Abstract
Objective
In patients with chronic hepatitis B virus (HBV) infection, it is not known whether altered serum iron markers are directly because of the infection or the associated liver injury. We determined the serum iron status of patients with chronic HBV infection, and investigated whether it is HBV infection or HBV-related liver injury that likely causes abnormal serum iron markers in chronic HBV infection.
Materials and methods
For a retrospective study, chronic HBV-infected patients (80 patients with cirrhosis and 76 patients without cirrhosis) and 58 healthy controls were enrolled. Serum alanine transaminase levels were measured to ascertain liver damage. Indicators of iron status included serum iron, ferritin, and transferrin.
Results
Compared with noncirrhotic patients and healthy controls, the serum transferrin of cirrhotic patients was lower and the serum iron and ferritin values were higher (P<0.001, all). In cirrhotic patients, the serum iron and ferritin levels correlated positively with serum alanine transaminase levels and the transferrin levels were inversely related to both end-stage liver disease scores and iron levels (all P<0.01).
Conclusion
Serum iron markers tended to be aberrant in chronic HBV-infected patients with cirrhosis. The liver injury associated with HBV infection, but not chronic HBV infection directly, is likely the main cause for iron metabolism disorder.
Keywords: ferritin, liver cirrhosis, liver injury, serum iron, transferrin
Introduction
Iron is crucial to humans’ biological functions and cellular biochemical processes 1,2. Both iron deficiency and iron overload pose significant and potentially fatal health risks, and the homeostasis of iron is tightly regulated. When this regulation is disrupted, for example because of hereditary hemochromatosis, excessive absorption of iron by the intestine leads to cellular toxicity, tissue injury, and organ fibrosis. Such harmful effects are mediated through the deposition of iron in parenchymal cells of a number of vital organs, including the heart, pancreas, and liver. Iron-generated oxyradicals contribute toward the peroxidation of lipid membranes, leading to organelle fragility and cellular toxicity.
The liver is a major storage organ for iron. Approximately one-third of the body’s total iron is deposited in hepatocytes, sinusoidal mesenchymal cells, and reticuloendothelial cells 3,4. The liver also plays a fundamental role in recycling iron as the organ synthesizes both transferrin (the main transporting protein) and ferritin (the major storage protein). Hepatic injury and dysfunction can disturb iron homeostasis. Excessive iron deposition in the liver leads to further injuries by triggering hepatocellular necrosis 5, inflammation 6, fibrosis 7,8, and even carcinoma 9. Many experimental and clinical studies suggest that chronic iron deposition promotes the progression of liver damage and increases the risk of fibrosis, cirrhosis, and hepatocellular carcinoma in chronic hepatitis C patients 10–13. Furthermore, some studies suggest that excess iron in the liver may induce adverse effects on patients’ response to antiviral therapy for chronic hepatitis C 14,15.
Data on iron metabolism and its clinical significance are relatively scarce in hepatitis B virus (HBV)-related liver disease. Blumberg et al. 16 were the first to describe an association between changes in iron levels and hepatitis B infection in 1981. They reported that 33 patients who were persistent HBV carriers had significantly higher levels of serum iron than 34 patients who cleared the viral infection. Their data implied that high levels of serum iron may be related to the presence of chronic HBV infection. However, they did not report serum ferritin or transferrin levels, or total iron-binding capacity 16. More recently, Martinelli et al. 17 found elevated transferrin saturation and increased liver iron deposition in 27.1 and 48.7%, respectively, of HBV patients investigated. Yonal et al. 18 reported elevated serum transferrin saturation and ferritin concentration in patients with HBV-related liver disease. Ohkoshi et al. 19 suggested that a successful treatment with lamivudine may correlate with reduction in serum ferritin levels in patients with chronic hepatitis and liver cirrhosis type B.
Conclusive data on the prevalence and clinical significance of disturbed iron metabolism in patients with HBV-related cirrhosis are still lacking. Especially, it remains unclear whether changed serum iron markers observed in HBV infection are related to HBV infection or to liver injury that is associated with chronic HBV infection. In this study, we determined the serum iron status of patients with HBV-related cirrhosis and investigated whether it is the direct HBV infection or HBV-related liver injury that leads to changes in serum iron markers.
