Abstract
Hepatocellular carcinoma (HCC) is the third most common cause of cancer death worldwide and most patients with HCC have limited treatment options. Focal Adhesion Kinase (FAK) is overexpressed in many HCC specimens, offering a potential target for HCC treatment. However, the role of FAK in hepatocarcinogenesis remains elusive. Establishing whether FAK expression plays a role in HCC development is necessary to determine whether it is a viable therapeutic target. In this study, we generated mice with hepatocyte-specific deletion of Fak and investigated the role of Fak in an oncogenic (c-MET/β-catenin, MET/CAT)-driven HCC model. We found that deletion of Fak in hepatocytes did not affect morphology, proliferation or apoptosis. However, Fak deficiency significantly repressed MET/CAT-induced tumor development and prolonged survival of animals with MET/CAT-induced HCC. In mouse livers and HCC cell lines, Fak was activated by MET, which induced the activation of Akt/Erk and up-regulated Cyclin D1 and tumor cell proliferation. CAT enhanced MET-stimulated FAK activation and synergistically induced the activation of the AKT/ERK-Cyclin D1 signaling pathway in a FAK kinase-dependent manner. In addition, FAK was required for CAT-induced Cyclin D1 expression in a kinase-independent fashion.
Conclusion
Fak is required for c-Met/β-catenin-driven hepatocarcinogenesis. Inhibition of FAK provides a potential strategy to treat HCC.
Keywords: Hepatocellular carcinoma, Sleeping Beauty Transposon system, Proliferation, AKT, ERK
Introduction
Hepatocellular carcinoma (HCC) is the most common type of liver cancer. HCC is the fifth leading cancer and the third most common cause for cancer death in the world (1). The overall survival of patients with HCC is less than 10%. HCC is also the most rapidly increasing cancer in the United States, resulting in more than 12,000 deaths per year (1). The major causes of HCC are liver disease due to viral hepatitis, alcohol use, and non-alcoholic steatohepatitis (NASH) (2).
HCC can be treated to achieve long-term survival by surgical resection, liver transplantation and locoregional therapy if patients are diagnosed at an early stage (3). However, a majority of patients with HCC present with advanced disease and treatment options are limited (4). The current most effective targeted therapeutic agent for advanced HCC, Sorafenib, only increased survival from 7.9 months to 10.7 months (5). Therefore, it is urgent to develop newer and more effective therapeutic strategies and agents to treat HCC. To achieve this goal, it is necessary to elucidate the molecular signaling pathways which drive or mediate the development of HCC.
FAK is a non-receptor tyrosine kinase, which can be phosphorylated and activated by integrins and growth factors (6, 7). Activation of FAK can target multiple downstream signaling pathways (e.g., protein kinase B (PKB or AKT), extracellular receptor kinase (ERK) and Ras-related C3 botulinum toxin substrate (Rac)), thereby regulating different cellular functions, including cell survival, proliferation and cell migration (8). FAK has been shown to play an important role in tumorigenesis and cancer progression in several tissues, including breast (9), intestine (10), and brain (11). FAK was reported to be overexpressed in HCC specimens (12, 13), offering a potential target to treat HCC. However, the role of FAK in hepatocarcinogenesis has not been established. Studying the role of FAK in hepatocarcinogenesis by in vivo models is critical to determine whether FAK is a suitable candidate target for treating HCC.
The oncoproteins c-MET and β-catenin play critical roles in hepatocarcinogenesis and their signaling pathways are frequently dysregulated in HCC (14–16). Co-activation of c-MET and β-catenin often occurs in HCC (15). Co-delivery of c-MET (MET) and constitutively active β-catenin (ΔN90-β-catenin, CAT) into mouse livers using the Sleeping Beauty Transposon system efficiently and rapidly induces primary hepatic tumors (15, 17, 18). Therefore, this model (referred to here as MET/CAT) is useful to study the functions of genes in hepatocarcinogenesis because of its clinical relevance and efficiency.
In this study, we investigated the role of Fak in MET/CAT-induced mouse models of hepatocarcinogenesis. We found that specific deletion of Fak in hepatocytes decreases HCC development and tumor proliferation in MET/CAT-induced HCC. Importantly, Fak deletion prolongs survival in animals with MET/CAT-induced HCC. Furthermore, we demonstrated that the deletion of Fak in hepatocytes significantly decreases activation of Akt, Erk and Cyclin D1 in MET/CAT-treated livers. Our data suggest that Fak is involved in the pathogenesis of HCC and inhibition of FAK may be a promising strategy to treat HCC.
Materials and Methods
Mice and Treatment
All animals received humane care according to the “Guide for the Care and Use of Laboratory Animals” (http://oacu.od.nih.gov/ac_cbt/guide3.htm). The procedures for all animal experiments were approved by the Institutional Animal Care and Use Committee at Loyola University Chicago. To generate mice with hepatocyte-specific Fak-deficiency, Fakflox/flox mice (purchased from MMRRC, Cat # 009967) were mated to Alb-Cre mice (JAX, Cat # 003574). The resulting offspring Alb-Cre; Fakflox/+ mice were then mated to generate the Alb-Cre and Alb-Cre; Fakflox/flox littermates. Detailed information on genotyping of the mice can be found in the supporting information. The mice were housed in micro-isolator cages in a room illuminated from 7:00 AM to 7:00 PM (12:12-hr light-dark cycle), and allowed access to water and chow ad libitum.
For the MET/CAT-induced HCC model (19), 55 µg of total plasmids, encoding the Sleeping Beauty transposase (HSB2) and transposons with oncogenes MET/CAT and gaussia luciferase (Gluc) (22.5µg pT3-EF1α-c-MET(human) + 22.5µg pT3-EF1α-ΔN90-β-catenin (human)+ 5µg pT3-Gluc+ 5µg HSB2) (15, 17), were injected hydrodynamically into age- and gender-matched mice. Mice were maintained on the standard diet and sacrificed after 3 days or 7 weeks. The liver weight and body weight of each mouse were measured. Some mice were injected hydrodynamically with MET (22.5µg pT3-EF1α-c-MET (human) + 22.5µg pT3-EF1α + 5ug pT3-Gluc + 5µg HSB2) or CAT (22.5µg pT3-EF1α-Δ90-β-catenin (human) + 22.5µg pT3-EF1α + 5µg pT3-Gluc + 5µg HSB2) to study activation of FAK by MET or CAT. Some mice were injected hydrodynamically with pT3 (45µg pT3-EF1α + 5µg pT3-Gluc + 5µg HSB2) to serve as controls.
Cells and treatment
Huh7, HepG2, and HEP3B cells were maintained in Dulbecco's modified Eagle's (DMEM) high glucose medium (Thermo scientific, Waltham, MA) supplemented with 10% FBS, penicillin, and streptomycin (Sigma-Aldrich, St. Louis, MO) in a humidified atmosphere of 5% CO2 at 37°C. Cells were plated in 6-well plates at 30–40% density 24h before treatment with 20ng/ml human growth factor (HGF) (Thermo scientific, Waltham, MA) for 15 minutes and 1h. Total cell lysates were prepared and used for Western blotting.
For FAK shRNA experiments, cells were infected with lentiviral pLKO.1 puro particles which contain FAK or scrambled shRNA and selected with 10µg /ml puromycin for 5 days. They were then subjected to 20ng/ml HGF treatment for 15minutes or 1h. Lentiviral pLKO.1 puro particles against human FAK shRNA (Table S1), and control scrambled shRNA (Sigma-Aldrich) were packaged with pCMV-dR8.2 dvpr (Addgene) and pCMV-VSVG (Addgene).
For β-catenin transfection experiments, Huh7 or HEP3B cells were transfected with pT3-EF1α-Δ90Ncatenin or control pT3-EF1α plasmids by lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions. Twenty-four hr after transfection, some cells were treated with 20ng/ml HGF for 1h. Total cell lysates were collected 24 hours after transfection and used in Western blotting.
Isolation and culture of primary mouse hepatocytes
Hepatocytes from Alb-Cre and Alb-Cre;Fakflox/flox mice were isolated by the non-recirculating two-step perfusion method as previously described (20). The hepatocytes were then cultured in Williams’ medium E supplemented with Hepatocyte Maintenance Supplement Pack (Life Technologies, Grand Island, NY) overnight and Western blotting was done to examine expression of Fak and Gapdh.
