Supplemental Digital Content is Available in the Text.
Preoperative widespread pressure pain sensitivity is not predictive of the amount of pain relief that patients gain from total hip or knee replacement.
Keywords: Knee, Hip, Replacement, Chronic postsurgical pain, Prediction, Quantitative sensory testing, Pain sensitization
Abstract
Chronic pain after joint replacement is common, affecting approximately 10% of patients after total hip replacement (THR) and 20% of patients after total knee replacement (TKR). Heightened generalized sensitivity to nociceptive input could be a risk factor for the development of this pain. The primary aim of this study was to investigate whether preoperative widespread pain sensitivity was associated with chronic pain after joint replacement. Data were analyzed from 254 patients receiving THR and 239 patients receiving TKR. Pain was assessed preoperatively and at 12 months after surgery using the Western Ontario and McMaster Universities Osteoarthritis Pain Scale. Preoperative widespread pain sensitivity was assessed through measurement of pressure pain thresholds (PPTs) at the forearm using an algometer. Statistical analysis was conducted using linear regression and linear mixed models, and adjustments were made for confounding variables. In both the THR and TKR cohort, lower PPTs (heightened widespread pain sensitivity) were significantly associated with higher preoperative pain severity. Lower PPTs were also significantly associated with higher pain severity at 12 months after surgery in the THR cohort. However, PPTs were not associated with the change in pain severity from preoperative to 12 months postoperative in either the TKR or THR cohort. These findings suggest that although preoperative widespread pressure pain sensitivity is associated with pain severity before and after joint replacement, it is not a predictor of the amount of pain relief that patients gain from joint replacement surgery, independent of preoperative pain severity.
1. Background
Osteoarthritis is a leading cause of chronic pain, and when painful osteoarthritis cannot be managed in primary care, joint replacement surgery is often performed. Total hip replacement (THR) and total knee replacement (TKR) are 2 of the most commonly performed elective surgical procedures, with increased projected demand over the coming decades.46 Although a successful intervention for pain relief for many patients, around 10% of patients with THR and 20% of patients with TKR experience chronic postsurgical pain.8 This pain is distressing to many of those patients affected21 and potentially has a considerable financial implication to health care providers.24
Given the prevalence and impact of this condition, there is a need for research to provide evidence to guide the development of a preoperative screening protocol that could be used to identify patients at high risk of not benefitting from joint replacement. These patients could then be informed of the risk and targeted with interventions to reduce their risk factor(s) or offered an alterative treatment. Research has been undertaken to identify risk factors for the development of chronic pain after joint replacement. However, this work has highlighted that little of the variation in pain severity after joint replacement can be explained by preoperative risk factors such as gender, depression, pain severity, and body mass index.22,23 This work also highlights the need to investigate other preoperative risk factors. Preliminary research suggests that preoperative widespread pain sensitization is associated with chronic pain after joint replacement.31,58 Central pain sensitization involves amplification in neuronal activity that occurs at a generalized level leading to increased sensitivity to nociceptive input at sites distant to the painful area. It is now well established that some patients with painful osteoarthritis have central pain sensitization.4,16,25,26,29–31,50,57 These patients may be at higher risk of experiencing chronic pain after joint replacement, as removal of the peripheral pain source may not reverse augmented central pain-processing changes.
Widespread pain sensitization can be assessed using quantitative sensory testing (QST). This is a noninvasive method that measures participants' responses to external stimuli of controlled intensity. Quantitative sensory testing can be used to detect a range of pain perception abnormalities, although pain sensitization is most frequently assessed through pain thresholds. A number of modalities can be used to assess pain sensitization, including mechanical, thermal, electrical, and chemical modalities.41 However, only pressure pain thresholds (PPTs) have demonstrated good short-term test–retest reliability56 and sensitivity for evaluating pain sensitization50 in patients with osteoarthritis.
The primary aim of this study was to investigate the association between preoperative widespread pressure pain sensitivity and long-term pain outcomes after THR and TKR. A secondary aim was to determine whether preoperative PPTs were an independent predictor of chronic postsurgical pain severity after THR and TKR.
2. Patients and methods
Between 2009 and 2012, 322 patients undergoing THR and 316 patients undergoing TKR were recruited into 2 double-blind, single-center, randomized controlled trials that investigated the effect of intraoperative local anesthetic wound infiltration on pain severity at 12 months after joint replacement. A detailed description of the design of the Arthroplasty Pain Experience (APEX) trials has been previously reported.54 Briefly, the inclusion criteria were being listed for primary unilateral THR or TKR for osteoarthritis. Exclusion criteria included inability to provide informed consent or complete questionnaires. In addition, patients with medical comorbidity which precluded the use of spinal anesthesia, regional blocks, or strong opioids postoperatively were excluded because inability to tolerate these pain-relief strategies may have influenced the trial results. The primary outcome of the trials was pain severity in the replaced joint at 12 months postoperative, measured using the Western Ontario and McMaster Universities Osteoarthritis (WOMAC) pain scale.6 The cohort for the current analysis comprises all participants with complete information before surgery and at 12-month follow-up.
The APEX trials were approved by Southampton and South West Hampshire Research Ethics Committee (09/H0504/94), and all participants provided informed written consent.
2.1. Measurements
2.1.1. Exposure
Widespread pain sensitivity, measured by the assessment of forearm PPTs, was the primary exposure of interest. Preoperative PPTs were assessed at the pain-free volar forearm using a digital algometer (Somedic, Hörby, Sweden) with a 1-cm probe. Pressure pain thresholds were measured at a pain-free site distant to the osteoarthritic joint to measure widespread pain sensitivity. The probe was held perpendicular to the skin, and force was applied at a constant rate of 10 kPa/s. This rate of stimulus intensity change was used to minimize the impact of the examiners' reaction time on the recorded pain threshold values.40 The participant was instructed by the research nurse to say “stop” when the sensation of pressure became the very first sensation of pain. Pressure algometry was repeated 3 times, and between each reading, the position of the algometer on the skin was altered very slightly to avoid sensitization of the test area. The primary exposure was a standardized average of the 3 PPT measurements. Lower PPTs represent increased widespread pressure pain sensitivity.