Materials and methods
Participants
The Ethics Committee of the First Affiliated Hospital of Zhejiang University College of Medicine approved all procedures, and the study was carried out according to the Declaration of Helsinki. Each study participant provided written informed consent before enrollment. The chronic hepatitis B (CHB) diagnosis was made in accordance with the criteria recommended by the Viral Hepatitis Management Guidelines, which were adopted by the Chinese Society of Infectious Diseases and Parasitology, and the Chinese Society of Hepatology, of the Chinese Medical Association 20 in the 2000 Xi’an Conference. Briefly, CHB is diagnosed when an HBV carrier experiences a clinical course of hepatitis B infection for more than 6 months, and may have shown symptoms or signs of hepatitis and abnormal hepatic function, or with histological changes. Patients with any of the following were excluded from the study: acute hepatitis; hematologic disorders; malignancies such as hepatocellular carcinoma; pregnancy; on-going steroid or pegylated interferon or nucleoside analog therapy; homozygous or compound heterozygous hemochromatosis (HFE) gene mutations; concurrence of hepatitis C virus, hepatitis D virus, hepatitis G virus, HIV infection, or autoimmune or other liver diseases; alcohol abuse (>60 g/day) or biochemical or histological features of alcoholic liver disease; or large-volume blood transfusion (>6 U). There were no exclusions on the basis of age or sex. Patients with chronic HBV infection (n=156) who were referred to the Clinic of Liver Diseases between July 2012 and December 2013 and fulfilled the above criteria were enrolled in this study. Eighty of the 156 patients were diagnosed with HBV-related cirrhosis and the remaining 76 patients were chronically HBV-infected, but noncirrhotic. All patients received standard medical treatments including energy supplements, intravenous infusion of albumin and plasma, and preventive treatment of complications in accordance with the Strategy for Prevention and Therapy of Viral Hepatitis reported in 2000 20. All the patients were positive for hepatitis B surface antigen (HBsAg) for at least 6 months and all patients were positive for HBV DNA.
Healthy controls
A group of 58 healthy controls (HCs) were also included in the study that included healthcare staff with no known liver disease and no HBV infection. Each volunteer consumed less than 20 g/day of alcohol, and had normal iron indices and liver function tests at the time of evaluation. The timing of blood collection for HCs was the same as that for patients.
Laboratory analysis
Within 24 h after admission, after an overnight fast, blood samples were collected from all patients. Blood samples were taken from the 58 HCs at the time of recruitment. Serum iron indices (ferritin, iron, and transferrin) were measured for each participant. Serum iron was tested using an automated Roche analyzer system (Roche Diagnostic, Mannheim, Germany). Serum ferritin was determined using an electrochemiluminescence assay (Abbott Laboratories, Chicago, Illinois, USA) and serum transferrin using an immunoturbidimetric method (Roche Diagnostic). Serum albumin, total bilirubin, and alanine transaminase (ALT) levels were measured using a Hitachi 704 Analyzer (Roche Diagnostic). The ALT upper limit of normal is 40 U/l. International normalized ratios (INRs) were measured using a Sysmex CA1500 full-automatic analyzer (Sysmex Corp, Kobe, Japan). HBV serological markers (HBsAg and HBeAg) were assayed using commercial enzyme immunoassay kits (Abbott Laboratories). Serum HBV DNA levels were determined using a fluorescence quantitative PCR method (PG, Shenzhen, China). Patients with serum HBV DNA greater than 500 IU/ml were referred to as HBV DNA positive.
The diagnosis of cirrhosis was supported by liver biopsy in 40 patients (50%). The diagnosis in the remaining 40 patients (50%) was made on the basis of clinical (e.g. physical stigmata of cirrhosis) or biochemical manifestations (decreased serum albumin and increased serum globulin), and imaging findings of ultrasonography or computed tomography (nodular liver surface, coarsened echogenicity of liver parenchyma, enlarged spleen, or ascites). At the same time, 26 of 76 patients with noncirrhosis were diagnosed histologically and the remaining patients by clinical, endoscopic, or ultrasound evaluation. Baseline demographic and clinical characteristics were collected, including the model for end-stage liver disease (MELD) score, in which higher scores reflect more severe liver disease.
Molecular analysis of HFE gene mutations
All patients were investigated for C282Y and H63D mutations of HFE. Cellular DNA was extracted from peripheral blood leukocytes for PCR, cleaved by restriction enzymes RsaI or BclI (for C282Y and H63D mutation analyses, respectively), and visualized by agarose gel migration (State Key Laboratory, Zhejiang University, China). Primers and PCR conditions have been described previously 21.
