Abstract
Background
The Clinical Assessment Program and Teflaro® Utilization Registry is designed to collect information on the clinical use of ceftaroline fosamil in the Unites States. This report presents data on the treatment of patients with Staphylococcus aureus bacteremia (SAB) secondary to acute bacterial skin and skin structure infections (ABSSSIs) or community-acquired bacterial pneumonia (CABP).
Methods
Patients diagnosed with ABSSSI or CABP were identified through sequential review of randomly ordered charts generated from pharmacy listings from August 2011 to February 2013. Data were collected by chart review 30 days or more after completion of ceftaroline fosamil therapy.
Results
Secondary SAB was reported in a total of 48 of 1428 evaluable patients (27 with ABSSSI, 21 with CABP). The mean (SD) patient age was 61 (15) years. At least 1 comorbidity was recorded for 74% of patients with ABSSSI and 81% with CABP. Methicillin-resistant S. aureus was isolated from 59% of patients with ABSSSI and 76% with CABP. The mean (SD) duration of ceftaroline fosamil therapy was 5.8 (4.8) days for ABSSSI and 7.0 (3.8) days for CABP. Clinical success among all patients with SAB treated with ceftaroline fosamil was 58% (52% for SAB secondary to ABSSSI, 67% for SAB secondary to CABP). Clinical success rates of methicillin-resistant S. aureus SAB were 50% (8/16) for ABSSSI and 63% (10/16) for CABP.
Conclusions
This study supports the use of ceftaroline fosamil as a viable treatment option in hospitalized patients with SAB secondary to ABSSSI or CABP. Further studies evaluating the use of ceftaroline fosamil for the treatment of SAB are warranted.
Key Words: Ceftaroline fosamil, Bacteremia, Staphylococcus aureus, CAPTURE, ABSSSI, CABP
Staphylococcus aureus is a leading cause of community-associated and health care–associated bacteremia and is also associated with high morbidity and mortality.1 Although most cases of S. aureus bacteremia (SAB) result from medical interventions such as surgical procedures and intravascular catheters,2 SAB arising from community infections such as acute bacterial skin and skin structure infections (ABSSSIs) or community-acquired bacterial pneumonia (CABP), especially postinfluenza or postviral pneumonias, are frequently reported.2,3 Comorbidities including diabetes, obesity, alcoholism, structural lung disease, or cardiovascular disease predispose patients to secondary SAB, which frequently results in poor outcomes and increased mortality rates.4,5
Therapeutic options for patients with SAB are limited, and among those available, treatment-limiting adverse effects can further limit those options. Success rates for the available therapeutic agents for both methicillin-susceptible S. aureus (MSSA) and methicillin-resistant S. aureus (MRSA) are variable, with success rates of up to 60% reported by a number of studies.6–8 However, failure rates as low as 13% have also been reported.9 β-Lactams are considered the agents of choice when the causative pathogen is known to be MSSA. For MRSA, treatment options include either vancomycin or daptomycin,10 and trimethoprim-sulfamethoxazole is also considered an alternative to vancomycin for the treatment of ABSSSI.11 There have been reports describing the development of in vivo resistance in S. aureus to these antibiotics.12–15 In addition, there are potential limitations associated with vancomycin (in terms of target attainment for minimum inhibitory concentration >1 mg/L, ototoxicity and nephrotoxicity)16 and daptomycin (inactivation by pulmonary surfactant rendering this an inappropriate agent for treatment of pneumonia, rhabdomyolysis).17,18 Therefore, there are challenges in the selection of appropriate empiric therapy for serious bacterial infections due to S. aureus and an unmet need for new antibiotics that are efficacious in the treatment of serious systemic infections due to SAB.
Ceftaroline is a broad-spectrum cephalosporin, which is active in vitro against Gram-positive pathogens such as S. aureus and Streptococcus pneumoniae as well as their resistant phenotypes (eg, MRSA, vancomycin-resistant S. aureus, and multidrug-resistant S. pneumoniae).19–21 It is also active in vitro against common Gram-negative pathogens such as Escherichia coli, Klebsiella pneumoniae, and Haemophilus influenzae; however, it is not active against Gram-negative organisms that produce extended spectrum β-lactamases.
