Abstract
Background
Studies investigating structural brain abnormalities in depression have typically employed a categorical rather than dimensional approach to depression [i.e. comparing subjects with Diagnostic and Statistical Manual of Mental Disorders (DSM)-defined major depressive disorder (MDD) v. healthy controls]. The National Institute of Mental Health, through their Research Domain Criteria initiative, has encouraged a dimensional approach to the study of psychopathology as opposed to an over-reliance on categorical (e.g. DSM-based) diagnostic approaches. Moreover, subthreshold levels of depressive symptoms (i.e. severity levels below DSM criteria) have been found to be associated with a range of negative outcomes, yet have been relatively neglected in neuroimaging research.
Method
To examine the extent to which depressive symptoms – even at subclinical levels – are linearly related to gray matter volume reductions in theoretically important brain regions, we employed whole-brain voxel-based morphometry in a sample of 54 participants.
Results
The severity of mild depressive symptoms, even in a subclinical population, was associated with reduced gray matter volume in the orbitofrontal cortex, anterior cingulate, thalamus, superior temporal gyrus/temporal pole and superior frontal gyrus. A conjunction analysis revealed concordance across two separate measures of depression.
Conclusions
Reduced gray matter volume in theoretically important brain regions can be observed even in a sample that does not meet DSM criteria for MDD, but who nevertheless report relatively elevated levels of depressive symptoms. Overall, these findings highlight the need for additional research using dimensional conceptual and analytic approaches, as well as further investigation of subclinical populations.
Keywords: Depressive symptoms, DSM, gray matter volume, research domain criteria, voxel-based morphometry
Introduction
A large body of research has explored functional and structural brain abnormalities associated with major depressive disorder (MDD). Most previous studies examining structural abnormalities in depression have utilized a region-of-interest (ROI) rather than a whole-brain approach. Findings from such studies may be biased towards investigating only a subset of brain regions, such as those that are more easily defined via anatomical scans or those of primary theoretical interest (e.g. amygdala and hippocampus; Koolschijn et al. 2009; Bora et al. 2012). An important benefit of voxel-based morphometry (VBM) is that it allows for a more comprehensive whole-brain assessment of structural abnormalities without the a priori selection of ROIs (Ashburner & Friston, 2000). Structural magnetic resonance imaging (MRI) studies have the additional benefit of facilitating comparison across studies, as they are paradigm-free, unlike functional MRI studies in which cross-study comparisons may be obscured due to differences in the paradigms employed.
A growing number of VBM studies of MDD have been conducted, allowing for the aggregation of findings using meta-analytic methods. Across three separate and recent meta-analyses of VBM studies, the most consistent finding reported was that subjects with MDD had reduced gray matter volume in the anterior cingulate cortex (ACC) relative to those without MDD (Bora et al. 2012; Du et al. 2012; Lai, 2013; for examples of individual studies, see Tang et al. 2007; Treadway et al. 2009). The ACC is a particularly large and heterogeneous structure in terms of function, receptor architecture and cytology (Vogt et al. 2005; Palomero-Gallagher et al. 2008; Pizzagalli, 2011), and has been subdivided into a more dorsal portion (dorsal ACC; dACC) and a more ventral subregion, the latter of which has been further divided into the rostral ACC (rACC; also referred to as the ‘pregenual’ or ‘perigenual’ ACC) and the subgenual ACC (sgACC; Etkin et al. 2011). Broadly speaking, a distinction may be made between the more ‘cognitive’ dACC versus the more ‘affective’ ventral subdivision encompassing the rACC and sgACC. Indeed, the ventral portion of the ACC has rich interconnections with limbic and paralimbic structures [e.g. amygdala, nucleus accumbens, orbitofrontal cortex (OFC), periaqueductal gray], whereas the dACC has strong connections with regions in the dorsolateral prefrontal cortex (DLPFC), parietal cortex and supplementary motor areas. Consistent with these differential connectivity patterns, the ventral ACC has been implicated in emotion expression and regulation, as well as assigning emotional valence to both internal and external stimuli. In contrast, the dACC has been associated with the processing of cognitively demanding information and response selection (Etkin et al. 2011; Pizzagalli, 2011).
Given these patterns of findings, it is interesting to note that gray matter volume reductions in the ACC in MDD subjects seem to be largely concentrated in a relatively focal region of the rACC (Bora et al. 2012), a core hub in Mayberg’s limbic–cortical dysregulation model of depression (Mayberg, 1997). The structural deficit in the rACC observed in MDD is intriguing given that this region has been implicated in cognitive and emotional processes that are believed to be abnormal in many depressed individuals. These include: (1) emotion regulation and assigning emotional valence to stimuli (Pizzagalli, 2011; D’Avanzato et al. 2013); (2) self-referential processing (Northoff et al. 2006; Yoshimura et al. 2009); (3) inhibitory processes (e.g. Bush et al. 2000; Shafritz et al. 2006; Eugene et al. 2010); (4) error processing (e.g. Holmes et al. 2010; Santesso et al. 2012); and (5) optimistic biases (i.e. relatively high probability estimates of positive events occurring to oneself compared with others; Sharot et al. 2007; Blair et al. 2013). Indeed, hyper-responsiveness of this region has been shown to correlate with subclinical depressive mood in adolescent children (Killgore & Yurgelun-Todd, 2006). It is important to note that in addition to ACC abnormalities, other areas found to have reduced gray matter volume within MDD subjects in the above-mentioned meta-analyses include the middle and inferior frontal gyrus, hippocampus and thalamus (Kim et al. 2008; Vasic et al. 2008; Zou et al. 2010; Wagner et al. 2011; Du et al. 2012), as well as the DLPFC and dorsomedial prefrontal cortex (Bora et al. 2012). Given that the thalamus connects the cortex to negative emotion-generating limbic structures such as the amygdala (Price & Drevets, 2009), relatively reduced thalamic gray matter volume may help account for deficits in top-down regulation of negative affect among individuals more prone to experiencing depressive symptoms. Moreover, reduced hippocampal volume in depression may be attributable to stress-related or neurotoxic processes associated with cumulative exposure to stress and depressive symptomatology (MacQueen et al. 2003; Warner-Schmidt & Duman, 2006).
As is the case with the bulk of functional MRI research, studies investigating structural brain abnormalities in depression have typically employed a categorical rather than dimensional approach to conceptualizing depression. Namely, studies have usually compared a group of subjects diagnosed with MDD, as defined by current Diagnostic and Statistical Manual of Mental Disorders (DSM) criteria, with a group of individuals who do not meet MDD diagnostic criteria [also see the International Classification of Diseases (ICD) diagnostic system; World Health Organization, 1993]. The extent to which depressive symptoms – irrespective of DSM-defined MDD status – are linearly related to gray matter volume reductions in theoretically important brain regions (e.g. the ACC) is not well understood. Indeed, the National Institute of Mental Health (NIMH), through their Research Domain Criteria (RDoC) initiative, has encouraged a dimensional approach to the study of psychopathology as opposed to an over-reliance on traditional diagnostic categories (e.g. see NIMH funding opportunity ‘Dimensional Approaches to Research Classification in Psychiatric Disorders’ no. RFA-MH-13-080). Previous studies comparing dimensional versus categorical models of depression, including findings from taxometric analyses, have tended to support dimensional conceptualizations of depressive symptoms (Prisciandaro & Roberts, 2009), and it is likely that such approaches may ultimately lead to a more valid understanding of the underlying neurobiological basis of depression than research focused on categorical diagnostic distinctions.
