Abstract
To better understand the mechanisms responsible for exercise intolerance in heart failure with reduced ejection fraction (HFrEF), the present study sought to evaluate the hemodynamic responses to small muscle mass exercise in this cohort. In 25 HFrEF patients (64 ± 2 yr) and 17 healthy, age-matched control subjects (64 ± 2 yr), mean arterial pressure (MAP), cardiac output (CO), and limb blood flow were examined during graded static-intermittent handgrip (HG) and dynamic single-leg knee-extensor (KE) exercise. During HG exercise, MAP increased similarly between groups. CO increased significantly (+1.3 ± 0.3 l/min) in the control group, but it remained unchanged across workloads in HFrEF patients. At 15% maximum voluntary contraction (MVC), forearm blood flow was similar between groups, while HFrEF patients exhibited an attenuated increase at the two highest intensities compared with controls, with the greatest difference at the highest workload (352 ± 22 vs. 492 ± 48 ml/min, HFrEF vs. control, 45% MVC). During KE exercise, MAP and CO increased similarly across work rates between groups. However, HFrEF patients exhibited a diminished leg hyperemic response across all work rates, with the most substantial decrement at the highest intensity (1,842 ± 64 vs. 2,675 ± 81 ml/min; HFrEF vs. control, 15 W). Together, these findings indicate a marked attenuation in exercising limb perfusion attributable to impairments in peripheral vasodilatory capacity during both arm and leg exercise in patients with HFrEF, which likely plays a role in limiting exercise capacity in this patient population.
heart failure with reduced ejection fraction (HFrEF) is associated with debilitating dyspnea and fatigue triggered by exercise, leading to a limited ability to perform everyday tasks and an impaired quality of life (53). Although central cardiac limitations are the paramount characteristic of HFrEF, impaired cardiac function does not fully explain the degree of exercise intolerance and symptom status in this patient population (8, 22, 35, 54, 61). This has steered studies focusing on exercise limitations in HFrEF toward potentially limiting factors in the periphery, with particular emphasis on disease-related changes in the regulation of skeletal muscle blood flow. Initial studies examining the hyperemic response to exercise in the skeletal muscle vasculature of HFrEF patients and healthy individuals utilized cycle ergometry, a large muscle mass exercise paradigm, which results in the recruitment of the vast majority of lower limb muscle. These studies observed a marked reduction in blood flow, associated with an attenuation in leg vascular conductance, in HFrEF patients during exercise compared with their healthy counterparts (53, 63, 65–68). However, because of the cardiac limitations associated with HFrEF, the engagement of such a large muscle mass during exercise may have outstripped cardiac pumping capacity (31), likely contributing to the observed attenuation in leg blood flow and vascular conductance in this patient population.
These initial studies utilizing cycle ergometry led to the recognition that skeletal muscle hyperemic and vasodilatory capacity in HFrEF might be better studied by utilizing an exercise paradigm employing a smaller muscle mass, to control for cardiac limitations. In an animal model of heart failure, this was accomplished by utilizing an in situ spinotrapezius muscle preparation and investigating capillary red blood cell flux, which is indicative of capillary blood flow, during electrically elicited muscle contractions (47). Results from this study convincingly displayed an attenuation in capillary red blood cell flux in HF animals compared with control animals. In human HF, the two most widely incorporated exercise modalities to study the peripheral hemodynamic responses to exercise while controlling for confounding cardiac limitations are static-intermittent handgrip (HG) and dynamic single-leg knee-extensor (KE) exercise. Despite the implementation of these exercise modalities in a small number of studies, results have not definitively determined the extent of peripheral hemodynamic limitations in this cohort. Indeed, studies using static-intermittent HG exercise have documented both similar (51) and blunted (23, 70) hyperemic responses, linked to impairments in vasodilation, in HFrEF patients compared with healthy individuals, differences likely attributable to the variations in rhythmicity, duration, and intensity of the exercise performed. Using the dynamic single-leg KE exercise model, Esposito et al. (15) identified an attenuated hyperemia during maximal effort in HFrEF patients, a response that was likely due to the significantly lower maximal KE work rate in the patient group compared with healthy individuals. In one of the only studies to use KE exercise at submaximal work rates in HFrEF patients, Magnusson et al. (31) reported similar increases in leg blood flow and vascular conductance between HFrEF and healthy individuals, suggesting that impaired peripheral hemodynamics may not contribute to the exercise intolerance in this cohort. Thus, significant controversy remains regarding the HFrEF-associated alteration of peripheral hemodynamics during exercise modalities that minimally challenge central hemodynamics.
It is noteworthy that significant advances in pharmacotherapy have been made since these previous small muscle mass exercise studies were performed (20, 21, 28, 56). Indeed, the average HFrEF patient now receives a pharmacological regimen that includes an average of 10 medications, with β-blockers and a host of peripheral vasodilators now included as a standard of care (46). Thus, while exercise intolerance remains a key clinical presentation in HFrEF, uncertainty exists regarding the possible role of impaired peripheral hemodynamics in patients who are optimally medicated in this “modern era” of differentiated drug treatment.
Consequently, we sought to systematically examine peripheral responses to small muscle mass exercise in optimally medicated HFrEF patients. To comprehensively assess the hemodynamic response to small muscle mass exercise in HFrEF patients and healthy age-matched control subjects, we utilized both upper and lower limb exercise paradigms across a wide range of intensities. We hypothesized that compared with healthy controls, HFrEF patients would exhibit an attenuated hyperemic response driven by an impaired vasodilatory capacity during both static-intermittent HG and single-leg KE exercise.
METHODS
Subjects
A total of 25 New York Heart Association class II-III HFrEF patients (24 males and 1 female) and 17 healthy, age-matched controls (16 males and 1 female) were recruited to partake in this study either by word of mouth or in the HF clinics at the University of Utah and the Salt Lake City VA Medical Center. All age-matched control subjects were nonsmokers, normally active, not taking any prescription medication, and were free of overt cardiovascular disease, as indicated by a health history questionnaire. Protocol approval and written informed consent were obtained according to University of Utah and Salt Lake City Veterans Affairs Medical Center Institutional Review Board requirements. All data collection took place at the Utah Vascular Research Laboratory located at the Veterans Affairs Salt Lake City Geriatric, Research, Education, and Clinical Center. All studies were performed in a thermoneutral environment, with subjects reporting to the laboratory fasted, and not having performed any exercise within 24 h of the study. Subjects reported to the laboratory on a preliminary day to complete health histories and physical examinations and perform a graded single-leg, knee-extensor test to determine maximal work rate.
Handgrip Exercise
Hemodynamic responses to static-intermittent HG exercise were assessed in 15 control subjects and 23 patients. Subjects rested in the supine position for ≈20 min prior to the start of data collection with the right arm abducted at 90°. The elbow joint was extended at heart level to allow subjects to perform HG exercise. First, maximal voluntary contraction (MVC) was established by taking the highest value recorded of three maximal contractions using a handgrip dynamometer (Biopac Systems, Goleta, CA). Static-intermittent HG exercise was performed at three workloads based on each subject's respective MVC (15, 30, and 45% of MVC). Each exercise level was performed for 3 min to ensure the attainment of steady-state hemodynamics. The subjects squeezed the dynamometer to the sound of a metronome at a rate of 1 Hz, and real-time force output was displayed to provide visual feedback to the subjects. A 5-min recovery period was given between each exercise bout.