The mechanism of action of ceftaroline is common to that of other β-lactam antibiotics, inhibiting bacterial cell wall synthesis by irreversibly binding to several penicillin-binding proteins, but unlike other β-lactams, it has a high affinity for penicillin-binding protein 2a in MRSA.22,23
Ceftaroline fosamil was approved by the US Food and Drug Administration in October 2010 for the treatment of ABSSSI and CABP caused by susceptible organisms, including MSSA in the case of CABP and MSSA and MRSA in the case of ABSSSI. It is approved for similar indications in Europe. Ceftaroline fosamil is not currently approved for the treatment of S. aureus bacteremia secondary to ABSSSI or CABP. It is well tolerated, having a safety profile reflective of the cephalosporin class and similar to that of comparator agents used in the phase 3 clinical trials.24
The Clinical Assessment Program and Teflaro® Utilization Registry (CAPTURE) is designed to collect information on the contemporary clinical use of ceftaroline fosamil in the United States. In this report, we present and discuss data from CAPTURE on the use of ceftaroline fosamil for the treatment of patients with SAB secondary to ABSSSI or CABP.
PATIENTS AND METHODS
Study Design
Patients diagnosed with ABSSSI or CABP were identified through sequential review of randomly ordered charts generated from pharmacy listings at participating institutions from August 2011 to February 2013. Inpatients and outpatients were permitted into the study. Acute bacterial skin and skin structure infection was diagnosed where infection involved deep soft tissues or required significant surgical intervention. Community-acquired bacterial pneumonia was diagnosed as an acute illness with clinical signs and symptoms consistent with a lower respiratory tract infection along with imaging consistent with bacterial pneumonia.
Eligible patients aged 18 years or older received 2 or more consecutive doses of ceftaroline fosamil between August 2011 and July 2012, and following a protocol amendment received 4 or more consecutive doses thereafter. Data collection was by review of charts 30 days or more after the completion of the ceftaroline fosamil therapy to increase the likelihood of a complete chart for data abstraction.
Patients were excluded when the information on dosing was missing or if data had previously been extracted for this study. Data were excluded from patients with additional or underlying infections other than SAB resulting from ABSSSI or CABP.
This registry study was carried out across study centers in the United States, was approved by each institution’s ethics committee or institutional review board, and was conducted in compliance with the International Conference on Harmonisation E6 Good Clinical Practice Guidance, 1976.25
Data Collection
Data collected for each eligible patient diagnosed with SAB secondary to ABSSSI or CABP included demographics, relevant medical and surgical history, location of care, clinical signs and symptoms (both at the time of diagnosis and at end of treatment with ceftaroline fosamil), and microbiology (methicillin susceptibility and source of S. aureus isolates). Data were also recorded on the clinical response to ceftaroline fosamil when used as either first-line or second-line therapy, as well as monotherapy or concurrent therapy with other antibiotics.
As a result of the study protocol amendment in July 2012, additional data including ceftaroline fosamil dosage, dosing frequency, laboratory data including serum creatinine level at diagnosis and end of treatment with ceftaroline fosamil, readmission within 30 days of discharge, and reason for readmission were collected.
Data Analysis and Clinical Outcome
Statistical analyses were performed on the data using SAS Version 9.2. The data were summarized using descriptive statistics, including demographic data, disease and pathogen characteristics, ceftaroline fosamil dose, frequency and duration, outcome, and hospital discharge. Numeric data were summarized with mean (SD) and median values, where categorical data were presented by frequency and percentage.
Evaluable patients were those for whom a clinical outcome (success or failure) could be determined. Clinical success was defined as either clinical cure with no further need for antibiotic therapy or as a clinical improvement with a switch to oral antibiotic treatment. Clinical failure was determined where patients experienced an adverse event leading to discontinuation or where insufficient therapeutic effect was documented. In some cases, after a review of information, treatment was also deemed to be successful where patients were confirmed to be improving at the time of discontinuation of ceftaroline fosamil.
