Abstract
Scent marking is often assumed to be a secondary sexual trait that increases males’ mating and reproductive success, although direct evidence for this hypothesis is lacking. We conducted a study with wild-derived house mice, Mus musculus musculus, to test whether scent marking increases males’ reproductive success when females can freely choose between two territorial males. We also experimentally manipulated males’ competitive scent marking by exchanging scent-marked tiles between the neighbouring males’ territories (intrusion treatment) or relocating males’ tiles within their own territory (control). Experimental animals were tested twice and we examined whether individual males were consistent in their marking. We found that males marked more in the intrusion treatment than controls and more at shared territorial borders than elsewhere. We found high day-to-day variation in most individuals’ scent marking, and yet the sum of individuals’ scent marking was consistent over time and across different social conditions. Genetic paternity analyses revealed that males’ scent marking significantly increased their reproductive success in both the intrusion treatment and the controls. Surprisingly, however, female social preference was not positively correlated with male scent marking. These results provide direct evidence that scent marking increases males’ reproductive success when females can choose their mates, even though it did not increase females’ social preferences.
Keywords: house mouse, male–male competition, marking consistency, Mus musculus musculus, reproductive success, scent marking, social preference
Many animal species deposit scent marks, which mediate intraspecific communication and sexual selection, and scent marking has been suggested to function to advertise males’ territory ownership and social dominance status and to attract females (Ralls 1971). Darwin (1874, page 809) originally suggested that the evolution of odour glands in male mammals is ‘intelligible through sexual selection, if the most odouriferous males are the most successful in winning the females and in leaving offspring to inherit their gradually perfected glands and odours’. Many studies have subsequently found evidence that scent marking functions to intimidate rivals and attract females (Ralls 1971; Gosling 1982; Hurst 1990a; Gosling & Roberts 2001). For example, male house mice, Mus musculus, produce many small urinary scent marks and they increase the quantity of scent mark deposition in the presence of sexually mature females (Ralls 1971; Reynolds 1971; Maruniak et al. 1974; Arakawa et al. 2007) or female scent (Wolff & Powell 1984; Hurst 1989; Zala et al. 2004). Moreover, female mice are capable of assessing males’ quality (Lenington 1983; Kavaliers & Colwell 1995; Penn et al. 1998) and compatibility (Yamazaki et al. 1976) from their scent. Therefore, males’ scent and scent marks appear to be a secondary sexual trait, analogous to colourful visual displays of birds and fish (Penn & Potts 1998), although unlike conventional displays, scent marks provide extended phenotypes (Penn 2006) or extrabodily ornaments (Schaedelin & Taborsky 2009).
Although many studies provide evidence that females can assess potential mates by the quality of their urinary scent or bedding odour, it is unclear why males produce so many scent marks, as there are few studies on the benefits of scent marking per se. In house mice, infection reduces the amount of males’ scent marking and the attractiveness of their scent marks to females (Zala et al. 2004). Moreover, females prefer the scent marks of males genetically engineered (transgenic ‘knock-in’) to be immunologically resistant to infection compared to susceptible controls (Zala et al. 2008), but it is unclear whether females prefer to mate with high versus low markers. Only two studies to our knowledge have tested whether female mate choice is influenced by the quantity of males’ marking, and they found no evidence that males’ scent marking affects female social preferences or mate choice (Thomas 2002; Mech et al. 2003). However, these studies on prairie voles, Microtus ochrogaster, measured males’ scent marking for only 30 min, which may have been insufficient to assess quantitative differences in males’ marking accurately, and they did not test whether scent marking increased males’ actual reproductive success (paternity).
Most studies on scent marking have examined their function in male–male interactions, and these studies show that male marking provides an honest indicator of males’ territorial ownership and social status (Ralls 1971; Gosling 1982; Hurst 1990a; Gosling & Roberts 2001). In house mice, for example, dominant males mark more than subordinates (Desjardins et al. 1973; Drickamer 2001) and territory owners countermark the scent marks of intruders (Hurst 1990a; Rich & Hurst 1999). Scent marking is a cheat-proof indicator of a male’s social status because it is physically impossible for subordinates to fake territory ownership and occupation (Hurst & Rich 1999; Gosling & Roberts 2001). Scent marking mediates competitor assessment, and a high level of marking is suspected to intimidate rivals and prevent unnecessary fights (Gosling 1982; Hurst 1990a; Gosling & Roberts 2001). Dominant male mice aggressively defend their territories (Crowcroft 1955; Crowcroft & Rowe 1963; Hurst 1990a), and subordinate males avoid areas marked by dominant individuals (Jones & Nowell 1973), probably to prevent agonistic encounters. Competition in many species induces males to increase their signalling effort and to produce more conspicuous and costly displays. For example, in Thomson’s gazelles, Gazella thomsoni, scent marks are more dense in territories under the threat of intrusion than elsewhere (Walther 1978) and male oribi, Ourebia ourebi, mark more at common boundaries where other males are located (Brashares & Arcese 1999). However, these findings are observational, and we know of no study that has experimentally tested whether male–female conflict increases males’ scent marking, and if so, whether increased scent marking subsequently improves males’ reproductive success.
Scent-marking behaviour probably evolves through both inter- and intrasexual selection, and female choice may be influenced by male–female competition (Wong & Candolin 2005). For example, females may be able to assess male quality by eavesdropping on a male’s competitive scent marking, and females may change their preferences after observing male–female interactions. Studies investigating female preferences for male scent usually control for male–female effects, although several studies indicate that female preferences for male odour are influenced by male–female interactions. Female mice preferentially mate with territorial, socially dominant males (Rolland et al. 2003) or winners in agonistic encounters (DeFries & McClearn 1970; Parmigiani et al. 1982b). Dominant males produce more scent marks than subordinates (Desjardins et al. 1973) and females prefer the odour of dominant males (Drickamer 1992). Female mice also prefer the odour of males that countermark the scent marks of competitors (Rich & Hurst 1999); however, it is unclear whether females use males’ scent marks (quality or quantity) to choose between dominant territorial males. Thus, studies are needed to test whether interactions among territorial males affect female preferences and males’ actual reproductive success.
