Abstract
To understand how different parts of the frontal cortex control the timing of action, we characterized the firing patterns of single neurons in two areas of rodent frontal cortex—dorsomedial prefrontal cortex (dmPFC) and motor cortex—during a simple reaction time task. Principal component analysis was used to identify major patterns of delay-related activity in frontal cortex: ramping activity and sustained delay activity. These patterns were similar in dmPFC and motor cortex and did not change as animals learned to respond at novel delays. Many neurons in both areas were modulated early in the delay period. Other neurons were modulated in a persistent manner over the duration of the delay period. Delay-related modulations started earlier in motor cortex than in dmPFC and terminated around different task events (at the time of release in dmPFC, just before release of the lever in motor cortex). A subpopulation of neurons was found in dmPFC, but not motor cortex, that fired in response to the trigger stimulus. These results suggest that populations of neurons in rodent frontal cortex are coordinated during delay periods to enable proactive inhibitory control of action.
INTRODUCTION
Medial regions of frontal cortex in rats, monkeys, and human beings have been implicated in controlling the timing of action (Botvinick et al. 2004; Li et al. 2006; Naito et al. 2000; Rushworth et al. 2004). These cortical regions are densely interconnected (Fisk and Wyss 1999) and send massive projections to neuromodulatory systems in the brain stem (Gabbott et al. 2005; Sesack et al. 1989). Through these connections, medial frontal areas may form a brain system that exerts top-down control over the motor system to suppress responding during delay periods and update the frontal cortex about the consequences of actions (Boulinguez et al. 2008; Brunia 1999; Churchland and Shenoy 2007; Hanakawa et al. 2008; Narayanan and Laubach 2006; Picton et al. 2006, 2007; Schall et al. 2002; Stuphorn and Schall 2006).
Disruptions of neural activity in the medial frontal cortex lead to lasting deficits in the temporal control of action (Broersen and Uylings 1999; Muir et al. 1996; Narayanan and Laubach 2006; Narayanan et al. 2006; Risterucci et al. 2003). By contrast, inactivations of motor cortex do not increase premature responding (Martin et al. 1993; Matsumura et al. 1991; Narayanan et al. 2006). In a recent study, we found that inactivation of the dorsomedial part of the rat prefrontal cortex (dmPFC; this region is comprised of the pregenual anterior cingulate area and the dorsal part of the prelimbic area) led to reduced delay period firing in the motor cortex (Narayanan and Laubach 2006). This finding suggests that medial frontal neurons exert proactive control over the motor system to inhibit responding until the right time or right stimulus has occurred (Boulinguez et al. 2008).
Neurons in the medial frontal cortex show persistent firing during delay periods in primates (Niki and Watanabe 1976a,b, 1979) and rodents (Baeg et al. 2001, 2003; Batuev et al. 1990; Cowen and McNaughton 2007; Narayanan and Laubach 2006). For example, Narayanan and Laubach (2006) found that one third of dmPFC neurons are modulated (i.e., fire at increased or decreased rates) during the delay period of a simple reaction time (RT) task. The firing rates of these neurons were predictive of successful waiting behavior and were significantly correlated with firing rates of neurons in the motor cortex (Narayanan and Laubach 2006). We also found that many delay-related neurons in dmPFC and motor cortex are sensitive to errors in the task (Laubach et al. 2000; Narayanan and Laubach 2006; Narayanan et al. 2005) and, most recently, we reported that neurons in dmPFC, but not in motor cortex, fire persistently through the intertrial interval following an error until the beginning of the next trial (Narayanan and Laubach 2008).
In the present study, we describe how neuronal activity in dmPFC and motor cortex is modulated during delay periods. As in a recent study by Paz et al. (2005), we used principal component analysis (Reyment and Joreskog 1996) to characterize major patterns of firing in populations of neurons from dmPFC and the motor cortex during the delay period. This analysis enabled us to measure potential differences in the timing of task-related activity in the two cortical areas, which is a prerequisite for top-down influences of dmPFC on motor cortex (Narayanan and Laubach 2006). Our results suggest that the time courses of neuronal activation in dmPFC and motor cortex are mutually dependent and that persistent firing during the delay period may arise from task-related activity associated with lever pressing at the start of the trial.
A second goal of this study was to assess the role of delay-period activity in dmPFC and the motor cortex in preparing for the forthcoming stimulus and response. Neurons could be involved in maintaining the motor response during the delay period (Narayanan et al. 2006; Ollman and Billington 1972) or could be sensitive to the expected timing of the stimulus (Baeg et al. 2001; Kornblum 1973; Los and Van Den Heuvel 2001; Naatanen 1971, 1972; Nobre et al. 2001). To address this issue, we compared task-related activity on trials with 1.0-s delays in sessions with one (1.0 s) and two (0.4 or 1.0 s) delay periods. If frontal neurons are involved in proactively inhibiting responding during the delay period, then there should be no major differences in delay-period activity in sessions with one and two delay periods. On the other hand, if frontal neurons are involved in anticipating the timing of the forthcoming stimulus and the subsequent response, then there should be modulations of neural activity around the time of the short delay period (i.e., 0.4 s), even when the stimulus does not occur at that time. If these effects reflect changes in preparatory activity, then they should be apparent at the population level (Brunia 1999). We found no evidence for modulation of neuronal activity at the time of the short delay period and suggest that single-neuron activity in rodent frontal cortex is involved in proactive inhibitory control of responding.
METHODS
Subjects
Twelve male Long–Evans rats (3–4 mo old; Harlan) were trained to perform a simple RT task and were then implanted with arrays of recording electrodes in dmPFC (n = 8), motor cortex (n = 3), or both cortical areas (n = 1). Rats were motivated by water regulation, whereas food was always available. Rats consumed 10–15 ml of water during each behavioral session and additional water (5–10 ml) was provided 1–3 h after each behavioral session in the home cage. Rats were maintained at roughly 90% of their free-access body weights during these experiments and received 1 day of free access to water per week. The Animal Care and Use Committee at the John B. Pierce Laboratory approved all procedures.
Simple reaction time task
Rats were trained to perform a simple reaction time task using standard operant procedures (Fig. 1A). Trials were initiated when rats pressed a lever. Response force was measured using a thin-film load cell (S100, Strain Measurement Devices, Meriden, CT), rated to 1 N, which was mounted on the lever. The lever press was maintained for a 1.0-s delay period. A tone (frequency: 8 kHz; intensity: 72 dBA; duration: 100 ms) was presented at the end of the delay period. To receive liquid rewards, animals had to release the lever with RT <0.6 s (correct responses). If the lever was released before the trigger stimulus (premature error responses) or after the response window (late error responses), there was a timeout period. Animals typically made premature responses on 25–35% of trials and late responses on <10% of trials. Neuronal activity from these error trials was excluded from all analyses in this study.
FIG. 1.
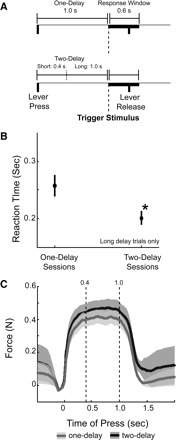
Simple reaction time task. A: rats were trained to perform a simple reaction time task. Lever presses were maintained over a 1.0-s delay period (“one-delay” sessions) until a tone (frequency: 8 kHz; intensity: 72 dBA; duration: 100 ms) was presented. To receive liquid rewards, animals released the lever within 0.6 s of stimulus onset. After implantation of recording electrodes and testing in 3 sessions with a one-delay period, animals were run in 3 additional sessions with delays of 0.4 or 1.0 s (“two-delay” sessions). B: reaction times are shown for the one-delay and two-delay sessions. Rats responded faster at the long delay in sessions with 2 delays, suggesting that the addition of the early delay period led to “delay-dependent speeding” of reaction times. Asterisk indicates significance at P < 0.01 by a paired t-test (see text for details). C: lever force in one-delay and two-delay sessions for trials with correct responses and long delays. Animals maintained similar lever force during the delay period in one-delay and two-delay sessions.
