Abstract
Objective
To evaluate the long-term cost-effectiveness of endoscopic sinus surgery (ESS) compared to continued medical therapy for patients with refractory chronic rhinosinusitis (CRS).
Study Design
Cohort-style Markov decision tree economic evaluation
Methods
The economic perspective was the US third party payer with a 30 year time horizon. The two comparative treatment strategies were: 1) ESS followed by appropriate postoperative medical therapy and 2) continued medical therapy alone. Primary outcome was the incremental cost per quality adjusted life year (QALY). Costs were discounted at a rate of 3.5% in the reference case. Multiple sensitivity analyses were performed including differing time-horizons, discounting scenarios, and a probabilistic sensitivity analysis (PSA).
Results
The reference case demonstrated that the ESS strategy cost a total of $48,838.38 and produced a total of 20.50 QALYs. The medical therapy alone strategy cost a total of $28,948.98 and produced a total of 17.13 QALYs. The incremental cost effectiveness ratio (ICER) for ESS versus medical therapy alone is $5,901.90 per QALY. The cost-effectiveness acceptability curve from the PSA demonstrated that there is 74% certainty that the ESS strategy is the most cost-effective decision for any willingness to pay threshold greater then $25,000. The time horizon analysis suggests that ESS becomes the cost-effective intervention within the 3rd year after surgery.
Conclusion
Results from this study suggest that employing an ESS treatment strategy is the most cost-effective intervention compared to continued medical therapy alone for the long-term management of patients with refractory CRS.
Keywords: Chronic rhinosinusitis, sinusitis, endoscopic sinus surgery, medical therapy, Markov, decision tree, economic evaluation, cost effectiveness
Introduction
Chronic rhinosinusitis (CRS) is a common disabling illness affecting approximately 6 to 16% of the population1,2. CRS is characterized by diffuse sinonasal inflammation producing symptoms of nasal congestion, facial pain, reduction or complete loss of smell, headache, and fatigue3. Furthermore, there are substantial negative impacts on sleep4 and daily productivity5. The economic burden of CRS is substantial with annual direct costs exceeding $8.6 billion, which can be predominantly attributed to, physician office visits, emergency department encounters, and medication use6.
Following a diagnosis of CRS, the accepted primary management strategy begins with medical therapy to reduce mucosal inflammation and improve sinonasal function. Despite best medical efforts, a subset of patients will have persistent symptoms and are considered refractory. Strong evidence supports the use of endoscopic sinus surgery (ESS) in this cohort of patients with refractory CRS to improve clinical outcomes; however, the costs of surgery have not been justified through a rigorous economic evaluation with a long-term time horizon. Therefore, it is unknown whether ESS or continued medical therapy alone is the most cost-effective option in managing patients with refractory CRS over a life-time.
The purpose of this economic evaluation is to evaluate the cost-effectiveness of an ESS treatment strategy compared to continued medical therapy alone for patients with refractory CRS. A cost-utility analysis (CUA) was performed using a cohort-style Markov decision tree model to determine if the short-term increase in costs associated with performing ESS is justified during the long-term management of refractory CRS.
Methods
The perspective of this economic evaluation was from the United States (US) government payer. All costs are expressed in US dollars (USD) as of June 2013 (published costs prior to 2013 were adjusted to account for inflation). The primary outcome is the cost per quality adjusted life year (QALY). Since refractory CRS is a chronic non-terminal condition, normal life expectancy was assumed based on US population norms and a 30-year time horizon considered for this analysis.
All costs and effects are presented in disaggregated and aggregated form and incremental cost effectiveness ratios (ICERs) are presented for the primary outcome. The ICER is a commonly used equation in health economics to provide important information to resource allocation decision makers. It is the ratio of change in costs between two strategies to the change in effectiveness between the two strategies: (Cost strategy A - Cost strategy B)/(Effectiveness strategy A - Effectiveness strategy B)7. Therefore, the ICER provides the additional cost associated with the additional benefit of the new intervention being evaluated.
Costs were discounted at a rate of 3.5% for the reference case and multiple forms of sensitivity analysis were performed to account for inherent data uncertainty. The reporting of this economic evaluation followed the 2013 Consolidated Health Economic Evaluation Reporting Standards (CHEERS) guidelines8,9.
