Abstract
Introduction
Survivors of sepsis report persistent problems that can last years after hospital discharge. The main aim of this study was to investigate long-term health-related quality of life in survivors of SIRS and sepsis compared with Welsh normative data, controlling for age, length of stay and pre-existing conditions. The second aim was to investigate any differences in long-term health-related quality of life specifically with the patients categorised into three groups; SIRS, uncomplicated sepsis and severe sepsis/septic shock.
Methods
A prospective study design was used in order to investigate all sepsis patients either presenting to the Emergency Department or admitted to the Intensive Care Unit of a regional trauma centre. Baseline demographics, clinical characteristics and outcomes were collected and surviving patients were sent a SF-12v2 survey at between six months to two years post-hospital discharge.
Results
Quality of life was significantly reduced in all patients when compared to local normative data (all p<0.0001). Reductions in the physical components of health-related quality of life were more pronounced in severe sepsis/septic shock patients when compared to uncomplicated sepsis and SIRS patients, when controlling for age, pre-existing conditions, hospital and ICU length of stay.
Conclusions
This is the first observational study to specifically focus on the different groups of SIRS and sepsis patients to assess long-term quality of life. Local population norms were used for comparison, rather than UK-wide norms that fail to reflect the intricacies of a country’s population.
Introduction
The incidence of sepsis has increased despite advances in supportive care and a number of national and international campaigns aimed at developing guidelines for the management of the condition. [1], [2] In the UK, severe sepsis is estimated to kill 37,000 patients annually, and consume 50% of critical care resources. [2] Severe sepsis is a common cause of Intensive Care Unit (ICU) admission and patients with sepsis are also at risk of other complications such as multi-organ failure and acute lung injury. [3] Pathophysiological mechanisms are not fully understood however severe infection initiates an inflammatory process that compromises the immune system, leading to more invasive infection, altered coagulation and cardiovascular instability and ultimately causing organ failure or death [3].
Survivors of sepsis report persistent problems that can last years after hospital discharge. These include physical, (weakness and dyspnoea) psychological (post-traumatic stress syndrome and depression), cognitive (poor concentration and memory loss) and social issues (delayed return to work and loss of earnings) [3]–[7].
Heyland et al (2000) reported that the long-term health related quality of life of survivors of sepsis was significantly lower than that of the general US population. [5] A more recent study reported that severe sepsis survivors have a significantly lower physical quality of life compared to population norms but mental quality of life scores were only slightly below population norms up to five years after hospital discharge. [4] Similar results were reported in another study, in which severe sepsis was independently associated with substantial and persistent new cognitive impairment and functional disability among survivors. [6] Hofhuis et al (2008) reported that physical and general health recovery is incomplete in survivors of severe sepsis at six months post-ICU discharge when compared with preadmission status [7].
Comparison between studies investigating long-term health-related quality of life outcomes in sepsis survivors is difficult. One of the main limitations described in the systematic review by Winters et al (2010) was that previous studies do not control for comorbidities when comparing to a control population. Another limitation they described was a lack of biomarkers for the sepsis patients investigated in the included studies. [3] They stated that because other conditions such as non-infectious systemic inflammatory response syndrome (SIRS) may masquerade as sepsis, they could not rule out the possibility of misclassification bias. [3] The main aim of this study therefore was to investigate long-term health-related quality of life in survivors of SIRS and sepsis compared with Welsh normative data, controlling for age, length of stay and pre-existing conditions. The second aim was to investigate any differences in long-term health-related quality of life specifically with the patients categorised into three groups; SIRS, uncomplicated sepsis and severe sepsis/septic shock.
Materials and Methods
Ethical Approval
Full ethical approval was given by the South West Wales Research Ethics Committee. Informed 2-stage written consent was given by patients with capacity to do so. Assent was obtained from personal or legal representation in cases where capacity to give informed consent was lacking.
Setting and sample
A prospective study design was used in order to investigate all sepsis patients either presenting to the Emergency Department (ED) or admitted to the Intensive Care Unit (ICU) (from a hospital ward or peripheral hospital) of a large regional trauma centre in South West Wales from October 2011 to November 2013. Morriston hospital has approximately 90,000 presentations to the ED per year and serves a population of 450,000 people. A total of 106 patients were recruited and all patients were considered eligible as per the SIRS and sepsis criteria defined in 2003. [9] Using these criteria, SIRS was defined as a systemic inflammatory response that can be triggered by a variety of infectious and non-infectious conditions; uncomplicated sepsis is defined the presence of both infection and a systemic inflammatory response; severe sepsis was defined as sepsis complicated by organ dysfunction; and septic shock was defined as a state of acute circulatory failure characterized by persistent arterial hypotension unexplained by other causes.
