Abstract
To examine whole body protein turnover and muscle protein fractional synthesis rate (MPS) following ingestions of protein in mixed meals at two doses of protein and two intake patterns, 20 healthy older adult subjects (52–75 yr) participated in one of four groups in a randomized clinical trial: a level of protein intake of 0.8 g (1RDA) or 1.5 g·kg−1·day−1 (∼2RDA) with uneven (U: 15/20/65%) or even distribution (E: 33/33/33%) patterns of intake for breakfast, lunch, and dinner over the day (1RDA-U, 1RDA-E, 2RDA-U, or 2RDA-E). Subjects were studied with primed continuous infusions of l-[2H5]phenylalanine and l-[2H2]tyrosine on day 4 following 3 days of diet habituation. Whole body protein kinetics [protein synthesis (PS), breakdown, and net balance (NB)] were expressed as changes from the fasted to the fed states. Positive NB was achieved at both protein levels, but NB was greater in 2RDA vs. 1RDA (94.8 ± 6.0 vs. 58.9 ± 4.9 g protein/750 min; P = 0.0001), without effects of distribution on NB. The greater NB was due to the higher PS with 2RDA vs. 1RDA (15.4 ± 4.8 vs. −18.0 ± 8.4 g protein/750 min; P = 0.0018). Consistent with PS, MPS was greater with 2RDA vs. 1RDA, regardless of distribution patterns. In conclusion, whole body net protein balance was greater with protein intake above recommended dietary allowance (0.8 g protein·kg−1·day−1) in the context of mixed meals, without demonstrated effects of protein intake pattern, primarily through higher rates of protein synthesis at whole body and muscle levels.
Keywords: sarcopenia, essential amino acids, protein turnover, stable isotope tracers
it is well established that protein or amino acid ingestion (AA) stimulates protein synthesis (PS), for which essential amino acids (EAA) are mainly responsible (31, 33). There is a potential decline in muscle protein synthetic efficiency with an advancing age when a small amount of EEAs are ingested (14), but a larger intake of EAAs (i.e., 15 g in the form of beef) resulted in a similar stimulation of muscle protein synthesis (MPS) in young and old adults (27). Previous studies have shown that ingestion of ∼20–35 g of high-quality protein results in a near-maximal stimulation of MPS (18, 28). More specifically, Moore et al. (17) have shown in a breakpoint analysis using published fractional synthesis rate data from their laboratories that the minimum amount of protein intake per meal to achieve a maximal fractional synthesis rate is 0.40 g/kg body wt for healthy older (71 ± 1 yr) and 0.24 g/kg body wt for healthy younger (22 ± 4 yr) individuals. According to these data, the daily protein intake required to maximally stimulate the 24-h integrated MPS response for older individuals should be higher than 1.2 g·kg−1·day−1. However, the recommended dietary allowance (RDA) for protein is 0.8 g protein·kg−1·day−1 regardless of age in adults despite the demonstrated anabolic resistance with an advancing age. Individuals typically consume the majority of protein intake (and daily calories) with the dinner meal. The amount of protein intake ingested in this manner could theoretically maximize MPS. Thus, we postulated that a more frequent stimulation of protein anabolism, through ingestion of sufficient protein at each meal to elicit a maximal response, would result in a greater MPS than the more conventional pattern of ingestion.
Because the body protein pool size is the balance between PS and protein breakdown (PB), determination of only MPS is insufficient for a complete understanding of muscle protein turnover. From the practical standpoint, however, it is difficult to perform a simultaneous determination of muscle protein breakdown and MPS in a same experimental setting (35). Furthermore, although muscle anabolic response has been typically equated with anabolic response at the whole body level (11, 35), the determination of net balance of muscle protein may underestimate the total anabolic response over time, since part of the anabolic response takes place in the gut (10, 11). Therefore, we chose to determine MPS and protein turnover (synthesis and breakdown) at the whole body level to investigate the effects of both the pattern and amount of protein intake on net protein balance. Prior protein dose-response studies have generally investigated effects of AA/protein alone (37) or protein in a particular food source such as beef (24, 28) or egg (18) on muscle anabolic response. However, the pragmatic approach to protein ingestion is in the context of a mixed-nutrient meal. Both carbohydrate and fat components of a meal might be expected to affect the response to the protein in the meal. Therefore, we have investigated the response to different quantities and patterns of intake in the context of complete meals. We hypothesized that, in the context of mixed meal intake, 1) the even distribution of both protein intake levels would result in a greater cumulative anabolic response at both muscle and whole body levels compared with the uneven pattern, and 2) the anabolic response would be greater following 2RDA (1.5 g·kg−1·day−1) compared with 1RDA (0.8 g·kg−1·day−1) at both muscle and whole body levels.
SUBJECTS AND METHODS
Subjects.
Twenty-four healthy older subjects were recruited from the Little Rock area using local newspaper advertisements and flyers posted around the University of Arkansas for Medical Sciences campus and the Little Rock area (August 2011 through August 2012). Written informed consent was obtained from all subjects, and the study was approved by the Institutional Review Board at the University of Arkansas for Medical Sciences. All 24 subject were randomized, and upon their first visit to the lab, a battery of medical tests was performed for subject eligibility, including medical history, blood count, plasma electrolytes, blood glucose concentration, and liver and renal function tests. Exclusion criteria ruled out subjects with diabetes, active malignancy within the past 6 mo, gastrointestinal bypass surgery, a chronic inflammatory disease, low hematocrit or hemoglobin concentration, low platelets, concomitant use of corticosteroids, any unstable medical conditions, and subjects who took part in regular resistance exercise more than one time per week. Twenty older adult subjects [range of age: 52–75 yr] were included in the final analyses for this study (Table 1) due to subject dropout (n = 2) and insufficient numbers of blood samples due to technical issues during the infusion study (n = 2). In addition, we found that a subject who had been initially assigned to 1RDA-uneven (U) actually ate foods for 2RDA-U; thus, the final groups were 1RDA-U (n = 4), 1RDA-even (E) (n = 5), 2RDA-U (n = 6), and 2RDA-E (n = 5). Subjects, with the exception of one woman, were not sarcopenic by the definition of Baumgartner et al. (4).
Table 1.
Group characteristics
| Groups | 1RDA-U | 1RDA-E | 2RDA-U | 2RDA-E |
|---|---|---|---|---|
| n (M/F) | 4 (3/1) | 5 (1/4) | 6 (4/2) | 5 (2/3) |
| Age, yr | 64.0 ± 3.6 | 66.4 ± 1.7 | 68.4 ± 2.2 | 64.0 ± 27 |
| Height, cm | 175 ± 11 | 168 ± 8 | 168 ± 13 | 163 ± 8 |
| Total mass, kg | 88.9 ± 10.7 | 81.4 ± 5.7 | 79.9 ± 4.3 | 74.4 ± 7.9 |
| BMI, kg/m2 | 28.7 ± 2.0 | 28.8 ± 1.7 | 28.1 ± 1.0 | 26.6 ± 1.7 |
| LBM, kg | 52.8 ± 8.1 | 47.3 ± 4.0 | 51.0 ± 3.9 | 47.1 ± 7.3 |
| Body fat mass, % | 33.4 ± 4.3 | 38.6 ± 1.8 | 33.3 ± 2.6 | 34.6 ± 4.0 |
Values are expressed as means ± SE. M/F is the no. of male and female subjects in the study;
BMI, body mass index; LBM, lean body mass; 1RDA, 0.8 g protein intake; 2RDA, 1.5 g·kg−1·day−1 protein intake; U, eneven; E, even.