In this study, we investigated wild-derived house mice, M. m. musculus, in large enclosures to test whether scent marking increases males’ reproductive success when females can freely choose their mates, which, to our knowledge, has never been tested before. We also measured individual variation in scent marking over time (daily amount of marking over 5 days) and analysed individual consistency in marking (in another 5 days with a different female and male competitor), which, to our knowledge, has not been studied before. In addition, we experimentally introduced scent marks from neighbouring males into males’ territories (‘territorial intrusion’), which increases male–female interactions, and we tested whether territorial intrusion affected males’ marking, females’ social preferences and males’ reproductive success. In addition to eliciting male countermarking, territorial intrusion probably has additional effects on males’ behaviour and females’ perception of males’ competitive ability. We expected that increased scent marking would improve males’ reproductive success when females can select their mates, and we tested whether this effect was eliminated or increased by male–female interactions in our intrusion treatment.
METHODS
Animals and Housing
Experimental animals were F1 offspring of wild-caught house mice, which were trapped at 14 locations within a 500 m radius in Vienna (48°12′38″N, 16°16′54″E) and crossed between sites to avoid inbreeding. The F1 mice were weaned at the age of 21 ± 1 days and then housed individually in standard mouse cages (type II, 26.5 × 20.5 cm and 14 cm high) containing wooden bedding (ABEDD), wood shavings and a nestbox. Food (Altromin rodent diet 1324) and water were provided ad libitum and a 12:12 h light:dark cycle was maintained. The room temperature was 22 ± 2 °C. At weaning all animals received an ear punch which was necessary for individual identification. Animals were 3–5 months old when the experiment began.
Mate Choice Assay
Each female (N = 32) was released into a large (3.4 m2) enclosure where she could choose to interact and mate with either one or both of two unrelated males (N = 64). The enclosure was divided in half by an opaque plastic wall (divider), which separated the males on either side of the neighbouring compartments (1.7 m2 each). Females could move freely between the males’ compartments through a small passage tube at the base of the divider. Males were prevented from entering the passage by small collars to ensure that both established their own territory and to avoid injuries from fighting. At the base of the divider, four mesh-sealed holes (4 cm diameter) allowed visual and olfactory contact between the neighbouring males to stimulate their signalling effort towards competitors and females. Each male compartment contained one nestbox and one shelter both equipped with bedding and nesting material, one water dispenser and randomly distributed food. We provided females with a cage within each male’s compartment (including separate water and food), which was accessible only to females through another passage tube, and thus allowed them to escape male harassment.
To assess females’ ‘social preferences’, we recorded females’ presence in the males’ compartments once per day (at 1030 hours ± 30 min) on 6 days a week to determine how often a female was located in each male’s compartment over the course of the experiment. To assess females’ actual mating preferences, we conducted paternity analyses on offspring.
Male Scent Marks and Experimental Treatment
To manipulate and quantify males’ scent marking, we placed 18 PVC tiles (10 × 10 cm) on the floor of each of the males’ compartments before males were introduced. Tiles were arranged along the periphery of their compartments and next to nesting sites, covering approximately 11% of the compartments’ total area. Each tile was assigned to a predefined position within the compartment. Males were released into the enclosure 2 days before females were introduced to establish a territory and to allow us to assess the amount of males’ initial scent marking. To simulate territorial intrusion, all of the tiles in a male’s compartment were collected and exchanged with his neighbour’s tiles (‘intrusion treatment’), whereas for the control group, the male’s tiles were collected and relocated within his own territory. A study by Desjardins et al. (1973) revealed that males in neighbouring compartments that are separated from each other by a mesh both become dominant markers. The tile relocation started on day 3 of the experiment, shortly before females were introduced and was conducted on a daily basis at the same time (1100 hours ± 30 min to minimize disturbance) until the end of the experiment. To quantify males’ scent marking, we took photographs of all tiles before relocating them for 6 consecutive days starting on the day of female introduction. To avoid spreading scent between the tiles or the enclosures, observers wore one-way plastic shoe covers and tiles were handled with one-way latex gloves.
Experimental Design
Each of the 32 females was used for two trials, once in the intrusion treatment and once with a different pair of males under control conditions (within-subject design). The 64 males were also tested twice; however, males were assigned to new pairs for the second trial. Male pairs were always size-matched within 0.5 g body mass and we ensured that none of the experimental animals were familiar with or related to one another. Because of space limitations we could not test all 32 females and 64 males at the same time, so we ran two groups per trial, in each of which we tested 16 females and 32 males. The number of treatments and controls was balanced within each group. The time between the first and the second trial was 2 months.
All females had given birth to one litter before the experiment began to control for potential order effects when comparing virgin versus nonvirgin females. Males were sexually inexperienced in their first trial, and at least 61% of them were sexually experienced in the second trial (61% of males sired offspring in the first trial but the number of males that mated may have been higher). The mice in each group were allowed to interact in the experiment for 18 days and then all animals were returned to the colony. We measured male body mass prior to the experiment shortly before they received the collar and once again immediately after the experiment when we removed the collars to calculate male body mass change during the experiment. Females were placed individually in type IIL mouse cages (32 × 20.5 cm and 14 cm high) to give birth under controlled conditions. Reproductive success was measured from genetic paternity analyses on the 57 litters produced.
Scent Mark Analysis
To quantify males’ scent marking, we took photographs of scent-marked tiles (Fig. 1) each day for 6 consecutive days. The first photograph was taken shortly before females were introduced to the experiment to quantify the amount of male ‘initial scent marking’ (sum of male scent marking within the first 2 days of the experiment). The other photographs were used to assess the daily amount of newly marked tile area and to calculate the sum of marked tile area for each male after female introduction (‘sum of male scent marking’). We did not analyse the number of marks to quantify male scent marking because we found that when males added new marks, previous marks often merged into a single mark, erroneously reducing the number of marks. This merging probably explains why we found no significant increase and in fact a negative trend between the number of marks and the total area marked (Pearson correlation: r94 = −0.175, P = 0.088). Photographs of tiles were taken inside a dark box (60 × 60 cm and 80 cm high) under UV light, emitted by two 18 W strip lights (90 cm, OMNILUX) attached to the ceiling of the box. The 18 tiles within each territory were photographed in two sets of nine tiles each (Fig. 1). Set 1 included five tiles that were located at the males’ shared territorial border with their neighbouring competitor and four tiles along a nonshared territorial border (‘shared border’), whereas the second set included nine tiles from nonshared territorial borders (‘non-shared border’). To photograph tiles, we placed each set centrally on the bottom of the box in the same order and exactly the same position. Digital photographs were recorded (Canon EOS 400 D, 0.8′ exposure time and 4.5 aperture value) from a fixed position on top of the box. The box was cleaned with 70% ethanol between photographs to prevent odour contamination. We took the photographs of the first group in trial 1 (see Experimental Design) in JPG format, but we excluded these data from our analyses as the image quality was inadequate and we only analysed subsequent photographs which were recorded in CR2 format. Photographs were imported into Adobe Photoshop CS 5.1 for image analyses and interpolated (10 cm ≙ 1000 pixels) before a fixed threshold was assigned to determine the proportion of marked tile area. We assessed our measurement error by photographing the same set of tiles three times and found that the sum of the marked area differed by ≤0.05% between the three photographs (mean proportion marked ± SE: 0.0693 ± 1.12 × 105).
