Abstract
MicroRNAs (miRNAs or miRs) are endogenous, small RNA molecules that suppress expression of targeted mRNA. miR-21, one of the most extensively studied miRNAs, is importantly involved in divergent pathophysiological processes relating to ischemia/reperfusion (I/R) injury, such as inflammation and angiogenesis. The role of miR-21 in renal I/R is complex, with both protective and pathological pathways being regulated by miR-21. Preconditioning-induced upregulation of miR-21 contributes to the protection against subsequent renal I/R injury through the targeting of genes such as the proapoptotic gene programmed cell death 4 and interactions between miR-21 and hypoxia-inducible factor. Conversely, long-term elevation of miR-21 may be detrimental to the organ by promoting the development of renal interstitial fibrosis following I/R injury. miR-21 is importantly involved in several pathophysiological processes related to I/R injury including inflammation and angiogenesis as well as the biology of stem cells that could be used to treat I/R injury; however, the effect of miR-21 on these processes in renal I/R injury remains to be studied.
Keywords: microRNA, hypoxia, apoptosis, inflammation, angiogenesis
ischemia/reperfusion (i/r) injury is a complex phenomenon in which lack of blood supply results in hypoxia/anoxia (ischemia) and is then followed by resumption of blood flow (reperfusion). I/R injury causes a series of pathological responses including early- and late-phase inflammation and fibrosis, which leads to cell and organ damage that may or may not be reversible (108). Prevention and treatment of I/R injury are an important area of research in many disease processes, including myocardial infarction, stroke, and acute kidney injury. In recent years a particular interest in preconditioning and stem cell therapies for I/R injury has increased greatly.
Emerging evidence suggests that miRNAs could contribute to regulating processes involved in I/R injury. MicroRNAs (miRNAs) are endogenous, small (18–22 nucleotides) RNA molecules that play an important and ubiquitous role in regulating genes expression. miRNAs typically bind to the 3′-untranslated region of their mRNA targets and downregulate gene expression via mRNA degradation or translational inhibition (8, 36, 68). miRNAs are known to play a significant role in a variety of cellular processes such as cell differentiation (40), proliferation (35), and apoptosis (18).
microRNA (miR)-21 is one of the first human miRNA genes whose regulation was extensively studied. The gene encoding pri-miR-21 is located on human chromosome 17 in the intronic region of the TMEM49 gene. Despite its position within TMEM, pri-miR-21 has its own promoter region allowing it to be individually transcribed (67). miR-21 has been reported to be involved with the regulation of I/R injury and associated processes; however, in some cases it is unclear if that regulation is beneficial, deleterious, or both. The regulatory mechanisms of miR-21 mediated pathophysiology in acute kidney injury including apoptosis, inflammation, and fibrosis have been reviewed elsewhere (77). Here we will expand the description of evidence supporting the involvement of miR-21 in renal I/R. Additionally, because knowledge in this area is emerging, the relationship of miR-21 to I/R in other organs will also be discussed when appropriate. Through this review article we aim to provide a comprehensive description of what is known in the following areas: 1) the relationship between miR-21 and hypoxia, 2) the role of miR-21 in I/R and related pathophysiological processes including inflammation, fibrosis, angiogenesis, and cell survival, and 3) the beneficial role of miR-21 in preconditioning and stem cell therapy for I/R injury.
miR-21 AND HYPOXIA
Hypoxia plays an important role in I/R injury including renal I/R injury. Under hypoxic conditions, tissues and cells produce a series of hypoxic responses involving complex molecular mechanisms. Hypoxia-inducible factor (HIF) is at the center of cellular hypoxia responses (66). HIF is a heterodimeric complex composed of a HIF-α subunit and a HIF-β subunit. While HIF-β remains constitutively high, HIF-α is tightly regulated to control the HIF response. HIF-α has three isoforms (HIF-1α, HIF-2α, and HIF-3α), of which HIF-1α is expressed ubiquitously and has been studied most extensively. Stable expression of HIF-α is an important adaptive response of cells to hypoxia. In the presence of O2, HIF-α is degraded by the ubiquitin-proteasome pathway with the prolyl hydroxylase (PHD). Under hypoxic conditions, the hydroxylation and degradation of HIF-α are inhibited.
The accumulated HIF-α translocates into cell nucleus, and together with HIF-β, binds to the hypoxia-response elements of hypoxia responsive genes, such as vascular endothelial growth factor (VEGF), erythropoietin, glucose transporter 1, and heme oxygenase-1 to promote their transcription. These gene products could increase oxygen supply and capacity for oxygen transport or reduce the oxygen consumption of cells. Reconciliation of the discrepancy between oxygen supply and demand would help to maintain a stable internal environment during ischemia and hypoxia (74).
In recent years many studies have confirmed that miRNAs play an important regulatory role in hypoxic responses as well. A series of hypoxia-related miRNA (HRMs) have been identified in cancer studies, including miRNAs that were upregulated (miR-210, 21, 30, and 192) or downregulated (miR -15b, 20a, and 122a) (70, 71). Fasanaro et al. (34) found that HIF binding sites are present in most HRM promoters, suggesting that HIF may be the key regulator of HRM expression under hypoxic conditions. These findings suggest that HIF regulates alterations in gene expression, not only from direct regulation of hypoxia-responsive genes, but also from indirect regulation of mRNAs targeted by HRMs (52).
miR-21 is one of the HRMs upregulated in tumor tissues, and some evidence suggests that HIF and miR-21 regulation may be interdependent. HIF binding sites are found ∼2 kb upstream of the transcription start site of pri-miR-21 (71), which suggests that HIF may act to promote miR-21 expression under hypoxic conditions. We previously found that blockade of HIF abolished miR-21 upregulation in hypoxic human renal epithelial cells, demonstrating a significant role for HIF in the upregulation of miR-21 (120). Interestingly, miR-21 could also regulate HIF-1α expression. miR-21 target gene phosphatase and tensin homology deleted on chromosome 10 (PTEN) has been reported to be an inhibitor of phosphoinositide 3-kinase (PI3K)/Akt signaling pathway(60). The PI3K/Akt signaling pathway plays an important role in the expression and activation of HIF induced by hypoxia, growth factor, and nitric oxide (38, 99, 126). PTEN has been shown to attenuate hypoxia-mediated activation of Akt and HIF-1α stabilization and inhibit HIF-1-regulated gene expression (136). Later Liu et al. (81) demonstrated that miR-21 inhibited PTEN resulting in activation of Akt that can induce expression of HIF-1α and its target gene VEGF. These results indicate that miR-21 can indirectly promote HIF-1 stabilization and activity under hypoxic conditions and act with HIF-1 to form a positive feedback loop.
miR-21 can also be regulated by other mechanisms in hypoxia in addition to HIF. Polytarchou et al. (90) found that binding of nuclear factor kappaB (NF-κB), cAMP responsive element-binding protein (CREB), and CREB binding protein/p300 to the promoter of miR-21 was induced by the activation of Akt2 in hypoxia.
The studies reviewed here indicate that miR-21 is upregulated in hypoxia and then acts to perpetuate the actions of HIF by indirect activation of Akt via targeting PTEN. Additionally, miR-21 and HIF-1 are in a hypoxia-induced positive feedback loop in which HIF-1α drives miR-21 expression and miR-21 indirectly stabilizes and upregulates HIF-1α. Future directions for research are mutual regulatory mechanism of HIF-1α and miR-21 under hypoxic condition.
miR-21 IN RENAL I/R AND RELATED PROCESSES
The kidney is very sensitive to hypoxia and vulnerable to ischemic or hypoxic injury because of the renal vascular anatomy and the high energy consumption of renal tubular epithelial cells (12). The mechanism of renal I/R injury is extremely complex, however, much of the damage is mediated by reactive oxygen species (ROS) during reperfusion (26). ROS activate inflammatory cells resulting in the release of interleukin (IL), tissue necrosis factor, and other inflammatory factors, promoting cell apoptosis and increasing tissue damage during reperfusion. HIF-1α has also been detected in renal tubular epithelial cells during renal ischemia and postreperfusion (93). ROS could stimulate expression of HIF-1 and upregulate a variety of HRMs under hypoxic conditions through the PI3K/Akt signal pathway (41).