No statistically significant differences among treatments were found in all the variables.
Experimental protocol.
During the study screening for subject eligibility, dual-energy X-ray absorptiometry (QDR-4500A; Holologic, Waltham, MA) was performed for determination of body composition (Table 1). Subjects were instructed to abstain from strenuous physical activity for >72 h before starting the study. Subjects were studied in the Translational Research Institute (TRI) Clinical Research Services Core (CRSC) after an overnight fast. Subjects were randomly assigned by a study coordinator to one of four groups using a permuted block randomization procedure: 1RDA-U, 1RDA-E, 2RDA-U, or 2RDA-E. 1RDA-E and 1RDA-U are the groups in which subjects ate 0.8 g·kg−1·day−1 with even distribution (i.e., 33% of total protein amount at breakfast/lunch/dinner) and with uneven distribution (i.e., 15/20/65% of total protein amount at breakfast/lunch/dinner, respectively), and 2RDA-E and 2RDA-U are the groups in which subjects ate 1.5 g·kg−1·day−1 (∼1.9 × RDA) with the respective distribution. After screening, subjects were given a 3-day dietary record and instructions on its completion. The information from these records was used by the TRI Research Dietician to determine routine protein consumption, eating habits, food preferences, and caffeine consumption. The study dietician used the dietary records to ensure that meals provided in the run-in period before the metabolic study contained adequate caloric intake, and a daily vitamin/mineral supplement was included. Interventional diets are summarized in Table 2. All diets were prepared by the Metabolic Kitchen at the TRI to contain 0.8 g·kg−1·day−1 of protein. To achieve a protein intake of 1.5 g·kg−1·day−1 for the 2RDA groups, preweighed milk protein concentrate (equal parts of whey and casein) was included with the meals according to each subject's requirements. Diets were isocaloric such that the variable of protein intake could be appropriately tested. Each study paradigm (protein intake and distribution pattern) was consumed by the subjects for a total of 4 days. The first 3 days served as dietary habituation and a lead-in to the metabolic study performed on the 4th day. A previous study demonstrated that a dietary lead-in of 2 days was appropriate to determine differences in protein flux, as measured by phenylalanine tracer, from protein intake ranges from 0.8 to 2.0 g·kg−1·day−1 in healthy adults (29). Subjects obtained the 3-day meal allotments from our study coordinator and were also given a dietary record and point-and-shoot digital camera. Subjects were asked to record time of meal consumption, percentage of meal consumption, and photograph the meal prior to and after consumption. Subjects were instructed to return all unused or empty meal/supplement packaging and camera on the morning of the 4th day when they reported to the CRSC for the metabolic study. These data helped the Research Dietician ascertain caloric/protein intake as well as study compliance. Subjects who achieved a minimum compliance of 80% consumption of meals progressed to the metabolic study. During the dietary lead-in period, subjects were instructed to refrain from intense physical exercise.
Table 2.
Interventional diet during the study period (1–4 days)
| Protein |
Fat |
Carbohydrate |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amount | Pattern | Subjects Total (M/F) | Meal | Energy Intake, kcal/kg | g/kg | g | % | g/kg | % | g/kg | % | Fiber, g/kg | Leucine, g/kg |
| 1RDA | Uneven | 4 (3/1) | B | 6.10 ± 0.8 | 0.1 ± 0.0 | 11.1 ± 1.3 | 8 ± 1 | 0.2 ± 0.0 | 29 ± 0 | 1.0 ± 0.1 | 63 ± 1 | 0.05 ± 0.00 | 0.01 ± 0.00 |
| L | 8.0 ± 0.7 | 0.2 ± 0.0 | 14.9 ± 1.7 | 8 ± 1 | 0.2 ± 0.1 | 23 ± 5 | 1.4 ± 0.1 | 69 ± 5 | 0.16 ± 0.00 | 0.01 ± 0.00 | |||
| D | 17.7 ± 1.5 | 0.5 ± 0.0 | 47.8 ± 5.8 | 12 ± 1 | 0.9 ± 0.1 | 44 ± 1 | 2.0 ± 0.2 | 44 ± 1 | 0.15 ± 0.04 | 0.04 ± 0.01 | |||
| Total | 2,801 ± 312 | 0.8 ± 0.0 | 73.7 ± 8.8 | 10 ± 0 | 1.3 ± 0.1 | 36 ± 0 | 4.4 ± 0.1 | 54 ± 0 | 0.37 ± 0.05 | 0.05 ± 0.01 | |||
| Even | 5 (1/4) | B | 7.2 ± 1.0 | 0.3 ± 0.0 | 22.3 ± 1.6 | 15 ± 2 | 0.2 ± 0.1 | 29 ± 4 | 1.0 ± 0.1 | 56 ± 2 | 0.05 ± 0.01 | 0.02 ± 0.00 | |
| L | 10.2 ± 1.9 | 0.2 ± 0.1 | 21.5 ± 1.9 | 9 ± 2 | 0.4 ± 0.1 | 33 ± 8 | 1.5 ± 0.4 | 58 ± 6 | 0.16 ± 0.07 | 0.02 ± 0.01 | |||
| D | 11.2 ± 0.5 | 0.3 ± 0.0 | 22.0 ± 1.4 | 9 ± 0 | 0.6 ± 0.1 | 47 ± 3 | 1.3 ± 0.1 | 44 ± 3 | 0.13 ± 0.03 | 0.01 ± 0.01 | |||
| Total | 2,325 ± 174 | 0.8 ± 0.1 | 65.8 ± 4.8 | 11 ± 0 | 1.2 ± 0.1 | 37 ± 1 | 3.8 ± 0.4 | 52 ± 1 | 0.35 ± 0.06 | 0.05 ± 0.01 | |||
| 2RDA | Uneven | 6 (4/2) | B | 5.4 ± 0.5 | 0.2 ± 0.0 | 18.1 ± 1.1 | 15 ± 3 | 0.2 ± 0.0 | 26 ± 5 | 0.8 ± 0.0 | 59 ± 4 | 0.05 ± 0.01 | 0.01 ± 0.00 |
| L | 9.4 ± 0.6 | 0.3 ± 0.1 | 24.3 ± 1.6 | 12 ± 3 | 0.2 ± 0.0 | 24 ± 4 | 1.5 ± 0.1 | 64 ± 3 | 0.20 ± 0.03 | 0.02 ± 0.01 | |||
| D | 16.8 ± 1.7 | 0.9 ± 0.2 | 78.4 ± 5.3 | 22 ± 6 | 0.8 ± 0.2 | 42 ± 5 | 1.5 ± 0.2 | 36 ± 2 | 0.11 ± 0.04 | 0.06 ± 0.02 | |||
| Total | 2,466 ± 182 | 1.4 ± 0.3 | 120.8 ± 8.0 | 19 ± 0 | 1.2 ± 0.1 | 32 ± 0 | 3.9 ± 0.2 | 48 ± 0 | 0.35 ± 0.06 | 0.10 ± 0.03 | |||
| Even | 5 (2/3) | B | 8.0 ± 0.7 | 0.2 ± 0.0 | 38.0 ± 4.0 | 25 ± 3 | 0.2 ± 0.1 | 26 ± 2 | 1.0 ± 0.2 | 49 ± 4 | 0.06 ± 0.01 | 0.04 ± 0.00 | |
| L | 11.6 ± 1.7 | 0.5 ± 0.0 | 36.5 ± 3.8 | 17 ± 2 | 0.4 ± 0.1 | 26 ± 3 | 1.7 ± 0.3 | 57 ± 3 | 0.20 ± 0.02 | 0.04 ± 0.00 | |||
| D | 11.5 ± 1.0 | 0.5 ± 0.0 | 37.9 ± 3.9 | 18 ± 2 | 0.6 ± 0.0 | 45 ± 2 | 1.1 ± 0.2 | 38 ± 3 | 0.12 ± 0.03 | 0.03 ± 0.01 | |||
| Total | 2,301 ± 217 | 1.5 ± 0.1 | 112.4 ± 11.6 | 19 ± 1 | 1.2 ± 0.1 | 33 ± 1 | 3.8 ± 0.3 | 48 ± 1 | 0.11 ± 0.01 | 0.11 ± 0.01 | |||
Values are expressed as means ± SD; n, no of subjects.
