Abstract
Childhood and adolescence are critical periods of bone mineral content (BMC) accrual that may have long-term consequences for osteoporosis in adulthood. Adequate dietary calcium intake and weight-bearing physical activity are important for maximizing BMC accrual. However, the relative effects of physical activity and dietary calcium on BMC accrual throughout the continuum of pubertal development in childhood remains unclear. The purpose of this study was to determine the effects of self-reported dietary calcium intake and weight-bearing physical activity on bone mass accrual across the five stages of pubertal development in a large diverse cohort of US children and adolescents. The Bone Mineral Density in Childhood study was a mixed longitudinal study with 7393 observations on 1743 subjects. Annually, we measured BMC by DXA, physical activity and calcium intake by questionnaire, and pubertal development (Tanner stage) by examination, for up to seven years. Mixed-effects regression models were used to assess physical activity and calcium intake effects on BMC accrual at each Tanner stage. We found that self-reported weight-bearing physical activity contributed to significantly greater BMC accrual in both sexes and racial subgroups (black and non-black). In non-black males, the magnitude of the activity effect on total body BMC accrual varied among Tanner stages after adjustment for calcium intake; the greatest difference between high and low activity boys was largest in Tanner stage 3. Calcium intake had a significant effect on bone accrual only in non-black girls. This effect was not significantly different among Tanner stages. Our findings do not support differential effects of physical activity or calcium intake on bone mass accrual according to maturational stage. The study demonstrated significant longitudinal effects of weight bearing physical activity on bone mass accrual through all stages of pubertal development.
Keywords: bone mass accrual, puberty, adolescence, physical activity, calcium intake, Tanner stage
Introduction
Senile osteoporosis is a pediatric disease
Charles E. Dent, 1972 (1)
Today, more than 40 years since Dr. Dent suggested that prevention of osteoporosis should begin in childhood, it is widely accepted that bone mineral accrual during childhood and adolescence is a critical determinant of bone health in adulthood. Peak bone mass (PBM), the maximum amount of bone mineral an individual accrues, is considered the best predictor of osteoporotic fracture.(2-4) In developed countries, at least 90% of PBM is accrued by 19 to 20 years of age.(5-8) The relative risk of fracture increases as much as 2.6-fold for each 1 standard deviation decrease in bone mass,(9) and a 10% increase in PBM in the population is estimated to decrease risk of fracture in the elderly by 50%.(10) Furthermore, bone mass predicts fractures in children.(11)
Most randomized clinical trials show positive effects of higher dietary calcium or dairy food intake on bone mass accrual in childhood ,(12,13) although there is some evidence that the increases in BMD and BMC do not persist after the supplementation has been stopped.(14-16) Many randomized trials show positive effects of physical activity on bone mass accrual and bone structure during childhood.(17-25) Indeed, bone adaptation to mechanical loading is much greater during growth than after cessation of growth.(26,27) Physical activity and calcium intake are modifiable behaviors that affect bone mass accrual, but it remains uncertain whether they have greater effects during specific stages of pubertal development when bone might be most responsive to changes in diet and exercise.(24,28,29) If such a critical period exists, it would be an opportune time to promote increased dietary calcium intake and bone-building physical activity to maximize their positive impact on bone accrual.(30)
The objectives of this study were to determine whether self-reported dietary calcium intake and weight-bearing physical activity have greater effects on bone mass accrual at specific stages of pubertal development. We utilized data from the Bone Mineral Density in Childhood Study (BMDCS), a large diverse cohort of healthy US children and adolescents whose pubertal staging was done annually by pediatric endocrinologists and specially trained nurse practitioners. Pubertal development ranged from pre-pubertal to sexually mature in this cohort.
Methods
Participants
The National Institute of Child Health and Human Development BMDCS was a large, multi-center, multi-ethnic U.S. prospective study with a primary goal to develop bone density reference data for Dual-Energy X-Ray Absorptiometry (DXA) measures in children and adolescents.(31,32) Healthy children and adolescents were recruited from five centers: Children's Hospital of Los Angeles (Los Angeles, CA), Cincinnati Children's Hospital Medical Center (Cincinnati, OH), Creighton University (Omaha, NE), Children's Hospital of Philadelphia (Philadelphia, PA), and Columbia University (New York, NY). Recruitment of 1554 girls (6-15 yr) and boys (6-16 yr) occurred from July 2002 to November 2003. These participants were evaluated annually for 6 yr (7 visits). Additional participants, ages 5 or 19 y, (n=460) were recruited between August 2006 and November 2007 to provide a sufficient number of observations to allow development of reference data for the ages 5 to 20 yr. These participants were evaluated at three annual visits. The final sample consisted of 2,014 participants with a total of 10,716 study visits.
Entry criteria have been reported previously.(31,32) Briefly, these included: anticipated residence in the United States for three years or longer; school placement within one year of expected for age; full-term birth; birth weight greater than 2.3 kg; and no evidence of precocious or delayed puberty. Children were excluded for history of two or more fractures if age ten years or younger, or three or more fractures if age older than ten years. This exclusion was based on evidence that refracture rates in children range from 5-33% (33,34) and that low bone mass values in girls who fracture persist four years later.(33) In fact, the 2013 Official Position Statement on Pediatric Health from the International Society of Clinical Densitometry posits, “A clinically significant fracture history is one or more of the following: 1) two or more long bone fractures by age 10 years; 2) three or more long bone fractures at any age up to age 19 years.”(35) Other exclusion criteria for the BMDCS study included current or previous medication or medical condition known to affect bone health; history of extended bed rest; height, weight, or body mass index outside the range of the 3rd to the 97th percentile for age; indwelling hardware; or scoliosis.
Following enrollment, data were excluded for use of medications including: steroid use, parenteral ≥ 1 week or non-parenteral ≥ 6 week; anticonvulsant and oral Isotrentinoin; DepoProvera or Norplant; psychiatric medication or stimulant use for more than 180 days. Also, subjects were excluded if they developed a medical condition known to affect bone health or had pregnancy with delivery of a full term baby.
Written assent/consent was obtained from study participants and also from parents/guardians if the participants were minors. The protocol was approved by the institutional review boards of each clinical center.
Measurements
DXA scans were obtained using Hologic, Inc. (Bedford, MA) bone densitometers (QDR4500A, QDR4500W, and Delphi A models). Cross-calibration of DXA devices and longitudinal calibration stability were performed as previously described.(31) Whole body, posteroanterior lumbar spine (L1-L4, fast array), non-dominant forearm, and left proximal femur (fast array) scans were acquired on each study participant following the manufacturer guidelines for patient positioning. The BMDCS DXA Core Laboratory (University of California, San Francisco) analyzed all scans using Hologic software release 12.3.
Weight (±0.1 kg) was measured on a digital scale and height (±0.1 cm) was measured using a stadiometer. Participants were dressed in examination gowns or lightweight clothing with shoes removed during the measurement. Information on population of origin (African American, Asian, etc.) and ethnicity (Hispanic/Latino vs. Non-Hispanic/Latino) was obtained by questionnaire using National Institutes of Health classifications.