MELD score
The severity of liver disease was evaluated by the MELD score, which uses the patient’s serum bilirubin and creatinine levels and the INR for prothrombin time to predict survival. The MELD score was calculated using the web site calculator (http://www.mayoclinic.org/gi-rst/mayomodel7.html).
Statistical analyses
All continuous variables are expressed as the mean±SD or median (range). Categorical data are reported as percentages. Differences in variables were analyzed using analysis of variance and Student’s t-tests (for normally distributed data) or the Kruskal–Wallis and Mann–Whitney U-tests (for non-normally distributed data). The χ2-test or Fisher’s exact test was used for categorical data, as appropriate. Correlations between variables were examined using Spearman’s correlation. Statistical analyses were performed using the statistical package SPSS version 12.0 (SPSS Inc., Chicago, Illinois, USA), and the level of statistical significance was set at a P value less than 0.05.
Results
Baseline characteristics of the study population
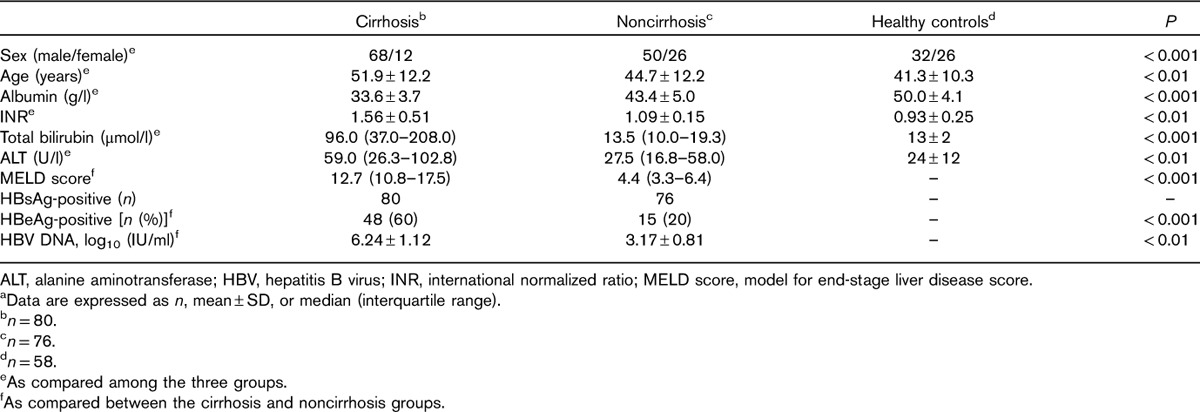
A total of 156 chronic HBV-infected patients (80 cirrhotic and 76 noncirrhotic) and 58 HCs were recruited into this study. The study population ranged in age from 22 to 71 years, mean 46 years. Compared with HCs and noncirrhotic patients, patients with cirrhosis tended to be older men, more likely to have severe liver disease, lower levels of serum albumin, and higher INR, total bilirubin, ALT, and MELD scores. The baseline characteristics are shown in Table 1.
Table 1.
Clinical characteristics of the participantsa studied
To exclude the possibility of skewed data for serum iron that could be because of sex, we first determined whether there was any difference in the serum iron levels between men and women. No significant differences in serum iron levels were observed between men and women in the three groups. Specifically, in the HC group, the serum iron levels of men (9.62±2.15 μmol/l, n=32) and women (9.65±2.16 μmol/l, n=26) were similar (P=0.361). In the CHB group, the serum iron levels of men (26.32±8.91 μmol/l, n=50) and women (24.42±9.01 μmol/l, n=26) were similar (P=0.526). In the cirrhosis group, the serum iron levels of men (29.74±11.6 μmol/l, n=68) and women (28.82±10.43 μmol/l, n=12) were similar (P=0.392).
