Abstract
Objective
TNFα antagonists are effective for treating rheumatoid arthritis and other inflammatory diseases, but their use can be complicated by lupus-like phenomena. We investigated the role of TNFα in murine lupus.
Methods
TLR7 ligand-driven lupus was induced in B6 and B6-TNFα−/− mice using pristane. Autoantibodies and type I interferon (IFN-I) production were measured and the effects on IFN-I-producing plasmacytoid dendritic cells (pDCs), Ly6Chi monocytes, and TNFα-producing neutrophils were determined.
Results
TNFα−/− mice did not spontaneously develop autoantibodies or clinical manifestations, suggesting that TNFα deficiency alone is insufficient to cause lupus. Although IFN-I levels were comparable in untreated TNFα−/− and B6 mice, untreated TNFα−/− mice had increased circulating pDCs and “pDC-like” cells, enhancing their potential to make IFN-I. When treated with pristane, TNFα−/− mice developed more severe lupus than controls with increased levels of anti-Sm/RNP autoantibodies, IFN-I, pDCs, and peritoneal inflammatory (Ly6Chi) monocytes. Neutrophils, which promoted resolution of inflammation, were decreased considerably in pristane-treated TNFα−/− mice, whereas the inflammatory monocyte and pDC responses and IFN-I were increased and prolonged.
Conclusions
Low levels of TNFα increased circulating pDC numbers, enhancing the potential to make IFN-I. But this did not lead to IFN-I production or autoimmunity unless there was concomitant exposure to endogenous TLR7 ligands released from dead cells following pristane treatment. In patients, the rate of clearance of dead cells along with TNFα levels may influence who will develop lupus when treated with TNFα inhibitors.
Type I interferon (IFN-I) is strongly implicated in the pathogenesis of SLE. Peripheral blood mononuclear cells (PBMC) from ~60% of adult and nearly all pediatric SLE patients over-express IFN-I regulated genes (interferon signature) (1), individuals with three copies of the type I interferon gene cluster develop lupus-like disease (2), and IFNα exacerbates lupus in NZB/W mice (3). In contrast, tumor necrosis factor α (TNFα) is critical to the pathogenesis of rheumatoid arthritis (RA), inflammatory bowel disease, and psoriasis (4), diseases that are treated with TNFα inhibitors (TNFIs).
Up to 60% of patients treated with TNFIs develop antinuclear antibodies (ANA) and 10–20% develop anti-double-stranded (ds) DNA autoantibodies, but <1% develop clinical lupus (5–7). The pathogenesis of TNFI-induced lupus remains uncertain and the risk factors are incompletely understood. Patients with Sjogren’s syndrome treated with a TNFI have increased IFN-I levels (8), suggesting that TNFα negatively regulates IFN-I production. Consistent with that possibility, TNFα inhibits the generation of plasmacytoid dendritic cells (pDCs) and IFN-I secretion by pDCs upon viral triggering. Further, in vitro culture of PBMCs with a TNFI increases IFNα expression (9). Here, we examined whether TNFα deficient (TNFα−/−) mice develop lupus. Although TNFα deficiency was associated with increased pDC generation, it did not induce autoantibodies or lupus-like manifestations spontaneously. However, when lupus was induced by the inflammatory hydrocarbon pristane (2,6,10,14 tetramethylpentadecane, TMPD), IFN-I and autoantibody levels were 5-fold higher in TNFα−/− vs. TNFα-intact mice. These observations may have implications for the pathogenesis of human TNFI-induced lupus.
Materials and Methods
Mice
Mice were bred and maintained under specific pathogen-free conditions at the University of Florida Animal Facility. C57BL/6 (B6) and B6,129S-Tnftm1Gkl/J (TNFα−/−) mice were from Jackson Laboratory (Bar Harbor, ME). To induce lupus, 0.5 ml pristane (Sigma-Aldrich) was injected i.p. Controls were left untreated (10, 11). Serum from the tail vein (0–24 weeks after pristane injection) was analyzed for autoantibodies (10). These studies were approved by the Institutional Animal Care and Use Committee.
ELISA
Total Ig levels were measured by ELISA as described (11, 12). Anti-RNP/Sm ELISA used 1:400 diluted mouse sera and 1:1000 goat anti-mouse IgG second Abs (10). Anti-dsDNA antibodies were quantified by ELISA (1:400 serum dilution) using S1 nuclease-treated calf thymus DNA as antigen (13). Anti-U1A (anti-RNP subset) antibodies were quantified by ELISA using recombinant U1A antigen, 1:400 diluted serum samples, and 1:1000 alkaline phosphatase-conjugated goat anti-mouse isotype antibodies (10).
To quantify anti-U1A autoantibodies in B6 vs. TNFα−/− mice, equal amounts of serum from each mouse in the two groups (B6 n=12, TNFα−/− n=12) were pooled and serially diluted (fold from 1:400 to 1:2,624,400). Optical densities (ELISA) using samples from the two groups were plotted as a function of serum dilution. To estimate the relative levels of anti-U1A, O.D. was plotted as a function of the log dilution at an O.D. of 0.7 and X-intercepts were determined.
Fluorescent ANA assay
Serum ANA were detected by Crithidia luciliae kinetoplast staining assay (Inova) following the manufacturer’s protocol. Serum dilution was 1:100 and secondary antibody was goat anti-mouse IgG (20 μg/ml).
Quantitative PCR (Q-PCR)
Q-PCR was performed as described (12, 14) using RNA extracted from 106 peritoneal cells using TRIzol (Invitrogen). Peripheral blood RNA was isolated with QIAamp RNA Blood Mini Kit (Qiagen). cDNA was synthesized using the Superscript II First-Strand Synthesis kit (Invitrogen) according to the manufacturer’s protocol. SYBR Green Q-PCR analysis was performed using an Opticon II thermocycler (Bio-Rad). Primer sequences were as follows: IFN-stimulated gene (ISG)-15 forward, 5′-GAGCTAGAGCCTGCAGCAAT and reverse, 5′-TAAGACCGTCCTGGAGCACT-3′; IRF7 forward, 5′-ACAGCACAGGGCGTTTTATC-3′ and reverse, 5′-GAGCCCAGCATTTTCTCTTG-3′; Mx-1 forward, 5′-GATCCGACTTCACTTCCAGATGG-3′ and reverse, 5′-CATCTCAGTGGTAGTCCAACCC-3′; CXCL5 forward, 5′-CCCCTTCCTCAGTCATAGCC-3′ and reverse, 5′-TGGATTCCGCTTAGCTTTCT-3′; TCF4 (E2-2) forward, 5′-GTGGACATTTCACTGGCTCA-3′ and reverse, 5′-CCCTGCTAGTCATGTGGTCA-3′; SPIB forward, 5′-AACCACCATGCTTGCTCTG-3′, and reverse, 5′-CTGGGTAACTGAAGGGCTTG-3′; CXCL2 forward: 5′-AAGTTTGCCTTGACCCTGAA-3′; reverse: 5′-CGAGGCACATCAGGTACGAT-3′ and CXCL3 forward: 5′-CCACTCTCAAGGATGGTCAA-3′, reverse: 5′-GGATGGATCGCTTTTCTCTG-3′; Dock2 forward: 5′-CTTCTTCCAAGTCTCAGATGG-3′, reverse: 5′-TTCCCACAGTGCTCGGCTCA-3′; IL1α forward: 5′-TGTGACTGCCCAAGATGAAG-3′ and reverse, 5′-CTTAGTGCCGTGAGTTTCCC-3′; IL1β forward: 5′-TGAAGCAGCTATGGCAACTG-3′ and reverse, 5′-AGGTCAAAGGTTTGGAAG-3′; BAFF forward: 5′-AGGGACCAGAGGAAACAGAA-3′ and reverse, 5′-AAAGCTGAGAAGCCATGGAA-3′.
