Abstract
Foxp3+ regulatory T cells (Tregs) maintain immune tolerance and play an important role in immunological diseases and cancers. Recent studies have revealed an intricate relationship between Treg biology and host and microbial metabolism. Various metabolites or nutrients produced by host and commensal microbes, such as vitamins and short chain fatty acids (SCFAs), regulate Treg generation, trafficking and function. Furthermore, cell-intrinsic metabolic programs, orchestrated by mTOR and other metabolic sensors, modulate Foxp3 induction and Treg suppressive activity. Conversely, Tregs are crucial in regulating obesity-associated inflammation and host metabolic balance, and shaping homeostasis of gut microbiota. This review discusses the interplay between Tregs and metabolism, with a particular focus on how host, commensal and cellular metabolism impinges upon Treg homeostasis and function.
Keywords: metabolism, vitamin, commensal microbiota, SCFA, mTOR, obesity
Introduction
Although long considered to be two separate disciplines, metabolism and immunology have been recently merged to form the rapidly advancing field of immunometabolism, which explores the interaction between metabolic activities and immune responses [1]. Upon cognate antigen stimulation, naïve CD4+ T cells are activated, differentiate into TH1, TH2, TH17 or follicular helper (Tfh) effector lineages dependent on instructive cytokine mileu, and some of the effector T cells eventually become memory T cells. Foxp3+ regulatory T cells (Tregs) are a specialized T cell population that provides dominant suppression over effector T cells as well as other immune cells. The majority of Tregs develop in the thymus (tTregs), where they are selected by strong or intermediate T cell receptor (TCR) signals while escaping negative selection [2]. Tregs can also be converted from naïve T cells in the periphery (pTregs), generally at mucosal surfaces that interface with the environment, or in in vitro assays (iTregs), particularly in the presence of the anti-inflammatory cytokine TGF-β. Expression of the transcription factor Foxp3 is essential for Treg development and function, and is regulated by genomic regulatory elements termed conserved noncoding DNA sequences (CNS) 1–3. CNS1 is dispensable for tTreg differentiation, but critical in pTreg generation in gut associated lymphoid tissues (GALT). CNS2 is required for Foxp3 expression in the progeny of dividing Tregs. CNS3, the pioneer element, controls de novo Foxp3 expression and tTreg differentiation [3]. Stimulation through the T cell receptor (TCR) induces Foxp3 expression and promotes Treg-specific CpG hypomethylation in Treg signature genes, and the combined actions of these independent events drive Treg development [4]. Thus, Treg lineage development is governed by both genetic and epigenetic programs.
Recent studies have revealed that metabolic factors derived from both extrinsic and intrinsic sources shape Treg abundance and activity. Host-derived nutrients and hormones play an important role in the generation, proliferation and survival of Tregs. Additionally, commensal microbiota-derived metabolites, such as short chain fatty acids (SCFAs), control Treg homeostasis and function in the GALT. Furthermore, compared to naïve T cells, Tregs exhibit unique metabolic activities, characterized by low to modest glycolysis and elevated mechanistic target of rapamycin (mTOR) activity and nutrient metabolism, and these Treg-intrinsic metabolic pathways program Treg generation and activity [5–7].
These exciting new studies indicate that Tregs could serve as a “liaison” between immunity and metabolism, that is, immune function is affected by metabolic fitness through modulation of Tregs at three levels of regulation: host nutritional status, commensal microbes and the cellular metabolism of Tregs themselves. Here, we first discuss how host metabolism, including vitamin and hormone production, affects Treg cellularity, trafficking and survival. Second, we summarize recent discoveries on how commensal microbial metabolites control colonic Treg generation and activity. Third, we describe how intracellular metabolic pathways program Treg homeostasis and function. Finally, it is also important to note that immune system could reciprocally regulate host, microbial and cellular metabolism through Tregs. Therefore, we briefly discuss the reciprocal interaction between Tregs and metabolic disease, and the implications of this interaction for Treg-based therapeutics.
Host metabolism and Tregs
Metabolism is a set of physical and chemical processes that derive energy and macromolecules from nutrients to sustain life. The interaction between malnutrition and impaired immunity was explored nearly 100 years ago [8], but it was not until late 1950s that malnutrition was firmly established as one of the causes of increased susceptibility to infection [9]. It is now recognized that both malnourishment and over-nutrition, exemplified by the ongoing epidemic of obesity, adversely impact immunity. Further, dysregulated immune system function contributes to many metabolic disorders including insulin resistance and diabetes [10]. Recent findings have revealed that host metabolic status and multiple nutrient metabolites impact Treg homeostasis, and this may in turn have bearing in metabolic disorders and associated inflammation.
Various vitamins and their metabolites control Treg trafficking, de novo generation and survival
Vitamins are essential organic compounds that are either synthesized or obtained through dietary sources. A variety of immunological disorders can result from deficiency of various vitamins [11]. Among these, vitamins A, D, B3 and B9 have been linked to Treg biology. Dietary sources of vitamin A include all-trans-retinol, retinyl esters and β-carotene. These are first converted to all-trans-retinal by alcohol dehydrogenases or short chain dehydrogenases/reductases, which are ubiquitously expressed. All-trans-retinal is then oxidized to all-trans retinoic acid (RA) by retinal dehydrogenases (RALDHs), which are selectively expressed by dendritic cells (DCs) in GALT [12]. RA has pleiotropic effects on the host immune system. Specifically, RA promotes the effector functions of CD4+ T cells [13], supports the generation of immunoglobulin A (IgA) secreting B cells in GALT [14], mediates the balance between innate lymphoid cell (ILC) 3 and ILC2 [15], and controls secondary lymphoid organ development [16]. RA also imprints gut-homing specificity on T cells and B cells by inducing the expression of α4β7 integrin and CCR9, two receptors critical for trafficking to the small intestine [12, 14], and this process is dependent on the activity of the p38 signaling pathway in mucosal DCs [17].
RA has an important role in shaping Treg development and function in the gut. Of note, different T cell lineages do not represent irreversibly differentiated endpoints. In particular, iTregs and TH17 subsets exhibit considerable plasticity, in that they can be reciprocally regulated by cytokines and cellular metabolism [7, 18, 19]. Mucida et al. showed that treatment of naïve CD4+ T cells in vitro with RA and TGF-β induced the expression of Foxp3 and gut-homing receptors, while suppressing TH17 differentiation [20]. Further studies attributed this Treg promoting effect in part to RA-mediated inhibition of proinflammatory cytokine production from CD4+CD44hi effector T cells [21]. RA synthesis from vitamin A in the intestine is dependent upon CD103+ DCs in the mesenteric lymph nodes (MLN) and the small intestine lamina propria (LP). CD103+ DC-derived RA, in combination with TGF-β, induces Foxp3 expression in naïve T cells, and these pTregs preferentially home to MLN and the small intestine [22–24]. This mechanism has significant physiological implications because it underlies tolerance induced by oral or food-derived antigens, known as oral tolerance, which is critically dependent on pTreg generation [25]. Further, TGF-β/RA converted Tregs ameliorate inflammatory responses in animal models of colitis [20]. Thus, RA promotes TGF-β-mediated pTreg generation and homing in GALT and maintains mucosa immune tolerance.
Vitamin D3 is synthetized in the skin from 7-dehydrochelesterol under sunlight, or acquired from the diet. Vitamin D3 is further metabolized into 25-dihydroxyvitamin D3 (25(OH)VD3), and the most biologically active form, 1,25 (OH)2VD3, in the liver, kidney and many immune cells. The cellular action of 1,25 (OH)2VD3 is mediated by the vitamin D receptor (VDR), a ligand-dependent transcription factor. 1,25 (OH)2VD3 inhibits T cell proliferation and cytokine production, as well as inducing Foxp3 expression and enhancing the suppressive activity of Tregs [26–29]. Moreover, it can induce tolerogenic DCs that enhance Treg generation and mediate transplantation tolerance [30]. Vitamin D response elements have been identified in the conserved noncoding sequence region of the human Foxp3 gene, and these could underlie how vitamin D3 induces Foxp3 expression [31]. However, vitamin D3 was shown to be required for human T cell activation [32]. Additionally, mice lacking VDR contain normal numbers of functional Tregs in spleen and thymus [33] and do not develop overt systemic autoimmune disorders [34]. Nonetheless, they do have low-grade inflammation in the colon manifested by increased expression of IL-1β and TNF-α [34]. Therefore, the physiological relevance of vitamin D3 metabolites on Treg induction remains to be ascertained.
Tetrahydrofolate, which is derived from vitamin B9 (also known as folic acid (FA)), is required for DNA synthesis, repair and methylation, and is particularly involved in cell proliferation and survival [35]. tTregs constitutively express high level of folate receptor 4 (FR4), which binds FA and delivers FA derivatives into cells [36]. The importance of dietary FA to Tregs is revealed by the selective reduction of intestinal Tregs in mice fed with FA-deficient diet or treated with an inhibitor that disrupts FA metabolism [37]. FA promotes Treg cellularity by inhibiting apoptosis [37, 38]. Further, FA deficiency leads to increased colonic inflammation, which can be ameliorated by transfer of FR4+ tTregs [37]. Tregs purified from human peripheral blood mononuclear cells (PBMC) also preferentially express FR4 relative to other lymphocytes [36], but the impact of FA on human Tregs remains to be examined. Thus, vitamin B9 metabolism maintains gut Treg survival and restricts intestinal inflammation.
Vitamin B3, also known as niacin and nicotinic acid, is an essential nutrient. Deficiency of vitamin B3 in human leads to pellagra, a disease characterized by intestinal inflammation, diarrhea, dermatitis and dementia [39]. Vitamin B3 signals through the G-protein-coupled receptor (GPR) 109a. This interaction induces anti-inflammatory properties, including the expression of RALDHs in colon macrophages and DCs, which in turn induce Treg differentiation. GPR109a-deficient mice contain reduced colonic Tregs and show increased susceptibility to colonic inflammation [40]. Thus, vitamin B3 promotes colonic Treg generation and maintains colon homeostasis.