Figure 1. Photograph of male scent marks on one set of nine tiles.
For photographs the nine tiles of each set were collected from the male’s compartment and placed centrally in the same order and the same position on the bottom of a dark box under UV light.
We could not control for female scent marking; however, it has been shown that female house mice show very low levels of scent marking (Kimura & Hagiwara 1985), independent of oestrous cycle or sexual experience (Maruniak et al. 1975). Even in response to males, females only mark very little (Hurst 1990b), and, therefore, they are expected to have a relatively small effect on the overall marking.
Genetic Paternity Analyses
For genetic paternity analyses DNA was extracted from ear punch samples using a proteinase K/isopropanol protocol (Sambrook et al. 1989) and individuals were genotyped at a minimum of six and a maximum of 16 microsatellite loci (D11Mit150, D9Mit34, D9Mit135, D17Saha, D17Mit28, D10Mit20, D2Mit252, D6Mit138, D15Mit16, D5Mit25, D19Mit39, D7Mit227, D1Mit456, D2Mit380, D17Mit21, D1Mit404, see Mouse Microsatellite Data Base of Japan, www.shigen.nig.ac.jp/mouse/mmdbj/). Whenever paternity could not be assigned by complete exclusion, we genotyped additional loci. Amplification mixes were subjected to a denaturation step at 94 °C for 15 min followed by 30 cycles at 94 °C for 30 s, 55 °C for 90 s and 72 °C for 60 s, followed by an elongation step at 72 °C for 10 min. PCR products were analysed using an automated sequencer (Beckman Coulter CEQ 8000) and allele scoring was performed using Beckman Coulter CEQ 8000 System software. Allele sizes were determined with SLS + 400 as size standard. Paternity results were confirmed using CERVUS 3.0.3 (Marshall et al. 1998; Kalinowski et al. 2007). As maternity was certain the program assigned paternity with a 95–99% confidence (given dam–sire–offspring relationship) based on male allele differences.
Statistical Analyses
We applied a linear mixed-effects model (LMM) to test whether the sum of male scent marking was affected by the intrusion treatment. We included the sum of male scent marking as the dependent variable, trial and treatment as fixed factors and male body mass and body mass change during the experiment as covariates. As males were tested in pairs we included male pair as a random factor to control for nonindependence. Male scent-marking data referred to proportions; thus, we performed arcsine square-root transformation on this variable.
To test whether males’ scent marking predicted their reproductive success we used a generalized mixed-effects model (GLMM) with a binomial distribution and a logit link function. We calculated two models, in which we assessed the influence of male marking under the intrusion treatment and controls separately as we found a significant interaction between treatment and female social preference (number of observations in a male territory). For both models, the number of offspring sired was included as the dependent variable and litter size as the binomial denominator. Trial was included as the fixed factor and the sum of male marking, male body mass change and female social preference were included as covariates. We included male pair as a random factor to control for nonindependence. To test whether female social preference was predicted by the sum of male marking, we applied a GLMM with a binomial distribution and a logit link function. We separately assessed female social preference for the intrusion treatment and controls as we found a significant interaction between the sum of male marking and treatment. For both models, female social preference was included as the dependent variable and the total number of observations (15) as the binomial denominator. We included trial as the fixed factor and the sum of male marking and body mass change as covariates. Again we included male pair as a random factor to control for nonindependence. For all models we tested for collinearity within predictor variables and ensured that model assumptions were fulfilled. We only included biologically meaningful two-way interactions in initial models and applied a backward stepwise removal procedure (Grafen & Hails 2002) to avoid problems from inclusion of nonsignificant terms (Engqvist 2005; model terms were removed when P > 0.05). Removed variables were re-entered one by one to the final model to obtain relevant statistics. Statistical analyses were performed using ‘R’ version 2.14.1. We implemented LMMs using the ‘lme’ function of the ‘nlme’ package, and GLMMs using the ‘lmer’ function in the ‘lme4’ package (R Development Core Team 2011).
Ethical Note
This study was approved by the University of Veterinary Medicine, Vienna, ethics committee in accordance with Good Scientific Practice guidelines and national legislation. We worked with F1 from wild-trapped house mice. Trapping of the founder individuals was conducted overnight with Sherman live traps. Each trap was equipped with a piece of apple and bread with peanut butter and nesting material (wood shavings and cotton). Traps were checked twice during the night for occupancy and trapped individuals were immediately removed and placed individually into standard mouse cages (type II, 26.5 × 20.5 cm and 14 cm high) for 3 weeks of quarantine before joining the colony. Quarantine conditions were identical to standard housing conditions in the colony. Trapping was in accordance with national legislation and was approved by the MA 22 (Municipality for Environment and Conservation of Vienna, Austria). At weaning all animals received an ear punch for individual identification. Ear punches were conducted with a small (2 mm diameter) hole punch device and we collected and stored ear punch tissues at −20 °C for genetic analyses, thereby avoiding any additional pain or distress to the animals by any other tissue sampling. We did not administer analgesics to the animals before the ear punch as this procedure would have caused additional handling and stress to the animals. All animals were inspected on a regular basis after the ear punch to monitor behaviour and health. No infection ever occurred after ear punching. Males received collars, which consisted of cable ties (2.5 mm wide) with two wires (2.5 cm, 1 mm diameter), to provide a mechanical barrier at the shared territory border. Male collaring was necessary to prevent males from fighting, as males aggressively defend their home range against intruders, and to protect females from male harassment and coercion by blocking males’ entrance into females’ cages (see Mate Choice Assay). Males received collars 2 days prior to the experiment to allow them to habituate to the collar in their home cage, while they were inspected at regular intervals. To put the collar on, we gently held the mouse in one hand and moved the collar over its head with the other hand to adjust it behind the ears. Collars were loose enough to be moved but tight enough to prevent its removal by the forepaws of the animal. They did not cause any tissue irritation or injury and collared males did not show altered behaviour. After the experiment all animals were returned to and kept in the colony. Experimental offspring were weaned at the age of 21 ± 1 days and then also integrated into the colony.