Interest in the relationship between miR-21 and I/R injury has increased in the recent years (25, 44, 56, 96, 120). In studies of renal I/R models by Godwin et al. (44) and Wei et al. (115) several miRNAs including miR-21, miR-7, and miR-192 were shown to be upregulated, while others such as miR-322 were downregulated. In the myocardium, miR-21 is also known to have an important role in cardiac ischemic injury (fibrosis) and myocardial remodeling following I/R (121, 130). The role of miR-21 in brain I/R (stoke) may be related to atherosclerosis (92), possibly involving angiogenesis. A role for miR-21 in lung and liver I/R injury organs has not been reported.
The functional role of miR-21 in renal I/R injury appears to be complex and few studies have explored its role in the many I/R related processes in the kidney. Upregulation of miR-21 by ischemic preconditioning (IPC) contributes to the protection of kidneys from subsequent I/R injury; however, knocking down miR-21 at the time of renal I/R injury has no effect on the I/R injury (120). In ischemia, an I/R-related condition, the overexpression of miR-21 in cultured mouse tubular epithelial cells does not prevent cell death following simulated ischemia (44). In addition, miR-21 may promote renal interstitial fibrosis that occurs after I/R injury (19). The diverse effects of miR-21 on I/R-related processes are likely due to temporal changes in expression of miR-21 target genes and pathway involvement through the different stages of I/R pathology. The relationship of miR-21 to these pathways and pathological processes, which are summarized in Fig. 1 and are discussed in detail in the following sections.
Fig. 1.
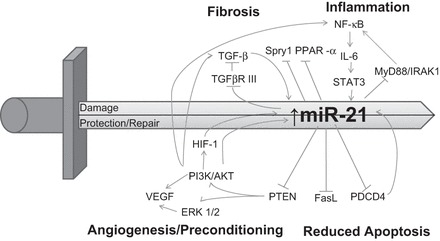
Double-edged sword effects of microRNA (miR)-21 on processes related to ischemia-reperfusion (I/R) injury. Pathways regulated by miR-21 may promote protection/repair or damage following renal I/R injury. In the initial stage of I/R, miR-21 upregulation provides protection in I/R injury. miR-21 protects against the stimulation of apoptosis by targeting programmed cell death 4 (PDCD4), Fas ligand (FasL), and phosphatase and tensin homology deleted on chromosome 10 (PTEN). Meanwhile, inflammation may also be reduced as a result of a decrease in NF-κB induced inflammation [via a reduction in PDCD4 or MyD88 and IL-1R-associated kinase 1 (IRAK1)] and miR-21 targeting of peroxisome proliferator activated receptor-α (PPAR-α). miR-21 is also involved in the angiogenic repair process of ischemic injury by regulation of PTEN-AKT/ERK1-VEGF pathway. Moreover, excessive upregulation of miR-21 induced by transforming growth factor (TGF)-β stimulation exacerbates tissue fibrosis via suppression Ppar-α or Sprouty homolog 1 (Spry1), resulting in chronic kidney injury.
miR-21 and Inflammation
The inflammatory response is initiated quickly after I/R, further exacerbating I/R injury (26). This response involves NF-κB, inflammatory cytokines, inflammatory cells, and several other factors (26). Studies have shown that suppression of the inflammatory response can reduce renal I/R injury and preserve renal function (15, 32).
miR-21 has been reported to be involved in many conditions in which inflammation is central, including lipopolysaccharide (LPS)-stimulated lung inflammation (87), allergic airway inflammation (83), osteoarthritis (104), psoriasis and atopic eczema (137), and many others. In these inflammatory diseases miR-21 may act to regulate immune-related target genes, including confirmed targets IL-12p25 (83) and transforming growth factor-β receptor 2 (TGFBR2) (20). miR-21 is also involved in common inflammatory pathways. IL-6, a proinflammatory cytokine activated by NF-κB, can drive miR-21 expression through a signal transducer and activator of transcription 3 (STAT3)-dependent mechanism and STAT3-activated miR-21 directly inhibits PTEN. Additionally, the inhibition of PTEN results in increased Akt activity and subsequent activation of NF-κB, which is required for sustaining the inflammatory positive feedback loop (53). Zhou et al. (129) found another autoregulatory feedback loop between peroxisome proliferator activated receptor-α (PPAR-α) and miR-21.
Despite the previously described studies suggesting that miR-21 is involved with proinflammtory signaling, several studies suggest that miR-21 may be negatively regulating inflammatory processes. Sheedy et al. (101) found that transfection of miR-21 precursor into cells exposed to LPS blocked NF-κB activity and promoted the production of anti-inflammatory IL-10 through miR-21 targeting of programmed cell death 4 (PDCD4). Similarly, Chen et al. (21) found that miR-21 inhibited two important factors in the Toll-like receptor signaling pathway MyD88 and IL-1R-associated kinase 1, thereby reducing NF-κB signaling. miR-21 is also upregulated during the process of T cell differentiation (117). In regulatory T cells (Treg) miR-21 was found to increase expression of forkhead box P3, an important characteristic of natural Tregs (94), that negatively regulate immune cells involved with the inflammatory process.
These miR-21-regulated genes are in pathways that have been well studied in I/R injury through a direct or indirect link to miR-21. Activation of NF-κB is a central part in the inflammation signaling pathway (134). We previously found that prolonged renal ischemia/hypoxia excessively activated NF-κB, increased expression of several proinflammtory factors, and exacerbated the infiltration of monocyte-macrophages, resulting in acute renal failure (58). IL-6 is considered to be an indicator of renal I/R-induced inflammation (113). Tregs have also been found to be protective in renal I/R injury (24). Despite reported interactions between miR-21 and NF-κB, cytokines (IL-6), and Tregs in inflammatory processes, further studies are required to understand how these miR-21 regulation in these pathways would impact the development of inflammation in renal I/R injury.
miR-21 and Angiogenesis
Animal experiments and clinical studies have shown that angiogenesis can be induced following ischemia (myocardium, kidney, and brain) (33, 39, 54). For example, Iruela-Arispe et al. (54) found that endothelial cell proliferation, migration, and new vasculature formation occurred 2–14 days after renal ischemic injury. Ischemia/hypoxia-induced angiogenesis is of great significance in the repair of the ischemic injury because it can help to restore a normoxic environment in the impacted tissue.
It has been reported that knockout of key enzymes in the biogenesis of miRNAs such as Dicer and Drosha could inhibit the generation and migration of capillary endothelial cells and alter factors that regulate angiogenesis, suggesting that dyspoiesis of miRNAs has a dramatic impact on angiogenesis (69, 104). In addition, miRNAs such as proangiogenic miR-92a and miR-210 and antiangiogenic miR-221 and miR-222 have been reported to regulate ischemia-induced angiogenesis (2, 103).
miR-21 is highly expressed in vascular endothelial cells (45, 55). The endothelial cell responses to shear stress or hypoxia, which are important in vascular remodeling are affected by miR-21 (116). In a recent study by Ji et al. (55), microarray analysis revealed that miR-21 was significantly upregulated in vascular walls following balloon injury. They also found that miR-21 knock-down decreased neointimal hyperplasia following angioplasty, pointing to a proangiogenic function of miR-21. This mechanism may be related to the ability of miR-21 to suppress target PTEN and to indirectly increase B-cell lymphoma 2 expression. Navarro et al. (88) found that miR-21 induced tumor angiogenesis through targeting of PTEN, leading to activation of Akt and extracellular regulated protein kinases 1/2 (ERK1/2) signaling pathways, and thereby enhancing HIF-1α and VEGF expression. A study of miR-21 in a cancer model showed that angiogenesis was dependent on the upregulation of miR-21 through regulating VEGF protein levels (127). In addition, miR-21 has been found to induce differentiation of induced pluripotent stem cells into endothelial cells via PTEN/Akt pathway and TGF-β2 activation, which might be involved in angiogenesis (29). Though several studies report a proangiogenic role for miR-21, it is also important to note that miR-21 has also been reported to inhibit angiogenesis by targeting genes RhoB (16) in endothelial cells and TGFBR2 in human adipose-derived stem cells, decreasing the tumor vascularity (102). In addition, Zhu et al. (131) also demonstrated that in endothelial progenitor cells, angiogenesis was inhibited via a miR21-Hmga2-p16Ink4a/p19Arf axis in vitro and in vivo.