B, breakfast; L, lunch; D, dinner. “Totals” for energy intake are total kcal units; those for macronutrients are mean (not total) percentages.
Tracer infusion protocol.
The 24-h tracer infusion protocol is presented in Fig. 1. On the 4th day, subjects reported to the TRI CRSC after an overnight (after 2200) fast. Each study paradigm was administered accordingly throughout the 24-h metabolic study. Two 18-gauge catheters were placed in each lower arm, one for the infusion of stable isotopes and the other for blood sampling. Before the initiation of the isotope tracer infusion study, a baseline blood sample was collected to determine background isotopic enrichments. For determination of the integrated PS, PB, and NB at whole body levels, primed continuous infusions of l-[ring-2H5]phenylalanine (prime, 3.07 μmol/kg; rate, 5.04 μmol·kg−1·h−1) and l-[3,3-2H2]tyrosine (prime, 0.47 μmol/kg; rate, 0.57 μmol·kg−1·h−1) were performed. To appropriately reach isotopic equilibrium of l-[ring-2H4]tyrosine enrichment derived from l-[ring-2H5]phenylalanine tracer infused, a priming dose of l-[ring-2H4]tyrosine was also injected (prime: 0.44 μmol/kg). All isotope tracers were purchased from Cambridge Isotope Laboratories (Andover, MA). The first muscle biopsy was taken from vastus lateralis muscle 2 h after the initiation of the tracer infusion (time at 120 min). Blood samples were taken at 120 min before breakfast (the fasted blood sample) and at 210, 240, 270, 300, 360, 480, 510, 540, 570, 630, 750, 780, 810, 840, 900, and 960 min to measure tracer enrichment and plasma responses of leucine, glucose, blood urea nitrogen (BUN), and insulin (Fig. 1). A total of 18 blood samples was taken during the stable isotope infusion study (∼140 ml), including the blood sample for the determination of background enrichment. Breakfast, lunch, and dinner were fed in the format commensurate with the group in which subjects were randomized. To ameliorate the catabolic effects of primary bed rest and to simulate daily activity, subjects walked on the treadmill for 15 min at 2.0 miles/h between lunch and dinner. Approximately 24 h after the start of the isotope tracer infusion (at 1,440 min), the second muscle biopsy with an additional blood sample was taken from the vastus lateralis muscle, and then the metabolic study was concluded.
Fig. 1.
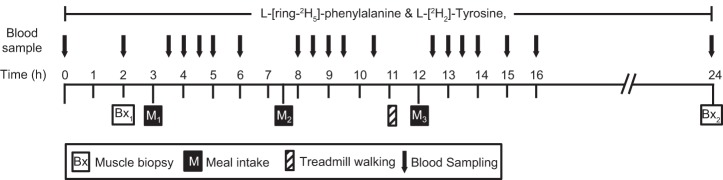
The infusion protocol.
Analytic methods.
Plasma samples were precipitated with 125 ul of 10% sulfosalicylic acid (SSA) and centrifuged. Simultaneously, a known amount (50 ul of 0.24 mM) of [13C6]leucine tracer was added as an internal standard for the determination of plasma leucine concentration. Plasma free AAs were extracted from 300 ul supernatant fluid by cation exchange chromatography (Strata-X-C; Phenomenex, Torrance, CA) and dried under Speed Vac (Savant Instruments, Farmingdale, NY). Enrichments of phenylalanine and tyrosine were measured on the tert-butyldimethylsilyl derivative with the use of gas chromatography-mass spectrometry (models 7890A/5975; Agilent Technologies, Santa Clara, CA) (20, 34). Ions of mass-to-charge ratio of 234, 235, and 239 for phenylalanine, of 302 and 308 for leucine, and of 466, 467, 468, and 470 for tyrosine were monitored with electron impact ionization and selective ion monitoring. Plasma glucose and BUN concentrations were measured spectrophotometrically on a Cobas c 111 analyzer (Roche, F. Hoffman-La Roche, Basel, Switzerland). Plasma insulin concentrations were measured by using a commercially available human insulin ELISA kit (Alpco Diagnostics).
Muscle biopsy tissue samples taken from the vastus lateralis muscle were cleaned of visible fat, blotted for excess blood, then frozen in liquid nitrogen, and stored in a −80°C freezer until later analysis. Upon thawing, muscle tissues were weighed, and tissue proteins were precipitated with 0.5 ml of 4% SSA. The tissues were then homogenized and then centrifuged for collection of supernatant. The procedure was repeated two more times, and tissue intracellular free AAs were extracted from the pooled supernatant via the same cation exchange chromatography stated in plasma analyses and then dried under the Speed Vac. The remaining muscle pellet was washed, dried, and hydrolyzed in 0.5 ml of 6 N HCl at 105°C for 24 h. Enrichments from muscle free and bound tracers were determined as in plasma analyses.
Calculations.
Calculations of whole body PS and PB rates were performed based on the determinations of the rate of appearance (Ra) into the plasma of phenylalanine and tyrosine and the fractional Ra of endogenous tyrosine converted from phenylalanine (35). Plasma enrichments of phenylalanine and tyrosine tracers were curve-fitted with a cubic spline method using Graphpad Prism 5 for Mac (Graphpad Software, La Jolla, CA). The cubic spline method was employed in the present study because this method accounted for variations in postmeal tracer kinetics (35). Although the cubic spline area under the curve (AUC) values tended to be smaller than those derived from using AUC of non-curve-fitted values, there were no statistical differences between two sets of values and did not affect the conclusion. Whole body protein turnover can be calculated by dividing kinetic values of a single EAA, i.e., phenylalanine, which cannot be synthesized in the body, by its fractional contribution to protein. For the calculations for whole body PB rate, contribution from exogenous meal and tracers infused was subtracted from total Ra. Appropriate skew correction was also made for 13C-labeled tracers (35).
Calculation of MPS was determined as the incorporation of the phenylalanine tracer over a 22-h time period (120–1,440 min) using a precursor-product model (3). For the calculation of MPS, plasma enrichment of phenylalanine tracer was used as a substitute for the true precursor enrichment from the charged tRNAs because plasma enrichment of phenylalanine tracer reflects the net gain or loss of protein, which can only come from the use of plasma AA precursors.