Dietary calcium intake (Ca, mg/d) was determined with a semi-quantitative food frequency questionnaire (FFQ) developed by Block Dietary Data Systems (BDDS, Berkeley, CA). The FFQ asked about the frequency of intake in the last week and the portion size of 45 food and beverage items. These 45 items were selected because they captured 90% of dietary calcium intake of individuals 6 to 19 yr of age participating in NHANES III. Food models and pictures were used to assist with estimation of portion sizes. Participants, with help of their parents if < 13 yr, completed the FFQ at each study visit. Ca was calculated from the questionnaire using an automated computer analysis program by BDDS.
Usual weight-bearing physical activity (PA, hours per week) was assessed with the modified self-report tool of Slemenda.(36) The questionnaire listed 37 weight-bearing activities, such as walking, basketball, dance, etc., in which children and adolescents are likely to participate. Participants indicated the time spent in activities in which they participated during the previous week. Space was provided to include activities that were not listed. Parents of younger children helped them complete the questionnaire. In a subset of ten children and adolescents in this cohort, the test-retest reliability of the tool within a two-week interval was 0.8.
Sexual maturation was assessed by physical exam by pediatric endocrinologists or nurse practitioners trained in endocrinology. Testicular volume (measured with an orchiometer) in boys and breast development in girls were classified according to criteria of Tanner. (37-39)
Statistical Analysis
BMC was measured at each annual visit. Annualized bone mineral content accrual rate (chgBMC) was calculated for each observation as current BMC (end of the interval) minus BMC at the previous annual visit (beginning of the interval) and divided by interval length. Observations were classified by the Tanner stage (TS) at the end of the interval. We calculated the mean and SD of annualized BMC accrued for each TS. Percent accrual (%chgBMC) was calculated as the annualized accrual divided by the BMC at previous visit multiplied by 100.
Some participants maintained the same TS for multiple visits. We calculated the mean value of PA and Ca within a TS for each subject so that there would be one value of PA and Ca for each TS for each subject. Group means and standard deviations by TS were calculated using these values. Pearson correlation coefficients were calculated to evaluate the consistency (tracking) of PA and Ca across TS; the individual means within a TS were used in these calculations. To evaluate differences among TS in PA and Ca, mixed model analysis of individual observations (not individual within-TS means) was assessed in two stages. In the first stage, we developed a base model in which annualized BMC change (chgBMC) was modeled for each skeletal site as a function of BMC at the beginning of the interval (previous visit BMC, pvBMC), annualized height change (chgHT, calculated like chgBMC described above), age at the beginning of the interval (previous visit age, pvAGE) and TS. Subject was included as a random effect, while all other predictors were fixed effects, as recommended.(40) All regressions were performed separately by four sex and race subgroups: nonblack (NB) males, black (BL) males, NB females, and BL females. This was done because sex/race differences in children and adolescents are seen in absolute BMC,(41,42) and at least one study suggests sex/racial differences in bone accrual.(42) Based on these differences, it has been recommended that race and sex be accounted for in analyses of bone mass accrual.(42,43)
Because of large differences among TS in pvBMC, velHT and pvAGE, these three continuous predictor variables were centered by subtracting the mean of the variable for that TS, sex and racial subgroup from each observation. This transformation enabled us to carry the maximum amount of information about biological age in the single variable (TS). All possible main effects and interactions were evaluated, and those that were statistically significant (p<0.05) were included in a final basic model. Ordinary residuals from this final basic model were interpreted as annualized BMC change adjusted for pvBMC, chgHT, pvAGE, and TS.
The second stage modeled the residuals, using a mixed model, as a function of PA and Ca. Interactions between PA and Ca, as well as interactions between PA or Ca and the factors in the basic model (pvBMC, chgHT, pvAGE, and TS) were evaluated. Type 3 tests were used for hypothesis testing, with p<0.05 as the criterion for significance. P values of 0.05 to > 0.01 are referred to as weak or marginal in this report since no adjustment was made for testing multiple hypotheses.
To illustrate observed effects, we calculated the predicted accrual, adjusted for pvBMC, chgHT and pvAGE, for the 25th percentile (Q1) and the 75th percentile (Q3) of PA and Ca for each race, sex, and TS subgroup. We used within-subject means by TS for these calculations, rather than individual observations.
Study data were collected at five centers. To evaluate the possibility that observed effects were due to confounding with center, the analysis was repeated with study site included in the basic model. Similarly, some observations were associated with intervals which deviated from the usual visit interval of one year. To evaluate the possibility that such visits affected the results, the analysis was repeated after excluding visits with intervals less than 11 months or more than 13 months. In both cases, if the reanalysis results did not differ from the original results in terms of which factors were significant, we concluded that the results were not sensitive to these phenomena.
Results
Descriptive results
After exclusions and data lost due to missed examinations, we included 7393 observations on 1743 subjects. Of these subjects, 696 were NB males, 214 were BL males, 645 were NB females, and 188 were BL females. The numbers of observations in each TS were: TS1, 1544 (20.9% of sample); TS2, 580 (7.8%); TS3, 601 (8.1%); TS4, 797 (10.8%); and TS5, 3871 (52.4%).
Table 1 shows BMC accrual rate (g/yr) and percent (%) accrual in each race/sex subgroup by TS. The greatest BMC accrual at all skeletal sites occurred at TS4, with the exception that the greatest % accrual in hip BMC in females was at TS3. Also apparent in Table 1 is the wide age range for any specific TS and the overlap in ages among TS.
Table 1.
Unadjusted BMC annual accrual (g chgBMC) and percent accrual (%chgBMC) in each race/sex subgroup by Tanner Stage: median and interquartile range (Q1-Q3).