It is also important to note that subthreshold levels of depressive symptoms (i.e. severity levels below DSM criteria) have been found to be associated with a range of negative outcomes (Cuijpers et al. 2004; Fergusson et al. 2005), including a significantly increased risk of developing MDD (Klein et al. 2013). These findings highlight the importance of investigating the neurobiological underpinnings of subclinical levels of depressive symptoms. A few previous studies have explored structural abnormalities in subclinical depression, but these studies differed widely in study samples (e.g. youth versus the elderly), analytic strategy (ROI versus whole-brain approach, group comparisons versus dimensional analyses) and depression measures employed. For example, in a ROI-based study focused on rACC volumes in children and adolescents (aged 7–17 years) with no history of MDD or psychiatric disorder, Boes et al. (2008) found that males with depressive symptoms had lower rACC volumes bilaterally than males without depressive symptoms. This pattern was not observed in females. In addition, in a recent VBM study comparing adults with elevated, but subthreshold, levels of depressive symptoms versus those with no depressive symptoms, Hayakawa et al. (2013) reported that subclinically depressed females had smaller gray matter volume in the bilateral ACC and in the right rectal gyrus. The pattern was not observed in males. There has also been a series of studies examining structural abnormalities in elderly populations with subthreshold depression (e.g. Taki et al. 2005; Dotson et al. 2009; Ries et al. 2009), with mixed findings regarding the ACC (Ries et al. 2009). None of the above-mentioned studies employed the commonly used Beck Depression Inventory (BDI; Beck & Steer, 1993; Beck et al. 1996).
In line with the NIMH’s RDoC initiative, the current study examines – in a dimensional rather than categorical manner – the association between gray matter volume and depressive symptoms in a non-clinical sample. We employed a whole-brain VBM regression approach with a sample of adults (aged 18–45 years) deemed free of any history of DSM-IV Axis I diagnoses. Importantly, to examine the consistency of any emerging findings, two separate instruments were used to assess depressive symptoms, including the commonly used BDI and the Depression subscale of the Personality Assessment Inventory (PAI-DEP; Morey, 1991). The PAI-DEP was selected in addition to the BDI for several reasons. First, the BDI, like the Hamilton Rating Scale for Depression (Hamilton, 1960), tends to exhibit very low mean scores and little variability in healthy samples. In contrast, the PAI-DEP includes items intended to assess the full range of depressive symptoms and with greater variability of scores across non-clinical samples (Morey, 2007). Moreover, whereas the BDI has a relatively heavy representation of cognitive aspects of depression, the PAI-DEP was designed to tap cognitive, affective and physiological symptoms of depression with generally equal weighting across these three domains.
Informed by the literature discussed above, we hypothesized that the severity of mild depressive symptoms, even in a healthy subclinical population, would be associated with reduced gray matter volume in the rACC, hippocampus, thalamus, as well as the DLPFC and dorsomedial prefrontal cortex.
Method
Participants
A total of 54 healthy participants (28 females), aged 18 to 45 years (mean=30.9 years, S.D.=8.13 years), were recruited from the greater Boston region through flyers posted in the area and from Internet advertisements. The sample was 69.1% Caucasian, 16.4% African–American, 9.1% Asian, 1.8% other, and 3.6% ‘more than one race’. In addition, 3.6% classified themselves as Hispanic. All participants identified English as their primary language. A trained Bachelor’s-level technician screened all participants for evidence of mental disorders and medical conditions using a structured series of questions adapted from the Structured Clinical Interview for DSM-IV, text revision (SCID-I; First et al. 2001). All participants included in the current study were determined to be free of any history of DSM-IV Axis I mental disorders, excessive substance use, drug or alcohol treatment, or severe medical or neurological conditions. The current study was embedded within a larger study on the neural correlates of emotional intelligence in which subjects completed a battery of self-report measures and behavioral tasks. Unrelated findings emerging from that larger study have been reported elsewhere (Killgore et al. 2012a,b, 2013; Webb et al. 2013) and will not be discussed further here. To reduce subject burden, self-report measures (including the BDI and PAI) were administered 1 day after the MRI scan.
BDI
The BDI (Beck & Steer, 1993; Beck et al. 1996) is a commonly used 21-item self-report measure of depressive symptoms with strong psychometric properties (Beck et al. 1988). The BDI has been shown to have acceptably high internal consistency reliability in psychiatric (α=0.86; Beck et al. 1988) and non-clinical (α=0.88; Killgore, 1999) samples.
Personality Assessment Inventory (PAI)
The PAI personality assessment (Morey, 1991) contains 344 statements that are rated using one of four response options (‘false, not at all true’, ‘slightly true’, ‘mainly true’, ‘very true’). The measure yields 11 clinical subscales (Somatic Complaints, Anxiety, Anxiety-Related Disorders, Depression, Mania, Paranoia, Schizophrenia, Borderline Features, Antisocial features, Drug-Related Problems, Alcohol-Related Problems). Given the current study’s focus on depressive symptoms, we restricted our analyses to the Depression subscale of the PAI (PAI-DEP). Raw scores were converted into T scores. The PAI-DEP scale shows high internal consistency reliability for clinical (α=0.87) and non-clinical (α=0.93) samples (Morey, 2007). The use of the PAI, in addition to the BDI, allows us to test the consistency of our findings across two measures of depression, which sample somewhat different aspects of depression (i.e. whereas the BDI weights cognitive items more heavily, the PAI is designed to tap cognitive, affective and physiological symptoms of depression with generally equal weighting across these three domains).
MRI parameters
Structural magnetic resonance images were acquired at 3.0 T using a 12-channel head coil (Siemens Tim Trio; Germany) and a T1-weighted three-dimensional magnetization-prepared rapid acquisition with gradient echo (MPRAGE) sequence using the following parameters: repetition time=2.1 s; echo time=2.25 ms; flip angle=12°; 128 sagittal slices; 256×256 matrix; in-plane resolution=1×1×1 mm; slice thickness=1.33 mm.