Leptin limits Treg proliferation
One of the characteristics of Tregs is their lack of proliferation upon TCR engagement alone in vitro, known as in vitro anergy. In an effort to explain this phenomenon, Matarese and colleagues found that human PBMC Tregs produce leptin (also known as OB) and constitutively express leptin receptor (LEPR, also known as OBR), which can be further increased upon TCR engagement [41, 42]. Leptin, a 16-kilodalton adipokine produced primarily by adipocytes, exerts its canonical function of restraining food intake and promoting energy expenditure by interacting with LEPR in the central nervous system [43]. In tTregs, leptin acts to inhibit TCR-induced proliferation; administration of an anti-leptin antibody breaks in vitro anergy, and tTregs from leptin or LEPR-deficient mice exhibit increased proliferative potential in response to TCR stimulation [41, 42]. These data indicate that leptin is partly responsible for the in vitro anergic phenotype of Tregs. In vivo, leptin or LEPR-deficient mice have reduced susceptibility to autoimmunity [44–47], which is associated with increased tTreg numbers as well as reduced naïve T cell proliferative potential [41]. Additionally, the interplay between leptin signaling and Tregs could underlie the immunosuppressive state induced by fasting or starvation [42]. Furthermore, these observations could potentially explain the significant decrease of visceral adipose tissue (VAT)-associated Tregs in obese mice, as these cells express increased level of leptin in the fat tissue [48, 49]. Whole-body energy metabolism also regulates Tregs independently of leptin. For instance, deletion of Sirt1 in hypothalamic agouti-related peptide-expressing (AgRP) neurons promotes appetite and decreases body metabolism, and this is associated with the reduced abundance of tTregs and impaired immunosuppressive activity in a leptin-independent manner [50].
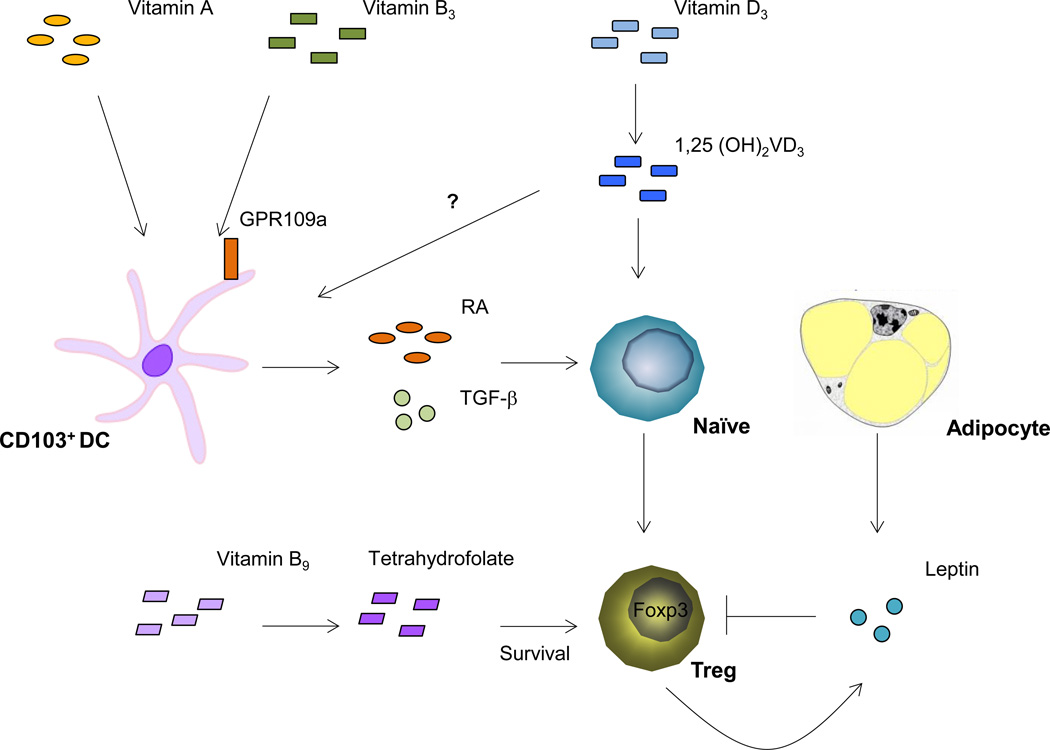
Taken together, host nutritional status, including the uptake and generation of various vitamin metabolites as well as the production of leptin in response to the accumulation of body fat mass, modulates Treg abundance and function. This interaction in turn affects immune homeostasis, particularly in GALT and adipose tissue (Figure 1). Moreover, GALT Tregs are further shaped by commensal microbiota metabolism, which will be described below.
Figure 1. Host metabolism modulates Treg homeostasis.
RA, a vitamin A metabolite produced by GALT CD103+ DC, induces the conversion of naïve CD4+ T cells to Tregs. Vitamin B3 binds to GPR109a on GALT DCs and other innate immune cells, allowing these cells to induce Foxp3 expression in naïve T cells. Vitamin D3, through its metabolite 1,25 (OH)2VD3 and the receptor VDR, enhances Foxp3 gene transcription by binding to the Foxp3 promoter locus. The vitamin B9 metabolite tetrahydrofolate maintains Treg survival. Leptin, produced by adipocyte and Tregs, restrains Treg proliferation and abundance.
Commensal microbial metabolism and Tregs
It is estimated that the human body harbors 100 trillion commensal microbes, exceeding the number of host cells by more than 10-fold. Multiple studies into how the microbiota and the host immune system interact have revealed the fundamental importance of the microbiome in shaping host immune responses, and how the immune system in turn impacts the composition of the microbiota; these concepts have been reviewed elsewhere [51]. Here we focus on the impact of microbial metabolism and the associated metabolites on the differentiation and function of Tregs.
Specific microbial species induce Treg generation in the colon
In mice, both tTreg and gut-induced pTregs are required to maintain intestinal immune tolerance [52–54]. The importance of commensal microbiota for mucosa Treg abundance is underscored by the severe reduction of Tregs in the colon LP in germ-free (GF) mice or Vancomycin-treated mice [55]. In addition, the beneficial effects of probiotics have been partly attributed to the induction of Tregs [56–59]. Given the critical role of Tregs in maintaining intestinal homeostasis, it is probably not surprising that many microbial species have the potential to induce Tregs [60, 61]. Consistent with preferential accumulation of Tregs in the colon during ontogeny [55], Treg expansion induced by microbial colonization occurs specifically in the colon LP [60, 61]. Atarashi et al. screened both mouse and human microbiota for specific microbe species that are capable of inducing Tregs in the colon. They discovered that colonization of mice with murine fecal-derived Clostridia clusters IV and XIVa, or clusters IV, XIVa and XVIII isolated from human feces, expands the population of Tregs and enhances their activity in colonic LP, as indicated by the increased expression of IL-10 and CTLA-4. These gram-positive, spore-forming bacteria preferentially colonize the cecum and proximal colon, consistent with their preferential impact on colonic Tregs. Mechanistically, they are attached to colon intestinal epithelium cells (IECs) and activate IECs to produce TGF-β, a major cytokine that promotes Treg differentiation in the intestine [55, 62]. In addition, colonization of GF mice with Clostridia stimulates colonic conventional T cells to produce IL-2, an essential cytokine for Treg proliferation. IL-2 induces expression of the epigenetic regulator Uhrf1 in colonic Tregs, which silences the expression of the cell cycle inhibitor p21 (encoded by Cdkn1a) by maintaining DNA methylation at the promoter region of Cdkn1a. Deletion of Uhrf1 in CD4+ T cells leads to reduced Tregs specifically in the colon LP and the development of spontaneous colitis [63]. How commensal mirobiota stimulate IL-2 production in conventional T cells remains to be defined. Hence, Clostridia represents a major commensal microbial species that promotes colonic Treg differentiation through TGF-β and IL-2 production from IECs and conventional T cells, respectively.
Polysaccharide A (PSA), produced by Bacteroides fragilis (B. fragilis), a prominent symbiotic anaerobe colonizing mammalian lower gastrointestinal tract, has important immunoregulatory functions [64]. B. fragilis-derived PSA engages Toll-like receptor 2 (TLR2) expressed by T cells, which promotes pTreg generation from naïve CD4+ T cells in GALT and enhances Treg production of regulatory cytokines including IL-10 and TGF-β2. Moreover, PSA promotes IL-10-producing Treg generation in peripheral lymphoid organs. Of note, PSA administration or B. fragilis colonization protects mice from intestinal inflammatory diseases induced by chemical agents or Helicobacter hepaticus colonization [65–67], and ameliorates experimental autoimmune encephalomyelitis (EAE), a murine model for human multiple sclerosis (MS) [68–70]. These findings suggest that PSA production by B. fragilis induces the generation of Tregs with enhanced immunoregulatory activity and protects the host from inflammatory diseases. However, the ability of B. fragilis to induce colonic Tregs is significantly less potent compared to that of Clostridia [55], suggesting that different bacteria may elicit distinct responses, in terms of colonic Treg induction, via potentially different mechanisms.
Specific microbes have also been linked to the generation of effector T cell lineages in the intestine. Colonization of mice with a single commensal microbe, segmented filamentous bacterium (SFB), induces TH17 cells but not other effector lineages or Tregs in the small intestine LP, and this in turn promotes anti-bacterial immunity as well as autoimmune arthritis in pathological settings [71–73]. Notably, commensal microbe-derived ATP drives intestinal TH17 generation, suggesting that microbiota metabolism controls TH17 lineage differentiation in the GALT [74]. The discovery of microbiota-dependent induction of specific T cell subsets suggests that different T cell subsets may recognize distinct commensal microbe antigens. Indeed, intestinal DCs present SFB antigens to drive mucosal TH17 cell differentiation at different lymphoid tissues in an MHC-II-dependent mechanism [75, 76]. Importantly, the TCR repertoire of gut TH17 cells is mostly restricted to SFB antigens, which implies that microbiota antigens could dictate the fate of antigen-specific T cells [77]. This raises the interesting possibility that the TCR repertoire of gut Tregs is also shaped by specific microbial antigens, and future work is required to test this model.