RESULTS
Variation in Male Scent Marking
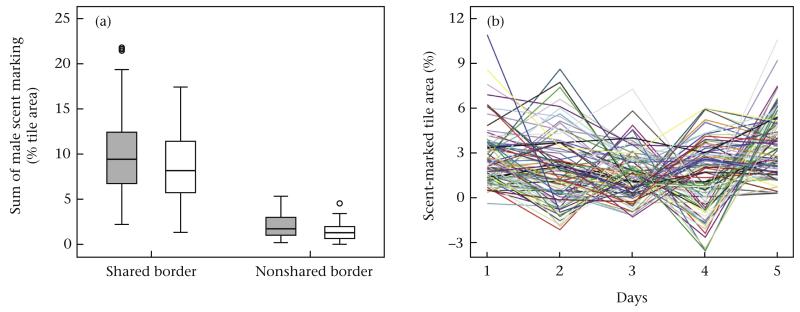
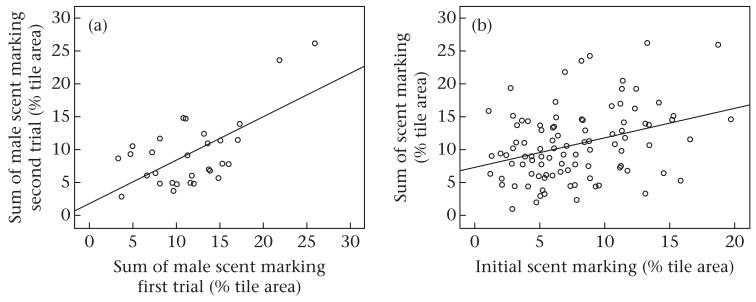
We tested whether the intrusion treatment elicited increased scent marking, and we confirmed that the sum of male scent marking was significantly increased in the intrusion treatment compared to controls (LMM: F1,46 = 5.132, P = 0.028; Fig. 2a). We found no evidence that male body mass (LMM: F1,47 = 0.050, P = 0.824), body mass change (LMM: F1,46 = 0.464, P = 0.499) or the sum of male trial (LMM: F1,47 = 1.027, P = 0.316) influenced scent marking. We found that males scent marked around the entire periphery, but they marked significantly more at the shared versus the nonshared border in both controls (paired t test: t49 = 18.46, P < 0.001; Fig. 2a) and the intrusion treatment (paired t test: t45 = 16.55, P < 0.001; Fig. 2a). In addition to this spatial pattern, we also found high within- and between-individual variation in the daily amount of male scent marking after female introduction (Fig. 2b). Despite this day-to-day variation, individual males’ sum of scent marking was correlated between the two trials (Pearson correlation: r28 = 0.584, P = 0.0007; Fig. 3a).
Figure 2. Variation in scent marking over time and space.
(a) Amount of male scent marking at the shared territorial borders versus the nonshared territorial borders under the intrusion treatment (grey) and control conditions (white). The box plots show the median and 25th and 75th percentiles; the whiskers indicate the values within 1.5 times the interquartile range and the circles are outliers. (b) Within- and between-individual variation in the daily amount of newly marked tile area after female introduction. Males were tested twice under different social conditions and both trials are individually plotted here.
Figure 3. Consistency of male scent marking.
(a) Relationship of the sum of male scent marking in their first and second trial of the experiment. (b) Relationship of males’ initial scent marking and their sum of scent marking.
Females’ introduction into the enclosures did not change males’ scent-marking rank: the amount of males’ initial marking was positively correlated with the sum of scent marking after female introduction (Pearson correlation: r94 = 0.311, P = 0.002; Fig. 3b).
Male Scent Marking and Reproductive Success
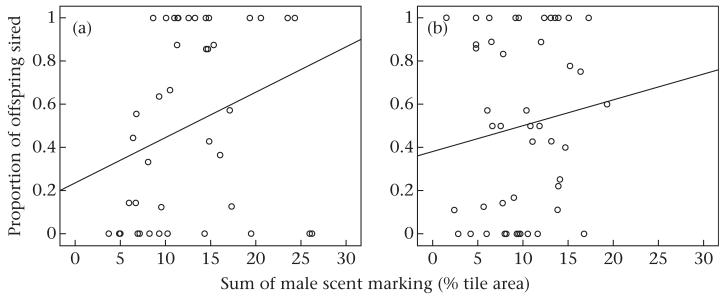
We found that the sum of male scent marking was positively and significantly associated with the number of offspring sired, and the beta values indicate that this effect was stronger in the intrusion treatment than the control (GLMM: intrusion treatment: z = 4.358, β = 0.095, SE = 0.021, N = 44, P < 0.001; control: z = 1.972, β = 0.055, SE = 0.028, N = 44, P = 0.049; Fig. 4). Females’ social preferences influenced male reproductive success, but this effect depended on treatment: under intrusion, female social preference was positively correlated with male reproductive success (GLMM: z = 3.299, β = 0.109, SE = 0.033, N = 44, P < 0.001; Fig. 5a), whereas in the controls, female social preference was negatively correlated with male reproductive success (GLMM: z = −2.242, β = −0.064, SE = 0.029, N = 44, P 0.025; Fig. 5b). We found no evidence that trial had any influence on male reproductive success in either the intrusion treatment (GLMM: z = 0.567, N = 44, P = 0.571) or the controls (GLMM: z = −0.954, N = 44, P = 0.340). Male reproductive success was not related to male body mass change in the intrusion treatment (GLMM: z = 0.118, N = 44, P = 0.906), whereas in the control, there was a positive correlation between male body mass gain and male reproductive success (GLMM: z = 2.683, β = 0.220, SE = 0.082, N = 44, P = 0.007). Individual male reproductive success was correlated across experimental trials (Spearman rank correlation: rS = 0.425, N = 62, P = 0.003).
Figure 4. Male scent marking and reproductive success.