VBM
Preprocessing of structural images was conducted using the VBM8 toolbox (http://dbm.neuro.uni-jena.de/vbm.html) in SPM8 (Wellcome Department of Imaging Neuroscience Group, UK; http://www.fil.ion.ucl.ac.uk/spm). The VBM8 default settings were used (i.e. modulated VBM – gray matter volume was corrected for total intracranial volume). Each structural image was DARTEL-normalized (diffeomorphic anatomical registration through exponentiated lie algebra) to Montreal Neurological Institute (MNI) space and subsequently segmented into gray matter, white matter and cerebrospinal fluid using a fully automated algorithm within SPM8. Standard quality checks of segmentation and sample homogeneity were conducted, before normalized gray matter images were smoothed with an 8 mm full-width at half-maximum (FWHM) Gaussian kernel. In other words, the data were preprocessed such that the dependent variable of interest was the local concentration of gray matter at each local voxel without concern for differences in morphological shape (Ashburner & Friston, 2000).
Statistical analysis
Scores on both depression scales were initially tested for normality. To assess the severity of skew and kurtosis, z scores were computed (Field, 2009a). Consistent with prior work examining the distribution of BDI scores in the population (Veerman et al. 2009), scores in the present sample were positively skewed (z=4.10, p<0.001; Kline, 2005; Field, 2009b). Accordingly, a square-root transformation was applied to reduce the positive skew (transformed BDI, z=0.861, p>0.05). For simplicity, the term BDI is used henceforth, rather than repeating the more accurate but unwieldy term ‘square-root transformed BDI’.
Normalized smoothed gray matter images were entered into a series of three random-effects multiple regression analyses in SPM8. A non-stationary cluster extent correction was implement in SPM8 according to the random field theory (RFT) framework (Hayasaka et al. 2004). In the first regression, we tested the association between BDI scores and voxelwise gray matter volume. Normalized smoothed gray matter images were entered as the dependent variable into a whole-brain general linear model. For statistical inference, a whole-brain false discovery rate (FDR)-corrected height threshold of p <0.05 was applied. A cluster extent threshold of k ≥81 (i.e. determined statistically as the number of voxels per cluster that would be expected by chance based on the theory of Gaussian random fields applied to this analysis as provided in standard VBM8 output) was also applied for the purpose of reducing noise in the images and to minimize reporting of clusters of little interest, but was not considered for statistical inference. For the second regression, we conducted a similar analysis with PAI-DEP as the covariate of interest (height threshold of FDR p<0.05 for statistical inference, with an empirically derived cluster extent of k ≥65). Finally, to examine the overlap in gray matter volume correlations across the BDI and PAI-DEP analyses, the activation clusters obtained from the first regression analysis (i.e. BDI) were used as a region of interest (ROI) mask in conjunction with the output from the second regression (i.e. PAI-DEP; height threshold FDR p < 0.05, cluster extent k ≥65). Thus, the resulting findings from the latter analysis reflect overlapping regions where gray matter volume correlated significantly with self-reported depression symptoms on both the BDI and PAI-DEP scales. Age and gender were included as nuisance covariates in all analyses.
Results
The mean raw BDI score was 3.89 (S.D.=4.56, range=0–17), with seven participants scoring above the suggested clinical criterion for ‘minimal depression’ (i.e. total BDI score >9; Beck et al. 1996). The mean PAI-DEP score was 46.20 (S.D.=7.97, range=36–71)1,†. The Pearson correlation between the BDI and PAI-DEP in the current sample was r=0.67 (p<0.001).
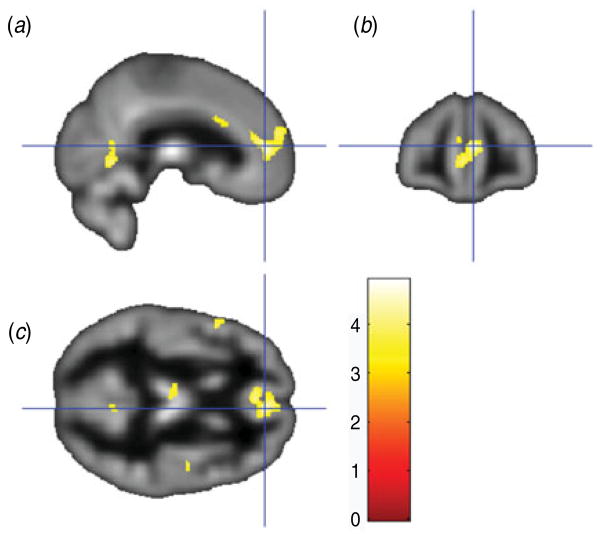
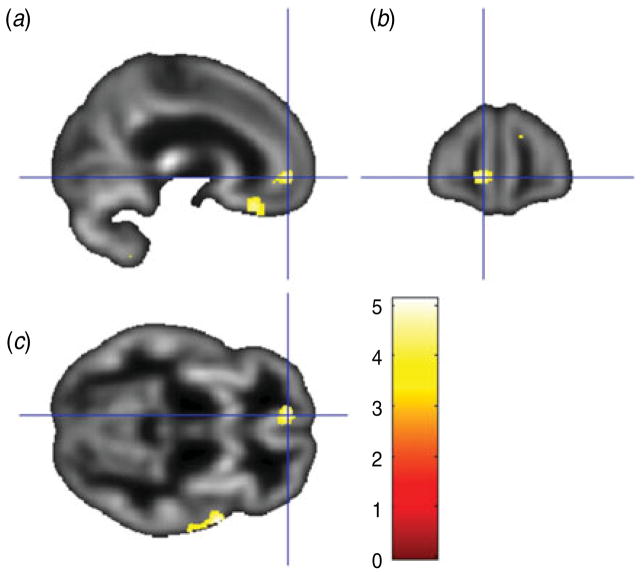
As detailed in Table 1, relatively higher BDI scores were associated with reduced gray matter volume in 16 clusters, including: (i) bilateral anterior cingulate, bilateral medial frontal cortex and left medial OFC (see Fig. 1); (ii) bilateral anterior/mid-cingulate; (iii) left thalamus; and (iv) left insula. As shown in Table 2, relatively higher PAI-DEP scores were associated with reduced gray matter volume in nine clusters, including: (i) left anterior cingulate and left medial OFC (see Fig. 2); (ii) bilateral medial OFC; and (iii) bilateral thalamus.
Table 1.
Brain regions with reduced gray matter volume associated with higher Beck Depression Inventory scores
| Region | Cluster size | Peak-level MNI coordinatesa
|
T | ||
|---|---|---|---|---|---|
| x | y | z | |||
| Bilateral anterior cingulate | 1669 | 6 | 46 | 10 | 4.33* |
| Right superior temporal/insula/Heschl’s gyrus | 737 | 51 | −9 | 4 | 4.35* |
| Bilateral dorsal anterior cingulate/mid-cingulate | 598 | 0 | 20 | 28 | 4.62† |
| Left superior temporal gyrus | 562 | −51 | 6 | −6 | 4.33† |
| Right middle frontal gyrus | 549 | 44 | 21 | 46 | 4.34* |
| Left middle cingulate gyrus | 346 | −8 | −33 | 49 | 4.30 |
| Right superior temporal pole/insula | 297 | 48 | 15 | −9 | 4.92† |
| Left inferior frontal operculum | 260 | −52 | 14 | 15 | 4.20 |
| Left postcentral gyrus | 190 | −50 | −18 | 43 | 4.16 |
| Left superior temporal pole | 168 | −32 | 14 | −30 | 4.38 |
| Right lingual gyrus | 135 | 8 | −61 | 0 | 4.03 |
| Left thalamus | 122 | −8 | −18 | 9 | 3.59 |
| Right precentral gyrus | 101 | 52 | −9 | 40 | 3.78 |
| Right middle temporal gyrus | 101 | 68 | −18 | −3 | 3.96 |
| Left insula | 84 | −33 | 18 | 4 | 3.71 |
| Left middle occipital gyrus | 83 | −44 | −81 | 0 | 4.16 |
MNI, Montreal Neurological Institute.