RESULTS
Patient and Disease Characteristics
Of 1500 patients with either ABSSSI or CABP enrolled across 40 study centers, 1428 were evaluable. A subset of 3% (48/1428) of evaluable patients had a diagnosis of secondary SAB, comprising 3% (27/1030) of patients with ABSSSI and 5% (21/398) of patients with CABP (Table 1). All patients in this analysis were inpatients. The patients with SAB had a near-even sex distribution and a mean (SD) age of 61 (15) years.
TABLE 1.
Patient Demographics and Location of Care—Evaluable Patients With SAB Secondary to ABSSSI or CABP
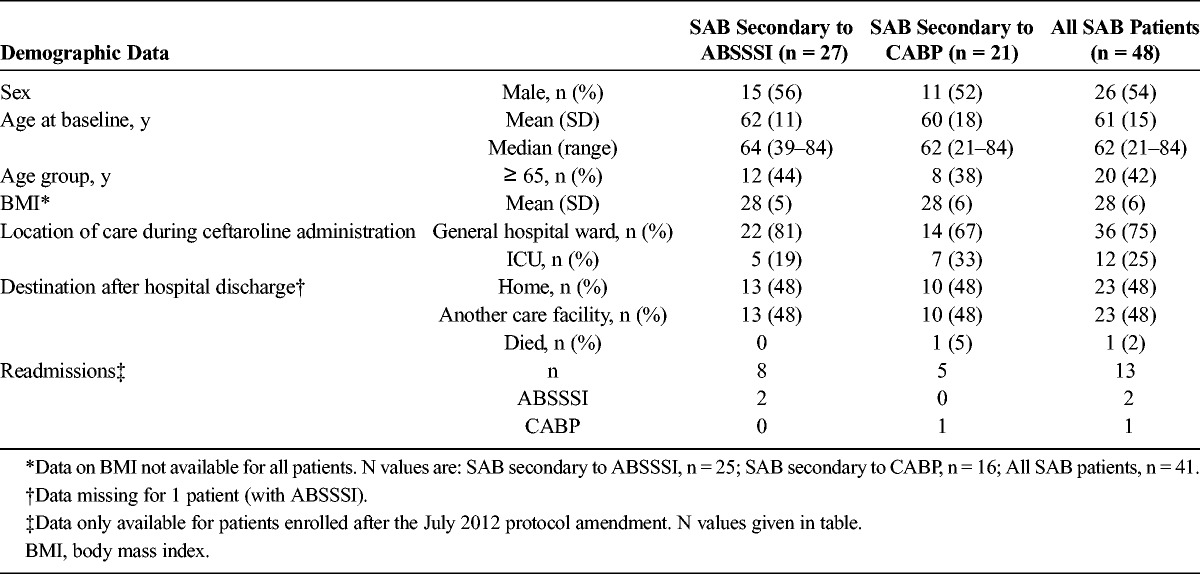
At least 1 comorbid condition was recorded for 74% of patients with ABSSSI and 81% with CABP. Analysis of patients’ medical history revealed the most common ABSSSI-associated comorbidity to be diabetes (70%), followed by obesity (33%) and peripheral vascular disease (22%). The most common comorbidities associated with CABP were structural lung disease (33%), gastroesophageal reflux disease (29%), and congestive heart failure (24%).
Among the patients with SAB, 13 patients (8 with ABSSSI, 5 with CABP) were enrolled subsequent to July 2012. The mean (SD) serum creatinine measurement for these 13 patients was 2.4 (2.4) mg/dL, with a median (range) of 1.5 (0.5-9.1) mg/dL before the start of ceftaroline fosamil treatment. Two patients (1 each, ABSSSI and CABP) received hemodialysis.
Readmission data (within 30 days of discharge) were collected after a protocol amendment in 2012; therefore readmission data were only available for 8 patients with ABSSSI, of which 2 patients were readmitted for ABSSSI. Readmission data were available for 5 patients with CABP of which one was readmitted for CABP.
Pathogen Characteristics
Of the 48 patients with SAB, MRSA was isolated in 67% (32/48) of cases. Among patients with SAB secondary to ABSSSI, MRSA was isolated in 59% (16/27) of cases and among patients with SAB secondary to CABP, in 76% (16/21) of cases.