Rats reached criterion (>60% correct) in 9.75 ± 1.5 sessions (1,748 ± 343 trials) and performed the task with one delay period (1.0 s) for a total of 24 ± 1.9 sessions (5,776 ± 564 trials). They were then implanted with recording electrodes. Neuronal data were collected in three sessions with one delay period. Then, three sessions (567 ± 98 trials) were run with two randomly interleaved and equally likely delays of 0.4 s (short) or 1.0 s (long). To make the data sets comparable between the one- and two-delay sessions, all analyses in this study were restricted to trials with delays of 1.0 s (all trials in the one-delay sessions and trials with long delays in the two-delay sessions).
Prior to these experiments, nine of these rats (six with recording electrodes in dmPFC, three with recording electrodes in motor cortex, and one with both) performed a modified version of the task, based on a study by Kornblum (1973). In these sessions, all trials had delays of 1.0 s and trigger stimuli were presented on one half of the trials (with pseudorandomly interleaved types of trials). On the other half of trials (called time production trials), no trigger stimulus was presented and rats were rewarded for releasing the trigger stimulus. Animal behavior was not different on these two trials (Narayanan and Laubach 2006; Narayanan et al. 2006). This task was used to assess tone-related firing in dmPFC and motor cortex.
Microwire electrode arrays
Twelve animals were implanted with 4 × 4 or 3 × 3 × 2 arrays of 50-μm stainless steel wires (250 μm between wires; impedance measured in vitro at 100–300 kΩ; Neurolinc, New York, NY) into the dorsal prelimbic region of rat frontal cortex (coordinates from bregma: anteroposterior [AP]: +3.2; mediolateral [ML]: ±1.4; dorsoventral [DV] −3.6 at 10° in the frontal plane) targeting coordinates of previous inactivation (Narayanan et al. 2006) or into rat motor cortex (coordinates from bregma: AP: −0.5; ML: ±2.5–3.5; DV: −1.5 at −25° in the frontal plane) according to methods described in detail previously (Laubach et al. 2000; Narayanan et al. 2005, 2006). Electrodes were implanted unilaterally into dmPFC and/or motor cortex contralateral to the rats’ dominant paw (i.e., the paw used to press the lever) or bilaterally into dmPFC (Fig. 2).
FIG. 2.
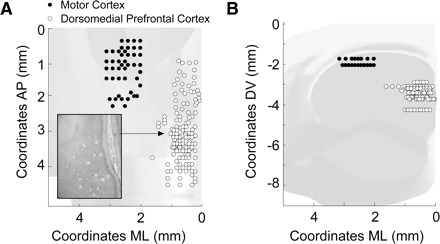
Location of microwire arrays. Recording sites are shown in dorsomedial prefrontal cortex (dmPFC; 9 animals) as white dots and in motor cortex (4 animals) as black dots. Reconstructions are shown for the horizontal (A) and frontal (B) planes. An example histological section is inset in A, as used to determine electrode location.
Surgery
Aseptic stereotaxic methods were used to implant microwire electrode arrays into dmPFC or into motor cortex (Fig. 2). Anesthesia was initiated with around 4% halothane and maintained with intraperitoneal injections of ketamine (100 mg/kg) and diazepam (10 mg/kg). A surgical level of anesthesia was maintained over the course of surgery with supplements (30 mg/kg) of ketamine every 45 min to 1 h. Under aseptic conditions, the scalp was retracted and the skull was leveled between bregma and lambda. A single craniotomy was drilled over the area above dmPFC cortex that spanned both hemispheres or over motor cortex. Microwire arrays were then inserted in dmPFC and/or motor cortex, with electrophysiological signals used to target placement into the deep layers of the cortex. Implants were then sealed with cyanoacrylate (“SloZap”; Pacer Technologies, Rancho Cucamonga, CA), an accelerator (“ZipKicker”; Pacer Technologies), and methyl methacrylate (AM Systems, Port Angeles, WA). Animals were allowed to recover for 1 wk and then acclimated to recording procedures over several sessions before recordings commenced in the simple RT task.
Electrophysiological recordings
Neuronal ensemble recordings were made using a Many Neuron Acquisition Program (Plexon, Dallas, TX). Putative single units were identified on-line using an oscilloscope and audio monitor. The Plexon off-line sorter was used to analyze the signals off-line and to remove artifacts due to cable noise and behavioral devices (pump that delivered fluid to the rats, click stimulus generated by a mechanical relay at the time of fluid delivery). Principal component analysis (PCA) and waveform shape were used for spike sorting. Single units were identified as having 1) consistent waveform shape, 2) separable clusters defined by waveform parameters and analysis with PCA (using the first two PCs), 3) average amplitude estimated at least threefold larger than background activity, 4) a consistent refractory period of ≥2 ms in interspike interval histograms, and 5) consistent firing rates around behavioral events (i.e., “stationarity,” as measured by a runs test of firing rates around behavioral events; neurons with |z| scores >4 were excluded). Using these techniques, in all 414 single units were included in this study. A quantitative analysis of these spike trains was carried out using NeuroExplorer (Nex Technologies, Littleton, MA) and custom routines for Matlab (The MathWorks, Natick, MA) and R (http://www.r-project.org/).
Analysis of task-related modulations in firing rates
Only data from trials with correct responses (sustained until stimulus and RT <0.6 s) and long delay periods (1.0 s) were analyzed in both one-delay and two-delay sessions. Perievent histograms were created for the epoch from 0.5 s before to 1.5 s after the lever press using 1-ms bins. Spike density functions were created as follows. Spike counts for each trial were stored in a vector. The vector was padded with 25 zeros at each end (zero padding). Each trial was then convolved with a 25-point Gaussian window, decimated 25 times (using the “decimate” function in Matlab), and the first and last points in the resulting vector were cut off (to eliminate the padding). This gave an estimate of spike density with an effective time resolution of 25 ms. Spike density functions were then converted to a measure of firing rate by dividing the vector by the effective bin size.
Population averages were calculated by simply averaging the raw firing rates of all neurons and by averaging together average firing rates that were normalized using z-scores. For these analyses, and those below, we included neurons in the data set only if they fired at an average rate that was >1 Hz over the perievent epoch (from 1 s before the lever press to 2 s after the lever press). All analyses were also confirmed with 10-ms bins; bin size did not influence the results in this study.
Correlations were performed between neural firing rate in the RT epoch (1.0–1.6 after lever press) and reaction times. Partial correlation was also performed between simultaneously recorded neurons controlling for reaction time using built-in functions in Matlab (partialcorr.m).
We used PCA (Paz et al. 2005; Reyment and Joreskog 1996) to capture major patterns of firing within the neuronal populations. For this analysis, we calculated average spike density functions (effective bin size of 25 ms) for all neurons in sessions with one- and two-delay periods, arranged the neurons in a matrix with neurons as rows and bins as columns, normalized the rows of the matrix as z-scores, and then performed PCA using singular value decomposition. For each area, neurons were combined into the same matrix for this analysis and potential differences between sessions with one- and two-delay periods were evaluated based on the scores for the PCs over neurons from each type of session. Importantly, neurons from dmPFC and motor cortex were analyzed using separate analyses. The covariance explained by each resulting principal component (PC) was explored by plotting the eigenvalues as scree plots. We found that each area (dmPFC and motor cortex) contained only a few components with large eigenvalues, each explaining >10% of covariance. The number of large components was further confirmed using functions in the Matlab Toolbox for Dimensionality Reduction (http://ticc.uvt.nl/∼lvdrmaaten/).
Analysis of stimulus-related activity
Exploratory analysis suggested that a subset of neurons in dmPFC, but not in motor cortex, fired in response to the trigger stimulus. Such neurons had sharp modulations in firing rate immediately after stimulus onset and well before the rats released the lever. To evaluate the fractions of neurons in the two cortical areas with such activity, we analyzed data collected during a modified task in which stimuli occurred on half of the trials and at a delay of 1.0 s. Stimulus-related modulations in these sessions were defined using a Wilcoxon rank-sum test (criterion of P < 0.05) that compared spike rates in a 200-ms epoch at 1.0 s into the delay period on trials with and without a trigger stimulus.