2.1.2. Outcomes
The primary outcome for this analysis was patient-reported pain severity in the replaced joint. Pain severity was assessed before surgery and at 12 months after surgery using the WOMAC pain scale.6 The scale was administered as part of a postal questionnaire, and participants who did not respond after 2 postal reminders were contacted and asked to complete the scale over the telephone. Administration of the WOMAC scale over the telephone has been validated for collection of outcomes data.7 The WOMAC pain scale is a validated tool that includes 5 questions asking about pain severity on (1) walking, (2) using stairs, (3) sitting or lying, (4) in bed, and (5) standing upright. Response options for each item are on a 5-point ordered response scale (none, mild, moderate, severe, and extreme). Total WOMAC pain scores were calculated as an average of all 5 items. Previous research has emphasized the importance of distinguishing between movement pain and rest pain,43,48 and therefore further analysis was conducted with these subcomponents of the WOMAC pain score. Movement pain was calculated as an average of WOMAC pain scale items 1, 2, and 5, and rest pain was calculated as an average of items 3 and 4.49
2.2. Confounding variables
Confounding factors that were adjusted for in the analyses included age at recruitment, gender, cohabitation (living alone), employment status, educational attainment (more than after 16 years of age), height, and weight. These factors were adjusted for based on the literature that suggests that demographics,45 socioeconomic status,5,15 and obesity39,42 influence patient-reported outcomes after joint replacement. In addition, as this analysis involved analyzing the APEX trials as cohort data, all analyses were adjusted for the treatment participants received in the trial. This approach ensures that any treatment effect from the intervention does not bias the results.10,33
2.3. Statistical methods
2.3.1. Descriptive statistics
Population characteristics and outcome measures are reported as means, SDs, and interquartile cut points for continuous measures and as frequencies for categorical variables. In addition, the SD of the individual 3 PPT measurements was calculated and summarized to indicate the variability of the QST measurements. T tests were used to compare scores between patients undergoing THR and those undergoing TKR.
2.3.2. Cross-sectional/prospective analysis
Simple linear regression was used to investigate the association between average preoperative pain (cross-sectional analysis) and postoperative pain (prospective analysis) and standardized PPTs. Three adjusted models were fitted: (1) minimally adjusted for gender and randomization, (2) more fully adjusted ie, model 1 and age, height, weight, cohabitation, employment, and education, and (3) baseline adjusted ie, model 2 and preoperative pain. The analyses were repeated using the average of all 5 WOMAC pain items, WOMAC pain items associated with movement pain, and WOMAC pain items associated with rest pain. Results are interpreted as per SD increase in PPT and its association with a 1-unit change in pain on the WOMAC pain scale either preoperatively or postoperatively while holding all other factors constant.
2.3.3. Longitudinal analysis
Using a multilevel model, a longitudinal analysis of pain preoperatively and at 12 months postoperatively was conducted. A multilevel approach allows simultaneous investigation of the effect of PPT on preoperative pain severity and change in pain after surgery. This approach is subtly different from model 3 described in the prospective analysis, as the effect of PPTs on preoperative pain severity is not modeled. The effect of PPTs on preoperative pain was investigated by the inclusion of an interaction between the preoperative measurement occasion and standardized PPTs. Results are interpreted as per SD increase in PPTs and the association with preoperative pain. In addition, the effect of PPTs on change in pain is also modeled by the inclusion of an interaction between standardized PPTs and time. Results are interpreted as per SD increase in PPTs and the association with change in pain from preoperative to postoperative while taking into account any effect of PPTs on preoperative pain.
To investigate the linearity of PPTs on preoperative pain and change in pain, 2 additional models were fitted. Using tertiles of preoperative PPTs, a longitudinal model was refitted with separate intercepts and a common slope. In addition, a fully stratified model of pain was fitted using 3 different intercepts and 3 different slopes. Models were compared using likelihood ratio tests.
All models are fitted using iterative generalized least squares in MLwiN (Bristol, UK)44 using Stata runmlwin command.28
3. Results
3.1. Descriptive data
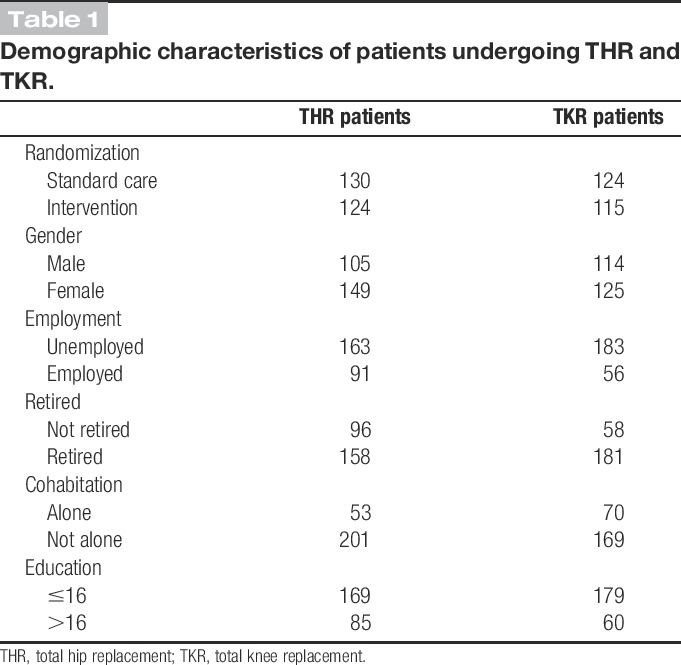
A total of 254 patients undergoing THR and 239 patients undergoing TKR had complete covariate information and were included in these analyses. Baseline characteristics of participants are provided in Table 1. There was a higher percentage of women undergoing THR than men (59:41), compared with the more equal percentage of women and men undergoing TKR (52:48). Patients undergoing THR had a mean age of 66.5 years (SD, 10), which was slightly less than the mean age of 69.1 years for patients undergoing TKR (SD, 8.2).
Table 1.
Demographic characteristics of patients undergoing THR and TKR.