Antibiotic Usage
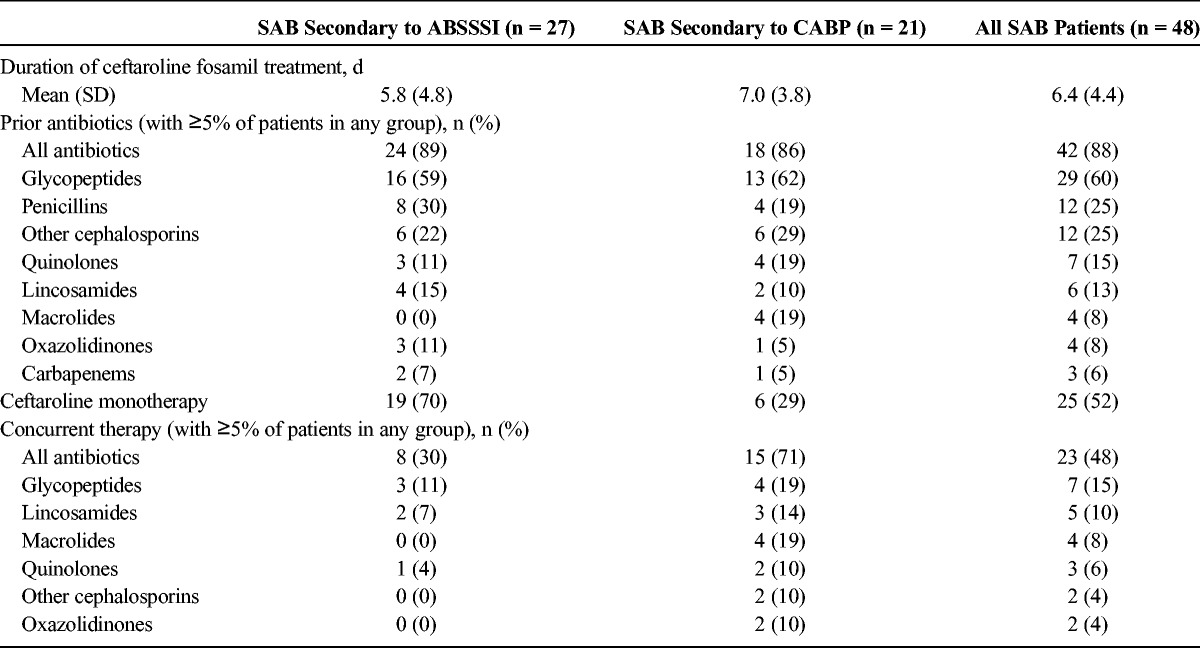
The mean (SD) duration of ceftaroline fosamil therapy was 5.8 (4.8) days for SAB secondary to ABSSSI and 7.0 (3.8) days for SAB secondary to CABP. Before the administration of ceftaroline fosamil, 89% of patients with SAB secondary to ABSSSI and 86% of patients with SAB secondary to CABP had received antibiotic therapy, of which glycopeptides, penicillins, and other cephalosporins were most commonly used (Table 2). Concurrent therapy was administered in 30% of patients with SAB secondary to ABSSSI (most commonly glycopeptides and lincosamides) and 71% of patients with SAB secondary to CABP (most commonly glycopeptides and macrolides). Monotherapy with ceftaroline fosamil was utilized in 70% of patients with SAB secondary to ABSSSI and in 29% of patients with SAB secondary to CABP. After July 2012, data were collected on frequency of infusion with ceftaroline fosamil. Among those patients for whom such data were available (n = 13), 1 patient with SAB secondary to CABP received ceftaroline fosamil by infusion every 8 hours.
TABLE 2.
Antibiotic Usage for Patients With SAB
Location of Care
One quarter of all patients with SAB were treated in the intensive care unit (ICU) while receiving ceftaroline fosamil. A higher proportion of patients with SAB secondary to CABP were treated in the ICU (33%) when compared with patients with SAB secondary to ABSSSI (19%).
After ceftaroline fosamil therapy, patients had similar rates of discharge to home or to another care facility (48%). One patient with CABP died, and postdischarge data for one patient with ABSSSI were missing.