Histology
Once experiments were complete, rats were killed via overdose with 100 mg/kg sodium pentobarbital and then transcardially perfused with either 10% formalin or 4% paraformaldehyde. Lesions were made at select recording sites by passing a unipolar current (100 μA) for 10 s. Brains were sectioned on a freezing microtome, mounted, and stained with thionin. Electrode locations were visualized using custom-written three-dimensional reconstruction software (Eyal Kimchi, Laubach Lab) based on an atlas of coronal sections by Swanson (1999). Software for electrode reconstruction is available at http://spikelab.jbpierce.org/Resources.
RESULTS
Behavioral data
Twelve rats were trained to perform a simple reaction time task (Fig. 1A). Standard methods for operant training were used in all studies (see Narayanan et al. 2006). Arrays of microwire electrodes were implanted into dmPFC (n = 8), motor cortex (n = 3), or both areas (n = 1) (Fig. 2). Recordings were made in nine animals (six animals in dmPFC, three animals in motor cortex, one animal with both) using the modified task.
In the standard reaction time task, animals performed on average 135 ± 40 correct trials, 38 ± 17 premature errors, and 28 ± 23 late errors. Animals made equivalent numbers of correct responses in one-delay and two-delay sessions [one-delay sessions: 70 ± 3%; two-delay sessions: 71 ± 2%; paired t(1,11) = 0.6, P < 0.56]. However, on trials with long delays, reaction times were faster in two-delay sessions (0.201 ± 0.12 s) compared with one-delay sessions [0.257 ± 0.19 s; paired t(1,11) = 2.99, P < 0.01] (Fig. 1B).
In six animals, we measured the force applied to the lever in one-delay and two-delay sessions. On trials with long delays, animals achieved the same maximal lever force in one-delay sessions (0.39 ± 0.02) compared with two-delay sessions [0.37 ± 0.02 N; paired t(1,5) = 0.72, P < 0.50] (Fig. 1C). These data indicate that animals’ movements were similar in one-delay and two-delay sessions and that lever pressing was a stable habit.
Neuronal database
During one-delay sessions, we recorded 144 dmPFC neurons (in nine animals, with an average of 16 ± 2.5 neurons per animal). In the same nine animals, we recorded 135 dmPFC neurons (15 ± 1.5 neurons per animal) after animals learned the two-delay task (i.e., after 3 days of training). We also investigated neural activity in motor cortex in four animals. We recorded from 65 motor cortex neurons (16.5 ± 1.3 neurons per animal) during one-delay sessions. In the same animals, we recorded 70 motor cortex neurons (17.5 ± 2.3 neurons per animal) in the third session with the two-delay design. Additional recordings of 88 neurons in dmPFC in six animals (17.3 ± 7.4 neurons per animal) and 50 neurons in motor cortex in four animals (12.5 ± 3.4 neurons per animal) were acquired during the modified reaction time task with time production trials (i.e., with stimuli presented on half of the trials).
Population averages were strongly modulated in motor cortex, but not in dmPFC
Neuronal activity was diverse across neurons in dmPFC. As a result, population averages of trial-averaged firing rates, synchronized to the time of the lever press, were not modulated around the task events (Fig. 3A). However, by normalizing firing rates using z-scores prior to averaging, we were able to detect modulations in dmPFC activity, especially around the time of lever pressing. By contrast, activity in motor cortex was more consistently modulated around lever pressing (0 s), during the delay period (between 0 and 1 s after lever press), and during the reaction time epoch (1.0 to 1.6 s after lever press) (Fig. 3B). As in dmPFC, averages of normalized firing rates also revealed modulation around these events and exposed a significant difference (based on comparisons of 95% confidence intervals, estimated using bootstrapping) in motor cortex population activity during the reaction time period in sessions with one and two delay periods.
FIG. 3.
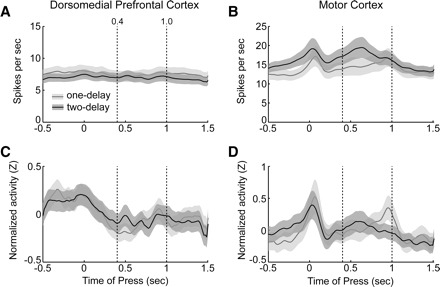
Task-related modulations in neuronal activity in rodent frontal cortex. Simple arithmetic averages of neuronal firing rates for dmPFC (A) and motor cortex (B) are shown as lines and the 95% confidence intervals around the average rates are shown as bands (sessions with one delay: gray lines, light gray bands; sessions with 2 delays: black lines, dark gray bands). Average firing rates in dmPFC were similar in sessions with one- and two-delay periods. However, motor cortical neurons were increasingly modulated during the reaction time epoch (from 1.0 to 1.6 s) in sessions with 2 delay periods compared with sessions with one delay period. This effect may have been due to the faster reaction times that were found in the two-delay sessions (see Fig. 1B). Averages of normalized firing rates, calculated as z-scores, are shown as in A and B for dmPFC (C) and motor cortex (D). Dashed lines depict the timing of delay periods (0.4 and 1.0 s). Note that all analyses are restricted to correct trials with a long delay.
Firing rates of rodent frontal cortex neurons could be correlated with behavioral reaction times during the reaction time window (1.0 to 1.6 s after lever press). In dmPFC, similar fractions of neurons had significant (P < 0.05) firing rate correlations in one-delay sessions and in two-delay sessions [35 of 144 (23%) neurons in one-delay sessions; 29 of 135 (22%) neurons in two-delay sessions; χ2 = 0.31, df = 1, P < 0.58], although correlations among these neurons were stronger in two-delay sessions (R = |0.24| ± 0.9 in one-delay sessions, R = |0.33| ± 0.14 in two-delay sessions, Wilcoxon rank-sum test, P < 0.002). In motor cortex, although fewer neurons had significant correlations in two-delay sessions than in one-delay sessions [43 of 65 (66%) neurons in one-delay sessions; 32 of 70 (45%) neurons in two-delay sessions; χ2 = 5.7, df = 1, P < 0.02], correlations among these neurons were stronger in two-delay sessions than in one-delay sessions (R = |0.31| ± 0.14 in one-delay sessions, R = |0.42| ± 0.16 in two-delay sessions, Wilcoxon rank-sum test, P < 0.002).
Furthermore, simultaneously recorded rodent frontal cortex neurons could be correlated with each other (Narayanan and Laubach 2009; Narayanan et al. 2005). We investigated this issue using the partial correlation method to control for correlation with reaction times (discussed earlier) and identified significantly correlated (P < 0.05 via a partial correlation) pairs of neurons. In dmPFC, similar numbers of simultaneously recorded pairs were correlated in one-delay [1,161 of 17,966 pairs (6.5%), R = |0.45|] and two-delay sessions [1,039 of 14,205 (7.3%), R = |0.45|; χ2 = 0.78, df = 1, P value = 0.3786]. However, in motor cortex, more neurons had pairwise correlations in two-delay sessions [876 of 6,495 (13.1%), R = |0.47|] compared with one-delay sessions [549 of 6,051 (9.1%), R = |0.45|, χ2 = 5.63, df = 1, P < 0.02]. Strengthening correlations in motor cortex may underlie firing rate differences observed in Fig. 3D.
