Abstract
Skeletal muscle satellite cells (SCs) are involved in remodeling and hypertrophy processes of skeletal muscle. However, little knowledge exists on extrinsic factors that influence the content of SCs in skeletal muscle. In a comparative human study, we investigated the muscle fiber type-specific association between emergence of satellite cells (SCs), muscle growth, and remodeling in response to 12 wk unilateral resistance training performed as eccentric (Ecc) or concentric (Conc) resistance training ± whey protein (Whey, 19.5 g protein + 19.5 g glucose) or placebo (Placebo, 39 g glucose) supplementation. Muscle biopsies (vastus lateralis) were analyzed for fiber type-specific SCs, myonuclei, and fiber cross-sectional area (CSA). Following training, SCs increased with Conc in both type I and type II fibers (P < 0.01) and exhibited a group difference from Ecc (P < 0.05), which did not increase. Myonuclei content in type I fibers increased in all groups (P < 0.01), while a specific accretion of myonuclei in type II fibers was observed in the Whey-Conc (P < 0.01) and Placebo-Ecc (P < 0.01) groups. Similarly, whereas type I fiber CSA increased independently of intervention (P < 0.001), type II fiber CSA increased exclusively with Whey-Conc (P < 0.01) and type II fiber hypertrophy correlated with whole muscle hypertrophy exclusively following Conc training (P < 0.01). In conclusion, isolated concentric knee extensor resistance training appears to constitute a stronger driver of SC content than eccentric resistance training while type II fiber hypertrophy was accentuated when combining concentric resistance training with whey protein supplementation.
Keywords: hyperplasia, eccentric training, pax7, whey protein
skeletal muscle maintenance is essential for preservation of metabolic health and contractile function, and relies on continuous remodeling of muscle tissue initiated through both extrinsic (e.g., physical activity or dietary supplements) and intrinsic (e.g., effects of endogenous molecules released from various tissues/cells) mechanisms. An essential aspect of muscle remodeling and repair is a functional population of quiescent muscle-specific stem cells, commonly referred to as satellite cells (SCs) (7, 46, 68). Since the discovery and proposed role as a resident pool of quiescent myogenic stem cells (40), the SCs have been shown to possess a critical role in animal skeletal muscle regeneration (34, 65), during which SCs are activated, proliferate, and differentiate to support regeneration and new myofiber formation. In agreement, several studies on human skeletal muscle have demonstrated a proliferative SC response during exercise-induced muscle regeneration and remodeling following high-intensity eccentric exercise (13, 14, 37, 44), in which the SC cell-cycle progression is regulated by transcription factors, such as the paired box transcription factor 7 (Pax7), known to induce expression of specific myogenic regulatory factors such as MyoD (55, 68).
While the role of SCs during skeletal muscle regeneration is generally accepted, the need for SC involvement in muscle hypertrophy in response to overloading (i.e., resistance exercise) is more debated (7, 24, 27, 41, 49). In earlier rodent studies, an impaired hypertrophic capacity following irradiation was observed to be associated with limited proliferation of SCs and addition of nuclei to existing myofibers (3, 49). However, these results have been challenged by recent study reports demonstrating that 2 wk of functional muscle overloading (synergist ablation) in rodents can promote hypertrophy despite conditional blockage of Pax7+ SCs (41). Yet, despite no immediate imperative need for SCs and accretion of nuclei to induce myofiber hypertrophy during the initial hypertrophic phase in rodents, other recent findings still render possible that the SCs may be involved during the later phases of myofiber hypertrophy (2, 24), possibly related to maintaining a constancy in myonuclei domain size (49, 52). Furthermore, as recently shown in rodents, the additional myonuclei generated from activated SCs following resistance exercise may possess functional importance by augmenting the regain of muscle mass following a period of muscle atrophy (9). The latter supports the notion that SCs can be regarded as important in promoting and sustaining myofiber hypertrophy.
The importance of SCs in myofiber hypertrophy and remodeling is indicated in human exercise studies as well, in which the SC pool has been shown to expand acutely following traditional hypertrophy-inducing exercise [i.e., within hours to days; (43, 56)] as well as following accumulated resistance exercise bouts [i.e., after weeks to months (32, 36, 38, 50, 52, 60)]. However, while some studies have reported accretion of myonuclei following resistance training in humans (48, 50, 52), others have failed to observe this accretion (32, 36, 38), which may be related to the relative magnitude of fiber hypertrophy induced by the training protocol (50, 52). Furthermore, whereas traditional resistance training is inherent of both eccentric and concentric muscle actions, the results of some studies indicate that isolated eccentric contractions may promote greater single-fiber hypertrophy than isolated concentric contractions in untrained (29) as well as trained (62) individuals. In particular, the type II muscle fibers have been shown to exhibit an augmented responsiveness to the eccentric resistance training (29, 62), a finding which is also common following a period of heavy traditional resistance training (19, 21). We have recently demonstrated that eccentric and concentric resistance exercise may acutely evoke divergent responses in relation to stretch-mediated signaling (64) as well as divergent regulation of atrogene/FOXO activity (57), which may collectively influence myofibrillar protein turnover. However, while exhibiting differences in the signaling responses following acute exercise (57, 64), the two muscle actions were equally capable of inducing whole muscle hypertrophy (as measured by magnetic resonance imaging) following 12 wk resistance training (20). Importantly, it is to our knowledge unknown if the two muscle actions differently regulate satellite cell proliferation and accretion of myonuclei. In relation to this, we speculate that the strain on the extracellular matrix, myofibers, and the satellite cells from eccentric muscle actions (13, 14, 35) may more profoundly influence satellite cell proliferation (59) than the stimuli from concentric muscle actions.
In addition to exercise contraction mode, protein supplementation combined with traditional resistance training is known to augment muscle growth (10). Moreover, we recently reported that whey protein supplementation augments whole muscle hypertrophy following both eccentric and concentric resistance training (20). While whey protein is known to stimulate muscle protein synthesis (6), a second mechanism through which whey protein may influence muscle growth is the proliferation of SCs. In regards to the latter speculation, the amino acid leucine is known to stimulate the kinase complex mTORC1 (16), and through this pathway leucine administration may, at least during in vitro conditions, stimulate SC proliferation (28). In vivo protein ingestion combined with long-term traditional resistance training may also augment skeletal muscle SC content (50). We therefore speculate that protein ingestion combined with eccentric resistance training may comprise a potent regulator of satellite cell proliferation and myonuclei accretion.
The objectives of the present study were 1) to investigate if eccentric resistance training [i.e., inherent of a high degree of mechanical stress (53) and/or strain (35)] modulates the SC pool differently compared with concentric resistance training [i.e., the latter being more metabolically demanding (23)]; 2) to investigate if the SC proliferation as well as the accretion of myonuclei is fiber type dependent; and 3) to investigate if intake of whey protein hydrolysate could augment the SC pool expansion, myonuclei accretion and myofiber hypertrophy.
We hypothesized 1) that eccentric exercise resistance training would induce a greater accumulation of SCs, greater accretion of myonuclei, and greater fiber CSA hypertrophy in type II muscle fibers compared with concentric resistance training; and 2) that the fiber type-specific response of SCs, myonuclei, and fiber CSA would be further augmented with provision of a whey protein hydrolysate supplementation.
METHODS
Ethical approval.
All subjects were informed of the purposes and risks of the study and provided written informed consent in accordance with the Declaration of Helsinki, and the study protocol was approved by The Central Denmark Region Committees on Health Research Ethics (j. no. M-20110003).
Participants.
Twenty-two healthy young men were included in the study (means ± SE: height 181.5 ± 1.5 cm, body mass 78.1 ± 1.8 kg, age 23.9 ± 0.8 yr, body fat 16.0 ± 0.9%). Exclusion criteria were 1) participation in systematic resistance or high-intensity training for lower extremity muscles within 6 mo prior to participation; 2) a history of musculoskeletal lower extremity injuries; 3) vegans; and 4) use of dietary supplements or prescription medication that potentially could influence muscle size or satellite cell function.