Western Blotting
Western blotting was performed as previously described (21). Detailed information can be found in the supporting information. Primary antibodies including those for c-MET, FAK, p-FAK (Y397), p-FAK (Y576/577), GAPDH, Src, p-Src, Pyk2, p-Pyk2, p-AKT (ser473), AKT, p-ERK, ERK, and Cyclin D1 were purchased from Cell Signaling (Danvers, MA). β-catenin antibody was purchased from BD Transduction Labs (San Jose, CA). p-β-catenin (Y654) antibody was purchased from Life Technology (Grand Island, NY).
TUNEL staining
TUNEL staining was performed as previously described (21). The apoptotic index was scored in at least 5 fields at 400× magnification/mouse and reported as mean ± SD. Three mice were used in each group.
Immunohistochemical (IHC) and immunofluorescence (IF) staining
IHC and IF staining were performed as previously described (21). Detailed information can be found in the supporting Information. Cells with positive staining were scored in at least 5 fields at ×400 or ×200 magnification and reported as mean ± SD. Three mice were used in each group.
Statistical Analysis
Statistical analysis was carried out using GraphPad Prism V software. Data are presented as mean ± standard deviation (SD). Statistical significance was calculated with a Student’s t test. P < 0.05 was considered to be significant. The means ± SD are shown in the figures where applicable.
Results
Deletion of Fak in hepatocytes does not affect morphology, histology, proliferation or apoptosis in mouse liver
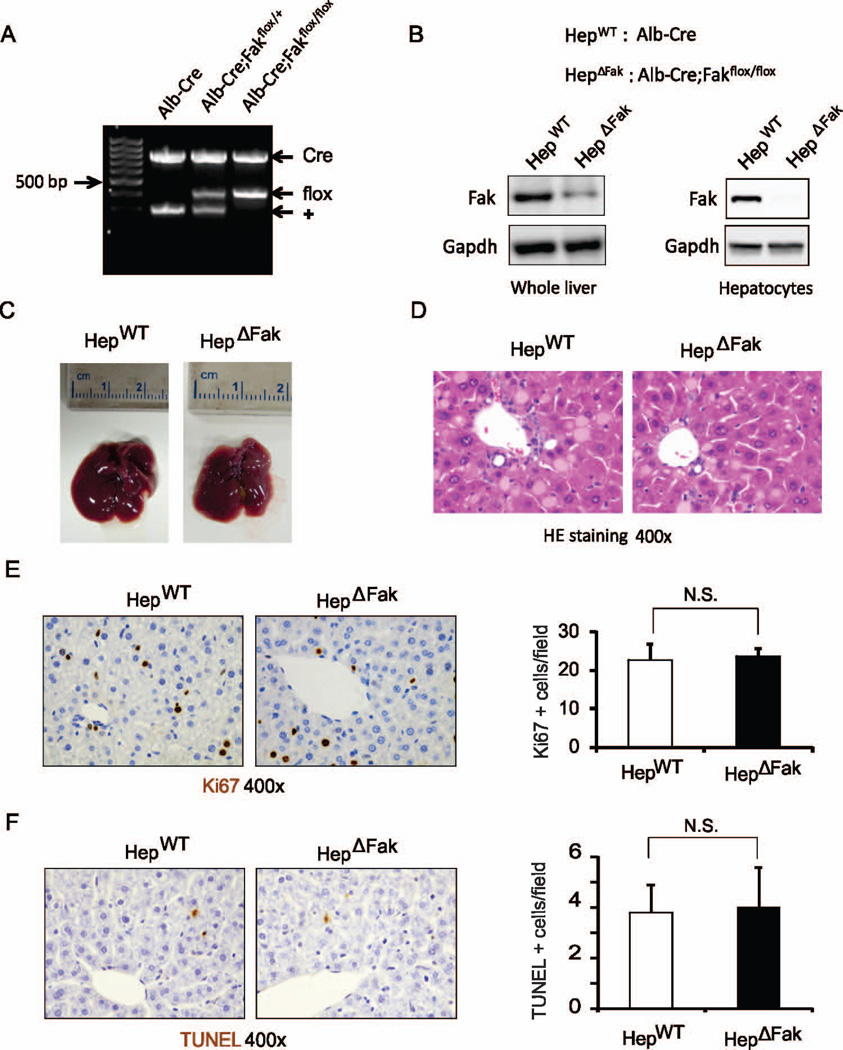
To determine the role of Fak in hepatocarcinogenesis in vivo, we first generated mice with hepatocyte-specific deletion of Fak (Alb-Cre; Fakflox/flox). Alb-cre; Fakflox/flox mice express Cre recombinase from the albumin promoter, which is specifically expressed in hepatocytes. Alb-Cre; Fakflox/flox mice are viable, fertile and indistinguishable from wild-type mice, suggesting FAK is not required for normal liver development. We confirmed that expression of Cre recombinase in hepatocytes removes the FAK allele between 2 loxP sites (Fig. 1A). As hepatocytes comprise about 80% of the total liver cell population, we expected to see deletion of FAK in the majority of liver cells in Alb-Cre; Fakflox/flox mice (referred to here as HepΔFak). Western blotting results confirmed our hypothesis (Fig. 1B). We further isolated hepatocytes from HepΔFak and control Alb-Cre mice (referred to here as HepWT) and verified complete deletion of Fak protein in the hepatocytes of HepΔFak mice (Fig. S1 and 1B). There was no significant difference in morphology and histology of livers between HepWT and HepΔFak mice (Fig. 1C, 1D, and S2). Furthermore, Fak deficiency did not affect cell proliferation or apoptosis in mouse liver (Fig. 1E and 1F). These results suggest that deletion of Fak in hepatocytes does not affect mouse liver homeostasis.
Figure 1. Deletion of Fak in hepatocytes does not affect morphology, histology, proliferation or apoptosis in mouse liver.
(A) Genotyping of Alb-Cre, Alb-Cre; FAKflox/+, and Alb-Cre; FAKflox/flox mice. (B) Protein expression of Fak and Gapdh in whole livers and isolated hepatocytes of Alb-Cre (HepWT) and Alb-Cre; Fakflox/flox (HepΔFak) mice was determined by Western blotting. (C) Photographs of livers from HepWT and HepΔFak mice at 7 weeks of age. (D) Representative pictures of H&E-stained sections for (C). (E) Left, hepatic proliferation in the livers of 7 week old HepWT and HepΔFak mice was examined by immunohistochemistry for Ki67 protein expression. Right, quantification of Ki67 staining (n=3). (F) Left, hepatic apoptosis in the livers of 7 week old HepWT and HepΔFak mice was examined by TUNEL staining. Right, quantification of TUNEL staining for (E) (n=3).