| TS | age | age | Spine g chgBMC | Hip g chgBMC | TB g chgBMC | Spine % chgBMC | Hip% chgBMC | TB% chgBMC | |
|---|---|---|---|---|---|---|---|---|---|
| mean | range | Median(Q1-Q3) | Median(Q1-Q3) | Median(Q1-Q3) | Median(Q1-Q3) | Median(Q1-Q3) | Median(Q1-Q3) | ||
| NB Male | |||||||||
| 1 | 9.1 | 5.9-14.1 | 2.05 (1.5-2.7) | 1.61 (1.1-2.2) | 95.19 (76.1-115.5) | 9.93 (7.2-13.2) | 12.22 (8.3-17.6) | 10.41 (8.2-12.5) | |
| 2 | 11.6 | 8.7-16.2 | 2.44 (1.5-3.4) | 2.13 (1.3-3.0) | 113.32 (78.7-148.0) | 9.61 (6.5-12.4) | 10.93 (7.3-15.4) | 9.62 (6.9-11.9) | |
| 3 | 12.9 | 10.1-17.3 | 4.02 (2.8-6.0) | 3.02 (2.0-4.4) | 160.85 (111.5-220.0) | 14.18 (9.6-19.8) | 14.71 (9.9-20.9) | 12.25 (9.0-15.9) | |
| 4 | 14.1 | 10.4-19.4 | 7.25 (4.7-9.9) | 4.64 (3.0-6.6) | 245.04 (175.2-309.1) | 21.12 (14.3-28.3) | 18.59 (12.1-26.7) | 15.79 (11.4-20.0) | |
| 5 | 17.5 | 11.8-23.3 | 4.06 (1.3-8.2) | 1.76 (0.1-4.2) | 126.17 (39.7-262.9) | 6.47 (2.0-16.2) | 4.25 (0.3-11.3) | 5.28 (1.6-13.0) | |
| BL Male | |||||||||
| 1 | 9 | 6.0-12.7 | 2.27 (1.5-3.0) | 1.90 (1.2-2.6) | 111.16 (87.2-138.6) | 10.82 (7.7-13.6) | 12.50 (8.1-17.9) | 11.02 (8.8-13.1) | |
| 2 | 11.7 | 9.3-14.1 | 2.65 (2.0-3.6) | 2.52 (1.5-3.1) | 128.72 (84.4-170.4) | 10.50 (8.0-13.0) | 11.24 (7.1-15.0) | 9.88 (6.5-12.8) | |
| 3 | 13.1 | 9.9-18.3 | 4.70 (3.0-7.3) | 3.77 (2.1-5.2) | 206.96 (133.3-262.1) | 14.80 (10.9-20.7) | 13.60 (8.8-19.6) | 13.79 (9.7-17.3) | |
| 4 | 14.4 | 10.8-20.7 | 7.70 (4.7-10.4) | 4.51 (3.0-6.0) | 262.13 (185.2-347.0) | 19.80 (11.5-26.9) | 15.07 (8.1-22.5) | 14.40 (10.8-19.9) | |
| 5 | 17.5 | 11.8-23.4 | 4.35 (1.4-8.3) | 2.24 (0.6-4.6) | 157.38 (64.6-275.8) | 6.91 (2.0-16.3) | 4.89 (1.3-11.5) | 5.74 (2.2-12.5) | |
| NB Female | |||||||||
| 1 | 8.6 | 5.9-13.7 | 1.93 (1.4-2.5) | 1.43 (1.0-2.0) | 90.14 (70.0-109.1) | 10.71 (8.0-13.5) | 12.65 (8.5-17.6) | 10.58 (8.3-12.8) | |
| 2 | 10.5 | 6.9-14.7 | 2.81 (1.7-3.6) | 1.87 (1.1-2.6) | 103.61 (81.8-126.0) | 12.54 (8.6-15.5) | 12.05 (8.0-17.1) | 10.55 (8.0-12.5) | |
| 3 | 11.8 | 8.9-15.8 | 4.82 (3.3-6.7) | 2.92 (2.0-3.8) | 156.95 (117.2-198.4) | 18.25 (13.7-24.3) | 16.20 (12.0-21.6) | 13.26 (10.2-16.9) | |
| 4 | 13.1 | 10.4-18.9 | 6.92 (4.9-9.1) | 3.40 (2.0-4.7) | 210.55 (152.8-267.1) | 22.73 (14.9-28.7) | 15.73 (9.5-22.6) | 15.57 (10.7-19.4) | |
| 5 | 16.4 | 9.9-22.1 | 2.18 (0.7-4.3) | 0.70 (−0.2-1.7) | 68.08 (23.0-126.1) | 4.11 (1.2-8.8) | 2.38 (−0.6-5.8) | 3.30 (1.1-6.8) | |
| BL Female | |||||||||
| 1 | 8.2 | 5.9-12.0 | 2.05 (1.4-2.7) | 1.51 (1.1-2.1) | 91.04 (73.5-113.4) | 10.67 (7.9-14.2) | 13.52 (8.3-17.6) | 10.47 (8.4-12.8) | |
| 2 | 9.8 | 7.9-11.9 | 2.36 (1.9-3.3) | 1.84 (1.4-3.0) | 117.39 (94.9-131.7) | 12.21 (8.9-14.2) | 12.61 (9.5-19.7) | 11.48 (10.3-12.7) | |
| 3 | 11.1 | 6.6-14.0 | 4.74 (2.9-6.9) | 2.61 (1.7-4.0) | 148.78 (117.1-208.3) | 17.93 (12.4-25.8) | 14.14 (9.7-20.6) | 12.23 (10.1-17.7) | |
| 4 | 12.5 | 9.0-17.1 | 6.89 (5.0-8.6) | 2.93 (2.1-4.2) | 229.83 (164.7-276.0) | 21.50 (15.7-26.3) | 14.25 (9.9-18.8) | 15.65 (11.8-20.0) | |
| 5 | 15.8 | 10.8-21.7 | 2.52 (1.0-4.6) | 0.84 (-0.1-2.0) | 88.16 (30.6-166.6) | 4.79 (1.9-9.5) | 2.89 (−0.5-6.7) | 4.19 (1.4-8.6) | |
Mean PA among race/sex subgroups and TS ranged from 10.3 to 15.9 hr/wk (Table 2). There were marginally significant differences (0.05 > p >0.01) in PA among TS in NB males and NB females. The percent of subjects below currently recommended PA (7 hr/wk)(44) ranged from 11.1% to 38.1% among the race/sex/TS subgroups. Mean Ca among race/sex subgroups and TS ranged from 576 to 989 mg/d. Mean values in males were consistent across TS, but highly significant differences were seen among TS in females, with considerably lower intakes in TS5.
Table 2.