Microbial metabolites promote Treg generation in the colon
Mammals depend upon bacteria to break down certain dietary components, including resistant starch and dietary fibers [78]. SCFAs are major metabolites from the bacterial fermentation of dietary fiber, and are highly enriched in the colon [79]. To explore the immunoregulatory functions of SCFAs, several groups performed a series of quantitative analysis of SCFA composition in caecal content between specific-pathogen free (SPF) mice and GF mice [80, 81] or between mice fed with high-fiber diet and low-fiber diet [82]. They reported that butyrate, acetate and propionate are the major bacteria-derived SCFAs that control the mucosa Treg differentiation and function.
SCFAs promote Treg differentiation through several mechanisms. First, signaling mediated by G protein-coupled receptors for SCFAs stimulates Treg differentiation. Expression of GPR43, which binds multiple SCFAs, on neutrophils and eosinophils has been implicated in dampening gut inflammatory responses [83]. Smith et al. found that colonic Tregs also express GPR43, and that this expression required the presence of gut microbiota. Furthermore, propionate-induced Treg differentiation and potentiation of suppressive activity is abolished in GPR43-deficient mice [81]. GPR109a, which specifically binds butyrate, is expressed on colon IECs and innate immune cells, but not lymphocytes [84]. The interaction between butyrate and GPR109a promotes production of IL-10 and RALDHs from macrophages and DCs, thereby inducing Treg generation [40]. Thus, SCFAs signal via distinct receptors expressed on Tregs and innate immune cells to induce Treg differentiation.
SCFAs also regulate Treg differentiation through epigenetic modifications. Butyrate is a known histone deacetylase (HDAC) inhibitor. When naïve CD4+ T cells are cultured in iTreg differentiation conditions, butyrate treatment enhances acetylation at histone H3 lysine 27 (H3K27) at the Foxp3 promoter and CNS1 and CNS3 enhancers. These epigenetic modifications lead to increased Foxp3 induction [80, 82]. Moreover, butyrate, through its HDAC inhibition activity, potentiates the capability of DCs to induce Treg differentiation by repressing expression of lipopolysaccharide (LPS) response genes [80], and also inhibits macrophage activation by rendering them hyporesponsive to TLR stimulation [85]. However, the developmental origin of SCFAs-induced colonic Tregs remains controversial. Although some studies found that SCFAs only promote the conversion of naïve CD4+ T cells to Tregs [80, 82], one group reported that SCFAs expand existing tTregs in the colon [81]. While further investigation is required to resolve the discrepancy, these studies collectively demonstrate that SCFAs, generated by gut microbial metabolism, promote colonic Treg differentiation and contribute to immune homeostasis in the colon (Figure 2).
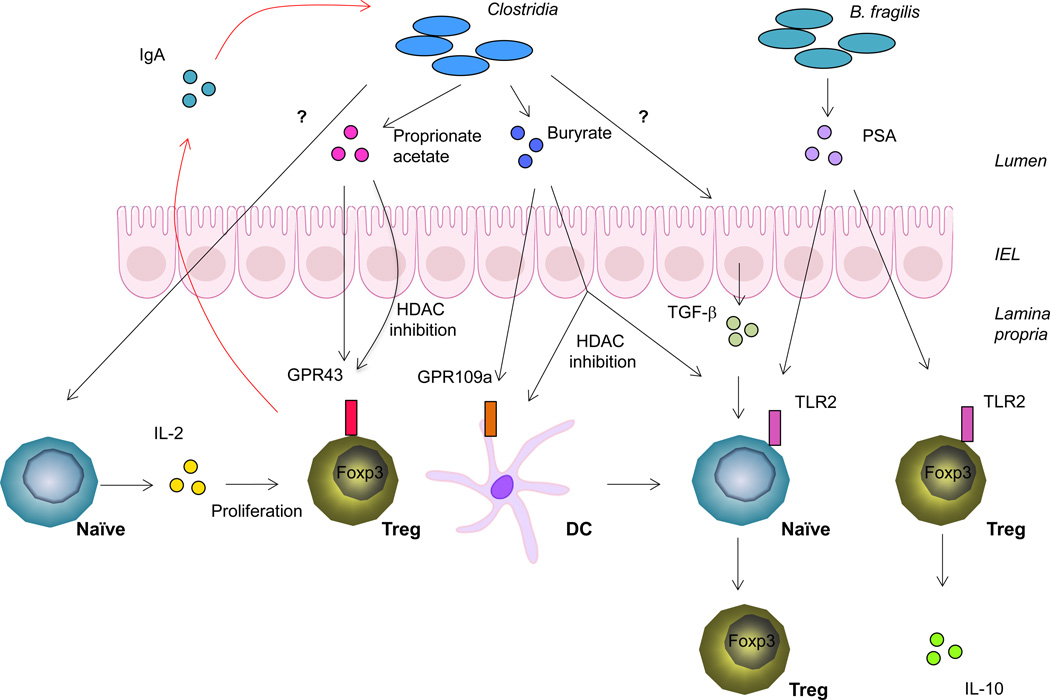
Figure 2. Commensal microbial metabolism controls Treg homeostasis in the colon.
Colonization of mice with Clostridia species from both mouse and human feces leads to increased Treg abundance in the colon. SCFAs, metabolites through bacteria fermentation of dietary fiber, promote Treg expansion and de novo generation. Clostridia species also stimulate conventional T cells to produce IL-2, which promotes colonic Treg proliferation. B. fragilis produces PSA that can induce Treg induction and potentiate Tregs to produce regulatory cytokines such as IL-10. In a feedback loop, Tregs contribute to diversification of Clostridia species by promoting generation and selection of IgA.
Cellular metabolism and Tregs
In addition to aforementioned extrinsic metabolic factors that control Treg differentiation and function, cell-intrinsic metabolic programing also plays a crucial role in Tregs. The progression of T cell activation, effector cell differentiation and memory cell formation is accompanied by a series of metabolic transitions [86, 87]. Furthermore, different T cell lineages display distinct metabolic features; in particular, Tregs exhibit high levels of fatty acid oxidation, nutrient metabolism and elevated mTOR activity as compared to naïve T cells [6, 87]. The evolutionarily conserved mTOR pathway senses diverse environmental cues, including nutrient availability and metabolic activities, to regulate cell growth and differentiation. The mTOR pathway consists of two complexes, mTORC1 and mTORC2, with scaffold proteins Raptor and Rictor as their defining components, respectively. Both mTORC1 and mTORC2 contain the catalytic subunit, the serine/threonine kinase mTOR. Another major upstream activator of mTORC1 is Rheb, a small GTPase, which mediates early, but not late, mTORC1 activation following T cell activation [88]. The immunosuppressive drug rapamycin preferentially inhibits mTORC1, but also interferes with mTORC2 activity. mTORC1 promotes anabolic metabolism especially protein and lipid biosynthesis and inhibits autophagy, whereas mTORC2 regulates cytoskeleton dynamics. The detailed composition and function of mTOR pathway have been reviewed elsewhere [89, 90]. Here we mainly focus on mTOR-dependent regulation of Treg generation and function.
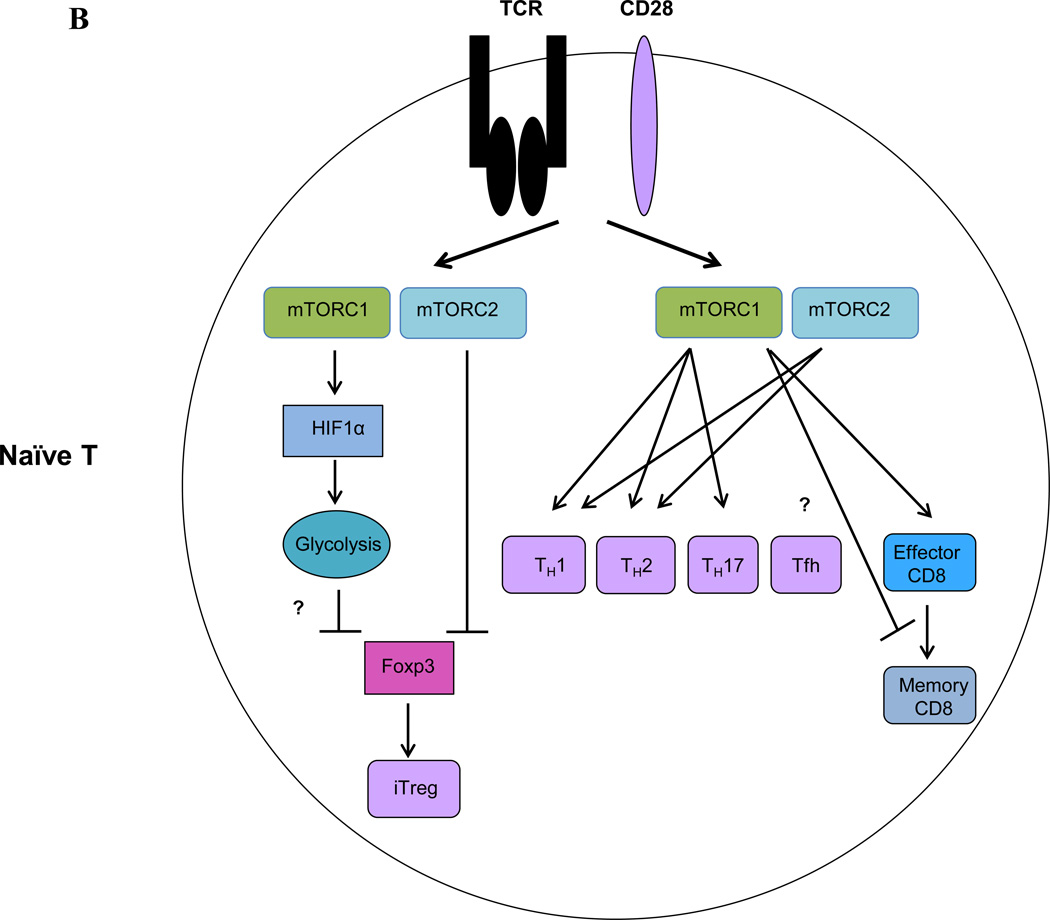
In cell culture experiments, inhibition of mTOR with rapamycin or by restricting essential amino acids (EAAs) induces Foxp3 expression and promotes Treg generation from naïve CD4+ T cells. Conversely, mTOR activation through the PI3K-AKT axis inhibits Foxp3 expression [91–95]. Genetic analyses have confirmed these findings. Deletion of mTOR and thus eliminating both mTORC1 and mTORC2 activity, leads to failure of TH1, TH2 or TH17 effector lineage differentiation, but spontaneous generation of Foxp3+ iTregs, even in the absence of Treg-polarizing cytokines [96] (see Box 1 and Figure 3B for mTOR signaling in conventional T cell differentiation). Importantly, mTOR-dependent induction of the transcription factor hypoxia-inducible factor 1α (HIF1α) suppresses iTreg generation while promoting TH17 differentiation through modulating the glycolytic pathway and transcriptional control [7, 97]. Mice with T cell-specific deletion of Rheb or Rictor remain healthy and their naïve CD4+ T cells do not spontaneously differentiate into Foxp3+ iTregs in vitro, suggesting both mTORC1 and mTORC2 are required to inhibit iTreg generation [96, 98]. Therefore, mTOR signaling is a negative regulator of de novo Foxp3 expression in naïve T cells.