Deficiency of Fak in hepatocytes suppresses MET/CAT-induced HCC development
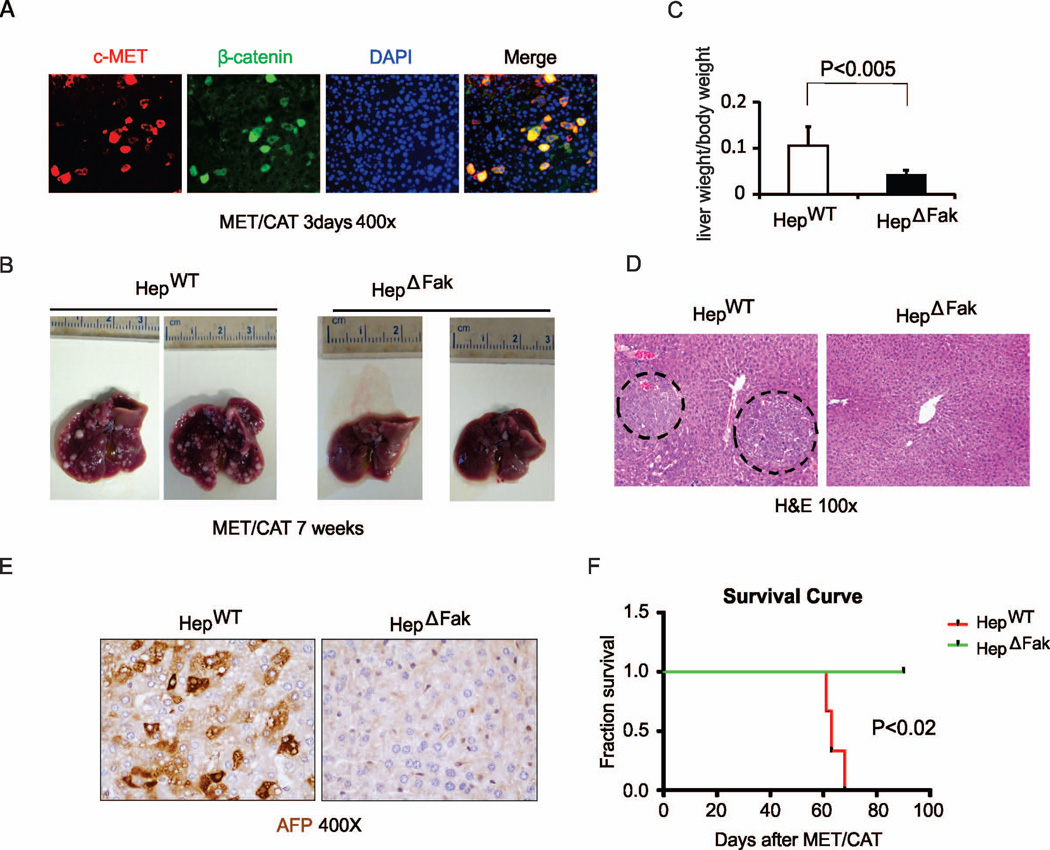
To study the role of Fak in hepatocarcinogenesis, we hydrodynamically injected age- and gender-matched HepWT and HepΔFak mice (Fig. S3A) with plasmids encoding the Sleeping Beauty transposase (HSB2) and transposons with oncogenes MET/CAT (15, 17). Immunohistochemical staining confirmed successful delivery of MET (recognized by an antibody specific for human c-MET) and CAT (with high expression or nuclear localization of β-catenin) in the mouse livers (Fig. S3B and Table S2). Interestingly, a majority of the hepatocytes with high MET expression co-expressed CAT (Fig. 2A and Table S2), suggesting that hydrodynamic injection equivalently delivered both plasmids into the same cells.
Figure 2. Deletion of Fak suppresses tumor development and prolongs survival in the MET/CAT-induced HCC mouse model.
(A) Representative pictures of co-staining of c-MET and β-catenin protein expression from the livers of HepWT mice 3 days after hydrodynamic injection of MET/CAT (magnification, ×200). (B) Photographs of livers of HepWT and HepΔFak mice 7 weeks after injection of MET/CAT. (C) Liver weight/body weight ratio was analyzed in the mice from (B) (n=6). (D) Histological analysis of the livers of HepWT and HepΔFak mice 7 weeks after injection of MET/CAT by H&E staining. (E) HCC in the livers of HepWT and HepΔFak mice 7 weeks after injection of MET/CAT was examined by immunohistochemistry for AFP. (F) Survival curves of HepWT and HepΔFak mice after of MET/CAT (n=4).
Mice were sacrificed 7 weeks after the injection and their livers were collected and analyzed. Intriguingly, we found that the overall tumor load in HepΔFak mice was significantly decreased compared with HepWT mice (Fig. 2B, and S3C). The relative liver weight versus body weight in HepΔFak mice was decreased by 1.5-fold compared to HepWT mice (0.1±0.34 vs.0.04±0.13) (Fig. 2C). The size of the tumors was also significantly decreased in the livers of HepΔFak mice compared to those of HepWT mice (Fig. 2B and 2D, and S3D). Immunohistochemical staining signals for alpha-fetoprotein (AFP), a common HCC marker, were significantly lower in the livers of HepΔFak mice compared to those of HepWT mice (Fig. 2E and S3E). Importantly, the HepWT mice with a liver tumor burden died at the age of 60–70 days, while all of HepΔFak mice were still alive at the age of 90 days (Fig. 2F). The data indicate that deficiency of FAK in hepatocytes suppresses MET/CAT-induced HCC development and prolongs survival of animals with HCC.
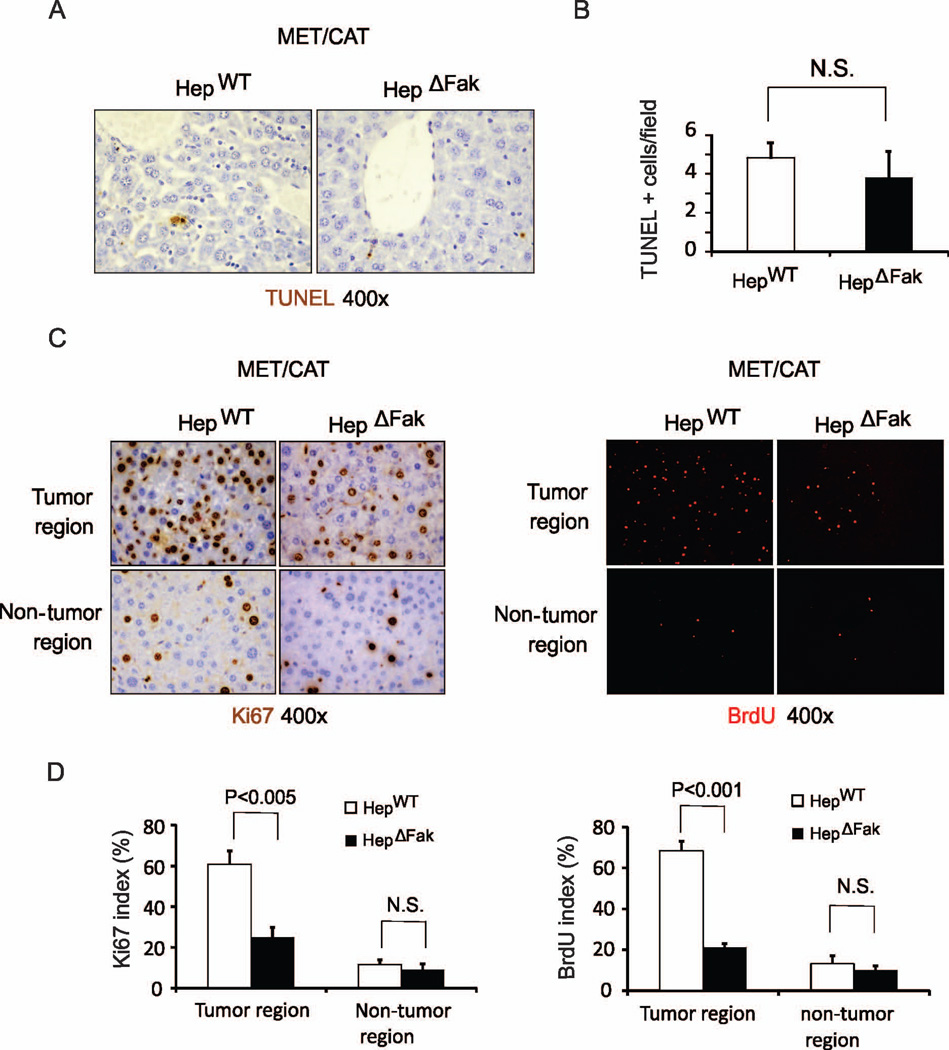
Fak deficiency in hepatocytes decreases proliferation in MET/CAT-induced HCC
Fak has been shown to regulate cell survival and proliferation (6, 22). Suppression of HCC development by deletion of Fak could be due to increased apoptosis or decreased proliferation of tumor cells (23). To determine whether deficiency of Fak affects apoptosis in MET/CAT-induced HCC, we performed TUNEL staining in the livers from HepWT and HepΔFak mice injected with MET/CAT. We did not find significant differences in apoptosis in livers when comparing HepWT and HepΔFak mice (Fig. 3A and 3B). We then analyzed proliferation in the MET/CAT-injected livers from HepWT and HepΔFak mice by Ki67 and BrdU staining. The number of Ki67 and BrdU positive cells was significantly decreased in tumors but not in areas without tumor in Fak-deficient livers compared to WT livers (Fig. 3C and 3D, and S4). These results demonstrate that Fak deficiency in hepatocytes decreases cell proliferation in MET/CAT-induced HCC.
Figure 3. Fak deficiency decreases hepatocyte proliferation but does not affect apoptosis in MET/CAT-induced liver tumors.
(A) Apoptosis in the livers of HepWT and HepΔFak mice 7 weeks after injection of MET/CAT was examined by TUNEL staining. (B) Quantification of TUNEL staining for (A) (n=3). (C) Hepatocyte proliferation in the livers of HepWT and HepΔFak mice 7 weeks after injection of MET/CAT was examined by immunohistochemistry for Ki67 and immunofluorescence for BrdU. (D) Quantification of Ki67 and BrdU staining for (C) (n=3).