Mean and standard deviation (sd) of activity (PA) and calcium (Ca) by Tanner stage (TS), sex and race, and percent (%) of group below recommended levels (44,45)
| Nonblack male | Black male | Nonblack female | Black female | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tanner | mean | sd | % | mean | sd | % | mean | sd | % | mean | sd | % |
| PA (hrs/wk) | ||||||||||||
| 1 | 11.94 | 7.26 | 24.3 | 12.84 | 7.90 | 26.5 | 10.65 | 8.12 | 35.9 | 13.69 | 10.73 | 24.6 |
| 2 | 12.75 | 8.92 | 24.8 | 14.05 | 9.80 | 20.4 | 10.32 | 7.15 | 38.1 | 11.96 | 6.50 | 26.7 |
| 3 | 13.62 | 11.00 | 27.4 | 15.89 | 10.16 | 21.3 | 11.95 | 9.79 | 27.1 | 13.44 | 8.97 | 25.0 |
| 4 | 13.85 | 9.84 | 22.1 | 15.80 | 11.62 | 31.5 | 12.43 | 8.45 | 26.8 | 14.69 | 8.24 | 15.2 |
| 5 | 13.11 | 7.75 | 22.5 | 15.53 | 8.46 | 11.1 | 11.30 | 6.20 | 25.2 | 12.93 | 10.92 | 27.4 |
| p (diff among TS) | 0.04 | 0.48 | 0.02 | 0.08 | ||||||||
| Ca (mg/day) | ||||||||||||
| 1 | 989 | 414 | 73.6 | 805 | 407 | 83.1 | 834 | 378 | 82.2 | 736 | 375 | 84.2 |
| 2 | 972 | 479 | 80.7 | 701 | 431 | 90.9 | 819 | 405 | 89.0 | 708 | 519 | 90.0 |
| 3 | 950 | 540 | 76.4 | 863 | 510 | 82.9 | 824 | 461 | 85.5 | 711 | 346 | 92.9 |
| 4 | 917 | 534 | 83.7 | 827 | 522 | 83.6 | 859 | 521 | 84.7 | 699 | 560 | 91.5 |
| 5 | 962 | 525 | 80.6 | 804 | 495 | 87.3 | 716 | 387 | 91.0 | 576 | 300 | 99.3 |
| p (diff among TS) | 0.29 | 0.66 | 0.0001 | 0.0001 | ||||||||
Tracking of calcium and activity
Correlations of Ca between adjacent TS were moderate in size, ranging from 0.39 to 0.69, and significant in nearly all race/sex subgroups (Table 3). Correlations of PA between adjacent TS were significant in NB males, ranging from 0.37 to 0.55. Tracking of PA was not consistent in BL males; significant correlations were observed only in higher TS. For both BL and NB females, correlations of PA between adjacent TS decreased with increasing TS.
Table 3.
Pearson correlation coefficients (r) between calcium intake (Ca) in adjacent Tanner stages (TS) and between physical activity (PA) in adjacent TS
| NB males | BL males | NB females | BL females | |||||
|---|---|---|---|---|---|---|---|---|
| R | P | r | p | r | p | r | p | |
| Ca | ||||||||
| TS1 to TS2 | 0.60 | 0.0001 | 0.66 | 0.0003 | 0.57 | 0.0001 | 0.69 | 0.0003 |
| TS2 to TS3 | 0.67 | 0.0001 | 0.55 | 0.011 | 0.65 | 0.0001 | 0.50 | 0.032 |
| TS3 to TS4 | 0.44 | 0.0001 | 0.51 | 0.011 | 0.70 | 0.0001 | 0.39 | 0.058 |
| TS4 to TS5 | 0.50 | 0.0001 | 0.42 | 0.006 | 0.61 | 0.0001 | 0.40 | 0.007 |
| PA | ||||||||
| TS1 to TS2 | 0.37 | 0.0001 | 0.14 | 0.51 | 0.37 | 0.0001 | 0.66 | 0.0007 |
| TS2 to TS3 | 0.55 | 0.0001 | 0.27 | 0.23 | 0.48 | 0.0001 | 0.50 | 0.029 |
| TS3 to TS4 | 0.52 | 0.0001 | 0.51 | 0.012 | 0.27 | 0.003 | 0.13 | 0.55 |
| TS4 to TS5 | 0.42 | 0.0001 | 0.44 | 0.003 | 0.00 | 0.99 | 0.27 | 0.072 |
Correlation between calcium and activity
Correlations between Ca and PA within TS were generally weak, ranging from −0.042 to 0.292. Correlations exceeded 0.25 in TS-5 in all males and BL females, and in TS-3 in NB males. (Data not shown)
Relationships among predictors in the basic model
As expected, there were marked differences among TS in the values of the other predictors (pvBMC, chgHT, and pvAGE). However, rescaling these other predictors by subtraction of the mean for each specific TS eliminated these differences.
Summary of mixed model regression results for the basic model
TS and interaction effects including TS were highly significant in most subgroups and skeletal sites. pvBMC and chgHT effects were universally significant, while pvAGE was significant at some skeletal sites, in some subgroups. Interactions among these latter three covariates were significant only in NB females. Because all subgroups showed a significant four-way (TS x pvBMC x pvAGE x chgHT) interaction at two or more skeletal sites, we accepted the complete basic model with all possible interaction effects as the best representation. The effects of PA and Ca at each skeletal site were assessed after adjustment for this complete model.
Dietary calcium and physical activity effects on BMC accrual
There was no evidence of an interaction between PA and Ca in predicting adjusted annualized BMC accrual rate at any skeletal site in any race/sex subgroup. Given the correlation between PA and Ca, we evaluated the effects on adjusted BMC accrual of each factor (PA and Ca) after adjustment for the other factor.
PA and Ca were evaluated first in models including interactions with TS. Significant PA x TS interactions were observed, only in NB males, and only for total body BMC. Details of the results are provided in Table 4 and Figure 1. PA by TS interaction effects in other subgroups and other skeletal sites were all non-significant (p>0.05; Figure 2 is shown as an example of non-significant interaction effects (in NB females). Ca by TS interaction effects were insignificant/marginally significant in all subgroups and sites.
Table 4.
Mixed model analysis results, the effect of weight-bearing physical activity (PA) on total body BMC accrual in nonblack males after adjustment for the basic model and for calcium intake.
| Effect | Tanner | Estimate | StdErr* | ProbF |
|---|---|---|---|---|
| Nonblack Males | ||||
| Intercept | −3.277 | 2.816 | ||
| PA | 0.248 | 0.170 | 0.00047 | |
| Tanner | 1 | 0.117 | 5.183 | |
| Tanner | 2 | 1.814 | 7.030 | |
| Tanner | 3 | −24.992 | 8.639 | |
| Tanner | 4 | 1.182 | 7.498 | |
| Tanner | 5 | 0 | 0.05913 | |
| PA*tanner | 1 | 0.020 | 0.346 | |
| PA*tanner | 2 | −0.135 | 0.437 | |
| PA*tanner | 3 | 1.887 | 0.523 | |
| PA*tanner | 4 | −0.098 | 0.441 | |
| PA*tanner | 5 | 0 | 0.00732 | |
StdErr: standard error.
Figure 1.
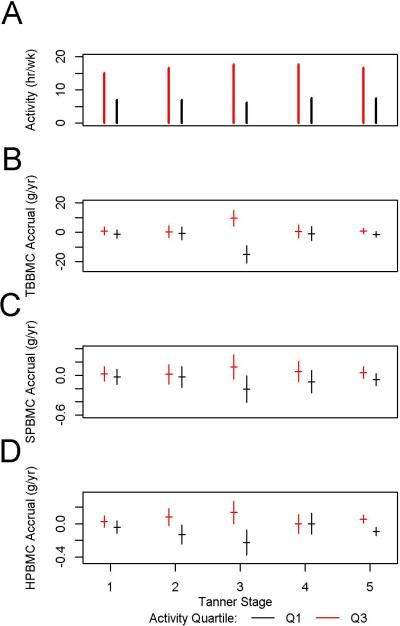
For NB males, for each TS, expected annualized BMC change (adjusted for the basic model and calcium intake) is shown, along with the +/− 1 standard error range, predicted for a high activity child (at Q3, the 75th percentile of PA for the subgroup and TS) and a low activity child (at Q1, the 25th percentile). The figure also shows activity hours, Q1 and Q3, for each TS. A: Q1 and Q3 of weight-bearing activity. B: TBBMC expected annualized accrual. C: SPBMC expected annualized accrual. D: HP BMC expected annualized accrual.
