926.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kuhn HG, Biebl M, Wilhelm D, Li M, Friedlander RM, Winkler J. Increased generation of granule cells in adult Bcl-2-overexpressing mice: a role for cell death during continued hippocampal neurogenesis. Eur J Neurosci. 2005;22:1907–1915. doi: 10.1111/j.1460-9568.2005.04377.x. [DOI] [PubMed] [Google Scholar]
- 35.Biebl M, Cooper CM, Winkler J, Kuhn HG. Analysis of neurogenesis and programmed cell death reveals a self-renewing capacity in the adult rat brain. Neuroscience letters. 2000;291:17–20. doi: 10.1016/s0304-3940(00)01368-9. [DOI] [PubMed] [Google Scholar]
- 36.Kim TI, McCall JG, Jung YH, Huang X, Siuda ER, Li Y, et al. Injectable, cellular-scale optoelectronics with applications for wireless optogenetics. Science. 2013;340:211–216. doi: 10.1126/science.1232437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yizhar O, Fenno LE, Davidson TJ, Mogri M, Deisseroth K. Optogenetics in neural systems. Neuron. 2011;71:9–34. doi: 10.1016/j.neuron.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 38.Zhu H, Roth BL. Silencing synapses with DREADDs. Neuron. 2014;82:723–725. doi: 10.1016/j.neuron.2014.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Robinson S, Todd TP, Pasternak AR, Luikart BW, Skelton PD, Urban DJ, et al. Chemogenetic silencing of neurons in retrosplenial cortex disrupts sensory preconditioning. J Neurosci. 2014;34:10982–10988. doi: 10.1523/JNEUROSCI.1349-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Loureiro M, Lecourtier L, Engeln M, Lopez J, Cosquer B, Geiger K, et al. The ventral hippocampus is necessary for expressing a spatial memory. Brain structure & function. 2012;217:93–106. doi: 10.1007/s00429-011-0332-y. [DOI] [PubMed] [Google Scholar]
- 41.Moser MB, Moser EI. Distributed encoding and retrieval of spatial memory in the hippocampus. J Neurosci. 1998;18:7535–7542. doi: 10.1523/JNEUROSCI.18-18-07535.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moser E, Moser MB, Andersen P. Spatial learning impairment parallels the magnitude of dorsal hippocampal lesions, but is hardly present following ventral lesions. J Neurosci. 1993;13:3916–3925. doi: 10.1523/JNEUROSCI.13-09-03916.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Crabbe JC, Phillips TJ, Kosobud A, Belknap JK. Estimation of genetic correlation: interpretation of experiments using selectively bred and inbred animals. Alcoholism, clinical and experimental research. 1990;14:141–151. doi: 10.1111/j.1530-0277.1990.tb00461.x. [DOI] [PubMed] [Google Scholar]