All false discovery rate-corrected p<0.05; k ≥81.
p<0.05,
p<0.10 cluster level, family-wise error corrected.
Fig. 1.
Sagittal view (a), coronal view (b) and axial view (c) of the anterior cingulate/medial orbitofrontal cortex cluster (overlaid on sample-specific T1 mean image) that was negatively correlated with depressive symptoms on the Beck Depression Inventory. Montreal Neurological Institute (MNI) coordinates x=6, y=46, z=10; cluster size=1669 voxels.
Table 2.
Brain regions with reduced gray matter volume associated with higher scores on the Depression subscale of the Personality Assessment Inventory
| Region | Cluster size | Peak-level MNI coordinatesa
|
T | ||
|---|---|---|---|---|---|
| x | y | z | |||
| Right cerebellum | 747 | 24 | −78 | −27 | 4.58 |
| Right superior temporal gyrus/temporal pole | 660 | 62 | 4 | −2 | 5.13* |
| Bilateral thalamus | 395 | 0 | −19 | 10 | 4.47 |
| Left superior orbitofrontal gyrus | 248 | −9 | 28 | −24 | 4.56 |
| Right superior frontal gyrus | 213 | 14 | 56 | 25 | 4.21 |
| Left medial OFC/ACC | 209 | −9 | 51 | −5 | 4.68 |
| Left cerebellum | 163 | −15 | −55 | −60 | 3.79 |
| Bilateral caudate | 96 | 0 | 10 | 1 | 4.61 |
| Bilateral medial OFC | 83 | 0 | 62 | −12 | 4.32 |
MNI, Montreal Neurological Institute; OFC, orbitofrontal cortex; ACC, anterior cingulate cortex.
All false discovery rate-corrected p<0.05; k ≥65.
p<0.05,
p<0.10 cluster level, family-wise error corrected.
Fig. 2.
Sagittal view (a), coronal view (b) and axial view (c) of the left anterior cingulate/medial orbitofrontal cortex cluster (overlaid on sample-specific T1 mean image) that was negatively correlated with depressive symptoms on the Depression subscale of the Personality Assessment Inventory. Montreal Neurological Institute (MNI) coordinates x=−9, y=51, z=−5; cluster size=209 voxels.
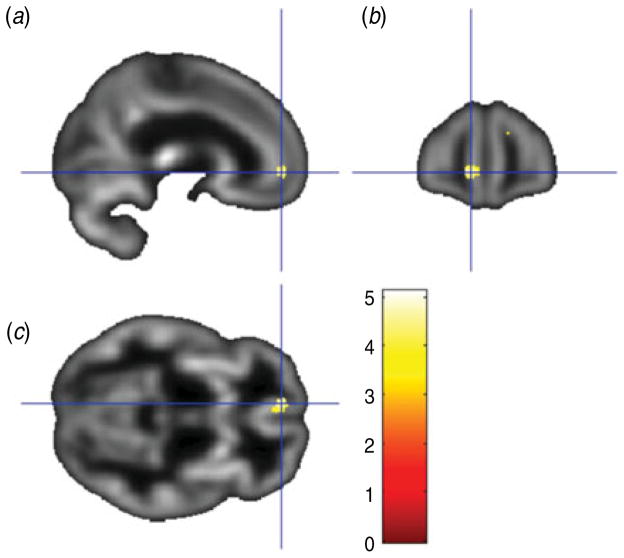
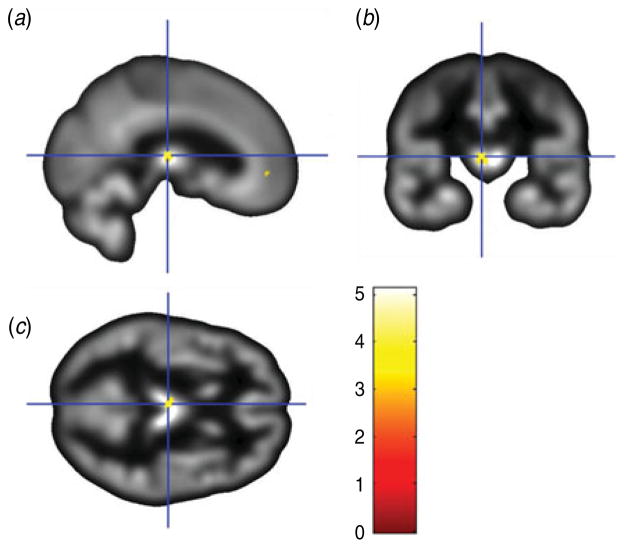
The conjunction between the two primary analyses was used to identify the regions showing common overlap between the gray matter volume correlations for the two depression measures used in the current study. As shown in Table 3, this analysis showed that the BDI and PAI-DEP scores were both associated with reduced gray matter volume in four common regions: (i) left medial OFC and anterior cingulate (Fig. 3); (ii) left thalamus (Fig. 4); (iii) right superior medial frontal gyrus/superior frontal gyrus; and (iv) right superior temporal gyrus extending to the superior temporal pole.
Table 3.
Brain regions with reduced gray matter volume associated with higher scores on both the Beck Depression Inventory and the Depression subscale of the Personality Assessment Inventory
| Region | Cluster size | Peak-level MNI coordinatesa
|
T | ||
|---|---|---|---|---|---|
| x | y | z | |||
| Right superior temporal gyrus/temporal pole | 251 | 62 | 4 | −2 | 5.13* |
| Right superior frontal gyrus | 153 | 14 | 56 | 25 | 4.21* |
| Left medial OFC/ACC | 116 | −9 | 51 | −5 | 4.68* |
| Left thalamus | 79 | −2 | −19 | 10 | 4.39† |
MNI, Montreal Neurological Institute; OFC, orbitofrontal cortex; ACC, anterior cingulate cortex.
All false discovery rate-corrected p<0.05; k ≥65.
p<0.05,
p<0.10 cluster level, family-wise error corrected.
Fig. 3.
Sagittal view (a), coronal view (b) and axial view (c) of the results of a conjunction analysis showing a cluster where gray matter volume in the left rostral anterior cingulate/medial orbitofrontal cortex (overlaid on sample-specific T1 mean image) was negatively correlated with depressive symptoms on both the Beck Depression Inventory and Depression subscale of the Personality Assessment Inventory. Montreal Neurological Institute (MNI) coordinates x=−9, y=51, z=−5; cluster size=116 voxels.