Figure 2.
For NB females, for each TS, expected annualized BMC change (adjusted for the basic model and calcium intake) is shown, along with the +/− 1 standard error range, predicted for a high activity child (at Q3, the 75th percentile of PA for the subgroup and TS) and a low activity child (at Q1, the 25th percentile). The figure also shows activity hours, Q1 and Q3, for each TS. A: Q1 and Q3 of weight-bearing activity. B: TBBMC expected annualized accrual. C: SPBMC expected annualized accrual. D: HP BMC expected annualized accrual.
Given the lack of clearly significant interactions with TS in most subgroups/skeletal sites, PA and Ca were each evaluated as the sole predictors in the model. Results from these analyses are presented in Table 5. PA was a significant predictor of annualized BMC accrual rate at all skeletal sites after adjustment for the basic model and Ca in NB males, at the lumbar spine and hip in BL males, at the total body and hip in NB females, and at the spine in BL females. Ca was a significant predictor of BMC accrual rate at the lumbar spine in NB females (p<0.002) after adjusting for PA (Table 5).
Table 5.
Mixed model analysis results, the effect of weight-bearing physical activity (PA) or calcium intake (Ca) on BMC accrual, adjusted for the basic model, and for Ca when evaluating PA effects, and for PA when evaluating Ca effects.
| Total body BMC | Spine BMC | Hip BMC | ||
|---|---|---|---|---|
| Nonblack Males | PA | 0.006* | 0.006 | 0.0001 |
| Ca | NS | NS | NS | |
| Black Males | PA | NS | 0.0278 | 0.0383 |
| Ca | NS | NS | NS | |
| Nonblack Females | PA | 0.0171 | NS | 0.0034 |
| Ca | NS | 0.0018 | NS | |
| Black Females | PA | NS | 0.0177 | NS |
| Ca | NS | NS | NS |
Significant PA by TS interaction
Sensitivity analyses indicated that neither including study site nor excluding observations with visit intervals less than 11 months or more than 13 months in the model affected the outcomes.
Discussion
This is the first long-term prospective study to compare the effects of self-reported weight-bearing physical activity and dietary calcium intake on rates of bone mass accrual throughout pubertal maturation in a large diverse cohort of boys and girls. We found that self-reported weight-bearing physical activity contributed to significantly greater BMC accrual after adjustment for age, height velocity, Tanner stage, prior visit BMC, and calcium intake in both sexes and both racial subgroups. Findings from our study do not support the hypothesis that the effects of PA and Ca on BMC accrual vary as a function of puberty, with the exception that the PA effect on total BMC accrual varied among TS in non-black males. The difference in predicted total body BMC accrual between high and low activity non-black boys was largest in TS-3. Calcium intake had a significant effect on adjusted BMC accrual only in non-black girls; this effect was not significantly different among TS. The lack of calcium and activity effects in BL boys and girls may be attributed partially to our much smaller number of observations in those groups.
Others have found greater effects of PA on percent bone accrual at various maturational times. MacKelvie et al recently conducted a systematic review of exercise interventions in children to determine whether a critical period exists for bone response to weight-bearing exercise.(28) Eight studies met their criteria of being an exercise intervention in healthy non-athletes less than 18 yr of age. Of those eight studies, all three interventions in pre-pubertal children, designated by the researchers as TS1 and 2, showed a significant effect on bone accrual at one or more skeletal sites.(18,21,25) The one study of early-pubertal (designated as TS1 – 3) girls by Morris et al(22) also demonstrated a significant effect on bone accrual. Neither study of post-menarcheal (designated as TS4-5) girls found an effect;(46,47) however, one of these studies had a very small sample size and used as an intervention weight training with hydraulic machines, which may not have provided enough additional strain on the skeleton.(47) A study by Heinonen et al designed to determine whether pre-menarcheal girls had a greater bone response to an exercise intervention than post-menarcheal girls found that the exercise significantly increased bone accrual in pre-, but not post-menarcheal girls.(29) In the latter study, pre-menarcheal girls were TS1-3 while post-menarcheal girls were TS2-5, so there was overlap in TS between the two menarcheal groups. Our findings that the effect of self-reported PA on BMC accrual does not vary with pubertal stage, compared to findings of randomized PA trials, suggests that more vigorous PA is needed to detect a difference in skeletal loading response across TS.
The PA levels in our study were self-selected and self-reported, which is less exact than PA administered in a treatment intervention. Nonetheless, mean PA values were significantly associated with BMC accrual in all subgroups of our cohort, reinforcing the importance of weight-bearing PA in development of optimal peak bone mass. In fact, as can be seen in Figure 1 showing NB boys as an example, those in the lowest activity group demonstrated lower than expected accrual at some stages. Thus it is critical that children and adolescents be encouraged to engage in weight-bearing physical activity throughout development.
The U.S. Department of Health and Human Services recommends that children and adolescents participate in at least one hour of PA per day (seven hours/week), including daily moderate- or vigorous-intensity aerobic physical activity. Bone and muscle-strengthening activity should be performed at least three days per week.(44) Mean PA in each race/sex subgroup of this cohort, using the Slemenda questionnaire, exceeded recommended overall hours of physical activity, except for TS2 in black females and TS5 in nonblack females. All subgroups exceeded recommended hours of weight-bearing PA to strengthen bones (three hours/week). Our self-report questionnaire did not ascertain level of intensity so that cannot be compared to recommended levels.
The level of PA correlated significantly between adjacent TS in all race/sex subgroups, albeit weaker in BL males, indicating that PA tracked as children grew older (See Table 3). This suggests that implementing PA routines in younger children may lead to higher physical activity levels later in childhood and through adolescence.