The ability of miR-21 to indirectly upregulate via targeting of PTEN may significantly affect the progression of I/R injury. VEGF is one of the most important factors in kidney vasculogenesis, acting through its receptor on the endothelial cells. Leonard et al. (75) found that treatment with synthetic VEGF-121 promoted regeneration of renal vessels and improved renal function in acute renal injury. Despite many studies reporting a role for miR-21 in the regulation of angiogenesis, it remains to be determined whether miR-21 regulates angiogenesis and repair processes through VEGF or other signaling mechanisms.
miR-21 and Cell Survival
miR-21 is a strong prosurvival and antiapoptotic miRNA especially in cancer. miR-21 has been found to be elevated in several types of solid tumors, including breast tumors, colon tumors, and gliomas (11, 18, 112). The antiapoptotic function of miR-21 is mediated, at least in part, by targeting a series of pro-apoptotic genes including PDCD4 (37), PTEN (86), tropomyosin 1 (132).
The antiapoptotic protection mediated by miR-21 has been studied in ischemic heart and cerebrovascular diseases. For example, miR-21 expression has been shown to be inversely correlated with PDCD4 expression and cellular apoptosis in the heart after I/R (23). Additionally, overexpression of miR-21 in the left ventricle reduced cardiomyocyte apoptosis following I/R (91). It has also been reported that miR-21 attenuates the death of ischemic cortical neurons by reducing expression of cell death inducing Fas ligand (FasL) gene (13). Overexpression of miR-21 in the mouse heart inhibited ischemia-induced upregulation of PTEN and FasL, reduced infarct size, and attenuated apoptosis (100). It is interesting to note that suppression of PDCD4 and PTEN could lead to activation of activator protein 1 or Akt signaling, respectively, which could in turn upregulate miR-21, forming positive feedback loops (30, 100, 105).
In renal I/R injury apoptosis contributes to tubular epithelial cell death (79, 97). It has been suggested that miR-21 may also play an important protective role in the renal I/R injury through antiapoptotic regulatory mechanisms (44). We found that in mouse kidneys exposed to 15 min of ischemia miR-21 was significantly upregulated after 4 h of reperfusion and remained significantly higher 4 days later. The preconditioning-induced upregulation of miR-21 protected mouse kidneys from subsequent I/R injury, which might in part be mediated by a decrease in cell apoptosis through miR-21-induced suppression of PDCD4 (120). The functional significance of miR-21 regulation of apoptosis in long-term I/R injury response is not known.
miR-21 in Fibrosis
I/R response is associated with chronic (days to weeks) molecular mechanisms of repair including pathways associated with fibrosis. I/R is known to incite fibrosis in the heart (110), kidney (46), and liver (22) through expression of a series of profibrositic genes, some of which may be regulated by miRNA. As discussed above, miR-21 is upregulated in response to I/R, and several studies have shown that miR-21 is involved with the regulation of fibrosis in lung (80), heart (1, 78, 106), liver (114), and kidney (43, 111, 128).
Renal TGF-β, a key fibrogenic protein, is upregulated in response to I/R. miR-21 is rapidly induced in the kidney after TGF-β treatment (44). Findings from studies in other disease processes suggests that miR-21 may be involved in TGF-β induced profibrotic signaling in renal I/R injury through several signaling pathways. Dey et al. (27) found that suppression of miR-21 attenuated phosphorylation of endogenous inhibitors of mammalian target of rapamycin complex 1 by inhibiting TGF-β-stimulated phosphorylation of Akt kinase. TGF-β signaling is also amplified by miR-21 through inhibition of Smad7 expression and reduced Smad2 phosphorylation, promoting fibrotic lung disease (80). Other studies have shown that miR-21 contributes to fibrosis by the PTEN/Akt pathway (7, 114). A positive feedback loop with miR-21 targeting of transforming growth factor beta receptor III (TGFBR3) may also be involved with cardiac fibrosis (78). In addition to the PTEN/Akt pathway, Sprouty homolog 1 (Spry1), a miR-21 target gene, in the ERK-MAPK signaling pathway is also involved in cardiac fibrosis. Overexpression of miR-21 promoted proliferation of cardiac fibroblasts through activation of MAPK which was inhibited by Spry1 (106). Roy et al. (95) showed that increased miR-21 was localized to cardiac fibroblasts in the infarct zone of mouse hearts subjected to I/R. They found that targeting of PTEN by miR-21 increased matrix metalloprotease-2 expression via alterations in the PTEN signaling pathway, contributing to fibrosis. The mode of action for miR-21 in renal fibrosis could be different from that in the heart or lung. Recently, Chau et al. (19) found that the ERK/MAPK activation by miR-21 in the renal fibrosis after I/R through target genes Ppar-α and Mpv171, respectively. In the unilateral ureteral obstruction model miR-21 is localized primarily to the tubular epithelial cells, In this model the tubulointerstitium remains ischemic/hypoxic following renal reperfusion, and this has been shown to promote renal fibrosis (73). Serum miR-21 level was increased and associated with the pathological renal fibrosis in renal transplant patients (43). Since miR-21 is hypoxia responsive, and elevations in circulating miR-21 are associated with renal fibrosis, the role that miR-21 plays in the occurrence and development of tubulointerstitial fibrosis and fibrotic repair processes following renal I/R is an important area for future research.
Role of miR-21 in Preconditioning
The response of a cell to hypoxia/ischemia is bimodal. Initially there is an adaptive protective conditioning reaction that transitions to cell death upon persistence of the insult. Different pretreatments including brief exposures to ischemia, mild heat shock, and certain drugs activate endogenous defense mechanisms, to protect cells or organs against subsequent, sustained ischemic insult. This is known as ischemic tolerance (10). Ischemic tolerance is, by far, the most powerful endogenous mechanism in the prevention of ischemic organ damage (42). Among studies on various pretreatments to induce ischemic tolerance, IPC was evaluated most frequently. Organ protection phenomenon of IPC has been confirmed in the heart, brain, liver, lung, and kidney (14, 49, 61). Our previous study found that IPC could effectively attenuate acute ischemic renal injury by reducing apoptosis and inflammation. We also found that the window of renal protection could be significantly prolonged by adjusting the form of ischemic pretreatment. IPC also inhibited transdifferentiation of glomerular mesangial cells and tubular epithelial cell and significantly reduced tubulointerstitial fibrosis during late renal I/R injury (57).
The protective mechanism of IPC appears to involve a series of protective mediators and/or effectors such as ROS, protein kinase C, HIF, inducible nitric oxide synthase, and heat shock protein (HSP) (42, 49). Recently miRNAs have been identified to be involved with the regulation of IPC. Several miRNAs such as miR-133, miR-200, and miR-23a have been reported to be involved in cardiac, brain, and hepatic IPC (72, 98, 119). A protective effect of miR-21 in cardiac IPC was reported by both Dong et al. (30) and Yin et al. (123). In these studies IPC inhibited the downregulation of miR-21 in the infarcted areas. Overexpression of miR-21 reduced cell apoptosis in the border and the infarcted areas, decreased myocardial infarct size, and improved left ventricle remodeling 2 wk following myocardial infarction. The protective role of miR-21 in cardiac IPC was further supported in a study by Cheng et al. (23), which found that miR-21 was upregulated early after cardiac IPC and protected against cardiac I/R injury. Consistent with these findings, exogenous miR-21 reduced infarct size following I/R by 64% and inhibition of miR-21 abolished the protective effect (124). In addition, Dharap et al. (28) analyzed the spectrum of miRNAs in brain after IPC and found that expression of miR-21 increased most significantly in the rat cerebral cortex 24 h after IPC.
In our study, we utilized a mouse model of delayed renal IPC and found that expression levels of miR-21 were significantly induced in kidneys with 15 min of IPC. The in vivo knockdown of miR-21 during IPC led to worsen renal functional and histological damage resulting from I/R injury 4 days later, indicating that miR-21 contributes to the protection conferred by delayed IPC (120).