BOX 1. mTOR in effector and memory T cell differentiation (Figure 3B).
While mTOR kinase activity is required for TH1, TH2 and TH17 differentiation [96], different mTOR complexes have differential functions in effector T cell differentiation, as discussed below.
TH1
T cell-specific deletion of Rheb or Rictor leads to impaired TH1 differentiation, suggesting a positive role of mTORC1 and mTORC2 in TH1 differentiation [98, 109]. However, Raptor-deficient T cells exhibit normal TH1 differentiation in vitro [110]. Further research is required to solve this discrepancy.
TH2
While Rheb-deficient T cells differentiate into TH2 cells normally [98], Raptor-deficient T cells have a profound defect in TH2 differentiation in vitro and in vivo [88]. This difference is ascribed to Rheb-independent, but Raptor-dependent mTORC1 activity [88]. Rictor-deficient T cells also have impaired TH2 differentiation [98, 109]. Thus both mTORC1 and mTORC2 promote TH2 differentiation.
TH17
Deletion of either Rheb or Raptor impairs TH17 differentiation [98, 110], while Rictor deletion does not affect this process [98, 109]. Thus, mTORC1 is critical for TH17 differentiation.
Tfh
It is currently unknown whether or how mTOR signaling regulates Tfh differentiation.
Effector and memory CD8+ T cells
Deletion of Raptor abolishes the generation of antigen specific effector CD8+ T cells in a Listeria monocytogenes infection model, while deletion of Rictor marginally reduces this process, indicating that mTORC1 plays a predominant role in effector CD8+ T cell formation [88]. Moreover, pharmacological study using rapamycin demonstrated that mTORC1 is required for effector CD8+ T cell differentiation through transcription factor HIF1 [111]. However, mTORC1 negatively controls memory CD8+ T cell generation, as rapamycin treatment or Raptor knockdown promotes the frequency and function of memory CD8+ T cells [112–114].
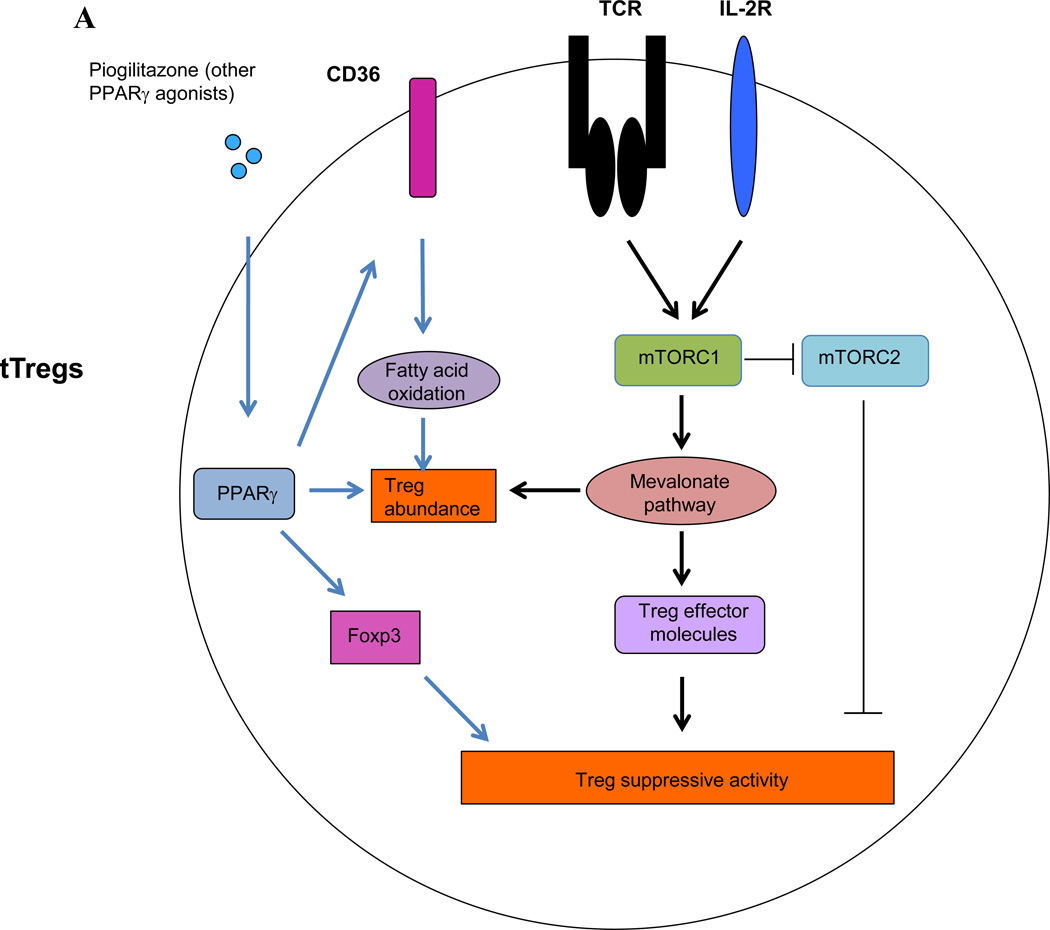
Figure 3. mTOR signaling in Tregs and conventional T cells.
(A) In Tregs, TCR and IL-2 signaling promotes Treg proliferation and function by activating mTORC1-dependent lipid biosynthesis, particularly the mevalonate pathway. mTORC1 also promotes Treg function through inhibition of mTORC2 activity. VAT-associated Tregs exhibit increased expression of PPAR-γ, which promotes fatty acid metabolism and hence stimulates the accumulation and suppressive phenotypes of Tregs residing in adipose tissue. (B) In conventional T cells, mTOR signaling inhibits Foxp3 induction partly by inducing HIF1α expression and HIF1α-dependent glycolysis. mTOR signaling promotes TH1, TH2, TH17 and effector CD8+ T cell differentiation. However, mTORC1 signaling negatively regulates memory CD8+ T cell differentiation. Whether and how mTOR signaling controls Tfh differentiation is currently unknown.
Although initial study using T cell-specific deletion of mTOR indicated that mTOR deficiency does not significantly impact Treg suppressive function in vitro [96], genetic deletion of Rptor (which encodes Raptor) specifically in Tregs revealed that mTORC1 has a surprisingly positive role in Treg function. Treg-specific deletion of Rptor leads to severe systemic autoimmune diseases and early death, while deletion of Rictor preserves immune homeostasis [5]. Raptor-deficient Tregs have intrinsic developmental defects, as they express reduced levels of the immune suppressive molecules CTLA-4 and ICOS and have severely impaired suppressive activity in vitro and in vivo. These functional defects are linked to impaired lipid biosynthesis, in particular the mevalonate pathway. Pharmacological inhibition of the mevalonate pathway in Tregs using statins, inhibitors of the rate-limiting enzyme 3-hydroxy-3-methylglutaryl-CoA reductase (HMGCR), impairs their suppressive activity, while addition of mevalonate, the product of HMGCR, restores Treg-mediated suppression. TCR and IL-2 stimulation potently activates mTORC1, suggesting that mTORC1 couples immune signals and lipid biosynthesis to establish Treg functional competency. The role of mTORC1 in additional metabolic pathways and other relevant processes remains to be explored. Interestingly, deletion of Rictor partially restores the defective suppressive activity of Raptor-deficient Tregs, and Treg-specific deletion of both Rptor and Rictor slightly improves autoimmune symptoms observed in mice lacking Rptor in Tregs. Therefore, mTORC1 promote Treg function partly through inhibition of mTORC2 activity, but mTORC1-dependent lipid metabolism plays a more dominant role [5]. Lipid metabolism, via PPAR-γ-dependent program, has been associated with the homeostasis of VAT-associated Tregs [48], which will be further discussed below. Taken together, these data indicate that T cell-intrinsic metabolic activity controls Foxp3 expression and Treg function, in that mTOR-mediated anabolism inhibits Foxp3 induction in naïve T cells, while mTOR-dependent lipid biosynthesis potentiates Treg function (Figure 3A).