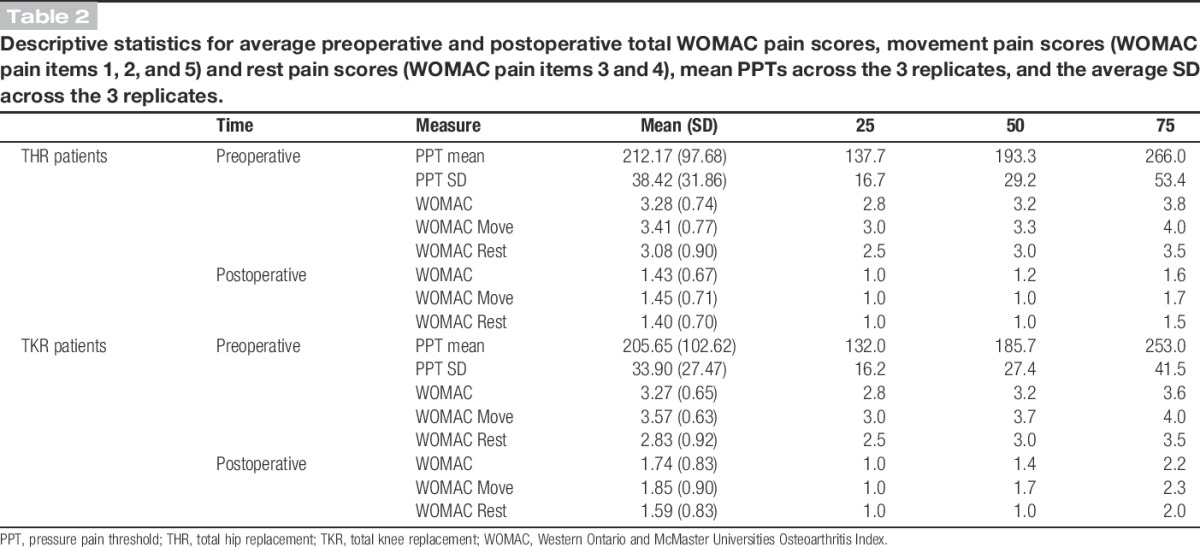
Preoperative total WOMAC pain scores were similar between patients undergoing THR and TKR (P = 0.87) (Table 2). However, patients undergoing TKR had more severe preoperative movement pain (P = 0.01) and patients undergoing THR had more severe preoperative rest pain (P = 0.0024). Pain severity at 12 months after surgery was significantly higher in patients undergoing TKR compared with those undergoing THR, whether considering overall pain severity, movement pain, or rest pain (P < 0.01). The mean preoperative PPT for patients undergoing THR was 212 (SD, 98), which was similar to the mean PPT of 206 (SD, 103) for patients undergoing TKR (P = 0.48) (Table 2).
Table 2.
Descriptive statistics for average preoperative and postoperative total WOMAC pain scores, movement pain scores (WOMAC pain items 1, 2, and 5) and rest pain scores (WOMAC pain items 3 and 4), mean PPTs across the 3 replicates, and the average SD across the 3 replicates.
3.2. Preoperative widespread pain sensitivity and preoperative pain severity
3.2.1. Total hip replacement
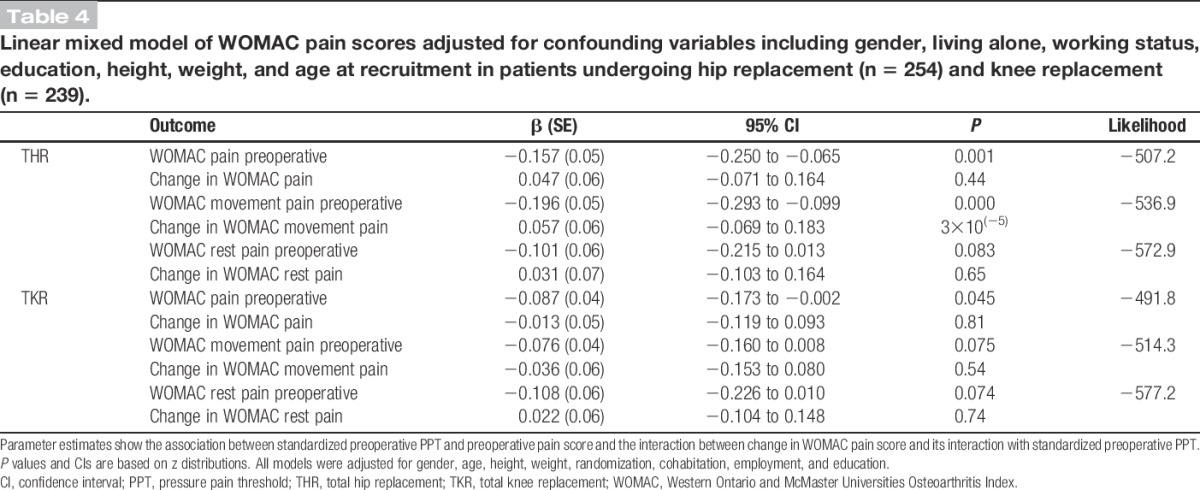
In both the minimally and fully adjusted linear regression models, preoperative PPTs were strongly associated with preoperative pain severity (P = 0.002 and P = 0.001, respectively; Table 3). The association was negative, reflecting that higher pain severity was associated with lower PPTs ie, greater widespread pressure pain sensitivity. The same pattern of association was found using a linear mixed model (P = 0.001; Table 4). When the preoperative WOMAC pain score was broken down in the subconstructs of movement pain and rest pain, preoperative PPTs were significantly associated with movement pain but not rest pain (Tables 3 and 4).
Table 3.
Simple linear regressions between average PPT and total WOMAC pain scores, movement pain scores and rest pain scores, adjusted for confounding variables including gender, living alone, working status, education, height, weight, and age at recruitment in patients undergoing hip replacement (n = 254) and knee replacement (n = 239).