Sepsis-related Organ Failure Assessment (SOFA) score was determined over the first 24 hours to assess organ function. [10] Patients were assigned to groups as follows: 1) Sterile SIRS 2) Uncomplicated sepsis 3) Severe sepsis or septic shock as per the criteria defined in 2003. [9] Assignment into groups was blinded and performed by an experienced intensive care specialist independent of the study.
A sample size of n = 100 was proposed for this study; calculated using a previously published Minimally Clinically Important Difference of 8.1 points (SD 9.7) using the SF-12v2 to assess quality of life. [8] The sample size, based on a three sample comparison of means was calculated at 17 per group for the SF-12v2 at 80% power with a two-sided, α error level of 0.05. This takes into consideration a 30% loss to follow up (mortality, drop-outs and non-response to the survey).
Study design and outcome measures
Baseline demographics, clinical characteristics and outcomes were collected during hospital stay. These included age, sex, SOFA scores, pre-existing conditions, primary admitting diagnosis, hospital and ICU length of stay and mortality. Pre-existing conditions were scored using a previously published electronic version of the Charlson Comorbidity Index. [11] Primary admitting diagnosis was categorised into groups; respiratory, neurologic, cardiovascular, endocrine, renal and gastro-intestinal. In terms of the assessment of quality of life, this study used a cross sectional design surveying the survivors of sepsis at a single point in time (there was no baseline measure of health-related quality of life). Surviving patients were sent a postal survey at between six months to two years post-hospital discharge. A second survey was sent out to non-responders after two months.
The Short Form-12 version 2 (SF-12v2) is a multipurpose survey with 12 questions, (all selected from the SF-36 Health Survey) which investigate mental and physical functioning and overall health-related quality of life. It has been developed to provide a shorter alternative to the SF-36. [12] The SF-12 is a valid and reliable measure that is weighted and summed to provide easily interpretable scales for physical and mental Health Composite Scores (PCS & MCS) in which scores range from 0 to 100 (100 = optimal) [12], [13].
This instrument contains eight multi-item dimensions (ie, physical functioning [PF], role limitation due to physical problems [RP], bodily pain [BP], general health [GH], vitality [VT], social functioning [SF], role limitation due to emotional problems [RE], and mental health [MH]). The physical health summary score (PCS) reflects PF, RP, BP, and GH. The mental health summary scale (MCS) reflects VT, SF, RE, and MH. Answers were transformed, weighed, subsequently scored and aggregated to summary measures according to predefined guidelines. [14] The US normative data normally used to complete this transformation and analysis were replaced with Welsh normative data produced in 2011 [15].
Statistical analysis
All statistical analyses were performed using IBM SPSS Statistics version 20 software. A baseline comparison of demographics, clinical characteristics and outcomes was completed with continuous data expressed as medians (interquartile ranges) and categorical data expressed as absolute values (percentages). Scores for each for the eight domains on the SF-12v2 were expressed as means (SD) as previously recommended. [16] These means (SD) were then compared to the Welsh normative data and also to Mid and West Wales normative data (geographical location of Morriston hospital) using independent t tests.
Data normality was assessed using the Kolmogorov-Smirnov and Shapiro-Wilk tests using an alpha value of 0.05. For the subgroup analysis, differences between the three groups were compared using Analysis of Covariance, in order to control for the possible confounding variables age, co-morbidity and hospital and ICU length of stay. Bonferroni correction was used to assess multiple comparisons across groups. All p values were two tailed and p values <0.05 were considered statistically significant.
Results
A total of 109 patients who met study inclusion criteria presented to the ED during the data collection period. A mortality rate of 34% was recorded in this study. Three patients were of no-fixed-abode therefore a total of 69 surveys were sent out for completion. A total of 50 surveys (response rate 72%) were received by the end of the data collection period. There was no significant difference in any of the variables analysed between the responders and non-responders. The patients’ demographic and clinical characteristics are highlighted in Table 1. The only significant difference between the three groups was hospital and ICU length of stay.