Experimental design.
This 12-wk training study was conducted in a double-blinded fashion in relation to dietary supplementation. Following inclusion, subjects were randomly allocated into either a whey protein hydrolysate group (Whey, n = 11) or an isocaloric carbohydrate placebo group (Placebo, n = 11). Regardless of supplementary intake, all subjects performed eccentric (Ecc) training with one leg and concentric (Conc) training with the other (i.e., total legs n = 44). Accordingly, the following four interventions were compared: Whey + Eccentric (Whey-Ecc), Placebo + Eccentric (Placebo-Ecc), Whey + Concentric (Whey-Conc), and Placebo + Concentric (Placebo-Conc). The eccentric leg was randomly chosen to be either the dominant (preferred kicking leg) or the nondominant leg, to exclude any potential pretraining difference between the two. Throughout the study period, the subjects were instructed to maintain habitual physical activity level and dietary intake. Subjects were instructed not to engage in high-intensity activities 48 h before pre- and posttraining tests/measurements, not to consume alcohol, and to maintain normal habitual dietary intake (to minimize fluid shift). All tests were performed at the same time of the day, pre- and posttraining, to control for potential effects of diurnal and circadian rhythm.
Resistance training and supplementation.
The details of the resistance training program and peptide/amino acid profile of the whey protein have been described previously (20). In brief, the subjects completed 33 resistance training sessions during the 12 wk, with each session consisting of 6–12 sets × 6–15 repetitions (with repetition loading equal to repetition maximum), with identical set and repetition numbers for the eccentric and concentric legs. The exercise consisted of isolated knee extensions performed in a Technogym knee extensor machine (Technogym-Selection line, Technogym, Italy) with all repetitions conducted in a 75–85° range of motion and a 2- to 3-s tempo. All training sessions were closely supervised and monitored to ensure proper execution and loading. The average training load was 11.0 ± 0.8% and 10.3 ± 0.8% greater in the eccentric leg compared with the concentric leg for the Whey and Placebo groups, respectively.
On training days the subjects received an 8% solution (663 kJ) drink containing either 19.5 g hydrolyzed whey protein + 19.5 g of carbohydrate (Whey group) or 39 g of carbohydrate (Placebo group). Half of the supplement was ingested immediately before exercise and the remaining half immediately after exercise. The whey protein (Arla Foods Ingredients Group P/S, Viby J, Denmark) contained 27.7% branched-chain amino acids (BCAA; leucine 14.2%, isoleucine 6.6%, valine 6.9%) and 53.3% essential amino acids.
Sample collection and preparation.
Pre- and posttraining muscle biopsies were obtained under local anesthesia (10 mg/ml lidocaine) from the middle lateral part of the vastus lateralis muscle by applying the Bergstrom needle technique as described previously (63). At pretraining, one biopsy was obtained representing the basal level from both legs (randomly distributed between the Ecc and Conc leg), while at posttraining a biopsy from each leg was extracted. All pre- and posttraining muscle biopsies were obtained between 8 am and 12 pm following an overnight fast, and posttraining biopsies were collected 3–6 days after the final training session. The samples were dissected free of visible fat and connective tissue. A well-aligned portion of the biopsy was immediately mounted in Tissue-Tek (Qiagen, Valencia, CA), frozen in isopentane precooled with liquid nitrogen, and stored at −80°C until further analysis.
All biopsies were assigned a random unique identification number, thereby blinding the investigator to subject identity and time point. Serial transverse sections (10 μm) were cut at −20°C using a cryostat and placed onto Superfrost Plus glass slides (Menzel-Gläser, Braunschweig, Germany) with both pre- and posttraining samples from one subject on the same slide.
Satellite cell and myonuclei analysis.
Muscle biopsy sections were fixed in Histofix (Histolab, Gothenborg, Sweden) followed by 1.5 h in blocking buffer (0.2% Triton X, 2% BSA, 5% FBS, 2% goat serum, and 0.1% sodium azide). The sections were incubated overnight at 4°C with primary antibody for Pax7 (1:500; cat. no MO15020, Neuromics, Edina, MN), followed by 1.5 h in secondary Alexa Fluor 568 goat anti-mouse antibody (Molecular Probes, cat no. A11034, Invitrogen A/S, Taastrup, Denmark). Following this, the sections were incubated with primary antibodies for Type I myosin [1:500; cat. no. A4.951, Developmental Studies Hybridoma Bank (DSHB)] and laminin (1:500; cat. no. Z0097, Dako Norden) for 2 h and secondary Alexa Fluor 488 goat anti-mouse green and Alexa Fluor 488 goat anti-rabbit green (Molecular Probes, cat no. A11031 and cat no. A11034, Invitrogen A/S, Taastrup, Denmark) antibodies for 1 h. Finally, a mounting media containing 4′,6-diamidino-2-phenylindole (DAPI) was utilized to visualize nuclei (Molecular Probes Prolog Gold anti-fade reagent, cat. no. P36935, Invitrogen A/S), and samples were stored at −20°C until final analyses. Staining was verified using appropriate negative controls to ensure specificity.
Images were obtained at 20× magnification using a Leica DM2000 microscope (Leica, Stockholm, Sweden) and a Leica Hi-resolution Color DFC camera (Leica, Stockholm, Sweden). The number of Pax7 positive (Pax7+) cells (SCs) associated with type I (A4.951+) or type II (A4.951−) fibers was quantified separately and expressed relatively to the total number of type I or II fibers and fiber area (SC/mm2). To ensure reliable numbers of SCs, in accordance with Mackey et al. (39), we counted a mean of 246 ± 3 fibers.
Finally, in accordance with Bruusgaard et al. (8), we utilized these sections to quantify total sublaminar nuclei (assumed to be myonuclei) by only counting Pax7 negative nuclei with a visible geometric center within the basal lamina to ensure that nonmyonuclei were not counted. The myonuclei content from total type I and type II fibers was enumerated and the SC content was additionally normalized to these.
Single-fiber area and phenotype.
Sections were stained as described above to display fiber basal lamina (laminin, Dako Norden) and enable categorization into type I (A4.951+, DHSB) or type II (A4.951−) fibers. The sections were visualized and analyzed using a Leica DM2000 microscope and a Leica Hi-resolution Color DFC camera combined with image-analysis software (Leica Qwin ver. 3.2, Leica, Stockholm, Sweden) with the investigator blinded to pre/post samples and subject information. Only fibers cut perpendicularly to their longitudinal axis were used in the determination of fiber size in accordance with Andersen and Aagaard (4). The mean number of fibers used for the area analysis was 280 ± 10.
Muscle fiber regeneration/remodeling.
Regeneration/remodeling was assessed by staining of biopsy sections for embryonic myosin heavy chain (F1.652, Developmental Studies Hybridoma Bank) or neonatal myosin heavy chain (NCL-MHCn, Novocastra, Newcastle upon Tyne, UK) combined with laminin (Dako Norden). Following 1.5 h in blocking buffer (0.2% Triton, 2% BSA, 5% FBS, 2% goat serum, and 0.1% sodium azide), the sections were incubated in primary antibody overnight at 4°C (F1.652 + laminin) or for 2 h at room temperature (NCL-MHCn + laminin), followed by secondary Alexa Fluor 568 goat anti-rabbit and Alexa Fluor 488 goat anti-mouse (Molecular Probes, cat no. A11011 and cat no. A11001) antibodies for 1.5 h, and were finally mounted in media containing DAPI to visualize nuclei. Again staining was verified using negative controls to ensure specificity. The number of fibers positive for embryonic or neonatal myosin heavy chain was expressed relative to total fiber number from the sections.
Magnetic resonance imaging.
