Abstract
Purpose
The aim of the study was to establish whether the duration of anticoagulant (AC) therapy can be tailored, on an objective basis, by using ventilation/perfusion single-photon emission computed tomography (V/P SPECT) and to assess the extent of residual perfusion defects over time. In particular, we addressed the following: (a) is the extent of perfusion recovery at 3 months of initial pulmonary embolism (PE) diagnosis a satisfactory criterion for deciding the duration of oral AC? (b) Is it safe to withdraw AC at 3 months if perfusion recovery is complete?
Patients and methods
Of 269 consecutive patients with suspected PE, 100 patients were diagnosed with PE using V/P SPECT. Sixty-seven patients with acute PE were followed up clinically and with V/P SPECT at 3 months. Sixty-four patients were subject to review and examination using V/P SPECT for a period of 6 months and 33 were followed up only clinically. Therapy was terminated after 3 months if perfusion was normalized, and patients were free of symptoms and the risk of hypercoagulability. Initial extension of PE did not have an impact on decision making.
Results
PE extension varied from 10 to 70% in the acute stage. After 3 months, complete resolution of PE was found in 48 patients. The treating pulmonologist decided to terminate therapy in 35 (73%) patients and to continue AC in 13 patients because of persistent risk factors. Six months later, at the second control stage, 53 patients had complete recovery of pulmonary perfusion. Eleven patients still had perfusion defects at 6 months. No recurrence was identified at 6 months in the 35 patients whose therapy was terminated after 3 months. No bleeding effects were observed in any of the patients during the 6-month follow-up.
Conclusion
This study shows that AC therapy can be tailored, on an objective basis, by using V/P SPECT. Normalization of perfusion at 3 months of initial PE diagnosis was a reliable indicator that AC could be safely withdrawn in patients who were without hypercoagulability risk.
Keywords: duration of anticoagulant therapy, follow-up, pulmonary embolism, ventilation/perfusion single-photon emission computed tomography
Introduction
Pulmonary embolism (PE) affects one in 1000 individuals every year and is one of the most common preventable causes of death among hospital in-patients 1.
PE cannot be diagnosed solely on clinical grounds because symptoms and signs are often nonspecific. However, clinical evaluation is vital in selecting patients for further objective testing. Furthermore, laboratory tests, such as chest radiographs, ECGs or the measurement of D-dimer, may assist in stratification of clinical probability.
To diagnose PE, objective diagnostic imaging is necessary. This has traditionally been carried out with pulmonary angiography, which can visualize intravascular filling defects due to emboli, or with lung scintigraphy, which shows the perfusion defects that are caused by emboli. However, because of its invasiveness and the need for high-level expertise, conventional pulmonary angiography is no longer widely used. Multidetector computed tomographic angiography is a fast procedure used in most centres worldwide to establish a diagnosis of PE. Yet, it is contraindicated in a substantial number of patients as shown in the study by Stein et al. 2 and by Gutte et al. 3. Multidetector computed tomographic angiography has a sensitivity to PE that, in some circumstances, is as low as 78%, which is too low to completely exclude the diagnosis 2. The high radiation dose absorbed in the breast is another major disadvantage 4.
Ventilation/perfusion single-photon emission computed tomography (V/P SPECT) is a recommended technique that establishes the diagnosis in most patients suspected of having PE 3,5–7. PE is excluded when perfusion is normal, whereas it is confirmed in the presence of segmental and/or subsegmental perfusion defects in areas with preserved ventilation (mismatch). Applying holistic interpretation criteria, V/P SPECT can be used to quantify the extent of PE as well as to establish alternative diagnoses 8–10.
The initial treatment of PE consists of unfractionated heparin or low-molecular-weight heparins (LMWH) for at least 5 days, in conjunction with oral anticoagulation (AC) during this period until the international normalized ratio attains the therapeutic range. The optimal duration of AC after PE is still a matter of debate. In general, the duration of treatment is determined by the balance between the subjective assessment of the risk of recurrent PE after discontinuing AC and the risk of AC-related bleeding while on vitamin K antagonists 11. The risk of recurrent PE is deemed low when PE is provoked by a reversible risk factor such as surgery or trauma. Under these circumstances, 3-month AC is regarded as sufficient. Conversely, the risk of recurrence is deemed high when PE is unprovoked or associated with persistent risk factors (e.g. cancer). In this case, prolonged AC (6 months or more) is warranted 12.
The objective of this study was to establish whether the duration of AC therapy can be tailored, on an objective basis, by using V/P SPECT and to assess the extent of residual perfusion defects over time. In particular, we addressed the following questions:
Is the normalized perfusion at 3 months of initial PE diagnosis a satisfactory indicator for deciding the duration of AC therapy?
Is it safe to withdraw AC at 3 months if perfusion recovery is complete in patients without hypercoagulability risk?
Patients and methods
Study protocol
The study was approved by the ethical committee in Sarajevo (Klinički Centar Univerziteta u Sarajevu). All patients signed an informed consent form before being included in the study. A total of 269 consecutive patients with suspected PE were examined from September 2011 to September 2012 at the Department of Nuclear Medicine University Hospital in Sarajevo using V/P SPECT. The referring physicians suspected PE on the basis of relevant clinical symptoms and signs, predisposing risk factors and elevated D-dimer levels (cutoff 0.55 mg/l).
On the basis of the design of the study patients who experienced earlier PE and those who could not be followed up were excluded from the study.
Patients with first time PE and who accepted to participate in the study were included.
Patients with a confirmed diagnosis of PE received AC therapy and were re-evaluated by V/P SPECT 3 months after the initial diagnosis. If V/P SPECT was normalized, AC therapy was discontinued if there were no other medical reasons to continue treatment. In patients with persistent perfusion defects, AC therapy was maintained, and the patients were re-examined by V/P SPECT after a further 3 months of treatment.
All the patients with PE at inclusion were instructed to contact their physicians if they experienced new symptoms suggesting a recurrent episode of PE. Bleeding events while on AC therapy were also recorded.
Quantitative V/P SPECT
V/P SPECT is performed as a 1-day protocol starting with inhalation of aerosolized 99mTc-Technegas (Cyclomedica Ltd, Lucas Heights, New South Wales, Australia) while in a supine position, until about 30 MBq has reached the lung. Immediately thereafter, and without patient movement, 120 MBq 99mTc-MAA (TechneScan LyoMAA; Mallinckrodt Medical BV, Petten, the Netherlands) is given intravenously for the perfusion study. Patients carefully maintain their supine position during V/P acquisition. Immobilization lasting 20 min is usually well tolerated even by critically ill patients. The methodology has been described in full elsewhere 8,13. Quantification of PE was made by counting segments or subsegments showing a complete or relative mismatch, and expressing this figure as a percentage of total lung parenchyma 5,14–17. A segmental reduction or a subsegmental total deficiency of function was attributed one point, and segmental total deficiency was attributed two points. Each lung comprises nine segments, representing 18 points. Mismatch defects were expressed as mismatch points, which after division by 36 give the percentage of the lung that is embolized. All regions with V/P defects were calculated to estimate the reduction in total lung function. Recurrent PE was defined if a new perfusion defect was objectively visualized by V/P SPECT in a patient at one of the control stages.
Statistical analysis
Differences between groups were assessed by Fisher’s exact test for categorical variables and by Mood’s median test for continuous variables. The Wilcoxon matched pairs signed-rank test was used to compare PE extent between initial examination and 3 and 6 months’ follow-up. Continuous variables in the text and in the tables are reported as median and interquartile range.
Results
Sample
PE was diagnosed by V/P SPECT in 37% (100/269) of the cases. Patients with PE did not differ significantly from those without PE as regards age, sex or prevalence of most comorbid conditions. However, patients with PE showed significantly higher incidence of symptoms such as acute dyspnoea, chest pain and syncope compared with those without. Of the 169 patients in whom PE was excluded by V/P SPECT, 95 had normal V/P SPECT and 74 had V/P abnormalities consistent with disorders other than PE, such as pneumonia, tumours, empyema, chronic obstructive pulmonary disease and left heart failure. The flow chart in Fig. 1 summarizes the number of patients included, treatment time, number of completed V/P SPECT examinations and results from V/P SPECT.
Fig. 1.
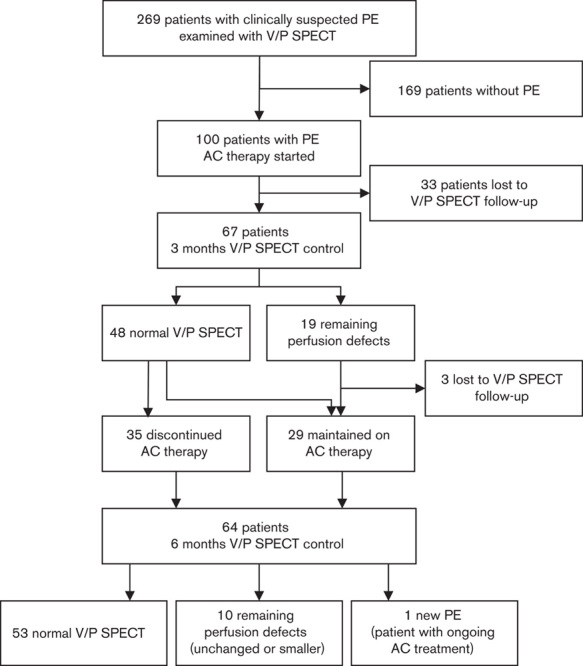
Flow chart illustrating the number of patients in the study, results from V/P SPECT follow-up examinations and AC therapy duration. AC, anticoagulant; PE, pulmonary embolism; V/P SPECT, ventilation/perfusion single-photon emission computed tomography.
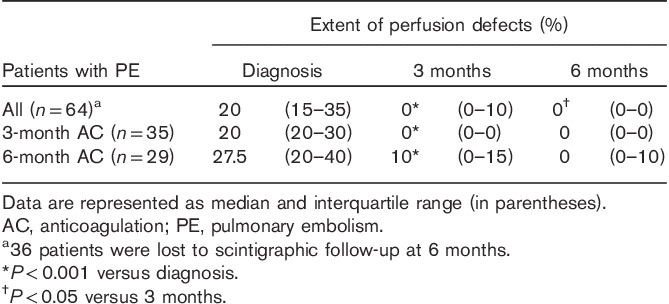
The median extent of the perfusion defects at the time of PE diagnosis was 20% (interquartile range, 15–35%) (Table 1). Of the 100 patients with PE, one of the patients received unfractionated heparin, and the remaining 99 patients received LMWH.
Table 1.
Extent of perfusion defects at diagnosis and after 3 and 6 months of AC therapy
Follow-up
Patients lost to V/P SPECT follow-up
As shown in Fig. 1, 36 patients were lost to V/P SPECT follow-up, three of them not until the 6-month control. The reason was that 20 patients moved away from Sarajevo, three were psychiatrically ill, three became pregnant, three died and seven decided not to participate in the study on grounds of inconvenience. The 20 patients who left Sarajevo were all followed up clinically and by telephone contact. All 20 patients were treated with AC therapy for at least 6 months. No adverse events were recorded among these 20 patients. The cause of death in the three patients who died was cardiac disease, unrelated to the PE event. The three pregnant women stayed on LMWH throughout their pregnancy.
Patients with completed V/P SPECT follow-up
Sixty-four of the patients with PE completed the 6-month period of scintigraphic follow-up. The timing of perfusion recovery for all patients is displayed in Fig. 2 and in Table 1.
Fig. 2.
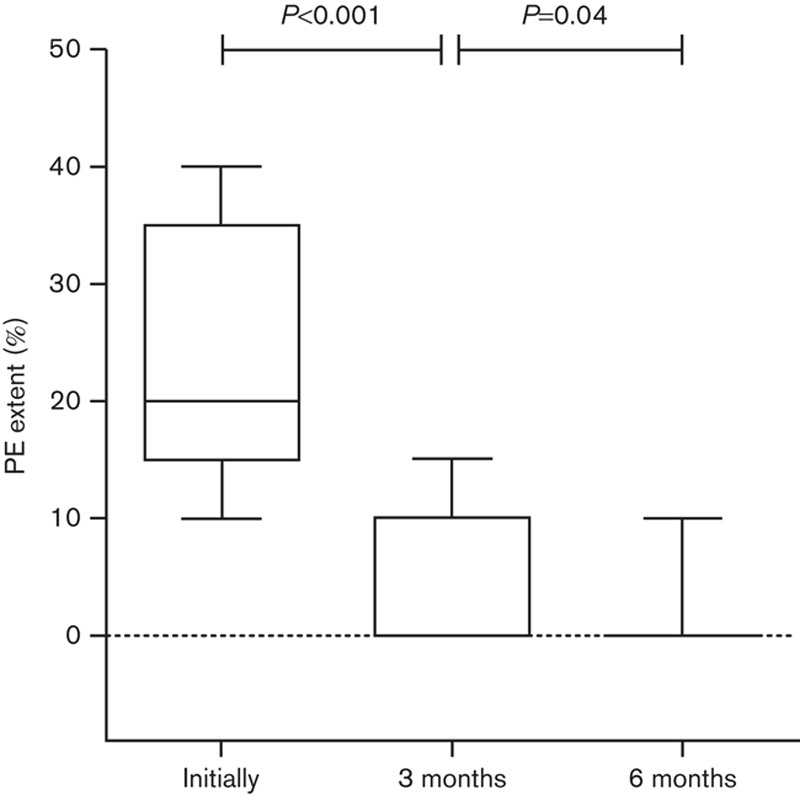
Box and whiskers plot of PE extent at the time of diagnosis and PE extent after 3 and 6 months for all patients. Whiskers represent the 10–90% percentile. PE, pulmonary embolism.
