Abstract
In clinical practice, an overwhelming majority of biopsied thyroid nodules are benign. Therefore, there is a need for a complementary and noninvasive imaging tool to provide clinically relevant diagnostic information about thyroid nodules to reduce the rate of unnecessary biopsies. The goal of this study was to evaluate the feasibility of utilizing Comb-push Ultrasound Shear Elastography (CUSE) to measure the mechanical properties (i.e., stiffness) of thyroid nodules and use this information to help classify nodules as benign or malignant. CUSE is a fast and robust 2D shear elastography technique in which multiple laterally distributed acoustic radiation force beams are utilized simultaneously to produce shear waves. Unlike other shear elasticity imaging modalities, CUSE does not suffer from limited field of view (FOV) due to shear wave attenuation and can provide a large FOV at high frame rates. To evaluate the utility of CUSE in thyroid imaging, a preliminary study was performed on a group of 5 healthy volunteers and 10 patients with ultrasound (US)-detected thyroid nodules prior to fine needle aspiration biopsy (FNAB). The measured shear wave speeds in normal thyroid tissue and thyroid nodules were converted to Young's modulus (E), indicating a measure of tissue stiffness. Our results indicate an increase in E for thyroid nodules compared to normal thyroid tissue. This increase was significantly higher in malignant nodules compared to benign. The Young's modulus in normal thyroid tissue, benign and malignant nodules were found to be 23.2±8.29 kPa, 91.2±34.8 kPa, and 173.0±17.1 kPa, respectively. Results of this study suggest the utility of CUSE in differentiating between benign and malignant thyroid nodules.
Keywords: Cancer, Elasticity, In vivo, Shear Wave Elastography, Thyroid, Ultrasound
I. Introduction
The incidence of thyroid cancer is increasing rapidly, more than that of any other cancer, in both males and females and within all ethnic backgrounds [1], including a drastic increased incidence in recent years [2-5]. While palpable thyroid nodules are very common [6, 7], studies have shown that as many as 68% of the general population are diagnosed with one or more thyroid nodule upon ultrasound (US) examination, which are not clinically apparent at physical examination. Ultrasound is the first line imaging tool for characterization of the thyroid and can accurately determine the number, locations, and sizes of nodules within the gland [7-10]. The presence of microcalcifications, ill-defined or spiculated margins, irregular shape, relative hypoechogenicity, mixed or solid echotexture and absent halo sign have significant association with malignancy [11-18]. However, each of these individual features, if used alone, is not an accurate predictor of thyroid cancer [19]. While the combination of features is highly specific for malignancy (80.8–96.1%), the sensitivity of US to detect specific features is low (9.7–48.3%) [13, 20, 21]. Consequently, US demonstrated relatively low specificity in detecting malignant nodules, which limits its ability to accurately distinguish malignant lesions from the sea of benign nodules [22]. Because only a minority of US detected thyroid nodules (5-12%) are malignant [9, 23], a large number of unnecessary biopsies are performed resulting in a substantial national financial health care burden. Moreover, many patients unnecessarily undergo biopsy and, potentially, surgery for benign disease. Therefore, there is a need for an alternative imaging modality that can improve the specificity of thyroid nodule diagnosis beyond conventional US imaging.
It is known that tissue stiffness may be a biomarker of pathology in various types of tissues [24-28]. Thyroid palpation has been the traditional method for assessment of thyroid nodules. with stiffer nodules associated with increased risk of malignancy [29, 30]. However, assessment of thyroid nodule stiffness with palpation depends on several factors such as size, depth, and location of the nodule, as well as experience of the examiner [31-33]. Elasticity imaging is an emerging field of medical imaging for noninvasive evaluation of tissue stiffness [24, 34]. For example, magnetic resonance elastography (MRE) has been shown to differentiate normal from pathological thyroid gland [35]. US-based elastography methods for thyroid nodule classification have been an interest for numerous researchers and clinicians because of several notable advantages of US imaging such as its being nonionizing, real-time, cost efficient, portable, and widely available in clinical practice [36]. Among US-based thyroid elastography methods, strain imaging or quasi-static elastography utilizes compression and strain imaging to obtain maps of relative stiffness [37-41]. However, strain imaging is a qualitative technique and its results are sensitive to operator experience in data acquisition and interpretation. A recent study reported the limitation of quasi-static elastography in screening thyroid nodules with indeterminate cytology and indicated the necessity for development of quantitative tools to better evaluate and differentiate the thyroid nodules [42]. Acoustic radiation force impulse (ARFI) shear wave imaging [43] and Supersonic Shear Imaging (SSI) [44] provide quantitative information about the tissue stiffness by generating shear waves within the tissue and measuring the propagation speed of the induced shear waves. Both methods use acoustic radiation force (ARF) to induce shear waves within the tissue of interest [43]. Both SSI and ARFI shear wave imaging provide 2D maps of shear elasticity and have shown promising results in thyroid nodule assessment [45-51]. In addition, point quantification ARFI has also been used in measurement of thyroid tissue stiffness [52, 53].
Shear waves produced by ARF are usually weak and do not propagate far due to high attenuation in tissue [54]. Therefore, multiple push-detect events at different areas under the transducer are required to piece together a 2D shear wave image with appropriate field-of-view (FOV). To address this limitation, our group has recently developed a novel ultrasound shear wave elastography called Comb-push Ultrasound Shear Elastography (CUSE) [55, 56]. CUSE utilizes multiple simultaneous ARF push beams spaced out laterally like a comb for shear wave production. In CUSE, the entire FOV under the US transducer is filled with shear waves produced by multiple “teeth” of the comb-push to produce high signal-to-noise-ratio (SNR) shear wave signals throughout the FOV. Therefore, the full FOV under the transducer can be reconstructed in one single comb-push data acquisition taking only about 25 milliseconds in most soft tissues. The wide FOV of CUSE is beneficial for thyroid imaging because multiple nodules separated by large distances can be captured in the same CUSE image. In addition, the fast acquisition feature of CUSE makes it less sensitive to interfering body or physiological motions (such as carotid artery pulsation) and allows averaging shear wave speed images from multiple acquisitions, further improving image SNR, and nodule boundary delineation.
In this study, we demonstrate the utility of CUSE to provide robust and accurate 2D maps of thyroid tissue elasticity. Further, we investigate the potential of using CUSE results to differentiate between benign and malignant thyroid nodules. To the best of our knowledge, this study is the first demonstration of in vivo application of CUSE for assessment of thyroid nodules.
II. Materials and Methods
A. Principles of CUSE Imaging
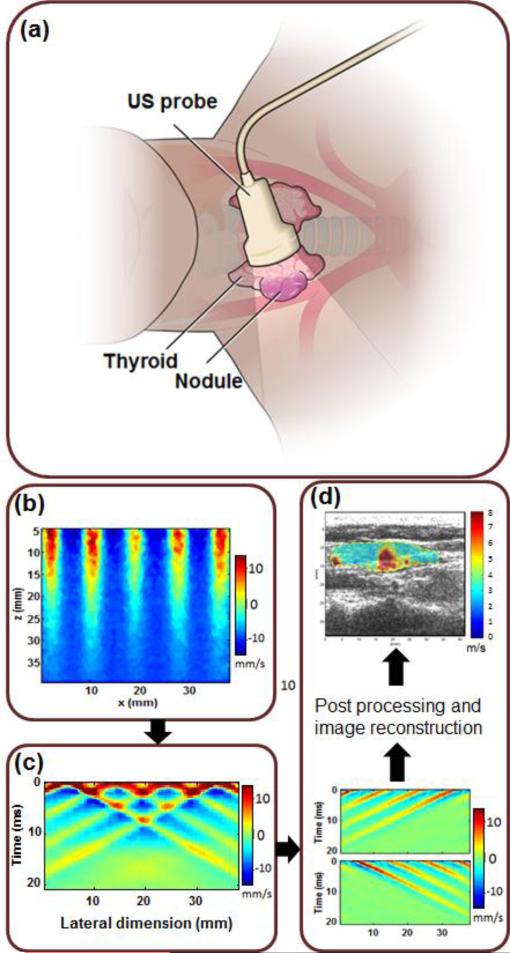
In CUSE, shear waves are produced by multiple laterally-spaced push beams (Fig. 1b). The ARF push beams could be either unfocused or focused depending on the required penetration depth (i.e. the depth of the thyroid nodule) [56]. Shear waves produced by each push beam can be treated as independent realizations of a single excitation push beam. Shear waves from different push beams interfere with each other and eventually fill the entire FOV (Fig. 1c). To achieve robust shear wave speed estimation, a directional filter is used to extract left-to-right (LR) propagating shear waves and right-to-left (RL) propagating shear waves from the interfering shear wave patterns [57, 58]. A time-of-flight based shear wave speed estimate method is used to recover local shear wave speed at each pixel from both LR waves and RL waves. A final shear wave speed map is then composed by averaging LR and RL speed maps (Fig. 1d). Additional technical details of CUSE imaging are described elsewhere [55, 56].