The details of this method have been reported previously (20). In brief, imaging was performed with a 1.5-T scanner (Philips Achieva, Best, Netherlands) in which the subjects were placed in supine position with the feet entering the scanner first. After an initial frontal survey scan, 50 transversal slices were acquired, with the first slice located 70 mm proximal the femur condyles and the other slices acquired proximally. A T1-weighted, fast spin echo sequence with the following parameters was used: scan matrix = 576 × 576, field of view = 46 × 46 cm, number of slices = 50, slice thickness = 7 mm, slice gap = 3 mm, repetition time = 2 s, echo train length = 18, number of signal averages = 2, TR = 500 ms, TE = 6.2 ms, and pixel size = 0.8 × 0.8 mm. The later offline analysis was conducted using a free software program (Osirix 4.1.1, Osirix Foundation, Geneva, Switzerland). Knee extensor muscle CSA was manually outlined at one-half of the femur length corresponding to the area from which the biopsies were obtained and indicative of the overall change in whole muscle size (20).
Muscle contractile performance.
Subsequent to a standardized warm-up consisting of 5 min light aerobic exercise (100 W) on a stationary bicycle (Monark, Varberg, Sweden), the subjects were seated in an isokinetic dynamometer (Humac Norm, CSMI, Stoughton) with 90° hip flexion and restraining straps crossing the torso and nontesting leg. The transverse axis of the subject's knee was aligned with the axis of the dynamometer. Subjects were instructed to grab the chair handles. The dynamometer was adjusted individually so the contact point between the subjects' leg and the dynamometer arm was 3 cm proximal to the malleolus medialis. Eccentric and concentric muscle strength was evaluated using a knee joint angular speed of 30°/s (5–90° range of motion, 0° equals full knee extension), and isometric strength was evaluated at 70°. All recorded peak moments were gravity corrected by measuring the passive moment exerted by the gravitational pull on lower leg around the knee joint. The mean peak torque obtained at three trials within each condition was used for further analysis. All trials were sampled at 1,500 Hz.
Data presentation and statistical analysis.
The sample size in the present study was, in part, based on the ability to detect a difference between interventions of 20–40% in myofiber hypertrophy and SC content (α = 0.05, power = 0.8). Utilizing these estimates and SDs from previous studies (38, 48, 50), a sample size of 9–12 subjects was sufficient to detect a difference. Following check for normality of distribution and tests of equal variance, data were expressed as means ± SE or individual plots and median bars for eMHC and nMHC data. The effect of time (Pre vs. Post), supplementation (Whey vs. Placebo), and contraction mode (Ecc vs. Conc), and their interactions on dependent variables, were assessed using a mixed-effect linear model with repeated measures for time and contraction mode by using subject ID as a random effect variable. The mixed-effect analyses for all biopsy-derived parameters were conducted on calculated prepost differences. When interactions were observed, linear comparison analysis, extracted from the mixed-effect linear model, was used to evaluate differences between conditions, and paired t-test was used to evaluate time effects within conditions. Since eMHC and nMHC data showed a nonparametric distribution, we performed a Kruskal-Wallis test to examine pre-post differences between groups and Wilcoxon-Mann-Whitney test to examine for differences across time. To describe the association between whole muscle and single-fiber cross-sectional area changes, a Pearson product-moment correlation analysis was employed. Alpha level was set to P ≤ 0.05. All statistical analyses were performed using Stata (Stata v 12.0, StataCorp LP), and all graphs were designed in GraphPad Prism (version 6.0, San Diego, CA).
RESULTS
Fiber type-specific SCs.
The SC response to the different exercise training modalities and supplementation regimes were quantified from the Pax7 (SCs)/MHC-I/Laminin/DAPI immunofluorescent staining (exemplified in Fig. 1). Conc training elicited increases in type I fiber SCs of 132 ± 21% and 78 ± 30% (P < 0.001) in the Whey and Placebo group (Fig. 2, A and C), respectively. This was greater than with Ecc training (P < 0.05), where tendencies (P = 0.08) toward increases were observed (110 ± 62% and 24 ± 32% in the Whey and Placebo group, respectively). For SCs associated with type II fibers, Conc training increased the SC content by 78 ± 26% and 81 ± 43% (P < 0.01) in the Whey and Placebo group, respectively, which was greater than Ecc training (P < 0.05), which did not evoke any changes (Fig. 2, B and D).
Fig. 1.
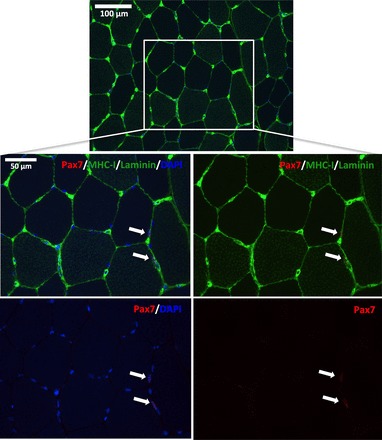
Representative image of a muscle cross-section stained for Pax7 (red), MHC-I (green), laminin (green), and nuclei (DAPI; blue). Boxed area is magnified in the bottom panels displaying two Pax7 nuclei (SCs) localized to a type I fiber and a type II fiber, respectively. Arrows indicate the location of the SCs.
Fig. 2.
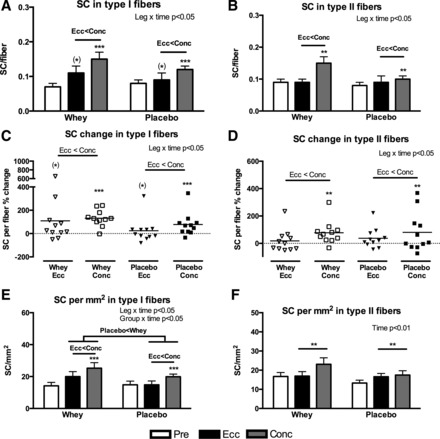
Satellite cells (SCs) associated with type I or II fibers expressed per fiber (A and B), as individual and mean relative change per fiber (C and D) or mm2 fiber area (E and F) evaluated from biopsies obtained pre and post 12 wk of eccentric (Ecc) or concentric (Conc) training combined with either whey protein (Whey) or isocaloric carbohydrate (Placebo) supplementation. Overall interactions and time effects are displayed in top right corner and overall differences between contraction modes (Ecc < Conc) or supplementation types (Whey > Placebo) are shown with “>” or “<” symbols. Significant difference from pretraining are denoted by ** (P < 0.01) or *** (P < 0.001) and tendencies by (*) (P < 0.1).
The number of SCs per square millimeter type I fiber area increased in the Conc leg by 90 ± 16% and 56 ± 23% (P < 0.001) in the Whey and Placebo groups, respectively, which was greater than the Ecc legs (P < 0.05, Fig. 2E), where no changes were observed. Furthermore, a greater overall increase in the Whey group compared with the Placebo group (P < 0.05) was noted. In contrast, no between-group differences were observed in SCs per square millimeter type II fiber area, in which we only found an overall training effect (P < 0.01) with Conc training increasing by 39 ± 14% and 72 ± 42% and Ecc training increasing by 14 ± 23% and 35 ± 16%, in the Whey and Placebo group, respectively (Fig. 2F).
As for SCs/myonuclei (data not shown), a leg × time (P < 0.05) and a group × time (P < 0.01) interaction was observed in I fibers. Accordingly, the Whey group increased by 109 ± 23% and 113 ± 55% (P < 0.001) in the Conc and Ecc leg, respectively, whereas no changes were observed in the Placebo group. Post hoc analysis revealed a greater overall increase in the Whey compared with the Placebo group (P < 0.01) and a greater overall increase in Conc compared with Ecc legs (P < 0.05). As for type II fibers, only a tendency toward a leg × time interaction (P = 0.06) was observed.
Fiber type-specific myonuclei.