Slow modulations of population activity during the trial
Several distinct firing patterns were revealed through application of PCA to matrices of neuronal firing rates during the delay period (from −0.5 s before to 1.5 s after the time of lever pressing). Two major components, each accounting for >10% of variance, were found in activity from dmPFC (Fig. 4). The first component (PC1), which accounted for 20.3% of variance, was a steadily decreasing function that spanned from the time of the lever press until the time of pump activation (top plot in Fig. 4A). More neurons had positive loadings on PC1 (i.e., their activity resembled this negatively decelerating function) than had negative loadings (top plot in Fig. 4B; proportions test: χ2 = 13.1, df = 1, P < 0.00001). There was no difference in loadings on this function in sessions with one and two delay periods (left plot in Fig. 4C). Examples of neurons with a large loading on PC1 (i.e., the coefficient of the neuron for the first eigenvector) are shown in Fig. 5, A and B. These neurons showed very slow rates of change in firing rate and fired in a reciprocal manner during the course of the trial.
FIG. 4.
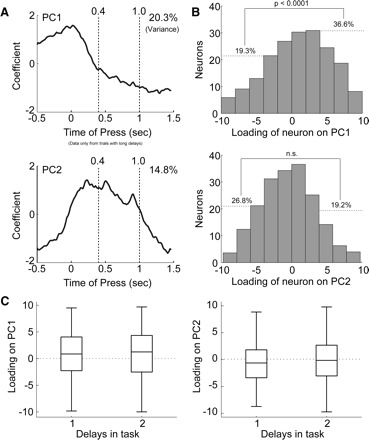
Analysis of firing patterns in dmPFC using principal component analysis (PCA). A: eigenvectors are shown for the first 2 principal components (PCs) relative to the time of lever press. The eigenvectors describe patterns of firing in the neuronal population. PC1 accounted for 20.3% of variance in the neuronal population. Eigenvectors for PC1 increased slightly at the lever press and then were negative during the delay period. PC2 accounted for 14.8% of variance in the neuronal population. Eigenvectors for PC2 increased around the lever press, were relatively constant during the delay period, and then were reduced during lever release. B: histograms of the loadings of neurons onto the PCs are shown. The loadings reflect the correlation between the neuron's firing pattern and the firing pattern described by the PC. More neurons had positive loadings on PC1 than had negative loadings (proportions test: χ2 = 13.1, df = 1, P < 0.0001); that is, more neurons “ramped down” than “ramped up” during the delay period (Fig. 5A). Loadings on PC2 were equally positive and negative. C: boxplots show that loadings on both PCs were equivalent for sessions with one- and two-delay periods. Note that all analyses are restricted to correct trials with a long delay.
FIG. 5.
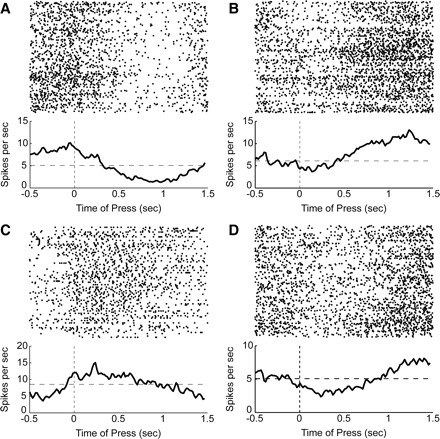
Examples of firing patterns in dmPFC. A: spike rasters and average perievent histograms (1-ms bins, smoothed with a 25-ms Gaussian) are shown for a neuron with one of the largest positive loadings (>3 standard units) on PC1. This neuron “ramped up” during the delay period. B: a neuron with one of the largest negative loadings (< −3 standard units) on PC1. This neuron “ramped down” during the delay period. C: a neuron with one of the largest positive loadings on PC2. This neuron fired persistently during the delay period. D: a neuron with one of the largest negative loadings on PC2. This neuron showed a persistent reduction in firing rate during the delay period. All rasters depict correct trials with a long delay.
The second component (PC2) accounted for 14.8% of variance. This function changed at the time of the lever press and then began to change in the opposite direction during the delay period until the time of the stimulus at 1.0 s (bottom plot in Fig. 4A). This component resembled the activity of many dmPFC neurons with sustained delay activity (Narayanan and Laubach 2006). There were equal numbers of neurons with positive and negative loadings on this component (bottom plot in Fig. 4B; proportions test: P > 0.05). As for PC1, the pattern of firing associated with PC2 was found in both the one-delay and two-delay sessions (right plot in Fig. 4C). Examples of neurons with a large loading on PC2 are shown in Fig. 5, C and D. As before, these neurons showed very slow rates of change in firing rate and fired in a reciprocal manner during the course of the trial.
In the motor cortex, there were three major components, each accounting for >10% of variance (Fig. 6). The first component (PC1), which accounted for 29.5% of variance, was a similar to PC1 from dmPFC and had a steadily decreasing form spanning from the lever press until just before the end of the delay period (top plot in Fig. 6A). Equal numbers of neurons had positive and negative loadings on PC1 (top plot in Fig. 6B; proportions test: P > 0.05) and there was no difference in loadings on this function in sessions with one and two delay periods (left plot in Fig. 6C). Examples of neurons with a large loading on PC1 (i.e., the coefficient of the neuron for the first eigenvector) are shown in Fig. 7, A and B. These neurons showed much sharper modulations around lever pressing than the neurons that were related to PC1 from dmPFC. However, as in dmPFC, we found neurons with strong reciprocal patterns of firing that were related to PC1.
FIG. 6.
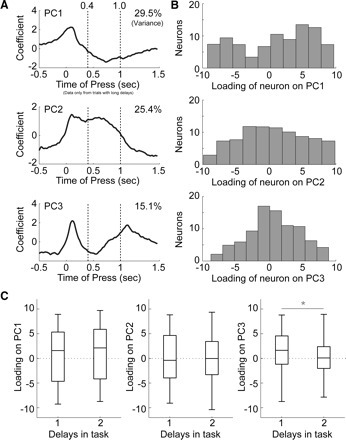
Analysis of firing patterns in motor cortex using PCA. A: eigenvectors are shown for the first 2 principal components relative to the time of lever press. PC1 accounted for 29.5% of variance and was modulated at the time of the lever press. PC2 accounted for 25.4% of variance and was modulated just before the lever press and then gradually was reduced in sign in the late delay period. PC3 accounted for 15.1% of variance and was sharply modulated around the press and release of the lever. B: histograms of the loadings of neurons onto the PCs showed that all 3 PCs had equally positive and negative loadings. C: boxplots show that loadings for PC1 and PC2 were equivalent for sessions with one- and two-delay periods. However, loadings for PC3 were significantly more positive in one-delay sessions compared with two-delay sessions (Wilcoxon rank-sum test, P < 0.0001). Note that all analyses are restricted to correct trials with a long delay.
FIG. 7.
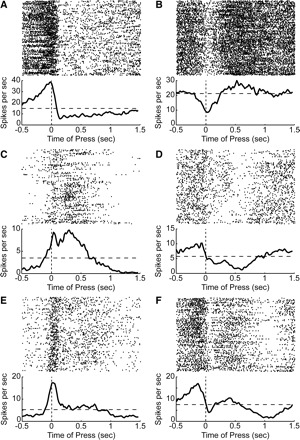
Examples of firing patterns in motor cortex. A: spike rasters and average perievent histograms (1-ms bins, smoothed with a 25-ms Gaussian) are shown for a neuron with one of the largest positive loadings (>3 standard units) on PC1. This neuron fired as the rat pressed the lever and then quickly came to fire at a lower rate. B: a neuron with one of the largest negative loadings (< −3 standard units) on PC1. This neuron fired at a reduced rate as the rat pressed the lever and then quickly came to fire at an elevated rate. C: a neuron with one of the largest positive loadings on PC2. This neuron fired at the time of the lever press and then fired persistently until just before the end of the delay period. D: a neuron with one of the largest negative loadings on PC2. This neuron fired at a reduced rate during the delay period and also during the lever press during the trials near the end of the session (bottom third of raster plot). E: a neuron with one of the largest positive loadings on PC3. This neuron fired at the time of the lever press and then at a lower rate during the rest of the trial. F: a neuron with one of the largest negative loadings on PC3. This neuron was strongly modulated at the time of the lever press and then again when the rat released the lever (after 1 s). All rasters depict correct trials with a long delay.