Fig. 4.
Sagittal view (a), coronal view (b) and axial view (c) of the results of a conjunction analysis showing a cluster where gray matter volume in the left thalamus (overlaid on sample-specific T1 mean image) was negatively correlated with depressive symptoms on both the Beck Depression Inventory and Depression subscale of the Personality Assessment Inventory. Montreal Neurological Institute (MNI) coordinates x=−2, y=19, z=10; cluster size=79 voxels.
Discussion
The present study complements prior research on structural brain abnormalities in depression. Specifically, previous studies examining structural deficits in depression have typically relied on categorical comparisons of subjects diagnosed with MDD versus healthy controls. The extent to which depressive symptoms in individuals who do not meet DSM criteria for MDD are linearly related to structural differences in key brain regions is not well understood. Inspired by the NIMH’s RDoC initiative, which highlights dimensional/continuous – rather than DSM-based categorical – conceptualization of psychopathology, we examined the linear associations between gray matter volume and depressive symptoms in a sample of adults deemed free of any history of DSM-IV Axis I diagnoses. Paralleling findings from prior studies employing a categorical (i.e. MDD versus healthy controls) analytic approach, we found that relatively higher depressive symptoms were associated with reduced gray matter volume in the left rACC (extending into the medial OFC). Perhaps the most consistently reported finding in the existing literature is that subjects with MDD have reduced gray matter volume in the ACC relative to those without MDD (for recent meta-analytic reviews, see Bora et al. 2012; Du et al. 2012; Lai, 2013). The current findings extend prior work by suggesting that reduced ACC/OFC gray matter volume can be observed even in a sample that does not meet DSM criteria for MDD, but who nevertheless report relatively elevated levels of depressive symptoms. In addition, we also observed an association between relatively elevated depressive symptoms and reduced gray matter volume in the left thalamus, as well as the right superior medial frontal gyrus/superior frontal gyrus, and right superior temporal gyrus extending to the superior temporal pole. The fact that this finding emerged across both of our depressive symptom measures, including the commonly used BDI, as well as the depression subscale of the PAI (PAI-DEP), suggests that these findings probably reflect true and robust associations between gray matter and depressive symptoms within the subclinical range.
Regarding our ACC finding, it is interesting to note that gray matter volume reductions were concentrated in the rACC. The rACC has been implicated in a range of cognitive/affective functions, including: (1) optimistic biases (e.g. Blair et al. 2013); (2) error processing (e.g. Holmes et al. 2010; Santesso et al. 2012); (3) self-referential processing (Northoff et al. 2006; Yoshimura et al. 2009); (4) inhibitory processes (e.g. Bush et al. 2000; Shafritz et al. 2006; Eugene et al. 2010); and (5) regulation of emotional conflict and dampening of amygdala hyperactivity (Etkin et al. 2006). Given these patterns of findings, one could speculate that structural abnormalities in the rACC in part underlie the biased information processing and negative maladaptive cognitive features described in the cognitive model of depression (Beck & Alford, 2009; DeRubeis et al. 2010). More specifically, to the extent that the rACC does play a role in the aforementioned functions, relatively reduced gray matter volume in this region may help account for the depressogenic, cognitive features of depression, including: (1) pessimistic (rather than optimistic) biases (e.g. Strunk et al. 2006); (2) hypersensitivity to perceived errors and negative feedback (e.g. Elliott et al. 1998; Pizzagalli et al. 2006); (3) maladaptive self-referential cognitive processes (e.g. Nolen-Hoeksema, 1991; Nolen-Hoeksema et al. 2008; Pizzagalli, 2011); (4) difficulties inhibiting negative information (e.g. Goeleven et al. 2006); and (5) emotion regulation deficits (D’Avanzato et al. 2013). These abnormal cognitive and emotion regulatory features of depression do not appear to be categorical in nature (i.e. present in MDD, yet absent in those who do not meet DSM criteria for the disorder). Rather, behavioral and neural findings suggest that at least some of these features are linearly related to depressive symptoms across a broad range of severity, from no or very low levels of depressive symptoms to very high levels (e.g. Joormann, 2004; Pizzagalli et al. 2006; Strunk et al. 2006). Overall, and consistent with the NIMH’s RDoC initiative, these findings may highlight the limitations of restricting the study of abnormal cognitive, emotional and behavioral processes in depression to DSM-based categorical comparisons, and the need for additional research using dimensional/continuous conceptual and analytic approaches.
Although perhaps not as consistently reported as rACC findings, prior studies have also identified gray matter reductions in the thalamus among MDD subjects (Bielau et al. 2005; Kim et al. 2008; Vasic et al. 2008; Lee et al. 2011). The current study suggests that reductions in thalamic gray matter volume can also be observed in individuals with subclinical, yet relatively elevated, levels of depressive symptoms. The latter finding is intriguing given that the thalamus connects the cortex to negative emotion-generating limbic structures such as the amygdala (Price & Drevets, 2009). Thus, relatively reduced thalamic gray matter volume, coupled with volumetric reductions in the rACC, may also help account for the deficits in top-down regulation of negative emotions among individuals more prone to experiencing depressive symptoms.
In addition, previous depression studies have also reported volume reductions in the superior temporal gyrus/temporal pole (e.g. Caetano et al. 2004; Abe et al. 2010) and superior frontal gyrus (e.g. Serra-Blasco et al. 2013). Interestingly, Caetano et al. (2004) found that right superior temporal gyrus volume was inversely correlated with length of depression illness. Similarly, the findings of Serra-Blasco et al. (2013) revealed that participants who suffered from chronic, treatment-resistant depression exhibited the smallest volumes in frontotemporal regions. It is tempting to attribute such volumetric reductions to neurotoxic effects of stress and depression accumulating over time. However, conclusions regarding the direction of effects are necessarily tempered when cross-sectional designs are employed.
It may be that reduced gray matter volume in the regions highlighted in the current study (e.g. rACC, thalamus) increase the risk of experiencing depressive symptoms. Conversely, these structural differences may also reflect the neurotoxic effects of prior depressive symptoms and stress. It should be noted that we intentionally restricted our sample to those without prior episodes of depression. Nevertheless, reduced gray matter volume could of course still be due to cumulative exposure to subthreshold depressive symptoms and neurotoxic stress processes. Methodologically sound longitudinal studies are needed to disentangle causal direction and test whether or not reduced gray matter volume in certain brain regions (e.g. rACC) prospectively predicts depression onset, and/or whether structural abnormalities are a consequence of depression due to particular neuropathological processes associated with the disorder. Longitudinal studies could help delineate factors that predict the development of these structural brain abnormalities over time. For example, prior research has found that smaller ACC volumes are associated with retrospective reports of early life stressors (Cohen et al. 2006). This finding highlights the possible neurotoxic effects of early life stress on the developing brain. Ideally, however, given that current symptomatology may bias recall of early life stressors (e.g. Southwick et al. 1997), prospective longitudinal, rather than retrospective studies would be needed to more conclusively establish the link between early life stress and structural brain abnormalities, ideally complementing self-report measures of stressors with more ‘objective’ measures (e.g. assessments of stress hormones).