Fig. 1.
US/CUSE imaging diagram. (a) In vivo US/CUSE thyroid nodule imaging. (b) An example of comb-push ARF excitation of the medium. The colors indicate the particle velocity at the time of application of comb-push ARF. (c) Shear wave generated in response to the comb-push excitation. The colors represent the particle velocity and thus indicate the propagation of generated shear waves. (d) CUSE processing showing separation of the LR and RL waves by means of directional filters, post-processing, image formation and basic image processing to provide the shear wave speed map of a region of interest (ROI) including a thyroid nodule and its surrounding background.
Assuming soft tissues are incompressible, linear, isotropic, and purely elastic, the Young's modulus (E) (representing the tissue stiffness, kPa) can be calculated as:
| (1) |
where ρ represents the density of the tissue (approximately 1000 kg/m3 for most soft tissues) and Cs is the shear wave propagation speed [59] and E is in kPa. It must be noted that the Eq. (1) holds for an elastic (non-viscous) material in which the frequency dependency of shear waves is not taken into account [60]. In other words, the shear wave speed measured by CUSE is group velocity and thus does not represent the dispersive behavior of shear waves.
B. US/CUSE Imaging System
CUSE was implemented on a fully programmable US platform (Verasonics V-1, Verasonics Inc., Redmond, WA) equipped with a linear array transducer L7–4 (Philips Healthcare, Andover, MA). The Verasonics system is integrated around a software-based beamforming system that performs a pixel-oriented processing algorithm. The center frequency for comb-push beams was set to 4.09 MHz. The push pulse duration was set to 600 μs. The maximum derated (at 0.3 dB/cm/MHz) mechanical index (MI,0.3) from all push beams was 1.81, maximum derated spatial peak time average intensity (ISPTA,0.3) was 434 mW/cm2, both below the FDA regulatory limits (MI = 1.9, ISPTA = 720 mW/cm2). Upon comb-push excitation of the thyroid tissue, the Verasonics system immediately switched to plane wave imaging [44, 61] mode with all transducer elements (center frequency 5 MHz) to track the propagation of shear waves [62]. The shear wave particle velocity is tracked by the compounding plane wave imaging method [61] and calculated by the 1-D autocorrelation method using the in-phase/quadrature (IQ) data [63]. Upon US/CUSE data acquisition, the data were stored for further post processing. A Matlab®-based (Mathworks Inc., MA) graphical user interface (GUI) was developed to process acquired CUSE data and reconstruct shear wave speed maps. The shear wave speed map calculated by CUSE was overlaid on the corresponding B-mode US image so that the CUSE speed information and the structural information in B-mode can be visualized in a single image. The combined image allows more accurate evaluation of measured stiffness within the nodules and in the normal thyroid tissue background. As a measure of quality control and ensuring the robustness of displacement calculations [64], normalized cross-correlation coefficients were generated during the local shear wave speed calculation. Then, a n×n (n=3 or 5 pixels) mean filter was applied on the final shear wave speed map. The normalized cross-correlation coefficient (CC) varied between 0 and 1, where a larger coefficient indicated higher reliability in displacement calculation, and thus, more reliable shear wave data. A threshold of CC=0.6 was imposed in all shear wave speed computations to reject the shear wave speed values calculated at pixels with CC<0.6. Therefore, the CUSE map was displayed only using pixels with reliable values. Each CUSE image was acquired in approximately 25 ms and no frame averaging was performed on the processed images.
C. Tissue-mimicking Phantom Study
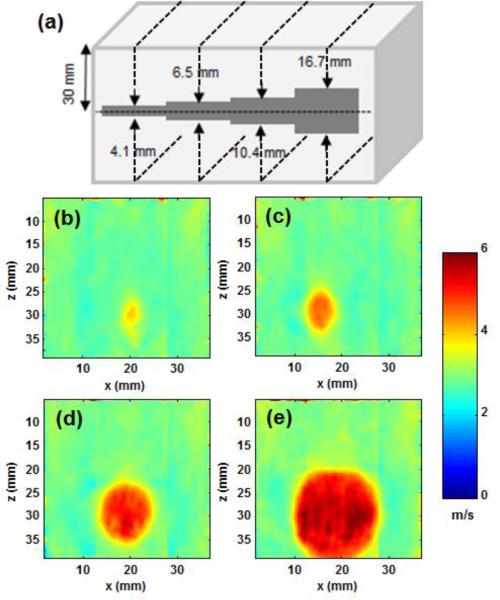
To evaluate the performance of CUSE in detecting lesions with clinically relevant sizes, we performed a tissue-mimicking phantom study on a standard elasticity phantom (CIRS model 049A, CIRS, Norfolk, VA, USA). This phantom contains 4 stepped cylindrical inclusions of different sizes ranging from 4.1 mm to 16.7 mm in diameters embedded in a background. All inclusions have the same stiffness and same acoustic attenuation of α=0.5 dB/cm/MHz. CUSE imaging was performed on inclusions of 4.1, 6.5. 10.4, and 16.7 mm in diameter, and the measured Young's modulus via CUSE was compared with the nominal values provided by the manufacturer. We chose phantom lesion type IV with nominal elasticity of 80 kPa for our analysis.
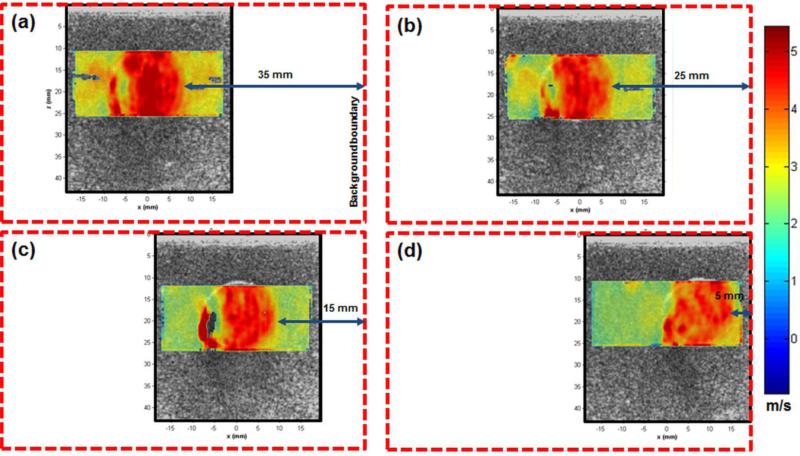
One of the most important technical considerations for shear wave imaging relates to the effect of tissue boundaries on shear wave speed measurements [57, 65]. In other words, boundary conditions can induce artifacts and affect the measurements. In the case of thyroid tissue, this problem can be severe due to the relative small size of the thyroid gland. In CUSE, the directional filters minimize the effect of reflected waves from the tissue boundaries and thus minimize such artifacts. To demonstrate performance of CUSE for lesions located at different distances from tissue boundaries, another set of tissue-mimicking phantoms were tested. Each phantom consisted of a gelatin cube (6×6×6 cm) with a cylindrical inclusion (with diameter of 20 mm). The background was made out of 8% wt/wt gelatin from porcine skin (300 bloom, Sigma Aldrich, MO, US), 10% v/v Glycerol (Sigma Aldrich, MO, US), and 0.5% laponite (Sigma Aldrich, MO, US). To add ultrasound backscatterers to the phantom, 1% cellulose (Sigma Aldrich, MO, US) was added to the mixture. The inclusion was made out of 10% wt/wt gelatin (similar to what was used to make the background) and 1.5% agar (Sigma Aldrich, MO, US) was added to increase the stiffness. 2% wt/wt cellulose particles were added to the inclusion material to produce US scatterers. To test the effect of phantom boundaries on the resulting image, the cylindrical inclusions were placed at different relative locations in each phantom. For this purpose, 4 phantoms were made in which the distance between the center of the cylindrical nodule-mimicking inclusion and boundary of the tissue-mimicking gelatin block were 35, 25, 15, and 5 mm. These phantoms were imaged by our US/CUSE imaging system.