Furthermore, numerous studies have shown that miR-21 regulates IPC through several signaling pathways. The role of PI3K/Akt signaling pathway has been well established in the protection against I/R injury conferred by early or delayed IPC, mainly through attenuation of apoptosis (64, 109, 118, 122). This suggests that the protective role of IPC-induced miR-21 may be related to its regulatory effects on the PTEN-PI3K/Akt signaling pathway. The miR-21 conferred protection with IPC is also conferred by the regulation of target gene PDCD4 in renal (120) or cardiac (23) tissues. In another study, miR-21 was shown to be involved in the resveratrol-induced cardioprotection against I/R injury through ERK/MAPK pathway (3). In addition to the established IPC-involved pathways, HIF is increasingly being considered as a mediator of ischemic tolerance (9, 31). It has been confirmed that high expression of HIF plays an important role in IPC in the heart, brain, liver, and kidney (14, 65, 82, 85). Our study showed that knock-down of miR-21 did not affect I/R injury in the absence of IPC, suggesting that the protective effect of miR-21 might depend on HIF-1 induction by IPC (120). In addition, xenon (84) or PHD inhibitor (50) pretreatment, which induces overexpression of HIF, also has a protective effect on renal ischemic injury. A study by Jia et al. (56), in our lab, showed miR-21 contributes to renal protection conferred by xenon preconditioning probably through the upregulation of HIF. miR-21 expression induced by cardiac IPC was also found to positively correlate with protective proteins including endothelial nitric oxide synthase, heat shock transcription factor 1, and HSP70 by Yin et al. (123), suggesting the involvement of other signaling pathways and molecules in this process.
miR-21 and Stem Cell Therapy in I/R
Unfractionated bone marrow stem cells (59), hematopoietic stem cells (76), and mesenchymal stem cells (MSC) (107, 133) have been studied as sources for stem cell-based repair after I/R injury. Currently, one of the major challenges to successful stem cell therapy in I/R injury is insuring cell survival and differentiation in the harsh microenvironment of damaged postischemic tissues or organs (4–6).
miR-21 was found to function in a lineage-specific manner during differentiation of stem cells into specialized cell types via PTEN/Akt or TGF-β signaling pathway (62, 63). Furthermore, Zou et al. (135) found that upregulation of miR-21 induced by TGF-β treatment in MSCs promoted skin wound healing by increasing proliferation and differentiation of these cells. These results indicate that induced miR-21 in renal I/R injury may regulate the differentiation of stem cell into renal tubule epithelial cell.
Transplanted donor stem cells are sensitive to serum and O2 deprivation in damaged tissues (47). Hypoxic preconditioning (HPC) of stem cells prior to transplantation is an effective protection strategy. In the heart stem cell therapy, HPC has been found to be able to enhance the stability of HIF-1 via the PI3K/Akt signaling pathway, enhancing the antiapoptotic state of MSCs (17, 51), which suggests that miR-21 may play a role in HPC for stem cell therapy by the HIF/miR-21/PTEN/Akt pathway. In addition, Haider et al. (48) found that HPC enhanced the proliferation and survival of injected stem cells in the infarcted hearts, which was associated with preconditioning-induced upregulation of miR-21 via activation of ERK1/2 and STAT3 signaling. Nie et al. (89) also reported that miR-21 was involved in the survival of MSCs under hypoxic condition with serum deprivation. Therefore, miR-21 could be used to improve feasibility and success of stem cell therapy in I/R injury.
SUMMARY
In summary, miR-21 clearly plays an important role in I/R injury (Fig. 1). Preconditioning-induced upregulation of miR-21 contributes to the protection against subsequent renal I/R injury. This protective effect of miR-21 may involve targeting of PDCD4 and the interaction between miR-21 and HIF. Long-term elevation of miR-21, however, may lead to the development of renal interstitial fibrosis following I/R injury. miR-21 is importantly involved in several pathophysiological processes related to I/R injury, including inflammation and angiogenesis as well as in the proliferation, differentiation, and survival of stem cells. Despite the reported role for miR-21 in those processes, it remains to be further investigated if and how miR-21 affects inflammation, angiogenesis, and stem cell biology in relationship to I/R injury and its treatment. In addition, it would be important to understand the role of miR-21 in different cell types relevant to I/R injury, including tubular epithelial cells, interstitial cells, endothelial cells, and infiltrating immune cells in the kidney.
GRANTS
This work was supported by National Natural Science Foundation of China Grants 81300570 (X. Xu) and 81270779 (X. Ding), American Heart Association Grant 13SDG17100095 (A. J. Kriegel), and US National Institutes of Health Grants HL-111580, HL-082798, HL-116264 (M. Liang), and 8UL1TR-000055 (A. J. Kriegel).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: X.X. conception and design of research; X.X. and X.J. prepared figures; A.J.K., H.L., X.B., J.O., M.L., and X.D. edited and revised manuscript; M.L. and X.D. approved final version of manuscript.
REFERENCES
- 1.Adam O, Lohfelm B, Thum T, Gupta SK, Puhl SL, Schafers HJ, Bohm M, Laufs U. Role of miR-21 in the pathogenesis of atrial fibrosis. Basic Res Cardiol 107: 278, 2012. [DOI] [PubMed] [Google Scholar]
- 2.Anand S, Cheresh DA. MicroRNA-mediated regulation of the angiogenic switch. Curr Opin Hematol 18: 171–176, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anversa P, Mukhopadhyay P, Mukherjee S, Ahsan K, Bagchi A, Pacher P, Das DK. Restoration of altered microRNA expression in the ischemic heart with resveratrol. PLoS One 5: e15705, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bai X, Alt E. Myocardial regeneration potential of adipose tissue-derived stem cells. Biochem Biophys Res Commun 401: 321–326, 2010. [DOI] [PubMed] [Google Scholar]
- 5.Bai X, Yan Y, Coleman M, Wu G, Rabinovich B, Seidensticker M, Alt E. Tracking long-term survival of intramyocardially delivered human adipose tissue-derived stem cells using bioluminescence imaging. Mol Imaging Biol 13: 633–645, 2011. [DOI] [PubMed] [Google Scholar]
- 6.Bai X, Yan Y, Song YH, Seidensticker M, Rabinovich B, Metzele R, Bankson JA, Vykoukal D, Alt E. Both cultured and freshly isolated adipose tissue-derived stem cells enhance cardiac function after acute myocardial infarction. Eur Heart J 31: 489–501, 2010. [DOI] [PubMed] [Google Scholar]
- 7.Bao H, Hu S, Zhang C, Shi S, Qin W, Zeng C, Zen K, Liu Z. Inhibition of miRNA-21 prevents fibrogenic activation in podocytes and tubular cells in IgA nephropathy. Biochem Biophys Res Commun 444: 455–460, 2014. [DOI] [PubMed] [Google Scholar]
- 8.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, function. Cell 116: 281–297, 2004. [DOI] [PubMed] [Google Scholar]
- 9.Bernhardt WM. Preconditional activation of hypoxia-inducible factors ameliorates ischemic acute renal failure. J Am Soc Nephrol 17: 1970–1978, 2006. [DOI] [PubMed] [Google Scholar]
- 10.Bonventre JV. Kidney ischemic preconditioning. Curr Opin Nephrol Hypertens 11: 43–48, 2002. [DOI] [PubMed] [Google Scholar]