The following equations were used for the calculations of whole body and muscle protein kinetics:
| (1) |
| (2) |
| (3) |
| (4) |
| (5) |
| (6) |
| (7) |
where enrichment (E) is expressed as tracer-to-tracee ratio (TTR) for calculation of PB or mole percent excess for calculation of MPS, calculated as TTR/(TTR + 1). E is enrichment of respective tracers at plateau. F is respective tracer infusion rate into a venous side: FPhe for phenylalanine tracer. ETyr M+4 and EPhe M+5 are plasma enrichments of tyrosine tracers at M+4 and M+5 relative to M+0, respectively. Correction factor of 25 is for conversion of value for phenylalanine to protein based on the assumption that contribution of phenylalanine to protein is 4% (100/4 = 25) (6). PROMEAL is the amount of exogenous protein (g) that appeared in the circulation, which was calculated as total amount of protein in the three meals, based on the assumption that 85% of the protein ingested was absorbed (9). Phenylalanine hydroxylation is the Ra of tyrosine derived from phenylalanine through hydroxylation. All whole body protein kinetics values are expressed as changes from the fasted (120 min) to the fed conditions. EP1 and EP2 are the enrichments of bound l-[ring-2H5]phenylalanine in the first and second biopsies, respectively, and Em is the calculated mean value of the enrichments of [ring-2H5]phenylalanine in the plasma pool. t is the time in minutes elapsed between the first and second muscle biopsy. Factors 60 and 100 were used to express MPS in percent per hour.
Statistical analysis.
Analysis of covariance models were used to evaluate the effect of protein amount (RDA and 2RDA) and distribution pattern (E and U) on measures of NB, PS, and PB after adjusting for the effects of age and gender. In addition, each model contained an amount-by-distribution interaction term to test whether the effect of protein amount changed with distribution pattern and vice versa. Linear regression analyses were performed to examine the association between protein intake and NB after each meal. Furthermore, the Pearson product-moment correlation coefficient was performed to examine the association between changes of plasma mean leucine concentrations and NB or MPS. A repeated-measures ANOVA model was used to investigate how plasma leucine changes with protein amount and sampling time. Finally, a two-factor (amount and pattern) ANOVA model was used to compare means of AUC of plasma leucine concentrations. Statistical significance was declared when the P value was less than the 5% level. This analysis was performed using SAS (version 9.3; SAS Institute, Cary, NC).
RESULTS
Whole body protein kinetics.
Whole body PS, PB, and NB were calculated as AUCs over the 16-h experimental time period relative to the fasted state (t = 120 min) and were expressed as changes from the fasted to fed states (Fig. 2). The intake of the meals leads to a large reduction in PB, whereas PS was only marginally stimulated with 2RDA. We found no protein amount-by-distribution interaction for NB, PS, and PB (P > 0.557). Furthermore, there was no main effect of distribution pattern of protein intake on NB, PS, and PB (P > 0.528). However, the effect of protein amount was highly significant for both NB and PS (P < 0.002), with 2RDA being significantly higher than 1RDA for both responses. To determine if the ingestion of a significant amount of protein in the previous meals affects the net anabolic response to the subsequent meals, we normalized NB to the amount of protein intake for each meal and compared this normalized NB across meals. We found that there was no difference in the normalized NB (0.73 ± 0.06, 0.86 ± 0.04, and 0.82 ± 0.01 for breakfast/lunch/dinner, respectively). Furthermore, linear regression analyses revealed that there were linear positive associations between protein intake and NB for breakfast, lunch, and dinner, even at high protein intake (for all; P < 0.001) (Fig. 3).
Fig. 2.
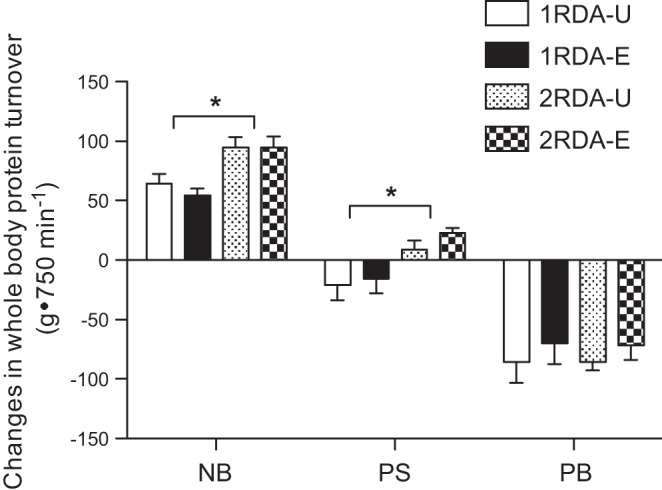
Changes in rates of the 16-h whole body protein synthesis (PS), breakdown (PB), and net balance (NB) from the fasted state. Two-factor ANOVAs indicate that there were no protein amount × distribution interactions for NB, PS, and PB. There were significant main effects of protein amount on NB and PS: 2RDA was significantly higher than 1RDA for NB (P = 0.0001) and for PS (P = 0.0018). However, there were no main effects of distribution pattern of protein intake on NS, PS, and PB. Values are expressed as means ± SE and calculated as changes from the fasted to the fed states. *Significantly higher in 2RDA than in 1RDA.
Fig. 3.
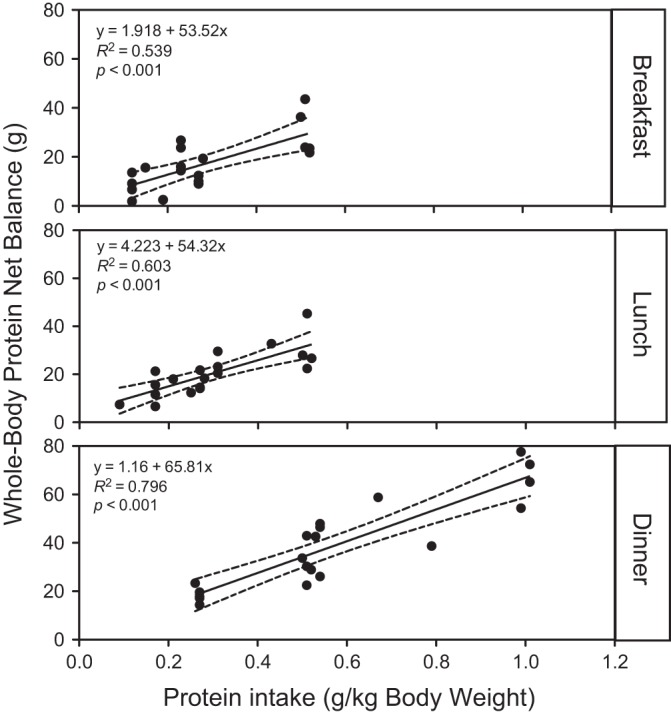
Whole body protein net balance (NB) as a function of protein intake (g protein intake/kg body wt) following an individual meal intake: breakfast, lunch, or dinner. Linear regression analyses indicate that there were significant associations between the amount of protein intake per each meal and NB response for breakfast, lunch, and dinner (for all, P < 0.001). Each solid circle indicates NB following respective meal intake.
MPS.
There was no protein amount-by-distribution interaction (P = 0.960) or protein distribution effect (P = 0.125) for the 22-h MPS. However, we found that there was a significant, albeit small, protein amount effect for the MPS (P = 0.033): 2RDA was significantly higher than 1RDA (Fig. 4).
Fig. 4.