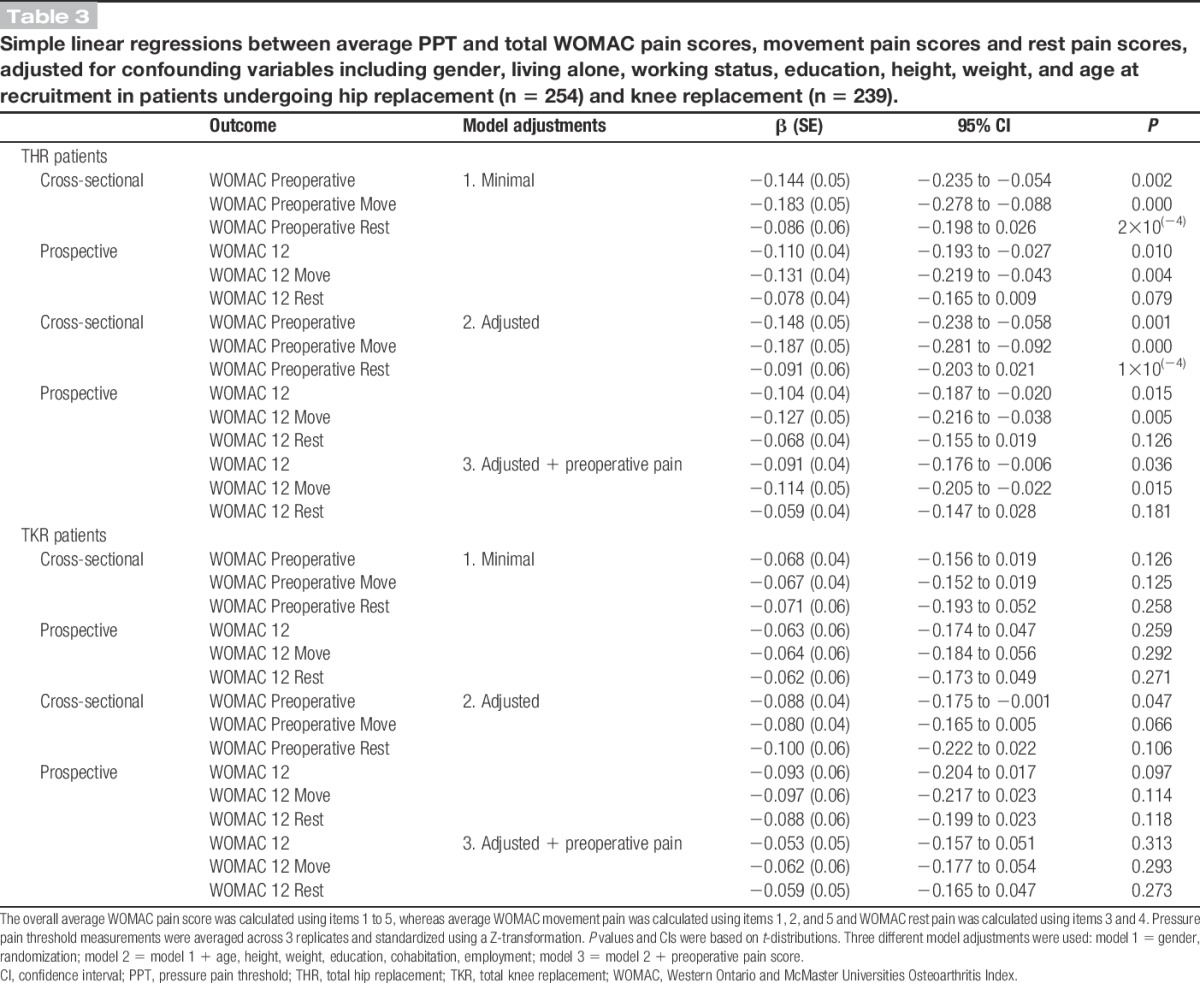
Table 4.
Linear mixed model of WOMAC pain scores adjusted for confounding variables including gender, living alone, working status, education, height, weight, and age at recruitment in patients undergoing hip replacement (n = 254) and knee replacement (n = 239).
3.2.2. Total knee replacement
The patterns of associations were much weaker in patients listed for TKR than THR (Table 3). In the minimally adjusted models, there was no evidence of an association of PPTs with total, movement, or rest pain before surgery (P > 0.1). However, following more complete adjustment, the strength of the association increased to borderline significance for total pain severity (P = 0.047). Similarly, a weak association between PPTs and preoperative total pain severity (P = 0.045), but not movement pain or rest pain, was found in the linear mixed model (Table 4).
3.3. Preoperative widespread pain sensitivity and postoperative pain severity
3.3.1. Total hip replacement
In the minimally and fully adjusted linear regression models, there was strong evidence of an association between preoperative PPTs and pain severity at 12 months after surgery (P = 0.01 and P = 0.015, respectively; Table 3). These models showed that lower PPTs were associated with more severe pain at 12 months after surgery. When the analyses were repeated with movement pain and rest pain, PPTs were associated with movement pain, but not rest pain, at 12 months after surgery (Table 3).
3.3.2. Total knee replacement
There was no evidence of an association between preoperative PPTs and pain severity at 12 months after surgery in any of the linear regression models (Table 3). Similarly, further analysis found that PPTs were not associated with rest pain or movement pain at 12 months postoperative (Table 3).
3.4. Preoperative widespread pain sensitivity and change in pain severity
3.3.3. Total hip replacement
After adjusting the prospective analysis for preoperative pain severity (Table 3), the associations in the linear regression models between PPTs and pain severity at 12 months postoperative were mildly attenuated. Using a linear mixed model to fully adjust any differences in preoperative PPTs, the association between PPTs and change in WOMAC pain scores from preoperative to 12 months postoperative was investigated (Table 4). There was no evidence of an association between PPTs and change in total pain (P = 0.44), movement pain (P = 0.37), or rest pain score (P = 0.65). Further analyses using PPT tertiles to explore the linearity of the relationship between preoperative PPTs and change in pain scores showed similar results for total, movement, and rest pain (Web Appendix, available as Supplemental Digital Content, at http://links.lww.com/PAIN/A0).
3.3.4. Total knee replacement
There was no evidence of any association between PPTs and change in pain score from preoperative to 12 months after TKR, using either the prospective analysis adjusted for preoperative PPTs or the linear mixed model approach (Tables 3 and 4). This finding was the same when the analyses were repeated for movement pain and rest pain. Further analyses using PPT tertiles to explore the relationship between preoperative PPTs and change in pain scores showed similar results (Web appendix, available as Supplemental Digital Content, at http://links.lww.com/PAIN/A0).
Additionally, analyses were conducted in both the TKR and THR cohort to explore the interaction of gender with preoperative pain severity, change in pain severity, PPTs and preoperative pain severity, and PPTs and change in pain severity. No strong evidence of an interaction was observed (data not shown).
4. Discussion
This study provides novel insight into the impact of preoperative widespread pain sensitivity on chronic pain after THR and TKR. We found a strong association between widespread pressure pain sensitivity and preoperative pain severity in a large sample of patients with advanced hip osteoarthritis (n = 254) and a weaker association in patients with advanced knee osteoarthritis (n = 239). Our longitudinal study design allowed us to find that the association between preoperative PPTs and pain severity still persists at 12 months after THR, but not in TKR. However, despite the association between PPTs and pain severity, we demonstrated that preoperative PPTs did not influence the efficacy of THR or TKR in providing pain relief. This finding suggests that preoperative widespread pressure pain sensitivity does not influence the amount of pain relief that patients gain from joint replacement, independent of preoperative pain severity.