D. In vivo Human Study
Prior to in vivo patient studies, the US/CUSE imaging system was optimized and its performance was evaluated on five healthy volunteers with no history of thyroid disease. The optimizations include adjusting CUSE parameters such as push ARF frequency, number of push beams, push beam width, the gap between the push beams, and US detection parameters including pulse-echo frequency and angle compounding in CUSE, as well as the practical aspects of thyroid imaging such as probe placement and minimizing the applied compression force on patients. After appropriate optimization, CUSE settings and imaging procedures were held constant in order to perform a consistent study and avoid undesired variations caused by discrepancies in imaging conditions. To evaluate the performance of the US/CUSE imaging system for in vivo imaging of patients with known thyroid nodules, the optimized system was used to image thyroid lobes in 10 patients (7 females and 3 males) diagnosed with suspicious thyroid nodules prior to ultrasound guided fine needle aspiration biopsy (FNAB). Patients selected for evaluation were volunteers, age 18 and up, who had suspicious thyroid nodules on their conventional thyroid US imaging. In addition to the patients, 5 healthy volunteers with no history of thyroid disease were also included in our CUSE study. Both left and right thyroid lobes were imaged in the healthy volunteers. The study was conducted under a protocol approved by the Mayo Clinic Institutional Review Board (IRB). Informed consent was obtained prior to imaging. FNAB of suspicious thyroid lesions occurred after US/CUSE imaging procedures in all cases, and the results were used to validate the acquired CUSE results. In five cases with suspicious or indeterminate FNAB results, patients underwent surgical biopsy. In this study, all 3 malignant nodules and 2 benign were proven by surgical pathology.
Patients and healthy volunteers were scanned in the supine position with their necks slightly tilted back and turned to the left or right (depending on the position of the suspicious nodule) to provide full exposure of the gland to the sonographer. The procedure was similar to the clinical practice of conventional thyroid US imaging (Fig. 1a). In most cases, a longitudinal plane was selected for both US and CUSE imaging. The longitudinal view was preferred because it showed more normal adjacent thyroid tissue for comparison, and avoided the carotid artery. However, in a few cases and due to the size and location of the nodule, imaging was performed on a transverse plane. Clinical ultrasound was utilized for nodule location and CUSE imaging was performed on both the nodule and background healthy thyroid tissue for the sake of comparison between the stiffness of the nodule and the normal tissue. A highly experienced sonographer performed the examination and care was taken to minimize pressure on the skin to avoid the possible influence of compression [66] in elasticity measurements by CUSE.
III. Results
US and CUSE results for tissue-mimicking phantoms and in vivo thyroid tissues are presented in this section. For CUSE results, we demonstrated the shear wave speed maps, which are directly proportional to the material stiffness according to Eq. 1.
A. Phantom study results
CUSE images of the CIRS phantom with cylindrical nodule-mimicking inclusions of various sizes were acquired to investigate the accuracy of CUSE in imaging lesions of different sizes. Figure 2 demonstrates the results of CUSE in the CIRS phantom. A diagram indicating the phantom structure and dimensions is shown in Figure 2a. Figures 2b-2e show 2D images of shear wave speed obtained by CUSE in our CIRS phantom (Fig. 2a). These images show that all inclusions and the background are clearly defined. No image denoising, smoothing, or frame averaging was used. These results demonstrate the capabilities of CUSE in detecting inclusions as small as 4.1 mm in diameter. Often in practice, routine FNAB of nodules less than 10 mm is discouraged [2], because only a minority of smaller nodules, as small as 5 mm, are aggressive [67]. To further quantify the accuracy of CUSE measurements compared to the nominal stiffness of the inclusions, CUSE measurements were repeated 5 times for each inclusion and a “bias” parameter was defined as:
Fig 2.
(a) Schematic of the cross-section of the tissue mimicking phantom with cylindrical inclusions of different diameters . CUSE cross-sectional imaging planes are indicated by the dashed black lines. (b-e) Shear wave speed map of the phantom including the inclusions with sizes of 4.1, 6.5, 10.4, and 16.7 mm in diameter measured by CUSE.
The bias values for inclusions of 4.1, 6.5, 10.4, and 16.7 mm in diameter were calculated as 23%, 12%, 5% and 3.9%. Therefore, CUSE measurements of elasticity in inclusions larger than 10 mm have approximately 5% error compared to the nominal values (measured by independent mechanical testing). Clinically, nodules larger than 10 mm in diameter are considered to be most significant. The nominal values of Young's Modulus are given by the phantom manufacturer (CIRS, Norfolk, VA, USA). Eq. 1 was used to calculate the shear wave speeds from the given Young's moduli.
Figure 3 demonstrates the results of the second phantom study to evaluate the effect of the nodule distance to the tissue boundary. Figures 3a to 3d indicate the combined shear wave speed maps measured by CUSE and overlaid on the B-mode US images of the phantom in cases where the boundary of the inclusion was 35, 25, 15, and 5 mm from the background boundary, respectively. The inclusion is clearly identified in all 4 scenarios. The mean shear wave speed in CUSE images of the inclusion at 35, 25, 15, and 5 mm away from the boundary of the background was measured as 4.7, 4.4, 4.4, and 4.2 m/s, respectively. These results demonstrate that CUSE images of the inclusions are consistent in shape and contrast at all distances from the boundary. Hence, boundary effects are not a significant factor in the quality of CUSE images.
Fig. 3.
CUSE shear wave speed maps overlaid on B-mode US images of a gelatin phantom with a cylindrical inclusion. The inclusion distance from the left boundary was (a) 35, (b) 25, (c) 15, and (d) 5 mm. Boundaries of each phantom is shown with a red dashed-line box. Due to limited azimuthal field of view, the images do not cover the boundaries of phantoms.
B. Human In vivo Results
Here, we present results utilizing CUSE in the evaluation of thyroid tissue from 10 patients and 5 healthy volunteers to demonstrate the feasibility of using the developed US/CUSE imaging system to detect and measure the shear wave speed (or equivalently the Young's modulus) of different types of thyroid nodules. Amongst the selected examined patients, 7 thyroid nodules were benign and 3 nodules were diagnosed as papillary carcinomas. The pathology (FNAB) results of each case are included with the CUSE results.
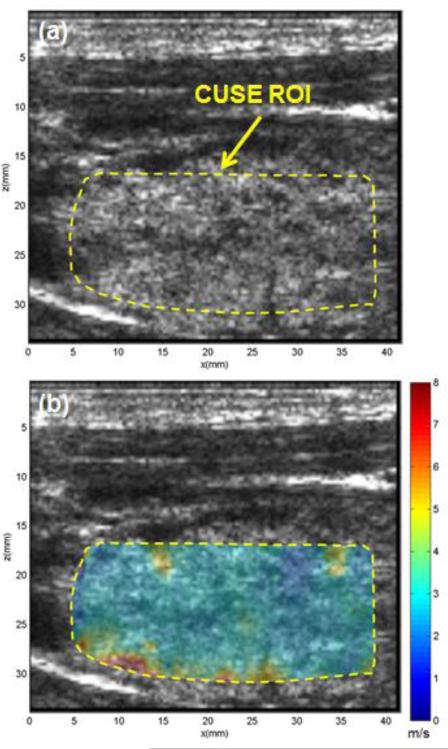
Figure 4 illustrates the US and CUSE shear wave speed maps of a healthy thyroid lobe. The thyroid tissue is located beneath a muscular layer and the CUSE ROI is marked with a yellow dashed line in Fig. 4a. The shear wave speed map in Fig. 4b shows the normal thyroid tissue as homogenously, low in stiffness (i.e. low shear wave speed). The mean and standard deviation of shear wave speed was measured to be 2.83±0.4 m/s within the thyroid tissue marked in panel a by the yellow boarder. There are a few pixels with high shear wave speed in the deeper part of the thyroid and adjacent to the thyroid tissue boundary. These artifacts apparently resulted from the adjacent high-stiffness muscle above and below the thyroid tissue.