From the immunofluorescent SC staining we determined the number of myonuclei in a fiber type-dependent manner. In accordance with the changes in type I fiber area, we observed an overall accretion of myonuclei in type I fibers (P < 0.01) with no differences between groups (Fig. 3, A and B). In contrast, in the Whey group, an accretion of myonuclei in type II fibers was observed (Fig. 3, C and D) with Conc training (28 ± 9%, P < 0.01), while Ecc training did not influence myonuclei content. As a consequence, the increase with Conc training was greater than with Ecc training (P < 0.01). Additionally, in the Placebo group, an accretion of myonuclei was observed in type II fibers with Ecc training only (29 ± 8%, P < 0.01).
Fig. 3.
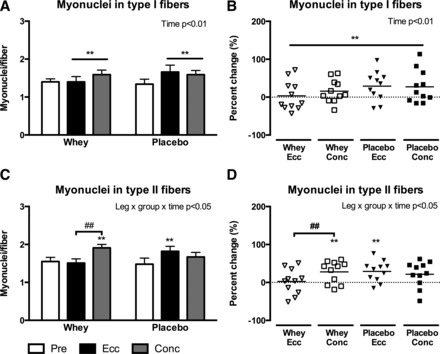
Myonuclei per fiber in type I (A and B) and type II (C and D) fibers evaluated from biopsies obtained pre and post 12 wk of eccentric (Ecc) or concentric (Conc) training combined with either whey protein (Whey) or isocaloric carbohydrate (Placebo) supplementation. Data are presented as means ± SE (A and C) or relative individual change and mean bars (B and D). Overall interactions or time effects are displayed in top right corner. Significant differences from pretraining is denoted by ** (P < 0.01) and significant difference between groups are denoted by ## (P < 0.01).
Mean fiber and fiber area frequency distribution.
To describe the fiber cross-sectional area (CSA) changes in detail, we quantified fiber type-specific mean fiber area and area frequency distribution. As depicted in Fig. 4, type I fibers increased similarly with Conc training (22 ± 6% and 12 ± 5%) as well as Ecc training (14 ± 6% and 16 ± 8%) (P < 0.001) in the Whey and Placebo group, respectively. In contrast type II fiber CSA increased exclusively in the Whey-Conc group by 25 ± 7% (P < 0.01) and this was significantly greater than both the Whey-Ecc (P < 0.01) and Placebo-Conc (P < 0.05) groups.
Fig. 4.
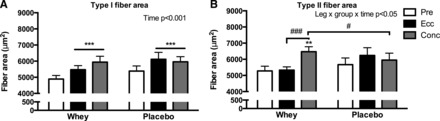
Fiber area (μm2) in type I (A) and type II (B) fibers evaluated from biopsies obtained pre and post 12 wk of eccentric (Ecc) or concentric (Conc) training combined with either whey protein (Whey) or isocaloric carbohydrate (Placebo) supplementation. Data are presented as means ± SE. Overall interactions or time effects are displayed in top right corner. Significant difference from pretraining are denoted by ** (P < 0.01) or *** (P < 0.001) and significant difference between groups are denoted by # (P < 0.05) or ### (P < 0.001).
Using area-frequency plots (Fig. 5, A and B), a greater proportion of larger type I fibers were noted as reflected by a right shift of the mean area-frequency curve, in all groups from pre- to posttraining. However, as for type II fibers in the whey group, the Conc post training leg contained a relatively higher quantity of large fibers, which resulted in a right shift of the area-frequency curve compared with the Ecc post training leg (Fig. 5, C and D). To examine if these observations differed significantly between the different exercise modes, we quantified the percentage of large (>8,000 μm2) and small (fibers >1,000 μm2 and < 5,000 μm2) fibers. As shown in Fig. 5E, the frequency of large type II fibers increased selectively with Whey-Conc from 5 ± 2% to 20 ± 4% (P < 0.01), which was greater than with Whey-Ecc (P < 0.01), where no changes were observed. In contrast, the proportion of small fibers (Fig. 5F) remained relatively constant with Whey-Ecc (pre 45 ± 9% vs. post 45 ± 6%) while a decrease was noted in the Whey-Conc (pre 45 ± 9% and post 21 ± 4%, P < 0.05) group. Hence the proportion of small fibers was greater in the Whey-Ecc than the Whey-Conc at posttraining (P < 0.001).
Fig. 5.
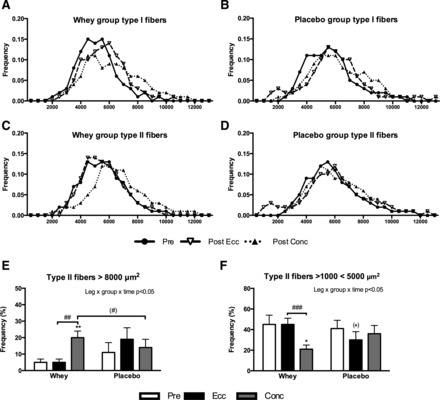
Fiber area frequency distribution shown as mean curves in type I (A and B) and type II (C and D) fibers or as percentage of “large” (>8,000 μm2) type II fibers (E) or “small” (> 1,000 < 5,000 μm2) type II fibers (F) evaluated from biopsies obtained pre and post 12 wk of eccentric (Ecc) or concentric (Conc) training combined with either whey protein (Whey) or isocaloric carbohydrate (Placebo) supplementation. A–D are presented as mean curves, and E and F are presented as means ± SE. Overall interactions are displayed in top right corner. Significant difference from pretraining are denoted by * (P < 0.05) or ** (P < 0.01) and tendencies by (*) (P < 0.1). Significant difference between groups are denoted by ## (P < 0.01) or ### (P < 0.001) and tendencies by (#) (P < 0.1).
Changes in single-fiber CSA vs. whole muscle CSA.
The changes in whole muscle CSA have been reported by our group previously (20). To investigate whether single-fiber hypertrophy correlated with whole muscle hypertrophy following Conc and Ecc resistance training, we correlated the relative change in mean and type II fiber CSA with the relative change in whole muscle CSA (quantified by MRI scans). As shown in Fig. 6, A and C, significant correlations were observed in the Conc leg for both mean fiber (r = 0.45, P < 0.05) and type II fibers (r = 0.56, P < 0.01), while for the Ecc leg (Fig. 6, B and D), no association was observed with mean fiber (r = −0.03, P = 0.90) or type II fibers (r = 0.04, P = 0.87).
Fig. 6.
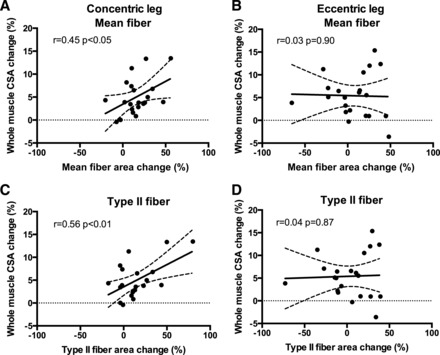
Correlations and scatterplots of relative changes in whole muscle cross-sectional area (CSA) and relative changes in mean (A and B) or type II (C and D) fiber CSA evaluated from magnetic resonance imaging and muscle biopsies obtained pre and post 12 wk of Eccentric (B and D) or Concentric (A + C) training combined with either whey protein or isocaloric carbohydrate supplementation (data are merged for supplementation). Dashed line denotes the 95% confidence intervals. Pearson correlation coefficients and significance are shown for each correlation.
Muscle fiber regeneration/remodeling.
To evaluate muscle fiber regeneration, remodeling and de novo fiber formation, we quantified the content of embryonic (F1.652) and neonatal (NCL-MHCn) MHC-positive fibers (Fig. 7, C and D).
Fig. 7.