Approximately 10% of patients with THR and 20% of patients with TKR report an unfavorable pain outcome between 3 months and 5 years after surgery. Why some patients develop chronic postsurgical pain is not yet clear because of the complex nature of this condition.8 In addition to the contribution of demographic and socioeconomic factors, research has highlighted the importance of a number of potentially modifiable factors in chronic postsurgical pain, including other chronic pain conditions,55 severity of acute postoperative pain,2 surgical factors,12,51 pain perception abnormalities,31,58 and psychosocial factors, such as depression, anxiety, stress, and pain catastrophizing.12,20,35 Given the multifactorial nature of chronic postsurgical pain, future research into prevention through preoperative screening to identify patients at high risk would need to incorporate a range of potential risk factors. However, as this is an emerging field of research, the individual risk factors need to be explored in detail to determine their potential predictive value and optimal assessment methods.
The findings from our analysis of preoperative data add to the growing literature that demonstrates that higher intensity joint pain is associated with greater widespread pain sensitivity in patients with osteoarthritis.3,4,16,37 However, we are unable to draw conclusions as to the direction of this relationship. The observed association could be interpreted in 2 ways: the severity of joint pain is heightened because of widespread pain sensitization, or widespread pain sensitization is driven by peripheral nociceptive input from the osteoarthritic joint. In support of the first theory, a study of patients with osteoarthritis found that patients with mild structural joint change but high pain had more widespread pain sensitization than patients with severe joint change and high pain, suggesting that some patients with osteoarthritis may have pain that is more driven by changes within the central nervous system than peripheral factors.16 Another study found that there was no association between duration of osteoarthritis symptoms and the extent of widespread pain sensitization, and the authors propose the possibility that pain sensitization is a “trait” rather than a state induced by osteoarthritis pain.37 However, the data to date have been cross-sectional and further longitudinal research is needed before causality can be determined.
There has been increasing interest in the application of QST in a surgical context. Many studies in this field have focused on the potential for preoperative QST to predict the severity of acute postoperative pain.17 Specific to orthopedics, studies have demonstrated that preoperative QST parameters are predictive of acute postoperative pain severity52 and morphine consumption.34 However, with the growing recognition of chronic postsurgical pain,32 the focus has turned toward investigating whether preoperative pain perception abnormalities are predictive of chronic postsurgical pain. This relationship has been explored in a wide range of surgical settings, including hernia repair,1 laparoscopic cholecystectomy,9 shoulder surgery,19 thoracotomy,59 and hysterectomy.11 The findings from these studies are mixed, although some report an association between preoperative QST and chronic postsurgical pain, suggesting that increased preoperative widespread pain sensitivity may be a risk factor for chronic postsurgical pain.19,59 Within the context of orthopedic surgery, a number of studies have demonstrated that preoperative pain perception abnormalities normalize after successful joint replacement surgery.3,18,26,27 Regarding the investigation of the predictive value of preoperative QST parameters, only a limited number of small studies have been conducted and these have demonstrated an association between measures of widespread pain sensitization and chronic pain after joint replacement.31,58 However, our study demonstrates that the observed associations between preoperative QST results and the severity of chronic pain after joint replacement are because of preoperative pain severity and that widespread pain sensitization does not influence the amount of long-term pain relief that patients gain from surgery.
Our findings suggest that inclusion of widespread pain sensitization, assessed by measurement of PPTs at the forearm, would add little predictive value to a preoperative screening protocol to identify patients at high risk of not responding to joint replacement surgery. However, these findings only apply to a single QST parameter and further research is warranted to explore the potential value of other measures of pain perception abnormalities. For example, less-efficient preoperative descending pain control, measured through assessment of diffuse noxious inhibitory controls, has been found to be associated with chronic postsurgical pain after thoracotomy59 and abdominal surgery.53 Studies involving patients with established chronic postsurgical pain have demonstrated that these patients have evidence of hypersensitivity, facilitated temporal summation, and impaired descending pain control.38,47 Also, there is some evidence supporting the effectiveness of pharmacological interventions for chronic postsurgical pain that prevent the development of postoperative pain sensitization.13,14 Therefore, further research is needed to investigate the role of other pain perception abnormalities in the development and maintenance of chronic pain after joint replacement.
Strengths of this study include the large sample size, long-term postoperative follow-up, use of validated outcome measures to assess pain, and good rates of data collection for the PPTs and questionnaire data. Our longitudinal study design allowed us to prospectively collect data and therefore draw conclusions on causality, an advantage over previous cross-sectional studies.38,47 Demographic and socioeconomic factors known to influence the pain experience, such as age, gender, cohabitation, and educational attainment, were adjusted for in the analyses. The study sample population is representative of the population undergoing THR and TKR as a whole with a similar disease profile, gender mix, and age range as reported by the National Joint Registry of England and Wales,36 and thus we believe the results to be generalizable. However, it is important to acknowledge the limitations of the study when interpreting the results. Pressure pain thresholds were measured as they have been shown to be a reliable and sensitive measure of pain sensitization in patients with osteoarthritis,50,56 and measurements are quick and easy to perform in a clinical setting. However, pain thresholds are a “static” measure and only assess a single point on a continuum of the pain experience.17 Assessing dynamic responses to pain, such as through assessing temporal summation or conditioned pain modulation, may provide further insight into pain modulation processes. In terms of the statistical analysis, many patients reported no pain at 12 months after surgery; it is possible that this ceiling effect observed on the WOMAC pain score may have been masking an effect modification of QST. In addition, the creation of tertiles is somewhat arbitrary; however, there was no evidence of nonlinearity following the inclusion of higher-order quadratic terms. Finally, there are many factors that can influence chronic postoperative pain, and while theoretically, we could have accounted for more of these factors in our analyses, such as psychosocial factors, existence of other chronic pain conditions, previous joint surgery, use of analgesics and acute postoperative pain severity, model convergence becomes difficult. Therefore, we controlled for key confounding variables including demographic and socioeconomic factors.
These findings have both methodological and clinical implications. In terms of methodology, our study highlights that simple analyses investigating the association between preoperative widespread pain sensitivity and postoperative pain severity need to be interpreted with caution, as they fail to fully account for the influence of preoperative pain severity. Using longitudinal multilevel modeling approach allows for analyses to investigate the change in pain severity over time, independent of preoperative pain severity. In terms of clinical implications, our findings provide novel evidence that preoperative widespread pressure pain sensitivity is not associated with the amount of pain relief that patients gain from joint replacement, independent of preoperative pain severity. Previous research has found that preoperative pain-processing abnormalities normalize after joint replacement, suggesting that these abnormalities are maintained by peripheral nociceptive input.3,18,26 Our findings support this hypothesis and provide evidence that preoperative widespread pain sensitivity is not a predictor of response to joint replacement, suggesting that this particular QST parameter would add little value to a preoperative screening protocol to identify patients at high risk of not responding to joint replacement surgery. Further research is needed to confirm these findings and explore whether other measures of altered preoperative pain processing demonstrate similar results.
Conflict of interest statement
The authors have no conflicts of interest to declare. This article presents independent research funded by the National Institute for Health Research (NIHR) under its Programme Grants for Applied Research program (RP-PG-0407-10070). The views expressed in this article are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health. The research team acknowledges the support of the NIHR, through the Comprehensive Clinical Research Network.
Supplementary Material
Acknowledgements
The authors thank all patients and staff at the Avon Orthopaedic Centre who were involved in the APEX trials. Particular thanks go to the following consultant orthopedic surgeon and anesthetists: Gordon Bannister, John Church, Steve Eastaugh-Waring, Alan Gibson, Williams Harries, Paul Harvie, Katherine Jenkins, Michael Kelly, Nick Koehli, John Leigh, Sanchit Mehendale, Ronelle Mouton, Andrew Porteous, and Jason Webb. The authors also thank the following people who were involved in data collection: Debbie Delgado, Louise Hawkins, Cindy Mann, Leigh Morrison, Dave Rea, Jenny Tyler, and Connie Jamera.
Appendix A. Supplemental Digital Content
Supplemental Digital Content associated with this article can be found online at http://links.lww.com/PAIN/A0.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Author Contributions: V. Wylde, R. Gooberman-Hill, A. D. Beswick, M. Pyke, P. Dieppe, and A. W. Blom were involved in the design, management, and delivery of the study. E. Lenguerrand was involved in data management, and A. Sayers performed the statistical analyses. V. Wylde and A. Sayers contributed equally to this work and drafted the article. All authors revised the article critically for important intellectual content and gave final approval of the version to be submitted.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.painjournalonline.com).
References
- [1].Aasvang EK, Gmaehle E, Hansen JB, Gmaehle B, Forman JL, Schwarz J, Bittner R, Kehlet H. Predictive risk factors for persistent postherniotomy pain. Anesthesiology 2010;112:957–69. [DOI] [PubMed] [Google Scholar]
- [2].Althaus A, Arranz Becker O, Neugebauer E. Distinguishing between pain intensity and pain resolution: using acute post-surgical pain trajectories to predict chronic post-surgical pain. Eur J PAIN 2014;18:513–21. [DOI] [PubMed] [Google Scholar]
- [3].Aranda-Villalobos P, Fernandez-de-Las-Penas C, Navarro-Espigares JL, Hernandez-Torres E, Villalobos M, Arendt-Nielsen L, Arroyo-Morales M. Normalization of widespread pressure pain hypersensitivity after total hip replacement in patients with hip osteoarthritis is associated with clinical and functional improvements. Arthritis Rheum 2013;65:1262–70. [DOI] [PubMed] [Google Scholar]
- [4].Arendt-Nielsen L, Nie H, Laursen MB, Laursen BS, Madeleine P, Simonsen OH, Graven-Nielsen T. Sensitization in patients with painful knee osteoarthritis. PAIN 2010;149:573–81. [DOI] [PubMed] [Google Scholar]
- [5].Barrack RL, Ruh EL, Chen J, Lombardi AV, Jr, Berend KR, Parvizi J, Della Valle CJ, Hamilton WG, Nunley RM. Impact of socioeconomic factors on outcome of total knee arthroplasty. Clin Orthop Relat Res 2014;472:86–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol 1988;15:1833–40. [PubMed] [Google Scholar]
- [7].Bellamy N, Campbell J, Hill J, Band P. A comparative study of telephone versus onsite completion of the WOMAC 3.0 osteoarthritis index. J Rheumatol 2002;29:783–6. [PubMed] [Google Scholar]
- [8].Beswick AD, Wylde V, Gooberman-Hill R, Blom A, Dieppe P. What proportion of patients report long-term pain after total hip or knee replacement for osteoarthritis? A systematic review of prospective studies in unselected patients. BMJ Open 2012;2:e000435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Bisgaard T, Rosenberg J, Kehlet H. From acute to chronic pain after laparoscopic cholecystectomy: a prospective follow-up analysis. Scand J Gastroenterol 2005;40:1358–64. [DOI] [PubMed] [Google Scholar]
- [10].Black JA, Sharp SJ, Wareham NJ, Sandbaek A, Rutten GE, Lauritzen T, Khunti K, Davies MJ, Borch-Johnsen K, Griffin SJ, Simmons RK. Change in cardiovascular risk factors following early diagnosis of type 2 diabetes: a cohort analysis of a cluster-randomised trial. Br J Gen Pract 2014;64:e208–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Brandsborg B, Dueholm M, Kehlet H, Jensen TS, Nikolajsen L. Mechanosensitivity before and after hysterectomy: a prospective study on the prediction of acute and chronic postoperative pain. Br J Anaesth 2011;107:940–7. [DOI] [PubMed] [Google Scholar]
- [12].Bruce J, Thornton AJ, Powell R, Johnston M, Wells M, Heys SD, Thompson AM, Cairns Smith W, Chambers WA, Scott NW; Recovery Study Group. Psychological, surgical, and sociodemographic predictors of pain outcomes after breast cancer surgery: a population-based cohort study. PAIN 2014;155:232–43. [DOI] [PubMed] [Google Scholar]
- [13].Chaparro LE, Smith SA, Moore RA, Wiffen PJ, Gilron I. Pharmacotherapy for the prevention of chronic pain after surgery in adults. Cochrane Database Syst Rev 2013;7:CD008307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Clarke H, Bonin RP, Orser BA, Englesakis M, Wijeysundera DN, Katz J. The prevention of chronic postsurgical pain using gabapentin and pregabalin: a combined systematic review and meta-analysis. Anesth Analg 2012;115:428–42. [DOI] [PubMed] [Google Scholar]
- [15].Clement ND, Jenkins PJ, MacDonald D, Nie YX, Patton JT, Breusch SJ, Howie CR, Biant LC. Socioeconomic status affects the Oxford knee score and short-form 12 score following total knee replacement. Bone Joint J 2013;95-B:52–8. [DOI] [PubMed] [Google Scholar]
- [16].Finan PH, Buenaver LF, Bounds SC, Hussain S, Park RJ, Haque UJ, Campbell CM, Haythornthwaite JA, Edwards RR, Smith MT. Discordance between pain and radiographic severity in knee osteoarthritis: findings from quantitative sensory testing of central sensitization. Arthritis Rheum 2013;65:363–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Granot M. Can we predict persistent postoperative pain by testing preoperative experimental pain? Curr Opin Anaesthesiol 2009;22:425–30. [DOI] [PubMed] [Google Scholar]
- [18].Graven-Nielsen T, Wodehouse T, Langford RM, Arendt-Nielsen L, Kidd BL. Normalization of widespread hyperesthesia and facilitated spatial summation of deep-tissue pain in knee osteoarthritis patients after knee replacement. Arthritis Rheum 2012;64:2907–16. [DOI] [PubMed] [Google Scholar]
- [19].Gwilym SE, Oag HC, Tracey I, Carr AJ. Evidence that central sensitisation is present in patients with shoulder impingement syndrome and influences the outcome after surgery. J Bone Joint Surg Br 2011;93:498–502. [DOI] [PubMed] [Google Scholar]
- [20].Hinrichs-Rocker A, Schulz K, Jarvinen I, Lefering R, Simanski C, Neugebauer EA. Psychosocial predictors and correlates for chronic post-surgical pain (CPSP): a systematic review. Eur J PAIN 2009;13:719–30. [DOI] [PubMed] [Google Scholar]
- [21].Jeffery AE, Wylde V, Blom AW, Horwood JP. “It's there and I'm stuck with it”: patients' experiences of chronic pain following total knee replacement surgery. Arthritis Care Res (Hoboken) 2011;63:286–92. [DOI] [PubMed] [Google Scholar]
- [22].Judge A, Arden NK, Cooper C, Kassim Javaid M, Carr AJ, Field RE, Dieppe PA. Predictors of outcomes of total knee replacement surgery. Rheumatology (Oxford) 2012;51:1804–13. [DOI] [PubMed] [Google Scholar]
- [23].Judge A, Batra RN, Thomas GE, Beard D, Javaid MK, Murray DW, Dieppe PA, Dreinhoefer KE, Peter-Guenther K, Field R, Cooper C, Arden NK. Body mass index is not a clinically meaningful predictor of patient reported outcomes of primary hip replacement surgery: prospective cohort study. Osteoarthritis Cartilage 2014;22:431–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kassam A, Dieppe P, Toms AD. An analysis of time and money spent on investigating painful total knee replacements. Br J Med Pract 2012;5:a526. [Google Scholar]
- [25].King CD, Sibille KT, Goodin BR, Cruz-Almeida Y, Glover TL, Bartley E, Riley JL, Herbert MS, Sotolongo A, Schmidt J, Fessler BJ, Redden DT, Staud R, Bradley LA, Fillingim RB. Experimental pain sensitivity differs as a function of clinical pain severity in symptomatic knee osteoarthritis. Osteoarthritis Cartilage 2013;21:1243–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Kosek E, Ordeberg G. Abnormalities of somatosensory perception in patients with painful osteoarthritis normalize following successful treatment. Eur J PAIN 2000;4:229–38. [DOI] [PubMed] [Google Scholar]
- [27].Kosek E, Ordeberg G. Lack of pressure pain modulation by heterotopic noxious conditioning stimulation in patients with painful osteoarthritis before, but not following, surgical pain relief. PAIN 2000;88:69–78. [DOI] [PubMed] [Google Scholar]
- [28].Leckie G, Charlton C. Runmlwin: a program to run the MLwiN multilevel modelling software from within Stata. J Stat Softw 2013;52:1–40.23761062 [Google Scholar]
- [29].Lee YC, Lu B, Bathon JM, Haythornthwaite JA, Smith MT, Page GG, Edwards RR. Pain sensitivity and pain reactivity in osteoarthritis. Arthritis Care Res (Hoboken) 2011;63:320–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Lluch E, Torres R, Nijs J, Van Oosterwijck J. Evidence for central sensitization in patients with osteoarthritis pain: a systematic literature review. Eur J PAIN 2014;18:1367–75. [DOI] [PubMed] [Google Scholar]
- [31].Lundblad H, Kreicbergs A, Jansson KA. Prediction of persistent pain after total knee replacement for osteoarthritis. J Bone Joint Surg Br 2008;90-B:166–71. [DOI] [PubMed] [Google Scholar]
- [32].Macrae WA. Chronic post-surgical pain: 10 years on. Br J Anaesth 2008;101:77–86. [DOI] [PubMed] [Google Scholar]
- [33].Martin RM, Kramer MS, Dahhou M, Platt RW, Patel R, Bogdanovich N, Matush L, Davey Smith G; Promotion of Breastfeeding Intervention Trial Study Group. Do gastrointestinal tract infections in infancy increase blood pressure in childhood? A cohort study. J Epidemiol Community Health 2010;64:1068–73. [DOI] [PubMed] [Google Scholar]
- [34].Martinez V, Fletcher D, Bouhassira D, Sessler DI, Chauvin M. The evolution of primary hyperalgesia in orthopedic surgery: quantitative sensory testing and clinical evaluation before and after total knee arthroplasty. Anesth Analg 2007;105:815–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Masselin-Dubois A, Attal N, Fletcher D, Jayr C, Albi A, Fermanian J, Bouhassira D, Baudic S. Are psychological predictors of chronic postsurgical pain dependent on the surgical model? A comparison of total knee arthroplasty and breast surgery for cancer. J Pain 2013;14:854–64. [DOI] [PubMed] [Google Scholar]