Fig. 4.
US image and CUSE shear wave speed map of a selected ROI within thyroid tissue in a healthy volunteer. (a) B-mode US image. (b) CUSE shear wave speed map. Both B-mode US and CUSE images were acquired in longitudinal planes.
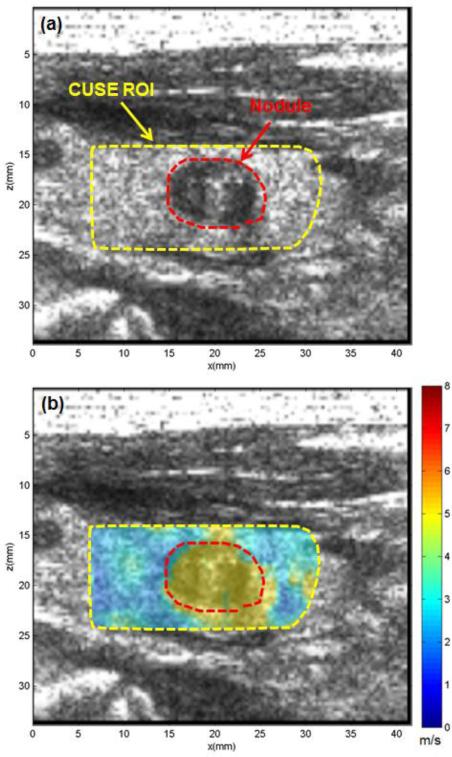
Figure 5 represents the US image and CUSE shear wave speed map of the thyroid of a patient diagnosed with a benign nodule. The patient was a 62-year-old male with a suspicious nodule in his left thyroid lobe. The CUSE ROI is marked with a yellow dashed line in Fig. 5a. The nodule clearly appears in the US image as a round hypoechoic region marked with a dashed red contour. The CUSE image shown in Fig. 5b clearly indicates higher shear wave speed within the nodule compared to its surrounding normal background. The mean and standard deviation of shear wave speed in the nodule and thyroid background was measured as 4.96±0.28 m/s, and 2.25±0.89 m/s, respectively. The US-guided FNAB revealed cytological features consistent with a benign thyroid nodule.
Fig. 5.
US and CUSE shear wave speed map of thyroid tissue with a benign nodule. (a) B-mode US image showing CUSE ROI (dashed yellow contour) and the nodule (red dashed contour). (b) CUSE shear wave speed map. The nodule is clearly visualized with a higher shear wave speed. Both B-mode US and CUSE images were acquired in longitudinal planes.
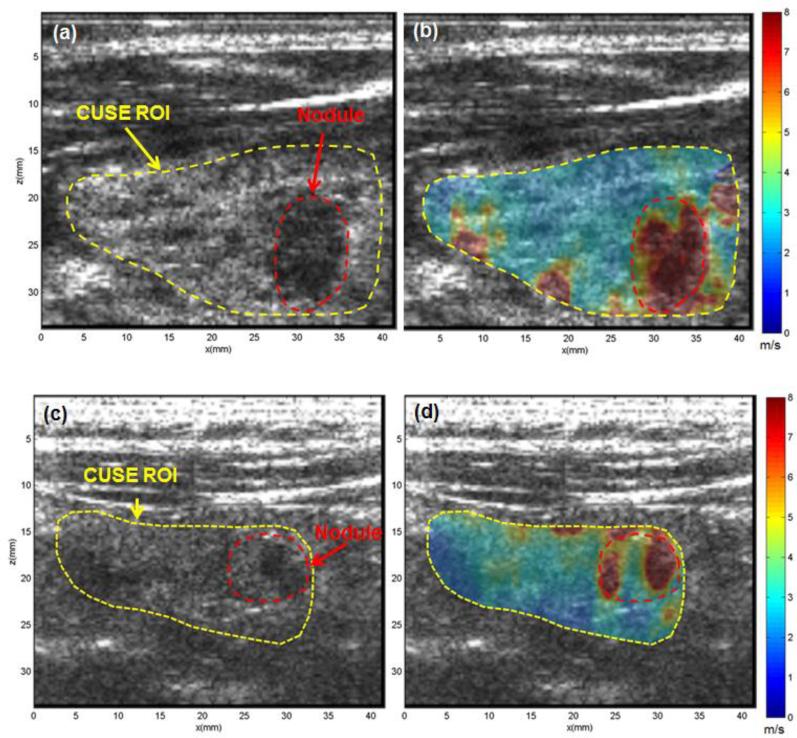
Figure 6 represents the US image and CUSE shear wave speed maps of thyroids in two patients diagnosed with papillary thyroid carcinomas (PTC). The first patient was an 18-year-old female with a suspicious nodule in her left thyroid lobe. The CUSE ROI is marked with a yellow dashed line in Fig. 6a. A suspicious 12 mm nodule located in the mid portion of the lobe clearly appears in the US image as a hypoechoic region, which is marked with a dashed red contour. The shear wave speed measured by CUSE within the nodule was significantly faster than the normal thyroid background (Fig. 6b). Other high-speed regions (red spots) seen in the lower part of the thyroid tissue near its border are artifacts resulting from the adjacent high-stiffness muscle. The mean and standard deviation of shear wave speed in the nodule and thyroid background was measured as 7.60±0.84 m/s, and 2.76±0.50 m/s, respectively. The second patient was a 74-year-old female with a suspicious 9 mm nodule in her right thyroid lobe. While higher shear wave speed was detected within the nodule, the measured shear wave speed, and accordingly tissue stiffness, of the nodule was heterogeneous with large variations. This could be because of the heterogeneity of the nodule structure. In other words, pathology-driven tissue stiffening in thyroid nodules can be spatially non-uniform. From a diagnostic point of view, the presence of any high-stiffness region within the nodule could be clinically significant and alarming. Therefore, to be conservative in differentiating nodules, it was decided to take into account the highest values of the stiffness rather than the mean stiffness within the nodule. For this purpose, a threshold was selected at 70% of the peak value of the E, and mean and standard deviation of the nodule stiffness was calculated in areas with stiffness higher than this threshold. After the thresholding algorithm, the mean and standard deviation of shear wave speed in the nodule was measured as 7.95±0.2 m/s. The mean and standard deviation of surrounding normal thyroid tissue was measured as 2.50±0.52 m/s. The US-guided FNAB result of this case reveals PTC.
Fig. 6.
US and CUSE shear wave speed map of two different thyroid glands, each containing a PTC nodule (red dashed contour). (a, c) B-mode US images showing CUSE ROIs (dashed yellow contours) and the nodules (red dashed contours). (b, d) CUSE shear wave speed map. The nodules are clearly visualized with a significantly higher shear wave speed than normal thyroid tissue. Both B-mode US and CUSE images were acquired in longitudinal planes.
Young's modulus is commonly used to represent the stiffness of soft tissues. To further demonstrate the potential utility of CUSE to identify and classify thyroid nodules, the shear wave speed values measured by CUSE were converted to Young's modulus (E) using Eq. (1). Table I summarizes the CUSE measurements of E in healthy thyroid tissue and benign and malignant nodules. For each group, mean and standard deviation, the range (minimum - maximum), and 95% confidence interval for mean of E are calculated. Although our study group is small for calculating the sensitivity and specificity of CUSE, we observed a distinct difference between the E values of benign and malignant cases.
Table I.