Single-Leg Knee-Extensor Exercise
Hemodynamic responses to dynamic single-leg KE exercise were assessed in 16 controls and 16 patients. The KE paradigm implemented in this study has been described in detail previously (3, 7, 27, 46). Briefly, subjects were seated in a semirecumbent position on an adjustable chair with a cycle ergometer (model 828E; Monark Exercise AB, Vansbro, Sweden) positioned behind them. Resistance was created by applying friction to the flywheel, which was turned by the subject via a metal bar connecting the crank arm of the ergometer to a metal boot in which the subject's foot was placed. Subjects exercised for 3 min at four work rates (0, 5, 10, and 15 W), while maintaining 60 contractions/min. A 5-min recovery period was given between each exercise bout.
Measurements
Ultrasound Doppler assessments.
Measurements of brachial and common femoral artery blood velocity and vessel diameter were performed using a Logiq 7 ultrasound Doppler system (GE Medical Systems, Milwaukee, WI) operating in duplex mode. The Logiq 7 is equipped with a linear array transducer operating at a Doppler frequency of 5 MHz in high-pulsed repetition frequency mode (2–25 kHz). All blood velocity measurements were obtained with the probe appropriately positioned to maintain an insonation angle of 60° or less. The sample volume was maximized according to vessel size and centered within the vessel based on real-time ultrasound visualization. Mean velocity values (angle-corrected, and intensity weighted area under the curve) were automatically calculated using commercially available software (Logiq 7). Vessel diameter was obtained during end diastole (corresponding to an R wave documented by the simultaneous ECG signal; Logiq 7) using the same transducer at an imaging frequency ranging from 9 to 14 MHz. The brachial artery of the right arm was insonated approximately midway between the antecubital and axillary regions, medial to the biceps brachii muscle, while the common femoral artery was insonated 2–3 cm proximal to the bifurcation of the common femoral artery into the superficial and deep branches. Vessel diameter was determined at a perpendicular angle along the central axis of the scanned area. Analysis of brachial artery diameter was performed using off-line automatic edge-detection brachial analyzer software (Medical Imaging Applications, LLC, Coralville, IA), which is described in detail elsewhere (40). Ultrasound Doppler measurements were performed continuously, with the last 60 s of each exercise intensity used for the determination of limb blood flow. Using arterial diameter and Vmean, forearm and leg blood flow were calculated: limb blood flow (ml/min) = (Vmean × π (arterial diameter/2)2 × 60).
Hemodynamic variables.
Stroke volume (SV), arterial blood pressure (ABP), and heart rate (HR) were determined noninvasively. SV was calculated using the Modelflow method, which includes age, sex, height, and weight in its algorithm (Beatscope version 1.1; Finapres Medical Systems BV, Amsterdam, The Netherlands) (9) and has been documented to accurately track SV during a variety of experimental protocols, including exercise (13, 14, 52). ABP was measured continuously via photoplethysmography (Finometer, Finapres Medical Systems BV, Amsterdam, The Netherlands), and mean arterial pressure (MAP) was calculated as MAP (mmHg) = diastolic arterial pressure + (pulse pressure × 0.33). Heart rate was monitored from a standard three-lead electrocardiogram recorded in duplicate on the data acquisition system (Biopac, Goleta, CA) and the Logiq 7. Cardiac output (CO) was calculated as CO (l/min) = SV × HR. Systemic vascular conductance (SVC) was calculated as SVC (ml·min−1·mmHg−1) = CO/MAP. Systemic vascular resistance (SVR) was calculated as SVR (mmHg−1·l−1·min) = MAP/CO. Forearm and leg vascular conductance were calculated as the following: Limb vascular conductance (ml·min−1·mmHg−1) = blood flow/MAP.
Statistical Analyses
Statistics were performed using commercially available software (SigmaStat 3.10; Systat Software, Point Richmond, CA). For HG exercise, 2 × 4 repeated-measures ANOVA (α < 0.05) (group, two levels: age-matched controls vs. HFrEF) (workload, 4 levels: rest, 15, 30, and 45% of MVC) were performed to determine the hemodynamic responses in age-matched controls and HFrEF during exercise of increasing intensity. For KE, 2 × 5 repeated-measures ANOVA (α < 0.05) (group, 2 levels: age-matched controls vs. HFrEF) (work rate, 5 levels: rest, 0, 5, 10, 15 W) were utilized to determine the hemodynamic responses in age-matched controls and HFrEF during exercise of increasing intensity. The Holm-Sidak method was used for alpha adjustment and post hoc analysis. A Person Product Moment Correlation (α < 0.05) was performed to evaluate the association between leg blood flow at 15 W and maximum KE work rate. All group data are expressed as means ± SE.
RESULTS
Subject Characteristics
Baseline characteristics of the control subjects and HFrEF patients are displayed in Table 1. Disease-specific characteristics and medications of patients with HFrEF are presented in Table 2.
Table 1.
Subject characteristics
| Control (n = 17) | HFrEF (n = 25) | |
|---|---|---|
| Age, yr | 64 ± 2 | 64 ± 2 |
| Height, cm | 177 ± 2 | 171 ± 3 |
| Weight, kg | 87 ± 7 | 85 ± 3 |
| Body mass index, kg/m2 | 25 ± 1 | 28 ± 1 |
| Systolic blood pressure, mmHg | 120 ± 3 | 117 ± 4 |
| Diastolic blood pressure, mmHg | 70 ± 2 | 67 ± 3 |
| Maximum voluntary contraction, kg | 27 ± 2 (n = 15) | 25 ± 2 (n = 23) |
| Knee-extensor maximum, W | 35 ± 2 (n = 16) | 19 ± 1 (n = 16)* |
| Glucose, mg/dl | 85 ± 5 | 99 ± 4* |
| Total cholesterol, mg/dl | 188 ± 12 | 151 ± 10* |
| Triglycerides, mg/dl | 130 ± 24 | 131 ± 11 |
| HDL, mg/dl | 48 ± 3 | 38 ± 2* |
| LDL, mg/dl | 123 ± 9 | 93 ± 7* |
Data are expressed as means ± SE. HFrEF, heart failure with reduced ejection fraction; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
Significant difference from control, P < 0.05.
Table 2.
Disease-specific characteristics and medications
| HFrEF (n = 25) | |
|---|---|
| Disease-specific characteristics | |
| Left ventricular ejection fraction, % | 23 ± 2 |
| Diagnosis (ischemic) | 16/25 |
| Diagnosis (nonischemic) | 9/25 |
| NYHA class II | 18/25 |
| NYHA class III | 7/25 |
| Diabetic | 5/25 |
| Medications | |
| β-Blocker | 25/25 |
| ACE inhibitor | 19/25 |
| Angiotensin receptor inhibitor | 4/25 |
| Statin | 20/25 |
| Diuretic | 20/25 |
| Aldosterone inhibitor | 4/25 |
| Calcium channel inhibitor | 1/25 |
| Digoxin | 4/25 |
| Anticoagulant | 15/25 |
| Antiarrhythmic | 1/25 |
| Erythropoiesis-stimulating agent | 1/25 |
HFrEF, heart failure with reduced ejection fraction; NYHA, New York Heart Association; ACE, angiotensin-converting enzyme.
Handgrip Exercise
During baseline, prior to HG exercise, there were no significant differences in resting MAP, forearm blood flow, forearm vascular conductance, brachial artery diameter, or CO between groups (Fig. 1 and Table 3). However, resting HR was significantly higher and SV was significantly lower in HFrEF patients compared with controls (Table 3).
Fig. 1.