Clinical Outcome
Clinical success among all patients with SAB treated with ceftaroline fosamil therapy was 58%. The success rate among patients with SAB secondary to ABSSSI was 52%, whereas the success rate among patients with SAB due to CABP was 67%. Clinical success rates of SAB associated with MRSA among patients with ABSSSI and with CABP were 50% (8/16) and 63% (10/16), respectively. Similarly, for SAB caused by MSSA and secondary to ABSSSI, the clinical success rate was 55% (6/11). For SAB caused by MSSA and secondary to CABP, 80% (4/5) were categorized as a clinical success.
Most patients received antibiotic therapy before receiving ceftaroline fosamil. Three patients with SAB secondary to ABSSSI and 3 patients with SAB secondary to CABP received ceftaroline fosamil as first-line therapy. Among patients who received ceftaroline fosamil as second-line therapy, the overall clinical success rate was 64% (27/42), with a clinical success rate of 58% (14/24) for SAB due to ABSSSI and 72% (13/18) for SAB due to CABP.
Among those patients who received ceftaroline fosamil as monotherapy, the overall clinical success rate was 64% (16/25). In patients with SAB secondary to ABSSSI, the clinical success rate was 58% (11/19). In patients with SAB secondary to CABP, 83% (5/6) were considered a clinical success.
Of the 23 patients that received concurrent antibiotic therapy, the clinical success rate was 52% (12/23). Among the 8 patients with SAB and ABSSSI who received concurrent therapy, 3 were a clinical success. For patients with SAB secondary to CABP, the clinical success rate was 60% (9/15).
In the ABSSSI group, 2 patients (7%) were classified as a clinical improvement and were switched to an oral antibiotic. For 8 patients (30%), there was insufficient therapeutic effect, and they were switched to an alternative intravenous antibiotic. Adverse events were given as the reason for discontinuation for 2 patients (7%) with SAB secondary to ABSSSI. For patients with SAB secondary to CABP, clinical improvement with switch to oral antibiotic was recorded for 1 patient, and for 4 patients, there was insufficient therapeutic effect, and they were switched to an alternative intravenous antibiotic. No adverse events were reported as a reason for discontinuation in this group.
DISCUSSION
Data for the treatment of SAB secondary to ABSSSI or CAP are not readily available in the literature, with few published studies. Khosrovaneh et al26 reported on a study of 50 cases of SAB originating from soft tissues; however, their study did not include an overall cure rate. Data on treatment outcomes using ceftaroline fosamil therapy for SAB are also limited.27,28 Analysis of data from the CAPTURE study reveals clinical success rates of 38% to 83%, which are comparable with the clinical success rates reported for vancomycin (52%) and linezolid (55%) in a pooled analysis of patients with SAB and daptomycin (44%) and vancomycin or an antistaphylococcal penicillin (42%) for patients with SAB with or without endocarditis.8,29 In studies for patients with MRSA bacteremia, the success rates ranged between 32% and 60% for various agents,6,8,29 and among patients with MSSA bacteremia, the success rates were between 45% and 49%.29 Late complications can occur with bacteremia; therefore the variable follow-up periods used in these published studies necessitates that some caution should be exercised when comparing studies. The follow-up period in the CAPTURE study was 30 days (with readmissions to the same hospital only recorded); complications of bacteremia resulting in readmission to hospital could occur either during this 30 days at another institution or after this time at the same institution and would not be recorded as part of CAPTURE, but as a registry chart review study rather than a prospective study with a late follow-up visit, this is a limitation of the study. Clinical response was limited to assessment at discontinuation of ceftaroline fosamil, and mortality was required to be reported either in-hospital or within 24 hours of discontinuation of ceftaroline fosamil.
The mean duration of treatment in this study was 6.4 days, which is shorter than would be expected for the treatment of SAB. However, the mean duration of treatment data presented is for ceftaroline fosamil only and does not include duration of prior or subsequent antibiotic therapy. Because many patients were treated with ceftaroline fosamil as second-line therapy and may have been switched to another agent after improving, the mean duration of treatment is not necessarily representative of the length of the entire treatment period. In addition, although case studies in the literature that present data on the use of ceftaroline fosamil in the treatment of bacteremia often use an 8-hour dosing regimen,27,28 dosing in this study most frequently followed a 12-hourly regimen. This most likely occurred because ceftaroline fosamil was being used in the treatment of a primary ABSSSI or CABP with secondary SAB.