- 11.Bourguignon LY, Spevak CC, Wong G, Xia W, Gilad E. Hyaluronan-CD44 interaction with protein kinase C(epsilon) promotes oncogenic signaling by the stem cell marker Nanog and the production of microRNA-21, leading to down-regulation of the tumor suppressor protein PDCD4, anti-apoptosis, and chemotherapy resistance in breast tumor cells. J Biol Chem 284: 26533–26546, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bracken CP. Cell-specific regulation of hypoxia-inducible factor (HIF)-1 and HIF-2 stabilization and transactivation in a graded oxygen environment. J Biol Chem 281: 22575–22585, 2006. [DOI] [PubMed] [Google Scholar]
- 13.Buller B, Liu X, Wang X, Zhang RL, Zhang L, Hozeska-Solgot A, Chopp M, Zhang ZG. MicroRNA-21 protects neurons from ischemic death. FEBS J 277: 4299–4307, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cai Z, Zhong H, Bosch-Marce M, Fox-Talbot K, Wang L, Wei C, Trush MA, Semenza GL. Complete loss of ischaemic preconditioning-induced cardioprotection in mice with partial deficiency of HIF-1. Cardiovasc Res 77: 463–470, 2007. [DOI] [PubMed] [Google Scholar]
- 15.Cao CC, Ding XQ, Ou ZL, Liu CF, Li P, Wang L, Zhu CF. In vivo transfection of NF-kappaB decoy oligodeoxynucleotides attenuate renal ischemia/reperfusion injury in rats. Kidney Int 65: 834–845, 2004. [DOI] [PubMed] [Google Scholar]
- 16.Capogrossi M, Sabatel C, Malvaux L, Bovy N, Deroanne C, Lambert V, Gonzalez MLA, Colige A, Rakic JM, Noël A, Martial JA, Struman I. MicroRNA-21 exhibits antiangiogenic function by targeting RhoB expression in endothelial cells. PLoS One 6: e16979, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chacko SM, Ahmed S, Selvendiran K, Kuppusamy ML, Khan M, Kuppusamy P. Hypoxic preconditioning induces the expression of prosurvival and proangiogenic markers in mesenchymal stem cells. Am J Physiol Cell Physiol 299: C1562–C1570, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chan JA, Krichevsky AM, Kosik KS. MicroRNA-21 is an antiapoptotic factor in human glioblastoma cells. Cancer Res 65: 6029–6033, 2005. [DOI] [PubMed] [Google Scholar]
- 19.Chau BN, Xin C, Hartner J, Ren S, Castano AP, Linn G, Li J, Tran PT, Kaimal V, Huang X, Chang AN, Li S, Kalra A, Grafals M, Portilla D, MacKenna DA, Orkin SH, Duffield JS. MicroRNA-21 promotes fibrosis of the kidney by silencing metabolic pathways. Sci Transl Med 4: 121ra118, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen RF, Huang HC, Ou CY, Hsu TY, Chuang H, Chang JC, Wang L, Kuo HC, Yang KD. MicroRNA-21 expression in neonatal blood associated with antenatal immunoglobulin E production and development of allergic rhinitis. Clin Exp Allergy 40: 1482–1490, 2010. [DOI] [PubMed] [Google Scholar]
- 21.Chen Y, Chen J, Wang H, Shi J, Wu K, Liu S, Liu Y, Wu J. HCV-induced miR-21 contributes to evasion of host immune system by targeting MyD88 and IRAK1. PLoS Pathog 9: e1003248, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheng F, Li Y, Feng L, Li S. Hepatic stellate cell activation and hepatic fibrosis induced by ischemia/reperfusion injury. Transplant Proc 40: 2167–2170, 2008. [DOI] [PubMed] [Google Scholar]
- 23.Cheng Y, Zhu P, Yang J, Liu X, Dong S, Wang X, Chun B, Zhuang J, Zhang C. Ischaemic preconditioning-regulated miR-21 protects heart against ischaemia/reperfusion injury via anti-apoptosis through its target PDCD4. Cardiovasc Res 87: 431–439, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cho WY, Choi HM, Lee SY, Kim MG, Kim HK, Jo SK. The role of Tregs and CD11c+ macrophages/dendritic cells in ischemic preconditioning of the kidney. Kidney Int 78: 981–992, 2010. [DOI] [PubMed] [Google Scholar]
- 25.D'Auria S, Du J, Cao X, Zou L, Chen Y, Guo J, Chen Z, Hu S, Zheng Z. MicroRNA-21 and risk of severe acute kidney injury and poor outcomes after adult cardiac surgery. PLoS One 8: e63390, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Degroot H, Rauen U. Ischemia-reperfusion injury: processes in pathogenetic networks: a review. Transpl P 39: 481–484, 2007. [DOI] [PubMed] [Google Scholar]
- 27.Dey N, Ghosh-Choudhury N, Kasinath BS, Choudhury GG. TGFbeta-stimulated microRNA-21 utilizes PTEN to orchestrate AKT/mTORC1 signaling for mesangial cell hypertrophy and matrix expansion. PLoS One 7: e42316, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dharap A, Bowen K, Place R, Li LC, Vemuganti R. Transient focal ischemia induces extensive temporal changes in rat cerebral microRNAome. J Cereb Blood Flow Metab 29: 675–687, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Di Bernardini E, Campagnolo P, Margariti A, Zampetaki A, Karamariti E, Hu Y, Xu Q. Endothelial lineage differentiation from induced pluripotent stem cells is regulated by microRNA-21 and transforming growth factor beta2 (TGF-beta2) pathways. J Biol Chem 289: 3383–3393, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dong S, Cheng Y, Yang J, Li J, Liu X, Wang X, Wang D, Krall TJ, Delphin ES, Zhang C. MicroRNA expression signature and the role of MicroRNA-21 in the early phase of acute myocardial infarction. J Biol Chem 284: 29514–29525, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eckle T, Kohler D, Lehmann R, El Kasmi KC, Eltzschig HK. Hypoxia-inducible factor-1 is central to cardioprotection: a new paradigm for ischemic preconditioning. Circulation 118: 166–175, 2008. [DOI] [PubMed] [Google Scholar]
- 32.Efrati S, Berman S, Hamad RA, Siman-Tov Y, Ilgiyaev E, Maslyakov I, Weissgarten J. Effect of captopril treatment on recuperation from ischemia/reperfusion-induced acute renal injury. Nephrol Dial Transplant 27: 136–145, 2012. [DOI] [PubMed] [Google Scholar]
- 33.Ergul A, Alhusban A, Fagan SC. Angiogenesis: a harmonized target for recovery after stroke. Stroke 43: 2270–2274, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fasanaro P, D'Alessandra Y, Di Stefano V, Melchionna R, Romani S, Pompilio G, Capogrossi MC, Martelli F. MicroRNA-210 modulates endothelial cell response to hypoxia and inhibits the receptor tyrosine kinase ligand Ephrin-A3. J Biol Chem 283: 15878–15883, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Feng S, Cong S, Zhang X, Bao X, Wang W, Li H, Wang Z, Wang G, Xu J, Du B, Qu D, Xiong W, Yin M, Ren X, Wang F, He J, Zhang B. MicroRNA-192 targeting retinoblastoma 1 inhibits cell proliferation and induces cell apoptosis in lung cancer cells. Nucleic Acids Res 39: 6669–6678, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Filipowicz W, Bhattacharyya SN, Sonenberg N. Mechanisms of post-transcriptional regulation by microRNAs: are the answers in sight? Nat Rev Genet 9: 102–114, 2008. [DOI] [PubMed] [Google Scholar]
- 37.Frankel LB, Christoffersen NR, Jacobsen A, Lindow M, Krogh A, Lund AH. Programmed cell death 4 (PDCD4) is an important functional target of the microRNA miR-21 in breast cancer cells. J Biol Chem 283: 1026–1033, 2007. [DOI] [PubMed] [Google Scholar]
- 38.Fu J, Chen Y, Cao J, Luo T, Qian YW, Yang W, Ren YB, Su B, Cao GW, Yang Y, Yan YQ, Shen F, Wu MC, Feng GS, Wang HY. p28GANK overexpression accelerates hepatocellular carcinoma invasiveness and metastasis via phosphoinositol 3-kinase/AKT/hypoxia-inducible factor-1alpha pathways. Hepatology 53: 181–192, 2011. [DOI] [PubMed] [Google Scholar]
- 39.Fu SP, He SY, Xu B, Hu CJ, Lu SF, Shen WX, Huang Y, Hong H, Li Q, Wang N, Liu XL, Liang F, Zhu BM. Acupuncture promotes angiogenesis after myocardial ischemia through h3k9 acetylation regulation at VEGF gene. PLoS One 9: e94604, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gagan J, Dey BK, Layer R, Yan Z, Dutta A. MicroRNA-378 targets the myogenic repressor myor during myoblast differentiation. J Biol Chem 286: 19431–19438, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Galanis A, Pappa A, Giannakakis A, Lanitis E, Dangaj D, Sandaltzopoulos R. Reactive oxygen species and HIF-1 signalling in cancer. Cancer Lett 266: 12–20, 2008. [DOI] [PubMed] [Google Scholar]