The amount of male scent marking in relation to the proportion of offspring sired within a litter under (a) the intrusion treatment and (b) control conditions. The proportion of offspring sired is shown, although male reproductive success was calculated as a binomial response with one male’s reproductive success being dependent on the second male’s reproductive success.
Figure 5. Female social preference (number of female observations in male territories) in relation to male reproductive success (number of offspring sired) under (a) the intrusion treatment and (b) control conditions.
The proportion of offspring sired is shown, although male reproductive success was calculated as a binomial response with one male’s reproductive success being dependent on the second male’s reproductive success.
Male Scent Marking and Female Social Preference
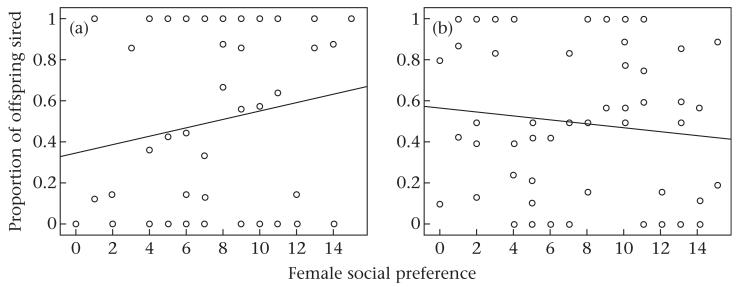
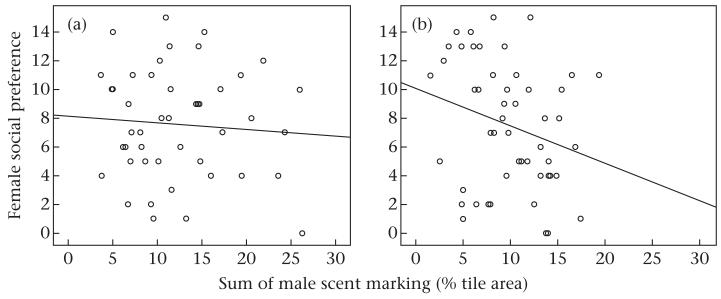
We conducted further analyses to assess whether marking may have increased males’ reproductive success by increasing female social preference. In the intrusion treatment, we found no evidence that the sum of male scent marking (GLMM: z = −0.926, N = 46, P = 0.354; Fig. 6a), male body mass change (GLMM: z = 0.764, N = 64, P = 0.445) or trial (GLMM: z = 0.275, N = 64, P = 0.784) influenced female social preference. In=the controls, female social preference was negatively associated with the sum of males’ scent marking (GLMM: z = −4.030, β = −0.071, SE = 0.018, N = 50, P < 0.001; Fig. 6b). Male body mass change (GLMM: z = 1.404, N = 48, P = 0.160) and trial (GLMM: z = −0.432, N = 50, P = 0.665) had no influence on female social preference.
Figure 6. Female social preference (number of female observations in male territories) in relation to male scent making under (a) the intrusion treatment and (b) control conditions.
DISCUSSION
Our results indicate that males that deposited more scent marks had higher reproductive success than other males, which, to our knowledge, provides the first direct evidence that scent marking is maintained by sexual selection. Our finding contrasts with previous studies that found no evidence in prairie voles that scent marking increased males’ mating success when females could choose between two males (Thomas 2002; Mech et al. 2003). However, as we explain below, our results suggest that the 30 min timescale used in these studies was probably insufficient to assess quantitative differences in males’ scent marking. Also, these studies did not measure paternity to assess male reproductive success directly. Our results might have been due to female mate choice (intersexual selection), as females could choose their mates without sexual coercion, but our observations of females’ social preferences provide only mixed evidence for this hypothesis. We address these results and our other main findings below in more detail.
Variation in Male Scent Marking
We analysed the quantitative variation in males’ scent marking, and we found several interesting results. First, we found that males significantly increased their marking in the intrusion treatment compared to controls. This result shows that the introduction of a competitor’s scent marks into a male’s territory elicited increased scent marking from territorial males. Second, we found that males’ marking was especially pronounced at the shared territory border in both the intrusion treatments and the controls. If scent marking functions to advertise the ownership of the resident male to potential intruders, then males are expected to mark mainly at their territorial borders where competition for ownership is most likely (Ralls 1971; Gosling 1982). To our knowledge, our study is the first that experimentally manipulated male scent marks to assess the influence of male–female competition on the amount of male marking, and the first to quantify the distribution of scent marks within a male’s territory. Our results provide additional support for the hypothesis that male marking and countermarking advertises a male’s territory ownership and mediates intrasexual competition (Gosling 1982; Hurst 1990a; Gosling & Roberts 2001). Third, we found that males’ scent marking showed surprisingly large differences between and within individuals over time. The high day-to-day variation in scent marking suggests that studies measuring scent marking for only a short period could erroneously classify a low marker as a high-marking individual and vice versa. The only other study to our knowledge that repeatedly measured male scent marking over time found that male mice (strain C57BL/J6) showed a rapid decline in marking when repeatedly exposed to the same individuals or the same environment (Arakawa et al. 2008), although we did not find evidence for such habituation effects. For some days, our estimates of marked area yielded negative values, indicating that more scent marks were lost during this period than were added, presumably because of the volatility of pheromones. For the intrusion treatment, negative values could also be the result of males removing the scent marks of competitors, although this hypothesis has been questioned for two reasons: (1) male scent marks are so broadly scattered that it is doubtful that residents can remove them (Hurst & Rich 1999); and (2) males may benefit from the presence of countermarks if they provide a record of the owner’s success in repelling intruders (Hurst & Rich 1999). None the less, it might be beneficial for males to remove competing scent marks to advertise their sole scent marks to others (Gosling 1982; Rich & Hurst 1998). Regardless of the causes of the negative values in marking, our findings help to explain why males must constantly scent mark their territory to advertise their social status and competitive ability honestly. Finally, although we found large fluctuations in most males’ daily scent marking, the sum of each male’s scent marking was correlated between the two experimental trials. This finding indicates that males show individual consistency in their overall scent marking, despite large day-to-day fluctuations and interactions with different individuals. Also, since males’ initial scent marking was correlated with their sum of marking, female introduction did not change the individual males’ rank order of marking. Low markers remained low markers regardless of the availability of females, which suggests that males’ low marking during females’ absence was not due to a strategic reduction of reproductive investment for the future, and that low-marking males may be poor quality and unable to afford higher marking rates.