Self-reported dietary calcium intake was a significant predictor of adjusted BMC accrual, independent of activity, only at the lumbar spine in nonblack females, where it had a small, but significant, positive effect. On the other hand, most randomized trials of calcium supplementation or dairy food in growing youth show a positive effect of calcium on BMC/BMD accrual. However, the effect is small, about 1-2%/y greater than the placebo group, (12,48) and there is some evidence that the increases in BMD and BMC do not persist after the supplementation has been stopped.(14-16,49) One explanation for the difference between our study and others may be the relatively low calcium intake in our study. Mean Ca among the four race/sex subgroups ranged from 576 to 989 mg/day. However, our calcium intake values are underestimates since the FFQ was restricted to the frequency of intake and the portion size of 45 food and beverage items, selected because they captured 90% of dietary calcium intake of individuals 6 to 19 yr of age participating in NHANES III. Mean Ca remained fairly constant across TS in males, but the Ca in females was lower in TS5 than in other TS, which is consistent with other findings and has been explained by dieting behavior as girls mature.(50)
There was no evidence of an interaction between Ca and PA in this observational study. Ca and PA were correlated, and controlling for the Ca in the analysis diminished the effect of PA. Likewise, controlling for PA diminished the effect of Ca. Contrary to our findings, a few studies have found additive or synergistic effects of PA and Ca.(51,52) For example, Bass et al(52) found that a calcium and exercise intervention in pre- and early pubertal boys resulted in greater increase in BMC at the femur and tibia-fibula than exercise or calcium alone, whereas no effect was seen at the radius or lumbar spine. In a 8.5-mo randomized trial of exercise and calcium in pre- and early pubertal girls,(51) an exercise/calcium interaction was seen in the femur. However, the interaction was not seen at other skeletal sites. Ward et al(53) found that there was no beneficial effect of calcium supplementation in pre-pubertal elite gymnasts who already consumed recommended levels of calcium. Thus, there is a need for more randomized trials to determine the combined effects of optimal calcium intake and weight bearing physical activity.
This analysis provided an opportunity to characterize BMC accrual by TS in race/sex subgroups. As can be seen in Table 2, the highest BMC accrual was in TS4 in all subjects and skeletal sites. This is to be expected since mineralization of the skeleton lags behind longitudinal growth,(54) and PHV occurs between TS2 and 3 in girls and between TS 3 and 4 in boys.(55)
Strengths and Limitations
A major strength of this study was the annual Tanner staging completed by endocrinologists or trained nurse practitioners. The large sample of both boys and girls of racial diversity from various regions of the U.S. is also important. Furthermore, the standardized acquisition, quality control and analysis of all DXA scans were performed by the DXA Core Laboratory at UCSF. A limitation of this study is that weight-bearing physical activity and calcium intake were ascertained by self-report. However, in order to maximize the accuracy of these reports, study staff members at all sites were trained on a common protocol for administration of these questionnaires. Also, parents were encouraged to help children complete the questionnaires, especially the younger ones. Since our methods of assessment are not precise, our effect size estimates are underestimates.
Summary
This study affirmed the importance of weight-bearing physical activity for bone mass accrual in children and adolescents. Moreover, there was no definitive Tanner stage at which weight-bearing physical activity or calcium intake had a significantly greater effect on BMC accrual at all skeletal sites or in all race/sex subgroups. Our study and those of others(56,57) found that PA levels track during childhood, which provides justification to stimulate interest in physical activity at an early age. Although we found an effect of self-reported dietary Ca on BMC accrual only at the lumbar spine in non-black females, calcium remains essential for growth and development of the skeleton, and adequate calcium intake should be encouraged in all individuals throughout the lifespan.
Acknowledgments
We acknowledge our collaborators in the pediatric endocrine divisions of each Bone Mineral Density in Childhood Study Clinical Center. We also acknowledge the contributions of the subjects and families who volunteered to make this study possible.
Authors’ Roles:
Study design – JL, PW, VG, TH, HK, JS, KW, BZ
Study conduct - JL, PW, VG, TH, HK, SO, JS, KW, BZ
Data collection - JL, VG, HK, SO, BZ
Data analysis – JL and PW
Data interpretation - JL, PW, VG, TH, HK, SO, JS, KW, BZ
Drafting manuscript – JL, PW
Revising manuscript content - JL, PW, VG, TH, HK, SO, JS, KW, BZ
Approving final version of manuscript - JL, PW, VG, TH, HK, SO, JS, KW, BZ
Responsibility for integrity of the data analysis – PW, JL
This project was supported by Eunice Kennedy Shriver National Institute of Child Health and Human Development Contracts NO1-HD-1-3228, NO1-HD-1-3329, NO1-HD-1-3330, NO1-HD-1-3331, NO1-HD-1-3332 and NO1-HD-1-3333 and the Clinical and Translational Research Center Grant 5-MO1-RR-000240 and UL1RR-026314.
Footnotes
Disclosures: The authors state that they have no conflicts of interest.
Contributor Information
Joan M. Lappe, Creighton University, Omaha, Nebraska.
Patrice Watson, Creighton University, Omaha, Nebraska.
Vicente Gilsanz, Children's Hospital Los Angeles, Los Angeles, California.
Thomas Hangartner, Wright State University, Dayton, Ohio.
Heidi J. Kalkwarf, Cincinnati Children's Hospital Medical Center, Cincinnati, Ohio.
Sharon Oberfield, Columbia University, New York, New York.
John Shepherd, University of California at San Francisco, San Francisco, California.
Karen K. Winer, Eunice Kennedy Shriver National Institute of Child Health and Human Development, Bethesda, Maryland.
Babette Zemel, Children's Hospital of Philadelphia, Philadelphia, Pennsylvania.