- 42.Gidday JM. Cerebral preconditioning and ischaemic tolerance. Nat Rev Neurosci 7: 437–448, 2006. [DOI] [PubMed] [Google Scholar]
- 43.Glowacki F, Savary G, Gnemmi V, Buob D, Van der Hauwaert C, Lo-Guidice JM, Bouye S, Hazzan M, Pottier N, Perrais M, Aubert S, Cauffiez C. Increased circulating miR-21 levels are associated with kidney fibrosis. PLoS One 8: e58014, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Godwin JG, Ge X, Stephan K, Jurisch A, Tullius SG, Iacomini J. Identification of a microRNA signature of renal ischemia reperfusion injury. Proc Natl Acad Sci USA 107: 14339–14344, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guduric-Fuchs J, O'Connor A, Cullen A, Harwood L, Medina RJ, O'Neill CL, Stitt AW, Curtis TM, Simpson DA. Deep sequencing reveals predominant expression of miR-21 amongst the small non-coding RNAs in retinal microvascular endothelial cells. J Cell Biochem 113: 2098–2111, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gueler F, Gwinner W, Schwarz A, Haller H. Long-term effects of acute ischemia and reperfusion injury. Kidney Int 66: 523–527, 2004. [DOI] [PubMed] [Google Scholar]
- 47.Haider HKh, Ashraf M. Preconditioning and stem cell survival. J Cardiovasc Transl Res 3: 89–102, 2009. [DOI] [PubMed] [Google Scholar]
- 48.Haider KH, Idris NM, Kim HW, Ahmed RPH, Shujia J, Ashraf M. MicroRNA-21 is a key determinant in IL-11/Stat3 anti-apoptotic signalling pathway in preconditioning of skeletal myoblasts. Cardiovasc Res 88: 168–178, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hausenloy DJ, Yellon DM. The second window of preconditioning (SWOP) where are we now? Cardiovasc Drugs Ther 24: 235–254, 2010. [DOI] [PubMed] [Google Scholar]
- 50.Hill P, Shukla D, Tran MGB, Aragones J, Cook HT, Carmeliet P, Maxwell PH. Inhibition of hypoxia inducible factor hydroxylases protects against renal ischemia-reperfusion injury. J Am Soc Nephrol 19: 39–46, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hu X, Yu SP, Fraser JL, Lu Z, Ogle ME, Wang JA, Wei L. Transplantation of hypoxia-preconditioned mesenchymal stem cells improves infarcted heart function via enhanced survival of implanted cells and angiogenesis. J Thorac Cardiovasc Surg 135: 799–808, 2008. [DOI] [PubMed] [Google Scholar]
- 52.Hua Z, Lv Q, Ye W, Wong CK, Cai G, Gu D, Ji Y, Zhao C, Wang J, Yang BB, Zhang Y. MiRNA-directed regulation of VEGF and other angiogenic factors under hypoxia. PLoS One 1: e116, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Iliopoulos D, Jaeger SA, Hirsch HA, Bulyk ML, Struhl K. STAT3 activation of miR-21 and miR-181b-1 via PTEN and CYLD are part of the epigenetic switch linking inflammation to cancer. Mol Cell 39: 493–506, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Iruela-Arispe L, Gordon K, Hugo C, Duijvestijn AM, Claffey KP, Reilly M, Couser WG, Alpers CE, Johnson RJ. Participation of glomerular endothelial cells in the capillary repair of glomerulonephritis. Am J Pathol 147: 1715–1727, 1995. [PMC free article] [PubMed] [Google Scholar]
- 55.Ji R, Cheng Y, Yue J, Yang J, Liu X, Chen H, Dean DB, Zhang C. MicroRNA expression signature and antisense-mediated depletion reveal an essential role of microRNA in vascular neointimal lesion formation. Circ Res 100: 1579–1588, 2007. [DOI] [PubMed] [Google Scholar]
- 56.Jia P, Teng J, Zou J, Fang Y, Zhang X, Bosnjak ZJ, Liang M, Ding X. miR-21 contributes to xenon-conferred amelioration of renal ischemia-reperfusion injury in mice. Anesthesiology 1193: 621–630, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jiang S, Chen Y, Zou J, Xu X, Zhang X, Liu C, Fang Y, Ding X. Diverse effects of ischemic pretreatments on the long-term renal damage induced by ischemia-reperfusion. Am J Nephrol 30: 440–449, 2009. [DOI] [PubMed] [Google Scholar]
- 58.Jiang SH, Liu CF, Zhang XL, Xu XH, Zou JZ, Fang Y, Ding XQ. Renal protection by delayed ischaemic preconditioning is associated with inhibition of the inflammatory response and NF-κB activation. Cell Biochem Funct 25: 335–343, 2007. [DOI] [PubMed] [Google Scholar]
- 59.Kale S. Bone marrow stem cells contribute to repair of the ischemically injured renal tubule. J Clin Invest 112: 42–49, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kato M, Putta S, Wang M, Yuan H, Lanting L, Nair I, Gunn A, Nakagawa Y, Shimano H, Todorov I, Rossi JJ, Natarajan R. TGF-beta activates Akt kinase through a microRNA-dependent amplifying circuit targeting PTEN. Nat Cell Biol 11: 881–889, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kim J, Jang HS, Park KM. Reactive oxygen species generated by renal ischemia and reperfusion trigger protection against subsequent renal ischemia and reperfusion injury in mice. Am J Physiol Renal Physiol 298: F158–F166, 2010. [DOI] [PubMed] [Google Scholar]
- 62.Kim N, Kim H, Jung I, Kim Y, Kim D, Han YM. Expression profiles of miRNAs in human embryonic stem cells during hepatocyte differentiation. Hepatol Res 41: 170–183, 2011. [DOI] [PubMed] [Google Scholar]
- 63.Kim YJ, Hwang SJ, Bae YC, Jung JS. miR-21 regulates adipogenic differentiation through the modulation of TGF-β signaling in mesenchymal stem cells derived from human adipose tissue. Stem Cells 27: 3093–3102, 2009. [DOI] [PubMed] [Google Scholar]
- 64.Kis A, Yellon DM, Baxter GF. Second window of protection following myocardial preconditioning: an essential role for PI3 kinase and p70S6 kinase. J Mol Cell Cardiol 35: 1063–1071, 2003. [DOI] [PubMed] [Google Scholar]
- 65.Knudsen AR, Kannerup AS, Gronbaek H, Andersen KJ, Funch-Jensen P, Frystyk J, Flyvbjerg A, Mortensen FV. Effects of ischemic pre- and postconditioning on HIF-1alpha, VEGF and TGF-beta expression after warm ischemia and reperfusion in the rat liver. Comp Hepatol 10: 3, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Koh M, Spivakkroizman T, Powis G. HIF-1 regulation: not so easy come, easy go. Trends Biochem Sci 33: 526–534, 2008. [DOI] [PubMed] [Google Scholar]
- 67.Krichevsky AM, Gabriely G. miR-21: a small multi-faceted RNA. J Cell Mol Med 13: 39–53, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Krol J, Loedige I, Filipowicz W. The widespread regulation of microRNA biogenesis, function and decay. Nat Rev Genet 11: 597–610, 2010. [DOI] [PubMed] [Google Scholar]
- 69.Kuehbacher A, Urbich C, Zeiher AM, Dimmeler S. Role of Dicer and Drosha for endothelial microRNA expression and angiogenesis. Circ Res 101: 59–68, 2007. [DOI] [PubMed] [Google Scholar]
- 70.Kulshreshtha R, Davuluri RV, Calin GA, Ivan M. A microRNA component of the hypoxic response. Cell Death Different 15: 667–671, 2008. [DOI] [PubMed] [Google Scholar]
- 71.Kulshreshtha R, Ferracin M, Wojcik SE, Garzon R, Alder H, Agosto-Perez FJ, Davuluri R, Liu CG, Croce CM, Negrini M, Calin GA, Ivan M. A microRNA signature of hypoxia. Mol Cell Biol 27: 1859–1867, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lee ST, Chu K, Jung KH, Yoon HJ, Jeon D, Kang KM, Park KH, Bae EK, Kim M, Lee SK, Roh JK. MicroRNAs induced during ischemic preconditioning. Stroke 41: 1646–1651, 2010. [DOI] [PubMed] [Google Scholar]
- 73.Legrand M, Mik EG, Johannes T, Payen D, Ince C. Renal hypoxia and dysoxia after reperfusion of the ischemic kidney. Mol Med 14: 502–516, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lendahl U, Lee KL, Yang H, Poellinger L. Generating specificity and diversity in the transcriptional response to hypoxia. Nat Rev Genet 10: 821, 2011. [DOI] [PubMed] [Google Scholar]