Male Reproductive Success
We found that male reproductive success was positively correlated with increased body mass during the experiment, although only under control conditions. This finding indicates that increasing body mass improved males’ reproductive success, which might have been because females preferred to mate with males in better condition or cryptically biased paternity towards these males. In this experiment, male pairs were closely matched for initial body mass, but females might have used changes in body mass during the experiment as an indicator of male quality. We would expect such a preference in both the intrusion treatment and controls, but perhaps females pay more attention to males’ condition under low levels of male–female competition. We cannot rule out effects of male–female competition, and a nonexclusive alternative explanation for this result could be that males that gained more body mass had more competitive sperm or were more effective in intimidating their rivals.
When we analysed how the sum of males’ scent marking influenced their reproductive success, we found a small but significant correlation between these variables, which supports the hypothesis that scent marking is a sexually selected trait. The correlation between male marking and reproductive success was stronger under the intrusion treatment compared to controls, although the increase was not significantly different. In this analysis, we also surprisingly found that female social preferences had different effects on male reproductive success depending on the experimental treatment: under intrusion, males’ reproductive success was positively correlated with female social preference, as expected, whereas in the controls, males’ reproductive success was negatively correlated with how often females were observed on their territories. In other words, under the threat of male intrusion, females were more likely to mate with their social partner than his rival, whereas without this threat, females spent more time with their social mates’ competitor. This result is difficult to understand, but perhaps females in control conditions, with reduced male–female interactions, were attempting to incite male–female competition, as observed in other mammals (Cox & Le Boeuf 1977). Alternatively, we cannot exclude that our experimental manipulation affected the establishment of male social dominance relations, which in turn, could have affected female social preferences. Regardless of the cause, this interaction shows that the effect of females’ social preferences on males’ reproductive success depended on male intrusion (male–female interactions; Wong & Candolin 2005). Thus, our findings emphasize that female social preferences are not as straightforward as often assumed, and that they do not necessarily correspond to actual mating preferences, which is why it is important to measure paternity and not only females’ social preferences (Gubernick & Addington 1994).
We conducted further analyses to test whether males’ scent marking increased their reproductive success by making themselves or their territories more attractive to females (female social preferences). Although male scent marking and female social preference both correlated with male reproductive success under intrusion, male marking had no effect on females’ social preferences under intrusion and, surprisingly, was associated with reduced social preferences in control conditions. This result suggests that even though males’ scent marking increased their reproductive success, this was not because it improved females’ social preferences. A previous study on wild-derived house mice, M. m. domesticus, found that females are attracted to the scent of competitively marking males (males in controls versus intrusion), but not to their territories (Rich & Hurst 1998). A possible explanation why females prefer to mate with high-marking males, but do not prefer their territories, might be that high markers are more aggressive (Drickamer 2001) and females are more likely to get attacked by these males. Female mice prefer dominant over subordinate male odours (Jones & Nowell 1974; Drickamer 1992); however, they prefer to dwell with subordinates when observed overnight, as subordinates are less aggressive towards them (Mainardi & Pasquali 1973; Parmigiani et al. 1982a).
Finally, although we controlled for male sexual coercion and allowed females to select among territorial males, we cannot rule out the possibility that females may have generally mated with both males and cryptically biased paternity towards the higher-marking males or that the reproductive benefits of scent marking were due to cryptic male–female interactions (intrasexual selection). For example, high markers may have produced more competitive ejaculates in sperm competition and thus sired more offspring. Dominant male mice have increased sperm motility, sperm density and preputial gland weight compared to subordinates (Koyama & Kamimura 1999, 2000) but it is not known whether differences in sperm production between dominant, territorial males is correlated with their scent marking. We also found that male reproductive success was correlated between the first and the second trial of the experiment. This result indicates that male reproductive success is robust as it is repeatable over time and across different social conditions. High-quality males might be constantly superior in male–female interactions including sperm competition or better at attracting females. Further studies are needed to determine whether male scent marking affects male reproductive success through female choice, male–female interactions or both, and how male–female interactions affect female choice for male scent marking (and vice versa).
Acknowledgments
We thank S. M. Zala for her valuable comments on the study and the manuscript and two anonymous referees for their insightful comments. We also thank T. Klaus and E. K. Straßer for assistance in data collection. Research was supported by the Austrian Science Fund (FWF): P 24711-B21; A.H. was supported by the ‘Lendület’ programme of the Hungarian Academy of Sciences (MTA, LP2012-24/2012; www.mta.hu) and S.R. by Swiss National Science Foundation fellowships (PBNEP3-132801 and PBNEP3-140190).