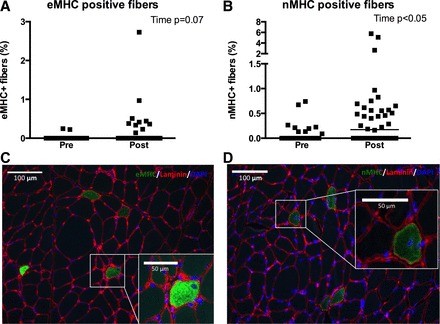
Fibers positive for embryonic myosin heavy chain (eMHC; A and C) or neonatal myosin heavy chain (nMHC; B and D) relative to total fibers counted (%) evaluated from biopsies obtained pre and post 12 wk of eccentric or concentric training combined with either whey protein or isocaloric carbohydrate supplementation. Since no group or leg interactions were observed data are collapsed for group and leg. Data are presented as individual values and median bars. Overall effects of time (or tendencies) are displayed in top right corner. Images of eMHC (C, green) and nMHC (D, green) positive fibers are obtained from one subject's eccentric posttraining leg containing high amounts of these fibers.
Quantification of eMHC+ or nMHC+ fibers revealed no statistical difference between the four groups and therefore the groups were collapsed graphically (Fig. 7, A and B). An overall tendency toward an increase in eMHC+ fibers (P = 0.07) and a significant increase in nMHC+ fibers (P < 0.05) from 0.0% at pretraining to 0.2% (median) posttraining was observed.
Dynamic and isometric muscle strength.
Maximum torque (N·m) obtained during eccentric, isometric, and concentric contractions from each group are shown in Table 1. Eccentric and isometric strength increased from pre- to posttraining by 13 ± 3% (P < 0.001) and 16 ± 4% (P < 0.001), respectively, with no effect of contraction mode or supplementation. Concentric strength showed a group × time interaction (P < 0.05) and increased by 13 ± 4% with Conc (P < 0.01), whereas no changes were observed with Ecc.
Table 1.
Peak torque (N·m) during eccentric (−30°/s), isometric (0°/s), or concentric (30°/s) contractions
| Whey |
Placebo |
|||||||
|---|---|---|---|---|---|---|---|---|
| Ecc |
Conc |
Ecc |
Conc |
|||||
| Pre | Post | Pre | Post | Pre | Post | Pre | Post | |
| Concentric (30°/s), N·m | 247 ± 10 | 253 ± 13 | 247 ± 13 | 264 ± 15* | 257 ± 16 | 268 ± 18 | 243 ± 19 | 291 ± 17* |
| Isometric (0°/s), N•m | 282 ± 15 | 299 ± 17† | 282 ± 16 | 330 ± 16† | 298 ± 17 | 329 ± 18† | 282 ± 19 | 341 ± 18† |
| Eccentric (−30°/s), N•m | 349 ± 19 | 384 ± 20† | 347 ± 17 | 376 ± 21† | 364 ± 25 | 394 ± 25† | 310 ± 32 | 370 ± 32† |
Values are means ± SE. Peak torque evaluated as a mean of three trials in an isokinetic dynamometer pre and post 12 wks of eccentric (Ecc) or concentric (Conc) resistance training combined with either whey protein (Whey) or isocaloric carbohydrate (Placebo) supplementation. Significant difference from pretraining:
P < 0.01,
P < 0.001.
DISCUSSION
An important aspect of skeletal muscle growth and remodeling processes is related to the content and function of the SCs. Therefore, identification of extrinsic factors, which can modulate SC proliferation as well as SC differentiation, is essential. Furthermore, as much of the current understanding on SCs is founded on in vitro and animal models, these important questions should also be pursued in vivo in human skeletal muscle.
In the present human study, we report on the effects of two extrinsic variables potentially affecting SCs, i.e., exercise contraction mode and dietary supplementation type. Here we report that Conc resistance exercise conducted during a prolonged period represents a potent modulator of skeletal muscle SC content. Accordingly, one main finding was the observation that Conc resistance training constitutes a stronger stimulator than Ecc resistance training for increasing the SC pool in both type I and II fibers. Another main finding was the specific increase in type II fiber CSA when Conc resistance exercise was combined with whey protein supplementation, which was accompanied by accretion of myonuclei. Finally, in opposition with our initial research hypothesis, in which we expected the Ecc resistance training to constitute a superior inducer of SC proliferation and type II fiber hypertrophy (compared with Conc resistance training), Ecc resistance training did not increase SC content or fiber CSA in type II fibers.
Resistance training contraction mode may modulate fiber type-specific SC content.
The observation that isolated Conc resistance training can act as a potent inducer of skeletal muscle SC content suggests that eccentric contractions per se are not necessary for increasing the skeletal muscle SC content. This finding is supported by a recent study in which training, characterized by concentric contractions (i.e., high-intensity cycling), increased SC proliferation, in particular in fibers coexpressing MHC-I and MHC-II (30). Combined with the results from the present study, this indicates that fiber growth and fiber remodeling in response to Conc contractions likely involve the SCs (30). The greater increase in SC content with Conc compared with Ecc resistance training is interesting and may adhere to several mechanisms; one such could be the greater metabolic requirement with concentric compared with eccentric exercise (1) leading to a greater lactate accumulation during concentric exercise (17), which has been shown to influence SC proliferation in vitro (67). Alternatively, expression of growth factors, known to influence SC proliferation [e.g., IGF-I (26, 28, 42)], may diverge between muscle actions. In this regard, we have previously shown that transcription of IGF-1 (specifically IGF-1Ec), a growth factor associated with SC proliferation and increases in myogenic regulatory factors (42), is more profoundly upregulated following concentric compared with eccentric resistance exercise (64).
When normalizing the SC content to fiber area (SC/mm2), Conc resistance training displayed a greater increase in SCs/mm2 in type I fibers compared with Ecc resistance training, whereas no group differences were observed in type II fibers. The interpretation of this fiber type-specific SC regulation is not straightforward. One explanation may relate to different SC subpopulations (e.g., more proliferating SCs at the time of biopsy sampling) for type I compared with type II fiber SCs. Alternatively, it could be speculated that a greater aerobic metabolism associated with type I fibers may increase reactive oxygen species (ROS) production, which again may influence SC activity as shown in vitro (67). As concentric exercise does require more ATP production and glycogen utilization compared with eccentric exercise (23), the former may be more prone to increasing the ROS production. However, these speculations will require more detailed investigation in vivo in humans to understand the mechanisms underlying the present study findings.
With regards to Ecc resistance training, the lack of SC pool expansion was in opposition to our initial hypothesis. Accordingly, eccentric contractions in human skeletal muscle are known to induce a mild-to-moderate muscle injury (13, 51) or even no injury (14), which in either scenario is associated with an increased proliferation of SCs in the days postexercise (13, 14, 37, 44, 45). The exercise-induced myofiber damage has been observed to be more prevalent in type II fibers in rodents (61), supported by an increased proliferation of SCs associated with type II fibers in humans 24 h post-eccentric exercise (11). Whether such acute changes can be extrapolated to chronic adaptations with prolonged Ecc training is, to our knowledge, unknown. However, the current results with Ecc resistance training do not seem to support this notion. Although we did not obtain biopsies during the early stages of the training period, with reference to the literature, we can reasonably assume that the SCs in the Ecc leg have been activated and undergone proliferation during the very early phase of the training period. Furthermore, the accretion of myonuclei in type II fibers, observed in the Placebo-Ecc group, which was accompanied by a tendency (P = 0.11) toward an increase in fiber CSA, also supports this notion. Surprisingly, we did not observe any changes in myonuclei or SC content in type II fibers in the Whey-Ecc group. The lack of increase in both myonuclei and SC content in the Whey-Ecc group is, however, in accordance with the lack of hypertrophy of type II fibers in the Whey-Ecc group. Notably, when normalizing the SC content to type II fiber area, all groups increased equally, indicating that fiber size may be a significant determinant of the SC content, in accordance with previous findings (58).
The mechanism underlying the lack of increase in SCs/fiber following eccentric resistance training, combined with the increase in SC/mm2, might relate to an enhanced ability of eccentric exercise to effectively promote SC differentiation by allowing the SCs to enter the myogenic differentiation program (68) and fuse with other myoblasts to form new myofibers. However, it is difficult to provide strong evidence to back this speculation based on an in vivo human model.
Whey protein supplementation may accentuate fiber CSA and SC content in a fiber type-specific manner.