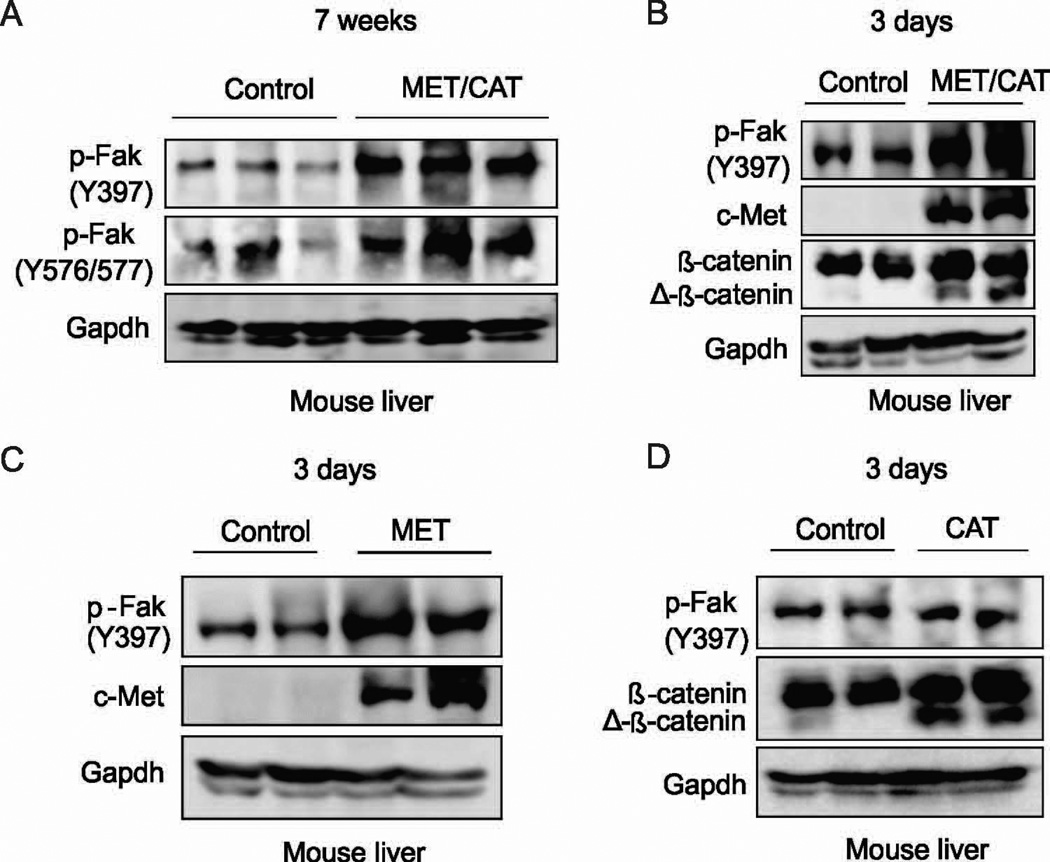
Fak is activated by MET but not CAT in mouse liver
The kinase activity of FAK plays a critical role in its functions (24, 25). Phosphorylation of FAK at Tyr397 can recruit and activate Src family kinases (22, 26). Src activation results in phosphorylation of additional sites on FAK and leads to its full activation (27). The mutually activated FAK/Src complex then initiates multiple downstream signaling pathways (6). We therefore examined whether Fak is phosphorylated by MET/CAT. We found that phosphorylation of Fak was significantly increased in the livers from mice which were injected with MET/CAT after 7 weeks compared to livers from control mice (Fig. 4A). To determine whether Fak could be activated by MET/CAT before tumor development, phosphorylation of Fak at Tyr397 was examined in the mouse livers 3 days after hydrodynamic injection of MET/CAT. Phosphorylation of Fak was significantly increased in those livers compared to controls (Fig. 4B). Furthermore, hydrodynamic injection of MET alone, but not CAT alone, was sufficient to activate FAK in mouse liver (Fig. 4C and 4D). Our data indicate that overexpression of MET activates FAK in mouse livers.
Figure 4. Fak activation is increased in mouse livers after delivery of MET/CAT or MET alone.
(A) Protein expression of p-Fak (Y397), p-Fak (Y576/577) and Gapdh in the livers of HepWT mice 7 weeks after hydrodynamic injection of MET/CAT or pT3 control. (B) Protein expression of p-Fak (Y397), c-Met, β-catenin and Gapdh in the livers of HepWT mice with or without introduction of MET/CAT assayed 3 days after hydrodynamic injection. (C) Protein expression of p-Fak (Y397), c-Met and Gapdh in the livers of HepWT mice 3 days after hydrodynamic injection with MET or pT3 control. (D) Protein expression of p-Fak (Y397), β-catenin and Gapdh in the livers of HepWT mice 3 days after hydrodynamic injection with CAT or pT3 control.
Fak deficiency in hepatocytes decreases activation of Akt and Erk in MET/CAT-induced HCC
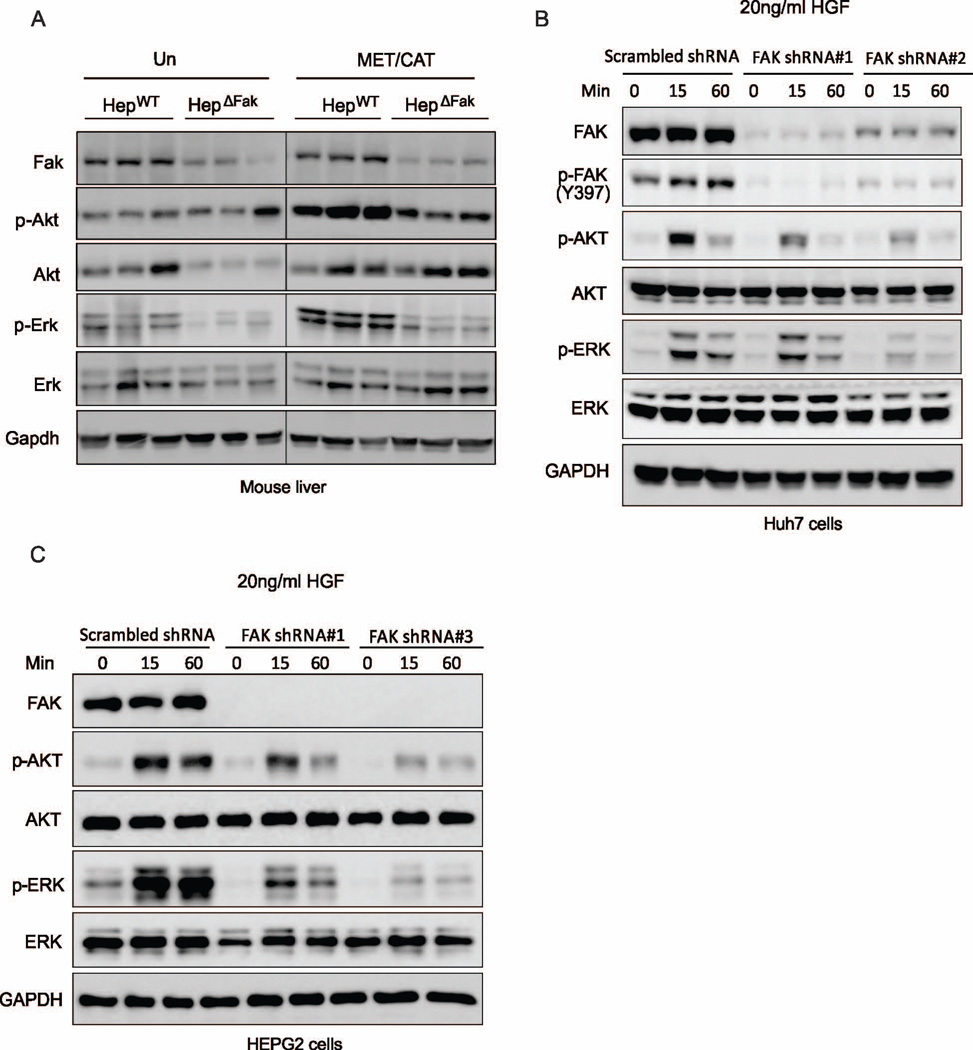
FAK can phosphorylate and activate a number of oncoproteins which promote cell growth and proliferation, such as AKT and ERK (6, 7). We reasoned that deficiency of Fak may inhibit cell proliferation and development of HCC by inactivating Akt or Erk, as it does in many other tissues (9–11). We therefore examined the expression of p-Akt and p-Erk in MET/CAT-treated livers collected from HepWT and HepΔFak mice. As expected, we found that the levels of both phosphorylated-Akt and -Erk were significantly increased in HepWT livers after MET/CAT-treatment. However, the levels of these two proteins were reduced in the livers of HepΔFak mice compared to those of HepWT mice (Fig. 5A), suggesting that deletion of FAK may suppress MET/CAT-induced HCC through inhibiting Akt and Erk.