References
- Arakawa H, Arakawa K, Blanchard DC, Blanchard RJ. Scent marking behavior in male C57BL/6J mice: sexual and developmental determination. Behavioural Brain Research. 2007;182:73–79. doi: 10.1016/j.bbr.2007.05.007. http://dx.doi.org/10.1016/j.bbr.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arakawa H, Arakawa K, Blanchard DC, Blanchard RJ. A new test paradigm for social recognition evidenced by urinary scent marking behavior in C57BL/6J mice. Behavioural Brain Research. 2008;190:97–104. doi: 10.1016/j.bbr.2008.02.009. http://dx.doi.org/10.1016/j.bbr.2008.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brashares JS, Arcese P. Scent marking in a territorial African antelope: I. The maintenance of borders between male oribi. Animal Behaviour. 1999;57:1–10. doi: 10.1006/anbe.1998.0941. http://dx.doi.org/10.1006/anbe.1998.0941. [DOI] [PubMed] [Google Scholar]
- Cox CR, Le Boeuf BJ. Female incitation of male competition: a mechanism in sexual selection. The American Naturalist. 1977;111:317–335. http://dx.doi.org/10.2307/2460066. [Google Scholar]
- Crowcroft P. Territoriality in wild house mice, Mus musculus L. Journal of Mammalogy. 1955;36:299–301. http://dx.doi.org/10.2307/1375908. [Google Scholar]
- Crowcroft P, Rowe FP. Social organization and territorial behaviour in the wild house mouse (Mus musculus L.) Proceedings of the Zoological Society of London. 1963;140:517–531. http://dx.doi.org/10.1111/j.1469-7998.1963.tb01871.x. [Google Scholar]
- Darwin C. The Descent of Man and Selection in Relation to Sex. 2nd edn. J. Murray; London: 1874. [Google Scholar]
- DeFries JC, McClearn GE. Social dominance and Darwinian fitness in the laboratory mouse. The American Naturalist. 1970;104:408–411. http://www.jstor.org/stable/2459127. [Google Scholar]
- Desjardins C, Maruniak JA, Bronson FH. Social rank in house mice: differentiation revealed by ultraviolet visualization of urinary marking patterns. Science. 1973;182:939–941. doi: 10.1126/science.182.4115.939. http://dx.doi.org/10.1126/science.182.4115.939. [DOI] [PubMed] [Google Scholar]
- Drickamer LC. Oestrous female house mice discriminate dominant from subordinate males and sons of dominant from sons of subordinate males by odour cues. Animal Behaviour. 1992;43:868–870. http://dx.doi.org/10.1016/S0003-3472(05)80212-X. [Google Scholar]
- Drickamer LC. Urine marking and social dominance in male house mice (Mus musculus domesticus) Behavioural Processes. 2001;53:113–120. doi: 10.1016/s0376-6357(00)00152-2. http://dx.doi.org/10.1016/S0376-6357(00)00152-2. [DOI] [PubMed] [Google Scholar]
- Engqvist L. The mistreatment of covariate interaction terms in linear model analyses of behavioural and evolutionary ecology studies. Animal Behaviour. 2005;70:967–971. http://dx.doi.org/10.1016/j.anbehav.2005.01.016. [Google Scholar]
- Gosling LM. A reassessment of the function of scent marking in territories. Zeitschrift für Tierpsychologie. 1982;60:89–118. http://dx.doi.org/10.1111/j.1439-0310.1982.tb00492.x. [Google Scholar]
- Gosling LM, Roberts SC. Scent-marking by male mammals: cheat-proof signals to competitors and mates. Advances in the Study of Behavior. 2001;30:169–217. http://dx.doi.org/10.1016/S0065-3454(01)80007-3. [Google Scholar]
- Grafen A, Hails R. Modern Statistics for the Life Sciences. Oxford University Press; Oxford: 2002. [Google Scholar]
- Gubernick DJ, Addington RL. The stability of female social and mating preferences in the monogamous California mouse, Peromyscus californicus. Animal Behaviour. 1994;47:559–567. http://dx.doi.org/10.1006/anbe.1994.1079. [Google Scholar]
- Hurst JL. The complex network of olfactory communication in populations of wild house mice Mus domesticus Rutty: urine marking and investigation within family groups. Animal Behaviour. 1989;37:705–725. http://dx.doi.org/10.1016/0003-3472(89)90057-2. [Google Scholar]
- Hurst JL. Urine marking in populations of wild house mice, Mus domesticus Rutty. I. Communication between males. Animal Behaviour. 1990a;40:209–222. http://dx.doi.org/10.1016/S0003-3472(05)80916-9. [Google Scholar]
- Hurst JL. Urine marking in populations of wild house mice, Mus domesticus Rutty. III. Communication between the sexes. Animal Behaviour. 1990b;40:233–243. http://dx.doi.org/10.1016/S0003-3472(05)80918-2. [Google Scholar]
- Hurst JL, Rich TJ. Scent marks as competitive signals of mate quality. In: Johnston RE, Müller-Schwarze D, Sorokin ES, editors. Advances in Chemical Signals in Vertebrates. Kluwer; Dordrecht: 1999. pp. 209–225. [Google Scholar]
- Jones RB, Nowell NW. Aversive effects of the urine of a male mouse upon the investigatory behaviour of its defeated opponent. Animal Behaviour. 1973;21:707–710. doi: 10.1016/s0003-3472(73)80095-8. http://dx.doi.org/10.1016/S0003-3472(73)80095-8. [DOI] [PubMed] [Google Scholar]
- Jones RB, Nowell NW. A comparison of the aversive and female attractant properties of urine from dominant and subordinate male mice. Animal Learning and Behavior. 1974;2:141–144. doi: 10.3758/bf03199141. http://dx.doi.org/10.3758/BF03199141. [DOI] [PubMed] [Google Scholar]
- Kalinowski ST, Taper ML, Marshall TC. Revising how the computer program CERVUS accommodates genotyping error increases success in paternity assignment. Molecular Ecology. 2007;16:1099–1106. doi: 10.1111/j.1365-294X.2007.03089.x. http://dx.doi.org/10.1111/j.1365-294X.2007.03089.x. [DOI] [PubMed] [Google Scholar]
- Kavaliers M, Colwell DD. Odours of parasitized males induce aversive response in female mice. Animal Behaviour. 1995;50:1161–1169. http://dx.doi.org/10.1016/0003-3472(95)80032-8. [Google Scholar]
- Kimura T, Hagiwara Y. Regulation of urine marking in male and female mice: effects of sex steroids. Hormones and Behavior. 1985;19:64–70. doi: 10.1016/0018-506x(85)90006-6. http://dx.doi.org/10.1016/0018-506X(85)90006-6. [DOI] [PubMed] [Google Scholar]
- Koyama S, Kamimura S. Lowered sperm motility in subordinate social status of mice. Physiology and Behavior. 1999;65:665–669. doi: 10.1016/s0031-9384(98)00205-4. http://dx.doi.org/10.1016/S0031-9384(98)00205-4. [DOI] [PubMed] [Google Scholar]