The results from our CSA analysis suggest that while type I fibers exhibit an increase in CSA following resistance training in general, the type II fiber CSA is exclusively increased when Conc resistance training is combined with whey protein supplementation. The differentiated fiber type response is interesting, although somewhat in contrast to the observation that traditional resistance training without protein supplementation predominantly increases type II fiber CSA (21). In this regard, whey protein supplementation has previously been shown to augment muscle hypertrophy induced by traditional resistance training (5, 6, 10, 18, 20). However, to our knowledge, it is unknown whether type II fibers are more influenced by amino acid availability immediately postexercise compared with type I fibers. Previous studies have indicated that limited amino acid availability in the hours before and after resistance exercise may limit myofiber growth (5, 18). Moreover, a recent observation in rodents suggests that protein synthesis is more decreased in the faster fiber types compared with the slower fiber types in response to food deprivation (25). Thus, in relation to the results from the present study, we speculate that type I fibers are less dependent on immediate supply of amino acids following resistance exercise, compared with type II fibers.
While whey protein combined with Conc resistance training was observed to increase type II fiber CSA, this was not the case when whey protein was combined with Ecc resistance training in contrast to some earlier studies (29, 62). We have previously shown that prolonged Conc and Ecc resistance training are equally capable of promoting whole muscle hypertrophy when combined with protein supplementation (20), indicating a discrepancy between the findings on whole muscle hypertrophy vs. single-fiber hypertrophy. The observed changes in type II fiber CSA were reflected in the fiber area frequency analysis, in which the Whey-Conc group displayed an increase in the percentage of larger type II fibers compared with the Whey-Ecc group, wherein the frequency of smaller fibers was maintained. Moreover, the correlation analysis displayed a significant association between myofiber and whole muscle hypertrophy following Conc training, which was not present following Ecc training. A similar finding was recently reported in a human training study on elderly subjects (47) in which whole muscle hypertrophy (evaluated by DXA-scan) was positively associated with single-fiber hypertrophy following traditional resistance training, while no association was present following eccentric ergometer training. Based on these findings, it was proposed that intense training, and perhaps in particular eccentric training may promote whole muscle growth by sarcomere addition or hyperplasia, whereas traditional resistance training may primarily increase the fiber CSA (47). Hyperplasia has been demonstrated in rodent and avian skeletal muscle following overload or chronic long-term stretch (33). However, direct evidence for the hypothesis in human skeletal muscle is very limited, since only reports on indirect measures, such as fascicle length or correlation between myofiber and whole muscle hypertrophy, is currently available from in vivo human studies (15, 22, 47, 54).
In an effort to provide information on the extent of regeneration, remodeling and de novo fiber synthesis we quantified fibers expressing eMHC and nMHC, since extensive injury or de novo synthesis in rodent skeletal muscle is associated with increased expression of these MHC isoforms (12, 31, 66). Prior to initiation of training, very few eMHC and nMHC positive fibers were found and while we did observe an increase with training, these changes were not dependent on the type of intervention. However, as this study only provides data before and after 12 wk training, this may limit the ability to detect changes in eMHC or nMHC positive fibers. Furthermore, even when positive for eMHC or nMHC, we cannot specifically determine if this is related to regeneration, remodeling, or de novo fiber synthesis.
As for added effects of supplementation on SC content, a greater overall increase in SCs/mm2 and SCs/ myonuclei were observed with Whey compared with Placebo supplementation in type I fibers, but not in type II fibers. Currently, the literature on the effects of protein ingestion in combination with resistance training on SCs is very scarce. In one of the few studies, Olsen et al. (50) observed an increased SC content (no discrimination between fiber types) when combining resistance exercise with protein supplementation compared with resistance exercise alone. However, whether this increase was related to a direct effect of the amino acids on the SCs or indirectly related to the greater fiber hypertrophy from the protein supplementation is not entirely clear. Conversely, Snijders et al. (56) observed no difference in SC proliferation between a very low and a normal protein intake group 12–72 h following traditional resistance exercise; however, such acute observations are not necessarily indicative of the long-term effects.
In conclusion, unilateral isolated concentric resistance training displayed a greater capacity for increasing the SC pool in both type I and type II fibers compared with eccentric resistance training, independent of supplementary protocol. Conversely, type II fiber hypertrophy was exclusively observed when concentric resistance training was combined with whey protein supplementation, suggesting that both contraction mode and whey supplementation influence type II fiber growth. Finally, while type II fiber hypertrophy appeared to be influenced by exercise contraction mode and whey supplementation, type I fiber CSA and myonuclei content increased independent of intervention type.
GRANTS
The study was funded by Arla Foods Ingredients Group P/S, DK.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.F., S.K.R., M.H.V., F.V.d.P., and K.V. conception and design of research; J.F., S.K.R., M.H.V., F.V.d.P., and K.V. performed experiments; J.F. and S.R. analyzed data; J.F., S.R., and K.V. interpreted results of experiments; J.F. prepared figures; J.F. and K.V. drafted manuscript; J.F., S.K.R., S.R., M.H.V., F.V.d.P., and K.V. edited and revised manuscript; J.F., S.K.R., S.R., M.H.V., F.V.d.P., and K.V. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank the participants for efforts in the project. S. Ringgard is thanked for assistance with magnetic resonance imaging, and J. M. Jensen is thanked for assistance in biopsy preparation. Associate Professor B. M. Bibby is thanked for statistical guidance. The F1.652 and the A4.591 monoclonal antibodies developed by H. M. Blau were obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by The University of Iowa, Department of Biological Sciences, Iowa City, IA.