Summary of E (kPa) measured in 20 healthy thyroid tissues, 7 benign and 3 malignant thyroid nodules. All values are in kPa.
| Mean | Standard Deviation | Range (Min- Max) | 95% confidence interval for Mean | |
|---|---|---|---|---|
| Normal | 23.2 | 8.29 | 9.18-46.09 | 15.09-31.31 |
| Benign | 91.2 | 34.8 | 57.55-161.18 | 77.15-105.2 |
| Malignant | 173 | 17.1 | 155.52-189.60 | 151.3-194.3 |
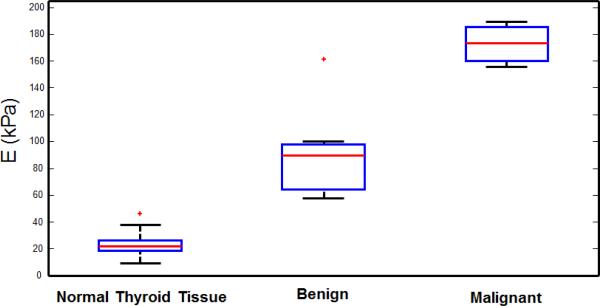
Figure 7 represents the box plot of calculated E in 20 normal thyroid tissues, 7 benign thyroid nodules and 3 malignant nodules. The normal group includes 10 from 5 healthy volunteers (both left and right thyroid lobes), and 10 from the healthy part of thyroid tissue in 10 patients. The mean and standard deviation of E for healthy thyroid tissue, benign, and malignant nodules was calculated as 23.2±8.29 kPa, 91.2±34.8 kPa, and 173±17.1 kPa, respectively. Our results indicate that the tissue elasticity (i.e. E) is significantly higher in malignant nodules compared to benign nodules. Despite the small sample size, the Wilcoxon rank sum test indicates that the measured stiffness of benign and malignant groups are significantly different (Exact P-value=0.033). The measured E in one of the benign cases is significantly higher than the other 6 cases and is shown as an outlier marked with a red plus in Fig. 7.
Fig 7.
Box plots show interquartile range (blue box), median (red line within box), range (whisker), and outliers (red +) of normal thyroid tissue, as well as benign and malignant thyroid nodules, measured by CUSE.
IV. Discussion
CUSE is a novel 2-D shear elasticity imaging method with two major advantages over other current shear elasticity imaging techniques. First, because CUSE uses multiple ARF push beam excitations, the whole FOV is filled with shear waves leading to more accurate and reliable measurements of shear wave speed [55, 56]. Consequently, CUSE is less affected by significant shear wave attenuation in areas that are far from the push beam. Secondly, and more importantly, in CUSE, a full FOV 2-D reconstruction of shear wave speed map can be obtained with only one fast data acquisition (about 25 ms). Such fast acquisition can play an important role for thyroid imaging applications where the carotid pulsation can result in significant interference. Because the CUSE acquisition time (~25 ms) is much shorter than the carotid pulsation (~1-2 pulse/s), no significant interference and consequent motion artifact is anticipated. The notable advantages of CUSE suggest its potential utility as a complementary tool to B-mode imaging, providing additional clinically important information about the internal composition of thyroid nodules.
Ex vivo tissue mimicking phantom studies revealed two important features of CUSE imaging. First, our results indicated high accuracy of CUSE in measuring the stiffness of nodules larger than 10 mm. In clinical practice, thyroid nodules smaller than 10 mm are considered less suspicious. Consequently, we believe CUSE would provide useful complementary diagnostic information to B-mode US imaging in clinical settings. Second, our tissue mimicking phantom results indicate the robustness of CUSE measurements of a stiffer inclusion located within a softer background and at different distances (5 to 35 mm) to the boundary of the background. Therefore, CUSE should be capable of assessing the stiffness of the thyroid nodules that are close to the thyroid capsule. In the preliminary assessment of thyroid, all nodules subjected to CUSE analysis were all larger than 10 mm in diameter and were recommended for FNAB. In most cases, we tried to find a view in which the lesion is surrounded by normal tissue on both sides, however, in two patients, including the case shown in Fig 6c and 6d, the nodule was fairly close to the boundary of the thyroid tissue. Similar to the phantom studies, we could obtain a clear shear wave speed map of each nodule.
All CUSE maps shown in this work indicate the shear wave speed. However, since reporting the Young's modulus is more common in the literature [28, 48, 49], we converted the shear wave speed to the Young's modulus in the summarized results (Table I and boxplot in Fig. 7) to facilitate easier comparison with literature results. Our preliminary in vivo results indicate two important findings. First, the CUSE shear wave speed (or stiffness) map can accurately localize the thyroid nodules. In both benign and malignant cases, the higher shear wave speed was measured in regions that are associated with nodules. Second, the shear wave speed map, and thus the measured E, is significantly higher in malignant nodules compared to benign. This second finding suggests the potential utility of CUSE measurements to classify thyroid nodules and potentially distinguishing nodules that should be biopsied from those that should be observed. For example, our results (Fig. 7), suggest that E values higher than 120 kPa correlate with higher probability of malignancy (sensitivity: 100%, specificity: 87%). Although the number of cases in this pilot study is limited, our results indicate a distinct difference between the measured stiffness in benign and malignant nodules. Other studies have also reported higher stiffness in malignant nodules compared to benign nodules. However, an overlap between shear wave elasticity measurements of benign and malignant thyroid nodules is reported in the same reports [51, 68]. While a study on a larger group of patients is required to further evaluate the abilities of CUSE, the clear distinction between the measured stiffness in benign and malignant nodules shown in Figure 7 could be the result of thresholding higher stiffness values (as opposed to the mean) within the nodule. The high speed of CUSE, which minimizes errors caused by tissue motion, may be another reason leading to clearly defined nodules and a good separation between benign and malignant nodules. The clinical report for the benign case in which a high stiffness (E=161 kPa) was measured, indicates a focally calcified nodule. We speculate the higher stiffness measured in this case could be due to the presence of calcifications, as observed in [69].
One limitation of our study is the lack of comparison between CUSE and other similar shear-wave based elastography techniques, a potential topic for a future study. The B-mode US images presented in this study are of lower quality as compared to clinical US images. This limitation is due to the utilization of plane-wave (ultrafast) US imaging [61, 70] in our US/CUSE system that leads to a high frame rate US imaging, which is essential for reliable tracking of shear waves but results in lower quality B-mode US. A potential future optimization of CUSE imaging is to use conventional B-mode US imaging [71] techniques with line-by-line scanning in combination with CUSE shear wave speed maps tracked by plane-wave imaging. We did not use ECG gating to minimize the effect of carotid pulsation in this study. However, we did not notice significant interference from carotid pulsation, likely due to the short CUSE acquisition time. Nevertheless, it is possible to use ECG gating to perform the CUSE data acquisition between carotid pulses to minimize the effect of intrinsic tissue movements.
V. Conclusion
We have demonstrated the feasibility of utilizing a novel shear elastography technique, comb-push ultrasound shear elastography (CUSE), to measure the stiffness of thyroid nodules in vivo. The significant advantages of CUSE, such as large FOV and fast acquisition, provide a reliable shear wave speed map, and thus, accurate and reliable measurement of stiffness in thyroid nodules. Our preliminary in vivo results indicate significant differences between benign and malignant nodules in terms of CUSE stiffness measurements. These findings suggest that CUSE could potentially serve as a noninvasive tool for classifying thyroid nodules based on their stiffness (or shear wave speed). Our results should provide a foundation for further investigation of thyroid nodules using this technology in a larger patient population.
Acknowledgements
The authors are grateful to Ms. Mercy Asiedu for her help in processing CUSE data, to Dr. Rickey E. Carter and Dr. Matthew W. Urban for their helpful discussions, to Ms. Theresa Nielson for her assistance with the patient study, to Mr. Thomas Kinter for computer support, and to Ms. Jennifer Milliken for administrative support.
This study was supported by National Institute of Health (NIH) grants R01 EB017213, R01CA148994, and R01DK082408. The content is solely the responsibility of the authors and does not necessarily represent the official views of NIH. Disclosure of Conflict of Interest: Mayo Clinic and some of the authors have a potential financial interest related to a device or technology referenced in this paper.
Contributor Information
Mohammad Mehrmohammadi, Department of Physiology and Biomedical Engineering, Mayo Clinic College of Medicine, Rochester, MN.
Pengfei Song, Department of Physiology and Biomedical Engineering, Mayo Clinic College of Medicine, Rochester, MN.
Duane D. Meixner, Department of Diagnostic Radiology, Mayo Clinic College of Medicine, Rochester, MN
Robert T. Fazzio, Department of Diagnostic Radiology, Mayo Clinic College of Medicine, Rochester, MN
Shigao Chen, Department of Physiology and Biomedical Engineering, Mayo Clinic College of Medicine, Rochester, MN.
James F. Greenleaf, Department of Physiology and Biomedical Engineering, Mayo Clinic College of Medicine, Rochester, MN.
Mostafa Fatemi, Department of Physiology and Biomedical Engineering, Mayo Clinic College of Medicine, Rochester, MN.
Azra Alizad, Department of Physiology and Biomedical Engineering and with the Department of Internal Medicine, Mayo Clinic College of Medicine, Rochester, MN (Alizad.azra@mayo.edu)..
References
- 1.Pellegriti G, Frasca F, Regalbuto C, Squatrito S, Vigneri R. Worldwide Increasing Incidence of Thyroid Cancer: Update on Epidemiology and Risk Factors. Journal of cancer epidemiology. 2013;2013 doi: 10.1155/2013/965212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, Mandel SJ, Mazzaferri EL, McIver B, Sherman SI, Tuttle RM. Management guidelines for patients with thyroid nodules and differentiated thyroid cancer: The American Thyroid Association Guidelines Taskforce. Thyroid. 2006;16:109–142. doi: 10.1089/thy.2006.16.109. [DOI] [PubMed] [Google Scholar]
- 3.Brito JP, Morris JC, Montori VM. Thyroid cancer: zealous imaging has increased detection and treatment of low risk tumours. BMJ: British Medical Journal. 2013;347 doi: 10.1136/bmj.f4706. [DOI] [PubMed] [Google Scholar]
- 4.Chen AY, Jemal A, Ward EM. Increasing incidence of differentiated thyroid cancer in the United States, 1988–2005. Cancer. 2009;115:3801–3807. doi: 10.1002/cncr.24416. [DOI] [PubMed] [Google Scholar]
- 5.Jemal A, Simard EP, Dorell C, Noone A-M, Markowitz LE, Kohler B, Eheman C, Saraiya M, Bandi P, Saslow D. Annual report to the Nation on the Status of cancer, 1975–2009, Featuring the Burden and trends in Human Papillomavirus (HPV)–Associated cancers and HPV Vaccination coverage levels. Journal of the National Cancer Institute. 2013;105:175–201. doi: 10.1093/jnci/djs491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tunbridge W, Evered D, Hall R, Appleton D, Brewis M, Clark F, Evans JG, Young E, Bird T, Smith P. The spectrum of thyroid disease in a community: the Whickham survey. Clinical endocrinology. 1977;7:481–493. doi: 10.1111/j.1365-2265.1977.tb01340.x. [DOI] [PubMed] [Google Scholar]
- 7.Guth S, Theune U, Aberle J, Galach A, Bamberger C. Very high prevalence of thyroid nodules detected by high frequency (13 MHz) ultrasound examination. European journal of clinical investigation. 2009;39:699–706. doi: 10.1111/j.1365-2362.2009.02162.x. [DOI] [PubMed] [Google Scholar]
- 8.Gharib H, Papini E, Paschke R, Duick DS, Valcavi R, Hegedus L. Medical guidelines for clinical practice for the diagnosis and management of thyroid nodules. Endocrine practice : official journal of the American College of Endocrinology and the American Association of Clinical Endocrinologists. 2006;12:63–93. doi: 10.4158/EP.12.1.63. [DOI] [PubMed] [Google Scholar]
- 9.Hegedüs L. The thyroid nodule. New England Journal of Medicine. 2004;351:1764–1771. doi: 10.1056/NEJMcp031436. [DOI] [PubMed] [Google Scholar]
- 10.Davies L, Welch HG. Increasing incidence of thyroid cancer in the United States, 1973-2002. JAMA: the journal of the American Medical Association. 2006;295:2164–2167. doi: 10.1001/jama.295.18.2164. [DOI] [PubMed] [Google Scholar]
- 11.Brkljacic B, Cuk V, Tomic-Brzac H, Bence-Zigman Z, Delic-Brkljacic D, Drinkovic I. Ultrasonic evaluation of benign and malignant nodules in echographically multinodular thyroids. Journal of clinical ultrasound. 1994;22:71–76. doi: 10.1002/jcu.1870220202. [DOI] [PubMed] [Google Scholar]
- 12.Leenhardt L, Hejblum G, Franc B, Fediaevsky LDP, Delbot T, Le Guillouzic D, Ménégaux F, Guillausseau C, Hoang C, Turpin G. Indications and limits of ultrasound-guided cytology in the management of nonpalpable thyroid nodules. Journal of Clinical Endocrinology & Metabolism. 1999;84:24–28. doi: 10.1210/jcem.84.1.5418. [DOI] [PubMed] [Google Scholar]
- 13.Moon W-J, Jung SL, Lee JH, Na DG, Baek J-H, Lee YH, Kim J, Kim HS, Byun JS, Lee DH. Benign and Malignant Thyroid Nodules: US Differentiation—Multicenter Retrospective Study1. Radiology. 2008;247:762–770. doi: 10.1148/radiol.2473070944. [DOI] [PubMed] [Google Scholar]
- 14.Nam-Goong IS, Kim HY, Gong G, Lee HK, Hong SJ, Kim WB, Shong YK. Ultrasonography-guided fine-needle aspiration of thyroid incidentaloma: correlation with pathological findings. Clinical endocrinology. 2004;60:21–28. doi: 10.1046/j.1365-2265.2003.01912.x. [DOI] [PubMed] [Google Scholar]
- 15.Cappelli C, Castellano M, Pirola I, Gandossi E, De Martino E, Cumetti D, Agosti B, Rosei EA. Thyroid nodule shape suggests malignancy. European Journal of Endocrinology. 2006;155:27–31. doi: 10.1530/eje.1.02177. [DOI] [PubMed] [Google Scholar]
- 16.Frates MC, Benson CB, Doubilet PM, Kunreuther E, Contreras M, Cibas ES, Orcutt J, Moore FD, Larsen PR, Marqusee E. Prevalence and distribution of carcinoma in patients with solitary and multiple thyroid nodules on sonography. Journal of Clinical Endocrinology & Metabolism. 2006;91:3411–3417. doi: 10.1210/jc.2006-0690. [DOI] [PubMed] [Google Scholar]
- 17.Frates MC, Benson CB, Charboneau JW, Cibas ES, Clark OH, Coleman BG, Cronan JJ, Doubilet PM, Evans DB, Goellner JR, Hay ID, Hertzberg BS, Intenzo CM, Jeffrey RB, Langer JE, Larsen PR, Mandel SJ, Middelton WD, Reading CC, Sherman SI, Tessler FN. Management of Thyroid Nodules Detected at US: Society of Radiologists in Ultrasound Consensus Conference Statement1. Radiology. 2005;237:794–800. doi: 10.1148/radiol.2373050220. [DOI] [PubMed] [Google Scholar]
- 18.Kim E-K, Park CS, Chung WY, Oh KK, Kim DI, Lee JT, Yoo HS. New sonographic criteria for recommending fine-needle aspiration biopsy of nonpalpable solid nodules of the thyroid. American Journal of Roentgenology. 2002;178:687–691. doi: 10.2214/ajr.178.3.1780687. [DOI] [PubMed] [Google Scholar]
- 19.Brito JP, Gionfriddo MR, Al Nofal A, Boehmer KR, Leppin AL, Reading C, Callstrom M, Elraiyah TA, Prokop LJ, Stan MN. The accuracy of thyroid nodule ultrasound to predict thyroid cancer: systematic review and meta-analysis. Journal of Clinical Endocrinology & Metabolism. 2013:2013–2928. doi: 10.1210/jc.2013-2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Popowicz B, Klencki M, Lewiński A, Słowińska-Klencka D. The usefulness of sonographic features in selection of thyroid nodules for biopsy in relation to the nodule's size. European Journal of Endocrinology. 2009;161:103–111. doi: 10.1530/EJE-09-0022. [DOI] [PubMed] [Google Scholar]
- 21.Sipos JA. Advances in ultrasound for the diagnosis and management of thyroid cancer. Thyroid. 2009;19:1363–1372. doi: 10.1089/thy.2009.1608. [DOI] [PubMed] [Google Scholar]
- 22.Deandrea M, Mormile A, Veglio M, Motta M, Pellerito R, Gallone G, Grassi A, Torchio B, Bradac R, Garberoglio R, Fonzo D. Fine-needle aspiration biopsy of the thyroid: comparison between thyroid palpation and ultrasonography. Endocrine practice : official journal of the American College of Endocrinology and the American Association of Clinical Endocrinologists. 2002 Jul-Aug;8:282–6. doi: 10.4158/EP.8.4.282. [DOI] [PubMed] [Google Scholar]
- 23.Wong K, Ahuja AT. Ultrasound of thyroid cancer. Cancer Imaging. 2005;5:157. doi: 10.1102/1470-7330.2005.0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gao L, Parker K, Lerner R, Levinson S. Imaging of the elastic properties of tissue—A review. Ultrasound in medicine & biology. 1996;22:959–977. doi: 10.1016/s0301-5629(96)00120-2. [DOI] [PubMed] [Google Scholar]
- 25.Sarvazyan A. Elastic properties of soft tissues. Handbook of elastic properties of solids, liquids and gases. 2001;3:107–127. [Google Scholar]
- 26.Monpeyssen H, Correas J, Tramalloni J, Poirée S, Voillemot N, Jais J. Correlations between shearwaveelastography of thyroid nodules and cytological data: study about 157 patients. Congress: ECR. 2011 [Google Scholar]
- 27.Krouskop TA, Wheeler TM, Kallel F, Garra BS, Hall T. Elastic moduli of breast and prostate tissues under compression. Ultrasonic imaging. 1998;20:260–274. doi: 10.1177/016173469802000403. [DOI] [PubMed] [Google Scholar]
- 28.Sebag F, Vaillant-Lombard J, Berbis J, Griset V, Henry J, Petit P, Oliver C. Shear wave elastography: a new ultrasound imaging mode for the differential diagnosis of benign and malignant thyroid nodules. Journal of Clinical Endocrinology & Metabolism. 2010;95:5281–5288. doi: 10.1210/jc.2010-0766. [DOI] [PubMed] [Google Scholar]
- 29.Gharib H, Papini E, Paschke R, Duick DS, Valcavi R, Hegedüs L, Vitti P. American Association of Clinical Endocrinologists, Associazione Medici Endocrinologi, and European Thyroid Association medical guidelines for clinical practice for the diagnosis and management of thyroid nodules. Endocrine Practice. 2010;16:1–43. [PubMed] [Google Scholar]
- 30.Christensen S, Bondeson L, Ericsson U, Lindholm K. Prediction of malignancy in the solitary thyroid nodule by physical examination, thyroid scan, fine-needle biopsy and serum thyroglobulin. A prospective study of 100 surgically treated patients. Acta chirurgica scandinavica. 1983;150:433–439. [PubMed] [Google Scholar]
- 31.Bojunga J, Herrmann E, Meyer G, Weber S, Zeuzem S, Friedrich-Rust M. Real-time elastography for the differentiation of benign and malignant thyroid nodules: a meta-analysis. Thyroid. 2010;20:1145–1150. doi: 10.1089/thy.2010.0079. [DOI] [PubMed] [Google Scholar]
- 32.Rago T, Vitti P. Role of thyroid ultrasound in the diagnostic evaluation of thyroid nodules. Best Practice & Research Clinical Endocrinology & Metabolism. 2008;22:913–928. doi: 10.1016/j.beem.2008.09.016. [DOI] [PubMed] [Google Scholar]
- 33.Tan GH, Gharib H, Reading CC. Solitary thyroid nodule: comparison between palpation and ultrasonography. Archives of internal Medicine. 1995;155:2418. doi: 10.1001/archinte.155.22.2418. [DOI] [PubMed] [Google Scholar]
- 34.Sarvazyan A, Hall TJ, Urban MW, Fatemi M, Aglyamov SR, Garra BS. An overview of elastography–an emerging branch of medical imaging. Current medical imaging reviews. 2011;7(4):255–282. doi: 10.2174/157340511798038684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bahn MM, Brennan MD, Bahn RS, Dean DS, Kugel JL, Ehman RL. Development and application of magnetic resonance elastography of the normal and pathological thyroid gland in vivo. Journal of Magnetic Resonance Imaging. 2009;30:1151–1154. doi: 10.1002/jmri.21963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Szabo TL. Diagnostic ultrasound imaging: inside out: 2004. Access Online via Elsevier.
- 37.Bae U, Dighe M, Dubinsky T, Minoshima S, Shamdasani V, Kim Y. Ultrasound thyroid elastography using carotid artery pulsation: preliminary study. Journal of ultrasound in medicine : official journal of the American Institute of Ultrasound in Medicine. 2007 Jun;26:797–805. doi: 10.7863/jum.2007.26.6.797. [DOI] [PubMed] [Google Scholar]
- 38.Dighe M, Bae U, Richardson ML, Dubinsky TJ, Minoshima S, Kim Y. Differential diagnosis of thyroid nodules with US elastography using carotid artery pulsation. Radiology. 2008 Aug;248:662–9. doi: 10.1148/radiol.2482071758. [DOI] [PubMed] [Google Scholar]
- 39.Lyshchik A, Higashi T, Asato R, Tanaka S, Ito J, Hiraoka M, Brill AB, Saga T, Togashi K. Elastic moduli of thyroid tissues under compression. Ultrasonic imaging. 2005 Apr;27:101–10. doi: 10.1177/016173460502700204. [DOI] [PubMed] [Google Scholar]
- 40.Lyshchik A, Higashi T, Asato R, Tanaka S, Ito J, Mai JJ, Pellot-Barakat C, Insana MF, Brill AB, Saga T, Hiraoka M, Togashi K. Thyroid gland tumor diagnosis at US elastography. Radiology. 2005 Oct;237:202–11. doi: 10.1148/radiol.2363041248. [DOI] [PubMed] [Google Scholar]
- 41.Rago T, Santini F, Scutari M, Pinchera A, Vitti P. Elastography: new developments in ultrasound for predicting malignancy in thyroid nodules. The Journal of clinical endocrinology and metabolism. 2007 Aug;92:2917–22. doi: 10.1210/jc.2007-0641. [DOI] [PubMed] [Google Scholar]
- 42.Lippolis PV, Tognini S, Materazzi G, Polini A, Mancini R, Ambrosini CE, Dardano A, Basolo F, Seccia M, Miccoli P, Monzani F. Is elastography actually useful in the presurgical selection of thyroid nodules with indeterminate cytology? The Journal of clinical endocrinology and metabolism. 2011 Nov;96:E1826–30. doi: 10.1210/jc.2011-1021. [DOI] [PubMed] [Google Scholar]
- 43.Nightingale K, McAleavey S, Trahey G. Shear-wave generation using acoustic radiation force:< i> in vivo</i> and< i> ex vivo</i> results. Ultrasound in medicine & biology. 2003;29:1715–1723. doi: 10.1016/j.ultrasmedbio.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 44.Bercoff J, Tanter M, Fink M. Supersonic shear imaging: a new technique for soft tissue elasticity mapping. Ultrasonics, Ferroelectrics and Frequency Control, IEEE Transactions on. 2004;51:396–409. doi: 10.1109/tuffc.2004.1295425. [DOI] [PubMed] [Google Scholar]