The second component (PC2) from the motor cortex accounted for 25.4% of variance. As in PC2 from dmPFC, this function changed at the time of the lever press and began to change in the opposite direction during the delay period (middle plot in Fig. 6A). The rate of change for PC2 from motor cortex was more gradual than the rate of change for PC2 from dmPFC and the change in rate continued through the end of the delay period. This component resembled the activity of many motor cortical neurons with sustained delay activity (Narayanan and Laubach 2006). As for PC1, there were equal numbers of neurons with positive and negative loadings on this component (middle plot in Fig. 6B; proportions test: P > 0.05) and the same range of loadings was found in sessions with one and two delay periods (right plot in Fig. 4C). Examples of neurons with a large loading on PC2 are shown in Fig. 7, C and D. As for PC1, the neurons were more sharply modulated than those found to weigh on PC2 in dmPFC.
The third component (PC3) from the motor cortex accounted for 15.1% of variance. This function was modulated around both lever events, i.e., press and release (bottom plot in Fig. 6A). This component resembled the activity of many movement-related neurons in the motor cortex (Narayanan and Laubach 2006). As for PC1 and PC2, there were equal numbers of neurons with positive and negative loadings on this component (bottom plot in Fig. 6B; proportions test: P > 0.05). However, this was the only component that showed a significant difference in loadings from neurons in sessions with one and two delay periods (Wilcoxon rank-sum test, P < 0.00001). Examples of neurons with a large loading on PC3 are shown in Fig. 7, E and F. Both of these neurons were modulated around lever pressing; however, other neurons were found that modulated around lever release (not shown). These data correspond to differences in firing rate modulation observed in motor cortex around lever events in Fig. 3, B and D.
Comparisons of the time course of the PCs derived from activity in dmPFC and motor cortex revealed that population activity in the two cortical regions changed at approximately the same times during the trial (Fig. 8). Moreover, the rates at which the PCs in each area changed over time were highly related. In both areas, plots of the cumulative sum of PC1 were highly similar to the time course of PC2 (correlation coefficient >0.9; Fig. 8, A and B). For all PCs, modulations occurred earlier in motor cortex compared with dmPFC (Fig. 8, C and D).
FIG. 8.
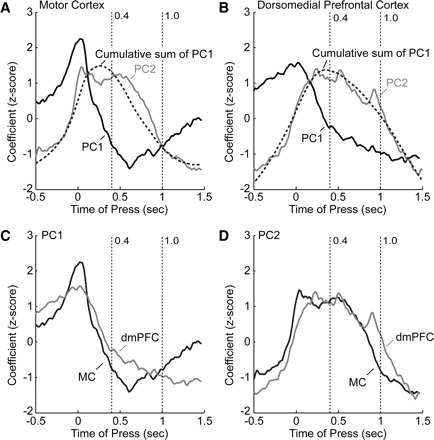
Comparison of firing patterns in motor cortex and dmPFC. A: eigenvectors for the first 2 PCs from motor cortex are shown as black and gray lines, respectively. In addition, the cumulative sum of PC1 is plotted as the dashed line and was highly similar to PC2, both in its time course and extent of modulation. B: eigenvectors for the first 2 PCs are shown from dmPFC. As in motor cortex, the cumulative sum of PC1 is plotted as the dashed line and was highly similar to PC2. In both areas, modulation of PC1 preceded modulation of PC2 and PC2 was subsequently “persistent.” C: a comparison of the time course of modulation in PC1 from motor cortex and dmPFC shows that both areas modulated at about the same time, just before the lever press. The modulation in motor cortex was sharper than that in dmPFC and decayed at a greater rate following the lever press. D: PC2 was similar from the 2 cortical areas, but the increase in PC2 from motor cortex preceded the increase in PC2 from dmPFC.
Stimulus-related activity in dmPFC
A subpopulation of dmPFC neurons was modulated around the trigger stimulus (Fig. 9, A and B). To determine whether these neurons fired to the stimulus or were modulated by other factors, such as response preparation, we recorded from 88 dmPFC in six rats performing a modified reaction time task (based on Kornblum 1973), in which stimuli occurred on half of the trials (on the other half of the trials, called time production trials, no stimulus occurred) and all delays were 1.0 s in duration. Rats were rewarded for responding at a latency of 1–1.6 s in these sessions regardless of whether a stimulus was presented. In these sessions, stimulus-driven firing was apparent for neurons that fired immediately after the onset of the stimulus and that did not fire on time production trials (i.e., when no stimulus was presented). Twelve such neurons (of 88, or 14%) were observed with significant stimulus-responsive activity (Wilcoxon rank-sum test, P < 0.05, comparing activity on trials with trigger stimuli to trials without for the epoch of 1.0 to 1.2 s after lever press). Each animal had at least one stimulus-responsive neuron (range: one to six neurons per animal). As in our previous study (Narayanan and Laubach 2006), we did not observe stimulus- responsive firing in the motor cortex.
FIG. 9.
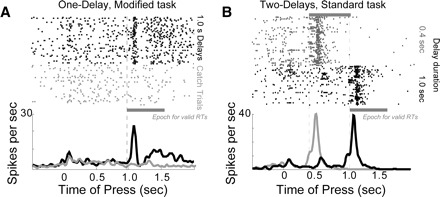
Stimulus-related activity in dmPFC.A: peristimulus rasters and histograms from a neuron with stimulus-related firing in dmPFC. Activity is shown for trials with a stimulus presented at a delay of 1.0 s (black) and for time production (or “catch”) trials (gray), on which the stimulus was not presented and rats were rewarded if they held the lever down for ≥1.0 s. Approximately 14% of neurons in dmPFC showed this pattern of firing and no neurons like this were found in motor cortex. B: activity from a dmPFC neuron in two-delay sessions that fired in response to the stimulus, similar to the neuron in A (0.4-s delays: gray; 1.0-s delays: black). This neuron also fired on some trials with long delays at the time of the short delay (black arrow) and these spikes are suggestive of stimulus-anticipatory activity. Note that all analyses are restricted to correct trials with a long delay.
DISCUSSION
In the present study, we described how neuronal activity in rodent frontal cortex is modulated during delay periods. We used principal component analysis to identify two prominent patterns of firing during the delay period: ramping activity and sustained delay activity. These patterns were common to both dmPFC and motor cortex and did not change as animals learned to respond at new delays. We also investigated whether delay period activity in rodent frontal cortex reflected proactive inhibition of responding or anticipation of forthcoming trigger stimuli. Neurons in dmPFC and motor cortex did not change after animals learned to respond at the novel delay, suggesting that frontal processing is involved in proactively inhibiting delay-related behavior and is not sensitive to the timing of stimuli.
Delay activity has been reported throughout the frontal cortex of primates, especially in premotor and prefrontal regions (Brody et al. 2003; Churchland and Shenoy 2007; Churchland et al. 2006; di Pellegrino and Wise 1991; Evarts et al. 1984; Mauritz and Wise 1986; Niki and Watanabe 1976b, 1979; Tanji and Evarts 1976). The present study, together with other recent reports (Baeg et al. 2001, 2003; Batuev et al. 1990; Cowen and McNaughton 2007; Narayanan and Laubach 2006), shows that nonmnemonic delay activity is also found in the rodent prefrontal cortex. These data support the hypothesis that across species, prefrontal regions are critical to the temporal organization of behavior (Fuster 1997), establishing that the capacity for frontal cortical neurons to fire in a persistent manner during delay periods is not a unique property of the primate prefrontal cortex.