Figure 5. FAK inhibition abrogates HGF/c-MET-mediated AKT and ERK activation in HCC cells.
(A) Protein expression of Fak, p-Akt, Akt, p-Erk, Erk and Gapdh in the livers of HepWT and HepΔFak mice 7 weeks after injection of MET/CAT. (B) Protein expression of FAK, p-FAK, p-AKT, AKT, p-ERK, ERK and GAPDH in Huh7 cells infected with scrambled shRNA, FAK shRNA#1 or FAK shRNA#2 lentiviral particles and then treated with 20ng/ml HGF for 15 or 60 minutes. (C) Protein expression of FAK, p-AKT, AKT, p-ERK, ERK and GAPDH in HEPG2 cells treated as in (B), except with FAK shRNA#3 instead of FAK shRNA#2.
MET has been shown to activate AKT and ERK in HCC cells (28). We therefore assessed whether FAK mediates activation of AKT and ERK by MET. We found that MET or its ligand HGF can induce activation of Fak in mouse livers (Fig. 4C) or the liver cancer cell lines Huh7 and HepG2 (Fig. 5B and S5). Furthermore, knockdown of FAK attenuated activation of AKT and ERK by HGF in Huh7 and HepG2 cells (Fig. 5B and 5C). These data suggest that FAK mediates the activation of AKT and ERK induced by MET in HCC cells.
Src plays an important role in regulating the activation of AKT and ERK (29). We thus examined whether deletion of FAK suppresses activation of AKT and ERK through inhibiting Src activation. Interestingly, we found that phosphorylation of Src was comparable in the livers between HepΔFak and HepWT mice (Fig. S6), suggesting that suppression of AKT and ERK activation caused by Fak deletion may not be caused by the inhibition of Src activation.
The combination of CAT and MET has a synergistic effect on activation of FAK in HCC cells
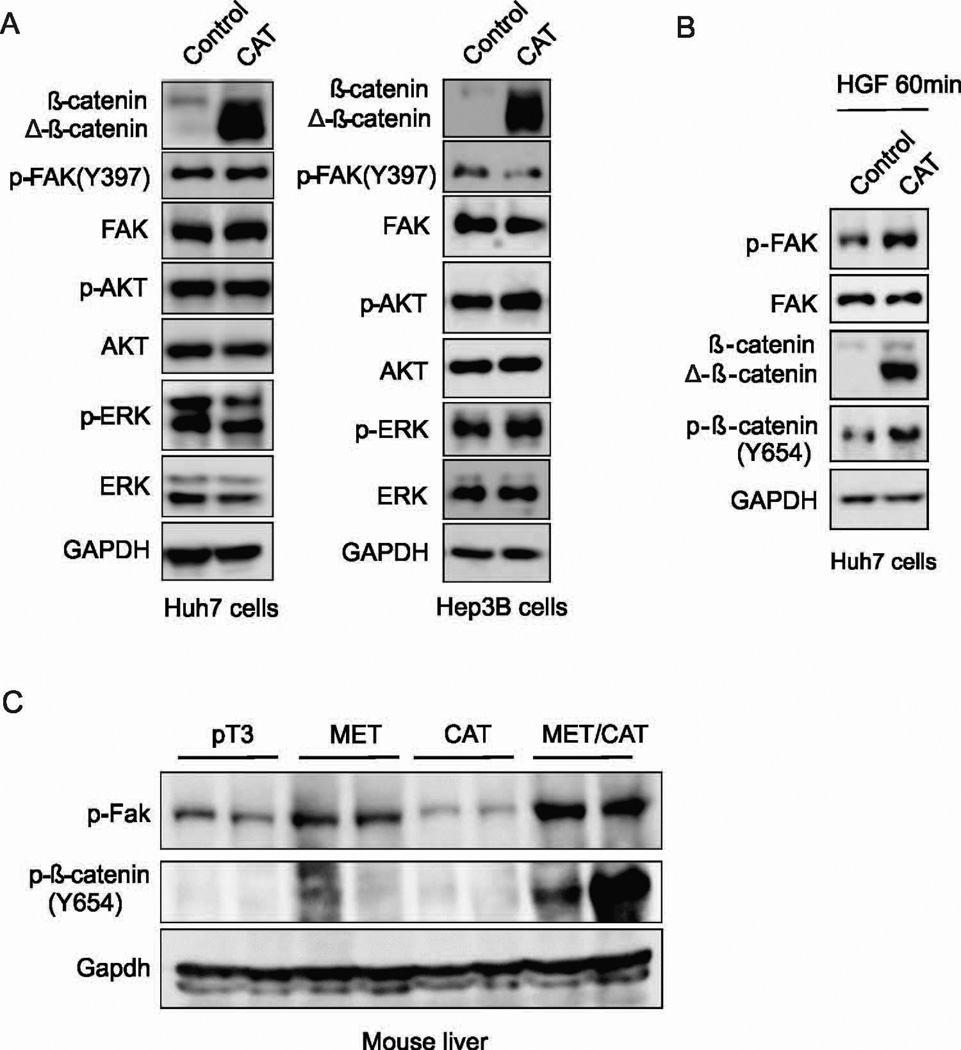
Fak has been shown to be required downstream of Wnt/c-Myc signaling for Akt activation, intestinal regeneration and tumorigenesis (10). We thus examined whether β-catenin activates FAK in HCC cells. We found that overexpression of CAT did not enhance the phosphorylation of FAK, AKT or ERK in Huh7 or Hep3B cells (Fig. 6A). These data suggest that CAT may not be sufficient to activate FAK in HCC cells.
Figure 6. The combination of CAT and MET has a synergistic effect on FAK activation in mouse liver and HCC cells.
(A) Protein expression of p-FAK, FAK, p-AKT, AKT, p-ERK, ERK and GAPDH in Huh7 and HEP3B cells transfected with CAT or control plasmids. (B) Protein expression of FAK, p-FAK, β-catenin, p-β-catenin (Y654) and GAPDH in Huh7 cells transfected with CAT or control plasmids, and after 24 hours treated with 20ng/ml HGF for 60 minutes. (C) Protein expression of p-Fak, p-β-catenin (Y654), and Gapdh in the livers of HepWT and HepΔFak mice 3 days after injection of pT3, MET, CAT or MET/CAT.
An interaction of c-MET with β-catenin has been reported in liver cells (30, 31). HGF/C-MET can phosphorylate β-catenin at tyrosine 654 and induce β-catenin nuclear translocation and activation (30, 31). On the other hand, activation of β-catenin can increase c-MET protein expression (32). Therefore, it is possible that the combination of MET and CAT might have a synergistic effect on FAK activation. Indeed, we found that the combination of CAT and HGF or MET synergistically activates FAK in Huh7 cells (Fig. 6B) or mouse liver (Fig. 6C). Correspondingly, phosphorylation of β-catenin at tyrosine 654 was also enhanced by co-delivery of HGF or MET and CAT in Huh7 cells or mouse liver (Fig. 6B and 6C). These results suggest that the combination of MET and CAT has a synergistic effect on FAK activation in HCC cells.
FAK mediates Cyclin D1 regulation by MET or CAT in HCC cells
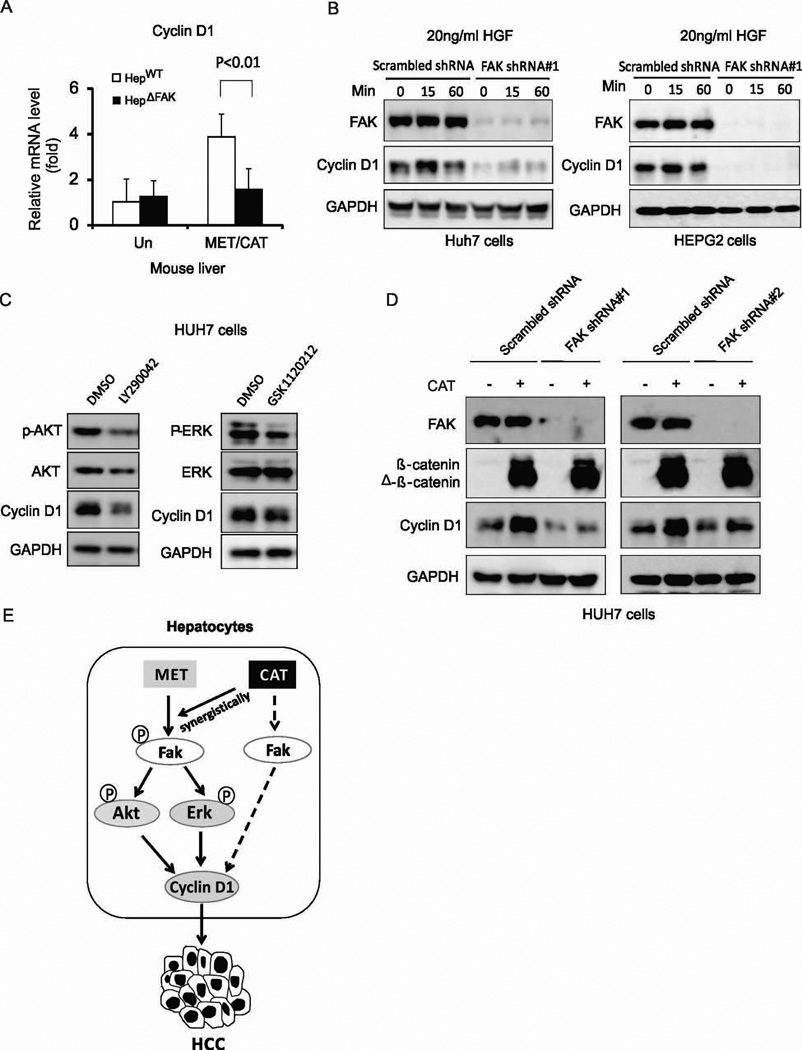
Cyclin D1, a downstream target of the PI3K/AKT, MAPK and Wnt/β-catenin pathways, is a key regulator of cell proliferation (33). Overexpression of Cyclin D1 has been related to the development and progression of HCC (34). Cyclin D1 is increased in MET/CAT-induced tumors and has been shown to mediate MET/CAT-induced hepatocarcinogenesis (18). Therefore, we speculated that suppression of AKT and ERK caused by FAK deletion might decrease Cyclin D1 expression. Indeed, CyclinD1 induction by MET/CAT was significantly attenuated by a deficiency of Fak in mouse livers (Fig. 7A). In addition, knockdown of FAK significantly suppressed Cyclin D1 induction by HGF in Huh7 and HEPG2 cells (Fig. 7B). Furthermore, the PI3K/AKT inhibitor, LY294002, or the MEK/ERK inhibitor, GSK1120212, decreased Cyclin D1 expression in Huh7 and HEPG2 cells (Fig. 7C and S7). These data suggest that FAK mediates Cyclin D1 induction by HGF/c-MET through the AKT and ERK pathways. Finally, as Cyclin D1 is a well-characterized target of activated β-catenin (35), we tested whether FAK mediates Cyclin D1 induction by CAT. Knockdown of FAK abrogated the induction of Cyclin D1 by CAT in HCC cells (Fig. 7D), although FAK activity and expression were not enhanced by CAT (Fig. 6A). These results suggest that a basal level of FAK is required for CAT-stimulated Cyclin D1 expression (Fig. 7D), which might be independent of AKT or ERK as neither AKT nor ERK was activated by CAT (Fig. 6A).
Figure 7. FAK mediates Cyclin D1 regulation by MET or CAT in HCC cells.
(A) Cyclin D1 mRNA expression level in the livers of HepWT and HepΔFAK mice 7 weeks after injection of MET/CAT. (B) Protein expression of FAK, Cyclin D1 and GAPDH in Huh7 and HEPG2 cells infected with scrambled shRNA or FAK shRNA#1 lentiviral particles, then treated with 20ng/ml HGF for 15 or 60 minutes. (C) Protein expression of p-AKT, AKT, p-ERK, ERK, Cyclin D1 and GAPDH in Huh7 cells treated with 10 µM LY294002 or 500 nM GSK1120212 for 24 hours. (D) Protein expression of FAK, Cyclin D1 and GAPDH in Huh7 cells infected with scrambled shRNA, FAK shRNA#1 or FAK shRNA#2 lentiviral particles, then transfected with CAT or control plasmids. (E) A schematic model: hydrodynamic injection of MET into hepatocytes results in Fak activation. CAT does not activate FAK directly but the combination of CAT and MET has a synergistic effect on Fak activation. Fak activation regulates MET- or CAT-mediated Cyclin D1 induction, acting through either Akt- and Erk-dependent or -independent pathways, thereby promoting hepatocarcinogenesis.
Fak deficiency in hepatocytes does not result in compensatory expression of Pyk2 in mouse liver
Pyk2 is the other member of the FAK subfamily of cytoplasmic tyrosine kinases that shares significant sequence homology and a similar structural organization with FAK (36). Several reports showed that deletion of FAK can lead to increased expression of endogenous Pyk2, which compensates for Fak functions in embryonic fibroblasts, adult endothelial cells or mammary cancer stem cells (37–39). We therefore examined whether Fak deletion results in compensatory expression of Pyk2 in mouse livers. There were no significant changes in the expression of Pyk2 or p-Pyk2 in the livers between HepΔFak and HepWT mice (Fig. S8). These data suggest that Fak deficiency in hepatocytes does not lead to a compensatory expression of Pyk2.
Discussion
Effective therapeutic strategies are desperately needed for the treatment of HCC. c-MET and β-catenin signaling pathways are frequently dysregulated in human HCC samples (14, 15, 40). Co-activation of c-MET and β-catenin often occurs in HCC (15) and co-delivery of MET and CAT using the Sleeping Beauty Transposon system efficiently and rapidly induced HCC in mice (15, 17, 18). HCC development in these mice resembles the pathogenesis of human HCC, providing a useful model system to study the role of signaling pathways in HCC initiation, progression and drug-response. In this study, we found that Fak deficiency suppresses MET/CAT-induced hepatocarcinogenesis and delays animal mortality by MET/CAT-induced HCC, suggesting that inhibition of FAK may be a promising strategy to treat HCC. It remains to be determined whether inhibition of Fak suppresses MET/CAT-induced tumor initiation, tumor progression, or both. Future studies will address this question using a hepatocyte-specific inducible Fak knockout mice.
The molecular mechanisms by which MET/CAT induces HCC are largely unknown. Our study has demonstrated that Fak deficiency in hepatocytes largely blocks HCC development induced by MET/CAT. This indicate that Fak is a key mediator of MET/CAT-induced hepatocarcinogenesis. c-MET directly interacts with the FERM domain of FAK and phosphorylates FAK in MEFs and HEK293 cells (41). Our data indicate that c-MET also phosphorylates FAK in mouse liver and HCC cells. In addition, we found that knockdown of FAK attenuates the activation of AKT and ERK, the two major targets of c-MET, with the treatment of HGF in HCC cells. Therefore, we suggest that in HCC cells c-MET might directly bind to and activate FAK, which is critical for activating AKT and ERK. We also found that Cyclin D1 induction by MET/CAT is significantly attenuated by Fak deficiency in mouse liver. As Cyclin D1 is a downstream target of the PI3K/AKT and MAPK pathways and plays a key role as a regulator of cell proliferation (33), these data suggest that FAK might mediate c-MET-driven tumor proliferation and development by regulating Cyclin D1 through the AKT and ERK pathways.
Our results showed that CAT alone does not enhance expression of p-FAK (Y397), p-AKT, or p-ERK in mouse liver and HCC cells, suggesting CAT does not directly phosphorylate FAK to enhance its kinase activity in liver cells. However, we found that the combination of CAT and MET has a synergistic effect on FAK activation. The underlying mechanisms remain to be determined. However, c-MET has been reported to interact with and phosphorylate β-catenin in liver cells (30, 31), and we found that the combination of MET and CAT enhances phosphorylation of β-catenin at tyrosine 654. Therefore, it is possible that activation FAK by c-Met phosphorylates β-catenin and enhances β-catenin’s nuclear translocation and function (42–44). Phosphorylated β-catenin might also facilitate c-Met activation to promote FAK activity. Thus Met-FAK-CAT might form a feed-forward loop in Met/CAT-induced HCC. Inactivation of FAK might break such a loop and inhibit HCC. However, without c-Met, basal levels of FAK are required for CAT-induced Cyclin D1 expression in HCC cells, which might be independent of its kinase activity. Interestingly, some specific Wnt targets, including glutamine synthetase, regucalcin, and axin-2, were not affected by Fak deletion in MET/CAT-induced tumors (Fig. S9). These data suggest that FAK might specifically regulate some downstream targets of β-catenin, such as Cyclin D1, through both kinase-dependent (such as in Met/CAT treatment) and kinase-independent (such as in CAT-only transduction) pathways. Activated β-catenin binds to TCF4 and transcriptionally activates Cyclin D1 in tumor cells (45). Future studies are needed to determine whether the kinase-dependent and independent activities of FAK play a role in the transcriptional regulation of Cyclin D1 by the β-catenin/TCF4 complex, and whether a FAK kinase-independent function is involved in HCC development.
In conclusion, our study shows that Fak plays a critical role in MET/CAT-driven hepatocarcinogenesis (Fig. 7E). Inhibition of FAK may offer an effective strategy to protect against HCC development. The normal liver function of mice with hepatocyte-specific Fak deficiency is reassuring. A number of FAK inhibitors have been developed and are being studied in phase I or phase II clinical trials for multiple solid tumors (46, 47). These inhibitors might be useful for treating HCC, especially in patients showing overexpression of c-MET and activated β-catenin.
Supplementary Material
Acknowledgements
We thank Drs. Manuel Diaz, Peter Breslin, Mitchell Denning, and Tarun Patel (Loyola University Chicago) for helpful discussions and advice, Drs. Robert Wiltrout (NCI) and Xin Chen (UCSF) for providing plasmids for the MET/CAT model, and Dr. Fillippo Giancotti (SKI) for providing FAK shRNA and lentiviral packaging plasmids.
Financial Support: This work is supported in part by AASLD Liver Scholar Award (W Qiu), NIH grants RO1HL095896 (J Zhang) and CA184652 (W Qiu).
Abbreviations
- FAK
focal adhesion kinase
- HCC
hepatocellular carcinoma
- CAT
constitutively active β-catenin
- TUNEL
terminal deoxynucleotidyl transferase-mediated deoxyuridinetriphosphate nick end labeling
- WT
wild type
- HGF
human growth factor
- H&E
hematoxylin and eosin
- PKB
protein kinase B
- ERK
extracellular receptor kinase
- Rac
Ras-related C3 botulinum toxin substrate
Footnotes
Disclosures: The authors have no conflicts to disclose. All authors agreed on the submission.
Author contributions:
NS performed experiments, analyzed data and wrote the paper.
MA bred mice and performed experiments.
AZ performed experiments.
JWZ and JS provided key reagents.
NZL wrote the paper.
SC analyzed pathology of mouse livers and wrote the paper.
WQ designed experiments, analyzed data and wrote the paper.
Contributor Information
Na Shang, Email: nshang@luc.edu.
Maribel Arteaga, Email: marteaga@luc.edu.
Ali Zaidi, Email: zaidiali5@yahoo.com.
Jimmy Stauffer, Email: stauffej@mail.nih.gov.
Scott J. Cotler, Email: scotler@lumc.edu.
Nancy Zeleznik-Le, Email: nzelezn@luc.edu.
Jiwang Zhang, Email: jzhang@luc.edu.
Wei Qiu, Email: wqiu@luc.edu.
References
- 1.Bruix J, Sherman M Practice Guidelines Committee AAftSoLD. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–1236. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 2.Aravalli RN, Cressman EN, Steer CJ. Cellular and molecular mechanisms of hepatocellular carcinoma: an update. Arch Toxicol. 2013;87:227–247. doi: 10.1007/s00204-012-0931-2. [DOI] [PubMed] [Google Scholar]
- 3.Belghiti J, Kianmanesh R. Surgical treatment of hepatocellular carcinoma. HPB (Oxford) 2005;7:42–49. doi: 10.1080/13651820410024067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnson PJ. Non-surgical treatment of hepatocellular carcinoma. HPB (Oxford) 2005;7:50–55. doi: 10.1080/13651820410024076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 6.Mitra SK, Hanson DA, Schlaepfer DD. Focal adhesion kinase: in command and control of cell motility. Nat Rev Mol Cell Biol. 2005;6:56–68. doi: 10.1038/nrm1549. [DOI] [PubMed] [Google Scholar]
- 7.Zhao X, Guan JL. Focal adhesion kinase and its signaling pathways in cell migration and angiogenesis. Adv Drug Deliv Rev. 2011;63:610–615. doi: 10.1016/j.addr.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schlaepfer DD, Hauck CR, Sieg DJ. Signaling through focal adhesion kinase. Prog Biophys Mol Biol. 1999;71:435–478. doi: 10.1016/s0079-6107(98)00052-2. [DOI] [PubMed] [Google Scholar]
- 9.Pylayeva Y, Gillen KM, Gerald W, Beggs HE, Reichardt LF, Giancotti FG. Ras- and PI3K-dependent breast tumorigenesis in mice and humans requires focal adhesion kinase signaling. J Clin Invest. 2009;119:252–266. doi: 10.1172/JCI37160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ashton GH, Morton JP, Myant K, Phesse TJ, Ridgway RA, Marsh V, Wilkins JA, et al. Focal adhesion kinase is required for intestinal regeneration and tumorigenesis downstream of Wnt/c-Myc signaling. Dev Cell. 2010;19:259–269. doi: 10.1016/j.devcel.2010.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee J, Borboa AK, Chun HB, Baird A, Eliceiri BP. Conditional deletion of the focal adhesion kinase FAK alters remodeling of the blood-brain barrier in glioma. Cancer Res. 2010;70:10131–10140. doi: 10.1158/0008-5472.CAN-10-2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Itoh S, Maeda T, Shimada M, Aishima S, Shirabe K, Tanaka S, Maehara Y. Role of expression of focal adhesion kinase in progression of hepatocellular carcinoma. Clin Cancer Res. 2004;10:2812–2817. doi: 10.1158/1078-0432.ccr-1046-03. [DOI] [PubMed] [Google Scholar]
- 13.Fujii T, Koshikawa K, Nomoto S, Okochi O, Kaneko T, Inoue S, Yatabe Y, et al. Focal adhesion kinase is overexpressed in hepatocellular carcinoma and can be served as an independent prognostic factor. J Hepatol. 2004;41:104–111. doi: 10.1016/j.jhep.2004.03.029. [DOI] [PubMed] [Google Scholar]
- 14.Kaposi-Novak P, Lee JS, Gomez-Quiroz L, Coulouarn C, Factor VM, Thorgeirsson SS. Met-regulated expression signature defines a subset of human hepatocellular carcinomas with poor prognosis and aggressive phenotype. J Clin Invest. 2006;116:1582–1595. doi: 10.1172/JCI27236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tward AD, Jones KD, Yant S, Cheung ST, Fan ST, Chen X, Kay MA, et al. Distinct pathways of genomic progression to benign and malignant tumors of the liver. Proc Natl Acad Sci U S A. 2007;104:14771–14776. doi: 10.1073/pnas.0706578104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cieply B, Zeng G, Proverbs-Singh T, Geller DA, Monga SP. Unique phenotype of hepatocellular cancers with exon-3 mutations in beta-catenin gene. Hepatology. 2009;49:821–831. doi: 10.1002/hep.22695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stauffer JK, Scarzello AJ, Andersen JB, De Kluyver RL, Back TC, Weiss JM, Thorgeirsson SS, et al. Coactivation of AKT and beta-catenin in mice rapidly induces formation of lipogenic liver tumors. Cancer Res. 2011;71:2718–2727. doi: 10.1158/0008-5472.CAN-10-2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patil MA, Lee SA, Macias E, Lam ET, Xu C, Jones KD, Ho C, et al. Role of cyclin D1 as a mediator of c-Met- and beta-catenin-induced hepatocarcinogenesis. Cancer Res. 2009;69:253–261. doi: 10.1158/0008-5472.CAN-08-2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bai L, Ni HM, Chen X, DiFrancesca D, Yin XM. Deletion of Bid impedes cell proliferation and hepatic carcinogenesis. Am J Pathol. 2005;166:1523–1532. doi: 10.1016/S0002-9440(10)62368-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li WC, Ralphs KL, Tosh D. Isolation and culture of adult mouse hepatocytes. Methods Mol Biol. 2010;633:185–196. doi: 10.1007/978-1-59745-019-5_13. [DOI] [PubMed] [Google Scholar]