I. Economic Model
A cohort-style state transition Markov decision tree model was constructed to simulate the clinical management patients with refractory CRS. Refractory CRS was defined as persistent disease despite a minimum of 3 months topical sinonasal corticosteroid therapy along with a minimum of a 7-day course of systemic corticosteroids +/− 2-week course of broad-spectrum antibiotics10.
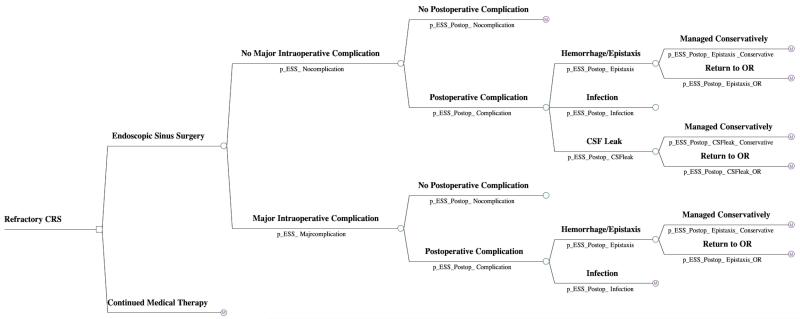
In the model, the two comparative treatment groups for managing refractory CRS included: 1) ESS followed by postoperative medical therapy and 2) continued medical therapy alone. For the ESS group, the first portion of the model involves a decision tree analysis which moves the patient through potential perioperative outcome pathways, including intraoperative and postoperative complications (Figure 1). In this model, a ‘major intraoperative complication’ involved experiencing one of three events: intraoperative CSF leak, intraoperative orbital injury, and major vascular injury. We did not include the probability of having a second CSF leak (in the postoperative period) in patients who sustained an major intraoperative complication to prevent the risk of inappropriately double counting. The risk of death from routine ESS was not included since it was felt to be negligible.
Figure 1.
Economic decision tree for intra- and post-operative outcomes
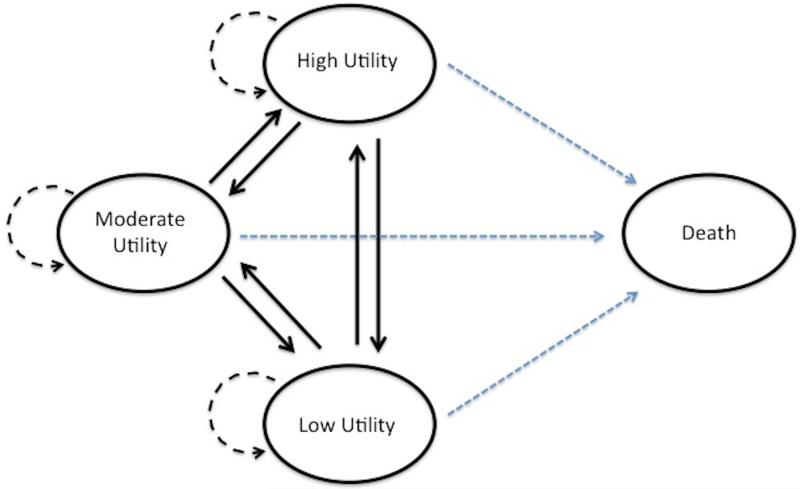
Since refractory CRS is a non-terminal chronic condition, the second portion involves performing a Markov model. The cycle duration was defined as one year. Based on national US life expectancy statistics11 and using an average age of CRS patients of 45-years old10, this model used a total Markov model duration of 30 cycles to reach an average age of 75 years old (rounded down from 75.4). Half-cycle corrections were added for all initial and final reward values. The medical therapy alone group entered directly into the Markov model, whereas the ESS group entered into the Markov model after they completed the ESS decision tree. Patients entered the Markov model into one of the following four refractory CRS health states based on their utility score: low, moderate, or high utility state or death. Following each cycle, patients either stayed in their current health state or transitioned into one of the other three states, based on transition probabilities (Figure 2).
Figure 2.
Markov model bubble diagram for refractory CRS health states
II. Effectiveness - Utility Values
Health state utility data for this economic evaluation was obtained using the SF-6D instrument. Health state utility scores (SF-6D) are derived from responses to 6 separate items indicated on both the SF-36 and SF-12 using a commercially available weighted algorithm derived by the Department of Health Economics and Decision Science at the University of Sheffield, Sheffield, United Kingdom. This algorithm application was used to calculate standardized health state utility values (range: 0.0= “death” - 1.0= “perfect health”) from follow-up survey responses provided by each study subject. One disadvantage of using the SF-6D utility scoring system is that it is based on standard gamble valuation technique performed in the United Kingdom general population, and therefore may not truly reflect US population preferences.