Table 1. Demographic and clinical characteristics.
| Characteristic | All patients n = 50 | Group 1 n = 19 | Group 2 n = 16 | Group 3 n = 15 |
| Age, years | 58 (30) | 52 (33) | 46.5 (30) | 68 (17) |
| Male, % | 23 (46) | 6 (32) | 8 (50) | 9 (60) |
| Female, % | 27 (54) | 13 (68) | 8 (50) | 6 (40) |
| SOFA score | 3 (4) | 3 (3) | 3 (3) | 5 (9) |
| CharlsonComorbidity Index | 3 (4) | 4 (5) | 2 (4) | 4 (5) |
| ICU LOS, days | 0 (4) | 0 (1) | 0 (5) | 4 (21)Δ ‡ |
| Hospital LOS, days | 7 (15) | 6 (16) | 5.5 (10) | 19 (29)Δ ‡ |
| Diagnostic groups, % | ||||
| Respiratory | 18 (36) | 3 (16) | 8 (50) | 7 (47) |
| Gastrointestinal | 9 (18) | 4 (21) | 1 (6) | 4 (27) |
| Neurologic | 1 (2) | 1 (5) | 0 (0) | 0 (0) |
| Endocrine | 10 (20) | 9 (47) | 0 (0) | 1 (7) |
| Renal | 8 (16) | 2 (11) | 5 (31) | 1 (7) |
| Other | 4 (8) | 0 (0) | 2 (13) | 2 (13) |
denotes a significant difference between group 1 and 3. (p<0.05).
denotes a significant difference between group 2 and 3. (p<0.05).
All results are presented as medians (IQR) or absolute values (%).
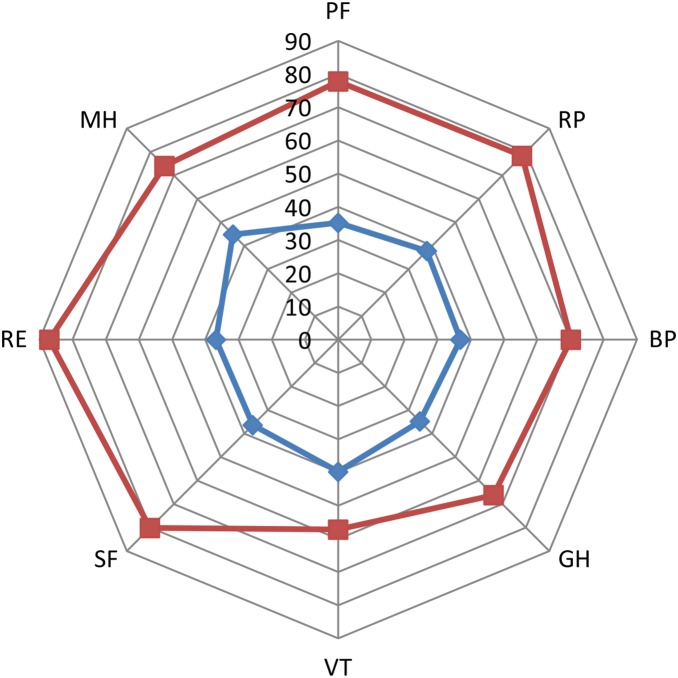
Table 2 illustrates the results of the SF-12v2 survey, comparing all the SIRS and sepsis patients (n = 50) with the Welsh population and Mid/West Wales population. Significant differences were recorded for each of the eight health domains, between the SIRS and sepsis patients and both populations. Means and standard deviations are presented for each domain.
Table 2. Short form-12 mean scores (SD) for survivors of SIRS/sepsis compared to Welsh population and Mid/West Wales population.
| Domains | All sepsis patients | All Welsh Population | p value | Mid/West Wales Population | p value |
| Physical functioning | 35.2 (11.3) | 77.8 (30.0) | <0.0001 | 77.5 (30.8) | <0.0001 |
| Role physical | 37.8 (9.5) | 78.3 (32.3) | <0.0001 | 78.1 (31.9) | <0.0001 |
| Bodily pain | 36.8 (12.7) | 70.1 (28.9) | <0.0001 | 69.4 (28.9) | <0.0001 |
| General health | 34.8 (10.9) | 66.2 (24.0) | <0.0001 | 66.2 (23.9) | <0.0001 |
| Vitality | 39.8 (10.0) | 57.2 (22.3) | <0.0001 | 57.7 (22.6) | <0.0001 |
| Social functioning | 36.4 (12.0) | 80.2 (28.1) | <0.0001 | 80.4 (27.9) | <0.0001 |
| Role emotional | 36.8 (12.8) | 87.0 (26.0) | <0.0001 | 87.4 (25.4) | <0.0001 |
| Mental health | 44.8 (13.5) | 74.0 (18.9) | <0.0001 | 74.7 (18.7) | <0.0001 |
All results are presented as means (SD).