- 21.Qiu W, Wang X, Leibowitz B, Yang W, Zhang L, Yu J. PUMA-mediated apoptosis drives chemical hepatocarcinogenesis in mice. Hepatology. 2011;54:1249–1258. doi: 10.1002/hep.24516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schaller MD, Hildebrand JD, Shannon JD, Fox JW, Vines RR, Parsons JT. Autophosphorylation of the focal adhesion kinase, pp125FAK, directs SH2-dependent binding of pp60src. Mol Cell Biol. 1994;14:1680–1688. doi: 10.1128/mcb.14.3.1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Evan GI, Vousden KH. Proliferation, cell cycle and apoptosis in cancer. Nature. 2001;411:342–348. doi: 10.1038/35077213. [DOI] [PubMed] [Google Scholar]
- 24.Gilmore AP, Romer LH. Inhibition of focal adhesion kinase (FAK) signaling in focal adhesions decreases cell motility and proliferation. Mol Biol Cell. 1996;7:1209–1224. doi: 10.1091/mbc.7.8.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Frisch SM, Vuori K, Ruoslahti E, Chan-Hui PY. Control of adhesion-dependent cell survival by focal adhesion kinase. J Cell Biol. 1996;134:793–799. doi: 10.1083/jcb.134.3.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lietha D, Cai X, Ceccarelli DF, Li Y, Schaller MD, Eck MJ. Structural basis for the autoinhibition of focal adhesion kinase. Cell. 2007;129:1177–1187. doi: 10.1016/j.cell.2007.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brunton VG, Avizienyte E, Fincham VJ, Serrels B, Metcalf CA, 3rd, Sawyer TK, Frame MC. Identification of Src-specific phosphorylation site on focal adhesion kinase: dissection of the role of Src SH2 and catalytic functions and their consequences for tumor cell behavior. Cancer Res. 2005;65:1335–1342. doi: 10.1158/0008-5472.CAN-04-1949. [DOI] [PubMed] [Google Scholar]
- 28.You H, Ding W, Dang H, Jiang Y, Rountree CB. c-Met represents a potential therapeutic target for personalized treatment in hepatocellular carcinoma. Hepatology. 2011;54:879–889. doi: 10.1002/hep.24450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koga F, Xu W, Karpova TS, McNally JG, Baron R, Neckers L. Hsp90 inhibition transiently activates Src kinase and promotes Src-dependent Akt and Erk activation. Proc Natl Acad Sci U S A. 2006;103:11318–11322. doi: 10.1073/pnas.0604705103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Monga SP, Mars WM, Pediaditakis P, Bell A, Mule K, Bowen WC, Wang X, et al. Hepatocyte growth factor induces Wnt-independent nuclear translocation of beta-catenin after Met-beta-catenin dissociation in hepatocytes. Cancer Res. 2002;62:2064–2071. [PubMed] [Google Scholar]
- 31.Zeng G, Apte U, Micsenyi A, Bell A, Monga SP. Tyrosine residues 654 and 670 in beta-catenin are crucial in regulation of Met-beta-catenin interactions. Exp Cell Res. 2006;312:3620–3630. doi: 10.1016/j.yexcr.2006.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rasola A, Fassetta M, De Bacco F, D'Alessandro L, Gramaglia D, Di Renzo MF, Comoglio PM. A positive feedback loop between hepatocyte growth factor receptor and beta-catenin sustains colorectal cancer cell invasive growth. Oncogene. 2007;26:1078–1087. doi: 10.1038/sj.onc.1209859. [DOI] [PubMed] [Google Scholar]
- 33.Nishida N, Fukuda Y, Komeda T, Kita R, Sando T, Furukawa M, Amenomori M, et al. Amplification and overexpression of the cyclin D1 gene in aggressive human hepatocellular carcinoma. Cancer Res. 1994;54:3107–3110. [PubMed] [Google Scholar]
- 34.Musgrove EA, Caldon CE, Barraclough J, Stone A, Sutherland RL. Cyclin D as a therapeutic target in cancer. Nat Rev Cancer. 2011;11:558–572. doi: 10.1038/nrc3090. [DOI] [PubMed] [Google Scholar]
- 35.Shtutman M, Zhurinsky J, Simcha I, Albanese C, D'Amico M, Pestell R, Ben-Ze'ev A. The cyclin D1 gene is a target of the beta-catenin/LEF-1 pathway. Proc Natl Acad Sci U S A. 1999;96:5522–5527. doi: 10.1073/pnas.96.10.5522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Avraham H, Park SY, Schinkmann K, Avraham S. RAFTK/Pyk2-mediated cellular signalling. Cell Signal. 2000;12:123–133. doi: 10.1016/s0898-6568(99)00076-5. [DOI] [PubMed] [Google Scholar]
- 37.Sieg DJ, Ilic D, Jones KC, Damsky CH, Hunter T, Schlaepfer DD. Pyk2 and Src-family protein-tyrosine kinases compensate for the loss of FAK in fibronectin-stimulated signaling events but Pyk2 does not fully function to enhance FAK- cell migration. EMBO J. 1998;17:5933–5947. doi: 10.1093/emboj/17.20.5933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weis SM, Lim ST, Lutu-Fuga KM, Barnes LA, Chen XL, Gothert JR, Shen TL, et al. Compensatory role for Pyk2 during angiogenesis in adult mice lacking endothelial cell FAK. J Cell Biol. 2008;181:43–50. doi: 10.1083/jcb.200710038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fan H, Guan JL. Compensatory function of Pyk2 protein in the promotion of focal adhesion kinase (FAK)-null mammary cancer stem cell tumorigenicity and metastatic activity. J Biol Chem. 2011;286:18573–18582. doi: 10.1074/jbc.M110.200717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Anson M, Crain-Denoyelle AM, Baud V, Chereau F, Gougelet A, Terris B, Yamagoe S, et al. Oncogenic beta-catenin triggers an inflammatory response that determines the aggressiveness of hepatocellular carcinoma in mice. J Clin Invest. 2012;122:586–599. doi: 10.1172/JCI43937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen SY, Chen HC. Direct interaction of focal adhesion kinase (FAK) with Met is required for FAK to promote hepatocyte growth factor-induced cell invasion. Mol Cell Biol. 2006;26:5155–5167. doi: 10.1128/MCB.02186-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fonar Y, Frank D. FAK and WNT signaling: the meeting of two pathways in cancer and development. Anticancer Agents Med Chem. 2011;11:600–606. doi: 10.2174/187152011796817673. [DOI] [PubMed] [Google Scholar]
- 43.Santos A, Bakker AD, Zandieh-Doulabi B, de Blieck-Hogervorst JM, Klein-Nulend J. Early activation of the beta-catenin pathway in osteocytes is mediated by nitric oxide, phosphatidyl inositol-3 kinase/Akt, and focal adhesion kinase. Biochem Biophys Res Commun. 2010;391:364–369. doi: 10.1016/j.bbrc.2009.11.064. [DOI] [PubMed] [Google Scholar]
- 44.Chen XL, Nam JO, Jean C, Lawson C, Walsh CT, Goka E, Lim ST, et al. VEGF-induced vascular permeability is mediated by FAK. Dev Cell. 2012;22:146–157. doi: 10.1016/j.devcel.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tetsu O, McCormick F. Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature. 1999;398:422–426. doi: 10.1038/18884. [DOI] [PubMed] [Google Scholar]
- 46.Schultze A, Fiedler W. Therapeutic potential and limitations of new FAK inhibitors in the treatment of cancer. Expert Opin Investig Drugs. 2010;19:777–788. doi: 10.1517/13543784.2010.489548. [DOI] [PubMed] [Google Scholar]
- 47.Infante JR, Camidge DR, Mileshkin LR, Chen EX, Hicks RJ, Rischin D, Fingert H, et al. Safety, pharmacokinetic, and pharmacodynamic phase I dose-escalation trial of PF-00562271, an inhibitor of focal adhesion kinase, in advanced solid tumors. J Clin Oncol. 2012;30:1527–1533. doi: 10.1200/JCO.2011.38.9346. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.