Our results support previous lesion and inactivation studies (Broersen and Uylings 1999; Narayanan and Laubach 2006; Narayanan et al. 2006; Risterucci et al. 2003) that demonstrate that rodent medial prefrontal regions are critical to inhibiting premature responses and to maintaining delay-related behavior. Together, these studies suggest that dmPFC neural activity is involved in inhibiting temporally inappropriate responses until the end of the delay period (Narayanan and Laubach 2006; Narayanan et al. 2006; Ollman and Billington 1972). Our data are also convergent with neuroimaging studies in human subjects that have implicated medial frontal regions in the prepotent inhibition of impending actions (Lewis and Miall 2006; Onoe et al. 2001; Rao et al. 2001; Vallesi et al. 2007).
We found no evidence that dmPFC or the motor cortex is sensitive to the expected timing of the trigger stimulus. This kind of processing is assumed in many models of simple reaction time performance (Los 2004; Los and Van Den Heuvel 2001; Los et al. 2001). One reason for our not finding such “orienting” activity might be that our animals had limited experience with stimuli presented at short delays (they experienced stimuli at that time in only two behavioral sessions prior to our recordings), although 3 days of two-delay task performance were sufficient to speed reaction times. Another possibility is that we are limited by insufficient statistical power, although we did observe differences in motor cortex activity as animals learned to respond to new delays. Alternatively, it may be that temporal processing is represented by some other measure of activity in frontal cortex (e.g., through correlations between neurons or through correlations between spike trains and field potentials). Although anticipatory activity has been previously reported in rodent frontal cortex in a classical conditioning paradigm (Baeg et al. 2001), our data suggest that in an operant context, such activity does not change as animals learn to anticipate novel delays.
Analysis of neuronal population activity revealed two prominent patterns of firing during the delay period—ramping activity and sustained delay activity—that were found in both dmPFC and motor cortex. Ramping activity has been shown in several theoretical studies to be capable of representing the passage of time (Durstewitz 2003; Reutimann et al. 2004). For example, Durstewitz (2003) proposed that temporal control could be achieved through interactions between a population of neurons with ramping neural activity and a population of stimulus-modulated neurons. Ollman and Billington (1972) and Kornblum (1973) made similar suggestions based on data from human subjects performing simple reaction time tasks. It is possible that dynamic changes in network activity (Haider et al. 2006), and not in the activity of individual neurons, are critical to temporal preparation. Evidence for such interactions might be found through analysis of noise correlations (Averbeck and Lee 2003) between groups of simultaneously recorded neurons or through studies of interactions between spike and field activity (Donoghue et al. 1998).
A second major pattern among dmPFC neurons was sustained, persistent delay-related activity. It is possible that this pattern of activity is directly responsible for inhibiting lever-response motor programs during the delay period (Narayanan and Laubach 2006) via top-down control over responding according to task rules (Miller 2000), such as inhibiting responses until the longest delay at which a stimulus has been presented. Indeed, our study establishes that the medial frontal cortex has the necessary elements for implementing a “deadline model” (Ollman and Billington 1972) for controlling responding during the delay period.
We previously proposed a model for the top-down control of action (Narayanan and Laubach 2006), based on classic work on human simple reaction time performance by Ollman and Billington (1972) and Kornblum (1973). The model proposed that dmPFC serves to temporally inhibit responding during the delay period and does so by having access to information about temporal and sensory events in the task. Inhibitory control was proposed to be due to a decaying inhibition over action during the delay period. This inhibition would be reduced by “excitatory” signals related to the prepotent tendency to release the lever (PC3 from the motor cortex, Fig. 6) to obtain a reward and the presentation of the trigger stimulus (stimulus-responsive cells in Fig. 9). Based on neuronal data in the present study, on our previous recordings from rodent frontal cortex (Narayanan and Laubach 2006, 2008; Narayanan et al. 2005), and on recent studies in human subjects (Boulinguez et al. 2008; Jaffard et al. 2008; van Elswijk et al. 2008), we are now able to revise this model as shown in Fig. 10. Using terms from Jaffard et al. (2008), we propose that lever pressing triggers two competing processes, represented by two pools of neurons, that prepare for the forthcoming action (“the prepotent response”) and that inhibit action during the delay period (“the proactive inhibition”). These two pools of neurons would excite themselves and inhibit the other pool. Based on theoretical work by Wang et al. (2002), Reutimann et al. (2004), Machens et al. (2005), and others, such mutually inhibitory neuronal pools would allow for the accumulation of ongoing activity in the network. Such network interactions may arise through an accumulation of press-related activity (Fig. 8) and would be manifest as maintained persistent firing that includes cells in both dmPFC and motor cortex.
FIG. 10.
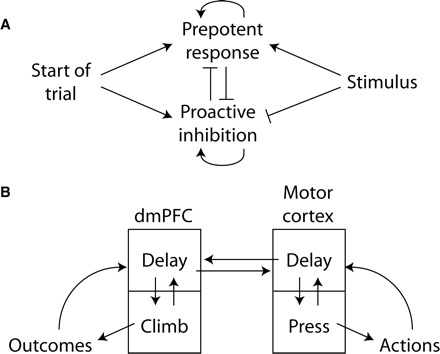
Conceptual model for top-down control over action by rodent frontal cortex. A: brain networks spanning rodent frontal cortex encode prepotent responses and proactive inhibition. This activity is terminated by the trigger stimulus, which facilitates rapid responding. When neurons mediating prepotent responding dominate, animals respond quickly and commit more premature errors. When neurons mediating proactive inhibition dominate, animals respond more slowly and make fewer premature errors. B: our data suggest a model of how neural activity within rodent frontal cortex interacts to control the timing of action. Ramping and sustained delay activity in rodent dorsomedial prefrontal cortex encodes the timing of rewards (Narayanan and Laubach 2008), whereas delay and pressing-related activity in motor cortex encodes the timing of actions (Laubach et al. 2000; Narayanan et al. 2005). Delay-related networks in rodent prefrontal cortex are correlated with and control delay-related activity in motor cortex (Narayanan and Laubach 2006).
Potential anatomical routes exist that could enable this kind of processing. Direct corticocortical connections have been reported between dmPFC and the motor cortex, although the connections are very sparse (Wang and Kurata 1998). Indirect corticocortical connections between dmPFC and motor cortex are also known to exist by way of the rostral forelimb area (Rouiller et al. 1993). Finally, interactions could arise through a thalamic route, for example, involving connections between dmPFC and the rostral forelimb areas by way of the medial dorsal nucleus (Conde et al. 1990).
Based on the present study and on Narayanan and Laubach (2006), we propose that there are functional interactions between dmPFC and motor cortex that are crucial for persistent delay activity. The present study (Fig. 8) suggests that population activity in both cortical areas is modulated at the lever press and that this change in network activity leads to lasting persistent activity during the delay period. In this view, persistent firing may be generated by the animal's own actions in the task.
Crucially, neurons in dmPFC are highly sensitive to the outcome of the current trial (Narayanan and Laubach 2006) and immediately preceding trial (Narayanan and Laubach 2008). As such, we suggest that the slow modulation of dmPFC population activity reflects the recent history of the animal's success in performing the simple reaction time task and, through integration with network activity in the motor cortex, delay-related activity in dmPFC allows animals to control their waiting behavior based on current goals and the recent history of task performance.
GRANTS
This work was supported by funds from the National Science Foundation, Kavli Institute at Yale, and the John B. Pierce Laboratory for M. Laubach and from the Army Research Office for N. S. Narayanan.
Acknowledgments
We thank G. Felsen, N. Horst, and J. Schall for helpful comments on an earlier version of this manuscript; X.-J. Wang, N. Smith, and two anonymous reviewers for comments on the present version of the manuscript; and personnel at the Instruments Shop at the John B. Pierce Laboratory for outstanding technical support.