As described previously for the overall population, clinical success rates among patients who received monotherapy and concurrent therapy were generally favorable. Clinical success rates were greater than 52%, with the exception of patients with SAB secondary to ABSSSI, who received concurrent therapy, although the numbers of patients in this subgroup was small (3 of 8 patients determined as clinical success).
Readmission data were collected after July 2012, leading to a small number of patients for whom readmission data are available (n = 13). This limits the conclusions that can be drawn from these data. A total of 5 patients were readmitted, 4 who had been treated for SAB secondary to ABSSSI and one who had been treated for SAB secondary to CABP. Of the 4 patients who were treated for SAB secondary to ABSSSI, 2 were readmitted for ABSSSI and 2 for non–ABSSSI-associated reasons. The patient with SAB associated with CABP was readmitted for CABP.
The phase 3 studies of ceftaroline fosamil showed a clinical success rate of 89% (16/18) among patients with SAB secondary to ABSSSI.24 This is markedly higher than the data reported in the CAPTURE study and may, in part, be due to the restricted use of prior antibiotic therapy in the phase 3 studies and the higher rate of comorbidities among patients in the CAPTURE study. In addition, the phase 3 studies had multiple other exclusionary criteria, which included the exclusion of patients directly admitted to the ICU. This discrepancy highlights a major difference between controlled phase 3 studies and registry studies. Despite the high incidence of comorbidities, clinical response rates in the CAPTURE study were generally favorable. Furthermore, the high proportion of patients treated in the ICU indicates a high acuity of illness with presence of severe disease in the patients with SAB in CAPTURE. Ceftaroline fosamil was frequently used as a second-line or salvage antimicrobial therapy (>85% of patients), and the glycopeptides (eg, vancomycin) were the most commonly administered antibiotic class before the initiation of ceftaroline fosamil (60% overall), implying glycopeptide failure. This further frames the clinical outcomes in a population of patients who were likely treatment failures to begin with and at higher risk for subsequent clinical failure.
As discussed previously, registry studies such as CAPTURE may have inherent limitations such as lack of randomization or blinding to control for bias; however, they have the potential to provide useful information on the contemporary use of antibiotics, including their spectrum of use, duration of treatment, effectiveness, safety, and cost.30,31
In conclusion, the results from this study support the clinical effectiveness of ceftaroline fosamil in the treatment of ABSSSI or CABP associated with secondary SAB. Ceftaroline fosamil was an effective treatment option, despite the wide range of comorbidities, including those patients who had likely received prior unsuccessful antibiotic therapy. These data support the use of ceftaroline fosamil as an effective treatment option in hospitalized patients with SAB secondary to ABSSSI and CABP. Furthermore, these results suggest that further clinical studies evaluating the use of ceftaroline fosamil for SAB are warranted.
ACKNOWLEDGMENTS
The authors would like to thank all the sites involved in this study. They also wish to acknowledge Micron Research Ltd (Ely, UK), who provided writing assistance.
Footnotes
Conflict of interest and source of funding: the Clinical Assessment Program and Teflaro® Utilization Registry is funded by Cerexa, Inc, a wholly owned subsidiary of Forest Laboratories, Inc. J.A.V. is a member of the Speakers’ Bureau for Astellas Pharma Inc, Forest Laboratories Inc, and Pfizer Inc and has received grants from Astellas Pharma Inc, Merck and Co Inc, and Pfizer Inc. C.M. serves as a clinical investigator for Cerexa, Inc. P.C., A.S., A.J., and H.D.F. are employees of Cerexa, Inc.