Serum iron markers in patients with and without cirrhosis and HCs
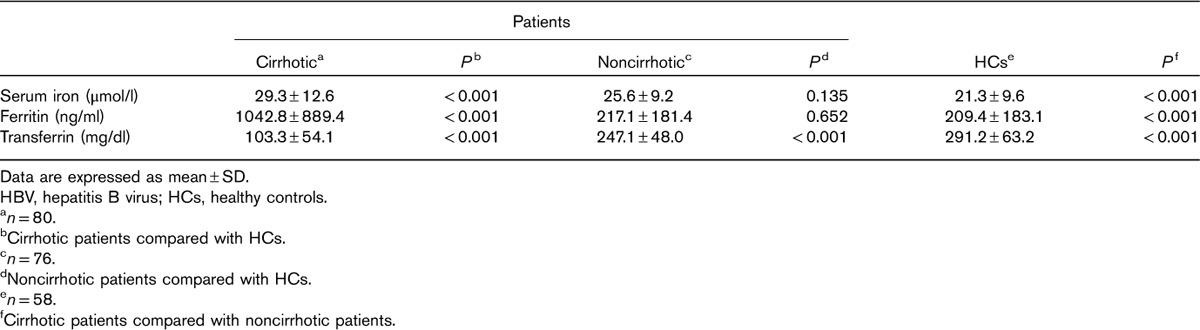
The serum transferrin of the cirrhotic patients was significantly lower than that of the HCs and noncirrhotic patients (P<0.001, all; Table 2). Noncirrhotic patients had lower serum transferrin values than HCs (P<0.001). Serum iron concentrations and ferritin values of the cirrhotic patients were higher than those of the noncirrhotic patients and HCs (P<0.001, all). However, between the noncirrhotic patients and HCs, there was no significant difference in serum iron concentrations (P=0.135) or ferritin values (P=0.652).
Table 2.
Iron-associated parameters measured in cirrhotic and noncirrhotic HBV-infected patients and HCs
Associations between iron markers, serum ALT levels, and MELD scores
To distinguish a potential link between iron markers and chronic HBV infection from that of iron markers and HBV-related liver injury, we first analyzed whether serum iron, ferritin, or transferrin levels were related to ALT in HBV-infected patients (Fig. 1). In cirrhotic patients, serum iron and ferritin levels correlated positively with serum ALT levels (serum iron: r=0.496, P<0.001; ferritin: r=0.402, P<0.001). Transferrin levels were inversely related to MELD scores (r=–0.395, P<0.001), although there was no correlation between transferrin levels and ALT (r=–0.084, P=0.416). Further analysis showed a negative association between transferrin and serum iron levels in these patients (r=–0.309, P=0.007). In noncirrhotic patients, there was no correlation between serum iron and transferrin levels and ALT levels, and transferrin showed no correlation with MELD scores, but ferritin was correlated positively with serum ALT (r=0.353, P<0.001; data not shown).
Fig. 1.
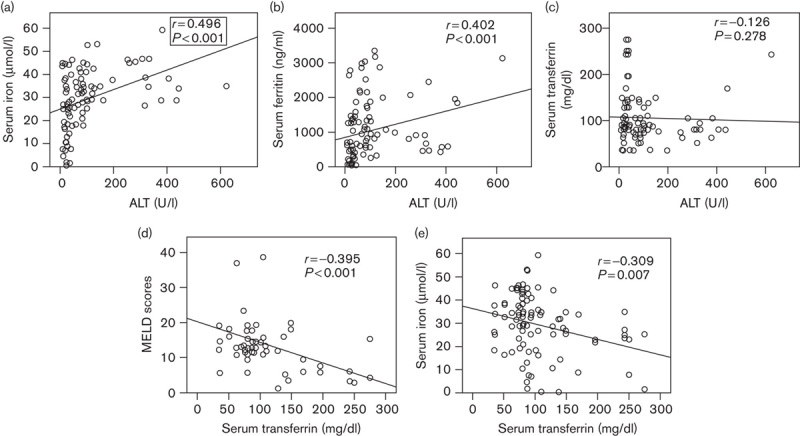
Correlations among serum parameters in patients with HBV-related cirrhosis. Serum concentrations of (a) iron, (b) ferritin, (c) transferrin, and (d) MELD scores correlated with ALT levels; (e) correlation between iron and transferrin. ALT, alanine aminotransferase; HBV, hepatitis B virus; MELD score, model for end-stage liver disease score.
Comparison of serum iron parameters in cirrhotic patients with different ALT levels
We stratified cirrhotic patients by ALT levels: low (<40 U/l) and high (≥40 U/l; Fig. 2). Compared with cirrhotic patients with low ALT, those with high ALT had significantly higher levels of serum iron (P<0.001), ferritin values (P<0.001), and MELD scores (P<0.035). By contrast, the serum transferrin levels of the high-ALT patients were significantly lower than those of the low-ALT patients (P<0.001).
Fig. 2.
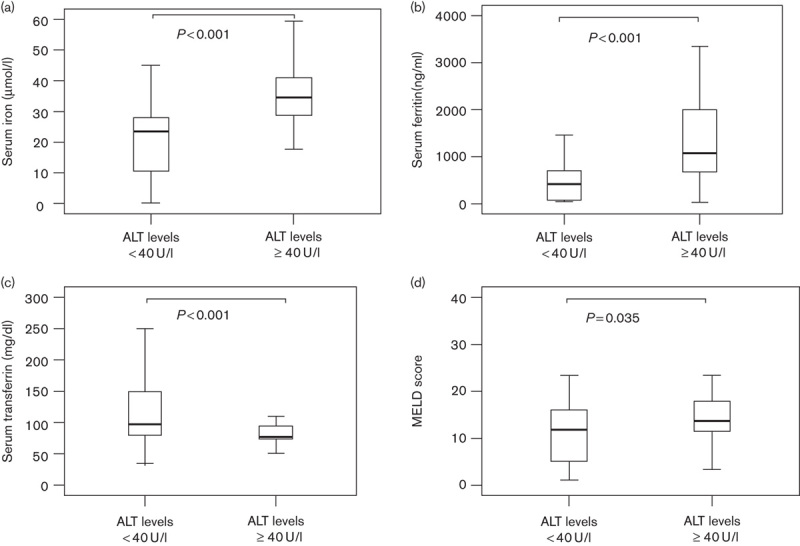
Comparison of iron-associated parameters in cirrhotic patients according to low and high ALT levels. Serum concentrations of (a) iron, (b) ferritin, (c) transferrin, and (d) MELD scores in cirrhotic patients and compared with low (<40 U/l) and high (≥40 U/l) ALT levels. Data are expressed as median and interquartile range. ALT, alanine aminotransferase; MELD score, model for end-stage liver disease score.
Discussion
Abnormal serum iron markers have been observed in chronic HBV infection 16–19, but earlier findings seemed to suggest that altered serum iron levels were associated with chronic HBV infection 16,17. Chronic HBV infection may be or may not be accompanied by liver injury, whereas altered iron metabolism could be a reflection of pathological changes in the organs, such as the liver, involved in iron metabolism. This raises the question of whether the altered iron level is associated with direct HBV infection or the related liver injury. In this study, we determined the serum iron status of patients with chronic HBV, and investigated whether it is direct HBV infection or HBV-related liver injury that leads to changes in serum iron markers.
In this study, our results showed that in HBV-related cirrhotic patients compared with noncirrhotic patients and the HCs, serum transferrin was significantly lower and serum iron was significantly higher. We also observed a higher level of serum ferritin in cirrhotic patients, which is in agreement with the results of Yonal et al. 18 and Wu et al. 22. However, other authors did not observe alterations in serum iron levels 23, or they reported a reduction, in liver cirrhosis and hepatocellular carcinoma patients 24. These discrepancies may be because of the differences in stages of liver diseases among the patients in the various studies.
Our results address two unresolved issues in understanding abnormal iron metabolism in chronic HBV infection. First, why were the changes in the serum iron markers observed in HBV cirrhotic patients? Furthermore, is HBV infection or HBV-related liver injury more directly responsible for alterations in serum iron markers? Our cirrhotic patients had on-going liver injury, as evidenced by elevated ALT levels, whereas the noncirrhotic patients did not. By examining the associations between ALT levels and serum iron markers in cirrhotic patients, we found that serum iron and ferritin levels correlated positively with ALT levels. However, there was no correlation between ALT levels and serum iron and transferrin levels in noncirrhotic patients, suggesting the concurrence of liver injury and elevated serum iron and ferritin levels. The liver is an important organ in iron homeostasis. Hepatic inflammation and dysfunction may interfere with iron metabolism (including synthesis and clearance). Liver injury can result in the release of more ferritin into plasma and reduce the production of transferrin, leading to lower serum transferrin levels. This in turn will reduce the transport of iron to the liver, erythrocytes, or both and increase serum iron. The severity of liver dysfunction can be quantified by MELD scores. In the present study, transferrin levels correlated negatively with the MELD scores and serum iron levels of cirrhotic patients. This supports our reasoning that it is liver injury that causes reduced serum transferrin levels. Furthermore, serum iron increased with MELD scores. Therefore, increased hepatic necroinflammatory activity accompanied by worsening liver function may be one explanation for aberrant serum iron parameters in cirrhotic patients. Our data clearly indicate that HBV-related liver injury, but not direct chronic HBV infection, is likely responsible for the changes in the serum iron markers.
Indeed, several studies have shown that removing excess iron through therapeutic phlebotomy reduces the severity of hepatic inflammation associated with chronic HCV infection 10. In addition, Bayraktar et al. 25 reported that desferrioxamine infusion to achieve a normal serum ferritin level increased the likelihood of a favorable response to interferon in patients with chronic hepatitis B. Accordingly, routine monitoring of serum iron and other iron-associated parameters during clinical management of chronic HBV infection will be helpful in understanding alterations in iron metabolism in HBV and their influence on further liver injury. Elucidation of the association between abnormal serum iron and liver injury may suggest an additional therapeutic approach, such as iron-removal therapy, that could improve the overall efficacy and outcomes of current management of chronic HBV infection with liver injury.
Our data only indicate, but not yet prove, the causal relationships between serum iron markers and liver injury in HBV-infected patients. We do not have serum samples available for analysis of other biochemical markers such as aspartate aminotransferase and serum lactate dehydrogenase for liver injury and this was a single-center study; the sample size was relatively small. The current findings need to be confirmed in a large prospective multicenter study.
Conclusion
We found that in HBV-infected patients with cirrhosis, serum transferrin levels were lower and serum iron and ferritin were higher compared with both noncirrhotic HBV patients and HCs. We conclude that the main cause of iron metabolism disorder in cirrhotic HBV-infected patients is liver injury, and most likely not chronic HBV infection directly.
Acknowledgements
Conflicts of interest
There are no conflicts of interest.
References
- 1.Tavill AS.Schiff L, Schiff ER. Hemochromatosis. Diseases of the liver. Philadelphia: JB Lippincott; 1993. pp. 669–691. [Google Scholar]
- 2.Anderson GJ. Things that go BMP in the liver: bone morphogenetic protein 6 and the control of body iron homeostasis. Hepatology 2009; 50:316–319. [DOI] [PubMed] [Google Scholar]
- 3.Franchini M, Targher G, Capra F, Montagnana M, Lippi G. The effect of iron depletion on chronic hepatitis C virus infection. Hepatol Int 2008; 2:335–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mitsuyoshi H, Yasui K, Yamaguchi K, Minami M, Okanoue T, Itoh Y. Pathogenic role of iron deposition in reticuloendothelial cells during the development of chronic hepatitis C. Int J Hepatol 2013; 2013:686420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Olynyk J, Hall P, Reed W, Williams P, Kerr R, Mackinnon M. A long-term study of the interaction between iron and alcohol in an animal model of iron overload. J Hepatol 1995; 22:671–676. [DOI] [PubMed] [Google Scholar]
- 6.Deugnier YM, Loréal O, Turlin B, Guyader D, Jouanolle H, Moirand R, et al. Liver pathology in genetic hemochromatosis: a review of 135 homozygous cases and their bioclinical correlations. Gastroenterology 1992; 102:2050–2059. [DOI] [PubMed] [Google Scholar]
- 7.Arezzini B, Lunghi B, Lungarella G, Gardi C. Iron overload enhances the development of experimental liver cirrhosis in mice. Int J Biochem Cell Biol 2003; 35:486–495. [DOI] [PubMed] [Google Scholar]
- 8.Gardi C, Arezzini B, Fortino V, Comporti M. Effect of free iron on collagen synthesis, cell proliferation and MMP-2 expression in rat hepatic stellate cells. Biochem Pharmacol 2002; 64:1139–1145. [DOI] [PubMed] [Google Scholar]
- 9.Niederau C, Fischer R, Sonnenberg A, Stremmel W, Trampisch HJ, Strohmeyer G. Survival and causes of death in cirrhotic and in noncirrhotic patients with primary hemochromatosis. N Engl J Med 1985; 313:1256–1262. [DOI] [PubMed] [Google Scholar]