- 75.Leonard EC, Friedrich JL, Basile DP. VEGF-121 preserves renal microvessel structure and ameliorates secondary renal disease following acute kidney injury. Am J Physiol Renal Physiol 295: F1648–F1657, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li B, Cohen A, Hudson TE, Motlagh D, Amrani DL, Duffield JS. Mobilized human hematopoietic stem/progenitor cells promote kidney repair after ischemia/reperfusion injury. Circulation 121: 2211–2220, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li YF, Jing Y, Hao J, Frankfort NC, Zhou X, Shen B, Liu X, Wang L, Li R. MicroRNA-21 in the pathogenesis of acute kidney injury. Prot Cell 4: 813–819, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liang H, Zhang C, Ban T, Liu Y, Mei L, Piao X, Zhao D, Lu Y, Chu W, Yang B. A novel reciprocal loop between microRNA-21 and TGFbetaRIII is involved in cardiac fibrosis. Int J Biochem Cell Biol 44: 2152–2160, 2012. [DOI] [PubMed] [Google Scholar]
- 79.Lieberthal W, Levine JS. Mechanisms of apoptosis and its potential role in renal tubular epithelial cell injury. Am J Physiol Renal Fluid Electrolyte Physiol 271: F477–F488, 1996. [DOI] [PubMed] [Google Scholar]
- 80.Liu G, Friggeri A, Yang Y, Milosevic J, Ding Q, Thannickal VJ, Kaminski N, Abraham E. miR-21 mediates fibrogenic activation of pulmonary fibroblasts and lung fibrosis. J Exp Med 207: 1589–1597, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Liu LZ, Li C, Chen Q, Jing Y, Carpenter R, Jiang Y, Kung HF, Lai L, Jiang BH. MiR-21 induced angiogenesis through AKT and ERK activation and HIF-1alpha expression. PLoS One 6: e19139, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liu M, Alkayed NJ. Hypoxic preconditioning and tolerance via hypoxia inducible factor (HIF) 1alpha-linked induction of P450 2C11 epoxygenase in astrocytes. J Cereb Blood Flow Metab 25: 939–948, 2005. [DOI] [PubMed] [Google Scholar]
- 83.Lu TX, Munitz A, Rothenberg ME. MicroRNA-21 is up-regulated in allergic airway inflammation and regulates IL-12p35 expression. J Immunol 182: 4994–5002, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ma D, Lim T, Xu J, Tang H, Wan Y, Zhao H, Hossain M, Maxwell PH, Maze M. Xenon preconditioning protects against renal ischemic-reperfusion injury via HIF-1alpha activation. J Am Soc Nephrol 20: 713–720, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mahfoudh-Boussaid A, Zaouali M, Hadj-Ayed K, Miled AH, Saidane-Mosbahi D, Rosello-Catafau J, Abdennebi H. Ischemic preconditioning reduces endoplasmic reticulum stress and upregulates hypoxia inducible factor-1α in ischemic kidney: the role of nitric oxide. J Biomed Sci 19: 7, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Meng F, Henson R, Wehbe-Janek H, Ghoshal K, Jacob ST, Patel T. MicroRNA-21 regulates expression of the PTEN tumor suppressor gene in human hepatocellular cancer. Gastroenterology 133: 647–658, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Moschos SA, Williams AE, Perry MM, Birrell MA, Belvisi MG, Lindsay MA. Expression profiling in vivo demonstrates rapid changes in lung microRNA levels following lipopolysaccharide-induced inflammation but not in the anti-inflammatory action of glucocorticoids. BMC Genom 8: 240, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Navarro A, Liu LZ, Li C, Chen Q, Jing Y, Carpenter R, Jiang Y, Kung HF, Lai L, Jiang BH. MiR-21 induced angiogenesis through AKT and ERK activation and HIF-1α expression. PLoS One 6: e19139, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nie Y, Han BM, Liu XB, Yang JJ, Wang F, Cong XF, Chen X. Identification of MicroRNAs involved in hypoxia- and serum deprivation-induced apoptosis in mesenchymal stem cells. Int J Biol Sci 7: 762–768, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Polytarchou C, Iliopoulos D, Hatziapostolou M, Kottakis F, Maroulakou I, Struhl K, Tsichlis PN. Akt2 regulates all Akt isoforms and promotes resistance to hypoxia through induction of miR-21 upon oxygen deprivation. Cancer Res 71: 4720–4731, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Qin Y, Yu Y, Dong H, Bian X, Guo X, Dong S. MicroRNA 21 inhibits left ventricular remodeling in the early phase of rat model with ischemia-reperfusion injury by suppressing cell apoptosis. Int J Med Sci 9: 413–423, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rink C, Khanna S. MicroRNA in ischemic stroke etiology and pathology. Physiol Genomics 43: 521–528, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rosenberger C. Expression of hypoxia-inducible factor-1 and -2 in hypoxic and ischemic rat kidneys. J Am Soc Nephrol 13: 1721–1732, 2002. [DOI] [PubMed] [Google Scholar]
- 94.Rouas R, Fayyad-Kazan H, El Zein N, Lewalle P, Rothe F, Simion A, Akl H, Mourtada M, El Rifai M, Burny A, Romero P, Martiat P, Badran B. Human natural Treg microRNA signature: role of microRNA-31 and microRNA-21 in FOXP3 expression. Eur J Immunol 39: 1608–1618, 2009. [DOI] [PubMed] [Google Scholar]
- 95.Roy S, Khanna S, Hussain SRA, Biswas S, Azad A, Rink C, Gnyawali S, Shilo S, Nuovo GJ, Sen CK. MicroRNA expression in response to murine myocardial infarction: miR-21 regulates fibroblast metalloprotease-2 via phosphatase and tensin homologue. Cardiovasc Res 82: 21–29, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Saikumar J, Hoffmann D, Kim TM, Gonzalez VR, Zhang Q, Goering PL, Brown RP, Bijol V, Park PJ, Waikar SS, Vaidya VS. Expression, circulation, and excretion profile of microRNA-21, -155, and -18a following acute kidney injury. Toxicol Sci 129: 256–267, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Salas MA, Valverde CA, Sanchez G, Said M, Rodriguez JS, Portiansky EL, Kaetzel MA, Dedman JR, Donoso P, Kranias EG, Mattiazzi A. The signalling pathway of CaMKII-mediated apoptosis and necrosis in the ischemia/reperfusion injury. J Mol Cell Cardiol 48: 1298–1306, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Salloum FN, Yin C, Kukreja RC. Role of microRNAs in cardiac preconditioning. J Cardiovasc Pharmacol 56: 581–588, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sandau KB, Faus HG, Brune B. Induction of hypoxia-inducible-factor 1 by nitric oxide is mediated via the PI 3K pathway. Biochem Biophys Res Commun 278: 263–267, 2000. [DOI] [PubMed] [Google Scholar]
- 100.Sayed D, He M, Hong C, Gao S, Rane S, Yang Z, Abdellatif M. MicroRNA-21 is a downstream effector of Akt that mediates its antiapoptotic effects via suppression of Fas ligand. J Biol Chem 285: 20281–20290, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sheedy FJ, Palsson-McDermott E, Hennessy EJ, Martin C, O'Leary JJ, Ruan Q, Johnson DS, Chen Y, O'Neill LAJ. Negative regulation of TLR4 via targeting of the proinflammatory tumor suppressor PDCD4 by the microRNA miR-21. Nat Immunol 11: 141–147, 2009. [DOI] [PubMed] [Google Scholar]
- 102.Shin KK, Lee AL, Kim JY, Lee SY, Bae YC, Jung JS. miR-21 modulates tumor outgrowth induced by human adipose tissue-derived mesenchymal stem cells in vivo. Biochem Biophys Res Commun 422: 633–638, 2012. [DOI] [PubMed] [Google Scholar]
- 103.Staszel T, Zapala B, Polus A, Sadakierska-Chudy A, Kiec-Wilk B, Stepien E, Wybranska I, Chojnacka M, Dembinska-Kiec A. Role of microRNAs in endothelial cell pathophysiology. Pol Arch Med Wewn 121: 361–366, 2011. [PubMed] [Google Scholar]
- 104.Suarez Y, Fernandez-Hernando C, Pober JS, Sessa WC. Dicer dependent microRNAs regulate gene expression and functions in human endothelial cells. Circ Res 100: 1164–1173, 2007. [DOI] [PubMed] [Google Scholar]