REFERENCES
- 1.Naber CK, Baddour LM, Giamarellos-Bourboulis EJ, et al. Clinical consensus conference: survey on Gram-positive bloodstream infections with a focus on Staphylococcus aureus. Clin Infect Dis. 2009; 48: S260– S270. [DOI] [PubMed] [Google Scholar]
- 2.Weinstein MP, Towns ML, Quartey SM, et al. The clinical significance of positive blood cultures in the 1990s: a prospective comprehensive evaluation of the microbiology, epidemiology, and outcome of bacteremia and fungemia in adults. Clin Infect Dis. 1997; 24: 584– 602. [DOI] [PubMed] [Google Scholar]
- 3.Mongardon N, Max A, Bouglé A, et al. Epidemiology and outcome of severe pneumococcal pneumonia admitted to intensive care unit: a multicenter study. Crit Care. 2012; 16: R155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Laupland KB, Ross T, Gregson DB. Staphylococcus aureus bloodstream infections: risk factors, outcomes, and the influence of methicillin resistance in Calgary, Canada, 2000–2006. J Infect Dis. 2008; 198: 336– 343. [DOI] [PubMed] [Google Scholar]
- 5.Shurland S, Zhan M, Bradham DD, et al. Comparison of mortality risk associated with bacteremia due to methicillin-resistant and methicillin-susceptible Staphylococcus aureus. Infect Control Hosp Epidemiol. 2007; 28: 273– 279. [DOI] [PubMed] [Google Scholar]
- 6.Rehm SJ, Boucher H, Levine D, et al. Daptomycin versus vancomycin plus gentamicin for treatment of bacteraemia and endocarditis due to Staphylococcus aureus: subset analysis of patients infected with methicillin-resistant isolates. J Antimicrob Chemother. 2008; 62: 1413– 1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sakoulas G, Moise-Broder PA, Schentag J, et al. Relationship of MIC and bactericidal activity to efficacy of vancomycin for treatment of methicillin-resistant Staphylococcus aureus bacteremia. J Clin Microbiol. 2004; 42: 2398– 2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shorr AF, Kunkel MJ, Kollef M. Linezolid versus vancomycin for Staphylococcus aureus bacteraemia: pooled analysis of randomized studies. J Antimicrob Chemother. 2005; 56: 923– 929. [DOI] [PubMed] [Google Scholar]
- 9.Stryjewski ME, Szczech LA, Benjamin DK, Jr, et al. Use of vancomycin or first-generation cephalosporins for the treatment of hemodialysis-dependent patients with methicillin-susceptible Staphylococcus aureus bacteremia. Clin Infect Dis. 2007; 44: 190– 196. [DOI] [PubMed] [Google Scholar]
- 10.Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis. 2011; 52: e18– e55. [DOI] [PubMed] [Google Scholar]
- 11.Markowitz N, Quinn EL, Saravolatz LD. Trimethoprim-sulfamethoxazole compared with vancomycin for the treatment of Staphylococcus aureus infection. Ann Intern Med. 1992; 117: 390– 398. [DOI] [PubMed] [Google Scholar]
- 12.Finks J, Wells E, Dyke TL, et al. Vancomycin-resistant Staphylococcus aureus, Michigan, USA, 2007. Emerg Infect Dis. 2009; 15: 943– 945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Hal SJ, Paterson DL, Gosbell IB. Emergence of daptomycin resistance following vancomycin-unresponsive Staphylococcus aureus bacteraemia in a daptomycin-naïve patient—a review of the literature. Eur J Clin Microbiol Infect Dis. 2011; 30: 603– 610. [DOI] [PubMed] [Google Scholar]
- 14.Cui L, Tominaga E, Neoh HM, et al. Correlation between reduced daptomycin susceptibility and vancomycin resistance in vancomycin Staphylococcus aureus. Antimicrob Agents Chemother. 2006; 50: 1079– 1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pate AJ, Terribilini RG, Ghobadi F, et al. Antibiotics for methicillin-resistant Staphylococcus aureus skin and soft tissue infections: the challenge of outpatient therapy. Am J Emerg Med. 2014; 32: 135– 138. [DOI] [PubMed] [Google Scholar]