- 10.Jaeschke H, Gores GJ, Cederbaum AI, Hinson JA, Pessayre D, Lemasters JJ. Mechanisms of hepatotoxicity. Toxicol Sci 2002; 65:166–176. [DOI] [PubMed] [Google Scholar]
- 11.Fattovich G, Stroffolini T, Zagni I, Donato F. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology 2004; 127Suppl 1S35–S50. [DOI] [PubMed] [Google Scholar]
- 12.Ufearo H, Kambal K, Onojobi GO, Nouraie M, Agbemabiese C, Diaz S, et al. Complete blood count, measures of iron status and inflammatory markers in inner-city African Americans with undiagnosed hepatitis C seropositivity. Clin Chim Acta 2010; 4119–10653–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Venturini D, Simão AN, Barbosa DS, Lavado EL, Narciso VE, Dichi I, Dichi JB. Increased oxidative stress, decreased total antioxidant capacity, and iron overload in untreated patients with chronic hepatitis C. Dig Dis Sci 2010; 55:1120–1127. [DOI] [PubMed] [Google Scholar]
- 14.Girelli D, Pasino M, Goodnough JB, Nemeth E, Guido M, Castagna A, et al. Reduced serum hepcidin levels in patients with chronic hepatitis C. J Hepatol 2009; 51:845–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Olynyk JK, Reddy KR, Di Bisceglie AM, Jeffers LJ, Parker TI, Radick JL, et al. Hepatic iron concentration as a predictor of response to interferon alfa therapy in chronic hepatitis C. Gastroenterology 1995; 108:1104–1109. [DOI] [PubMed] [Google Scholar]
- 16.Blumberg BS, Lustbader ED, Whitford PL. Changes in serum iron levels due to infection with hepatitis B virus. Proc Natl Acad Sci USA 1981; 78:3222–3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martinelli AL, Filho AB, Franco RF, Tavella MH, Ramalho LN, Zucoloto S, et al. Liver iron deposits in hepatitis B patients: association with severity of liver disease but not with hemochromatosis gene mutations. J Gastroenterol Hepatol 2004; 19:1036–1041. [DOI] [PubMed] [Google Scholar]
- 18.Yonal O, Akyuz F, Demir K, Ciftci S, Keskin F, Pinarbasi B, et al. Decreased prohepcidin levels in patients with HBV-related liver disease: relation with ferritin levels. Dig Dis Sci 2010; 55:3548–3551. [DOI] [PubMed] [Google Scholar]
- 19.Ohkoshi S, Yoshimura A, Yamamoto S, Yano M, Kurita S, Yamazaki K, et al. Successful treatment with lamivudine may correlate with reduction of serum ferritin levels in the patients with chronic hepatitis and liver cirrhosis type B. Hepatol Int 2008; 2:382–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anonymous. Management scheme of diagnostic and therapy criteria of viral hepatitis. Zhonghua Gan Zang Bing Za Zhi 2000; 6:324–329. [Google Scholar]
- 21.Merryweather-Clarke AT, Pointon JJ, Shearman JD, Robson KJ. Global prevalence of putative haemochromatosis mutations. J Med Genet 1997; 34:275–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu J, Chen L, Chen Y, Yang J, Wu D. Serum ferritin concentration predicts mortality in patients with hepatitis B virus-related acute on chronic liver failure. Arch Med Res 2014; 45:251–256. [DOI] [PubMed] [Google Scholar]
- 23.Boige V, Castéra L, de Roux N, Ganne-Carrié N, Ducot B, Pelletier G, et al. Lack of association between HFE gene mutations and hepatocellular carcinoma in patients with cirrhosis. Gut 2003; 52:1178–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Büyükaşik NS, Nadır I, Akin FE, Cakal B, Kav T, Ersoy O, Büyükaşik Y. Serum iron parameters in cirrhosis and chronic hepatitis: detailed description. Turk J Gastroenterol 2011; 22:606–611. [PubMed] [Google Scholar]
- 25.Bayraktar Y, Koseoglu T, Somner C, Kayhan B, Temizer A, Uzunalimoglu B, et al. The use of deferoxamine infusions to enhance the response rate to interferon-alpha treatment of chronic viral hepatitis B. J Viral Hepat 1996; 3:129–135. [DOI] [PubMed] [Google Scholar]