REFERENCES
- 1.Abbott BC, Bigland B, Ritchie JM. The physiological cost of negative work. J Physiol 117: 380–390, 1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adams GR, Bamman MM. Characterization and regulation of mechanical loading-induced compensatory muscle hypertrophy. Comp Physiol 2: 2829–2870, 2012. [DOI] [PubMed] [Google Scholar]
- 3.Adams GR, Caiozzo VJ, Haddad F, Baldwin KM. Cellular and molecular responses to increased skeletal muscle loading after irradiation. Am J Physiol Cell Physiol 283: C1182–C1195, 2002. [DOI] [PubMed] [Google Scholar]
- 4.Andersen JL, Aagaard P. Myosin heavy chain IIX overshoot in human skeletal muscle. Muscle Nerve 23: 1095–1104, 2000. [DOI] [PubMed] [Google Scholar]
- 5.Andersen LL, Tufekovic G, Zebis MK, Crameri RM, Verlaan G, Kjaer M, Suetta C, Magnusson P, Aagaard P. The effect of resistance training combined with timed ingestion of protein on muscle fiber size and muscle strength. Metabolism 54: 151–156, 2005. [DOI] [PubMed] [Google Scholar]
- 6.Atherton PJ, Smith K. Muscle protein synthesis in response to nutrition and exercise. J Physiol 590: 1049–1057, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brack AS, Rando TA. Tissue-specific stem cells: lessons from the skeletal muscle satellite cell. Cell Stem Cell 10: 504–514, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bruusgaard JC, Egner IM, Larsen TK, Dupre-Aucouturier S, Desplanches D, Gundersen K. No change in myonuclear number during muscle unloading and reloading. J Appl Physiol 113: 290–296, 2012. [DOI] [PubMed] [Google Scholar]
- 9.Bruusgaard JC, Johansen IB, Egner IM, Rana ZA, Gundersen K. Myonuclei acquired by overload exercise precede hypertrophy and are not lost on detraining. Proc Natl Acad Sci USA 107: 15111–15116, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cermak NM, Res PT, de Groot LC, Saris WH, van Loon LJ. Protein supplementation augments the adaptive response of skeletal muscle to resistance-type exercise training: a meta-analysis. Am J Clin Nutr 96: 1454–1464, 2012. [DOI] [PubMed] [Google Scholar]
- 11.Cermak NM, Snijders T, McKay BR, Parise G, Verdijk LB, Tarnopolsky MA, Gibala MJ, Loon LJ. Eccentric exercise increases satellite cell content in type II muscle fibers. Med Sci Sports Exerc 45: 230–237, 2012. [DOI] [PubMed] [Google Scholar]
- 12.Conboy IM, Conboy MJ, Wagers AJ, Girma ER, Weissman IL, Rando TA. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature 433: 760–764, 2005. [DOI] [PubMed] [Google Scholar]
- 13.Crameri RM, Aagaard P, Qvortrup K, Langberg H, Olesen J, Kjaer M. Myofibre damage in human skeletal muscle: effects of electrical stimulation versus voluntary contraction. J Physiol 583: 365–380, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crameri RM, Langberg H, Magnusson P, Jensen CH, Schroder HD, Olesen JL, Suetta C, Teisner B, Kjaer M. Changes in satellite cells in human skeletal muscle after a single bout of high intensity exercise. J Physiol 558: 333–340, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.D'Antona G, Lanfranconi F, Pellegrino MA, Brocca L, Adami R, Rossi R, Moro G, Miotti D, Canepari M, Bottinelli R. Skeletal muscle hypertrophy and structure and function of skeletal muscle fibres in male body builders. J Physiol 570: 611–627, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dibble CC, Manning BD. Signal integration by mTORC1 coordinates nutrient input with biosynthetic output. Nat Cell Biol 15: 555–564, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Durand RJ, Castracane VD, Hollander DB, Tryniecki JL, Bamman MM, O'Neal S, Hebert EP, Kraemer RR. Hormonal responses from concentric and eccentric muscle contractions. Med Sci Sports Exerc 35: 937–943, 2003. [DOI] [PubMed] [Google Scholar]
- 18.Esmarck B, Andersen JL, Olsen S, Richter EA, Mizuno M, Kjaer M. Timing of postexercise protein intake is important for muscle hypertrophy with resistance training in elderly humans. J Physiol 535: 301–311, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Farup J, Kjolhede T, Sorensen H, Dalgas U, Moller AB, Vestergaard PF, Ringgaard S, Bojsen-Moller J, Vissing K. Muscle morphological and strength adaptations to endurance vs. resistance training. J Strength Cond Res 26: 398–407, 2012. [DOI] [PubMed] [Google Scholar]
- 20.Farup J, Rahbek SK, Vendelbo MH, Matzon A, Hindhede J, Bejder A, Ringgard S, Vissing K. Whey protein hydrolysate augments tendon and muscle hypertrophy independent of resistance exercise contraction mode. Scand J Med Sci Sports 2013 May 7. 10.1111/sms.12083 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 21.Folland JP, Williams AG. The adaptations to strength training: morphological and neurological contributions to increased strength. Sports Med 37: 145–168, 2007. [DOI] [PubMed] [Google Scholar]
- 22.Franchi MV, Atherton PJ, Reeves ND, Fluck M, Williams J, Mitchell WK, Selby A, Beltran-Valls RM, Narici MV. Architectural, functional, and molecular responses to concentric and eccentric loading in human skeletal muscle. Acta Physiol (Oxf) 210: 642–654, 2014. [DOI] [PubMed] [Google Scholar]
- 23.Fredsted A, Clausen T, Overgaard K. Effects of step exercise on muscle damage and muscle Ca2+ content in men and women. J Strength Cond Res 22: 1136–1146, 2008. [DOI] [PubMed] [Google Scholar]
- 24.Fry CS, Lee JD, Jackson JR, Kirby TJ, Stasko SA, Liu H, Dupont-Versteegden EE, McCarthy JJ, Peterson CA. Regulation of the muscle fiber microenvironment by activated satellite cells during hypertrophy. FASEB J 28: 1654–1665, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goodman CA, Kotecki JA, Jacobs BL, Hornberger TA. Muscle fiber type-dependent differences in the regulation of protein synthesis. PLos One 7: e37890, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grubb A, Joanisse S, Moore DR, Bellamy LM, Mitchell CJ, Phillips SM, Parise G. IGF-1 colocalizes with muscle satellite cells following acute exercise in humans. Appl Physiol Nutr Metab 39: 514–518, 2014. [DOI] [PubMed] [Google Scholar]
- 27.Guerci A, Lahoute C, Hebrard S, Collard L, Graindorge D, Favier M, Cagnard N, Batonnet-Pichon S, Precigout G, Garcia L, Tuil D, Daegelen D, Sotiropoulos A. Srf-dependent paracrine signals produced by myofibers control satellite cell-mediated skeletal muscle hypertrophy. Cell Metab 15: 25–37, 2012. [DOI] [PubMed] [Google Scholar]
- 28.Han B, Tong J, Zhu MJ, Ma C, Du M. Insulin-like growth factor-1 (IGF-1) and leucine activate pig myogenic satellite cells through mammalian target of rapamycin (mTOR) pathway. Mol Reprod Devel 75: 810–817, 2008. [DOI] [PubMed] [Google Scholar]
- 29.Hortobagyi T, Hill JP, Houmard JA, Fraser DD, Lambert NJ, Israel RG. Adaptive responses to muscle lengthening and shortening in humans. J Appl Physiol 80: 765–772, 1996. [DOI] [PubMed] [Google Scholar]
- 30.Joanisse S, Gillen JB, Bellamy LM, McKay BR, Tarnopolsky MA, Gibala MJ, Parise G. Evidence for the contribution of muscle stem cells to nonhypertrophic skeletal muscle remodeling in humans. FASEB J 27: 4596–4605, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnston AP, Bellamy LM, Lisio MD, Parise G. Captopril treatment induces hyperplasia but inhibits myonuclear accretion following severe myotrauma in murine skeletal muscle. Am J Physiol Regul Integr Comp Physiol 301: R363–R369, 2011. [DOI] [PubMed] [Google Scholar]
- 32.Kadi F, Schjerling P, Andersen LL, Charifi N, Madsen JL, Christensen LR, Andersen JL. The effects of heavy resistance training and detraining on satellite cells in human skeletal muscles. J Physiol 558: 1005–1012, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kelley G. Mechanical overload and skeletal muscle fiber hyperplasia: a meta-analysis. J Appl Physiol (1985) 81: 1584–1588, 1996. [DOI] [PubMed] [Google Scholar]
- 34.Lepper C, Partridge TA, Fan CM. An absolute requirement for Pax7-positive satellite cells in acute injury-induced skeletal muscle regeneration. Development 138: 3639–3646, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lieber RL, Friden J. Muscle damage is not a function of muscle force but active muscle strain. J Appl Physiol (1985) 74: 520–526, 1993. [DOI] [PubMed] [Google Scholar]