A strength of the current study is that we tested the consistency of our findings across two separate measures of depressive symptoms. In addition, in contrast to many prior studies, we used a whole-brain VBM approach, rather than an ROI approach, the latter of which may bias investigators towards examining only a subset of brain regions and possibly ignoring other relevant regions (Ashburner & Friston, 2000). However, despite these strengths, several limitations of the current study should be noted. As discussed above, our findings were cross-sectional in nature and we cannot draw strong inferences regarding causal direction between structural brain differences and depressive symptoms. Moreover, our study focused on a sample without a history of Axis I pathology. It will be important for future studies to examine the full continuum from no depression to very severe levels of depressive symptoms, to fully examine the extent to which depressive symptoms are linearly related with structural brain differences. In addition, given our non-clinical sample, variability in depression scores was, as expected, limited. Thus, restriction in the range of depression scores may have limited our ability to detect significant effects in other brain regions. It should be noted that the current findings indicate that we did have sufficient variability to detect reduced gray matter volume in a number of regions, across two separate depression measures.
Acknowledgments
This study was supported by a US Army Medical Research Acquisition Activity (USAMRAA) grant (no. W81XWH-09-1-0730 to W.D.S.K.). The grant provided support for subject remuneration, scan costs, supplies and salary.
Footnotes
The notes appear after the main text.
Two outliers were identified. Specifically, one subject was an outlier on both the BDI (z = 4.68) and PAI Depression subscale (z = 4.00). This subject’s data were deleted from the current dataset.
Declaration of Interest
None.
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
References
- Abe O, Yamasue H, Kasai K, Yamada H, Aoki S, Inoue H, Takei K, Suga M, Matsuo K, Kato T, Masutani Y, Ohtomo K. Voxel-based analyses of gray/white matter volume and diffusion tensor data in major depression. Psychiatry Research. 2010;181:64–70. doi: 10.1016/j.pscychresns.2009.07.007. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Voxel-based morphometry –the methods. NeuroImage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Beck AT, Alford BA. Depression: Causes and Treatments. University of Pennsylvania Press; Philadelphia: 2009. [Google Scholar]
- Beck AT, Steer RA. Beck Depression Inventory Manual. Harcourt Brace; San Antonio: 1993. [Google Scholar]
- Beck AT, Steer RA, Brown GK. Beck Depression Inventory Manual. 2. Harcourt Brace; San Antonio: 1996. [Google Scholar]
- Beck AT, Steer RA, Carbin MG. Psychometric properties of the Beck Depression Inventory: twenty-five years of evaluation. Clinical Psychology Review. 1988;8:77–100. [Google Scholar]
- Bielau H, Trubner K, Krell D, Agelink MW, Bernstein HG, Stauch R, Baumann B. Volume deficits of subcortical nuclei in mood disorders: a postmortem study. European Archives of Psychiatry and Clinical Neuroscience. 2005;255:401–412. doi: 10.1007/s00406-005-0581-y. [DOI] [PubMed] [Google Scholar]
- Blair KS, Otero M, Teng C, Jacobs M, Odenheimer S, Pine DS, Blair RJ. Dissociable roles of ventromedial prefrontal cortex (vmPFC) and rostral anterior cingulate cortex (rACC) in value representation and optimistic bias. NeuroImage. 2013;78:103–110. doi: 10.1016/j.neuroimage.2013.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boes AD, McCormick LM, Coryell WH, Nopoulos P. Cortex volume correlates with depressed mood in normal healthy children. Biological Psychiatry. 2008;63:391–397. doi: 10.1016/j.biopsych.2007.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bora E, Fornito A, Pantelis C, Yucel M. Gray matter abnormalities in major depressive disorder: a meta-analysis of voxel based morphometry studies. Journal of Affective Disorders. 2012;138:9–18. doi: 10.1016/j.jad.2011.03.049. [DOI] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends in Cognitive Sciences. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Caetano SC, Hatch JP, Brambilla P, Sassi RB, Nicoletti M, Mallinger AG, Frank E, Kupfer DJ, Keshaven MS, Soares JC. Anatomical MRI study of hippocampus and amygdala in patients with current and remitted major depression. Psychiatry Research. 2004;132:141–147. doi: 10.1016/j.pscychresns.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Cohen RA, Grieve S, Hoth KF, Paul RH, Sweet L, Tate D, Williams LM. Early life stress and morphometry of the adult anterior cingulate cortex and caudate nuclei. Biological Psychiatry. 2006;59:975–982. doi: 10.1016/j.biopsych.2005.12.016. [DOI] [PubMed] [Google Scholar]
- Cuijpers P, de Graaf R, van Dorsselaer S. Minor depression: risk profiles, functional disability, health care use and risk of developing major depression. Journal of Affective Disorders. 2004;79:71–79. doi: 10.1016/S0165-0327(02)00348-8. [DOI] [PubMed] [Google Scholar]
- D’Avanzato C, Joormann J, Siemer M, Gotlib IH. Emotion regulation in depression and anxiety: examining diagnostic specificity and stability of strategy use. Cognitive Therapy and Research. 2013;37:968–980. [Google Scholar]
- DeRubeis RJ, Webb CA, Tang TZ, Beck AT. Cognitive therapy. In: Dobson KS, editor. Handbook of Cognitive–Behavioral Therapies. 3. Guilford; New York: 2010. pp. 277–316. [Google Scholar]
- Dotson VM, Davatzikos C, Kraut MA, Resnick SM. Depressive symptoms and brain volumes in older adults: a longitudinal magnetic resonance imaging study. Journal of Psychiatry and Neuroscience. 2009;34:367–375. [PMC free article] [PubMed] [Google Scholar]
- Du MY, Wu QZ, Yue Q, Li J, Liao Y, Kuang WH, Gong QY. Voxelwise meta-analysis of gray matter reduction in major depressive disorder. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2012;36:11–16. doi: 10.1016/j.pnpbp.2011.09.014. [DOI] [PubMed] [Google Scholar]
- Elliott R, Sahakian BJ, Michael A, Paykel ES, Dolan RJ. Abnormal neural response to feedback on planning and guessing tasks in patients with unipolar depression. Psychological Medicine. 1998;28:559–571. doi: 10.1017/s0033291798006709. [DOI] [PubMed] [Google Scholar]