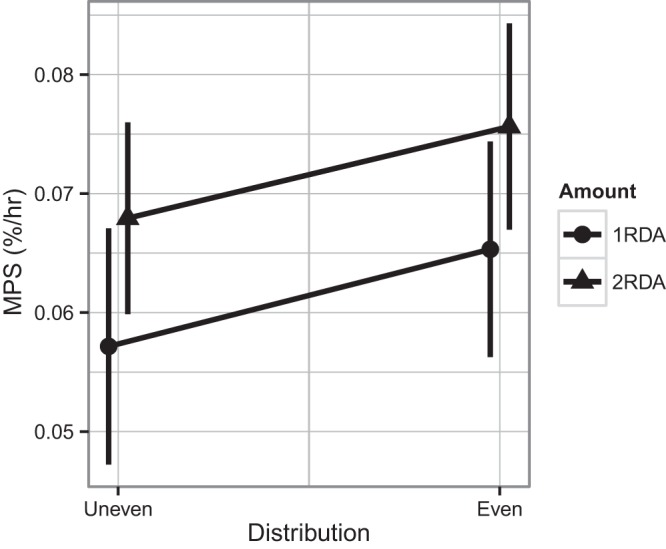
Muscle protein fractional synthesis rate (MPS) with 1RDA or 2RDA protein intake in isocaloric mixed meal intakes. Two-factor ANOVAs indicate that there was no protein amount × distribution interaction. There was a significant main effect of protein amount: 2RDA was significantly different from 1RDA (P = 0.033). Values are expressed as means ± SE.
Plasma concentrations.
Plasma glucose, insulin, and BUN responses are presented in Table 3. There were no protein amount-by-distribution interactions for glucose, insulin, and BUN. Furthermore, there was no significant treatment or distribution effect for glucose and insulin responses. However, we found that there was a significant treatment effect of protein amount for BUN: 2RDA was significantly higher than 1RDA (P = 0.0017). Mean plasma insulin concentrations in the fed state were significantly increased from the fasted states (P < 0.0001). For plasma leucine concentrations, there was a significant time-by-amount interaction (P < 0.005) (Fig. 5). There were also significant effects for protein amount and distribution pattern: 2RDA was significantly higher than 1RDA (P < 0.001), and the even intake pattern resulted in higher plasma leucine responses compared with the uneven pattern (P < 0.05). For AUC of plasma leucine concentrations (119 ± 10 for 1RDA-U, 126 ± 9 for 1RDA-E, 148 ± 5 for 2RDA-U, and 189 ± 15 for 2RDA-E), there was a trend for protein amount-by-distribution interaction (P = 0.111).
Table 3.
Plasma responses of glucose, insulin, and BUN in response to mixed meals
| Glucose |
Insulin, μIU/ml |
BUN, mmol/l |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | n (M/F) | Mean, mmol/l | AUC, mmol·l−1·h−1 | F | B1 | L1 | D1 | F | B1 | B2 | L1 | L2 | D1 | D2 |
| 1RDA-U | 4 (3/1) | 6.48 ± 0.4 | 390 ± 25 | 6.6 ± 1.8 | 54.2 ± 19.6 | 61.8 ± 22.8 | 56.1 ± 17.4 | 8.5 ± 0.7 | 8.3 ± 0.6 | 8.0 ± 0.8 | 7.9 ± 0.6 | 7.5 ± 0.5 | 7.0 ± 0.6 | 6.8 ± 0.7 |
| 1RDA-E | 5 (1/4) | 7.21 ± 0.4 | 436 ± 25 | 10.9 ± 3.6 | 53.7 ± 11.2 | 53.5 ± 29.1 | 37.2 ± 9.2 | 8.6 ± 0.6 | 8.3 ± 0.5 | 8.09 ± 0.6 | 7.91 ± 0.5 | 8.5 ± 0.4 | 8.1 ± 0.3 | 7.7 ± 0.5 |
| 2RDA-U | 6 (4/2) | 6.72 ± 0.5 | 413 ± 32 | 7.3 ± 1.8 | 61.7 ± 9.4 | 66.9 ± 20.8 | 56.0 ± 17.8 | 11.2 ± 1.5 | 10.6 ± 1.4 | 10.3 ± 1.5 | 10.2 ± 1.6 | 9.9 ± 1.4 | 9.8 ± 1.4 | 9.7 ± 1.2 |
| 2RDA-E | 5 (2/3) | 6.11 ± 0.2 | 366 ± 11 | 4.0 ± 0.9 | 48.0 ± 9.9 | 42.7 ± 5.7 | 32.9 ± 10.4 | 12.1 ± 0.6 | 12.0 ± 0.5 | 11.5 ± 0.8 | 12.65 ± 1.0 | 12.7 ± 0.9 | 12.5 ± 1.3 | 12.6 ± 1.0 |
Values are expressed as means ± SE; n, no of subjects. B1, B2, L1, L2, D1, and D2 are postmeal measurements at time points of 210, 240, 480, 510, 750, and 780 min, respectively, for breakfast (B), lunch (L), and dinner (D); F, fasted; BUN, blood urea nitrogen; AUC, area under curve. Mean differences for glucose (1 factor) and insulin and BUN (2 factor) were compared using repeated measures of ANOVA. There were no protein amount × distribution interactions for glucose, insulin, and BUN. Furthermore, there was no significant treatment or distribution effect for glucose and insulin responses. However, there was a significant treatment effect of protein amount for BUN: 2RDA was significantly higher than 1RDA, P = 0.0017.
Fig. 5.
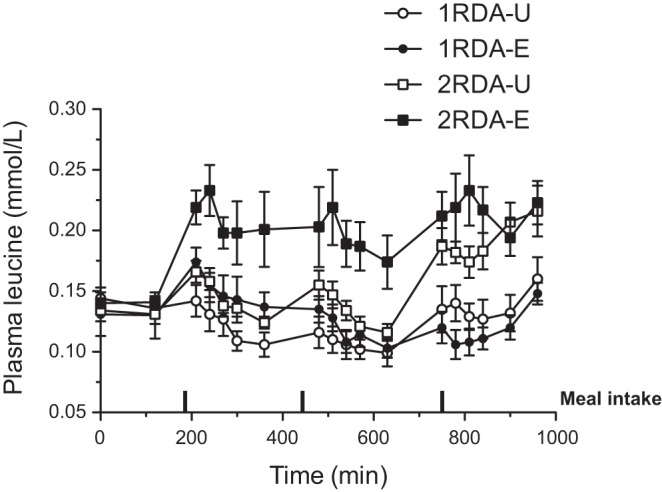
Time course responses of plasma leucine (Leu) over the 16-h experimental time period during 1RDA or 2RDA protein intake in isocaloric mixed meal intake. Two-factor ANOVAs indicate that there was a significant time-by-amount interaction (P < 0.005). There were also significant effects for protein amount and distribution pattern: 2RDA was significantly higher than 1RDA (P < 0.001), and the even intake pattern resulted in higher plasma leucine responses compared with the uneven pattern (P < 0.05). Values are expressed as means ± SE. Rectangles indicates time for breakfast, lunch, and dinner meals, taken at 180, 450, and 720 min, respectively.
Correlations.
There were significant correlations between mean leucine concentration and PS, both expressed as changes from the fasted to fed states (r2 = 0.51, P < 0.001) and between mean leucine concentration and MPS (r2 = 0.22, P = 0.036).