Tregs influence host metabolism and microbiota diversity
The interaction between metabolism and Tregs is a two-way street: host and commensal microbiota metabolism regulates Treg homeostasis, while Tregs also play an essential role in controlling host metabolic disorders and commensal microbial diversity. For example, normal mouse VAT is enriched with Tregs compared to other lymphoid organs, but these VAT-associated Tregs are significantly reduced in obese mice. Loss- and gain-of-function studies demonstrated that VAT-associated Tregs protect against obesity-associated inflammation, insulin resistance and improve metabolic parameters [49]. A later study showed that PPAR-γ, a master transcription factor for adipogenesis, is highly expressed in VAT-associated Tregs and critically regulates their homeostasis. Treg-specific deletion of PPAR-γ depletes Tregs specifically in VAT, but not in other organs. Strikingly, the insulin sensitizing effect of the commonly used drug pioglitazone, a PPAR-γ agonist, is largely dependent on PPAR-γ expression by VAT-associated Tregs. Furthermore, pioglitazone promotes lipid uptake by VAT-associated Tregs by upregulating the expression of the fatty acid transporter CD36 and potentially activating fatty acid oxidation [48]. The protective role of VAT-associated Tregs on metabolic syndromes is also evident from studies on human subjects [99–101]. These findings highlight a potential therapeutic value of modulating Tregs to improve obesity-associated metabolic disorders.
Tregs also exert an important regulatory role on the composition of gut microbiota. In mice deficient of Foxp3 CNS1, which is required for pTreg induction in mucosal environment, there is a reduction of relative abundance of the phylum Firmicutes, which includes Clostridia, in the gut [53]. A recent study demonstrates that Tregs contribute to diversification of commensal microbiota. Tregs support production and selection of immunoglobulin A (IgA) in germinal center of Peyer’s patches. Diversified IgA maintains a diverse microbial community in gut, particularly the diversification of Clostridia cluster IV and XIVa, which, as discussed above, induce gut Treg differentiation. Importantly, Treg-regulated microbial diversity is required for maturation of mucosal immune system [102]. Thus, Tregs and gut microbiota form a regulatory loop to mediate host-microbial symbiosis and immune homeostasis (Figure 2).
Although Tregs control metabolic disorders or dysbiosis-induced inflammatory diseases through inhibition of pro-inflammatory immune cells, Tregs also stimulate the generation and activity of anti-inflammatory M2 macrophages [103]. Moreover, Tregs could alter local amino acid availability by stimulating DCs to express enzymes that catabolize EAAs, and this leads to impaired T cell activation and increased Treg conversion from naïve T cells [94]. For example, the interaction between CTLA-4 on Tregs and CD80/CD86 on DCs induces DC expression of indoleamine 2, 3-dioxygenase (IDO), an enzyme that consumes tryptophan [104]. Treg-mediated limitation of nutrient availability in the immune microenvironment contributes to “infectious tolerance”, which posits that Tregs enforce immune tolerance partly through converting conventional T cells into Tregs. This provides a feed-forward mechanism to amplify the immune regulatory effects of Tregs through metabolic means, adding another layer of complexity to the interplay between metabolism and Tregs.
Concluding remarks
Emerging studies highlight the complex interplay between Tregs and extrinsic and intrinsic metabolic pathways with significant impacts on immune responses and diseases. Recent investigation of how specific nutrient availability and systemic metabolism affect immune cell function underlies the connection between metabolic and immune disorders. Similarly, the latest unraveling of microbial metabolite-mediated control of specific T cell lineage differentiation opens a fascinating new field of research for investigators to decipher the host-commensal interactions. Further, in light of the recent revelation that Tregs at different anatomic locations exert tissue-specific physiological functions [48, 49, 105, 106], it is tempting to speculate that other microbiota-rich locations, such as skin and oral cavity, may harbor Tregs with tissue-specific activities that are modulated by distinct local microbes and metabolites [107]. The discovery that cell-intrinsic metabolism controls T cell lineage fate and Treg function suggests the potential for metabolic modulation of immune function. Moreover, the prominence of Tregs in controlling obesity-associated inflammation indicates that immunotherapy could benefit metabolic diseases [108]. Immunometabolism is a nascent field and many questions remain open (Box 2). The exciting advances and future development may provide new opportunities to develop novel Treg-based immunological interventions for treatment of inflammatory and metabolic diseases.
BOX 2. Important areas of future research.
Determination of whether and how vitamin D affects mucosal Treg differentiation and function
Analysis of TCR repertoire of colon Tregs to determine their antigen specificity
Mechanistic study of mTOR-mediated Foxp3 suppression during iTreg induction
Metabolic profiling of steady-state Tregs and functionally suppressive Tregs under inflammatory conditions.
Identification of mTOR-dependent and independent metabolic factors that support Treg development and function.
Elucidation of the mechanism underlying lipogenesis-mediated Treg functional maturation.
Loss and gain of function study on how mTOR signaling modulates iTreg function
Investigation of Tregs in other barrier environment, such as skin and oral cavity and their relationship with local microbiota.
Nutrients, vitamins, and hormones control the generation and homeostasis of Tregs.
Microbiota-derived metabolites impact intestinal Treg accumulation and function.
mTOR and metabolic programming orchestrates Treg differentiation and activity.
Tregs are crucial in maintaining host metabolic balance and microbiota diversity.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rathmell JC. Metabolism and autophagy in the immune system: immunometabolism comes of age. Immunol Rev. 2012;249:5–13. doi: 10.1111/j.1600-065X.2012.01158.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Josefowicz SZ, Lu LF, Rudensky AY. Regulatory T cells: mechanisms of differentiation and function. Annual review of immunology. 2012;30:531–564. doi: 10.1146/annurev.immunol.25.022106.141623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zheng Y, Josefowicz S, Chaudhry A, Peng XP, Forbush K, Rudensky AY. Role of conserved non-coding DNA elements in the Foxp3 gene in regulatory T-cell fate. Nature. 2010;463:808–812. doi: 10.1038/nature08750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ohkura N, Hamaguchi M, Morikawa H, Sugimura K, Tanaka A, Ito Y, Osaki M, Tanaka Y, Yamashita R, Nakano N, Huehn J, Fehling HJ, Sparwasser T, Nakai K, Sakaguchi S. T cell receptor stimulation-induced epigenetic changes and Foxp3 expression are independent and complementary events required for Treg cell development. Immunity. 2012;37:785–799. doi: 10.1016/j.immuni.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 5.Zeng H, Yang K, Cloer C, Neale G, Vogel P, Chi H. mTORC1 couples immune signals and metabolic programming to establish T-cell function. Nature. 2013 doi: 10.1038/nature12297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Michalek RD, Gerriets VA, Jacobs SR, Macintyre AN, MacIver NJ, Mason EF, Sullivan SA, Nichols AG, Rathmell JC. Cutting edge: distinct glycolytic and lipid oxidative metabolic programs are essential for effector and regulatory CD4+ T cell subsets. Journal of immunology. 2011;186:3299–3303. doi: 10.4049/jimmunol.1003613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shi LZ, Wang R, Huang G, Vogel P, Neale G, Green DR, Chi H. HIF1alpha-dependent glycolytic pathway orchestrates a metabolic checkpoint for the differentiation of TH17 and Treg cells. The Journal of experimental medicine. 2011;208:1367–1376. doi: 10.1084/jem.20110278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Effects of nutrition on growth and resistance to infection. American Journal of Public Health. 1926;162 doi: 10.2105/ajph.16.12.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keusch GT. The history of nutrition: malnutrition, infection and immunity. J Nutr. 2003;133:336S–340S. doi: 10.1093/jn/133.1.336S. [DOI] [PubMed] [Google Scholar]
- 10.Mathis D. Immunological goings-on in visceral adipose tissue. Cell Metab. 2013;17:851–859. doi: 10.1016/j.cmet.2013.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mora JR, Iwata M, von Andrian UH. Vitamin effects on the immune system: vitamins A and D take centre stage. Nature reviews. Immunology. 2008;8:685–698. doi: 10.1038/nri2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iwata M, Hirakiyama A, Eshima Y, Kagechika H, Kato C, Song SY. Retinoic acid imprints gut-homing specificity on T cells. Immunity. 2004;21:527–538. doi: 10.1016/j.immuni.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 13.Hall JA, Cannons JL, Grainger JR, Dos Santos LM, Hand TW, Naik S, Wohlfert EA, Chou DB, Oldenhove G, Robinson M, Grigg ME, Kastenmayer R, Schwartzberg PL, Belkaid Y. Essential role for retinoic acid in the promotion of CD4(+) T cell effector responses via retinoic acid receptor alpha. Immunity. 2011;34:435–447. doi: 10.1016/j.immuni.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mora JR, Iwata M, Eksteen B, Song SY, Junt T, Senman B, Otipoby KL, Yokota A, Takeuchi H, Ricciardi-Castagnoli P, Rajewsky K, Adams DH, von Andrian UH. Generation of gut-homing IgA-secreting B cells by intestinal dendritic cells. Science. 2006;314:1157–1160. doi: 10.1126/science.1132742. [DOI] [PubMed] [Google Scholar]