- [36].National Joint Registry. 10th annual report. Hemel Hempstead: NJR Centre, 2013. [Google Scholar]
- [37].Neogi T, Frey-Law L, Scholz J, Niu J, Arendt-Nielsen L, Woolf C, Nevitt M, Bradley L, Felson DT; for the Multicenter Osteoarthritis Study. Sensitivity and sensitisation in relation to pain severity in knee osteoarthritis: trait or state? Ann Rheum Dis Published Online Ahead of Print [18 December 2013] doi: 10.1136/annrheumdis-2013-204191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Nikolajsen L, Kristensen AD, Thillemann TM, Jurik AG, Rasmussen T, Kehlet H, Jensen TS. Pain and somatosensory findings in patients 3 years after total hip arthroplasty. Eur J Pain 2008;13:576–81. [DOI] [PubMed] [Google Scholar]
- [39].Nunez M, Lozano L, Nunez E, Segur JM, Sastre S, Macule F, Ortega R, Suso S. Total knee replacement and health-related quality of life: factors influencing long-term outcomes. Arthritis Rheum 2009;61:1062–9. [DOI] [PubMed] [Google Scholar]
- [40].Palmer ST, Martin DJ, Steedman WM, Ravey J. C- and Adelta-fibre mediated thermal perception: response to rate of temperature change using method of limits. Somatosens Mot Res 2000;17:325–33. [DOI] [PubMed] [Google Scholar]
- [41].Pavlakovic G, Petzke F. The role of quantitative sensory testing in the evaluation of musculoskeletal pain conditions. Curr Rheumatol Rep 2010;12:455–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Rajgopal V, Bourne RB, Chesworth BM, MacDonald SJ, McCalden RW, Rorabeck CH. The impact of morbid obesity on patient outcomes after total knee arthroplasty. J Arthroplasty 2008;23:795–800. [DOI] [PubMed] [Google Scholar]
- [43].Rakel BA, Blodgett NP, Bridget Zimmerman M, Logsden-Sackett N, Clark C, Noiseux N, Callaghan J, Herr K, Geasland K, Yang X, Sluka KA. Predictors of postoperative movement and resting pain following total knee replacement. PAIN 2012;153:2192–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Rasbash J, Charlton C, Browne W, Healey M, Cameron B. MLwiN version 2.24. Centre for Multilevel Modelling, University of Bristol, Bristol, 2009. [Google Scholar]
- [45].Santaguida PL, Hawker GA, Hudak PL, Glazier R, Mahomed NN, Kreder HJ, Coyte PC, Wright JG. Patient characteristics affecting the prognosis of total hip and knee joint arthroplasty: a systematic review. Can J Surg 2008;51:428–36. [PMC free article] [PubMed] [Google Scholar]
- [46].Singh JA. Epidemiology of knee and hip arthroplasty: a systematic review. Open Orthop J 2011;5:80–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Skou ST, Graven-Nielsen T, Rasmussen S, Simonsen OH, Laursen MB, Arendt-Nielsen L. Widespread sensitization in patients with chronic pain after revision total knee arthroplasty. PAIN 2013;154:1588–94. [DOI] [PubMed] [Google Scholar]
- [48].Srikandarajah S, Gilron I. Systematic review of movement-evoked pain versus pain at rest in postsurgical clinical trials and meta-analyses: a fundamental distinction requiring standardized measurement. PAIN 2011;152:1734–9. [DOI] [PubMed] [Google Scholar]
- [49].Stratford PW, Kennedy DM, Woodhouse LJ, Spadoni GF. Measurement properties of the WOMAC LK 3.1 pain scale. Osteoarthritis Cartilage 2007;15:266–72. [DOI] [PubMed] [Google Scholar]
- [50].Suokas AK, Walsh DA, McWilliams DF, Condon L, Moreton B, Wylde V, Arendt-Nielsen L, Zhang W. Quantitative sensory testing in painful osteoarthritis: a systematic review and meta-analysis. Osteoarthritis Cartilage 2012;20:1075–85. [DOI] [PubMed] [Google Scholar]
- [51].VanDenKerkhof E, Peters ML, Bruce J. Chronic pain after surgery: time for standardization? A framework to establish core risk factor and outcome domains for epidemiological studies. Clin J Pain 2013;29:2–8. [DOI] [PubMed] [Google Scholar]
- [52].Werner MU, Duun P, Kehlet H. Prediction of postoperative pain by preoperative nociceptive responses to heat stimulation. Anesthesiology 2004;100:115–9; discussion 115A. [DOI] [PubMed] [Google Scholar]
- [53].Wilder-Smith OH, Schreyer T, Scheffer GJ, Arendt-Nielsen L. Patients with chronic pain after abdominal surgery show less preoperative endogenous pain inhibition and more postoperative hyperalgesia: a pilot study. J Pain Palliat Care Pharmacother 2010;24:119–28. [DOI] [PubMed] [Google Scholar]
- [54].Wylde V, Gooberman-Hill R, Horwood J, Beswick A, Noble S, Brookes S, Smith AJ, Pyke M, Dieppe P, Blom AW. The effect of local anaesthetic wound infiltration on chronic pain after lower limb joint replacement: a protocol for a double-blind randomised controlled trial. BMC Musculoskelet Disord 2011;12:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Wylde V, Hewlett S, Learmonth ID, Dieppe P. Persistent pain after joint replacement: prevalence, sensory qualities, and postoperative determinants. PAIN 2011;152:566–72. [DOI] [PubMed] [Google Scholar]
- [56].Wylde V, Palmer S, Learmonth ID, Dieppe P. Test-retest reliability of quantitative sensory testing in knee osteoarthritis and healthy participants. Osteoarthritis Cartilage 2011;19:655–8. [DOI] [PubMed] [Google Scholar]
- [57].Wylde V, Palmer S, Learmonth ID, Dieppe P. Somatosensory abnormalities in knee OA. Rheumatology (Oxford) 2012;51:535–43. [DOI] [PubMed] [Google Scholar]
- [58].Wylde V, Palmer S, Learmonth ID, Dieppe P. The association between pre-operative pain sensitisation and chronic pain after knee replacement: an exploratory study. Osteoarthritis Cartilage 2013;21:1253–6. [DOI] [PubMed] [Google Scholar]
- [59].Yarnitsky D, Crispel Y, Eisenberg E, Granovsky Y, Ben-Nun A, Sprecher E, Best LA, Granot M. Prediction of chronic post-operative pain: pre-operative DNIC testing identifies patients at risk. PAIN 2008;138:22–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