DISCUSSION
In the context of mixed meals, whole body net protein balance and MPS were greater with protein intake above the RDA without demonstrable effects of protein intake patterns. The greater protein NB with the higher protein intake was achieved as a consequence of the increased PS. Furthermore, reductions in PB from the fasted to the fed states in all groups contributed to the positive net protein balance in both levels of protein doses, underscoring the important role of PB in the context of mixed meal intake.
Contrary to our initial hypothesis, we found no significant differences in protein kinetics at whole body or muscle level with respect to the effect of protein intake patterns. Our finding is in disagreement with a recent study of Mamerow et al. (16) who found that MPS was significantly higher with an even compared with uneven pattern of protein intake in mixed meals in healthy young individuals (intake patterns: 15/20/65% vs. 33/33/33% at 1.2 g protein·kg−1·day−1). It is difficult to explain the discrepancy between the two findings. The most likely explanation lies in the study population differences (older adults vs. young adults), since it has been shown that there is an age-associated resistance in protein synthetic response for a given small dose of protein/AA intake (8, 14). The underlying theory to explain a benefit of even compared with uneven distribution of protein intake is that any amount of protein eaten at a given meal that exceeds the amount at which maximal stimulation of MPS is achieved provides no further benefit. It may be that the maximal effective dose of protein intake at one meal is greater in older adults (our study) than in young adults (Mamerow study) due to anabolic resistance in adults. Consistent with the notion, Moore et al. (17) have recently performed a biphase linear regression and breakpoint analysis of their published MPS data in response to ingestion of varying amounts of high-quality protein as a single dose. They found that the minimum amount of protein that maximally stimulates MPS in older subjects was ∼70% greater than that in the young subjects (0.40 ± 0.19 and 0.24 ± 0.06 g·kg body wt−1·meal−1). To achieve this goal in the majority of older individuals may require ∼0.6 g·kg−1·meal−1 or approximately ∼1.8 g·kg−1·day−1. Furthermore, because the Moore data were obtained following ingestion of a bolus of “high-quality” protein, more protein may be actually required since people likely consume varying degrees of protein quality. In the context of mixed meal, the optimal amount of protein intake may also be greater due to the systemic insulin effects on decreasing AA availability (5). Another explanation for the absence of a pattern effect may include the differential effects of gender on MPS. Smith et al. have shown gender dimorphism with respect to MPS in both normal-weight (26) and obese older women (25). These studies demonstrated that MPS was not increased above fasted states during combined insulin, glucose, and AA infusion (total 0.35 g protein/kg fat-free mass) or following a liquid meal containing 15% of energy as protein (total ∼0.2 g protein/kg fat-free mass). If so, we would not expect increases in MPS with 2RDA compared with that with 1RDA in older women. However, we found in the present study that there were significant increases in MPS with 2RDA compared with 1RDA in older women (5/9 subjects for 1RDA groups vs. 6/11 subjects for 2RDA). Potential explanations for the discrepancy may include: 1) a difference in the method of protein ingestion (infusion or liquid meal vs. complete mixed meal) and/or 2) the amount of protein given (∼0.2–0.35 g in their studies vs. over ∼0.65 g·meal−1·lean body mass−1 in the present study, respectively). Future studies utilizing sufficient subject numbers of each gender would help elucidate this issue. It is also possible that variability resulting from the relatively small number of subjects in the current study obscured a difference in the pattern of protein intake that might have been revealed with a larger number of subjects. However, it is less likely the case because, according to the post hoc analysis for sample size, ∼72 subjects/group (total = 288) would be required to detect the observed difference for protein distribution effect on MPS. Finally, the present data point to the importance of measuring NB at the whole body level. The theory of a distribution effect has been predicated entirely on the measurement of MPS. This theory also supposes that the anabolic response can be maximized at a protein intake commonly referenced as 20–35 g (18, 28). However, the whole body data indicate that NB increases with increasing protein intake. The reason for the increased NB with protein intake is that whole body NB is contingent upon changes in both PS and PB. Therefore, while our results would have been strengthened with a greater number of subjects, whole body NB is consistent with a primary effect of protein intake. A progressive increase in NB at higher levels of protein intake (when the synthetic response has been maximized) argues against the expectation of an effect of distribution of protein intake throughout the day on net protein balance.
Although there was no clear effect of the pattern of protein intake in our study, we observed a definitive effect of a higher amount of protein intake in mixed meals on whole body NB and MPS. NB was greater with 2RDA compared with 1RDA. Our findings are in agreement with the findings from Pannemans et al. (21, 22) in both young and older adults given either ∼0.9 g or 1.5 g protein·kg−1·day−1. Interestingly, with the lower protein intake in the Pannemans studies nitrogen balance was negative in older adults while positive in young adults, confirming an age-associated anabolic resistance. In the present study, NB was positive in 1RDA. This is mostly likely due to the fact that our calculation of whole body protein turnover did not include the overnight time period (960–1,440 min), where PB may predominate. Although NB was positive with both levels of protein intake, the underlying physiological mechanisms were different between 1RDA and 2RDA. For example, PS was actually reduced below the basal value in 1RDA while increased in 2RDA. On the other hand, PB was reduced similarly in all groups, underscoring the important role of reduced PB in achieving positive NB. These findings are in contrast to studies demonstrating improved NB following ingestion of EAA/protein was attributed entirely to an increase in PS (14, 30, 32, 33). However, the previous data were obtained in the fasting state and in response to AAs or protein alone. There appears to be a different response when a mixed meal is ingested (7, 36). It has been shown that plasma AA concentrations rise to a greater extent following ingestion of protein/AA alone compared with a complete meal (19). Furthermore, it has been demonstrated that plasma leucine concentrations increase to a greater extent following ingestion/infusion of pure protein/AA (27, 33). In the present study, plasma leucine concentrations were significantly elevated with 2RDA but reduced with 1RDA. This reduction is not evident following an intake of a smaller amount of EAAs alone (∼7 g) (14). EAA are primarily responsible for the stimulation of PS following a protein/AA intake (31, 33), and leucine is a key activator for the translation initiation process (1, 15). Consistent with this notion, we found a significant positive correlation between plasma leucine response and PS at both whole body and muscle levels. The different effects on plasma leucine response following intake of a mixed meal compared with ingestion of protein/AA alone can be attributed to the effects of insulin on systemic stimulation of AA uptake and suppression of PB (5). Thus, when insulin is increased, there must be a relatively large intake of protein (2RDA in this study) to effectively increase plasma AA availability. Thus, the suppression of PB at both levels of protein intake (1RDA and 2RDA) may be driven by insulin responses, whereas the increased EAA/leucine availability in the 2RDA group increases NB through increased MPS. The latter point is supported by a significant correlation between changes in plasma leucine and MPS.
There has been considerable momentum for the concept that distributing protein intake evenly over the day will be more anabolic than an uneven distribution of the same amount of protein. This perspective is largely based on the limited extent to which MPS can increase in response to a pure protein meal (28). Exclusive reliance upon this methodology to assess the anabolic response has several limitations. Most importantly, since net anabolic response is the balance between PS and PB, measurement of only PS without considering PB could lead to an erroneous conclusion with respect to net anabolic response. Second, determination of PS only at the muscle level can underestimate the whole body anabolic response since more than half of the whole body protein turnover reflects proteins other than skeletal muscle (2, 11). By combining whole body with muscle protein turnover measurements we were not only able to distinguish the muscle response of synthesis from the whole body response but, more importantly, dynamically determine the rate of PB, and thus the NB, between synthesis and breakdown. NB increased with increasing amounts of protein intake without evidence of plateau (Fig. 3). This is consistent with recent studies demonstrating a linear relationship between protein intake and NB (12, 13). Although AA oxidation increases correspondingly with increasing amounts of protein/AA intake (23), some portion of AAs is still being incorporated into protein. Taken together, we extend the previous data demonstrating a linear relationship between NB and protein intake to a range encompassing 6.4∼91.7 g.