- 16.Stevens DL. The role of vancomycin in the treatment paradigm. Clin Infect Dis. 2006; 42 (suppl 1): S51– S57. [DOI] [PubMed] [Google Scholar]
- 17.Silverman JA, Mortin LI, VanPraagh ADG, et al. Inhibition of daptomycin by pulmonary surfactant: in vitro modeling and clinical impact. J Infect Dis. 2005; 191: 2149– 2152. [DOI] [PubMed] [Google Scholar]
- 18.Papadopoulos S, Ball AM, Liewer SE, et al. Rhabdomyolysis during therapy with daptomycin. Clin Infect Dis. 2006; 42: e108– e110. [DOI] [PubMed] [Google Scholar]
- 19.Richter SS, Heilmann KP, Dohrn CL, et al. Activity of ceftaroline and epidemiologic trends in Staphylococcus aureus isolates collected from 43 medical centers in the United States in 2009. Antimicrob Agents Chemother. 2011; 55: 4154– 4160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Farrell DJ, Castanheira M, Mendes RE, et al. In vitro activity of ceftaroline against multidrug-resistant Staphylococcus aureus and Streptococcus pneumoniae: a review of published studies and the AWARE Surveillance Program (2008–2010). Clin Infect Dis. 2012; 55 (suppl 3): S206– S214. [DOI] [PubMed] [Google Scholar]
- 21.Saravolatz LD, Pawlak J, Johnson LB. In vitro susceptibilities and molecular analysis of vancomycin-intermediate and vancomycin-resistant Staphylococcus aureus isolates. Clin Infect Dis. 2012; 55: 582– 586. [DOI] [PubMed] [Google Scholar]
- 22.Moisan H, Pruneau M, Malouin F. Binding of ceftaroline to penicillin-binding proteins of Staphylococcus aureus and Streptococcus pneumoniae. J Antimicrob Chemother. 2010; 65: 713– 716. [DOI] [PubMed] [Google Scholar]
- 23.de Lencastre H, Oliveira D, Tomasz A. Antibiotic resistant Staphylococcus aureus: a paradigm of adaptive power. Curr Opin Microbiol. 2007; 10: 428– 435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.File TM, Jr, Wilcox MH, Stein GE. Summary of ceftaroline fosamil clinical trial studies and clinical safety. Clin Infect Dis. 2012; 55 (suppl 3): S173– S180. [DOI] [PubMed] [Google Scholar]
- 25.International Conference on Harmonisation. E6 Good Clinical Practice Guidance. Rockville, MD: Drug Information Branch (HFD-210), Center for Drug Evaluation and Research; 1976. [Google Scholar]
- 26.Khosrovaneh A, Sharma M, Khatib R. Favorable prognosis of Staphylococcus aureus bacteremia originating from soft tissues: a prospective study of fifty cases. Scand J Infect Dis. 2005; 37: 6– 10 [DOI] [PubMed] [Google Scholar]
- 27.Ho TT, Cadena J, Childs LM, et al. Methicillin-resistant Staphylococcus aureus bacteraemia and endocarditis treated with ceftaroline salvage therapy. J Antimicrob Chemother. 2012; 67: 1267– 1270. [DOI] [PubMed] [Google Scholar]
- 28.Lin JC, Aung G, Thomas A, et al. The use of ceftaroline fosamil in methicillin-resistant Staphylococcus aureus endocarditis and deep-seated MRSA infections: a retrospective case series of 10 patients. J Infect Chemother. 2013, 19: 42– 49. [DOI] [PubMed] [Google Scholar]
- 29.Fowler VG, Jr, Boucher HW, Corey GR, et al. Daptomycin versus standard therapy for bacteremia and endocarditis caused by Staphylococcus aureus. N Engl J Med. 2006; 355: 653– 665. [DOI] [PubMed] [Google Scholar]
- 30.Dreyer NA, Tunis SR, Berger M, et al. Why observational studies should be among the tools used in comparative effectiveness research. Health Aff (Millwood). 2010; 29: 1818– 1825. [DOI] [PubMed] [Google Scholar]
- 31.Gliklich RE, Dreyer NA. eds. Registries for Evaluating Patient Outcomes: A User’s Guide. Rockville, MD: Agency for Healthcare Research and Quality; 2010. [PubMed] [Google Scholar]