Reference List
- 1.Dent C. Problems in metabolic bone disease. Clinical aspects of metabolic bone disease.. Proceedings of the international symposium on clinical aspects of metabolic bone disease; Detroit. 1972; Henry Ford Hospital; pp. 1–7. [Google Scholar]
- 2.Seeman E, Hopper J, Back L, Cooper M, Parkinson E, McKay J, et al. Reduced bone mass in daughters of women with osteoporosis. New Engl J Med. 1989;320:554–8. doi: 10.1056/NEJM198903023200903. [DOI] [PubMed] [Google Scholar]
- 3.Sandler R, Slemenda C, LaPorte R, Cauley J, Schramm M, Barresi M, et al. Postmenopausal bone density and milk consumption in childhood and adolescence. Am J Clin Nutr. 1985;42:270–4. doi: 10.1093/ajcn/42.2.270. [DOI] [PubMed] [Google Scholar]
- 4.NIH Consensus Development Panel on Optimal Calcium Intake. Optimal calcium intake. JAMA. 1994;272(24):1942–8. [PubMed] [Google Scholar]
- 5.Matkovic V, Ilich J, Skugor M. Calcium intake and skeletal formation. In: Burchhardt P, Heaney R, editors. Nutritional aspects of osteoporosis '94. Ares-Serono Symposia Pub.; Rome, Italy: 1995. pp. 129–45. [Google Scholar]
- 6.Matkovic V, Jelic T, Wardlaw G, Ilich J, Goel P, Wright J, et al. Timing of peak bone mass in Caucasian females and its implication for the prevention of osteoporosis: Inference from a cross-sectional model. J Clin Invest. 1994;93:799–808. doi: 10.1172/JCI117034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bonjour J, Theintz G, Buchs B, Slosman D, Rizoli R. Critical years and stages of puberty for spinal and femoral bone mass accumulation during adolescence. J Clin Endocrinol Metabol. 1991;73:555–63. doi: 10.1210/jcem-73-3-555. [DOI] [PubMed] [Google Scholar]
- 8.Baxter-Jones ADG, Faulkner RA, Forwood M, Mirwald RL, Bailey DA. Bone mineral accrual from 8 to 30 years of age: An estimation of peak bone mass. J Bone Miner Res. 2011;26(8):1729–39. doi: 10.1002/jbmr.412. [DOI] [PubMed] [Google Scholar]
- 9.Cummings C, Black D, Nevitt M, Browner W, Cauley J, Ensurd K, et al. bone density at various sites for prediction of hip fractures. Lancet. 1993;341:72–5. doi: 10.1016/0140-6736(93)92555-8. [DOI] [PubMed] [Google Scholar]
- 10.Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: Report of a WHO Study Group. World Health Organ Tech Rep Ser. 1994:1–129. [PubMed] [Google Scholar]
- 11.Clark EM, Ness AR, Bishop NJ, Tobias JH. Association Between Bone Mass and Fractures in Children: A Prospective Cohort Study. J Bone Miner Res. 2006;21(9):1489–95. doi: 10.1359/jbmr.060601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wosje K, Specker B. Role of calcium in bone health during childhood. Nutr Rev. 2000;58:253–68. doi: 10.1111/j.1753-4887.2000.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 13.Winzenberg T, Shaw K, Fryer J, Jones G. Effects of calcium supplementation on bone density in healthy children: meta-analysis of randomised controlled trials. BMJ. 2006;333(7572):775. doi: 10.1136/bmj.38950.561400.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnston C, Miller J, Slemenda C, Reister T, Hui S, Christian J, et al. Calcium supplementation and increases in bone mineral density in children. New Engl J Med. 1992;327:82–7. doi: 10.1056/NEJM199207093270204. [DOI] [PubMed] [Google Scholar]
- 15.Lloyd T, Rollings N, Andon M, Eggli D, Maugeri D, Chinchilli V. Enhanced bone gain in early adolescence due to calcium supplementation does not persist in late adolescence. J Bone Miner Res. 1996;11(S1):S154. [Google Scholar]
- 16.Lee W, Leung S, Leung D, Cheng J. A follow-up study on the effect of calcium-supplement withdrawal and puberty on bone acquisition in children. Am J Clin Nutr. 1996;64(1):71–7. doi: 10.1093/ajcn/64.1.71. [DOI] [PubMed] [Google Scholar]
- 17.Hind K, Burrows M. Weight-bearing exercise and bone mineral accrual in children and adolescents: a review of controlled trials. Bone. 2007;40(1):14–27. doi: 10.1016/j.bone.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 18.Fuchs R, Bauer JJ, Snow CM. Jumping improves hip and lumbar spine bone mass in prepubescent children: A randomized controlled trial. J Bone Miner Res. 2001;16(1):148–56. doi: 10.1359/jbmr.2001.16.1.148. [DOI] [PubMed] [Google Scholar]
- 19.MacKelvie K, Petit MA, Khan K, Beck T, McKay H. Bone mass and structure are enhanced following a 2-year randomized controlled trial of exercise in prepubertal boys. Bone. 2004;34(4):755–64. doi: 10.1016/j.bone.2003.12.017. [DOI] [PubMed] [Google Scholar]
- 20.MacKelvie K, Khan K, Petit MA, Janssen P, McKay H. A school-based exercise intervention elicits substantial bone health benefits: a 2-year randomized controlled trial in girls. Pediatr. 2003;112(6):e447–e452. doi: 10.1542/peds.112.6.e447. [DOI] [PubMed] [Google Scholar]
- 21.Bradney M, Pearce G, Naughton G, Sullivan C, Bass S, Beck T, et al. Moderate exercise during growth in prepubertal boys: Changes in bone mass, volumetric density, and bone strength: A controlled prospective study. J Bone Miner Res. 1998;13:1814–21. doi: 10.1359/jbmr.1998.13.12.1814. [DOI] [PubMed] [Google Scholar]
- 22.Morris F, Naughton G, Gibbs J, Carlson J, Wark J. Prospective ten-month exercise intervention in premenarcheal girls: positive effects on bone and lean mass. J Bone Miner Res. 1997;12:1453–62. doi: 10.1359/jbmr.1997.12.9.1453. [DOI] [PubMed] [Google Scholar]
- 23.Heinonen A, Sievanen H, Kannus P, Pasanen M, Vuori I. High-impact exercise and bones of growing girls: A 9-month controlled trial. Osteoporos Int. 2000;11:1010–7. doi: 10.1007/s001980070021. [DOI] [PubMed] [Google Scholar]
- 24.MacKelvie K, McKay H, Khan K, Crocker PRE. A school-based exercise intervention augments bone mineral accrual in early pubertal girls. J Pediatr. 2001;139:501–8. doi: 10.1067/mpd.2001.118190. [DOI] [PubMed] [Google Scholar]
- 25.McKay H, Petit MA, Schutz RW, Prior J, Barr S, Khan KM. Augmented trochanteric bone mineral density after modified physical education classes: A randomized school-based exercise intervention study in prepubescent and early pubescent children. J Pediatr. 2000;136(2):156–61. doi: 10.1016/s0022-3476(00)70095-3. [DOI] [PubMed] [Google Scholar]
- 26.Forwood M, Burr D. Physical activity and bone mass: Exercises in futility? Bone Miner. 1993;21:89–112. doi: 10.1016/s0169-6009(08)80012-8. [DOI] [PubMed] [Google Scholar]
- 27.Parfitt A. The two faces of growth: Benefits and risks to bone integrity. Osteoporos Int. 1994;4:382–98. doi: 10.1007/BF01622201. [DOI] [PubMed] [Google Scholar]
- 28.MacKelvie KJ, Khan KM, McKay HA. Is there a critical period for bone response to weight-bearing exercise in children and adolescents? a systematic review. British Journal of Sports Medicine. 2002;36(4):250–7. doi: 10.1136/bjsm.36.4.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heinonen A, Sievanen H, Kannus P, Oja P, Pasanen M, Vuori I. High-impact exercise and bones of growing girls: a 9-month controlled trial. Osteoporos Int. 2000;11:1010–7. doi: 10.1007/s001980070021. [DOI] [PubMed] [Google Scholar]