AC treatment was discontinued after 3 months in 35 (73%) of the 48 patients with normal perfusion (Fig. 1). None of these 35 patients had recurrent episodes of PE following withdrawal of AC.
Thirteen patients with normal perfusion were kept on AC therapy. In three of them AC was maintained due to hereditary thrombophilia (mutations in factor V Leiden or prothrombin gene). In the 10 other patients, the reasons for prolonged AC were incomplete recanalization of lower limb deep vein thrombosis (n=5) or immobilization due to severe trauma (n=5).
The 16 patients who had residual perfusion defects on V/P SPECT at 3 months after initial diagnosis were also kept on AC for at least 3 more months. However, one patient who received 6 months’ treatment had an additional PE event observed at 6 months’ follow-up.
Fifty-three patients showed complete recovery of pulmonary perfusion at 6 months. All patients with normal perfusion at this time and no persistent risk factors for recurrent PE stopped therapy. Eleven patients who had persistent perfusion defects at the end of the follow-up period were kept on indefinite AC therapy.
No bleeding events were observed in any of patients during the 6 months’ follow-up.
The extent of perfusion defects over time in the patients who were treated for 3 and 6 months is shown in Table 1. No significant difference in initial PE extent was found in patients treated for 3 months compared with those treated for 6 months (P=0.08).
Figure 3 shows V/P SPECT images of a patient with PE who had residual defects during the 6-month follow-up period. Figure 4 shows a patient in whom the perfusion returned to normal after an episode of acute PE.
Fig. 3.
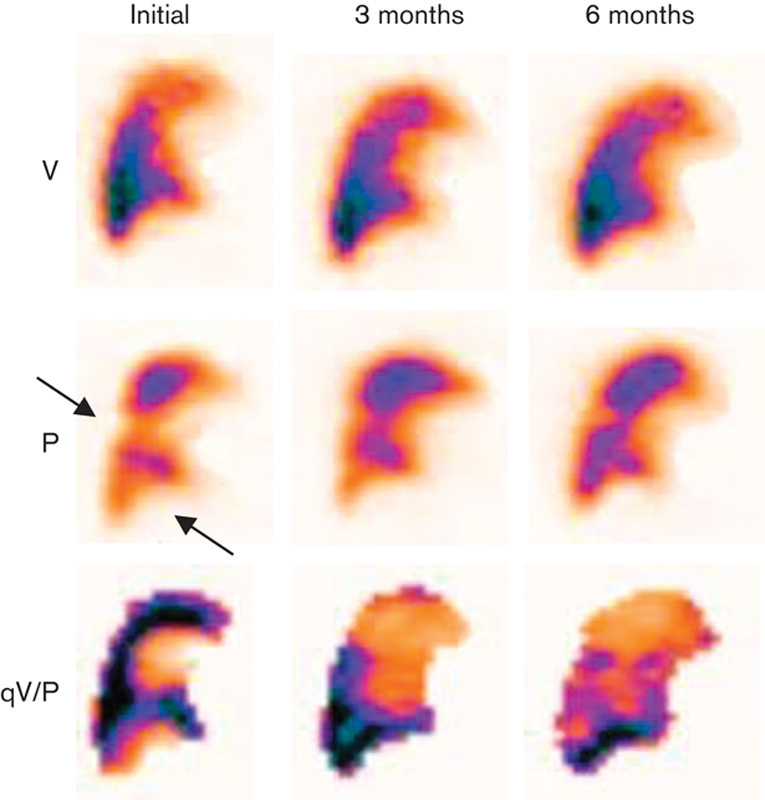
Sagittal V/P SPECT images of a patient with PE (arrow) at initial examination. Only partial regression of the perfusion defects is seen during the follow-up period and there remain perfusion defects at 6 months. P, perfusion; PE, pulmonary embolism; qV/P, V/P quotient; V, ventilation; V/P SPECT, ventilation/perfusion single-photon emission computed tomography.
Fig. 4.
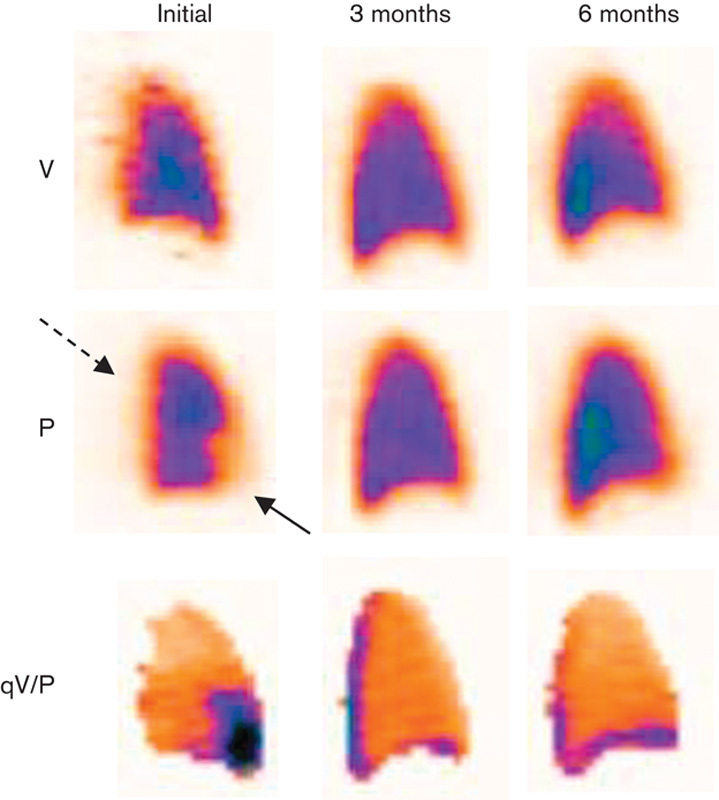
Sagittal V/P SPECT images of a patient with PE (arrow) and pleural fluid (intercepted arrow) at initial examination. Perfusion returns to normal at 3 months and remains normal at 6 months’ control. P, perfusion; PE, pulmonary embolism; qV/P, V/P quotient; V, ventilation; V/P SPECT, ventilation/perfusion single-photon emission computed tomography.
Discussion
This prospective study included patients with first episode of PE who underwent clinical and V/P SPECT follow-up. The objective was to establish whether the duration of AC therapy can be tailored, on an objective basis, by using V/P SPECT and to assess the extent of residual perfusion defects over time.
Complete resolution of PE occurred in 72% of the patients at the end of 3 months – that is, at the end of the first control stage. AC therapy was discontinued in these patients unless they had potential predisposing factors for PE recurrence, such as incomplete recanalization of deep vein thrombosis, prolonged immobilization or documented thrombophilia.
The proportion of patients observed in this study with normal perfusion after 3 months is in keeping with an earlier report in which as many as 70% of the sampled individuals had normal pulmonary perfusion at 3 months of PE diagnosis 15. In that study patients with perfusion defects at 3 months still had persistent perfusion defects at 6 months 15. In the present report five (35%) of 14 patients improved further at 6 months but none of them had perfusion defects larger than 15% at 3 months’ V/P SPECT control. One patient with residual perfusion defects had recurrent PE during follow-up.
By using lung scintigraphy, it was shown that perfusion improved in 78% of patients treated with LMWH after only 5 days regardless of initial PE extent 18. The average reduction of PE extent was 44%. Similar results were observed after treatment of acute PE with intravenous heparin for 7 days 18. A fast resolution of the embolic burden reflects an efficient thrombolytic capacity in most patients. However, the individual ability to lyse clots may differ markedly between patients. Anticoagulant therapy brings about bleeding complications in up to 5% of patients yearly 18. Thus, there is a need to achieve a better balance between therapeutic benefits and risk based on the time-course of PE resolution.
In the retrospective study by Alhadad et al. 14, PE recurrence was frequent in patients with residual perfusion defects. These findings underscore the importance of scintigraphic follow-up in patients with an established diagnosis of PE. In a study by Wartski et al. 18, the proportion of patients with residual perfusion defects at 3 months of PE diagnosis was higher than that in our study. Similarly, Miniati et al. 19 reported that pulmonary perfusion is completely restored in 65% of the patients who survived 1 year after acute, massive PE. These differences may reflect the baseline characteristics of the patients included in the studies.
Our study addresses the issue of what is the optimal duration of AC by incorporating V/P SPECT in follow-up. In our opinion, V/P SPECT is the imaging modality of choice as it carries a substantially lower radiation burden as compared with MD-CT. In addition, V/P SPECT is sensitive enough to detect small residual perfusion defects that are often missed on MD-CT.
Moreover, the study confirmed our earlier results of the importance of scintigraphically following up PE patients, not only to adjust the duration of AC but also to identify those patients who feature persistent, large perfusion defects and might be at risk of developing chronic thromboembolic pulmonary hypertension 18. In other words, the method had multiple benefits that other existing procedures do not, with potentially fatal consequences 18.
Study limitations
We acknowledge that our study has limitations. First, the sample is relatively small and originates from a single referral centre. Second, the follow-up period was limited to the 6 months following the initial diagnosis. Third, sequential V/P SPECT studies could not be obtained in all the patients with established PE. Therefore, our findings should be confirmed on a bigger cohort and preferably in broader multicentre studies.
Conclusion
Normalization of perfusion at 3 months of PE diagnosis was a reliable indicator that AC could be safely withdrawn in the case of patients who were without hypercoagulability risk. Tailoring AC treatment is feasible by incorporating V/P SPECT in the clinical decision tree. The short-term regimen appears safe whenever pulmonary perfusion is normal after 3 months of the embolic event in the absence of persistent risk factors for PE recurrence. The first results indicate that V/P SPECT can be successfully used for follow-up and for tailoring AC therapy after acute PE. The results merit further study.
Acknowledgements
The authors acknowledge the support from the Cantonal Ministry of health, Sarajevo. This study was partly financed by the Region of Scania (ALF and SUS funds), Sweden.
Authors’ contributions: A.B. carried out the study and interpreted the results. E.O. contributed to data acquisition and analysis and provided administrative support. V.C. and M.R. were the clinicians responsible for patient care. A.B. interpreted the results. M.M. was involved in study design and in critical revision of the manuscript. J.J. contributed to the study design, statistical analysis and manuscript drafting. M.B. designed the study and drafted the manuscript. All authors read and approved the final version of the manuscript.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Silverstein MD, Heit JA, Mohr DN, Petterson TM, O’Fallon WM, Melton LJ., 3rd Trends in the incidence of deep vein thrombosis and pulmonary embolism: a 25-year population-based study. Arch Intern Med 1998; 158:585–593. [DOI] [PubMed] [Google Scholar]
- 2.Stein PD, Fowler SE, Goodman LR, Gottschalk A, Hales CA, Hull RD, et al. PIOPED II Investigators. Multidetector computed tomography for acute pulmonary embolism. N Engl J Med 2006; 354:2317–2327. [DOI] [PubMed] [Google Scholar]
- 3.Gutte H, Mortensen J, Jensen CV, Johnbeck CB, von der Recke P, Petersen CL, et al. Detection of pulmonary embolism with combined ventilation-perfusion SPECT and low-dose CT: head-to-head comparison with multidetector CT angiography. J Nucl Med 2009; 50:1987–1992. [DOI] [PubMed] [Google Scholar]
- 4.Hurwitz LM, Yoshizumi TT, Reiman RE, Paulson EK, Frush DP, Nguyen GT, et al. Radiation dose to the female breast from 16-MDCT body protocols. Am J Roentgenol 2006; 186:1718–1722. [DOI] [PubMed] [Google Scholar]
- 5.Bajc M, Olsson B, Palmer J, Jonson B. Ventilation/perfusion SPECT for diagnostics of pulmonary embolism in clinical practice. J Intern Med 2008; 264:379–387. [DOI] [PubMed] [Google Scholar]
- 6.Leblanc M, Leveillée F, Turcotte E. Prospective evaluation of the negative predictive value of V/Q SPECT using 99mTc-Technegas. Nucl Med Commun 2007; 28:667–672. [DOI] [PubMed] [Google Scholar]
- 7.Lemb M, Pohlabeln H. Pulmonary thromboembolism: a retrospective study on the examination of 991 patients by ventilation/perfusion SPECT using Technegas. Nuklearmedizin 2001; 40:179–186. [PubMed] [Google Scholar]
- 8.Bajc M, Olsson C-G, Palmer J, Jonson B.Freeman LM. Quantitative ventilation/perfusion SPECT (QV/P SPECT): a primary method for diagnosis of pulmonary embolism. Nuclear medicine annual 2004Philadelphia: Lippincott Williams & Wilkins;173–186. [Google Scholar]
- 9.Jögi J, Ekberg M, Jonson B, Bozovic G, Bajc M. Ventilation/perfusion SPECT in chronic obstructive pulmonary disease: an evaluation by reference to symptoms, spirometric lung function and emphysema, as assessed with HRCT. Eur J Nucl Med Mol Imaging 2011; 38:1344–1352. [DOI] [PubMed] [Google Scholar]
- 10.Jögi J, Palmer J, Jonson B, Bajc M. Heart failure diagnostics based on ventilation/perfusion single photon emission computed tomography pattern and quantitative perfusion gradients. Nucl Med Commun 2008; 29:666–673. [DOI] [PubMed] [Google Scholar]
- 11.Savina EN, Couturaud F. Optimal duration of anticoagulation of venous thromboembolism. J Mal Vasc 2011; 36Suppl 1S28–S32. [DOI] [PubMed] [Google Scholar]
- 12.Boutitie F, Pinede L, Schulman S, Agnelli G, Raskob G, Julian J, et al. Influence of preceding length of anticoagulant treatment and initial presentation of venous thromboembolism on risk of recurrence after stopping treatment: analysis of individual participants’ data from seven trials. BMJ 2011; 342:d3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Palmer J, Bitzén U, Jonson B, Bajc M. Comprehensive ventilation/perfusion SPECT. J Nucl Med 2001; 42:1288–1294. [PubMed] [Google Scholar]
- 14.Alhadad A, Miniati M, Alhadad H, Gottsäter A, Bajc M. The value of tomographic ventilation/perfusion scintigraphy (V/P SPECT) for follow-up and prediction of recurrence in pulmonary embolism. Thromb Res 2012; 130:877–881. [DOI] [PubMed] [Google Scholar]
- 15.Begic A, Jögi J, Hadziredzepovic A, Kucukalic-Selimović E, Begovic-Hadzimuratovic S, Bajc M. Tomographic ventilation/perfusion lung scintigraphy in the monitoring of the effect of treatment in pulmonary embolism: serial follow-up over a 6-month period. Nucl Med Commun 2011; 32:508–514. [DOI] [PubMed] [Google Scholar]
- 16.Olsson CG, Bitzén U, Olsson B, Magnusson P, Carlsson MS, Jonson B, Bajc M. Outpatient tinzaparin therapy in pulmonary embolism quantified with ventilation/perfusion scintigraphy. Med Sci Monit 2006; 12:PI9–P13. [PubMed] [Google Scholar]
- 17.Baile EM, King GG, Müller NL, D’Yachkova Y, Coche EE, Paré PD, Mayo JR. Spiral computed tomography is comparable to angiography for the diagnosis of pulmonary embolism. Am J Respir Crit Care Med 2000; 161Pt 11010–1015. [DOI] [PubMed] [Google Scholar]
- 18.Wartski M, Collignon MA. Incomplete recovery of lung perfusion after 3 months in patients with acute pulmonary embolism treated with antithrombotic agents. THESEE Study Group. Tinzaparin ou Heparin Standard: Evaluation dans l’Embolie Pulmonaire Study. J Nucl Med 2000; 41:1043–1048. [PubMed] [Google Scholar]
- 19.Miniati M, Monti S, Bottai M, Scoscia E, Bauleo C, Tonelli L, et al. Survival and restoration of pulmonary perfusion in a long-term follow-up of patients after acute pulmonary embolism. Medicine (Baltimore) 2006; 85:253–262. [DOI] [PubMed] [Google Scholar]