Fig. 1 is a radar chart which illustrates the difference in each of the eight health domains between the sepsis patients and the Welsh population.
Figure 1. Health-Related Quality of Life in sepsis survivors at six months to two years compared to the Welsh Population.
Radar chart of the health related quality of life in long term survivors of sepsis compared to Welsh population. Blue line with squares: all sepsis group results. Red line with diamonds: Welsh population results. PF: physical functioning, RP: role limitation physical, BP: bodily pain, GH: general health, VT: vitality, SF: social functioning, RE: role limitation emotional, MH: mental health.
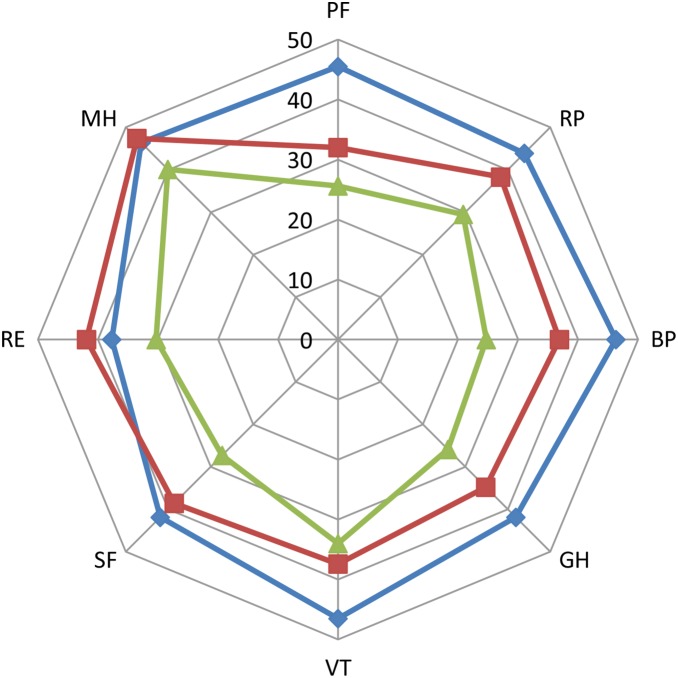
The ANCOVA results in Table 3 highlight the Short form-12 mean scores comparing the SIRS, uncomplicated sepsis and severe sepsis/septic shock patients, when controlling for the variables age, pre-existing conditions, hospital length of stay and ICU length of stay. Significant differences were found between groups 1 and 3 all the domains accept the mental health, role emotional and mental component scores. Significant differences were reported between groups 2 and 3 on the role emotional, bodily pain, general health, vitality, social functioning, role emotional and physical component scores.
Table 3. Short form-12 mean scores comparing SIRS, uncomplicated sepsis and severe sepsis/septic shock.
| Domains | Sterile SIRS | Uncomplicated sepsis | Severe sepsis/septic shock |
| Physical functioning | 45.5 (9.9) | 32.0 (7.7)* | 25.6 (7.4)Δ |
| Role physical | 43.9 (10.0) | 38.3 (6.3) | 29.5 (4.7)Δ ‡ |
| Bodily pain | 46.3 (10.3) | 36.9 (10.8)* | 24.7 (4.4)Δ ‡ |
| General health | 41.9 (11.3) | 34.8 (8.8) | 25.9 (4.1)Δ ‡ |
| Vitality | 46.5 (8.6) | 37.4 (8.2)* | 34.0 (9.0)Δ |
| Social functioning | 41.9 (13.3) | 38.6 (10.7) | 27.3 (5.5)Δ ‡ |
| Role emotional | 37.7 (13.7) | 41.9 (10.9) | 30.3 (11.5)‡ |
| Mental health | 46.4 (12.5) | 47.4 (15.6) | 40.1 (11.7) |
| Physical componentscore | 47 (10.3) | 31.4 (7.4)* | 20.9 (4.7)Δ ‡ |
| Mental component score | 41.3 (13.8) | 43.4 (12.4) | 35.5 (10.2) |
*denotes a significant difference between group 1 and 2. (p<0.05).
denotes a significant difference between group 1 and 3. (p<0.05).
denotes a significant difference between group 2 and 3. (p<0.05).
All results are presented as means (SD).
Fig. 2 is a radar chart that illustrates the difference in each of the eight health domains between the three different groups.