- Etkin A, Egner T, Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends in Cognitive Sciences. 2011;15:85–93. doi: 10.1016/j.tics.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Egner T, Peraza DM, Kandel ER, Hirsch J. Resolving emotional conflict: a role for the rostral anterior cingulate cortex in modulating activity in the amygdala. Neuron. 2006;51:871–882. doi: 10.1016/j.neuron.2006.07.029. [DOI] [PubMed] [Google Scholar]
- Eugene F, Joormann J, Cooney RE, Atlas LY, Gotlib IH. Neural correlates of inhibitory deficits in depression. Psychiatry Research. 2010;181:30–35. doi: 10.1016/j.pscychresns.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fergusson DM, Horwood LJ, Ridder EM, Beautrais AL. Subthreshold depression in adolescence and mental health outcomes in adulthood. Archives of General Psychiatry. 2005;62:66–72. doi: 10.1001/archpsyc.62.1.66. [DOI] [PubMed] [Google Scholar]
- Field A. Discovering Statistics Using SPSS. 3. Sage Publications, Inc; Thousand Oaks, CA: 2009a. pp. 138–139. [Google Scholar]
- Field A. Discovering Statistics Using SPSS. 3. Sage Publications, Inc; Thousand Oaks, CA: 2009b. pp. 154–156. [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams J. Structured Clinical Interview for DSM-IV-TR – Axis I Disorders, Research Version, Patient Edition with Psychotic Screen (SCID-I/P W/PSY SCREEN) Biometrics Research, New York State Psychiatric Institute; New York: 2001. [Google Scholar]
- Goeleven E, De Raedt R, Baert S, Koster EH. Deficient inhibition of emotional information in depression. Journal of Affective Disorders. 2006;93:149–157. doi: 10.1016/j.jad.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery, and Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa YK, Sasaki H, Takao H, Mori H, Hayashi N, Kunimatsu A, Ohtomo K. Structural brain abnormalities in women with subclinical depression, as revealed by voxel-based morphometry and diffusion tensor imaging. Journal of Affective Disorders. 2013;144:263–268. doi: 10.1016/j.jad.2012.10.023. [DOI] [PubMed] [Google Scholar]
- Hayasaka S, Phan KL, Liberzon I, Worsley KJ, Nichols TE. Nonstationary cluster-size inference with random field and permutation methods. NeuroImage. 2004;22:676–687. doi: 10.1016/j.neuroimage.2004.01.041. [DOI] [PubMed] [Google Scholar]
- Holmes AJ, Bogdan R, Pizzagalli DA. Serotonin transporter genotype and action monitoring dysfunction: a possible substrate underlying increased vulnerability to depression. Neuropsychopharmacology. 2010;35:1186–1197. doi: 10.1038/npp.2009.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joormann J. Attentional bias in dysphoria: the role of inhibitory processes. Cognition and Emotion. 2004;18:125–147. [Google Scholar]
- Killgore WD. Empirically derived factor indices for the Beck Depression Inventory. Psychological Reports. 1999;84:1005–1013. doi: 10.2466/pr0.1999.84.3.1005. [DOI] [PubMed] [Google Scholar]
- Killgore WD, Schwab ZJ, Kipman M, DelDonno SR, Weber M. Voxel-based morphometric gray matter correlates of daytime sleepiness. Neuroscience Letters. 2012a;518:10–13. doi: 10.1016/j.neulet.2012.04.029. [DOI] [PubMed] [Google Scholar]
- Killgore WD, Schwab ZJ, Weber M, Kipman M, Deldonno SR, Weiner MR, Rauch SL. Daytime sleepiness affects prefrontal regulation of food intake. NeuroImage. 2013;71:216–223. doi: 10.1016/j.neuroimage.2013.01.018. [DOI] [PubMed] [Google Scholar]
- Killgore WD, Schwab ZJ, Weiner MR. Self-reported nocturnal sleep duration is associated with next-day resting state functional connectivity. Neuroreport. 2012b;23:741–745. doi: 10.1097/WNR.0b013e3283565056. [DOI] [PubMed] [Google Scholar]
- Killgore WD, Yurgelun-Todd DA. Ventromedial prefrontal activity correlates with depressed mood in adolescent children. Neuroreport. 2006;17:167–171. doi: 10.1097/01.wnr.0000198951.30939.73. [DOI] [PubMed] [Google Scholar]
- Kim MJ, Hamilton JP, Gotlib IH. Reduced caudate gray matter volume in women with major depressive disorder. Psychiatry Research. 2008;164:114–122. doi: 10.1016/j.pscychresns.2007.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein DN, Glenn CR, Kosty DB, Seeley JR, Rohde P, Lewinsohn PM. Predictors of first lifetime onset of major depressive disorder in young adulthood. Journal of Abnormal Psychology. 2013;122:1–6. doi: 10.1037/a0029567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline RB. Principles and Practice of Structural Equation Modeling. 2. Guilford Press; New York: 2005. pp. 50–51. [Google Scholar]
- Koolschijn PC, van Haren NE, Lensvelt-Mulders GJ, Hulshoff Pol HE, Kahn RS. Brain volume abnormalities in major depressive disorder: a meta-analysis of magnetic resonance imaging studies. Human Brain Mapping. 2009;30:3719–3735. doi: 10.1002/hbm.20801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai CH. Gray matter volume in major depressive disorder: a meta-analysis of voxel-based morphometry studies. Psychiatry Research. 2013;211:37–46. doi: 10.1016/j.pscychresns.2012.06.006. [DOI] [PubMed] [Google Scholar]
- Lee HY, Tae WS, Yoon HK, Lee BT, Paik JW, Son KR, Ham BJ. Demonstration of decreased gray matter concentration in the midbrain encompassing the dorsal raphe nucleus and the limbic subcortical regions in major depressive disorder: an optimized voxel-based morphometry study. Journal of Affective Disorders. 2011;133:128–136. doi: 10.1016/j.jad.2011.04.006. [DOI] [PubMed] [Google Scholar]
- MacQueen GM, Campbell S, McEwen BS, Macdonald K, Amano S, Joffe RT, Nahmias C, Young LT. Course of illness, hippocampal function, and hippocampal volume in major depression. Proceedings of the National Academy of Sciences USA. 2003;100:1387–1392. doi: 10.1073/pnas.0337481100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayberg HS. Limbic–cortical dysregulation: a proposed model of depression. Journal of Neuropsychiatry. 1997;9:471–481. doi: 10.1176/jnp.9.3.471. [DOI] [PubMed] [Google Scholar]
- Morey LC. Personality Assessment Inventory. Psychological Assessment Resources Inc; Lutz, FL: 1991. [Google Scholar]
- Morey LC. Personality Assessment Inventory: Professional Manual. 2. Psychological Assessment Resources Inc; Lutz, FL: 2007. [Google Scholar]
- Nolen-Hoeksema S. Responses to depression and their effects on the duration of depressive episodes. Journal of Abnormal Psychology. 1991;100:569–582. doi: 10.1037//0021-843x.100.4.569. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S, Wisco BE, Lyubomirsky S. Rethinking rumination. Perspectives on Psychological Science. 2008;3:400–424. doi: 10.1111/j.1745-6924.2008.00088.x. [DOI] [PubMed] [Google Scholar]