- 15.Spencer SP, Wilhelm C, Yang Q, Hall JA, Bouladoux N, Boyd A, Nutman TB, Urban JF, Jr, Wang J, Ramalingam TR, Bhandoola A, Wynn TA, Belkaid Y. Adaptation of innate lymphoid cells to a micronutrient deficiency promotes type 2 barrier immunity. Science. 2014;343:432–437. doi: 10.1126/science.1247606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van de Pavert SA, Ferreira M, Domingues RG, Ribeiro H, Molenaar R, Moreira-Santos L, Almeida FF, Ibiza S, Barbosa I, Goverse G, Labao-Almeida C, Godinho-Silva C, Konijn T, Schooneman D, O'Toole T, Mizee MR, Habani Y, Haak E, Santori FR, Littman DR, Schulte-Merker S, Dzierzak E, Simas JP, Mebius RE, Veiga-Fernandes H. Maternal retinoids control type 3 innate lymphoid cells and set the offspring immunity. Nature. 2014;508:123–127. doi: 10.1038/nature13158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang G, Wang Y, Chi H. Control of T cell fates and immune tolerance by p38alpha signaling in mucosal CD103+ dendritic cells. Journal of immunology. 2013;191:650–659. doi: 10.4049/jimmunol.1300398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou L, Lopes JE, Chong MM, Ivanov II, Min R, Victora GD, Shen Y, Du J, Rubtsov YP, Rudensky AY, Ziegler SF, Littman DR. TGF-beta-induced Foxp3 inhibits T(H)17 cell differentiation by antagonizing RORgammat function. Nature. 2008;453:236–240. doi: 10.1038/nature06878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang XO, Nurieva R, Martinez GJ, Kang HS, Chung Y, Pappu BP, Shah B, Chang SH, Schluns KS, Watowich SS, Feng XH, Jetten AM, Dong C. Molecular antagonism and plasticity of regulatory and inflammatory T cell programs. Immunity. 2008;29:44–56. doi: 10.1016/j.immuni.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mucida D, Park Y, Kim G, Turovskaya O, Scott I, Kronenberg M, Cheroutre H. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317:256–260. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- 21.Hill JA, Hall JA, Sun CM, Cai Q, Ghyselinck N, Chambon P, Belkaid Y, Mathis D, Benoist C. Retinoic acid enhances Foxp3 induction indirectly by relieving inhibition from CD4+CD44hi Cells. Immunity. 2008;29:758–770. doi: 10.1016/j.immuni.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coombes JL, Siddiqui KR, Arancibia-Carcamo CV, Hall J, Sun CM, Belkaid Y, Powrie F. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. The Journal of experimental medicine. 2007;204:1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun CM, Hall JA, Blank RB, Bouladoux N, Oukka M, Mora JR, Belkaid Y. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. The Journal of experimental medicine. 2007;204:1775–1785. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kang SG, Lim HW, Andrisani OM, Broxmeyer HE, Kim CH. Vitamin A metabolites induce gut-homing FoxP3+ regulatory T cells. Journal of immunology. 2007;179:3724–3733. doi: 10.4049/jimmunol.179.6.3724. [DOI] [PubMed] [Google Scholar]
- 25.Weiner HL, da Cunha AP, Quintana F, Wu H. Oral tolerance. Immunol Rev. 2011;241:241–259. doi: 10.1111/j.1600-065X.2011.01017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Penna G, Roncari A, Amuchastegui S, Daniel KC, Berti E, Colonna M, Adorini L. Expression of the inhibitory receptor ILT3 on dendritic cells is dispensable for induction of CD4+Foxp3+ regulatory T cells by 1,25-dihydroxyvitamin D3. Blood. 2005;106:3490–3497. doi: 10.1182/blood-2005-05-2044. [DOI] [PubMed] [Google Scholar]
- 27.Gorman S, Kuritzky LA, Judge MA, Dixon KM, McGlade JP, Mason RS, Finlay-Jones JJ, Hart PH. Topically applied 1,25-dihydroxyvitamin D3 enhances the suppressive activity of CD4+CD25+ cells in the draining lymph nodes. Journal of immunology. 2007;179:6273–6283. doi: 10.4049/jimmunol.179.9.6273. [DOI] [PubMed] [Google Scholar]
- 28.Daniel C, Sartory NA, Zahn N, Radeke HH, Stein JM. Immune modulatory treatment of trinitrobenzene sulfonic acid colitis with calcitriol is associated with a change of a T helper (Th) 1/Th17 to a Th2 and regulatory T cell profile. The Journal of pharmacology and experimental therapeutics. 2008;324:23–33. doi: 10.1124/jpet.107.127209. [DOI] [PubMed] [Google Scholar]
- 29.Jeffery LE, Burke F, Mura M, Zheng Y, Qureshi OS, Hewison M, Walker LS, Lammas DA, Raza K, Sansom DM. 1,25-Dihydroxyvitamin D3 and IL-2 combine to inhibit T cell production of inflammatory cytokines and promote development of regulatory T cells expressing CTLA-4 and FoxP3. Journal of immunology. 2009;183:5458–5467. doi: 10.4049/jimmunol.0803217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Adorini L, Penna G, Giarratana N, Uskokovic M. Tolerogenic dendritic cells induced by vitamin D receptor ligands enhance regulatory T cells inhibiting allograft rejection and autoimmune diseases. J Cell Biochem. 2003;88:227–233. doi: 10.1002/jcb.10340. [DOI] [PubMed] [Google Scholar]
- 31.Kang SW, Kim SH, Lee N, Lee WW, Hwang KA, Shin MS, Lee SH, Kim WU, Kang I. 1,25-Dihyroxyvitamin D3 promotes FOXP3 expression via binding to vitamin D response elements in its conserved noncoding sequence region. Journal of immunology. 2012;188:5276–5282. doi: 10.4049/jimmunol.1101211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.von Essen MR, Kongsbak M, Schjerling P, Olgaard K, Odum N, Geisler C. Vitamin D controls T cell antigen receptor signaling and activation of human T cells. Nature immunology. 2010;11:344–349. doi: 10.1038/ni.1851. [DOI] [PubMed] [Google Scholar]
- 33.Yu S, Bruce D, Froicu M, Weaver V, Cantorna MT. Failure of T cell homing, reduced CD4/CD8alphaalpha intraepithelial lymphocytes, and inflammation in the gut of vitamin D receptor KO mice. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:20834–20839. doi: 10.1073/pnas.0808700106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Froicu M, Weaver V, Wynn TA, McDowell MA, Welsh JE, Cantorna MT. A crucial role for the vitamin D receptor in experimental inflammatory bowel diseases. Molecular endocrinology. 2003;17:2386–2392. doi: 10.1210/me.2003-0281. [DOI] [PubMed] [Google Scholar]
- 35.Stover PJ. Physiology of folate and vitamin B12 in health and disease. Nutrition reviews. 2004;62:S3–S12. doi: 10.1111/j.1753-4887.2004.tb00070.x. discussion S3. [DOI] [PubMed] [Google Scholar]
- 36.Yamaguchi T, Hirota K, Nagahama K, Ohkawa K, Takahashi T, Nomura T, Sakaguchi S. Control of immune responses by antigen-specific regulatory T cells expressing the folate receptor. Immunity. 2007;27:145–159. doi: 10.1016/j.immuni.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 37.Kinoshita M, Kayama H, Kusu T, Yamaguchi T, Kunisawa J, Kiyono H, Sakaguchi S, Takeda K. Dietary folic acid promotes survival of Foxp3+ regulatory T cells in the colon. Journal of immunology. 2012;189:2869–2878. doi: 10.4049/jimmunol.1200420. [DOI] [PubMed] [Google Scholar]
- 38.Kunisawa J, Hashimoto E, Ishikawa I, Kiyono H. A pivotal role of vitamin B9 in the maintenance of regulatory T cells in vitro and in vivo. PloS one. 2012;7:e32094. doi: 10.1371/journal.pone.0032094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hegyi J, Schwartz RA, Hegyi V. Pellagra: dermatitis, dementia, and diarrhea. International journal of dermatology. 2004;43:1–5. doi: 10.1111/j.1365-4632.2004.01959.x. [DOI] [PubMed] [Google Scholar]
- 40.Singh N, Gurav A, Sivaprakasam S, Brady E, Padia R, Shi H, Thangaraju M, Prasad PD, Manicassamy S, Munn DH, Lee JR, Offermanns S, Ganapathy V. Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity. 2014;40:128–139. doi: 10.1016/j.immuni.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.De Rosa V, Procaccini C, Cali G, Pirozzi G, Fontana S, Zappacosta S, La Cava A, Matarese G. A key role of leptin in the control of regulatory T cell proliferation. Immunity. 2007;26:241–255. doi: 10.1016/j.immuni.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 42.Procaccini C, De Rosa V, Galgani M, Abanni L, Cali G, Porcellini A, Carbone F, Fontana S, Horvath TL, La Cava A, Matarese G. An oscillatory switch in mTOR kinase activity sets regulatory T cell responsiveness. Immunity. 2010;33:929–941. doi: 10.1016/j.immuni.2010.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Myers MG, Cowley MA, Munzberg H. Mechanisms of leptin action and leptin resistance. Annual review of physiology. 2008;70:537–556. doi: 10.1146/annurev.physiol.70.113006.100707. [DOI] [PubMed] [Google Scholar]
- 44.Sanna V, Di Giacomo A, La Cava A, Lechler RI, Fontana S, Zappacosta S, Matarese G. Leptin surge precedes onset of autoimmune encephalomyelitis and correlates with development of pathogenic T cell responses. The Journal of clinical investigation. 2003;111:241–250. doi: 10.1172/JCI16721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Siegmund B, Sennello JA, Jones-Carson J, Gamboni-Robertson F, Lehr HA, Batra A, Fedke I, Zeitz M, Fantuzzi G. Leptin receptor expression on T lymphocytes modulates chronic intestinal inflammation in mice. Gut. 2004;53:965–972. doi: 10.1136/gut.2003.027136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Matarese G, Giacomo A, Sanna V, Lord GM, Howard JK, Tuoro A, Bloom SR, Lechler RI, Zappacosta S, Fontana S. Requirement for leptin in the induction and progression of autoimmune encephalomyelitis. The Journal of Immunology. 2001;166:5909–5916. doi: 10.4049/jimmunol.166.10.5909. [DOI] [PubMed] [Google Scholar]