Figure 2. Health-Related Quality of Life in sterile SIRS, uncomplicated sepsis and severe sepsis/septic shock.
Radar chart of the health-related quality of life in long-term survivors of Sterile SIRS (group 1– blue line with diamonds), uncomplicated sepsis (group 2– red line with squares) and severe sepsis/septic shock (group 3– green line with triangles). PF: physical functioning, RP: role limitation physical, BP: bodily pain, GH: general health, VT: vitality, SF: social functioning, RE: role limitation emotional, MH: mental health.
Discussion
The results of our study have demonstrated that survivors of SIRS and sepsis continue to experience a reduced quality of life from between six months to two years post hospital discharge. The SIRS and sepsis patients reported a significantly lower quality of life than the Welsh population on all eight health domains in the SF-12v2 survey. There was no comparative data for the physical and mental component scores for the Welsh population data as this was generated using the earlier SF-36 version 1 survey which does not calculate these component scores. [15] The results of this study support previous research which has reported that quality of life is lower in survivors of sepsis [3]–[7].
Our results also reported significantly lower quality of life than the Mid/West Wales population and this is the first study to use such local geographical normative data for comparisons. Previous research has stated that it is imperative that accurate normative data is used in population studies in order to provide an indication of the health status of particular populations. [15] It is only using such specific normative data that the impact of clinical interventions can be accurately measured.
This is the first study investigating long-term quality of life in sepsis survivors that has categorised patients into SIRS, uncomplicated sepsis and severe sepsis/septic shock in order to reduce misclassification bias. The results of our study demonstrated that severe sepsis/septic shock patients have significantly lower physical health related quality of life than SIRS and uncomplicated sepsis patients. These results are significant when controlling for possible confounding variables age, pre-existing conditions, hospital length of stay and ICU length of stay. This supports previous research which found that sepsis survivors demonstrated similar decrements in quality of life long-term to other chronic diseases such as chronic obstructive pulmonary disease or congestive heart failure3 and also acute lung injury [5].
The mental component score did not demonstrate any significant difference between groups. Previous research has reported that mental component scores do not significantly differ between sepsis patients and the US normative data. [5], [17] Our results found that although there were no significant differences between the three groups, the mental component scores were still significantly lower compared to the Welsh population norms.
Knowledge of the reduced quality of life in sepsis survivors facilitates a more accurate targeting of patients for interventions such as ICU follow up clinics. The physical components of the survey in our respondents illustrated the most profound reductions in quality of life which suggests that rehabilitation programmes should be considered for these patients. Hofhuis et al (2008) reported that recovery of quality of life in ICU survivors starts as early as the time of ICU discharge and therefore rehabilitation programmes should start early [7].
There are a number of limitations of this study that should be considered. The main limitation is the lack of a baseline measurement of quality of life for the patients. It has been suggested that ICU patients’ pre-morbid quality of life has a large effect on quality of life after critical illness. [18] It could be argued therefore that the reduced quality of life reported in this study merely reflects a poor baseline, rather than being an effect of the sepsis. To overcome this limitation the patients could have been requested to complete a survey retrospectively. We believe that a retrospective assessment of pre-sepsis quality of life would have not improved this study however due to the issues of recall bias. [18] The results of this study found no difference in the pre-existing conditions between the three groups. This may suggest therefore that there was no difference in pre-morbid quality of life between the three groups and the differences reported long-term were actually due to sepsis.
Another limitation concerns the size of the groups as they did not quite reach the number stated in the sample size calculation which may have influenced the power of the study. A response rate of 80% is ideal for a quality of life study and that was not quite achieved in this study. [18] However analysis of non-responders and responders found no differences between the two groups. Furthermore, the follow up period in our study ranged from six months to two years and this may have influenced the study’s findings. There is no universal definition for ‘long-term’ in follow-up studies and time periods previously investigated range from three months [3] through to five years. [4] It has been recommended that a follow up period of one to two years will probably capture the most accurate data and it may be the limit for improvement in most quality of life dimensions [18].
In conclusion, our study is the first observational study to specifically focus on the different groups of SIRS and sepsis patients to assess long-term quality of life. Local population norms were used for comparison, rather than United Kingdom wide geographical norms that fail to reflect the intricacies of a country’s population. Quality of life was reported to be significantly reduced in SIRS, uncomplicated sepsis and severe sepsis/septic shock patients when compared to local normative data. As expected, more significant reductions in quality of life were found in severe sepsis/septic shock patients than in uncomplicated sepsis and SIRS patients, when controlling for age, pre-existing conditions, hospital and ICU length of stay. Despite the limitations, this study encourages further multi-centred investigation into the long-term quality of life in survivors of SIRS and sepsis.