REFERENCES
- Averbeck and Lee 2003.Averbeck BB, Lee D. Neural noise and movement-related codes in the macaque supplementary motor area. J Neurosci 23: 7630–7641, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baeg et al. 2003.Baeg EH, Kim YB, Huh K, Mook-Jung I, Kim HT, Jung MW. Dynamics of population code for working memory in the prefrontal cortex. Neuron 40: 177–188, 2003. [DOI] [PubMed] [Google Scholar]
- Baeg et al. 2001.Baeg EH, Kim YB, Jang J, Kim HT, Mook-Jung I, Jung MW. Fast spiking and regular spiking neural correlates of fear conditioning in the medial prefrontal cortex of the rat. Cereb Cortex 11: 441–451, 2001. [DOI] [PubMed] [Google Scholar]
- Batuev et al. 1990.Batuev AS, Kursina NP, Shutov AP. Unit activity of the medial wall of the frontal cortex during delayed performance in rats. Behav Brain Res 41: 95–102, 1990. [DOI] [PubMed] [Google Scholar]
- Botvinick and Cohen 2004.Botvinick MM, Cohen JD, Carter CS. Conflict monitoring and anterior cingulate cortex: an update. Trends Cogn Sci 8: 539–546, 2004. [DOI] [PubMed] [Google Scholar]
- Boulinguez et al. 2008.Boulinguez P, Jaffard M, Granjon L, Benraiss A. Warning signals induce automatic EMG activations and proactive volitional inhibition: evidence from analysis of error distribution in simple RT. J Neurophysiol 99: 1572–1578, 2008. [DOI] [PubMed] [Google Scholar]
- Brody et al. 2003.Brody CD, Hernández A, Zainos A, Romo R. Timing and neural encoding of somatosensory parametric working memory in macaque prefrontal cortex. Cereb Cortex 13: 1196–1207, 2003. [DOI] [PubMed] [Google Scholar]
- Broersen and Uylings 1999.Broersen LM, Uylings HB. Visual attention task performance in Wistar and Lister hooded rats: response inhibition deficits after medial prefrontal cortex lesions. Neuroscience 94: 47–57, 1999. [DOI] [PubMed] [Google Scholar]
- Brunia 1999.Brunia CH. Neural aspects of anticipatory behavior. Acta Psychol (Amst) 101: 213–242, 1999. [DOI] [PubMed] [Google Scholar]
- Churchland et al. 2006.Churchland MM, Santhanam G, Shenoy KV. Preparatory activity in premotor and motor cortex reflects the speed of the upcoming reach. J Neurophysiol 96: 3130–3146, 2006. [DOI] [PubMed] [Google Scholar]
- Churchland and Shenoy 2007.Churchland MM, Shenoy KV. Delay of movement caused by disruption of cortical preparatory activity. J Neurophysiol 97: 348–359, 2007. [DOI] [PubMed] [Google Scholar]
- Conde et al. 1990.Conde F, Audinat E, Maire-Lepoivre E, Crepel F. Afferent connections of the medial frontal cortex of the rat. A study using retrograde transport of fluorescent dyes. I. Thalamic afferents. Brain Res Bull 24: 341–354, 1990. [DOI] [PubMed] [Google Scholar]
- Cowen and McNaughton 2007.Cowen SL, McNaughton BL. Selective delay activity in the medial prefrontal cortex of the rat: contribution of sensorimotor information and contingency. J Neurophysiol 98: 303–316, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- di Pellegrino and Wise 1991.di Pellegrino G, Wise SP. A neurophysiological comparison of three distinct regions of the primate frontal lobe. Brain 114: 951–978, 1991. [DOI] [PubMed] [Google Scholar]
- Donoghue et al. 1998.Donoghue JP, Sanes JN, Hatsopoulos NG, Gaal G. Neural discharge and local field potential oscillations in primate motor cortex during voluntary movements. J Neurophysiol 79: 159–173, 1998. [DOI] [PubMed] [Google Scholar]
- Durstewitz 2003.Durstewitz D. Self-organizing neural integrator predicts interval times through climbing activity. J Neurosci 23: 5342–5353, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evarts et al. 1984.Evarts E, Shinoda Y, Wise SP. Neurophysiological Approaches to Higher Brain Functions. New York: Wiley, 1984.
- Fisk and Wyss 1999.Fisk GD, Wyss JM. Associational projections of the anterior midline cortex in the rat: intracingulate and retrosplenial connections. Brain Res 825: 1–13, 1999. [DOI] [PubMed] [Google Scholar]
- Fuster 1997.Fuster JM. The Prefrontal Cortex. New York: Raven Press, 1997.
- Gabbott et al. 2005.Gabbott PL, Warner TA, Jays PR, Salway P, Busby SJ. Prefrontal cortex in the rat: projections to subcortical autonomic, motor, and limbic centers. J Comp Neurol 492: 145–177, 2005. [DOI] [PubMed] [Google Scholar]
- Haider et al. 2006.Haider B, Duque A, Hasenstaub AR, McCormick DA. Neocortical network activity in vivo is generated through a dynamic balance of excitation and inhibition. J Neurosci 26: 4535–4545, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanakawa et al. 2008.Hanakawa T, Dimyan MA, Hallett M. Motor planning, imagery, and execution in the distributed motor network: a time-course study with functional MRI. Cereb Cortex 18: 2775–2788, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffard et al. 2008.Jaffard M, Longcamp M, Velay JL, Anton JL, Roth M, Nazarian B, Boulinguez P. Proactive inhibitory control of movement assessed by event-related fMRI. Neuroimage 42: 1196–1206, 2008. [DOI] [PubMed] [Google Scholar]
- Kornblum 1973.Kornblum S. Simple reaction time as a race between signal detection and time estimation: a paradigm and a model. Percept Psychophys 13: 108–112, 1973. [Google Scholar]
- Laubach et al. 2000.Laubach M, Wessberg J, Nicolelis MA. Cortical ensemble activity increasingly predicts behaviour outcomes during learning of a motor task. Nature 405: 567–571, 2000. [DOI] [PubMed] [Google Scholar]
- Lewis and Miall 2006.Lewis PA, Miall RC. A right hemispheric system for cognitive time measurement. Behav Process 71: 226–234, 2006. [DOI] [PubMed] [Google Scholar]
- Li et al. 2006.Li CS, Huang C, Constable RT, Sinha R. Imaging response inhibition in a stop-signal task: neural correlates independent of signal monitoring and post-response processing. J Neurosci 26: 186–192, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Los 2004.Los SA. Inhibition of return and nonspecific preparation: separable inhibitory control mechanisms in space and time. Percept Psychophys 66: 119–130, 2004. [DOI] [PubMed] [Google Scholar]
- Los et al. 2001.Los SA, Knol DL, Boers RM. The foreperiod effect revisited: conditioning as a basis for nonspecific preparation. Acta Psychol (Amst) 106: 121–145, 2001. [DOI] [PubMed] [Google Scholar]
- Los and Van Den Heuvel 2001.Los SA, Van Den Heuvel CE. Intentional and unintentional contributions to nonspecific preparation during reaction time foreperiods. J ExpPsychol Hum Percept Perform 27: 370–386, 2001. [DOI] [PubMed] [Google Scholar]
- Machens et al. 2005.Machens CK, Romo R, Brody CD. Flexible control of mutual inhibition: a neural model of two-interval discrimination. Science 307: 1121–1124, 2005. [DOI] [PubMed] [Google Scholar]
- Martin et al. 1993.Martin JH, Cooper SE, Ghez C. Differential effects of local inactivation within motor cortex and red nucleus on performance of an elbow task in the cat. Exp Brain Res 94: 418–428, 1993. [DOI] [PubMed] [Google Scholar]
- Matsumura et al. 1991.Matsumura M, Sawaguchi T, Oishi T, Ueki K, Kubota K. Behavioral deficits induced by local injection of bicuculline and muscimol into the primate motor and premotor cortex. J Neurophysiol 65: 1542–1553, 1991. [DOI] [PubMed] [Google Scholar]
- Mauritz and Wise 1986.Mauritz KH, Wise SP. Premotor cortex of the rhesus monkey: neuronal activity in anticipation of predictable environmental events. Exp Brain Res 61: 229–244, 1986. [DOI] [PubMed] [Google Scholar]
- Miller 2000.Miller EK. The prefrontal cortex and cognitive control. Nat Rev Neurosci 1: 59–65, 2000. [DOI] [PubMed] [Google Scholar]
- Muir et al. 1996.Muir JL, Everitt BJ, Robbins TW. The cerebral cortex of the rat and visual attentional function: dissociable effects of mediofrontal, cingulate, anterior dorsolateral, and parietal cortex lesions on a five-choice serial reaction time task. Cereb Cortex 6: 470–481, 1996. [DOI] [PubMed] [Google Scholar]
- Naatanen 1971.Naatanen R. Non-aging fore-periods and simple reaction time. Acta Psychol 35: 316–327, 1971. [Google Scholar]
- Naatanen 1972.Naatanen R. Time uncertainty and occurrence uncertainty of the stimulus in a simple reaction time task. Acta Psychol 36: 492–503, 1972. [Google Scholar]
- Naito et al. 2000.Naito E, Kinomura S, Geyer S, Kawashima R, Roland PE, Zilles K. Fast reaction to different sensory modalities activates common fields in the motor areas, but the anterior cingulate cortex is involved in the speed of reaction. J Neurophysiol 83: 1701–1709, 2000. [DOI] [PubMed] [Google Scholar]
- Narayanan et al. 2006.Narayanan NS, Horst NK, Laubach M. Reversible inactivations of rat medial prefrontal cortex impair the ability to wait for a stimulus. Neuroscience 139: 865–876, 2006. [DOI] [PubMed] [Google Scholar]
- Narayanan et al. 2005.Narayanan NS, Kimchi EY, Laubach M. Redundancy and synergy of neuronal ensembles in motor cortex. J Neurosci 25: 4207–4216, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan and Laubach 2006.Narayanan NS, Laubach M. Top-down control of motor cortex ensembles by dorsomedial prefrontal cortex. Neuron 52: 921–931, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan and Laubach 2008.Narayanan NS, Laubach M. Neuronal correlates of post-error slowing in the rat dorsomedial prefrontal cortex. J Neurophysiol 100: 520–525, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan and Laubach 2009.Narayanan NS, Laubach M. Methods for studying functional interactions among neuronal populations. Methods Mol Biol 489: 135–165, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niki and Watanabe 1976a.Niki H, Watanabe M. Prefrontal unit activity and delayed response: relation to cue location versus direction of response. Brain Res 105: 79–88, 1976a. [DOI] [PubMed] [Google Scholar]
- Niki and Watanabe 1976b.Niki H, Watanabe M. Cingulate unit activity and delayed response. Brain Res 110: 381–386, 1976b. [DOI] [PubMed] [Google Scholar]
- Niki and Watanabe 1979.Niki H, Watanabe M. Prefrontal and cingulate unit activity during timing behavior in the monkey. Brain Res 171: 213–224, 1979. [DOI] [PubMed] [Google Scholar]
- Nobre et al. 2001.Nobre AC, Coelho GR, Coutinho MC, Silva MM, Angelim EV, Menezes DB, Fonteles MC, Monteiro HS. The role of phospholipase A(2) and cyclooxygenase in renal toxicity induced by microcystin-LR. Toxicon 39: 721–724, 2001. [DOI] [PubMed] [Google Scholar]
- Ollman and Billington 1972.Ollman RT, Billington MJ. The deadline model for simple reaction times. Cogn Psychol 3: 311–336, 1972. [Google Scholar]
- Onoe et al. 2001.Onoe H, Komori M, Onoe K, Takechi H, Tsukada H, Watanabe Y. Cortical networks recruited for time perception: a monkey positron emission tomography (PET) study. Neuroimage 13: 37–45, 2001. [DOI] [PubMed] [Google Scholar]
- Paz et al. 2005.Paz R, Natan C, Boraud T, Bergman H, Vaadia E. Emerging patterns of neuronal responses in supplementary and primary motor areas during sensorimotor adaptation. J Neurosci 25: 10941–10951, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picton et al. 2007.Picton TW, Stuss DT, Alexander MP, Shallice T, Binns MA, Gillingham S. Effects of focal frontal lesions on response inhibition. Cereb Cortex 17: 826–838, 2007. [DOI] [PubMed] [Google Scholar]
- Picton et al. 2006.Picton TW, Stuss DT, Shallice T, Alexander MP, Gillingham S. Keeping time: effects of focal frontal lesions. Neuropsychologia 44: 1195–1209, 2006. [DOI] [PubMed] [Google Scholar]
- Rao et al. 2001.Rao SM, Mayer AR, Harrington DL. The evolution of brain activation during temporal processing. Nat Neurosci 4: 317–323, 2001. [DOI] [PubMed] [Google Scholar]
- Reutimann et al. 2004.Reutimann J, Yakovlev V, Fusi S, Senn W. Climbing neuronal activity as an event-based cortical representation of time. J Neurosci 24: 3295–3303, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyment and Joreskog 1996.Reyment R, Joreskog KG. Applied Factor Analysis in the Natural Sciences. Cambridge, UK: Cambridge Univ. Press, 1996.
- Risterucci et al. 2003.Risterucci C, Terramorsi D, Nieoullon A, Amalric M. Excitotoxic lesions of the prelimbic-infralimbic areas of the rodent prefrontal cortex disrupt motor preparatory processes. Eur J Neurosci 17: 1498–1508, 2003. [DOI] [PubMed] [Google Scholar]
- Rouiller et al. 1993.Rouiller EM, Moret V, Liang F. Comparison of the connectional properties of the two forelimb areas of the rat sensorimotor cortex: support for the presence of a premotor or supplementary motor cortical area. Somatosens Mot Res 10: 269–289, 1993. [DOI] [PubMed] [Google Scholar]
- Rushworth et al. 2004.Rushworth MF, Walton ME, Kennerley SW, Bannerman DM. Action sets and decisions in the medial frontal cortex. Trends Cogn Sci 8: 410–417, 2004. [DOI] [PubMed] [Google Scholar]
- Schall et al. 2002.Schall JD, Stuphorn V, Brown JW. Monitoring and control of action by the frontal lobes. Neuron 36: 309–322, 2002. [DOI] [PubMed] [Google Scholar]
- Sesack et al. 1989.Sesack SR, Deutch AY, Roth RH, Bunney BS. Topographical organization of the efferent projections of the medial prefrontal cortex in the rat: an anterograde tract-tracing study with Phaseolus vulgaris leucoagglutinin. J Comp Neurol 290: 213–242, 1989. [DOI] [PubMed] [Google Scholar]
- Stuphorn and Schall 2006.Stuphorn V, Schall JD. Executive control of countermanding saccades by the supplementary eye field. Nat Neurosci 9: 925–931, 2006. [DOI] [PubMed] [Google Scholar]
- Tanji and Evarts 1976.Tanji J, Evarts EV. Anticipatory activity of motor cortex neurons in relation to direction of an intended movement. J Neurophysiol 39: 1062–1068, 1976. [DOI] [PubMed] [Google Scholar]
- Vallesi et al. 2007.Vallesi A, Shallice T, Walsh V. Role of the prefrontal cortex in the foreperiod effect: TMS evidence for dual mechanisms in temporal preparation. Cereb Cortex 17: 466–474, 2007. [DOI] [PubMed] [Google Scholar]
- van Elswijk et al. 2008.van Elswijk G, Schot WD, Stegeman DF, Overeem S. Changes in corticospinal excitability and the direction of evoked movements during motor preparation: a TMS study. BMC Neurosci 9: 51, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang 2002.Wang XJ. Probabilistic decision making by slow reverberation in cortical circuits. Neuron 36: 955–968, 2002. [DOI] [PubMed] [Google Scholar]
- Wang and Kurata 1998.Wang Y, Kurata K. Quantitative analyses of thalamic and cortical origins of neurons projecting to the rostral and caudal forelimb motor areas in the cerebral cortex of rats. Brain Res 781: 135–147, 1998. [DOI] [PubMed] [Google Scholar]