- Northoff G, Heinzel A, de Greck M, Bermpohl F, Dobrowolny H, Panksepp J. Self-referential processing in our brain – a meta-analysis of imaging studies on the self. NeuroImage. 2006;31:440–457. doi: 10.1016/j.neuroimage.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Palomero-Gallagher N, Mohlberg H, Zilles K, Vogt B. Cytology and receptor architecture of human anterior cingulate cortex. Journal of Comparative Neurology. 2008;508:906–926. doi: 10.1002/cne.21684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli DA. Frontocingulate dysfunction in depression: toward biomarkers of treatment response. Neuropsychopharmacology. 2011;36:183–206. doi: 10.1038/npp.2010.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli DA, Peccoralo LA, Davidson RJ, Cohen JD. Resting anterior cingulate activity and abnormal responses to errors in subjects with elevated depressive symptoms: a 128-channel EEG study. Human Brain Mapping. 2006;27:185–201. doi: 10.1002/hbm.20172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price JL, Drevets WC. Neurocircuitry of mood disorders. Neuropsychopharmacology. 2009;35:192–216. doi: 10.1038/npp.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prisciandaro JJ, Roberts JE. A comparison of the predictive abilities of dimensional and categorical models of unipolar depression in the National Comorbidity Survey. Psychological Medicine. 2009;39:1087–1096. doi: 10.1017/S0033291708004522. [DOI] [PubMed] [Google Scholar]
- Ries ML, Wichmann A, Bendlin BB, Johnson SC. Posterior cingulate and lateral parietal gray matter volume in older adults with depressive symptoms. Brain Imaging and Behavior. 2009;3:233–239. doi: 10.1007/s11682-009-9065-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santesso DL, Bogdan R, Birk JL, Goetz EL, Holmes AJ, Pizzagalli DA. Neural responses to negative feedback are related to negative emotionality in healthy adults. Social Cognitive and Affective Neuroscience. 2012;7:794–803. doi: 10.1093/scan/nsr054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serra-Blasco M, Portella MJ, Gómez-Ansón B, de Diego-Adeliño J, Vives-Gilabert Y, Puigdemont D, Granell E, Santos A, Alvarez E, Pérez V. Effects of illness duration and treatment resistance on grey matter abnormalities in major depression. British Journal of Psychiatry. 2013;202:434–440. doi: 10.1192/bjp.bp.112.116228. [DOI] [PubMed] [Google Scholar]
- Shafritz KM, Collins SH, Blumberg HP. The interaction of emotional and cognitive neural systems in emotionally guided response inhibition. NeuroImage. 2006;31:468–475. doi: 10.1016/j.neuroimage.2005.11.053. [DOI] [PubMed] [Google Scholar]
- Sharot T, Riccardi MA, Raio CM, Phelps EA. Neural mechanisms mediating optimism bias. Nature. 2007;450:102–105. doi: 10.1038/nature06280. [DOI] [PubMed] [Google Scholar]
- Southwick SM, Morgan CA, 3rd, Nicolaou AL, Charney DS. Consistency of memory for combat-related traumatic events in veterans of Operation Desert Storm. American Journal of Psychiatry. 1997;154:173–177. doi: 10.1176/ajp.154.2.173. [DOI] [PubMed] [Google Scholar]
- Strunk DR, Lopez H, DeRubeis RJ. Depressive symptoms are associated with unrealistic negative predictions of future life events. Behaviour Research and Therapy. 2006;44:861–882. doi: 10.1016/j.brat.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Taki Y, Kinomura S, Awata S, Inoue K, Sato K, Ito H, Fukuda H. Male elderly subthreshold depression patients have smaller volume of medial part of prefrontal cortex and precentral gyrus compared with age-matched normal subjects: a voxel-based morphometry. Journal of Affective Disorders. 2005;88:313–320. doi: 10.1016/j.jad.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Tang Y, Wang F, Xie G, Liu J, Li L, Su L, Liu Y, Hu X, He Z, Blumberg HP. Reduced ventral anterior cingulate and amygdala volumes in medication-naive females with major depressive disorder: a voxel-based morphometric magnetic resonance imaging study. Psychiatry Research. 2007;156:83–86. doi: 10.1016/j.pscychresns.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Treadway MT, Grant MM, Ding Z, Hollon SD, Gore JC, Shelton RC. Early adverse events, HPA activity and rostral anterior cingulate volume in MDD. PLoS One. 2009;4:e4887. doi: 10.1371/journal.pone.0004887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasic N, Walter H, Hose A, Wolf RC. Gray matter reduction associated with psychopathology and cognitive dysfunction in unipolar depression: a voxel-based morphometry study. Journal of Affective Disorders. 2008;109:107–116. doi: 10.1016/j.jad.2007.11.011. [DOI] [PubMed] [Google Scholar]
- Veerman JL, Dowrick C, Ayuso-Mateos JL, Dunn G, Barendregt JJ. Population prevalence of depression and mean Beck Depression Inventory score. British Journal of Psychiatry. 2009;195:516–519. doi: 10.1192/bjp.bp.109.066191. [DOI] [PubMed] [Google Scholar]
- Vogt B, Vogt L, Farber NB, Bush G. Architecture and neurocytology of monkey cingulate gyrus. Journal of Comparative Neurology. 2005;485:218–239. doi: 10.1002/cne.20512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner G, Koch K, Schachtzabel C, Schultz CC, Sauer H, Schlsser RG. Structural brain alterations in patients with major depressive disorder and high risk for suicide: evidence for a distinct neurobiological entity? NeuroImage. 2011;54:1607–1614. doi: 10.1016/j.neuroimage.2010.08.082. [DOI] [PubMed] [Google Scholar]
- Warner-Schmidt JL, Duman RS. Hippocampal neurogenesis: opposing effects of stress and antidepressant treatment. Hippocampus. 2006;16:239–249. doi: 10.1002/hipo.20156. [DOI] [PubMed] [Google Scholar]
- Webb CA, Schwab ZJ, Weber M, DelDonno S, Kipman M, Weiner MR, Killgore WDS. Convergent and divergent validity of integrative versus mixed model measures of emotional intelligence. Intelligence. 2013;41:149–156. [Google Scholar]
- World Health Organization. The ICD-10 Classification of Mental and Behavioural Disorders: Diagnostic Criteria for Research. World Health Organization; Geneva: 1993. [Google Scholar]
- Yoshimura S, Ueda K, Suzuki S, Onoda K, Okamoto Y, Yamawaki S. Self-referential processing of negative stimuli within the ventral anterior cingulate gyrus and right amygdala. Brain and Cognition. 2009;69:218–225. doi: 10.1016/j.bandc.2008.07.010. [DOI] [PubMed] [Google Scholar]
- Zou K, Deng W, Li T, Zhang B, Jiang L, Huang C, Sun X. Changes of brain morphometry in first-episode, drug-naive, non-late-life adult patients with major depression: an optimized voxel-based morphometry study. Biological Psychiatry. 2010;67:186–188. doi: 10.1016/j.biopsych.2009.09.014. [DOI] [PubMed] [Google Scholar]