- 47.Faggioni R, Jones-Carson J, Reed DA, Dinarello CA, Feingold KR, Grunfeld C, Fantuzzi G. Leptin-deficient (ob/ob) mice are protected from T cell-mediated hepatotoxicity: role of tumor necrosis factor alpha and IL-18. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:2367–2372. doi: 10.1073/pnas.040561297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cipolletta D, Feuerer M, Li A, Kamei N, Lee J, Shoelson SE, Benoist C, Mathis D. PPAR-gamma is a major driver of the accumulation and phenotype of adipose tissue Treg cells. Nature. 2012;486:549–553. doi: 10.1038/nature11132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Feuerer M, Herrero L, Cipolletta D, Naaz A, Wong J, Nayer A, Lee J, Goldfine AB, Benoist C, Shoelson S, Mathis D. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat Med. 2009;15:930–939. doi: 10.1038/nm.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Matarese G, Procaccini C, Menale C, Kim JG, Kim JD, Diano S, Diano N, De Rosa V, Dietrich MO, Horvath TL. Hunger-promoting hypothalamic neurons modulate effector and regulatory T-cell responses. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:6193–6198. doi: 10.1073/pnas.1210644110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Belkaid Y, Hand TW. Role of the Microbiota in Immunity and Inflammation. Cell. 2014;157:121–141. doi: 10.1016/j.cell.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cebula A, Seweryn M, Rempala GA, Pabla SS, McIndoe RA, Denning TL, Bry L, Kraj P, Kisielow P, Ignatowicz L. Thymus-derived regulatory T cells contribute to tolerance to commensal microbiota. Nature. 2013;497:258–262. doi: 10.1038/nature12079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Josefowicz SZ, Niec RE, Kim HY, Treuting P, Chinen T, Zheng Y, Umetsu DT, Rudensky AY. Extrathymically generated regulatory T cells control mucosal TH2 inflammation. Nature. 2012;482:395–399. doi: 10.1038/nature10772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lathrop SK, Bloom SM, Rao SM, Nutsch K, Lio CW, Santacruz N, Peterson DA, Stappenbeck TS, Hsieh CS. Peripheral education of the immune system by colonic commensal microbiota. Nature. 2011;478:250–254. doi: 10.1038/nature10434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Atarashi K, Tanoue T, Shima T, Imaoka A, Kuwahara T, Momose Y, Cheng G, Yamasaki S, Saito T, Ohba Y, Taniguchi T, Takeda K, Hori S, Ivanov II, Umesaki Y, Itoh K, Honda K. Induction of colonic regulatory T cells by indigenous Clostridium species. Science. 2011;331:337–341. doi: 10.1126/science.1198469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Di Giacinto C, Marinaro M, Sanchez M, Strober W, Boirivant M. Probiotics ameliorate recurrent Th1-mediated murine colitis by inducing IL-10 and IL-10-dependent TGF-beta-bearing regulatory cells. Journal of immunology. 2005;174:3237–3246. doi: 10.4049/jimmunol.174.6.3237. [DOI] [PubMed] [Google Scholar]
- 57.Feleszko W, Jaworska J, Rha RD, Steinhausen S, Avagyan A, Jaudszus A, Ahrens B, Groneberg DA, Wahn U, Hamelmann E. Probiotic-induced suppression of allergic sensitization and airway inflammation is associated with an increase of T regulatory-dependent mechanisms in a murine model of asthma. Clinical and experimental allergy: journal of the British Society for Allergy and Clinical Immunology. 2007;37:498–505. doi: 10.1111/j.1365-2222.2006.02629.x. [DOI] [PubMed] [Google Scholar]
- 58.Karimi K, Inman MD, Bienenstock J, Forsythe P. Lactobacillus reuteri-induced regulatory T cells protect against an allergic airway response in mice. American journal of respiratory and critical care medicine. 2009;179:186–193. doi: 10.1164/rccm.200806-951OC. [DOI] [PubMed] [Google Scholar]
- 59.Lyons A, O'Mahony D, O'Brien F, MacSharry J, Sheil B, Ceddia M, Russell WM, Forsythe P, Bienenstock J, Kiely B, Shanahan F, O'Mahony L. Bacterial strain-specific induction of Foxp3+ T regulatory cells is protective in murine allergy models. Clinical and experimental allergy: journal of the British Society for Allergy and Clinical Immunology. 2010;40:811–819. doi: 10.1111/j.1365-2222.2009.03437.x. [DOI] [PubMed] [Google Scholar]
- 60.Faith JJ, Ahern PP, Ridaura VK, Cheng J, Gordon JI. Identifying gut microbe-host phenotype relationships using combinatorial communities in gnotobiotic mice. Science translational medicine. 2014;6:220ra11. doi: 10.1126/scitranslmed.3008051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Geuking MB, Cahenzli J, Lawson MA, Ng DC, Slack E, Hapfelmeier S, McCoy KD, Macpherson AJ. Intestinal bacterial colonization induces mutualistic regulatory T cell responses. Immunity. 2011;34:794–806. doi: 10.1016/j.immuni.2011.03.021. [DOI] [PubMed] [Google Scholar]
- 62.Atarashi K, Tanoue T, Oshima K, Suda W, Nagano Y, Nishikawa H, Fukuda S, Saito T, Narushima S, Hase K, Kim S, Fritz JV, Wilmes P, Ueha S, Matsushima K, Ohno H, Olle B, Sakaguchi S, Taniguchi T, Morita H, Hattori M, Honda K. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature. 2013;500:232–236. doi: 10.1038/nature12331. [DOI] [PubMed] [Google Scholar]
- 63.Obata Y, Furusawa Y, Endo TA, Sharif J, Takahashi D, Atarashi K, Nakayama M, Onawa S, Fujimura Y, Takahashi M, Ikawa T, Otsubo T, Kawamura YI, Dohi T, Tajima S, Masumoto H, Ohara O, Honda K, Hori S, Ohno H, Koseki H, Hase K. The epigenetic regulator Uhrf1 facilitates the proliferation and maturation of colonic regulatory T cells. Nat Immunol. 2014 doi: 10.1038/ni.2886. [DOI] [PubMed] [Google Scholar]
- 64.Mazmanian SK, Liu CH, Tzianabos AO, Kasper DL. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell. 2005;122:107–118. doi: 10.1016/j.cell.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 65.Mazmanian SK, Round JL, Kasper DL. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature. 2008;453:620–625. doi: 10.1038/nature07008. [DOI] [PubMed] [Google Scholar]
- 66.Round JL, Mazmanian SK. Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:12204–12209. doi: 10.1073/pnas.0909122107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Round JL, Lee SM, Li J, Tran G, Jabri B, Chatila TA, Mazmanian SK. The Tolllike receptor 2 pathway establishes colonization by a commensal of the human microbiota. Science. 2011;332:974–977. doi: 10.1126/science.1206095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ochoa-Reparaz J, Mielcarz DW, Ditrio LE, Burroughs AR, Begum-Haque S, Dasgupta S, Kasper DL, Kasper LH. Central nervous system demyelinating disease protection by the human commensal Bacteroides fragilis depends on polysaccharide A expression. Journal of immunology. 2010;185:4101–4108. doi: 10.4049/jimmunol.1001443. [DOI] [PubMed] [Google Scholar]
- 69.Ochoa-Reparaz J, Mielcarz DW, Ditrio LE, Burroughs AR, Foureau DM, Haque-Begum S, Kasper LH. Role of gut commensal microflora in the development of experimental autoimmune encephalomyelitis. Journal of immunology. 2009;183:6041–6050. doi: 10.4049/jimmunol.0900747. [DOI] [PubMed] [Google Scholar]
- 70.Ochoa-Reparaz J, Mielcarz DW, Wang Y, Begum-Haque S, Dasgupta S, Kasper DL, Kasper LH. A polysaccharide from the human commensal Bacteroides fragilis protects against CNS demyelinating disease. Mucosal immunology. 2010;3:487–495. doi: 10.1038/mi.2010.29. [DOI] [PubMed] [Google Scholar]
- 71.Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, Wei D, Goldfarb KC, Santee CA, Lynch SV, Tanoue T, Imaoka A, Itoh K, Takeda K, Umesaki Y, Honda K, Littman DR. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139:485–498. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ivanov II, Frutos Rde L, Manel N, Yoshinaga K, Rifkin DB, Sartor RB, Finlay BB, Littman DR. Specific microbiota direct the differentiation of IL-17-producing T-helper cells in the mucosa of the small intestine. Cell host & microbe. 2008;4:337–349. doi: 10.1016/j.chom.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wu HJ, Ivanov II, Darce J, Hattori K, Shima T, Umesaki Y, Littman DR, Benoist C, Mathis D. Gut-residing segmented filamentous bacteria drive autoimmune arthritis via T helper 17 cells. Immunity. 2010;32:815–827. doi: 10.1016/j.immuni.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Atarashi K, Nishimura J, Shima T, Umesaki Y, Yamamoto M, Onoue M, Yagita H, Ishii N, Evans R, Honda K, Takeda K. ATP drives lamina propria T(H)17 cell differentiation. Nature. 2008;455:808–812. doi: 10.1038/nature07240. [DOI] [PubMed] [Google Scholar]
- 75.Goto Y, Panea C, Nakato G, Cebula A, Lee C, Diez MG, Laufer TM, Ignatowicz L, Ivanov II. Segmented filamentous bacteria antigens presented by intestinal dendritic cells drive mucosal th17 cell differentiation. Immunity. 2014;40:594–607. doi: 10.1016/j.immuni.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lecuyer E, Rakotobe S, Lengline-Garnier H, Lebreton C, Picard M, Juste C, Fritzen R, Eberl G, McCoy KD, Macpherson AJ, Reynaud CA, Cerf-Bensussan N, Gaboriau-Routhiau V. Segmented filamentous bacterium uses secondary and tertiary lymphoid tissues to induce gut IgA and specific T helper 17 cell responses. Immunity. 2014;40:608–620. doi: 10.1016/j.immuni.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 77.Yang Y, Torchinsky MB, Gobert M, Xiong H, Xu M, Linehan JL, Alonzo F, Ng C, Chen A, Lin X, Sczesnak A, Liao JJ, Torres VJ, Jenkins MK, Lafaille JJ, Littman DR. Focused specificity of intestinal T17 cells towards commensal bacterial antigens. Nature. 2014 doi: 10.1038/nature13279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ley RE, Peterson DA, Gordon JI. Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell. 2006;124:837–848. doi: 10.1016/j.cell.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 79.Cummings JH, Pomare EW, Branch WJ, Naylor CP, Macfarlane GT. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut. 1987;28:1221–1227. doi: 10.1136/gut.28.10.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Arpaia N, Campbell C, Fan X, Dikiy S, van der Veeken J, deRoos P, Liu H, Cross JR, Pfeffer K, Coffer PJ, Rudensky AY. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature. 2013;504:451–455. doi: 10.1038/nature12726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly YM, Glickman JN, Garrett WS. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science. 2013;341:569–573. doi: 10.1126/science.1241165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, Nakanishi Y, Uetake C, Kato K, Kato T, Takahashi M, Fukuda NN, Murakami S, Miyauchi E, Hino S, Atarashi K, Onawa S, Fujimura Y, Lockett T, Clarke JM, Topping DL, Tomita M, Hori S, Ohara O, Morita T, Koseki H, Kikuchi J, Honda K, Hase K, Ohno H. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504:446–450. doi: 10.1038/nature12721. [DOI] [PubMed] [Google Scholar]