- 30.Bailey D, Baxter-Jones A, Mirwald R, Faulkner R. Bone growth and exercise studies: The complications of maturation. J Musculoskelet Neuronal Interact. 2003;3(4):335–7. [PubMed] [Google Scholar]
- 31.Kalkwarf H, Gilsanz V, Lappe J, Zemel B, Horlick M, Oberfield S, et al. The bone mineral density in childhood study (BMDCS): Bone mineral content and density according to age, sex, and race. J Clin Endocrinol Metab. 2007;92:2087–99. doi: 10.1210/jc.2006-2553. [DOI] [PubMed] [Google Scholar]
- 32.Zemel BS, Kalkwarf HJ, Gilsanz V, Lappe JM, Oberfield S, Shepherd JA, et al. Revised Reference Curves for Bone Mineral Content and Areal Bone Mineral Density According to Age and Sex for Black and Non-Black Children: Results of the Bone Mineral Density in Childhood Study. Journal of Clinical Endocrinology & Metabolism. 2011;96:3160–9. doi: 10.1210/jc.2011-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goulding A, Jones I, Williams S, Grant A, Taylor R, Manning P, et al. First fracture is associated with increased risk of new fractures during growth. J Pediatr. 2005;146:286–8. doi: 10.1016/j.jpeds.2004.09.029. [DOI] [PubMed] [Google Scholar]
- 34.Gruber R, von Laer L. Etiology of the refracture of the forearm in childhood. Aktuelle Traumatol. 1979;9:251–9. [PubMed] [Google Scholar]
- 35.Gordon C, Leonard M, Zemel B. 2013 Pediatric Position Development Conference: executive summary and reflections. J Clin Densitom. 2014;17:219–24. doi: 10.1016/j.jocd.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 36.Slemenda C, Miller J, Hui S, Reister T, Johnston C. Role of physical activity in the development of skeletal mass in children. J Bone Miner Res. 1991;6:1227–33. doi: 10.1002/jbmr.5650061113. [DOI] [PubMed] [Google Scholar]
- 37.Tanner J. Growth at adolescence. 2nd ed. Blackwell Scientific Publications; Oxford UK: 1962. [Google Scholar]
- 38.Zachmann M, Prader A, Kind H, Hafliger H, Budliger H. Testicular volume during adolescence. Cross-sectional and longitudinal studies. Helv Paediatr Acta. 1974;29:61–2. [PubMed] [Google Scholar]
- 39.Tanner J. Physical growth and development. Textbook of Pediatrics. 1978;2:249–303. [Google Scholar]
- 40.Alison P. Fixed Effects Regression methods for Longitudinal Data using SAS. SAS Institute Inc.; Cary NC: 2005. [Google Scholar]
- 41.Gilsanz V, Skaggs D, Kovanlikaya A, Sayre J, Loro M, Kaufman F, et al. Differential effect of race on the axial and appendicular skeletons of children. J Clin Endocrinol Metab. 1998;83:1420–7. doi: 10.1210/jcem.83.5.4765. [DOI] [PubMed] [Google Scholar]
- 42.Bachrach L, Hastie T, Wang M, Balasubramanian N, Marcus B. Bone mineral acquisition in healthy Asian, Hispanic, Black, and Caucasian youth: A longitudinal study. J Clin Endocrinol Metab. 1999;84:4702–12. doi: 10.1210/jcem.84.12.6182. [DOI] [PubMed] [Google Scholar]
- 43.Norris S, Micklesfield L, Pettifor J. Ethnic differences in bone acquisition. In: Rosen editor-in-chief C, editor. Primer on the metabolic bone diseases and disorders of mineral metabolism. 8th ed. Wiley-Blackwell; Ames IA: 2013. pp. 135–41. [Google Scholar]
- 44.US Department of Health & Human Services. Lavizzo-Mourey R, Dorn JM, Fulton J, Janz K, Lee S, et al. Physical Activity Guidelines for Americans Midcourse Report: Strategies to Increase Physical Activity Among Youth. US Department of Health & Human Services; Washington, DC: 2012. [Google Scholar]
- 45.Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK, et al. The 2011 Report on Dietary Reference Intakes for Calcium and Vitamin D from the Institute of Medicine: What Clinicians Need to Know. Journal of Clinical Endocrinology Metabolism. 2010 doi: 10.1210/jc.2010-2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Witzke K, Snow C. Lean body mass and leg power best predict bone mineral density in adolescent girls. Med Sci Sports Exerc. 1999;31:1558–63. doi: 10.1097/00005768-199911000-00010. [DOI] [PubMed] [Google Scholar]
- 47.Blimkie CJ, Rice S, Webber CE, Martin J, Levy D, Gordon CL. Effects of resistance training on bone mineral content and density in adolescent females. Can J Physiol Pharmacol. 1996;74(9):1025–33. [PubMed] [Google Scholar]
- 48.Weaver C. Osteoporosis: The early years. In: Coulstron A, Boushey C, editors. Nutrition in the prevention and treatment of disease. 2nd ed. Academic Press; New York: 2008. [Google Scholar]
- 49.Ward K, Cole T, Laskey M, Ceesay M, Mendy M, Sawo Y, et al. The effect of prepubertal calcium carbonate supplementation on skeletal development in Gambian boys: a 12-year follow up study. J Clin Endocrinol Metab. 2014 doi: 10.1210/jc.2014-1150. early release on line(April):1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weaver C. Adolescence: The period of dramatic bone growth. Endocrine. 2002;17:43–8. doi: 10.1385/ENDO:17:1:43. [DOI] [PubMed] [Google Scholar]
- 51.Iuliano-Burns S, Saxon L, Naughton G, Gibbons K, Bass S. Regional specificity of exercise and calcium during skeletal growth in girls: a randomized controlled trial. J Bone Miner Res. 2003;18(1):156–62. doi: 10.1359/jbmr.2003.18.1.156. [DOI] [PubMed] [Google Scholar]
- 52.Bass SL, Naughton G, Saxon L, Iuliano-Burns S, Daly R, Brignati EM, et al. Exercise and calcium combined results in a greater osteogenic effect than either factor alone: a blinded randomized placebo-controlled trial in boys. J Bone Miner Res. 2007;22(3):458–64. doi: 10.1359/jbmr.061201. [DOI] [PubMed] [Google Scholar]
- 53.Ward KA, Roberts SA, Adams JE, Lanham-New S, Mughal MZ. Calcium supplementation and weight bearing physical activity--do they have a combined effect on the bone density of pre-pubertal children? Bone. 2007;41(4):496–504. doi: 10.1016/j.bone.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 54.Bailey DA, Martin A, McKay H, Whiting S, Mirwald R. Calcium accretion in girls and boys during puberty: A longitudinal analysis. J Bone Miner Res. 2000;15(11):2245–50. doi: 10.1359/jbmr.2000.15.11.2245. [DOI] [PubMed] [Google Scholar]
- 55.Abbassi V. Growth and normal puberty. Pediatrics. 1998;102(2 Pt 3):507–11. [PubMed] [Google Scholar]
- 56.Ferrari S, Rizzoli R, Slosman D, Bonjour J. Familial resemblance for bone mineral mass is expressed before puberty. J Clin Endocrinol Metab. 1998;83:358–61. doi: 10.1210/jcem.83.2.4583. [DOI] [PubMed] [Google Scholar]
- 57.Loro ML, Sayre J, Roe TF, Goran MI, Kaufman FR, Gilsanz V. Early identification of children predisposed to low peak bone mass and osteoporosis later inff life. J Clin Endocrinol Metab. 2000;85:3908–18. doi: 10.1210/jcem.85.10.6887. [DOI] [PubMed] [Google Scholar]