- 36.Mackey AL, Andersen LL, Frandsen U, Sjogaard G. Strength training increases the size of the satellite cell pool in type I and II fibres of chronically painful trapezius muscle in females. J Physiol 589: 5503–5515, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mackey AL, Brandstetter S, Schjerling P, Bojsen-Moller J, Qvortrup K, Pedersen MM, Doessing S, Kjaer M, Magnusson SP, Langberg H. Sequenced response of extracellular matrix deadhesion and fibrotic regulators after muscle damage is involved in protection against future injury in human skeletal muscle. FASEB J 25: 1943–1959, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mackey AL, Holm L, Reitelseder S, Pedersen TG, Doessing S, Kadi F, Kjaer M. Myogenic response of human skeletal muscle to 12 weeks of resistance training at light loading intensity. Scand J Med Sci Sports 21: 773–782, 2010. [DOI] [PubMed] [Google Scholar]
- 39.Mackey AL, Kjaer M, Charifi N, Henriksson J, Bojsen-Moller J, Holm L, Kadi F. Assessment of satellite cell number and activity status in human skeletal muscle biopsies. Muscle Nerve 40: 455–465, 2009. [DOI] [PubMed] [Google Scholar]
- 40.Mauro A. Satellite cell of skeletal muscle fibers. J Biophys Biochem Cytol 9: 493–495, 1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McCarthy JJ, Mula J, Miyazaki M, Erfani R, Garrison K, Farooqui AB, Srikuea R, Lawson BA, Grimes B, Keller C, Van Zant G, Campbell KS, Esser KA, Dupont-Versteegden EE, Peterson CA. Effective fiber hypertrophy in satellite cell-depleted skeletal muscle. Development 138: 3657–3666, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McKay BR, O'Reilly CE, Phillips SM, Tarnopolsky MA, Parise G. Co-expression of IGF-1 family members with myogenic regulatory factors following acute damaging muscle-lengthening contractions in humans. J Physiol 586: 5549–5560, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McKay BR, Ogborn DI, Bellamy LM, Tarnopolsky MA, Parise G. Myostatin is associated with age-related human muscle stem cell dysfunction. FASEB J 26: 2509–2521, 2012. [DOI] [PubMed] [Google Scholar]
- 44.McKay BR, Toth KG, Tarnopolsky MA, Parise G. Satellite cell number and cell cycle kinetics in response to acute myotrauma in humans: immunohistochemistry versus flow cytometry. J Physiol 588: 3307–3320, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mikkelsen UR, Langberg H, Helmark IC, Skovgaard D, Andersen LL, Kjaer M, Mackey AL. Local NSAID infusion inhibits satellite cell proliferation in human skeletal muscle after eccentric exercise. J Appl Physiol 107: 1600–1611, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Montarras D, L'Honore A, Buckingham M. Lying low but ready for action: the quiescent muscle satellite cell. FEBS J 280: 4036–4050, 2013. [DOI] [PubMed] [Google Scholar]
- 47.Mueller M, Breil FA, Lurman G, Klossner S, Fluck M, Billeter R, Dapp C, Hoppeler H. Different molecular and structural adaptations with eccentric and conventional strength training in elderly men and women. Gerontology 57: 528–538, 2011. [DOI] [PubMed] [Google Scholar]
- 48.Nielsen JL, Aagaard P, Bech RD, Nygaard T, Hvid LG, Wernbom M, Suetta C, Frandsen U. Proliferation of myogenic stem cells in human skeletal muscle in response to low-load resistance training with blood flow restriction. J Physiol 590: 4351–4361, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.O'Connor RS, Pavlath GK. Point:Counterpoint: Satellite cell addition is/is not obligatory for skeletal muscle hypertrophy. J Appl Physiol (1985) 103: 1099–1100, 2007. [DOI] [PubMed] [Google Scholar]
- 50.Olsen S, Aagaard P, Kadi F, Tufekovic G, Verney J, Olesen JL, Suetta C, Kjaer M. Creatine supplementation augments the increase in satellite cell and myonuclei number in human skeletal muscle induced by strength training. J Physiol 573: 525–534, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Paulsen G, Mikkelsen UR, Raastad T, Peake JM. Leucocytes, cytokines and satellite cells: what role do they play in muscle damage and regeneration following eccentric exercise? Exerc Immunol Rev 18: 42–97, 2012. [PubMed] [Google Scholar]
- 52.Petrella JK, Kim JS, Mayhew DL, Cross JM, Bamman MM. Potent myofiber hypertrophy during resistance training in humans is associated with satellite cell-mediated myonuclear addition: a cluster analysis. J Appl Physiol 104: 1736–1742, 2008. [DOI] [PubMed] [Google Scholar]
- 53.Proske U, Allen TJ. Damage to skeletal muscle from eccentric exercise. Exerc Sport Sci Rev 33: 98–104, 2005. [DOI] [PubMed] [Google Scholar]
- 54.Reeves ND, Maganaris CN, Longo S, Narici MV. Differential adaptations to eccentric versus conventional resistance training in older humans. Exp Physiol 94: 825–833, 2009. [DOI] [PubMed] [Google Scholar]
- 55.Relaix F, Rocancourt D, Mansouri A, Buckingham M. A Pax3/Pax7-dependent population of skeletal muscle progenitor cells. Nature 435: 948–953, 2005. [DOI] [PubMed] [Google Scholar]
- 56.Snijders T, Verdijk LB, McKay BR, Smeets JSJ, van Kranenburg J, Groen BBB, Parise G, Greenhaff P, van Loon LJC. Acute dietary protein intake restriction is associated with changes in myostatin expression after a single bout of resistance exercise in healthy young men. J Nutr 144: 137–145, 2013. [DOI] [PubMed] [Google Scholar]
- 57.Stefanetti RJ, Lamon S, Rahbek SK, Farup J, Zacharewicz E, Wallace MA, Vendelbo MH, Russell AP, Vissing K. Influence of divergent exercise contraction mode and whey protein supplementation on atrogin-1, MuRF1 and FOXO1/3A in human skeletal muscle. J Appl Physiol (1985) 116: 1491–1502, 2014. [DOI] [PubMed] [Google Scholar]
- 58.Suetta C, Frandsen U, Mackey AL, Jensen L, Hvid LG, Bayer ML, Petersson SJ, Schroder HD, Andersen JL, Aagaard P, Schjerling P, Kjaer M. Ageing is associated with diminished muscle re-growth and myogenic precursor cell expansion early after immobility-induced atrophy in human skeletal muscle. J Physiol 591: 3789–3804, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Urciuolo A, Quarta M, Morbidoni V, Gattazzo F, Molon S, Grumati P, Montemurro F, Tedesco FS, Blaauw B, Cossu G, Vozzi G, Rando TA, Bonaldo P. Collagen VI regulates satellite cell self-renewal and muscle regeneration. Nat Commun 4: 1964, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Verdijk LB, Gleeson BG, Jonkers RA, Meijer K, Savelberg HH, Dendale P, van Loon LJ. Skeletal muscle hypertrophy following resistance training is accompanied by a fiber type-specific increase in satellite cell content in elderly men. J Gerontol A Biol Sci Med Sci 64: 332–339, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vijayan K, Thompson JL, Norenberg KM, Fitts RH, Riley DA. Fiber-type susceptibility to eccentric contraction-induced damage of hindlimb-unloaded rat AL muscles. J Appl Physiol 90: 770–776, 2001. [DOI] [PubMed] [Google Scholar]
- 62.Vikne H, Refsnes PE, Ekmark M, Medbo JI, Gundersen V, Gundersen K. Muscular performance after concentric and eccentric exercise in trained men. Med Sci Sports Exerc 38: 1770–1781, 2006. [DOI] [PubMed] [Google Scholar]
- 63.Vissing K, McGee SL, Farup J, Kjolhede T, Vendelbo MH, Jessen N. Differentiated mTOR but not AMPK signaling after strength vs endurance exercise in training-accustomed individuals. Scand J Med Sci Sports 23: 355–366, 2011. [DOI] [PubMed] [Google Scholar]
- 64.Vissing K, Rahbek SK, Lamon S, Farup J, Stefanetti RJ, Wallace MA, Vendelbo MH, Russell A. Effect of resistance exercise contraction mode and protein supplementation on members of the STARS signalling pathway. J Physiol 591: 3749–3763, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.von Maltzahn J, Jones AE, Parks RJ, Rudnicki MA. Pax7 is critical for the normal function of satellite cells in adult skeletal muscle. Proc Natl Acad Sci USA 110: 16474–16479, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Whalen RG. Myosin isoenzymes as molecular markers for muscle physiology. J Exp Biol 115: 43–53, 1985. [DOI] [PubMed] [Google Scholar]
- 67.Willkomm L, Schubert S, Jung R, Elsen M, Borde J, Gehlert S, Suhr F, Bloch W. Lactate regulates myogenesis in C2C12 myoblasts in vitro. Stem Cell Res 12: 742–753, 2014. [DOI] [PubMed] [Google Scholar]
- 68.Yin H, Price F, Rudnicki MA. Satellite cells and the muscle stem cell niche. Physiol Rev 93: 23–67, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]