Flow cytometry
Flow cytometry was performed as described (10, 15). Cells were incubated with anti-mouse CD16/32 (Fc Block; BD Biosciences) before staining with primary antibody or isotype control antibodies (10 min, 22°C). Ten-thousand to 50,000 events per sample were acquired using a CYAN ADP flow cytometer (Beckman-Coulter) and analyzed with FCS Express 3 (De Novo Software). The following antibodies were used: anti-ScaI-phycoerythrin (PE), anti-TNFα-allophycocyanin, anti-Ly6G-PE, anti-B220-allophycocyanin-CY7, anti-CD11c-allophycocyanin, anti-B220-FITC, anti-Ly6C-FITC, anti-CCR7-PE (BD Bioscience), avidin-PE-CY7, anti-CD11b-Brilliant Violet, anti-CCR9-FITC, and anti-PDCA-1-biotin (BioLegend). Intracellular TNFα staining was performed as described (16).
Monocyte depletion
Clodronate-containing liposomes (anionic, clo-lip) were purchased from Formumax (Palo Alto, CA) and administrated as described (17). To deplete peritoneal monocytes in TNFα−/− mice, 150 μl of clo-lip were injected i.p. into mice treated with pristane 2 weeks earlier. Peritoneal cells were collected two days later and percentages of Ly6Chi and Ly6Clo monocytes and pDCs were determined. Expression of the interferon-regulated markers Sca-1 (fluorescence intensity, flow cytometry) and IRF7 (Q-PCR) were quantified.
Neutrophil transfer
Neutrophil donors (B6 mice) were treated with 1.5 ml thioglycollate i.p. 5-hours before harvesting peritoneal cells. Clo-lip (0.1 ml i.p.) were injected 30 minutes after injecting the thioglycollate. Donor peritoneal cells were pooled. Splenocytes from the same donor mice served as a control and were treated similarly with clo-lip. RBCs were lysed with 10 ml RBC lysis buffer (Qiagene). Cells were pelleted at 4°C and immediately re-suspended at 5 × 106/ml in medium. Three TNFα−/− mice pretreated with pristane 2-weeks earlier received either 5 × 106 monocyte-depleted peritoneal cells (mainly neutrophils) or splenocytes (mainly lymphocytes) i.p. Composition of the donor cell populations was verified by flow cytometry. Positive control was TNFα−/− mice treated with pristane for 2-weeks but not injected with donor cells. Negative control was untreated TNFα−/− mice.
Cytokine ELISAs
CCL19 and CXCL5 ELISAs (R&D Systems) were performed following the manufacturer’s instructions. Optical density was converted to concentration using standard curves based on recombinant chemokines using Softmax Pro 4.3 (Molecular Devices).
Assessment of glomerulonephritis
Glomerular cellularity was evaluated by counting nuclei per glomerular cross-section (18). Renal immune complex deposition was evaluated by direct immunofluorescence of 4-μm frozen sections stained with FITC-conjugated rat anti-mouse C3 mAb (Cedarlane) (18).
Statistical analysis
Statistical analyses were performed using Prism 4.0 software (GraphPad). Differences between groups were analyzed by unpaired Student t test (normally distributed data) or Mann-Whitney U test (non-normally distributed data). Data are shown as mean ± SD for normally distributed data sets. Normality was determined by the D’agostino and Person omnibus test. All tests were two-sided; p < 0.05 was considered significant.
Results
Pristane-treated B6 mice develop a TLR7-driven lupus syndrome associated with IFN-I and TNFα production and accumulation of dead cells in the bone marrow (BM) (16, 19). Un-cleared dead cells are likely to be the source of endogenous TLR7 ligands driving the inflammatory response to pristane. We examined the effect of TNFα deficiency on the pathogenesis of lupus in pristane-treated mice as a means of modeling TNFI-induced lupus.
Increased autoantibody production in TNFα−/− mice
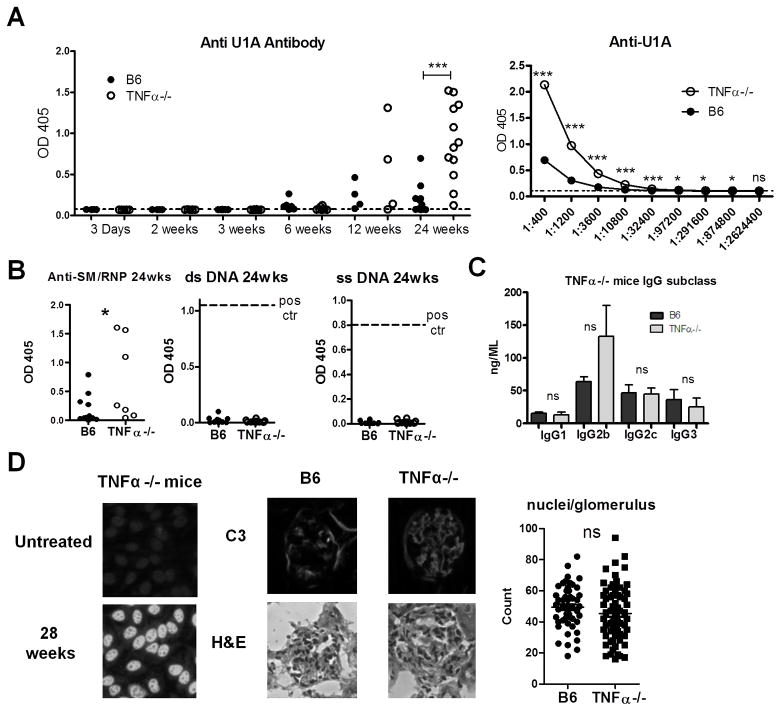
In B6-TNFα−/− and TNFα-intact B6 mice, anti-U1A autoantibodies were first detected 6–12 weeks after pristane treatment (Fig. 1A). Although the onset was similar, anti-U1A levels were increased 24-weeks post-pristane in TNFα−/− mice (P=0.0006, Student’s t-test). Moreover, 10/12 TNFα−/− mice had high-levels of anti-U1A autoantibodies vs. 2/12 controls at 6-months. Anti-U1A autoantibody titers (24 weeks) were substantially higher in pooled sera from TNFα−/− mice vs. controls (Fig. 1A, right panel). A significant difference was still observed at a serum dilution of 1:874,800. When the data were analyzed using a log plot, the mean autoantibody level in TNFα−/− mice was 5.37-fold higher in TNFα−/− mice than in wild type B6 mice (not shown).
Figure 1. Increased autoantibody production in TNFα−/− mice.
A, Left: B6-TNFα−/− (open circles) and TNFα-intact B6 mice (closed circles) were treated with pristane. Serum was collected at the indicated time-points and anti-U1A levels were measured: 3 days and 2, 6, and 12 weeks (N=4–6); 3 and 24 weeks (N=8–12). Sera from untreated B6 mice served as a control (dashed line). Right: Anti-U1A antibody titration curves of pooled (12 mice/group) week 24 sera from TNFα−/− (open circles) or B6 mice (closed circles). B, IgG anti-Sm/RNP autoantibody levels (ELISA) 24-weeks after pristane treatment. IgG anti-dsDNA and anti-ssDNA autoantibody levels (ELISA) 24-weeks after pristane treatment (12/group). Dashed line indicates positive control. C, Serum levels of total IgG1, IgG2b, IgG2c, and IgG3 in pristane-treated TNFα−/− and B6 mice (B6 N=12, TNFα−/− mice N=11). D, Left, Representative fluorescent ANA staining with sera from untreated and pristane-treated (28 weeks earlier) TNFα−/− mice (6 mice/group). Middle, Top, FITC-conjugated anti-mouse C3 antibody staining of renal tissue (4 μm sections) 3 months after pristane treatment. Bottom, staining with hematoxylin & eosin. Right, glomerular cellularity was evaluated by counting the number of nuclei per glomerulus. Similar counts were obtained by two independent observers. * P < 0.05; *** P <0.001 by unpaired Student’s t-test (A) or Mann-Whitney test (B). NS, not significant.