- 45.Bojunga J, Dauth N, Berner C, Meyer G, Holzer K, Voelkl L, Herrmann E, Schroeter H, Zeuzem S, Friedrich-Rust M. Acoustic radiation force impulse imaging for differentiation of thyroid nodules. PloS one. 2012;7:e42735. doi: 10.1371/journal.pone.0042735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Friedrich-Rust M, Romenski O, Meyer G, Dauth N, Holzer K, Grünwald F, Kriener S, Herrmann E, Zeuzem S, Bojunga J. Acoustic Radiation Force Impulse-Imaging for the evaluation of the thyroid gland: A limited patient feasibility study. Ultrasonics. 2012;52:69–74. doi: 10.1016/j.ultras.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 47.Gu J, Du L, Bai M, Chen H, Jia X, Zhao J, Zhang X. Preliminary Study on the Diagnostic Value of Acoustic Radiation Force Impulse Technology for Differentiating Between Benign and Malignant Thyroid Nodules. Journal of Ultrasound in Medicine. 2012;31:763–771. doi: 10.7863/jum.2012.31.5.763. [DOI] [PubMed] [Google Scholar]
- 48.Kim H, Kim J-A, Son EJ, Youk JH. Quantitative assessment of shear-wave ultrasound elastography in thyroid nodules: diagnostic performance for predicting malignancy. European radiology. 2013;23:1–6. doi: 10.1007/s00330-013-2847-5. [DOI] [PubMed] [Google Scholar]
- 49.Monpeyssen H, Correas J, Tramalloni J, Poiree S, Voillemot N, Jais J, Hélénon O. Shearwave Elastography of Thyroid Nodules: Correlations with Cytological Data: Study about 157 Patients. Ultrasound in Medicine and Biology. 2011;37:8. [Google Scholar]
- 50.Monpeyssen H, Tramalloni J, Poirée S, Hélénon O, Correas J-M. Elastography of the thyroid. Diagnostic and interventional imaging. 2013;94(5):535–544. doi: 10.1016/j.diii.2013.01.023. [DOI] [PubMed] [Google Scholar]
- 51.Sebag F, Vaillant-Lombard J, Berbis J, Griset V, Henry JF, Petit P, Oliver C. Shear wave elastography: a new ultrasound imaging mode for the differential diagnosis of benign and malignant thyroid nodules. The Journal of clinical endocrinology and metabolism. 2010 Dec;95:5281–8. doi: 10.1210/jc.2010-0766. [DOI] [PubMed] [Google Scholar]
- 52.Hou X-J, Sun A-X, Zhou X-L, Ji Q, Wang H-B, Wei H, Sun J-W, Liu H. The application of Virtual Touch tissue quantification (VTQ) in diagnosis of thyroid lesions: A preliminary study. European journal of radiology. 2013;82:797–801. doi: 10.1016/j.ejrad.2012.12.023. [DOI] [PubMed] [Google Scholar]
- 53.Zhang Y-F, Xu H-X, He Y, Liu C, Guo L-H, Liu L-N, Xu J-M. Virtual touch tissue quantification of acoustic radiation force impulse: a new ultrasound elastic imaging in the diagnosis of thyroid nodules. PloS one. 2012;7:e49094. doi: 10.1371/journal.pone.0049094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sarvazyan AP, Rudenko OV, Swanson SD, Fowlkes JB, Emelianov SY. Shear wave elasticity imaging: a new ultrasonic technology of medical diagnostics. Ultrasound in medicine & biology. 1998;24:1419–1435. doi: 10.1016/s0301-5629(98)00110-0. [DOI] [PubMed] [Google Scholar]
- 55.Song P, Zhao H, Manduca A, Urban MW, Greenleaf JF, Chen S. Comb-Push Ultrasound Shear Elastography (CUSE): A Novel Method for Two-Dimensional Shear Elasticity Imaging of Soft Tissues. Medical Imaging, IEEE Transactions on. 2012;31:1821–1832. doi: 10.1109/TMI.2012.2205586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Song P, Urban MW, Manduca A, Zhao H, Greenleaf J, Chen S. Comb-push Ultrasound Shear Elastography (CUSE) with Various Ultrasound Push Beams. Medical Imaging, IEEE Transactions on. 2013;32:1435–1447. doi: 10.1109/TMI.2013.2257831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Deffieux T, Gennisson J-L, Bercoff J, Tanter M. On the effects of reflected waves in transient shear wave elastography. Ultrasonics, Ferroelectrics and Frequency Control, IEEE Transactions on. 2011;58:2032–2035. doi: 10.1109/TUFFC.2011.2052. [DOI] [PubMed] [Google Scholar]
- 58.Manduca A, Lake DS, Kruse S, Ehman RL. Spatio-temporal directional filtering for improved inversion of MR elastography images. Medical image analysis. 2003;7:465–473. doi: 10.1016/s1361-8415(03)00038-0. [DOI] [PubMed] [Google Scholar]
- 59.Yamakoshi Y, Sato J, Sato T. Ultrasonic imaging of internal vibration of soft tissue under forced vibration. Ultrasonics, Ferroelectrics and Frequency Control, IEEE Transactions on. 1990;37:45–53. doi: 10.1109/58.46969. [DOI] [PubMed] [Google Scholar]
- 60.Chen S, Fatemi M, Greenleaf JF. Quantifying elasticity and viscosity from measurement of shear wave speed dispersion. The Journal of the Acoustical Society of America. 2004;115:2781–2785. doi: 10.1121/1.1739480. [DOI] [PubMed] [Google Scholar]
- 61.Tanter M, Bercoff J, Sandrin L, Fink M. Ultrafast compound imaging for 2-D motion vector estimation: application to transient elastography. Ultrasonics, Ferroelectrics and Frequency Control, IEEE Transactions on. 2002;49:1363–1374. doi: 10.1109/tuffc.2002.1041078. [DOI] [PubMed] [Google Scholar]
- 62.Pinton GF, Dahl JJ, Trahey GE. Rapid tracking of small displacements with ultrasound. Ultrasonics, Ferroelectrics and Frequency Control, IEEE Transactions on. 2006;53:1103–1117. doi: 10.1109/tuffc.2006.1642509. [DOI] [PubMed] [Google Scholar]
- 63.Kasai C, Namekawa K, Koyano A, Omoto R. Real-time two-dimensional blood flow imaging using an autocorrelation technique. IEEE Trans. Sonics Ultrason. 1985;32:458–464. [Google Scholar]
- 64.Cohn NA, Emelianov SY, Lubinski MA, O'Donnell M. An elasticity microscope. Part I: methods. Ultrasonics, Ferroelectrics and Frequency Control, IEEE Transactions on. 1997;44:1304–1319. [Google Scholar]
- 65.Sandrin L, Fourquet B, Hasquenoph J-M, Yon S, Fournier C, Mal F, Christidis C, Ziol M, Poulet B, Kazemi F. Transient elastography: a new noninvasive method for assessment of hepatic fibrosis. Ultrasound in medicine & biology. 2003;29:1705–1713. doi: 10.1016/j.ultrasmedbio.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 66.Barr RG, Zhang Z. Effects of Precompression on Elasticity Imaging of the Breast Development of a Clinically Useful Semiquantitative Method of Precompression Assessment. Journal of Ultrasound in Medicine. 2012;31:895–902. doi: 10.7863/jum.2012.31.6.895. [DOI] [PubMed] [Google Scholar]
- 67.McCartney CR, Stukenborg GJ. Decision analysis of discordant thyroid nodule biopsy guideline criteria. Journal of Clinical Endocrinology & Metabolism. 2008;93:3037–3044. doi: 10.1210/jc.2008-0448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Monpeyssen H, Correas J, Tramalloni J, Poiree S. Correlations between shearwave elastography of thyroid nodules and cytological data: study about 157 patients. Congress: ECR. 2011 [Google Scholar]
- 69.Barr RG, Memo R, Schaub CR. Shear wave ultrasound elastography of the prostate: initial results. Ultrasound quarterly. 2012;28:13–20. doi: 10.1097/RUQ.0b013e318249f594. [DOI] [PubMed] [Google Scholar]
- 70.Bercoff J. Ultrasound Imaging-Medical Applications. InTech Europe; 2011. Ultrafast Ultrasound Imaging; pp. 3–24. ISBN: 978-953-307-279-1. [Google Scholar]
- 71.Lu J.-y., Zou H, Greenleaf JF. Biomedical ultrasound beam forming. Ultrasound in medicine & biology. 1994;20:403–428. doi: 10.1016/0301-5629(94)90097-3. [DOI] [PubMed] [Google Scholar]