- 83.Maslowski KM, Vieira AT, Ng A, Kranich J, Sierro F, Yu D, Schilter HC, Rolph MS, Mackay F, Artis D, Xavier RJ, Teixeira MM, Mackay CR. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature. 2009;461:1282–1286. doi: 10.1038/nature08530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ganapathy V, Thangaraju M, Prasad PD, Martin PM, Singh N. Transporters and receptors for short-chain fatty acids as the molecular link between colonic bacteria and the host. Current opinion in pharmacology. 2013;13:869–874. doi: 10.1016/j.coph.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 85.Chang PV, Hao L, Offermanns S, Medzhitov R. The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:2247–2252. doi: 10.1073/pnas.1322269111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang R, Green DR. Metabolic checkpoints in activated T cells. Nature immunology. 2012;13:907–915. doi: 10.1038/ni.2386. [DOI] [PubMed] [Google Scholar]
- 87.Zeng H, Chi H. mTOR and lymphocyte metabolism. Curr Opin Immunol. 2013;25:347–355. doi: 10.1016/j.coi.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yang K, Shrestha S, Zeng H, Karmaus PW, Neale G, Vogel P, Guertin DA, Lamb RF, Chi H. T Cell Exit from Quiescence and Differentiation into Th2 Cells Depend on Raptor-mTORC1-Mediated Metabolic Reprogramming. Immunity. 2013;39:1043–1056. doi: 10.1016/j.immuni.2013.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chi H. Regulation and function of mTOR signalling in T cell fate decisions. Nature reviews. Immunology. 2012;12:325–338. doi: 10.1038/nri3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149:274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Haxhinasto S, Mathis D, Benoist C. The AKT-mTOR axis regulates de novo differentiation of CD4+Foxp3+ cells. The Journal of experimental medicine. 2008;205:565–574. doi: 10.1084/jem.20071477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Liu G, Yang K, Burns S, Shrestha S, Chi H. The S1P(1)-mTOR axis directs the reciprocal differentiation of T(H)1 and T(reg) cells. Nature immunology. 2010;11:1047–1056. doi: 10.1038/ni.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sauer S, Bruno L, Hertweck A, Finlay D, Leleu M, Spivakov M, Knight ZA, Cobb BS, Cantrell D, O'Connor E, Shokat KM, Fisher AG, Merkenschlager M. T cell receptor signaling controls Foxp3 expression via PI3K, Akt, and mTOR. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:7797–7802. doi: 10.1073/pnas.0800928105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cobbold SP, Adams E, Farquhar CA, Nolan KF, Howie D, Lui KO, Fairchild PJ, Mellor AL, Ron D, Waldmann H. Infectious tolerance via the consumption of essential amino acids and mTOR signaling. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:12055–12060. doi: 10.1073/pnas.0903919106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Liu G, Burns S, Huang G, Boyd K, Proia RL, Flavell RA, Chi H. The receptor S1P1 overrides regulatory T cell-mediated immune suppression through Akt-mTOR. Nature immunology. 2009;10:769–777. doi: 10.1038/ni.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Delgoffe GM, Kole TP, Zheng Y, Zarek PE, Matthews KL, Xiao B, Worley PF, Kozma SC, Powell JD. The mTOR kinase differentially regulates effector and regulatory T cell lineage commitment. Immunity. 2009;30:832–844. doi: 10.1016/j.immuni.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dang EV, Barbi J, Yang HY, Jinasena D, Yu H, Zheng Y, Bordman Z, Fu J, Kim Y, Yen HR, Luo W, Zeller K, Shimoda L, Topalian SL, Semenza GL, Dang CV, Pardoll DM, Pan F. Control of T(H)17/T(reg) balance by hypoxia-inducible factor 1. Cell. 2011;146:772–784. doi: 10.1016/j.cell.2011.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Delgoffe GM, Pollizzi KN, Waickman AT, Heikamp E, Meyers DJ, Horton MR, Xiao B, Worley PF, Powell JD. The kinase mTOR regulates the differentiation of helper T cells through the selective activation of signaling by mTORC1 and mTORC2. Nature immunology. 2011;12:295–303. doi: 10.1038/ni.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Deiuliis J, Shah Z, Shah N, Needleman B, Mikami D, Narula V, Perry K, Hazey J, Kampfrath T, Kollengode M, Sun Q, Satoskar AR, Lumeng C, Moffatt-Bruce S, Rajagopalan S. Visceral adipose inflammation in obesity is associated with critical alterations in tregulatory cell numbers. PloS one. 2011;6:e16376. doi: 10.1371/journal.pone.0016376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Eller K, Kirsch A, Wolf AM, Sopper S, Tagwerker A, Stanzl U, Wolf D, Patsch W, Rosenkranz AR, Eller P. Potential role of regulatory T cells in reversing obesity-linked insulin resistance and diabetic nephropathy. Diabetes. 2011;60:2954–2962. doi: 10.2337/db11-0358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wagner NM, Brandhorst G, Czepluch F, Lankeit M, Eberle C, Herzberg S, Faustin V, Riggert J, Oellerich M, Hasenfuss G, Konstantinides S, Schafer K. Circulating regulatory T cells are reduced in obesity and may identify subjects at increased metabolic and cardiovascular risk. Obesity. 2013;21:461–468. doi: 10.1002/oby.20087. [DOI] [PubMed] [Google Scholar]
- 102.Kawamoto S, Maruya M, Kato LM, Suda W, Atarashi K, Doi Y, Tsutsui Y, Qin H, Honda K, Okada T, Hattori M, Fagarasan S. Foxp3(+) T cells regulate immunoglobulin a selection and facilitate diversification of bacterial species responsible for immune homeostasis. Immunity. 2014;41:152–165. doi: 10.1016/j.immuni.2014.05.016. [DOI] [PubMed] [Google Scholar]
- 103.Tiemessen MM, Jagger AL, Evans HG, van Herwijnen MJ, John S, Taams LS. CD4+CD25+Foxp3+ regulatory T cells induce alternative activation of human monocytes/macrophages. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:19446–19451. doi: 10.1073/pnas.0706832104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Fallarino F, Grohmann U, Hwang KW, Orabona C, Vacca C, Bianchi R, Belladonna ML, Fioretti MC, Alegre ML, Puccetti P. Modulation of tryptophan catabolism by regulatory T cells. Nature immunology. 2003;4:1206–1212. doi: 10.1038/ni1003. [DOI] [PubMed] [Google Scholar]
- 105.Burzyn D, Kuswanto W, Kolodin D, Shadrach JL, Cerletti M, Jang Y, Sefik E, Tan TG, Wagers AJ, Benoist C, Mathis D. A special population of regulatory T cells potentiates muscle repair. Cell. 2013;155:1282–1295. doi: 10.1016/j.cell.2013.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Samstein RM, Josefowicz SZ, Arvey A, Treuting PM, Rudensky AY. Extrathymic generation of regulatory T cells in placental mammals mitigates maternal-fetal conflict. Cell. 2012;150:29–38. doi: 10.1016/j.cell.2012.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Naik S, Bouladoux N, Wilhelm C, Molloy MJ, Salcedo R, Kastenmuller W, Deming C, Quinones M, Koo L, Conlan S, Spencer S, Hall JA, Dzutsev A, Kong H, Campbell DJ, Trinchieri G, Segre JA, Belkaid Y. Compartmentalized control of skin immunity by resident commensals. Science. 2012;337:1115–1119. doi: 10.1126/science.1225152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Winer S, Chan Y, Paltser G, Truong D, Tsui H, Bahrami J, Dorfman R, Wang Y, Zielenski J, Mastronardi F, Maezawa Y, Drucker DJ, Engleman E, Winer D, Dosch HM. Normalization of obesity-associated insulin resistance through immunotherapy. Nat Med. 2009;15:921–929. doi: 10.1038/nm.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lee K, Gudapati P, Dragovic S, Spencer C, Joyce S, Killeen N, Magnuson MA, Boothby M. Mammalian target of rapamycin protein complex 2 regulates differentiation of Th1 and Th2 cell subsets via distinct signaling pathways. Immunity. 2010;32:743–753. doi: 10.1016/j.immuni.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kurebayashi Y, Nagai S, Ikejiri A, Ohtani M, Ichiyama K, Baba Y, Yamada T, Egami S, Hoshii T, Hirao A, Matsuda S, Koyasu S. PI3K-Akt-mTORC1-S6K1/2 axis controls Th17 differentiation by regulating Gfi1 expression and nuclear translocation of RORgamma. Cell reports. 2012;1:360–373. doi: 10.1016/j.celrep.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 111.Finlay DK, Rosenzweig E, Sinclair LV, Feijoo-Carnero C, Hukelmann JL, Rolf J, Panteleyev AA, Okkenhaug K, Cantrell DA. PDK1 regulation of mTOR and hypoxia-inducible factor 1 integrate metabolism and migration of CD8+ T cells. The Journal of experimental medicine. 2012;209:2441–2453. doi: 10.1084/jem.20112607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Pearce EL, Walsh MC, Cejas PJ, Harms GM, Shen H, Wang LS, Jones RG, Choi Y. Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature. 2009;460:103–107. doi: 10.1038/nature08097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Araki K, Turner AP, Shaffer VO, Gangappa S, Keller SA, Bachmann MF, Larsen CP, Ahmed R. mTOR regulates memory CD8 T-cell differentiation. Nature. 2009;460:108–1012. doi: 10.1038/nature08155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Rao RR, Li Q, Odunsi K, Shrikant PA. The mTOR kinase determines effector versus memory CD8+ T cell fate by regulating the expression of transcription factors T-bet and Eomesodermin. Immunity. 2010;32:67–78. doi: 10.1016/j.immuni.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]