- 105.Talotta F, Cimmino A, Matarazzo MR, Casalino L, De Vita G, D'Esposito M, Di Lauro R, Verde P. An autoregulatory loop mediated by miR-21 and PDCD4 controls the AP-1 activity in RAS transformation. Oncogene 28: 73–84, 2008. [DOI] [PubMed] [Google Scholar]
- 106.Thum T, Gross C, Fiedler J, Fischer T, Kissler S, Bussen M, Galuppo P, Just S, Rottbauer W, Frantz S, Castoldi M, Soutschek J, Koteliansky V, Rosenwald A, Basson MA, Licht JD, Pena JTR, Rouhanifard SH, Muckenthaler MU, Tuschl T, Martin GR, Bauersachs J, Engelhardt S. MicroRNA-21 contributes to myocardial disease by stimulating MAP kinase signalling in fibroblasts. Nature 456: 980–984, 2008. [DOI] [PubMed] [Google Scholar]
- 107.Togel F, Hu Z, Weiss K, Isaac J, Lange C, Westenfelder C. Administered mesenchymal stem cells protect against ischemic acute renal failure through differentiation-independent mechanisms. Am J Physiol Renal Physiol 289: F31–F42, 2005. [DOI] [PubMed] [Google Scholar]
- 108.Toledo-Pereyra LH, Toledo AH, Walsh J, Lopez-Neblina F. Molecular signaling pathways in ischemia/reperfusion. Exp Clin Transplant 2: 174–177, 2004. [PubMed] [Google Scholar]
- 109.Tong H, Imahashi K, Steenbergen C, Murphy E. Phosphorylation of glycogen synthase kinase-3beta during preconditioning through a phosphatidylinositol-3-kinase–dependent pathway is cardioprotective. Circ Res 90: 377–379, 2002. [DOI] [PubMed] [Google Scholar]
- 110.Vilahur G, Juan-Babot O, Pena E, Onate B, Casani L, Badimon L. Molecular and cellular mechanisms involved in cardiac remodeling after acute myocardial infarction. J Mol Cell Cardiol 50: 522–533, 2011. [DOI] [PubMed] [Google Scholar]
- 111.Wang J, Gao Y, Ma M, Li M, Zou D, Yang J, Zhu Z, Zhao X. Effect of miR-21 on renal fibrosis by regulating MMP-9 and TIMP1 in kk-ay diabetic nephropathy mice. Cell Biochem Biophys 67: 537–546, 2013. [DOI] [PubMed] [Google Scholar]
- 112.Wang P, Zou F, Zhang X, Li H, Dulak A, Tomko RJ, Lazo JS, Wang Z, Zhang L, Yu J. microRNA-21 negatively regulates cdc25a and cell cycle progression in colon cancer cells. Cancer Res 69: 8157–8165, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wang S, Diao H, Guan Q, Cruikshank WW, Delovitch TL, Jevnikar AM, Du C. Decreased renal ischemia-reperfusion injury by IL-16 inactivation. Kidney Int 73: 318–326, 2007. [DOI] [PubMed] [Google Scholar]
- 114.Wei J, Feng L, Li Z, Xu G, Fan X. MicroRNA-21 activates hepatic stellate cells via PTEN/Akt signaling. Biomed Pharmacother 67: 387–392, 2013. [DOI] [PubMed] [Google Scholar]
- 115.Wei Q, Bhatt K, He HZ, Mi QS, Haase VH, Dong Z. Targeted deletion of Dicer from proximal tubules protects against renal ischemia-reperfusion injury. J Am Soc Nephrol 21: 756–761, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wei Y, Schober A, Weber C. Pathogenic arterial remodeling: the good and bad of microRNAs. Am J Physiol Heart Circ Physiol 304: H1050–H1059, 2013. [DOI] [PubMed] [Google Scholar]
- 117.Wu H, Neilson JR, Kumar P, Manocha M, Shankar P, Sharp PA, Manjunath N. miRNA profiling of naive, effector and memory CD8 T cells. PLoS One 2: e1020, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Xie L, Zheng X, Qin J, Chen Z, Jin Y, Ding W. Role of PI3-kinase/Akt signalling pathway in renal function and cell proliferation after renal ischaemia/reperfusion injury in mice. Nephrology (Carlton) 11: 207–212, 2006. [DOI] [PubMed] [Google Scholar]
- 119.Xu CF, Yu CH, Li YM. Regulation of hepatic microRNA expression in response to ischemic preconditioning following ischemia/reperfusion injury in mice. OMICS 13: 513–520, 2009. [DOI] [PubMed] [Google Scholar]
- 120.Xu X, Kriegel AJ, Liu Y, Usa K, Mladinov D, Liu H, Fang Y, Ding X, Liang M. Delayed ischemic preconditioning contributes to renal protection by upregulation of miR-21. Kidney Int 82: 1167–1175, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ye Y, Perez-Polo JR, Qian J, Birnbaum Y. The role of microRNA in modulating myocardial ischemia-reperfusion injury. Physiol Genomics 43: 534–542, 2011. [DOI] [PubMed] [Google Scholar]
- 122.Yeh CH, Hsu SP, Yang CC, Chien CT, Wang NP. Hypoxic preconditioning reinforces HIF-alpha-dependent HSP70 signaling to reduce ischemic renal failure-induced renal tubular apoptosis and autophagy. Life Sci 86: 115–123, 2010. [DOI] [PubMed] [Google Scholar]
- 123.Yin C, Salloum FN, Kukreja RC. A novel role of microRNA in late preconditioning: upregulation of endothelial nitric oxide synthase and heat shock protein 70. Circ Res 104: 572–575, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yin C, Wang X, Kukreja R. Endogenous microRNAs induced by heat-shock reduce myocardial infarction following ischemia-reperfusion in mice. FEBS Lett 582: 4137–4142, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zarjou A, Yang S, Abraham E, Agarwal A, Liu G. Identification of a microRNA signature in renal fibrosis: role of miR-21. Am J Physiol Renal Physiol 301: F793–F801, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhang L, Liu Q, Lu L, Zhao X, Gao X, Wang Y. Astragaloside IV stimulates angiogenesis and increases hypoxia-inducible factor-1alpha accumulation via phosphatidylinositol 3-kinase/Akt pathway. J Pharmacol Exp Ther 338: 485–491, 2011. [DOI] [PubMed] [Google Scholar]
- 127.Zhao Y, Xu Y, Luo F, Xu W, Wang B, Pang Y, Zhou J, Wang X, Liu Q. Angiogenesis, mediated by miR-21, is involved arsenite-induced carcinogenesis. Toxicol Lett 223: 35–41, 2013. [DOI] [PubMed] [Google Scholar]
- 128.Zhong X, Chung AC, Chen HY, Meng XM, Lan HY. Smad3-mediated upregulation of miR-21 promotes renal fibrosis. J Am Soc Nephrol 22: 1668–1681, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zhou J, Wang KC, Wu W, Subramaniam S, Shyy JYJ, Chiu JJ, Li JYS, Chien S. MicroRNA-21 targets peroxisome proliferators-activated receptor- in an autoregulatory loop to modulate flow-induced endothelial inflammation. Proc Natl Acad Sci USA 108: 10355–10360, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhu H, Fan GC. Role of microRNAs in the reperfused myocardium towards post-infarct remodelling. Cardiovasc Res 94: 284–292, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zhu S, Deng S, Ma Q, Zhang T, Jia C, Zhuo D, Yang F, Wei J, Wang L, Dykxhoorn DM, Hare JM, Goldschmidt-Clermont PJ, Dong C. MicroRNA-10A* and microRNA-21 modulate endothelial progenitor cell senescence via suppressing high-mobility group A2. Circ Res 112: 152–164, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhu S, Si ML, Wu H, Mo YY. MicroRNA-21 targets the tumor suppressor gene tropomyosin 1 (TPM1). J Biol Chem 282: 14328–14336, 2007. [DOI] [PubMed] [Google Scholar]
- 133.Zhuo W, Liao L, Xu T, Wu W, Yang S, Tan J. Mesenchymal stem cells ameliorate ischemia-reperfusion-induced renal dysfunction by improving the antioxidant/oxidant balance in the ischemic kidney. Urol Int 86: 191–196, 2011. [DOI] [PubMed] [Google Scholar]
- 134.Ziegler-Heitbrock HW. Molecular mechanism in tolerance to lipopolysaccharide. J Inflamm 45: 13–26, 1995. [PubMed] [Google Scholar]
- 135.Zou Z, Zhang Y, Hao L, Wang F, Liu D, Su Y, Sun H. More insight into mesenchymal stem cells and their effects inside the body. Expert Opin Biol Ther 10: 215–230, 2010. [DOI] [PubMed] [Google Scholar]
- 136.Zundel W, Schindler C, Haas-Kogan D, Koong A, Kaper F, Chen E, Gottschalk AR, Ryan HE, Johnson RS, Jefferson AB, Stokoe D, Giaccia AJ. Loss of PTEN facilitates HIF-1-mediated gene expression. Genes Dev 14: 391–396, 2000. [PMC free article] [PubMed] [Google Scholar]