Acknowledgments
We would like to thank the Emergency Department and the Ed Major Critical Care Unit at Morriston Hospital who supported the completion of this study. We would also like to thank Dr Omar Bouamra for his statistical advice.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper.
Funding Statement
The authors have no support or funding to report.
References
- 1. Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, et al. (2013) Surviving Sepsis Campaign Guidelines Committee including The Pediatric Subgroup. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock. Intensive Care Med 39:165–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Daniels R, Nutbeam T, McNamara G, Galvin C (2011) The Sepsis Six and the Surviving Sepsis Campaign Resuscitation Bundle: a prospective observational cohort study. EMJ 28:507–12. [DOI] [PubMed] [Google Scholar]
- 3. Winters BD, Eberlein M, Leung J, Needham D, Pronovost PJ, et al. (2010) Long- term mortality and quality of life in sepsis: a systematic review. Crit Care Med 38:1276–83. [DOI] [PubMed] [Google Scholar]
- 4. Cuthbertson BH, Elders A, Hall S, Taylor J, MacLennan G, et al. and the Scottish Critical Care Trials Group and the Scottish Intensive Care Society Audit Group (2013) Mortality and quality of life in the five years after severe sepsis. Crit Care 17:R70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Heyland DK, Hopman W, Coo H, Tranmer J, McColl MA (2000) Long-term health-related quality of life in survivors of sepsis. Short Form 36: A valid and reliable measure of health-related quality of life. Crit Care Med 28:3599–605. [DOI] [PubMed] [Google Scholar]
- 6. Iwashyna TJ, Ely EW, Smith DM, Langa KM (2010) Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA 304:1787–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hofhuis JG, Spronk PE, van Stel HF, Schrijvers AJ, Rommes JH, et al. (2008) The impact of severe sepsis on health-related quality of life: a long-term follow-up study. Anesth Analg 107:1957–64. [DOI] [PubMed] [Google Scholar]
- 8. Parker SL, Godil SS, Shau DN, Mendenhall SK, McGirt MJ (2013) Assessment of the minimum clinically important difference in pain, disability and quality of life after anterior cervical discectomy and fusion: clinical article. J Neurosurg Spine 18:154–60. [DOI] [PubMed] [Google Scholar]
- 9. Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, et al. (2003) 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med 31:1250–1256. [DOI] [PubMed] [Google Scholar]
- 10. Vincent J, Moreno R, Takala J, Willatts S, de Mendonca A, et al. (1996) The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. Intensive care Med 22:707–10. [DOI] [PubMed] [Google Scholar]
- 11. Hall WH, Ramachandran R, Narayan S, Jani AB, Vijayakumar S (2004) An electronic application for rapidly calculating Charlson comorbidity score. BMC Cancer 4:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ware JE, Kosinski M, Keller SD (1996) A 12-item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Medical Care 34:220–233. [DOI] [PubMed] [Google Scholar]
- 13. Gandek B, Ware JE, Aaronson NK, Apolone G, Bjorner JB, et al. (1998) Cross-validation of item selection and scoring for the SF-12 Health Survey in nine countries: results from the IQOLA Project. J Clin Epidemiol 51:1171–8. [DOI] [PubMed] [Google Scholar]
- 14.Ware JE (1993) SF-36 Health Survey Manual and Interpretation Guide. Boston, The Health Institute, New England Medical Centre.
- 15. Burholt V, Nash P (2011) Short form 36 (SF-36) Health Survey Questionnaire: normative data for Wales. J Public Health 33:587–603. [DOI] [PubMed] [Google Scholar]
- 16. Dowdy DW, Eid MP, Sedrakyan A, Mendez-Tellez PA, Pronovost PJ, et al. (2005) Quality of life in adult survivors of critical illness: a systematic review of the literature. Intensive Care Med 31:611–20. [DOI] [PubMed] [Google Scholar]
- 17. Nessler N, Defontaine A, Launey Y, Morcet J, Malledant Y, et al. (2013) Long-term mortality and quality of life after septic shock: a follow up observational study. Intensive Care Med 39:881–8. [DOI] [PubMed] [Google Scholar]
- 18. Oeyen S, Vandijck DM, Benoit DD, Annemans L, Decruyenaere JM (2010) Quality of life after intensive care: a systematic review of the literature. Crit Care Med 38:2386–94. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper.