Abstract
Background
Fidaxomicin has been scrutinized because of its high acquisition cost. Real-world experience is needed to determine whether fidaxomicin has value in patients with Clostridium difficile–associated diarrhea (CDAD) and certain risk factors.
Methods
In this single-center, retrospective cohort study, patients 18 years or older with diarrheal symptoms and positive polymerase chain reaction assay for C. difficile toxin B gene or pseudomembranes were administered fidaxomicin between August 2011 and March 2013. Clinical success was defined as the resolution of signs and symptoms of disease and no further therapy required for CDAD as of the second day after cessation of fidaxomicin therapy. The recurrence of CDAD was defined by the reappearance of signs and symptoms of disease after the cessation of therapy, a new positive C. difficile polymerase chain reaction result, and the need for CDAD retreatment. Readmissions were tracked for 90 days after hospital discharge.
Results
Of the 60 patients who received fidaxomicin, 58 (96.7%) achieved clinical success. Twenty-six (43.3%) of the 60 patients were being treated for a second or greater episode. Six (10.3%) of the 58 patients had recurrence within 90 days after the initial treatment course, and 4 (6.9%) were readmitted within 30 days after hospital discharge.
Conclusions
In this real-world setting, fidaxomicin resulted in a high rate of clinical success, a low rate of recurrence, and a low readmission rate.
Key Words: Clostridium difficile infection, Clostridium difficile–associated diarrhea, fidaxomicin, recurrence
BACKGROUND
The incidence of Clostridium difficile infection (CDI) has increased significantly in the United States, with the number of CDI-associated hospital stays growing from approximately 86,000 in 1993 to approximately 337,000 in 2009. 1 The total cost of these hospital stays in 2009 was approximately $8.2 billion. 1 Clostridium difficile infection is an infectious disorder that results from the incorporation of C. difficile¸ a gram-positive spore-forming bacterium, into the normal colonic microbial ecology. Clostridium difficile–associated diarrhea (CDAD) is a component of CDI, with diarrhea as the primary presenting symptom. Despite appropriate treatment with standard therapies (ie, metronidazole and vancomycin), CDAD recurrence rates have remained high, with at least 1 recurrence observed in 27% of patients treated with metronidazole and 24% of patients treated with vancomycin. 2
In May 2011, the US Food and Drug Administration approved fidaxomicin for the treatment of CDAD. Fidaxomicin is a unique therapy for CDAD because it achieves high fecal concentrations with limited systemic absorption and limits C. difficile spore proliferation without pressuring the microbiome. 3–5 The recommended dose of fidaxomicin is 200 mg orally twice daily for 10 days with no dose reductions for special populations such as geriatrics or patients with renal or hepatic impairment. 6 Two phase 3 randomized controlled trials have confirmed the noninferiority of fidaxomicin 200 mg orally twice daily for 10 days compared with vancomycin 125 mg orally 4 times daily for 10 days for the treatment of CDAD, with a primary outcome of clinical cure (88% vs 86% and 88% vs 87% for the 2 trials, P = NS).6–8 Fidaxomicin had a lower recurrence rate, compared with vancomycin, and sustained clinical response (ie, cure without recurrence, originally termed “global cure”) was superior with fidaxomicin versus vancomycin at 25 days after the end of treatment in both trials (70% vs 57%, P = 0.0011; 72% vs 57%, P = 0.0004).6,9
The use of fidaxomicin has been scrutinized primarily because of an acquisition cost of approximately $2,100 for inpatients and $3,000 for outpatients for a 10-day course.10,11 Consequently, we developed criteria limiting the use of fidaxomicin to patients with numerous risk factors we felt predicted a higher likelihood of developing recurrent disease. We sought to determine whether fidaxomicin would be effective in this real-world setting among patients with confirmed disease, some of whom had previous CDAD recurrences. The study objective was to determine the clinical outcomes after fidaxomicin treatment of CDAD and to record subsequent readmissions.
MATERIALS AND METHODS
This was an Institutional Review Board–approved retrospective review of patients prescribed with fidaxomicin at Huntsville Hospital, an 881-bed community-teaching hospital in Huntsville, Alabama. Patients were identified based upon inpatient fidaxomicin use and charge data between August 2011 and March 2013. Patients 18 years or older were included if they were prescribed fidaxomicin for a diagnosis of CDAD, based upon clinical signs and symptoms of disease and a positive polymerase chain reaction (PCR) for C. difficile toxin B gene (Cepheid GeneXpert, Sunnyvale, CA) or endoscopically observed pseudomembranes. Institutional criteria for fidaxomicin use are shown in Table 1. All patients prescribed fidaxomicin met 5 or more of the criteria listed in the table. Patients were excluded if they received less than 2 days of fidaxomicin therapy. Data collection included patient demographics, CDAD episode treated, anti-infective agents administered for previous and current CDAD, PCR results, administration of concomitant systemic antibiotics, severity of illness, dose and duration of fidaxomicin therapy, and length of hospital stay.
TABLE 1.
Huntsville Hospital Criteria for Fidaxomicin Use
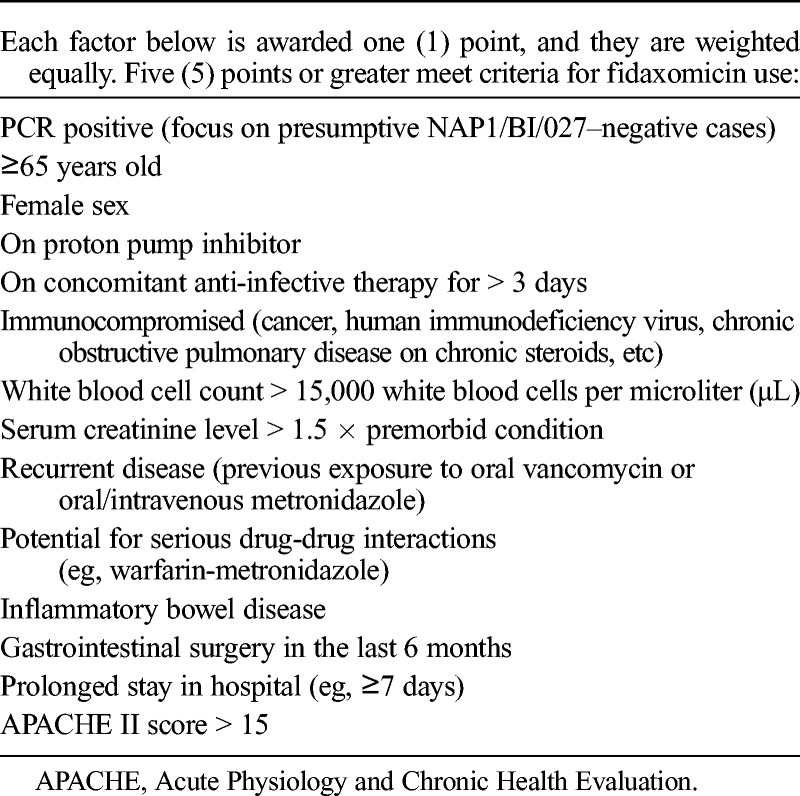
Primary end points were rates of clinical success; recurrence of CDAD up to 90 days after fidaxomicin treatment; and CDAD readmissions at 30, 60, and 90 days after hospital discharge. Clinical success was defined as the resolution of signs and symptoms of the disease and no further therapy required for CDAD as of the second day after cessation of fidaxomicin therapy. The recurrence of CDAD was defined by the reappearance of signs and symptoms of the disease after the cessation of therapy, a new positive C. difficile PCR result, and the need for CDAD retreatment. Only patients who were readmitted because of CDAD recurrence were included in the analysis; all-cause readmissions were not evaluated. All patients who completed fidaxomicin treatment were contacted 30 or more days after hospital discharge via telephone to confirm the total length of therapy, achievement of clinical success, and lack of CDAD recurrence.
RESULTS
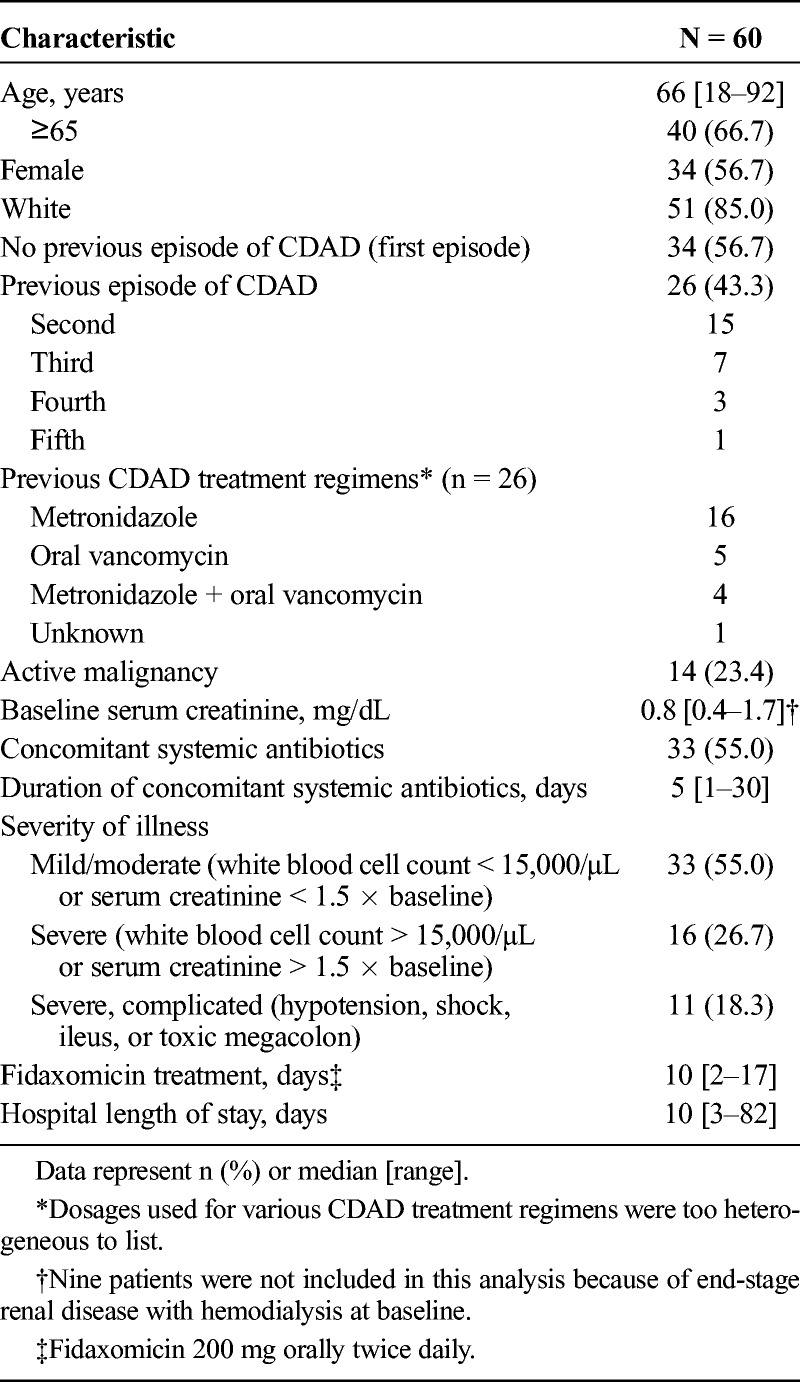
Sixty patients, representing 15% of all CDAD patients treated within our facility during the study time frame, received fidaxomicin and were analyzed. Fidaxomicin was prescribed in accordance with the guidelines developed by the hospital (Table 1). Patient demographics are listed in Table 2. A positive PCR was documented in 58 patients (96.7%), of which 4 patients were presumed to be infected with the NAP1/BI/027 strain. Pseudomembranes were identified in 2 patients (3.3%) without a positive PCR. Thirty-four patients (56.7%) were being treated for their first episode of CDAD, and 26 (43.3%) were being treated for their second or greater CDAD episode. Concomitant systemic antibiotics were administered in 33 patients (55.0%). A median of 10 days (range, 2–17 days) of fidaxomicin therapy was administered. Twenty-seven patients (45.0%) received more than 10 days of fidaxomicin therapy. Eight patients (13.3%) received metronidazole for more than 24 hours before fidaxomicin therapy began for a median of 2 days (range, 2–4 days). Five patients (8.3%) received combination treatment with metronidazole and fidaxomicin for a median of 3 days (range, 1–7 days). No other anti-CDAD treatments besides metronidazole and fidaxomicin were administered to patients.
TABLE 2.
Patient Characteristics
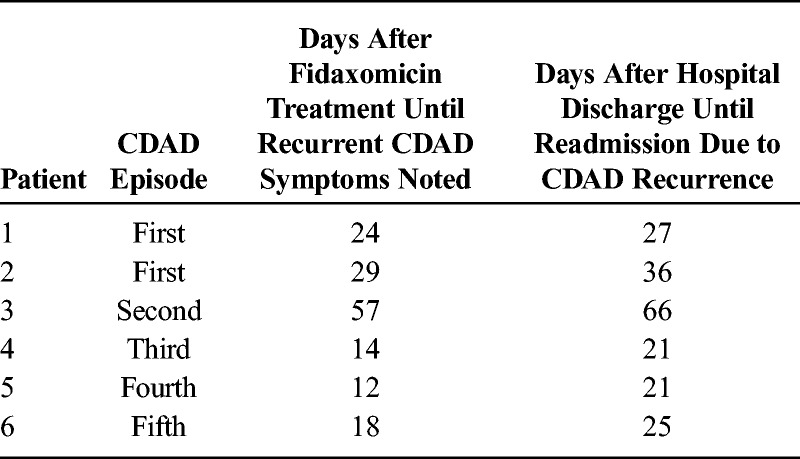
Fifty-eight (96.7%) of the 60 patients achieved clinical success. Two patients died before discharge; neither death was attributed to CDAD or fidaxomicin therapy. One patient achieved symptom resolution and was deemed a clinical success, yet the patient died because of cardiac arrest. The second patient received a total of 2 days of therapy before the implementation of comfort care measures resulting in the discontinuation of all therapies, including fidaxomicin, and was classified as a clinical failure. An additional patient, being treated for her third CDAD episode, experienced clinical failure because of lack of symptom resolution after 10 days of fidaxomicin therapy. Six (10.3%) of the 58 patients discharged experienced recurrence of CDAD within 90 days of completion of fidaxomicin treatment. Readmission to Huntsville Hospital for CDAD occurred for all patients with recurrent symptoms; 4 patients (6.9%) were readmitted within 30 days of discharge. As shown in Table 3, recurrence occurred on days 12, 14, 18, 24, 29, and 57 posttreatment for the 6 patients requiring readmission. Readmissions were recorded for the first, second, third, fourth, and fifth episodes of CDAD. One of the 4 patients presumably infected with the NAP1/BI/027 strain experienced recurrence and was readmitted.
TABLE 3.
Time to Recurrence and Readmission, Stratified by CDAD Episode Treated
DISCUSSION
Fidaxomicin possesses many characteristics that are desirable for the treatment of CDAD, such as a narrow spectrum of activity, low systemic absorption, twice-daily dosing, the absence of drug interactions, lack of dosing modifications in renal and hepatic impairment, and a limited adverse effect profile.3–6 Data from phase 3 clinical trials represent the largest prospective data published concerning C. difficile pharmacological treatment and report that fidaxomicin demonstrates superiority over oral vancomycin for sustained clinical response (ie, cure without recurrence) in adults with a first episode or first recurrence of CDAD.7,8 Despite these benefits, the high drug acquisition cost prevents extensive use of fidaxomicin for all CDAD episodes at our institution. Consequently, criteria were developed to identify patients with the highest risk of recurrence (Table 1). Because fidaxomicin use at our institution was not bound by the criteria defined in the phase 3 clinical trials, our experience provided the opportunity to evaluate outcomes of fidaxomicin use in a real-world setting.
As evidenced in our methodology, this analysis examined fidaxomicin use in clinical practice. We included all patients receiving fidaxomicin who were hospitalized with CDAD. Several of these patients were also exposed to metronidazole during their course of illness. Moreover, most patients received concomitant systemic antibiotic therapy while receiving fidaxomicin treatment. In addition, evaluated patients were not excluded if they received more than 24 hours of pretreatment with metronidazole before administration of fidaxomicin. Eleven (18.3%) of the 60 patients were receiving treatment for multiple recurrent CDAD (≥2 recurrences; ie, third episode or greater), and 27 patients (45.0%) received more than 10 days of fidaxomicin therapy when combining both inpatient and outpatient fidaxomicin exposure. Fidaxomicin treatment courses of more than 10 days were due to patients receiving a remaining supply of medication after enrollment in the manufacturer-sponsored patient access support service (DIFICID Rx Assist), with delivery of fidaxomicin to the patient’s residence (http://www.dificid.com/dificid-rx-assist). In some cases, submission of the documentation required for this service would not coincide with the forecasted date of hospital discharge, resulting in patients receiving more than 10 days of fidaxomicin therapy in aggregate.
In this retrospective analysis, fidaxomicin had a high rate of clinical success (96.7%), a low rate of recurrence (10.3%), and a low readmission rate (6.9%). The low overall CDAD recurrence rate was especially encouraging, given that 43% of patients were treated using fidaxomicin for their second or greater CDAD episode. The rate of recurrence in patients being treated for recurrent CDAD (second or greater episode) was 15% (4 recurrences in 26 patients), whereas the rate of recurrence in patients being treated for multiple recurrent CDAD (third or greater episode) was 27% (3 recurrences in 11 patients). For comparison, a recurrence rate of 35% to 65% is expected in patients who have had a previous recurrence and are treated with antibiotics other than fidaxomicin.12–14 Furthermore, the overall readmission rate (6.9%) observed in this analysis of fidaxomicin-treated patients compares favorably with data from the Healthcare Cost and Utilization Project, which found that 12.8% of patients with CDI during hospitalization were readmitted with a diagnosis of CDI within 30 days. 15
A number of limitations of our analysis can be identified. Given the retrospective nature of this analysis, it is possible that we failed to adequately identify all CDAD recurrences. Although this limitation would be present in all single-center studies, we expect this to be less of a concern within our health system. First, Huntsville Hospital is a regional referral center serving approximately 1.5 million patients and covering an extensive geographic area. This area does not have multiple acute care hospitals in close proximity, unlike many other urban settings. Second, patients who experienced CDAD as an outpatient are followed at outpatient centers associated with Huntsville Hospital in which PCR-positive cultures would have been recorded via the patient information system. Third, discharged patients were contacted 30 or more days after hospital discharge via telephone to confirm achievement of clinical success and lack of CDAD recurrence.
Finally, most of the patients described in this analysis were infected with non-NAP1/BI/027 strains. The subset of patients in the phase 3 trials infected with this strain demonstrated great benefit from receiving fidaxomicin.7,8 Although no difference in outcomes was observed between fidaxomicin and vancomycin in the subset of patients infected with a NAP1/BI/027 strain in the phase 3 trials, additional data beyond the 4 patients described in this analysis would be informative regarding fidaxomicin activity in this subset of patients.
Clostridium difficile—associated diarrhea continues to plague patients within the healthcare setting and leads to increased healthcare cost. In this evaluation, all but 2 of the 60 patients achieved clinical success with fidaxomicin, and the overall rates of recurrence and readmission were low. Although the phase 3 trials of fidaxomicin have been, by far, the largest prospective studies involving CDAD, the current analysis provides insight regarding fidaxomicin use in a real-world setting.
ACKNOWLEDGMENT
The authors wish to acknowledge StemScientific for providing medical writing and editorial support, funded by Cubist Pharmaceuticals.
Footnotes
Edward H. Eiland served on the Speakers Bureau for Optimer Pharmaceuticals. For the remaining authors, no conflicts of interest are declared.
REFERENCES
- 1. Lucado J, Gould C, Elixhauser A. Clostridium difficile infections (CDI) in hospital stays, 2009. Healthcare Cost and Utilization Project (HCUP) Statistical Brief #124. Available at: http://www.hcup-us.ahrq.gov/reports/statbriefs/sb124.pdf Accessed May 7, 2014. [PubMed]
- 2.Vardakas KZ, Polyzos KA, Patouni K, et al. Treatment failure and recurrence of Clostridium difficile infection following treatment with vancomycin or metronidazole: a systematic review of the evidence. Int J Antimicrob Agents. 2012;40 (1):1–8. [DOI] [PubMed] [Google Scholar]
- 3.Venugopal AA, Johnson S. Fidaxomicin: a novel macrocyclic antibiotic approved for treatment of Clostridium difficile infection. Clin Infect Dis. 2012;54 (4):568–574. [DOI] [PubMed] [Google Scholar]
- 4.Louie TJ, Cannon K, Byrne B, et al. Fidaxomicin preserves the intestinal microbiome during and after treatment of Clostridium difficile infection (CDI) and reduces both toxin reexpression and recurrence of CDI. Clin Infect Dis. 2012;55 (suppl 2):S132–S142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldstein EJC, Babakhani F, Citron DM. Antimicrobial activities of fidaxomicin. Clin Infect Dis. 2012;55 (suppl 2):S143–S148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dificid (Fidaxomicin) Prescribing Information. San Diego, CA: Optimer Pharmaceuticals, Inc; 2013. [Google Scholar]
- 7.Louie TJ, Miller MA, Mullane KM, et al. Fidaxomicin versus vancomycin for Clostridium difficile infection. N Engl J Med. 2011;364 (5):422–431. [DOI] [PubMed] [Google Scholar]
- 8.Cornely OA, Crook DW, Esposito R, et al. Fidaxomicin versus vancomycin for infection with Clostridium difficile in Europe, Canada, and the USA: a double-blind, non-inferiority, randomised controlled trial. Lancet Infect Dis. 2012;12 (4):281–289. [DOI] [PubMed] [Google Scholar]
- 9. Optimer Pharmaceuticals, Inc. Clostridium difficile-associated diarrhea (CDAD) in long-term care (LTC) facilities and the role of DIFICID® (fidaxomicin) tablets in the treatment of CDAD. Caring for the Ages. 2012;Nov(suppl):1–11. [Google Scholar]
- 10.Bookstaver PB, James TD, Siddiqui W, et al. Fidaxomicin: a new addition to the Clostridium difficile armamentarium. Curr Med Lit Gastroenterol. 2012;31 (4):95–103. [Google Scholar]
- 11.Ghose C. Clostridium difficile infection in the twenty-first century. Emerg Microbes Infect. 2013;2:e62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McFarland LV, Elmer GW, Surawicz CM. Breaking the cycle: treatment strategies for 163 cases of recurrent Clostridium difficile disease. Am J Gastroenterol. 2002;97 (7):1769–1775. [DOI] [PubMed] [Google Scholar]
- 13.McFarland LV, Surawicz CM, Greenberg RN, et al. A randomized placebo-controlled trial of Saccharomyces boulardii in combination with standard antibiotics for Clostridium difficile disease. JAMA. 1994;271 (24):1913–1918. [PubMed] [Google Scholar]
- 14.Cornely OA, Miller MA, Louie TJ, et al. Treatment of first recurrence of Clostridium difficile infection: fidaxomicin versus vancomycin. Clin Infect Dis. 2012;55(suppl 2):S154–S161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Elixhauser A, Steiner C, Gould C. Readmissions following hospitalizations with Clostridium difficile infections, 2009. Healthcare Cost and Utilization Project (HCUP) Statistical Brief #145. Available at: http://www.hcup-us.ahrq.gov/reports/statbriefs/sb145.pdf Accessed May 7, 2014. [PubMed]