TNFα−/− mice also had higher anti-Sm/RNP antibodies (Fig. 1B). However, consistent with previous observations (19), pristane-treated B6 mice did not produce anti-dsDNA or anti-ssDNA antibodies (Fig. 1B). Anti-DNA autoantibodies also were absent in pristane-treated TNFα−/− mice, suggesting that absence of TNFα did not break tolerance to DNA. Total IgG2b/IgG2c levels, the predominant isotypes of anti-Sm/RNP, were similar in TNFα−/− and B6 mice (Fig. 1C). Although TNFI therapy induces ANA in many patients, untreated TNFα-deficient mice were ANA negative up to 12-months of age (n=6) (Fig. 1D). In contrast, 28-weeks post-pristane, sera from 3/6 TNFα−/− mice were strongly positive.
Glomerulonephritis is generally mild in pristane-treated B6 mice (19). Although intensity of C3 staining 12-weeks after pristane treatment was somewhat increased in TNFα−/− vs. B6 controls, glomerular cellularity was similar (Fig. 1D).
Increased IFN-I production in pristane-treated TNFα−/− mice
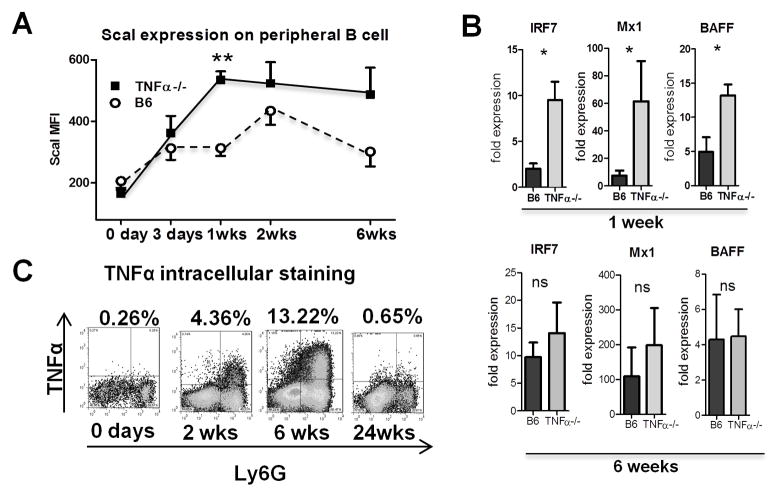
Since autoantibody production in pristane-treated mice is IFN-I dependent, we examined expression of the IFN-I inducible protein Sca-1 on circulating B cells (20). Sca-1 fluorescence intensity was increased in TNFα−/− mice as early as 1-week after pristane treatment (Fig. 2A). Similarly, Q-PCR showed increased expression of interferon-stimulated genes Irf7, Mx1, and Tnfsf13b (BAFF) in peritoneal cells from TNFα−/− mice 1-week after pristane treatment (Fig. 2B). Irf7 and Mx-1 expression further increased in TNFα−/− mice at 6-weeks, but were not significantly different than in B6. BAFF levels fell in TNFα−/− mice and did not differ between the strains at 6-weeks. CD11b+Ly6G+ peritoneal neutrophils from B6 mice exhibited increased intracellular TNFα 2-weeks after pristane treatment and staining increased up to 6-weeks before returning to baseline (Fig. 2C). This TNFα production was shown previously to require TLR7 (16).
Figure 2. Increased IFN-I production in pristane-treated TNFα−/− mice.
A, B6-TNFα−/− and TNFα-intact B6 mice were injected with pristane and peripheral blood was collected at different times (3–5 mice per data point). Erythrocytes were lysed and leukocytes were stained for Sca-1, gated on the CD19+ B cell population (solid squares, TNFα−/− mice; open circles, B6 mice). B, expression of interferon-stimulated genes Irf7, Mx-1, and BAFF (Tnfsf13b) in peripheral blood cells 1 week (top) and 6 weeks (bottom) after pristane treatment (Q-PCR). C, Total peritoneal exudate cells were surface-stained with anti-Ly6G and stained intracellularly for TNFα (TNFα+Ly6G+ cells as a % of total peritoneal exudate cells).
TNFα affects Ly6Chi (inflammatory) monocytes
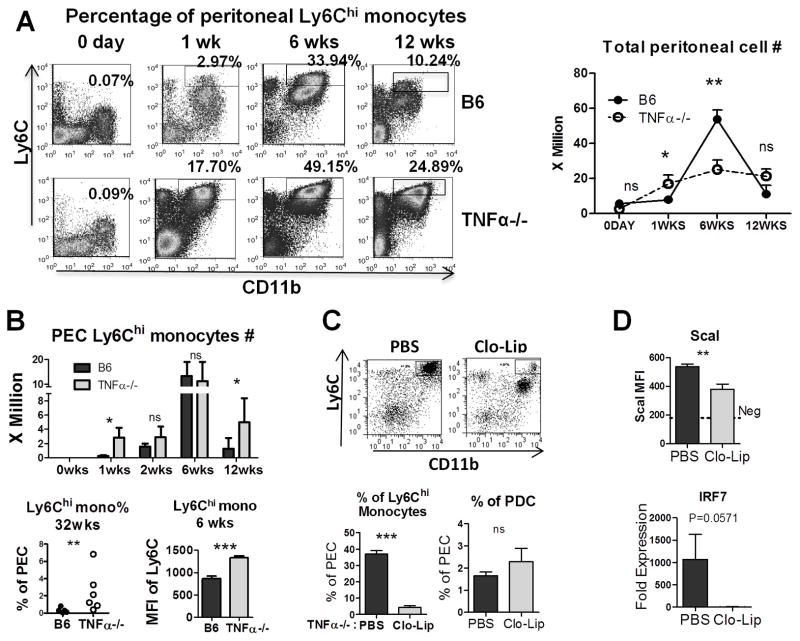
Total peritoneal cell number was determined at different time-points in B6 and TNFα−/− mice (Fig. 3A, Right). Ly6Chi monocytes are a major source of IFN-I in pristane-treated mice (21). In the peritoneum, IFNAR signaling blocks their maturation to Ly6Clo monocyte/macrophages. The percentage of peritoneal Ly6Chi monocytes (CD11b+Ly6G−Ly6Chi) was increased in TNFα−/− mice up to 12-weeks after pristane treatment (Fig. 3A, Left). Consistent with the increased IFN-I activity at 1-week (Fig. 2A, B), Ly6Chi monocyte counts also were increased in TNFα−/− mice (Fig. 3B). In contrast, at 2- and 6-weeks Ly6Chi monocyte counts were comparable in B6 and TNFα−/− mice (Fig. 3B) and IFN-I activity was less divergent (Fig. 2B). However, resolution of inflammation was delayed in TNFα−/− mice, as indicated by a higher percentage of Ly6Chi monocytes at 32 weeks post-pristane (Fig. 3B). Moreover, cell surface staining intensity of Ly6C (an interferon-inducible gene) was increased in the Ly6Chi monocytes from TNFα−/− mice (Fig. 3B, Lower)
Figure 3. TNFα affects Ly6Chi (inflammatory) monocytes.
A, Peritoneal cells were analyzed by flow cytometry 0–12 weeks after pristane injection. Left: Percentages of CD11b+Ly6Chi monocytes (rectangles) in total peritoneal exudate cells were assessed by flow cytometry. Right, total peritoneal cell counts in TNFα−/− (open circles) and B6 (closed circles) mice at 0–12 weeks after pristane treatment. B, Top: Comparison of CD11b+Ly6Chi monocytes at different time-points (3–5 mice per group). Bottom left, Ly6Chi monocytes (CD11b+Ly6ChiLy6G−) as a percentage of total peritoneal exudate cells at 32 weeks. Bottom right, Ly6C expression (MFI) on peritoneal Ly6Chi monocytes. C, Top: Flow cytometry of pristane-elicited peritoneal cells 2-days following treatment with clodronate liposomes (Clo-Lip) or PBS. Boxed areas indicate the percentage of Ly6Chigh monocytes (4 mice per group). Bottom left and right: Percentage of peritoneal Ly6Chi monocytes (left) and pDCs (right) in PBS or Clo-Lip treated mice. D: MFI of ScaI expression (flow cytometry) on peritoneal B cells and expression of IRF7 mRNA (Q-PCR) after PBS or Clo-Lip treatment in peritoneal exudate cells. * P < 0.05; ** P < 0.01; *** P < 0.001 unpaired Student’s t test.
Ly6Chi monocytes are a major source of IFN-I production in pristane-treated B6 mice. To evaluate the source of IFN-I production in the TNFα−/− mice, we depleted monocytes with clo-lip 2-weeks after pristane treatment (Fig. 3C). At 48-hours after pristane treatment the percentage of Ly6ChiCD11b+ cells was reduced by ~90% following i.p. injection of clo-lip, whereas there was little effect on CD11b−CD11cintB220+PDCA1+ pDCs, which are poorly phagocytic. Mice were pre-bled 24-hours before clo-lip treatment to verify that the two groups had similar expression of the interferon-inducible marker ScaI on peripheral blood B220+ cells (flow cytometry, not shown). Forty-eight hours after clo-lip treatment, expression of ScaI was reduced substantially in peritoneal exudate cells (PECs) by flow cytometry and there was a trend toward reduced IRF7 mRNA expression (Q-PCR) (Fig. 3D). Taken together, these data suggest that, as reported in wild-type B6 mice (20), Ly6Chi monocytes are responsible for much of the type I interferon produced in the peritoneum of pristane-treated TNFα−/− mice.
Defective neutrophil recruitment in TNFα−/− mice affects inflammation resolution
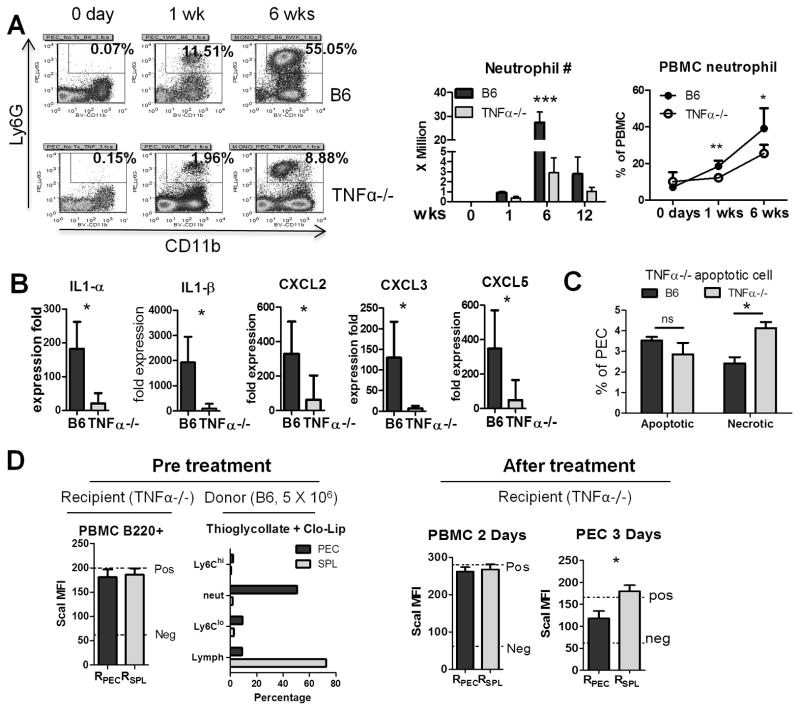
Since neutrophils are the main source of TNFα in the BM and peritoneum of pristane-treated B6 mice (16) and their apoptosis promotes resolution of inflammation (16), we examined CD11b+Ly6G+ peritoneal neutrophils (Fig. 4A). TNFα−/− mice had markedly fewer peritoneal neutrophils than controls by percentage and absolute count, especially near the peak of the acute inflammatory response (6-weeks) (Fig. 4A). Similarly, peripheral blood neutrophils were decreased (Fig. 4A, right). There was no significant change of the percentage of neutrophils in the BM 6 weeks after pristane treatment, suggesting that there is not an intrinsic deficiency in neutrophil maturation in the TNFα−/− mice (not shown). Peritoneal IL-1α and IL-1β transcripts as well as expression of the neutrophil chemokines CXCL2, CXCL3, and CXCL5 all were decreased in TNFα−/− mice (Fig. 4B). Decreased CXCL5 production was confirmed by ELISA (not shown).
Figure 4. Defective neutrophil recruitment in TNFα−/− mice.
Peritoneal cells from TNFα−/− and B6 mice were analyzed by flow cytometry at 0–12 weeks after pristane treatment. A, Left, percentages of CD11b+Ly6G+ neutrophils (rectangles). Middle, absolute CD11b+Ly6G+ neutrophil numbers at 0, 1, 6, and 12 weeks after pristane treatment (3–5 mice per group; absolute number = total peritoneal cell count X fraction of neutrophils). Right, percentages of CD11b+Ly6G+ neutrophils in peripheral blood at 0 to 6 weeks. B, Expression of IL1α and IL1β and neutrophil attractive chemokines CXCL2, CXCL3 in peritoneal exudate cells (Q-PCR) at 6 weeks. C, Percentages of apoptotic (annexin V+, 7AAD−) and necrotic (annexin V+, 7AAD+) cells in peritoneal exudate from B6 and TNFα−/− mice 2-weeks after pristane treatment. D, Left, recipient TNFα−/− mice 2-weeks post-pristane treatment were verified to have similar levels of ScaI expression in peripheral blood mononuclear cells (PBMC) before neutrophil transfer (RPEC, recipients of peritoneal neutrophils; RSPL, recipients of splenocytes). Composition of peritoneal donor cells from peritoneal exudate cells (PEC) and spleen (SPL) following treatment with clondronate liposomes (3 mice/group). Right, ScaI expression (mean fluorescence intensity by flow cytometry) on peripheral blood B220+ cells and B220+ peritoneal exudate cells (PEC) 2 days and 3 days after donor cell transfer. * P < 0.05; *** P <0.001 by unpaired Student’s t test. NS, not significant.
Apoptotic neutrophils are thought to play a critical role in the resolution phase of inflammation (22). To explore the possibility that a deficiency of peritoneal neutrophils contributes to the prolonged inflammatory response to pristane in TNFα−/− mice, we performed neutrophil transfer experiments and assessed apoptosis/necrosis. TNFα−/− mice and control B6 mice were injected with pristane and 1-week later PECs were stained for apoptotic and necrotic cells using annexin V and 7AAD (Fig. 4C). Although there was no significant difference in early apoptotic cells (annexin V+7AAD−), TNFα−/− mice had more late apoptotic/necrotic (annexin V+7AAD+) PECs than controls. Since necrotic cells tend to be pro-inflammatory, this may contribute to the increased inflammatory response in TNFα−/− mice.
To examine the functional role of neutrophils, we transferred enriched peritoneal neutrophils (elicited by thioglycollate injection) or splenic lymphocytes into pristane-treated TNFα−/− mice (Fig. 4D). Pre-treatment ScaI expression on peripheral blood B cells (B220+) was similar in all recipients (Fig. 4D, left). The recipients received either 5 × 106 Clo-Lip treated PECs (enriched in neutrophils) or Clo-Lip treated splenocytes (enriched in lymphocytes) as a control (Fig. 4D, Pre-treatment, right panel). Two-days after transfer, ScaI expression (flow cytometry) on recipient peripheral blood B cells was similar in the two treatment groups and comparable to non-transferred TNFα−/− mice (Fig. 4D, after treatment). However 3 days after transfer, TNFα−/− mice that were treated i.p. with B6 neutrophils exhibited significantly lower ScaI expression on peritoneal B cells than recipients of an equal number of splenocytes (Fig. 4D, after treatment). ScaI expression in the recipients of splenocytes was comparable to that in positive control mice (no cell transfer). Thus, increasing the number of peritoneal neutrophils suppressed peritoneal interferon levels (measured as expression of the interferon-inducible cell surface marker Sca-1) in pristane-treated mice, consistent with the proposal that neutrophils are involved in the resolution of inflammation. Taken together, these experiments suggest two potential mechanisms by which TNFα deficiency could promote chronic inflammation: 1) by increasing the number of necrotic cells, and 2) by decreasing the number of peritoneal neutrophils that promote resolution.
Increased pDCs in TNFα−/− mice
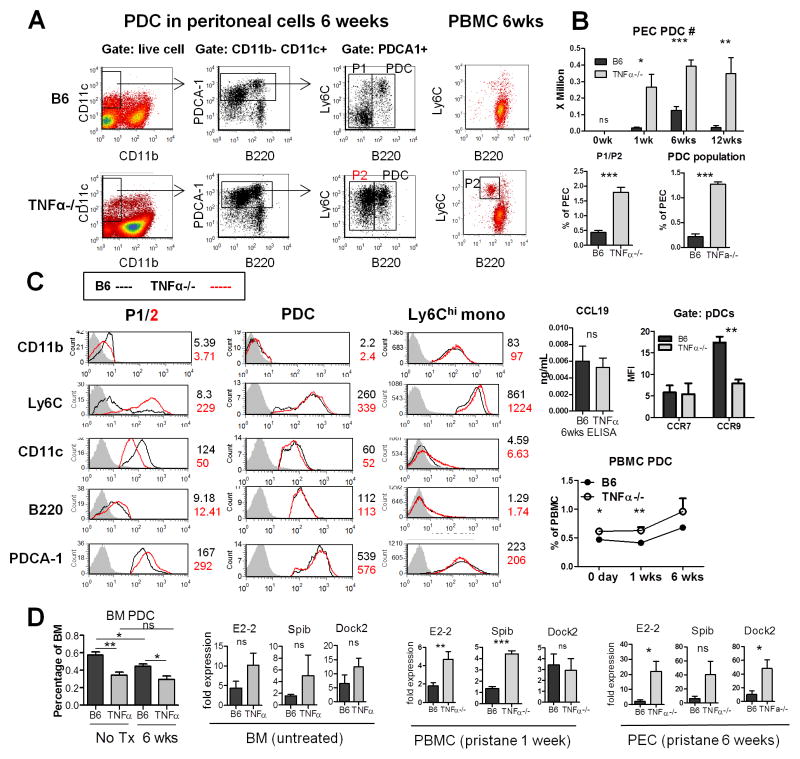
Although pDCs represent <3% of peritoneal cells, they produce up to 1000-fold more IFN-I than myeloid dendritic cells (23). Because TNFα inhibits pDC differentiation from CD34+ hematopoietic progenitor cells (9), we asked whether pDCs (CD11cint PDCA1hi B220+ Ly6C+ cells) were increased in TNFα−/− mice. Indeed, they were increased in PECs of TNFα−/− mice 6-weeks post-pristane (Fig. 5A, B, Table 1). PDCs increased as early as 1-week in both strains, but were dramatically elevated in TNFα−/− mice (Fig. 5B). In B6 mice, pDC levels peaked at ~6-weeks before declining. But in TNFα−/− mice they remained elevated through the 12-week time-point (Fig. 5B). Interestingly TNFα−/− mice exhibited a second population of cells (“P2”, Fig. 5A) with surface markers similar to pDCs except for lower B220 expression. This population expressed Ly6C and PDCA-1, two important pDC markers, comparably to regular pDCs. The P2 population was nearly absent in B6 mice, whereas a population of cells with higher CD11c and CD11b expression, lower PDCA-1, and negative Ly6C (“P1”, Figs. 5A, C) was seen instead. P1 may represent conventional dendritic cells (Table 1). P2 cells also were seen in peripheral blood of TNFα−/− mice 6-weeks after pristane treatment (Fig. 5A). P1 and P2 were readily distinguishable from Ly6Chi monocytes (CD11c− CD11b+ PDCA1int B220− Ly6Chi; Fig. 5C, Table 1).
Figure 5. Increased pDCs in TNFα−/− mice.
Peritoneal cells were collected from pristane-treated (6 weeks) TNFα−/− and B6 mice. A, left, pDC gating strategy. After gating on live cells, the CD11b−CD11c+ cell population (box) was analyzed for B220 and PDCA-1 staining (middle). PDCA-1+ cells (middle, box) were analyzed for Ly6C staining (right). pDCs, P1, and P2 populations are indicated. Right, pDCs (CD11b−CD11c+PDCA1+B220+) in peripheral blood (flow cytometry) of B6 mice (top) and TNFα−/− mice (bottom) 6-weeks after pristane injection. Population P2 is seen in the TNFα−/− but not B6 mice. B, upper panel, pDC numbers in peritoneal exudate cells (PEC) (3–6 per group). Lower panel, percentage of P1/P2 cells and pDCs as a percentage of total PEC. C, histograms showing surface staining of pDCs, population 1/2 (P1/2) cells, and Ly6Chi monocytes. Staining with isotype control is shown as a gray-filled histogram. Mean fluorescence intensity (MFI) of each surface stain is indicated for B6 (black) and B6-TNFα−/− (red) mice (see also Table 1). Right upper, level of CCL19 in peritoneal lavage 6 weeks after pristane treatment (ELISA) and CCR7 and CCR9 staining of pDC (MFI, flow cytometry). Right lower, percentage of pDCs in peripheral blood over time. D, percentage of pDCs (CD11b−CD11c+ PDCA1+B220+) in bone marrow (BM) of untreated and 6-week pristane-treated mice. Expression of pDC differentiation factors (E2-2, SpiB) and the pDC regulatory factor Dock2 was evaluated by Q-PCR in BM, peripheral blood mononuclear cells (PBMC), and peritoneal exudate cells (PEC) from untreated or pristane-treated (1-week, 6-weeks) mice (N = 3–4 in each group) * P < 0.05; ** P <0.01; *** P <0.001 by unpaired Student’s t test. NS, not significant.
Table 1.
Cell surface markers in TNFα−/− vs. wild-type mice (mean fluorescence intensity)
| Population 1 | PDC | Ly6C monocytes | ||||
|---|---|---|---|---|---|---|
| C57BL/6 P1 |
TNFα−/− P2 |
C57BL/6 | TNFα−/− | C57BL/6 | TNFα−/− | |
| CD11b | 4.9 ± 0.3 | 3.3 ± 0.2 | 2.6 ± 0.3 | 2.3 ± 0.1 | 120.6 ± 47.0 | 138.0 ± 26.9 |
| Ly6C | 8.4 ± 1.0 | 165.3 ± 47.8 | 270.2 ± 142.7 | 322.9 ± 47.8 | 862.9 ± 101.7 | 1327.7 ± 80 |
| CD11c | 126.6 ± 20.5 | 56.8 ± 3.2 | 56.5 ± 6.1 | 52.8 ± 4.6 | 6.8 ± 1.5 | 5.9 ± 0.4 |
| B220 | 10.1 ± 1.1 | 11.9 ± 0.3 | 140.6 ± 22.2 | 116.3 ± 7.7 | 6.0 ± 3.4 | 1.8 ± 0.2 |
| PDCA-1 | 125.9 ± 12.2 | 221.8 ± 12.1 | 515.9 ± 58.8 | 503.1 ± 43.0 | 251.2 ± 63.3 | 215.7 ± 27.2 |
The increased numbers of pDCs and P2 cells probably were unrelated to altered chemokine production, as the pDC-attractive chemokine CCL19 was present at similar levels in TNFα−/− and B6 mice (Fig. 5C). We also checked CCR7 and CCR9 expression on the surface of peritoneal pDCs (CD11b−CD11cintB220+PDCA1+) from TNFα−/− and B6 mice 6-weeks after pristane treatment and found no significant difference in the expression of CCR7 (Fig. 5C, right). However the percentage of CCR9+ pDCs was lower in TNFα−/− mice. Unexpectedly, the mean fluorescence intensity of CCR9 staining was lower on TNFα−/− pDCs than on pDCs from B6 controls, suggesting that decreased chemokine receptor expression is not responsible for the increased number of pDCs in the TNFα−/− mice (Fig. 5C). Even before treatment, TNFα−/− mice had a higher percentage of peripheral blood pDCs than controls (Fig. 5C, 0 days). This persisted up to 6-weeks after pristane treatment (Fig. 5C). In contrast, fewer pDCs were seen in BM from untreated and 6-week pristane-treated TNFα−/− mice vs. controls (Fig. 5D). Thus, pDCs may mature more rapidly and/or migrate more rapidly to sites of inflammation in TNFα−/− mice. Consistent with that possibility, mRNAs for E2-2 and SpiB, key transcription factors mediating pDC maturation (24), and Dock2, a hematopoietic cell-specific Rac GTPase activator essential for pDC and lymphocyte migration in response to CCL19 and other chemokines (25–27), were increased in TNFα−/− mice (Fig. 5D).
Discussion
ANAs and anti-dsDNA antibodies are common in patients treated with TNFIs but <1% develop clinical SLE (5–7, 28). Increased IFN-I levels may promote lupus in these patients, though the factors determining who will develop disease remain unclear. The view of lupus as strictly IFN-I-mediated may be overly simplistic, and multiple TLR7-stimulated cytokines, including IFN-I and TNFα, may help explain the diversity of clinical manifestations (16). Analogous to the polar forms of leprosy, which depend on the balance of IL-4 vs. IFNγ (29), autoantibodies/nephritis and hematological manifestations/arthritis may be at opposite ends of a clinical spectrum of SLE reflecting the balance of IFN-I vs. TNFα, respectively (16). We found that TNFα−/− mice did not spontaneously develop serological or clinical manifestations of lupus or the interferon signature (Figs. 1–2), despite increased numbers of circulating pDCs (Fig. 5). But when chronic TLR7-driven inflammation was induced with pristane, autoantibody, IFN-I, and Ly6Chi monocyte levels all were higher in TNFα−/− vs. B6 mice. In contrast, pristane-treated TNFα−/− mice do not develop hematological abnormalities (16). Thus, although TNFα-deficiency was insufficient alone to cause lupus, the increased numbers of pDCs may help polarize TLR7-stimulated cytokine production toward high IFN-I levels, promoting autoantibody production.
Absence of TNFα enhances autoantibody responses
Anti-Sm/RNP autoantibodies are reported in TNFI-induced lupus (6, 7), but anti-dsDNA antibodies are more common (though only present in ~10–20% of patients). Compared with controls, TNFα−/− mice had markedly higher levels of anti-Sm/RNP autoantibodies (more than 5-fold increased anti-U1A) after pristane treatment (Fig. 1A), suggesting that absence of TNFα promotes either the breaking of tolerance or the maturation of autoantibody-producing plasma cells. Pristane-treated B6-TNFα−/− mice, like B6 controls (19), did not produce anti-DNA autoantibodies (Figs. 1B), and anti-Sm/RNP autoantibody production was not accelerated in TNFα−/− mice (Fig. 1A), suggesting that TNFα-deficiency does not fundamentally alter B cell tolerance in pristane-induced lupus. Somewhat unexpectedly, pristane-treated TNFα−/− mice did not exhibit major differences in splenic B cell subsets in comparison with B6 controls (unpublished data).
TNFα−/− mice lack normal B cell follicles, organized follicular dendritic cell networks, and germinal centers since TNFα is required during development of secondary lymphoid tissue to create a milieu permissive of germinal center formation (30, 31). In contrast, germinal centers form normally after immunization of adult TNFI-treated mice. TNFI-treated mice have impaired T cell-dependent antibody responses, but TNFα−/− mice produce antigen-specific IgG after immunization with TNP-KLH, though at lower levels than wild-type mice, whereas IgG responses to the TI-2 antigen TNP-Ficoll are enhanced (30). Although lacking normal germinal centers, early T cell responses are intact in TNFα−/− mice following immunization (31). The enhanced IgG anti-Sm/RNP response in TNFα−/− mice (Fig. 1) is suggestive of a TI-2 response, but pristane does not induce anti-Sm/RNP in T cell-deficient mice (32). Thus, pristane may stimulate the extrafollicular differentiation of anti-Sm/RNP autoantibody-producing cells, as also has been reported for rheumatoid factor (33, 34). It will be of interest to see if IL-21-producing extrafollicular T cells (35) drive this anti-Sm/RNP response. Further studies are needed to definitively address the question of how TNFα deficiency enhances serum autoantibody levels.
Increased IFN-I and altered pDC homeostasis in TNFα−/− mice
Autoantibody production and renal disease in pristane-induced lupus is highly dependent on IFN-I production (19). In wild-type mice, much of this IFN-I is produced by Ly6Chi monocytes (20). However, pDCs produce up to 1000-fold more IFN-I than other cell types and have been implicated as key players in the pathogenesis of lupus (36). We show in Figure 3 that as in wild-type mice, Ly6Chi monocytes were a significant, but not the only, source of IFN-I in TNFα−/− mice. Untreated TNFα−/− mice had decreased BM pDCs and increased circulating pDCs (Fig. 5), which may be poised to over-produce IFN-I. However, IFN-I levels were comparable in untreated TNFα−/− and B6 mice (Fig. 2), suggesting that these circulating pDCs are not detrimental unless the mice are exposed to TLR7 ligands (37), probably derived from dead cells (16). Interestingly, the number of late apoptotic/necrotic cells was higher in pristane-treated TNFα−/− mice vs. controls (Fig. 4C), and could serve as a source of endogenous TLR7 ligands driving inflammatory cytokine production and possibly B cell differentiation.
We also identified a novel “pDC-like” cell population in TNFα−/− mice (P2, Fig. 5), the nature of which remains to be determined. We speculate that the P2 cells in blood and PECs of TNFα−/− mice are pDC precursors that prematurely exit the BM. The E2-2-inducible transcription factor SpiB regulates Ly49Q expression on naïve murine peripheral pDCs and mature BM pDCs and is necessary for optimal IFNα production (38, 39). Absence of SpiB causes premature export of immature (Ly49Q−) pDCs from the BM (40). Additional studies are needed to evaluate SpiB expression in BM pDCs and circulating P2 cells and whether or not the P2 cells are Ly49Q−.
Deficient neutrophil response may contribute to the enhanced inflammatory response in TNFα−/− mice
Neutrophils are recruited to the pristane-treated peritoneum in response to IL-1α and the neutrophil-attractive chemokine CXCL5 (21). This response was attenuated in TNFα−/− mice (Fig. 4). Although other neutrophil chemokines (CXCL2, CXCL3) also were reduced, CXCL5 production is TNFα-driven (41, 42), and CXCL5 mRNA and protein levels were decreased in TNFα−/− mice (Fig. 4B), suggesting that decreased CXCL5 was at least partly responsible for the attenuated peritoneal neutrophil influx.
Neutrophil extracellular traps (NETs) released by dying neutrophils are pro-inflammatory (43). However, neutrophil apoptosis also promotes resolution of inflammation by inducing the development of anti-inflammatory macrophages and by generating annexin A1 and other mediators, blocking further neutrophil recruitment (22). Thus, deficient neutrophil recruitment into the peritoneum could delay resolution of inflammation. Consistent with that possibility, transfer of neutrophils into the peritoneum of pristane-treated TNFα−/− mice inhibited local IFN-I production while having little effect on IFN-I levels in the peripheral blood (Fig. 4D). Finally, as IFN-I prevents Ly6Chi inflammatory monocytes from developing into non-inflammatory peritoneal macrophages (17), dysregulated IFN-I production in TNFα−/− mice may favor chronic inflammation over resolution.
Implications for TNFI-induced lupus
The mechanisms of TNFI-induced lupus are uncertain. One hypothesis is that TNFIs cause apoptosis of inflammatory cells, releasing endogenous immunostimulatory molecules (44, 45). Alternatively, TNFα antagonists may promote autoimmunity by increasing susceptibility to infections (46) or deviating cytokine production away from TH2 and toward a TH1 profile (44, 47). Finally, TNFI-lupus may be precipitated by removing the negative regulatory effects of TNFα on pDCs or other IFN-I producing cells (9). Our data are consistent with the latter hypothesis, but suggest that removal of TNFα alone is insufficient to induce autoimmunity. A hereditary or acquired inability to rapidly clear autologous TLR7 ligands may synergize with TNFα blockade in TNFI-treated patients to drive IFN-I production by an expanded pDC population. Consistent with that idea, the risk of developing lupus is higher in individuals who are ANA/anti-dsDNA autoantibody positive before treatment with TNFI (6, 48, 49). It may be useful to evaluate the clinical utility of pre-treatment screening for autoantibodies and/or delayed clearance of apoptotic cells as a means of assessing risk for TNFI-lupus.
Acknowledgments
Supported by research grants from the Lupus Research Institute, NIH/NIAMS (R01-AR44731), and NIH/NIAID (PO1-AI42288). HZ was an NIH T32 trainee (AR007603).
Footnotes
Conflict of Interest Disclosures: The authors declare no competing financial interests.
References
- 1.Baechler EC, Batliwalla FM, Karypis G, Gaffney PM, Ortmann WA, Espe KJ, et al. Interferon-inducible gene expression signature in peripheral blood cells of patients with severe lupus. Proc Natl Acad Sci U S A. 2003;100(5):2610–5. doi: 10.1073/pnas.0337679100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhuang H, Kosboth M, Lee P, Rice A, Driscoll DJ, Zori R, et al. Lupus-like disease and high interferon levels corresponding to trisomy of the type I interferon cluster on chromosome 9p. Arthritis Rheum. 2006;54(5):1573–9. doi: 10.1002/art.21800. [DOI] [PubMed] [Google Scholar]
- 3.Mathian A, Weinberg A, Gallegos M, Banchereau J, Koutouzov S. IFN-alpha induces early lethal lupus in preautoimmune (New Zealand Black x New Zealand White) F1 but not in BALB/c mice. J Immunol. 2005;174(5):2499–506. doi: 10.4049/jimmunol.174.5.2499. [DOI] [PubMed] [Google Scholar]
- 4.Feldmann M, Maini RN. Anti-TNF alpha therapy of rheumatoid arthritis: what have we learned? Annu Rev Immunol. 2001;19:163–96. doi: 10.1146/annurev.immunol.19.1.163. [DOI] [PubMed] [Google Scholar]
- 5.Williams VL, Cohen PR. TNF alpha antagonist-induced lupus-like syndrome: report and review of the literature with implications for treatment with alternative TNF alpha antagonists. Int J Dermatol. 2011;50(5):619–25. doi: 10.1111/j.1365-4632.2011.04871.x. [DOI] [PubMed] [Google Scholar]
- 6.Ramos-Casals M, Brito-Zeron P, Munoz S, Soria N, Galiana D, Bertolaccini L, et al. Autoimmune diseases induced by TNF-targeted therapies: analysis of 233 cases. Medicine (Baltimore) 2007;86(4):242–51. doi: 10.1097/MD.0b013e3181441a68. [DOI] [PubMed] [Google Scholar]
- 7.Costa MF, Said NR, Zimmermann B. Drug-induced lupus due to anti-tumor necrosis factor alpha agents. Seminars in arthritis and rheumatism. 2008;37(6):381–7. doi: 10.1016/j.semarthrit.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 8.Mavragani CP, Niewold TB, Moutsopoulos NM, Pillemer SR, Wahl SM, Crow MK. Augmented interferon-alpha pathway activation in patients with Sjogren’s syndrome treated with etanercept. Arthritis Rheum. 2007;56(12):3995–4004. doi: 10.1002/art.23062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Palucka AK, Blanck JP, Bennett L, Pascual V, Banchereau J. Cross-regulation of TNF and IFN-alpha in autoimmune diseases. Proc Natl Acad Sci U S A. 2005;102(9):3372–7. doi: 10.1073/pnas.0408506102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu Y, Zeumer L, Reeves WH, Morel L. Induced murine models of systemic lupus erythematosus. Methods in molecular biology. 2014;1134:103–30. doi: 10.1007/978-1-4939-0326-9_9. [DOI] [PubMed] [Google Scholar]
- 11.Perry D, Sang A, Yin Y, Zheng YY, Morel L. Murine models of systemic lupus erythematosus. Journal of biomedicine & biotechnology. 2011;2011:271694. doi: 10.1155/2011/271694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu Y, Lee PY, Li Y, Liu C, Zhuang H, Han S, et al. Pleiotropic IFN-dependent and -independent effects of IRF5 on the pathogenesis of experimental lupus. J Immunol. 2012;188(8):4113–21. doi: 10.4049/jimmunol.1103113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Richards HB, Satoh M, Shaw M, Libert C, Poli V, Reeves WH. Interleukin 6 dependence of anti-DNA antibody production: evidence for two pathways of autoantibody formation in pristane-induced lupus. J Exp Med. 1998;188(5):985–90. doi: 10.1084/jem.188.5.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Y, Lee PY, Kellner ES, Paulus M, Switanek J, Xu Y, et al. Monocyte surface expression of Fcgamma receptor RI (CD64), a biomarker reflecting type-I interferon levels in systemic lupus erythematosus. Arthritis research & therapy. 2010;12(3):R90. doi: 10.1186/ar3017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weinstein JS, Delano MJ, Xu Y, Kelly-Scumpia KM, Nacionales DC, Li Y, et al. Maintenance of anti-Sm/RNP autoantibody production by plasma cells residing in ectopic lymphoid tissue and bone marrow memory B cells. J Immunol. 2013;190(8):3916–27. doi: 10.4049/jimmunol.1201880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhuang H, Han S, Xu Y, Li Y, Wang H, Yang LJ, et al. Toll-like receptor 7-stimulated tumor necrosis factor alpha causes bone marrow damage in systemic lupus erythematosus. Arthritis Rheumatol. 2014;66(1):140–51. doi: 10.1002/art.38189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee PY, Li Y, Kumagai Y, Xu Y, Weinstein JS, Kellner ES, et al. Type I interferon modulates monocyte recruitment and maturation in chronic inflammation. Am J Pathol. 2009;175(5):2023–33. doi: 10.2353/ajpath.2009.090328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nacionales DC, Kelly-Scumpia KM, Lee PY, Weinstein JS, Lyons R, Sobel E, et al. Deficiency of the type I interferon receptor protects mice from experimental lupus. Arthritis Rheum. 2007;56(11):3770–83. doi: 10.1002/art.23023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reeves WH, Lee PY, Weinstein JS, Satoh M, Lu L. Induction of autoimmunity by pristane and other naturally occurring hydrocarbons. Trends Immunol. 2009;30(9):455–64. doi: 10.1016/j.it.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee PY, Weinstein JS, Nacionales DC, Scumpia PO, Li Y, Butfiloski E, et al. A novel type I IFN-producing cell subset in murine lupus. J Immunol. 2008;180(7):5101–8. doi: 10.4049/jimmunol.180.7.5101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee PY, Kumagai Y, Xu Y, Li Y, Barker T, Liu C, et al. IL-1alpha modulates neutrophil recruitment in chronic inflammation induced by hydrocarbon oil. J Immunol. 2011;186(3):1747–54. doi: 10.4049/jimmunol.1001328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ortega-Gomez A, Perretti M, Soehnlein O. Resolution of inflammation: an integrated view. EMBO molecular medicine. 2013;5(5):661–74. doi: 10.1002/emmm.201202382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu YJ. IPC: professional type 1 interferon-producing cells and plasmacytoid dendritic cell precursors. Annu Rev Immunol. 2005;23:275–306. doi: 10.1146/annurev.immunol.23.021704.115633. [DOI] [PubMed] [Google Scholar]
- 24.Ghosh HS, Cisse B, Bunin A, Lewis KL, Reizis B. Continuous expression of the transcription factor e2-2 maintains the cell fate of mature plasmacytoid dendritic cells. Immunity. 2010;33(6):905–16. doi: 10.1016/j.immuni.2010.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fukui Y, Hashimoto O, Sanui T, Oono T, Koga H, Abe M, et al. Haematopoietic cell-specific CDM family protein DOCK2 is essential for lymphocyte migration. Nature. 2001;412(6849):826–31. doi: 10.1038/35090591. [DOI] [PubMed] [Google Scholar]
- 26.Reif K, Cyster J. The CDM protein DOCK2 in lymphocyte migration. Trends in cell biology. 2002;12(8):368–73. doi: 10.1016/s0962-8924(02)02330-9. [DOI] [PubMed] [Google Scholar]
- 27.Gotoh K, Tanaka Y, Nishikimi A, Nakamura R, Yamada H, Maeda N, et al. Selective control of type I IFN induction by the Rac activator DOCK2 during TLR-mediated plasmacytoid dendritic cell activation. The Journal of experimental medicine. 2010;207(4):721–30. doi: 10.1084/jem.20091776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aringer M, Smolen JS. Efficacy and safety of TNF-blocker therapy in systemic lupus erythematosus. Expert Opin Drug Saf. 2008;7(4):411–9. doi: 10.1517/14740338.7.4.411. [DOI] [PubMed] [Google Scholar]
- 29.Modlin RL. The innate immune response in leprosy. Curr Opin Immunol. 2010;22(1):48–54. doi: 10.1016/j.coi.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pasparakis M, Alexopoulou L, Episkopou V, Kollias G. Immune and inflammatory responses in TNF alpha-deficient mice: a critical requirement for TNF alpha in the formation of primary B cell follicles, follicular dendritic cell networks and germinal centers, and in the maturation of the humoral immune response. The Journal of experimental medicine. 1996;184(4):1397–411. doi: 10.1084/jem.184.4.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cook MC, Korner H, Riminton DS, Lemckert FA, Hasbold J, Amesbury M, et al. Generation of splenic follicular structure and B cell movement in tumor necrosis factor-deficient mice. The Journal of experimental medicine. 1998;188(8):1503–10. doi: 10.1084/jem.188.8.1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nacionales DC, Weinstein JS, Yan XJ, Albesiano E, Lee PY, Kelly-Scumpia KM, et al. B cell proliferation, somatic hypermutation, class switch recombination, and autoantibody production in ectopic lymphoid tissue in murine lupus. J Immunol. 2009;182(7):4226–36. doi: 10.4049/jimmunol.0800771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.William J, Euler C, Christensen S, Shlomchik MJ. Evolution of autoantibody responses via somatic hypermutation outside of germinal centers. Science. 2002;297(5589):2066–70. doi: 10.1126/science.1073924. [DOI] [PubMed] [Google Scholar]
- 34.Shlomchik MJ. Sites and stages of autoreactive B cell activation and regulation. Immunity. 2008;28(1):18–28. doi: 10.1016/j.immuni.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 35.Odegard JM, Marks BR, DiPlacido LD, Poholek AC, Kono DH, Dong C, et al. ICOS-dependent extrafollicular helper T cells elicit IgG production via IL-21 in systemic autoimmunity. J Exp Med. 2008;205(12):2873–86. doi: 10.1084/jem.20080840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gilliet M, Cao W, Liu YJ. Plasmacytoid dendritic cells: sensing nucleic acids in viral infection and autoimmune diseases. Nat Rev Immunol. 2008;8(8):594–606. doi: 10.1038/nri2358. [DOI] [PubMed] [Google Scholar]
- 37.Lee PY, Kumagai Y, Li Y, Takeuchi O, Yoshida H, Weinstein J, et al. TLR7-dependent and FcgammaR-independent production of type I interferon in experimental mouse lupus. J Exp Med. 2008;205(13):2995–3006. doi: 10.1084/jem.20080462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tai LH, Goulet ML, Belanger S, Toyama-Sorimachi N, Fodil-Cornu N, Vidal SM, et al. Positive regulation of plasmacytoid dendritic cell function via Ly49Q recognition of class I MHC. J Exp Med. 2008;205(13):3187–99. doi: 10.1084/jem.20080718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Omatsu Y, Iyoda T, Kimura Y, Maki A, Ishimori M, Toyama-Sorimachi N, et al. Development of murine plasmacytoid dendritic cells defined by increased expression of an inhibitory NK receptor, Ly49Q. J Immunol. 2005;174(11):6657–62. doi: 10.4049/jimmunol.174.11.6657. [DOI] [PubMed] [Google Scholar]
- 40.Sasaki I, Hoshino K, Sugiyama T, Yamazaki C, Yano T, Iizuka A, et al. Spi-B is critical for plasmacytoid dendritic cell function and development. Blood. 2012;120(24):4733–43. doi: 10.1182/blood-2012-06-436527. [DOI] [PubMed] [Google Scholar]
- 41.Liu Y, Mei J, Gonzales L, Yang G, Dai N, Wang P, et al. IL-17A and TNF-alpha exert synergistic effects on expression of CXCL5 by alveolar type II cells in vivo and in vitro. Journal of immunology. 2011;186(5):3197–205. doi: 10.4049/jimmunol.1002016. [DOI] [PubMed] [Google Scholar]
- 42.Griffin GK, Newton G, Tarrio ML, Bu DX, Maganto-Garcia E, Azcutia V, et al. IL-17 and TNF-alpha sustain neutrophil recruitment during inflammation through synergistic effects on endothelial activation. J Immunol. 2012;188(12):6287–99. doi: 10.4049/jimmunol.1200385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Knight JS, Kaplan MJ. Lupus neutrophils: ‘NET’ gain in understanding lupus pathogenesis. Curr Opin Rheumatol. 2012;24(5):441–50. doi: 10.1097/BOR.0b013e3283546703. [DOI] [PubMed] [Google Scholar]
- 44.Bout-Tabaku S, Rivas-Chacon R, Restrepo R. Systemic lupus erythematosus in a patient treated with etanercept for polyarticular juvenile rheumatoid arthritis. J Rheumatol. 2007;34(12):2503–4. [PubMed] [Google Scholar]
- 45.D’Auria F, Rovere-Querini P, Giazzon M, Ajello P, Baldissera E, Manfredi AA, et al. Accumulation of plasma nucleosomes upon treatment with anti-tumour necrosis factor-alpha antibodies. J Intern Med. 2004;255(3):409–18. doi: 10.1111/j.1365-2796.2003.01298.x. [DOI] [PubMed] [Google Scholar]
- 46.Ferraccioli G, Mecchia F, Di Poi E, Fabris M. Anticardiolipin antibodies in rheumatoid patients treated with etanercept or conventional combination therapy: direct and indirect evidence for a possible association with infections. Ann Rheum Dis. 2002;61(4):358–61. doi: 10.1136/ard.61.4.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Steiner G, Smolen J. Autoantibodies in rheumatoid arthritis and their clinical significance. Arthritis Res. 2002;4 (Suppl 2):S1–5. doi: 10.1186/ar551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.De Bandt M, Sibilia J, Le Loet X, Prouzeau S, Fautrel B, Marcelli C, et al. Systemic lupus erythematosus induced by anti-tumour necrosis factor alpha therapy: a French national survey. Arthritis Res Ther. 2005;7(3):R545–51. doi: 10.1186/ar1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wetter DA, Davis MD. Lupus-like syndrome attributable to anti-tumor necrosis factor alpha therapy in 14 patients during an 8-year period at Mayo Clinic. Mayo Clin Proc. 2009;84(11):979–84. doi: 10.4065/84.11.979. [DOI] [PMC free article] [PubMed] [Google Scholar]