- Koyama S, Kamimura S. Influence of social dominance and female odor on the sperm activity of male mice. Physiology and Behavior. 2000;71:415–422. doi: 10.1016/s0031-9384(00)00361-9. http://dx.doi.org/10.1016/S0031-9384(00)00361-9. [DOI] [PubMed] [Google Scholar]
- Lenington S. Social preferences for partners carrying ‘good genes’ in wild house mice. Animal Behaviour. 1983;31:325–333. http://dx.doi.org/10.1016/S0003-3472(83)80050-5. [Google Scholar]
- Mainardi M, Pasquali A. Socio-sexual preferences of the female mouse (Mus musculus) in relation to the social status of the males. Ateneo Parmense. 1973;9:2–12. [Google Scholar]
- Marshall TC, Slate J, Kruuk LE, Pemberton JM. Statistical confidence for likelihood-based paternity inference in natural populations. Molecular Ecology. 1998;7:639–655. doi: 10.1046/j.1365-294x.1998.00374.x. http://dx.doi.org/10.1046/j.1365-294x.1998.00374.x. [DOI] [PubMed] [Google Scholar]
- Maruniak JA, Owen K, Bronson FK, Desjardins C. Urinary marking in male house mice: responses to novel environmental and social stimuli. Physiology and Behavior. 1974;12:1035–1039. doi: 10.1016/0031-9384(74)90151-6. http://dx.doi.org/10.1016/0031-9384(74)90151-6. [DOI] [PubMed] [Google Scholar]
- Maruniak JA, Owen K, Bronson FH, DesJardins C. Urinary marking in female house mice: effects of ovarian steroids, sex experience and type of stimulus. Behavioral Biology. 1975;13:211–217. doi: 10.1016/s0091-6773(75)91920-3. http://dx.doi.org/10.1016/S0091-6773(75)91920-3. [DOI] [PubMed] [Google Scholar]
- Mech SG, Dunlap AS, Wolff JO. Female prairie voles do not choose males based on their frequency of scent marking. Behavioural Processes. 2003;61:101–108. doi: 10.1016/s0376-6357(02)00128-6. http://dx.doi.org/10.1016/S0376-6357(02)00128-6. [DOI] [PubMed] [Google Scholar]
- Parmigiani S, Brunoni V, Pasquali A. Behavioural influences of dominant, isolated and subordinated male mice on female socio-sexual preferences. Bolletino di Zoologia. 1982a;49:31–35. http://dx.doi.org/10.1080/11250008209439369. [Google Scholar]
- Parmigiani S, Brunoni V, Pasquali A. Socio-sexual preferences of female mice (Mus musculus domesticus): the influence of social aggressive capacities of isolated or grouped males. Bolletino di Zoologia. 1982b;49:73–78. http://dx.doi.org/10.1080/11250008209439374. [Google Scholar]
- Penn DJ. Chemical communication: five major challenges in the post-genomics age. In: Dicke M, Takken W, editors. Chemical Ecology: From Gene to Ecosystem. Springer; Wageningen: 2006. pp. 9–18. [Google Scholar]
- Penn DJ, Potts WK. Chemical signals and parasite-mediated sexual selection. Trends in Ecology and Evolution. 1998;13:391–396. doi: 10.1016/s0169-5347(98)01473-6. http://dx.doi.org/10.1016/S0169-5347(98)01473-6. [DOI] [PubMed] [Google Scholar]
- Penn DJ, Schneider G, White K, Slev P, Potts W. Influenza infection neutralizes the attractiveness of male odor to female mice (Mus musculus) Ethology. 1998;104:685–694. http://dx.doi.org/10.1111/j.1439-0310.1998.tb00102.x. [Google Scholar]
- R Development Core Team . R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; Vienna: 2011. [Google Scholar]
- Ralls K. Mammalian scent marking. Science. 1971;171:443–449. doi: 10.1126/science.171.3970.443. http://dx.doi.org/10.1126/science.171.3970.443. [DOI] [PubMed] [Google Scholar]
- Reynolds E. Urination as a social response in mice. Nature. 1971;234:481–483. doi: 10.1038/234481a0. http://dx.doi.org/10.1038/234481a0. [DOI] [PubMed] [Google Scholar]
- Rich TJ, Hurst JL. Scent marks as reliable signals of the competitive ability of mates. Animal Behaviour. 1998;56:727–735. doi: 10.1006/anbe.1998.0803. http://dx.doi.org/10.1006/anbe.1998.0803. [DOI] [PubMed] [Google Scholar]
- Rich TJ, Hurst JL. The competing countermarks hypothesis: reliable assessment of competitive ability by potential mates. Animal Behaviour. 1999;58:1027–1037. doi: 10.1006/anbe.1999.1217. http://dx.doi.org/10.1006/anbe.1999.1217. [DOI] [PubMed] [Google Scholar]
- Rolland C, MacDonald DW, de Fraipont M, Berdoy M. Free female choice in house mice: leaving best for last. Behaviour. 2003;140:1371–1388. http://dx.doi.org/10.1163/156853903771980639. [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning. A Laboratory Manual. 2nd edn. Cold Spring Harbor Laboratory Press; Plainview, New York: 1989. [Google Scholar]
- Schaedelin FC, Taborsky M. Extended phenotypes as signals. Biological Reviews. 2009;84:293–313. doi: 10.1111/j.1469-185X.2008.00075.x. http://dx.doi.org/10.1111/j.1469-185X.2008.00075.x. [DOI] [PubMed] [Google Scholar]
- Thomas S. Scent marking and mate choice in the prairie vole,Microtus ochrogaster. Animal Behaviour. 2002;63:1121–1127. http://dx.doi.org/10.1006/anbe.2002.3014. [Google Scholar]
- Walther FR. Mapping the structure and the marking system of a territory of the Thomson’s gazelle. East African Wildlife Journal. 1978;16:167–176. http://dx.doi.org/10.1111/j.1365-2028.1978.tb00437.x. [Google Scholar]
- Wolff P, Powell A. Urine patterns in mice: an analysis of male/female counter-marking. Animal Behaviour. 1984;32:1185–1191. http://dx.doi.org/10.1016/S0003-3472(84)80235-3. [Google Scholar]
- Wong BBM, Candolin U. How is female mate choice affected by male competition? Biological Reviews of the Cambridge Philosophical Society. 2005;80:559–571. doi: 10.1017/S1464793105006809. http://dx.doi.org/10.1017/S1464793105006809. [DOI] [PubMed] [Google Scholar]
- Yamazaki K, Boyse EA, Mike V, Thaler HT, Mathieson BJ, Abbott J, Boyse J, Zayas ZA. Control of mating preferences in mice by genes in the major histocompatibility complex. The Journal of Experimental Medicine. 1976;144:1324–1335. doi: 10.1084/jem.144.5.1324. http://dx.doi.org/10.1084/jem.144.5.1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zala SM, Potts WK, Penn DJ. Scent-marking displays provide honest signals of health and infection. Behavioral Ecology. 2004;15:338–344. http://dx.doi.org/10.1093/beheco/arh022. [Google Scholar]
- Zala SM, Chan B, Bilbo SD, Potts WK, Nelson RJ, Penn DJ. Genetic resistance to infection influences a male’s sexual attractiveness and modulation of testosterone. Brain Behavior and Immunity. 2008;22:381–387. doi: 10.1016/j.bbi.2007.09.003. http://dx.doi.org/10.1016/j.bbi.2007.09.003. [DOI] [PubMed] [Google Scholar]