Several methodological issues warrant discussion. First, plasma phenylalanine enrichment was used as a surrogate precursor for the determination of MPS. When the intracellular enrichment is used as the precursor for the charged tRNA (3, 35), total synthesis is calculated (i.e., synthesis from plasma phenylalanine as well as the phenylalanine released from PB and directly reincorporated into protein without entering plasma). Utilizing plasma phenylalanine enrichment as the precursor yields a rate of PS derived from plasma AAs. We utilized the plasma enrichment because we were interested in the net gain or loss of protein over the study time period. The recycling of AAs from PB back into PS, represented by the calculation of MPS using the intracellular enrichment as precursor, results in neither a gain nor loss of protein. Furthermore, utilization of the intracellular enrichment provides the precursor enrichment only at the time of the biopsy. Utilization of the plasma enrichment gives an integrated picture of precursor enrichment over time. This is appropriate since the protocol involved both fasted and fed periods.
Second, for the accurate calculation of PB, it is crucial to know the rate of entry of exogenous AAs, in particular, phenylalanine. Thus, the calculation of PB requires an accurate assessment of the Ra of phenylalanine from PB and exogenous protein. This problem can be addressed by adding a different tracer of phenylalanine to the meal, but this approach has the limitation in that the free AA may be absorbed more rapidly than the protein-bound phenylalanine in the meal since digestion is not required. Furthermore, an exogenous tracer can be recycled and appear in the blood even after the absorption of AAs from the meal is complete (35), thereby leading to an underestimation of PB. Underestimation of PB is also possible due to the reincorporation of AAs released from PB, which may increase during mixed meal ingestion (5). To address these issues, we assumed that 85% of ingested protein was absorbed (9) and that phenylalanine constitute 4% of proteins (6). The difference in NB between the two doses was ∼45% of the difference in protein intake. This finding is consistent with the observation that ∼82% of total protein intake was retained at all levels of meals (Fig. 2). The high retention of dietary protein probably reflects that high-quality whey and casein proteins were used to make up differences in protein intake (31).
In conclusion, in the context of a mixed meal, whole body net protein balance increases in older individuals with protein intake above the RDA, without demonstrated effects of protein intake pattern. Greater net positive protein balance with protein intake above the RDA was primarily accomplished through increased whole body PS and MPS.
GRANTS
The project was financially supported by a grant from Dairy Research Institute. The project was partially supported by Pepper Center Grant PG30-AG-028718 and Award Number UL1-TR-000039 and KL2-TR-000063 from the National Center for Advancing Translational Sciences (NCATS).
DISCLOSURES
The content is solely the responsibility of the authors and does not necessarily represent the official view of the NCATS or the National Institutes of Health. Dr. Wolfe has received research grants and honoraria from the National Cattleman's Beef Checkoff program. Other authors have no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Author contributions: I.-Y.K., A.S., H.S., R.R.W., and A.A.F. analyzed data; I.-Y.K. and R.R.W. performed calculations of protein kinetics. I.-Y.K., R.R.W., A.S., H.S., and A.A.F. interpreted results of experiments; I.-Y.K., A.S., and H.S. prepared figures; I.-Y.K. drafted manuscript; I.-Y.K., R.R.W., and A.A.F. edited and revised manuscript; I.-Y.K., S.S., A.S., H.S., P.K., N.E.D., R.R.W., and A.A.F. approved final version of manuscript; S.S., P.K., and N.E.D. performed experiments; R.R.W. and A.A.F. research conception; R.R.W., A.A.F. and H.S. design of experiments.
ACKNOWLEDGMENTS
We thank the research subjects for participation in the study. We also thank the research staff/associates for support in conducting isotope tracer infusion protocols and sample analyses: Cosby J. Lasely for coordinating study subjects and helping the isotope infusion study, Dr. Lulu Xu for gas chromatography-mass spectrometry analysis, and Rick Williams and Joshua Spore for the determination of blood chemistry. Lastly, we thank the study dietician Amanda M. Dawson.
Present addresses: N. E. P. Deutz, Department of Health and Kinesiology, the Center for Translational Research in Aging and Longevity, Texas A&M University, College Station, TX; and P. Kortebein, Novartis Pharmaceuticals, East Hanover, NJ.
REFERENCES
- 1.Anthony JC, Anthony TG, Kimball SR, Vary TC, Jefferson LS. Orally administered leucine stimulates protein synthesis in skeletal muscle of postabsorptive rats in association with increased eIF4F formation. J Nutr 130: 139–145, 2000. [DOI] [PubMed] [Google Scholar]
- 2.Argilés JM, Busquets S, Felipe A, López-Soriano FJ. Molecular mechanisms involved in muscle wasting in cancer and ageing: cachexia versus sarcopenia. Int J Biochem Cell Biol 37: 1084–1104, 2005. [DOI] [PubMed] [Google Scholar]
- 3.Baumann PQ, Stirewalt WS, O'Rourke BD, Howard D, Nair KS. Precursor pools of protein synthesis: a stable isotope study in a swine model. Am J Physiol Endocrinol Metab 267: E203–E209, 1994. [DOI] [PubMed] [Google Scholar]
- 4.Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR, Garry PJ, Lindeman RD. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol 147: 755–763, 1998. [DOI] [PubMed] [Google Scholar]
- 5.Biolo G, Declan Fleming RY, Wolfe RR. Physiologic hyperinsulinemia stimulates protein synthesis and enhances transport of selected amino acids in human skeletal muscle. J Clin Invest 95: 811–819, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Biolo G, Fleming RY, Maggi SP, Wolfe RR. Transmembrane transport and intracellular kinetics of amino acids in human skeletal muscle. Am J Physiol Endocrinol Metab 268: E75–E84, 1995. [DOI] [PubMed] [Google Scholar]
- 7.Bohé J, Low A, Wolfe RR, Rennie MJ. Human muscle protein synthesis is modulated by extracellular, not intramuscular amino acid availability: a dose-response study. J Physiol (Lond) 552: 315–324, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burd NA, Gorissen SH, Van Loon LJC. Anabolic resistance of muscle protein synthesis with aging. Exerc Sport Sci Rev 41: 169–173, 2013. [DOI] [PubMed] [Google Scholar]
- 9.Deglaire A, Moughan PJ. Animal models for determining amino acid digestibility in humans - a review. Br J Nutr 108, Suppl 2: S273–S281, 2012. [DOI] [PubMed] [Google Scholar]
- 10.Deutz NE, Have Ten GA, Soeters PB, Moughan PJ. Increased intestinal amino-acid retention from the addition of carbohydrates to a meal. Clin Nutr 14: 354–364, 1995. [DOI] [PubMed] [Google Scholar]
- 11.Deutz NE, Wolfe RR. Is there a maximal anabolic response to protein intake with a meal? Clin Nutr (December1, 2012). doi: 10.1016/j.clnu.2012.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Engelen MPKJ, Com G, Wolfe RR, Deutz NEP. Dietary essential amino acids are highly anabolic in pediatric patients with cystic fibrosis. J Cyst Fibros (January25, 2013). doi: 10.1016/j.cf.2012.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jonker R, Deutz NEP, Erbland ML, Anderson PJ, Engelen MPKJ. Hydrolyzed casein and whey protein meals comparably stimulate net whole-body protein synthesis in COPD patients with nutritional depletion without an additional effect of leucine co-ingestion. Clin Nutr (July1, 2013). doi: 10.1016/j.clnu.2013.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Katsanos CS, Kobayashi H, Sheffield-Moore M, Aarsland A, Wolfe RR. Aging is associated with diminished accretion of muscle proteins after the ingestion of a small bolus of essential amino acids. Am J Clin Nutr 82: 1065–1073, 2005. [DOI] [PubMed] [Google Scholar]
- 15.Kimball SR, Shantz LM, Horetsky RL, Jefferson LS. Leucine regulates translation of specific mRNAs in L6 myoblasts through mTOR-mediated changes in availability of eIF4E and phosphorylation of ribosomal protein S6. J Biol Chem 274: 11647–11652, 1999. [DOI] [PubMed] [Google Scholar]
- 16.Mamerow MM, Mettler JA, English KL, Casperson SL, Arentson-Lantz E, Sheffield-Moore M, Layman DK, Paddon-Jones D. Dietary protein distribution positively influences 24-h muscle protein synthesis in healthy adults. J Nutr (January29, 2014). doi: 10.3945/jn.113.185280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moore DR, Churchward-Venne TA, Witard O, Breen L, Burd NA, Tipton KD, Phillips SM. Protein ingestion to stimulate myofibrillar protein synthesis requires greater relative protein intakes in healthy older versus younger men. J Gerontol A Biol Sci Med Sci (July23, 2014). doi: 10.1093/gerona/glu103. [DOI] [PubMed] [Google Scholar]
- 18.Moore DR, Robinson MJ, Fry JL, Tang JE, Glover EI, Wilkinson SB, Prior T, Tarnopolsky MA, Phillips SM. Ingested protein dose response of muscle and albumin protein synthesis after resistance exercise in young men. Am J Clin Nutr 89: 161–168, 2009. [DOI] [PubMed] [Google Scholar]
- 19.Paddon-Jones D, Sheffield-Moore M, Aarsland A, Wolfe RR, Ferrando AA. Exogenous amino acids stimulate human muscle anabolism without interfering with the response to mixed meal ingestion. Am J Physiol Endocrinol Metab 288: E761–E767, 2005. [DOI] [PubMed] [Google Scholar]
- 20.Paddon-Jones D, Sheffield-Moore M, Zhang XJ, Volpi E, Wolf SE, Aarsland A, Ferrando AA, Wolfe RR. Amino acid ingestion improves muscle protein synthesis in the young and elderly. Am J Physiol Endocrinol Metab 286: E321–E328, 2004. [DOI] [PubMed] [Google Scholar]
- 21.Pannemans DL, Halliday D, Westerterp KR, Kester AD. Effect of variable protein intake on whole-body protein turnover in young men and women. Am J Clin Nutr 61: 69–74, 1995. [DOI] [PubMed] [Google Scholar]
- 22.Pannemans DL, Halliday D, Westerterp KR. Whole-body protein turnover in elderly men and women: responses to two protein intakes. Am J Clin Nutr 61: 33–38, 1995. [DOI] [PubMed] [Google Scholar]
- 23.Pencharz PB, Elango R, Ball RO. An approach to defining the upper safe limits of amino acid intake. J Nutr 138: 1996S–2002S, 2008. [DOI] [PubMed] [Google Scholar]
- 24.Robinson MJ, Burd NA, Breen L, Rerecich T, Yang Y, Hector AJ, Baker SK, Phillips SM. Dose-dependent responses of myofibrillar protein synthesis with beef ingestion are enhanced with resistance exercise in middle-aged men. Appl Physiol Nutr Metab 38: 120–125, 2013. [DOI] [PubMed] [Google Scholar]
- 25.Smith GI, Atherton P, Villareal DT, Frimel TN, Rankin D, Rennie MJ, Mittendorfer B. Differences in muscle protein synthesis and anabolic signaling in the postabsorptive state and in response to food in 65–80 year old men and women. PLoS ONE 3: e1875, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith GI, Reeds DN, Hall AM, Chambers KT, Finck BN, Mittendorfer B. Sexually dimorphic effect of aging on skeletal muscle protein synthesis (Abstract). Biol Sex Differ 3: 11, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Symons TB, Schutzler SE, Cocke TL, Chinkes DL, Wolfe RR, Paddon-Jones D. Aging does not impair the anabolic response to a protein-rich meal. Am J Clin Nutr 86: 451–456, 2007. [DOI] [PubMed] [Google Scholar]
- 28.Symons TB, Sheffield-Moore M, Wolfe RR, Paddon-Jones D. A moderate serving of high-quality protein maximally stimulates skeletal muscle protein synthesis in young and elderly subjects. J Am Diet Assoc 109: 1582–1586, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thorpe JM, Roberts SA, Ball RO, Pencharz PB. Prior protein intake may affect phenylalanine kinetics measured in healthy adult volunteers consuming 1 g protein.kg-1 d-1. J Nutr 129: 343–348, 1999. [DOI] [PubMed] [Google Scholar]
- 30.Tipton KD, Børsheim E, Wolf SE, Sanford AP, Wolfe RR. Acute response of net muscle protein balance reflects 24-h balance after exercise and amino acid ingestion. Am J Physiol Endocrinol Metab 284: E76–E89, 2003. [DOI] [PubMed] [Google Scholar]
- 31.Tipton KD, Gurkin BE, Matin S, Wolfe RR. Nonessential amino acids are not necessary to stimulate net muscle protein synthesis in healthy volunteers. J Nutr Biochem 10: 89–95, 1999. [DOI] [PubMed] [Google Scholar]
- 32.Volpi E, Ferrando AA, Yeckel CW, Tipton KD, Wolfe RR. Exogenous amino acids stimulate net muscle protein synthesis in the elderly. J Clin Invest 101: 2000–2007, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Volpi E, Kobayashi H, Sheffield-Moore M, Mittendorfer B, Wolfe RR. Essential amino acids are primarily responsible for the amino acid stimulation of muscle protein anabolism in healthy elderly adults. Am J Clin Nutr 78: 250–258, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Volpi E, Mittendorfer B, Wolf SE, Wolfe RR. Oral amino acids stimulate muscle protein anabolism in the elderly despite higher first-pass splanchnic extraction. Am J Physiol Endocrinol Metab 277: E513–E520, 1999. [DOI] [PubMed] [Google Scholar]
- 35.Wolfe RR, Chinkes DL. Isotope Tracers in Metabolic Reserach (2nd ed.). Hoboken, NJ: Wiley, 2005. [Google Scholar]
- 36.Wolfe RR. Regulation of muscle protein by amino acids. J Nutr 132: 3219S–3224S, 2002. [DOI] [PubMed] [Google Scholar]
- 37.Yang Y, Breen L, Burd NA, Hector AJ, Churchward-Venne TA, Josse AR, Tarnopolsky MA, Phillips SM. Resistance exercise enhances myofibrillar protein synthesis with graded intakes of whey protein in older men. Br J Nutr 108: 1780–1788, 2012. [DOI] [PubMed] [Google Scholar]