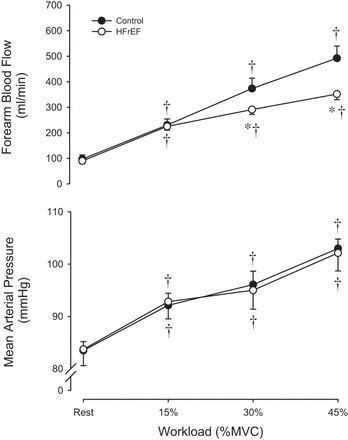
Forearm blood flow (top) and mean arterial pressure (bottom) at rest and during static-intermittent handgrip exercise. *Significant difference from control, P < 0.05. †Significant difference from rest, P < 0.05.
Table 3.
Central and peripheral hemodynamics at rest and during handgrip exercise
| Workload, % MVC | Rest | 15% | 30% | 45% |
|---|---|---|---|---|
| Control | ||||
| Forearm vascular conductance, ml·min−1·mmHg−1 | 1.1 ± 0.2 | 2.5 ± 0.3† | 3.8 ± 0.4† | 4.8 ± 0.4† |
| Brachial artery diameter, cm | 0.47 ± 0.02 | 0.48 ± 0.02† | 0.51 ± 0.02† | 0.52 ± 0.02† |
| Brachial artery blood velocity, cm/s | 9 ± 1 | 20 ± 1† | 30 ± 2† | 37 ± 2† |
| Heart rate, beats/min | 57 ± 2 | 64 ± 2† | 65 ± 2† | 68 ± 3† |
| Stroke volume, ml/beat | 109 ± 5 | 110 ± 5 | 111 ± 4 | 110 ± 4 |
| Cardiac output, l/min | 6.3 ± 0.3 | 7.0 ± 0.4† | 7.2 ± 0.4† | 7.4 ± 0.4† |
| Systemic vascular conductance, ml·min−1·mmHg−1 | 75 ± 4 | 77 ± 5 | 75 ± 4 | 72 ± 4 |
| Systemic vascular resistance, mmHg·l−1·min | 14 ± 1 | 14 ± 1 | 14 ± 1 | 14 ± 1 |
| HFrEF | ||||
| Forearm vascular conductance, ml·min−1·mmHg−1 | 1.1 ± 0.2 | 2.5 ± 0.2† | 3.2 ± 0.3† | 3.6 ± 0.3*† |
| Brachial artery diameter, cm | 0.47 ± 0.02 | 0.48 ± 0.02 | 0.48 ± 0.01† | 0.49 ± 0.01† |
| Brachial artery blood velocity, cm/s | 8 ± 1 | 21 ± 1† | 27 ± 1† | 31 ± 1*† |
| Heart rate, beats/min | 67 ± 2* | 69 ± 3† | 71 ± 2† | 73 ± 3† |
| Stroke volume, ml/beat | 83 ± 4* | 81 ± 4* | 79 ± 4* | 76 ± 4* |
| Cardiac output, l/min | 5.4 ± 0.3 | 5.5 ± 0.2* | 5.6 ± 0.3* | 5.4 ± 0.3* |
| Systemic vascular conductance, ml·min−1·mmHg−1 | 68 ± 4 | 62 ± 3*† | 61 ± 4*† | 55 ± 3*† |
| Systemic vascular resistance, mmHg·l−1·min | 17 ± 2 | 18 ± 2† | 20 ± 3*† | 21 ± 2*† |
Data are expressed as means ± SE.
MVC, maximum voluntary contraction.
Significant difference from control, P < 0.05.
Significant difference from rest, P < 0.05.
In both groups, MAP and HR increased from resting values in an exercise intensity-dependent manner, with no difference between groups (Fig. 1 and Table 3). CO increased from rest at all exercise intensities in the control group, but remained unchanged in the HFrEF patients (Table 3). This significant difference in the CO response in HFrEF patients appears to be the result of a tendency for decreased SV across workloads, although this reduction did not reach statistical significance (Table 3). During HG exercise, SVC decreased and SVR increased in HFrEF patients, but remained unchanged in the control group (Table 3).
At the lowest intensity (15% MVC), forearm blood flow and vascular conductance increased to a similar degree between groups; however, at the higher workloads (30 and 45% MVC), HFrEF patients exhibited significantly lower forearm blood flow (30 and 45% MVC) and vascular conductance (45% MVC) compared with controls (Fig. 1 and Table 3).
Single-Leg Knee-Extensor Exercise
During the baseline period prior to KE exercise, there were no significant differences in any indices of central or peripheral hemodynamics between groups (Fig. 2 and Table 4). In both groups, MAP and CO increased from resting values in an exercise intensity-dependent manner, with no differences between groups (Fig. 2 and Table 4). HR also increased in an intensity-dependent manner, with a significantly elevated HR in HFrEF patients compared with controls (5, 10, and 15 W) (Table 4). SV, SVC, and SVR remained unchanged in both groups across all KE exercise intensities (Table 4). In contrast to HG exercise, both leg blood flow and leg vascular conductance were markedly reduced at all exercise intensities in HFrEF patients compared with controls (Fig. 2 and Table 4), with the greatest reduction (25–35%) present at the highest exercise intensity (15 W). Additionally, there was a strong positive association, between, leg blood flow at 15 W and the maximum KE work rate in HFrEF, which was not apparent in the control group (Fig. 3).
Fig. 2.

Leg blood flow (top) and mean arterial pressure (bottom) at rest and during dynamic single-leg knee-extensor exercise. *Significant difference from control, P < 0.05 †Significant difference from rest, P < 0.05.
Table 4.
Central and peripheral hemodynamics at rest and during knee-extensor exercise
| Work rate (W) | Rest | 0 | 5 | 10 | 15 |
|---|---|---|---|---|---|
| Control | |||||
| Leg vascular conductance, ml·min−1·mmHg−1 | 3.5 ± 0.3 | 17.2 ± 0.7† | 20.6 ± 0.8† | 24.1 ± 0.9† | 26.8 ± 1.1† |
| Common femoral artery diameter, cm | 1.05 ± 0.03 | 1.05 ± 0.03 | 1.05 ± 0.03 | 1.05 ± 0.03 | 1.05 ± 0.03 |
| Common femoral artery blood velocity, cm/s | 6 ± 1 | 32 ± 2† | 34 ± 2† | 46 ± 3† | 53 ± 3† |
| Heart rate, beats/min | 64 ± 2 | 72 ± 2† | 74 ± 2† | 76 ± 2† | 78 ± 2† |
| Stroke volume, ml/beat | 88 ± 3 | 92 ± 4 | 95 ± 5 | 93 ± 6 | 97 ± 4 |
| Cardiac output, l/min | 5.5 ± 0.2 | 6.6 ± 0.3† | 7.0 ± 0.3† | 7.0 ± 0.4† | 7.5 ± 0.3† |
| Systemic vascular conductance, ml·min−1·mmHg−1 | 67 ± 2 | 71 ± 3 | 75 ± 4 | 73 ± 5 | 75 ± 3 |
| Systemic vascular resistance, mmHg·l−1·min | 15 ± 1 | 14 ± 1 | 14 ± 1 | 15 ± 1 | 14 ± 1 |
| HFrEF | |||||
| Leg vascular conductance, ml·min−1·mmHg | 2.7 ± 0.2 | 12.6 ± 0.8*† | 15.7 ± 0.7*† | 17.4 ± 0.8*† | 17.6 ± 0.7*† |
| Common femoral artery diameter, cm | 0.96 ± 0.03* | 0.96 ± 0.03* | 0.96 ± 0.03* | 0.96 ± 0.03* | 0.96 ± 0.03* |
| Common femoral artery blood velocity, cm/s | 6 ± 1 | 30 ± 3† | 36 ± 3† | 41 ± 4† | 43 ± 3† |
| Heart rate, beats/min | 68 ± 2 | 79 ± 4† | 85 ± 5*† | 89 ± 5*† | 94 ± 4*† |
| Stroke volume, ml/beat | 82 ± 9 | 92 ± 12 | 86 ± 11 | 83 ± 10 | 82 ± 10 |
| Cardiac output, l/min | 5.4 ± 0.5 | 6.8 ± 0.6† | 7.0 ± 0.8† | 7.0 ± 0.8† | 7.3 ± 0.7† |
| Systemic vascular conductance, ml·min−1·mmHg | 65 ± 6 | 70 ± 7 | 72 ± 8 | 72 ± 8 | 69 ± 6 |
| Systemic vascular resistance, mmHg·l−1·min | 19 ± 3 | 16 ± 2 | 18 ± 3 | 17 ± 2 | 17 ± 2 |
HFrEF, heart failure with reduced ejection fraction. Data are expressed as means ± SE.
Significant difference from control, P < 0.05.
Significant difference from rest, P < 0.05.
Fig. 3.
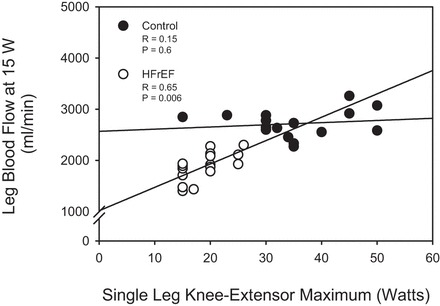
Relationship between leg blood flow at 15 W and maximal single-leg knee-extensor work rate. Significant Pearson Product Moment Correlation coefficient, P < 0.05.
DISCUSSION
This study sought to comprehensively evaluate the hemodynamic responses induced by limb-specific, small muscle mass exercise across a wide range of exercise intensities in HFrEF patients and healthy, age-matched controls. During HG exercise, a divergent hemodynamic response was observed across HG intensities in the HFrEF group. Specifically, both groups exhibited a similar forearm hyperemic and vasodilatory response during lower-intensity (15% MVC) HG exercise, but HFrEF patients exhibited a 15–25% attenuation in forearm blood flow at higher intensities (30 and 45% MVC), due to an impaired vasodilatory capacity. During KE exercise, HFrEF patients exhibited a 20–35% lower leg blood flow and vascular conductance during KE exercise compared with control subjects, with the most substantial decrements at the highest exercise intensity (15 W). Together, these findings indicate that HFrEF patients on modern, optimized pharmacotherapy exhibit a severely compromised ability to vasodilate vasculature of both the upper and lower limbs, thus restricting perfusion of the exercising skeletal muscle and likely limiting exercise capacity in this patient group.
Regulation of Skeletal Muscle Blood Flow During Exercise in HFrEF
A hallmark symptom of patients with HFrEF is an impaired exercise tolerance and an associated reduction in maximal exercise capacity (17, 41, 61, 64). Interestingly, the degree of left ventricular systolic dysfunction does not fully explain the degree of exercise intolerance or symptom status in this patient group (8, 22, 35, 54, 61), supporting the possibility that peripheral hemodynamic dysfunction may contribute to exercise intolerance. Evidence from previous studies (63, 65–68) indicates a functional role for impaired peripheral hemodynamics in limiting exercise capacity in this cohort during peak cycling exercise, where an apparent reduction in leg vascular conductance was observed in patients with HFrEF compared with healthy individuals, contributing to the documented impairment in perfusion. However, the use of a large muscle mass exercise paradigm presents limitations in distinguishing abnormalities in peripheral hemodynamics in HFrEF. During exercise that recruits a large fraction of total body muscle mass, central circulatory factors play an increasingly important role in the preservation of MAP (42, 48, 50). Because of an impaired left ventricular function during whole body exercise, patients with HFrEF largely depend on systemic vasoconstriction (i.e., reductions in SVC) to maintain MAP (53). This may indicate that the markedly lower leg vascular conductance and associated decrement in perfusion of the exercising skeletal muscle during cycling exercise might solely be due to the maintenance of MAP vs. limitations in vasodilatory capacity in this patient group (53, 68).
To investigate how altering the total amount of muscle recruited during exercise might affect the peripheral hemodynamic responses to exercise in this patient group, LeJemtel et al. (29) examined the differences in leg blood flow during maximal single- and double-legged upright cycling in HFrEF patients and healthy control subjects. Interestingly, they observed similar leg blood flow values in the HFrEF patients during both single- and double-legged exercise modalities, which they attributed to an impaired vasodilator response in the patient group (29). These results were indirectly supported by Jondeau et al. (24), who utilized maximal arm cycling exercise superimposed on maximal leg cycling to examine the impact of HF on cardiopulmonary reserve. In this study, the addition of arm exercise while cycling provoked an increase in peak oxygen (O2) consumption in severe HF patients, but not in healthy controls, which was interpreted as evidence for an inadequate vasodilator response to exercise resulting in impaired O2 delivery, as the arterial-venous O2 difference is near maximal in HFrEF during maximal leg cycling (26).
While these novel studies were some of the first to investigate the hemodynamic response to exercise, which recruited differing amounts of muscle mass, during all combinations of arm and leg cycling, it is likely that some degree of cardiac limitation still confound these results. This is even the case during maximum single-legged cycling; Martin et al. (32) has documented that the CO achieved in HFrEF patients is similar compared to CO values during maximum double-legged cycling in this patient group. Thus, in the present study, we used two limb-specific small muscle mass exercise modalities (static-intermittent HG and dynamic single-leg KE exercise) across a range of submaximal exercise intensities to more thoroughly investigate the peripheral hemodynamic response to exercise in HFrEF in isolation of the significant confounding effects of CO limitations and MAP regulation imposed by large muscle mass exercise.
Hemodynamic Responses to Handgrip Exercise
Because of the accessibility and limited cardiorespiratory stress associated with this modality, handgrip exercise has been used in a number of studies over the past several decades to investigate the peripheral hemodynamic response in HFrEF patients. Indeed, Zelis et al. (70) investigated the peripheral blood flow response to graded static-intermittent handgrip exercise in HFrEF patients compared with control subjects in the early 1970s and documented an impaired exercise hyperemia in this patient group. However, it is noteworthy that this study used venous plethysmography to measure blood flow during the 10-s relaxation phase between 5-s isometric contractions, an approach that precludes assessment of the phasic pattern of blood flow associated with rhythmic handgrip exercise. Thus, while this seminal work was among the first to examine disease-related changes in regional blood flow during small muscle mass exercise, the inherent limitations associated with plethysmographic determination of blood flow (30, 70) during low-cadence isometric handgrip exercise left some uncertainty regarding the true nature of the hyperemic response in this patient group.
In the current study, we used Doppler ultrasound to measure blood flow continuously during 1-Hz static-intermittent handgrip of graded intensity in HFrEF patients and age-matched controls. Interestingly, the HFrEF patients exhibited a divergent hyperemic and vasodilatory response to static-intermittent HG exercise. Specifically, we observed comparable changes in forearm blood flow and vascular conductance during low-intensity (15% MVC) exercise between HFrEF patients, and healthy controls, and a divergence at higher intensities, with HFrEF patients exhibiting an impaired ability to alter vasomotor tone and, therefore, increase blood flow (Fig. 1 and Table 3). Using a similar HG exercise model and a single, low-intensity workload (4.4 kg, ≈15% MVC), Shoemaker et al. (51) reported a similar hemodynamic response in HFrEF patients and controls, suggesting a preserved vasomotor regulation and hyperemic response in HFrEF patients when performing HG exercise. However, when viewed in the context of the current findings, across a wide range of exercise intensities (Fig. 1), this former study did not characterize the full scope of the hemodynamic and vasodilatory response in HFrEF. Thus, using beat-to-beat measurements, a dynamic (1 Hz) exercise cadence, and a wide range of exercise intensities, the present study both confirms and extends these previous findings, unmasking a marked impairment in forearm blood flow that may be attributed to a limited limb-specific vasodilatory capacity in the exercising skeletal muscle vasculature of HFrEF patients.
This reduction in forearm blood flow and vascular conductance in HFrEF patients was accompanied by a clear lack of an increase in CO across increasing intensities of exercise in this patient group (Table 3), which is in contrast to the robust (≈1 l/min; Table 3) increase observed in the age-matched control group at the highest exercise intensity. While the mechanisms responsible for this physiological adjustment to static-intermittent HG in HFrEF are unknown, a potential explanation for the absence of an exercise-induced increase in CO is the substantial reduction in SVC exhibited in this patient group (Table 3). Indeed, SVR (the inverse of SVC) is commonly used as a measurement of nonpulsatile arterial load on the left ventricle (36, 38, 60), and it is well established that patients with HFrEF are afterload-sensitive (6, 25, 49) and face impairments in left ventricular systolic function if arterial afterload is increased (25). However, it is possible that the unique cardiovascular adjustments associated with HG exercise in HFrEF might not elicit a sufficient stimulus to induce an increase in CO, thus requiring a reduction SVC to increase MAP in this patient group. Thus, it is tempting to speculate that during HG exercise, impaired blood flow within the exercising muscle vasculature may have indirectly limited the appropriate exercise-induced increase in CO in HFrEF patients. The “real world” implications of this response are not trivial; indeed, the cardiovascular adjustments observed with this HG exercise modality may also be present during tasks of daily living that utilize the upper limbs, such as carrying groceries. Thus, the observed impairment in vasodilation and the accompanying absence of central responses may be viewed as representing a previously unexplored aspect of exercise intolerance that could contribute to the diminished quality of life in this patient group.
Hemodynamic Responses to Knee-Extensor Exercise
While these data during HG exercise demonstrate a clear impairment in forearm hemodynamics, exercise intolerance in HFrEF patients has classically been documented during tasks primarily involving locomotion (17, 41, 61, 64), and, thus, further investigating the hemodynamic responses to physical exertion in the skeletal muscle vasculature of the legs is also warranted. During single-leg KE exercise, HFrEF patients exhibited a persistent impairment in the ability to overcome the tonic vasomotor restraint of the lower-limb vasculature across increasing exercise intensities (Table 4), thus limiting perfusion of the exercising limb (Fig. 2). These results are in contrast to Magnusson et al. (31), one of the only other studies to examine changes leg blood flow during submaximal KE exercise in this patient group. In this previous study, similar increases in leg blood flow and vascular conductance were observed across graded exercise intensities in HFrEF patients compared with controls. Although the reasons for this discrepancy between this former study and the present findings are not immediately obvious, one likely explanation is the evolution of pharmacotherapy associated with the treatment of HFrEF. Indeed, while a host of new drug classes have been developed and proven efficacious in the treatment of HFrEF since this previous study was undertaken, one noteworthy change in pharmacological standard of care for these patients over the past two decades is a reduction in the prevalence of positive inotropes. Indeed, the vast majority of patients in the aforementioned study were prescribed digoxin, a drug that has been documented to attenuate sympathetic nervous system activity (59), reduce circulating norepinephrine (2, 18, 55), and increase peripheral artery vasodilation (33) in HFrEF patients. This supports the concept that the higher exercising leg blood flow documented by Magnusson et al. (31) might be due to a digoxin-induced release of sympathetic restraint on peripheral vascular tone.
Additionally, there was a strong association between leg blood flow at a given absolute work rate (15 W) and the maximum KE work rate achieved in HFrEF patients, which was not present in the control group (Fig. 3). This is a unique finding, as blood flow is traditionally thought to match the metabolic demand of a given amount of work performed (45). Indeed, the current study (Fig. 3) and previous work (4) have documented similar leg blood flow values during KE exercise of a given absolute work rate in healthy individuals with varying maximal knee-extensor work rates. This HFrEF-specific relationship suggests that the more severe the impairment in exercise hyperemia, the more substantial the decrements in these patients' respective capacity to perform work.
In contrast to HG exercise, the exercise-induced changes in CO during KE exercise in the present study were remarkably similar between groups (Table 4), a differing response that is potentially attributed to a preserved SVC during KE exercise in HrEF (Table 4). This comparable central response serves to highlight the advantage of the KE exercise model, a small muscle mass modality that is capable of eliciting large, concomitant linear increases in skeletal muscle blood flow and CO without potential confounding factors, limiting the intensity-dependent increase in CO capacity performed at submaximal intensities. Indeed, at the highest exercise intensity (15 W), almost identical CO values were observed in HFrEF and control groups, while leg blood flow was ≈35% lower in the patient group. Together, these central and peripheral responses provide new evidence for a persistent restraint of skeletal muscle blood flow during leg exercise in optimally medicated HFrEF patients that cannot be explained by disease-related impairments in CO, implicating the lower limb peripheral vasculature as a significant contributor to the limited exercise capacity displayed by optimally medicated patients with HFrEF.
Perspectives
It is important to acknowledge that O2 transport during exercise is a multifaceted process, which includes both the bulk delivery of O2 to the exercising muscle (i.e., convective O2 transport) via increases in arterial blood flow, as well as the local O2 distribution to the exercising skeletal muscle via the unloading of O2 from hemoglobin to the skeletal muscle mitochondria (i.e., diffusional O2 conductance) (43, 44, 57, 58). Although the present study convincingly demonstrates an attenuated blood flow response during graded exercise in HFrEF patients, likely contributing to an impairment in convective O2 transport, deficiencies in diffusional conductance might be contributing to a systemic impairment in O2 transport in patients with HFrEF during exercise as well. Indeed, Esposito et al. (15) demonstrated an attenuated diffusional O2 conductance in HFrEF patients compared with control subjects during maximal KE exercise, although this response may have been attributable to the significantly lower maximal KE work rate in the patient group. Further studies are certainly warranted to investigate whether the impairments in diffusion conductance present in HFrEF during maximal exercise extends to submaximal exercise intensities.
It is widely accepted that one of the goals of pharmacotherapy for patients with HFrEF has been to relieve symptoms, many of which are associated with physical exertion and exercise intolerance (5, 10). While the early historical progression of the optimization of pharmacological therapy in HFrEF largely focused on improving left ventricular function through the use of positive inotropes (5, 19), the introduction of vasodilator therapy in the 1980s was a therapeutic milestone (5, 11). Indeed, the demonstrated beneficial effect of antihypertensive drugs, including nitrate-based medications (i.e., hydralazine) (12) and angiotensin-converting enzyme (ACE) inhibitors (1, 12, 16) on exercise intolerance and exercise-related symptoms has established important pleiotropic properties for these drug classes that are of significant value in the treatment of HFrEF. However, on the basis of the findings from the current study, the combined effect of vasodilators currently available and widely prescribed in HFrEF (i.e., ACE inhibitors, aldosterone receptor antagonists, angiotensin receptor blockers, and vasodilating β-adrenergic antagonists such as carvedilol) might not be sufficient to restore peripheral vasodilation during exercise.
Experimental Considerations
Several limitations to the present study are worth noting. While skeletal muscle vasodilation contributes significantly to the overall regulation of exercise hyperemia, we recognize that decrements in vasodilatory capacity are not the sole factor responsible for the observed impairment in blood flow during exercise in HFrEF. Indeed, disease-related changes in vascular architecture, including capillary rarefaction (37, 62, 69) and impairments in vascular flow capacity (34), may contribute to reduced limb blood flow independent of disease-related alterations in vascular control. We also recognize that the observed reduction in leg blood flow in HFrEF patients in the face of similar CO values between groups raises the question of whether blood flow distribution may be disturbed in the HFrEF cohort. Although the current study cannot answer this question, we acknowledge the possibility that increased blood flow to respiratory muscles may be partially responsible for our observed results during exercise in HFrEF. Indeed, during cycling exercise, Olson et al. (39) documented that HFrEF patients preferentially “steal” blood flow from the exercising skeletal muscle to accommodate their enhanced work of breathing. Additionally, in the present study, calculations of both systemic and regional vascular conductance were made using only arterial pressure, rather than the more conventional arterial-venous pressure difference. Thus, we cannot exclude the possibility that potential changes in venous pressure associated with HFrEF may have confounded our findings. However, we have recently reported similar venous pressures during maximal KE exercise in HFrEF and control subjects (15), an observation that somewhat mitigates this concern. We also acknowledge the lack of a direct assessment of exercise tolerance in the HFrEF group, although the observation that leg blood flow at a given absolute work rate was strongly associated with the maximum KE work rate in HFrEF patients (and not in healthy controls) is a strong indication that impairments in exercise hyperemia contributed to the limited work capacity in the patient group. Finally, on the basis of the differences in which workloads were determined between handgrip and KE exercise, we wish to emphasize that a direct comparison of hemodynamic responses between these two exercise modalities is not possible.
Conclusions
Using a wide range of exercise intensities, we have identified a clear impairment in perfusion of both the upper and lower limbs during small muscle mass exercise in HFrEF patients. These findings indicate that HFrEF patients on modern, optimized pharmacotherapy exhibit a severely compromised peripheral hyperemic response, implicating maladaptations in the peripheral vasculature and its regulation as potential factors contributing to the reduced exercise capacity in this patient group.
GRANTS
This study was funded, in part, by National Institutes of Health (NIH) Grant PO1 HL-091830, Veteran Affairs Rehabilitation Research and Development (VA RR&D) Grant E6910R, VA RR&D Grant 1I21RX001418-01, and NIH Grant R01 HL-118313.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: Z.B.-O., A.B., M.A.H.W., J.N.N., J.S., R.S.R., and D.W.W. conception and design of research; Z.B.-O., J.F.L., A.B., M.A.H.W., and D.W.W. performed experiments; Z.B.-O., A.B., M.A.H.W., and D.W.W. analyzed data; Z.B.-O., J.F.L., A.B., M.A.H.W., J.N.N., J.S., R.S.R., and D.W.W. interpreted results of experiments; Z.B.-O., J.F.L., and R.S.R. prepared figures; Z.B.-O. and D.W.W. drafted manuscript; Z.B.-O., J.F.L., A.B., M.A.H.W., J.N.N., J.S., R.S.R., and D.W.W. edited and revised manuscript; Z.B.-O., J.F.L., A.B., M.A.H.W., J.N.N., J.S., R.S.R., and D.W.W. approved final version of manuscript.
REFERENCES
- 1.Alicandri C, Fariello R, Boni E, Zaninelli A, Castellano M, Beschi M, Agabiti Rosei E, Muiesan G. Captopril versus digoxin in mild-moderate chronic heart failure: a crossover study. J Cardiovasc Pharmacol 9 Suppl 2: S61–S67, 1987. [DOI] [PubMed] [Google Scholar]
- 3.Andersen P, Adams RP, Sjogaard G, Thorboe A, Saltin B. Dynamic knee extension as model for study of isolated exercising muscle in humans. J Appl Physiol (1985) 59: 1647–1653, 1985. [DOI] [PubMed] [Google Scholar]
- 4.Andersen P, Saltin B. Maximal perfusion of skeletal muscle in man. J Physiol 366: 233–249, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Armstrong PW, Moe GW. Medical advances in the treatment of congestive heart failure. Circulation 88: 2941–2952, 1993. [DOI] [PubMed] [Google Scholar]
- 6.Asanoi H, Sasayama S, Kameyama T. Ventriculoarterial coupling in normal and failing heart in humans. Circ Res 65: 483–493, 1989. [DOI] [PubMed] [Google Scholar]
- 7.Barrett-O'Keefe Z, Ives SJ, Trinity JD, Morgan G, Rossman MJ, Donato AJ, Runnels S, Morgan DE, Gmelch BS, Bledsoe AD, Richardson RS, Wray DW. Taming the “sleeping giant”: the role of endothelin-1 in the regulation of skeletal muscle blood flow and arterial blood pressure during exercise. Am J Physiol Heart Circ Physiol 304: H162–H169, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benge W, Litchfield RL, Marcus ML. Exercise capacity in patients with severe left ventricular dysfunction. Circulation 61: 955–959, 1980. [DOI] [PubMed] [Google Scholar]
- 9.Bogert LW, van Lieshout JJ. Non-invasive pulsatile arterial pressure and stroke volume changes from the human finger. Exp Physiol 90: 437–446, 2005. [DOI] [PubMed] [Google Scholar]
- 10.Cohn JN. The management of chronic heart failure. N Engl J Med 335: 490–498, 1996. [DOI] [PubMed] [Google Scholar]
- 11.Cohn JN, Archibald DG, Ziesche S, Franciosa JA, Harston WE, Tristani FE, Dunkman WB, Jacobs W, Francis GS, Flohr KH, Goldman S, Cobb FR, Shah PM, Saunders R, Fletcher RD, Loeb HS, Hughes VC, Baker B. Effect of vasodilator therapy on mortality in chronic congestive heart failure. Results of a Veterans Administration Cooperative Study. N Engl J Med 314: 1547–1552, 1986. [DOI] [PubMed] [Google Scholar]
- 12.Cohn JN, Johnson G, Ziesche S, Cobb F, Francis G, Tristani F, Smith R, Dunkman WB, Loeb H, Wong M, Bhat G, Goldman S, Fletcher RD, Doherty J, Hughes CV, Carson P, Cintron G, Shabetai R, Haakenson C. A comparison of enalapril with hydralazine-isosorbide dinitrate in the treatment of chronic congestive heart failure. N Engl J Med 325: 303–310, 1991. [DOI] [PubMed] [Google Scholar]
- 12a.CONSENSUS Trial Study Group. Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). N Engl J Med 316: 1429–1435, 1987. [DOI] [PubMed] [Google Scholar]
- 13.de Vaal JB, de Wilde RB, van den Berg PC, Schreuder JJ, Jansen JR. Less invasive determination of cardiac output from the arterial pressure by aortic diameter-calibrated pulse contour. Br J Anaesth 95: 326–331, 2005. [DOI] [PubMed] [Google Scholar]
- 14.de Wilde RB, Geerts BF, Cui J, van den Berg PC, Jansen JR. Performance of three minimally invasive cardiac output monitoring systems. Anaesthesia 64: 762–769, 2009. [DOI] [PubMed] [Google Scholar]
- 15.Esposito F, Mathieu-Costello O, Shabetai R, Wagner PD, Richardson RS. Limited maximal exercise capacity in patients with chronic heart failure: partitioning the contributors. J Am Coll Cardiol 55: 1945–1954, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fletcher RD, Cintron GB, Johnson G, Orndorff J, Carson P, Cohn JN. Enalapril decreases prevalence of ventricular tachycardia in patients with chronic congestive heart failure. The V-HeFT II VA Cooperative Studies Group. Circulation 87: VI49–V155, 1993. [PubMed] [Google Scholar]
- 17.Franciosa JA, Ziesche S, Wilen M. Functional capacity of patients with chronic left ventricular failure. Relationship of bicycle exercise performance to clinical and hemodynamic characterization. Am J Med 67: 460–466, 1979. [DOI] [PubMed] [Google Scholar]
- 18.Gheorghiade M, Hall V, Lakier JB, Goldstein S. Comparative hemodynamic and neurohormonal effects of intravenous captopril and digoxin and their combinations in patients with severe heart failure. J Am Coll Cardiol 13: 134–142, 1989. [DOI] [PubMed] [Google Scholar]
- 19.Goldhaber JI, Hamilton MA. Role of inotropic agents in the treatment of heart failure. Circulation 121: 1655–1660, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gottlieb SS, McCarter RJ, Vogel RA. Effect of β-blockade on mortality among high-risk and low-risk patients after myocardial infarction. N Engl J Med 339: 489–497, 1998. [DOI] [PubMed] [Google Scholar]
- 21.Heidenreich PA, Lee TT, Massie BM. Effect of β-blockade on mortality in patients with heart failure: a meta-analysis of randomized clinical trials. J Am Coll Cardiol 30: 27–34, 1997. [DOI] [PubMed] [Google Scholar]
- 22.Higginbotham MB, Morris KG, Conn EH, Coleman RE, Cobb FR. Determinants of variable exercise performance among patients with severe left ventricular dysfunction. Am J Cardiol 51: 52–60, 1983. [DOI] [PubMed] [Google Scholar]
- 23.Imaizumi T, Hirooka Y, Takeshita A. Decreased skeletal muscle vasodilation in patients with congestive heart failure. Jpn Circ J 56: 500–503, 1992. [DOI] [PubMed] [Google Scholar]
- 24.Jondeau G, Katz SD, Zohman L, Goldberger M, McCarthy M, Bourdarias JP, LeJemtel TH. Active skeletal muscle mass and cardiopulmonary reserve. Failure to attain peak aerobic capacity during maximal bicycle exercise in patients with severe congestive heart failure. Circulation 86: 1351–1356, 1992. [DOI] [PubMed] [Google Scholar]
- 25.Kameyama T, Asanoi H, Ishizaka S, Sasayama S. Ventricular load optimization by unloading therapy in patients with heart failure. J Am Coll Cardiol 17: 199–207, 1991. [DOI] [PubMed] [Google Scholar]
- 26.Katz SD, Maskin C, Jondeau G, Cocke T, Berkowitz R, LeJemtel T. Near-maximal fractional oxygen extraction by active skeletal muscle in patients with chronic heart failure. J Appl Physiol (1985) 88: 2138–2142, 2000. [DOI] [PubMed] [Google Scholar]
- 27.Lawrenson L, Poole JG, Kim J, Brown C, Patel P, Richardson RS. Vascular and metabolic response to isolated small muscle mass exercise: effect of age. Am J Physiol Heart Circ Physiol 285: H1023–H1031, 2003. [DOI] [PubMed] [Google Scholar]
- 28.Lechat P, Packer M, Chalon S, Cucherat M, Arab T, Boissel JP. Clinical effects of β-adrenergic blockade in chronic heart failure: a meta-analysis of double-blind, placebo-controlled, randomized trials. Circulation 98: 1184–1191, 1998. [DOI] [PubMed] [Google Scholar]
- 29.LeJemtel TH, Maskin CS, Lucido D, Chadwick BJ. Failure to augment maximal limb blood flow in response to one-leg versus two-leg exercise in patients with severe heart failure. Circulation 74: 245–251, 1986. [DOI] [PubMed] [Google Scholar]
- 30.Longhurst J, Capone RJ, Mason DT, Zelis R. Comparison of blood flow measured by plethysmograph and flowmeter during steady state forearm exercise. Circulation 49: 535–540, 1974. [DOI] [PubMed] [Google Scholar]
- 31.Magnusson G, Kaijser L, Sylven C, Karlberg KE, Isberg B, Saltin B. Peak skeletal muscle perfusion is maintained in patients with chronic heart failure when only a small muscle mass is exercised. Cardiovasc Res 33: 297–306, 1997. [DOI] [PubMed] [Google Scholar]
- 32.Martin WH, 3rd, Berman WI, Buckey JC, Snell PG, Blomqvist CG. Effects of active muscle mass size on cardiopulmonary responses to exercise in congestive heart failure. J Am Coll Cardiol 14: 683–694, 1989. [DOI] [PubMed] [Google Scholar]
- 33.Mason DT, Braunwald E. Studies on digitalis. X. Effects of ouabain on forearm vascular resistance and venous tone in normal subjects and in patients in heart failure. J Clin Invest 43: 532–543, 1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McAllister RM, Laughlin MH, Musch TI. Effects of chronic heart failure on skeletal muscle vascular transport capacity of rats. Am J Physiol Heart Circ Physiol 264: H689–H691, 1993. [DOI] [PubMed] [Google Scholar]
- 35.Metra M, Raddino R, Dei Cas L, Visioli O. Assessment of peak oxygen consumption, lactate and ventilatory thresholds and correlation with resting and exercise hemodynamic data in chronic congestive heart failure. Am J Cardiol 65: 1127–1133, 1990. [DOI] [PubMed] [Google Scholar]
- 36.Nichols WW, Pepine CJ. Left ventricular afterload and aortic input impedance: implications of pulsatile blood flow. Prog Cardiovasc Dis 24: 293–306, 1982. [DOI] [PubMed] [Google Scholar]
- 37.Nusz DJ, White DC, Dai Q, Pippen AM, Thompson MA, Walton GB, Parsa CJ, Koch WJ, Annex BH. Vascular rarefaction in peripheral skeletal muscle after experimental heart failure. Am J Physiol Heart Circ Physiol 285: H1554–H1562, 2003. [DOI] [PubMed] [Google Scholar]
- 38.O'Rourke MF. Vascular impedance in studies of arterial and cardiac function. Physiol Rev 62: 570–623, 1982. [DOI] [PubMed] [Google Scholar]
- 39.Olson TP, Joyner MJ, Dietz NM, Eisenach JH, Curry TB, Johnson BD. Effects of respiratory muscle work on blood flow distribution during exercise in heart failure. J Physiol 588: 2487–2501, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Padilla J, Johnson BD, Newcomer SC, Wilhite DP, Mickleborough TD, Fly AD, Mather KJ, Wallace JP. Normalization of flow-mediated dilation to shear stress area under the curve eliminates the impact of variable hyperemic stimulus. Cardiovasc Ultrasound 6: 44, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Patterson JA, Naughton J, Pietras RJ, Gunnar RM. Treadmill exercise in assessment of the functional capacity of patients with cardiac disease. Am J Cardiol 30: 757–762, 1972. [DOI] [PubMed] [Google Scholar]
- 42.Pawelczyk JA, Hanel B, Pawelczyk RA, Warberg J, Secher NH. Leg vasoconstriction during dynamic exercise with reduced cardiac output. J Appl Physiol 73: 1838–1846, 1992. [DOI] [PubMed] [Google Scholar]
- 43.Poole DC, Hirai DM, Copp SW, Musch TI. Muscle oxygen transport and utilization in heart failure: implications for exercise (in)tolerance. Am J Physiol Heart Circ Physiol 302: H1050–H1063, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Richardson RS. Oxygen transport and utilization: an integration of the muscle systems. Adv Physiol Educ 27: 183–191, 2003. [DOI] [PubMed] [Google Scholar]
- 45.Richardson RS, Harms CA, Grassi B, Hepple RT. Skeletal muscle: master or slave of the cardiovascular system? Med Sci Sports Exerc 32: 89–93, 2000. [DOI] [PubMed] [Google Scholar]
- 46.Richardson RS, Saltin B. Human muscle blood flow and metabolism studied in the isolated quadriceps muscles. Med Sci Sports Exerc 30: 28–33, 1998. [DOI] [PubMed] [Google Scholar]
- 47.Richardson TE, Kindig CA, Musch TI, Poole DC. Effects of chronic heart failure on skeletal muscle capillary hemodynamics at rest and during contractions. J Appl Physiol (1985) 95: 1055–1062, 2003. [DOI] [PubMed] [Google Scholar]
- 48.Saltin B. Hemodynamic adaptations to exercise. Am J Cardiol 55: 42D–47D, 1985. [DOI] [PubMed] [Google Scholar]
- 49.Schwartzenberg S, Redfield MM, From AM, Sorajja P, Nishimura RA, Borlaug BA. Effects of vasodilation in heart failure with preserved or reduced ejection fraction implications of distinct pathophysiologies on response to therapy. J Am Coll Cardiol 59: 442–451, 2012. [DOI] [PubMed] [Google Scholar]
- 50.Secher NH, Clausen JP, Klausen K, Noer I, Trap-Jensen J. Central and regional circulatory effects of adding arm exercise to leg exercise. Acta Physiol Scand 100: 288–297, 1977. [DOI] [PubMed] [Google Scholar]
- 51.Shoemaker JK, Naylor HL, Hogeman CS, Sinoway LI. Blood flow dynamics in heart failure. Circulation 99: 3002–3008, 1999. [DOI] [PubMed] [Google Scholar]
- 52.Sugawara J, Tanabe T, Miyachi M, Yamamoto K, Takahashi K, Iemitsu M, Otsuki T, Homma S, Maeda S, Ajisaka R, Matsuda M. Non-invasive assessment of cardiac output during exercise in healthy young humans: comparison between Modelflow method and Doppler echocardiography method. Acta Physiol Scand 179: 361–366, 2003. [DOI] [PubMed] [Google Scholar]
- 53.Sullivan MJ, Hawthorne MH. Exercise intolerance in patients with chronic heart failure. Prog Cardiovasc Dis 38: 1–22, 1995. [DOI] [PubMed] [Google Scholar]
- 54.Szlachcic J, Massie BM, Kramer BL, Topic N, Tubau J. Correlates and prognostic implication of exercise capacity in chronic congestive heart failure. Am J Cardiol 55: 1037–1042, 1985. [DOI] [PubMed] [Google Scholar]
- 55.Tsakiris AG, Donald DE, Sturm RE, Wood EH. Volume, ejection fraction, and internal dimensions of left ventricle determined by biplane videometry. Fed Proc 28: 1358–1367, 1969. [PubMed] [Google Scholar]
- 56.van der Harst P, de Boer RA. Statins in the treatment of heart failure. Circ Heart Fail 3: 462–464, 2010. [DOI] [PubMed] [Google Scholar]
- 57.Wagner PD. Determinants of maximal oxygen transport and utilization. Ann Rev Physiol 58: 21–50, 1996. [DOI] [PubMed] [Google Scholar]
- 58.Wagner PD. Systemic oxygen transport and utilization. J Breath Res 2: 024001, 2008. [DOI] [PubMed] [Google Scholar]
- 59.Watanabe AM. Digitalis and the autonomic nervous system. J Am Coll Cardiol 5: 35A–42A, 1985. [DOI] [PubMed] [Google Scholar]
- 60.Weber KT, Janicki JS, Hunter WC, Shroff S, Pearlman ES, Fishman AP. The contractile behavior of the heart and its functional coupling to the circulation. Prog Cardiovasc Dis 24: 375–400, 1982. [DOI] [PubMed] [Google Scholar]
- 61.Weber KT, Kinasewitz GT, Janicki JS, Fishman AP. Oxygen utilization and ventilation during exercise in patients with chronic cardiac failure. Circulation 65: 1213–1223, 1982. [DOI] [PubMed] [Google Scholar]
- 62.Williams AD, Selig S, Hare DL, Hayes A, Krum H, Patterson J, Geerling RH, Toia D, Carey MF. Reduced exercise tolerance in CHF may be related to factors other than impaired skeletal muscle oxidative capacity. J Card Fail 10: 141–148, 2004. [DOI] [PubMed] [Google Scholar]
- 63.Wilson JR, Ferraro N. Effect of the renin-angiotensin system on limb circulation and metabolism during exercise in patients with heart failure. J Am Coll Cardiol 6: 556–563, 1985. [DOI] [PubMed] [Google Scholar]
- 64.Wilson JR, Ferraro N. Exercise intolerance in patients with chronic left heart failure: relation to oxygen transport and ventilatory abnormalities. Am J Cardiol 51: 1358–1363, 1983. [DOI] [PubMed] [Google Scholar]
- 65.Wilson JR, Ferraro N, Wiener DH. Effect of the sympathetic nervous system on limb circulation and metabolism during exercise in patients with heart failure. Circulation 72: 72–81, 1985. [DOI] [PubMed] [Google Scholar]
- 66.Wilson JR, Martin JL, Ferraro N. Impaired skeletal muscle nutritive flow during exercise in patients with congestive heart failure: role of cardiac pump dysfunction as determined by the effect of dobutamine. Am J Cardiol 53: 1308–1315, 1984. [DOI] [PubMed] [Google Scholar]
- 67.Wilson JR, Martin JL, Ferraro N, Weber KT. Effect of hydralazine on perfusion and metabolism in the leg during upright bicycle exercise in patients with heart failure. Circulation 68: 425–432, 1983. [DOI] [PubMed] [Google Scholar]
- 68.Wilson JR, Martin JL, Schwartz D, Ferraro N. Exercise intolerance in patients with chronic heart failure: role of impaired nutritive flow to skeletal muscle. Circulation 69: 1079–1087, 1984. [DOI] [PubMed] [Google Scholar]
- 69.Xu L, Poole DC, Musch TI. Effect of heart failure on muscle capillary geometry: implications for O2 exchange. Med Sci Sports Exerc 30: 1230–1237, 1998. [DOI] [PubMed] [Google Scholar]
- 70.Zelis R, Longhurst J, Capone RJ, Mason DT. A comparison of regional blood flow and oxygen utilization during dynamic forearm exercise in normal subjects and patients with congestive heart failure. Circulation 50: 137–143, 1974. [DOI] [PubMed] [Google Scholar]