Utility estimates were obtained from the prospective observational cohort study (Clinicaltrials# NCT00799097; NIH: R01 DC005805) evaluating clinical outcomes following ESS for the management of refractory CRS10. Utility scores on 168 patients with a mean follow-up of 1.5 years were obtained. Plotting the distribution of post-ESS utility scores demonstrated three naturally occurring tertiles based on the following utility cut-points: Low: 0.00 to 0.55, Moderate: 0.56 to 0.69, and High: 0.70 and 1.00. These utility cut-points were used to define the three refractory CRS Markov health states. Evaluation of potential confounding variables such as age, gender, allergies, asthma, and other comorbidities, demonstrated that there were no differences between the ESS and medical therapy cohorts12.
The effect value assigned to each Markov state was determined by calculating the mean utility score of all patients within each of the three health states. The mean utility value per health state was used to assign the effect per cycle. The average utility score for patients in the low state was 0.499, moderate group was 0.619, and high group was 0.786 (Table 1).
Table 1. Reference case model data.
| Parameter | Description | Mean Value |
Source |
|---|---|---|---|
| Effect | |||
| u_Healthstate_High | Annual utility value for patients in the High utility Markov state |
0.786 | Clinicaltrials# NCT00799097; NIH: R01 DC005805 |
| u_Healthstate_Moderate | Annual utility value for patients in the Moderate utility Markov state |
0.619 | Clinicaltrials# NCT00799097; NIH: R01 DC005805 |
| u_Healthstate_Low | Annual utility value for patients in the Low utility Markov state |
0.499 | Clinicaltrials# NCT00799097; NIH: R01 DC005805 |
| Probabilities | |||
| p_ESS_Majorcomplication | Probability of major intraoperative complication during ESS |
0.01 | Ramakrishnan et al.14 |
| p_ESS_Postop_Complication | Probability of any postop complication | 0.1727 | Stankiewicz et al.15; Dalziel et al.16; Ramakrishnan et al.14 |
| p_ESS_Postop_Hemorrhage | Probability that a postop complication is a hemorrhage (ie. Epistaxis) |
0.069 | Stankiewicz et al.15 |
| p_ESS_Postop_Hemorrhage_OR | Probability that postop epistaxis requires OR Tx | 0.73 | Stankiewicz et al.15 |
| p_ESS_Postop_Infection | Probability that a postop complication is an infection |
0.93 | Dalziel et al.16 |
| p_ESS_Postop_CSFleak | Probability that a postop complication is a CSF leak |
0.0041 | Ramakrishnan et al.14 |
| p_ESS_Postop_CSFleak_Conservative | Probability that a postop CSF leak is controlled with conservative Tx |
0.19 | Lindstrom et al.17 |
| Costs | |||
| c_Uncomplicated_ESS | Cost of ESS with no complication | $7,726 | Bhattacharyya et al.22 |
| c_Complicated_ESS | Cost of ESS with major intraoperative complication |
$16,877 | HCUPnet DRG #135 |
| c_Postop_Hemorrhage_Conservative | Cost of controlling a postoperative epistaxis with non-operative therapy |
$400 | (CPT# 99214), (CPT# 31231), (CPT# 30905) |
| c_Postop_Hemorrhage_OR | Cost of controlling a postoperative epistaxis requiring an OR |
$3,500 | Assumption based on original cost of ESS |
| c_Postop_Infection | Cost of a postoperative infection | $559.25 | (CPT# 99214), (CPT# 31231), Moxiflaxacin and prednisone |
| c_Postop_CSFleak_Conservative | Cost of managing a postoperative CSF leak conservatively |
$13,594 | DRG #52 following CCR conversion |
| c_Postop_CSFleak_OR | Cost of managing a postoperative CSF leak using operative repair |
$16,877 | DRG #136 following CCR conversion |
| c_Highutility | Annual cost for patients in the High utility Markov state |
$1,118 | Bhattacharyya et al. 15 Soler et al.20 |
| c_Moderateutility | Annual costs for patients in the Moderate utility Markov state |
$2,449 | Bhattacharyya et al. 15 Soler et al.20 |
| c_Lowutility | Annual costs for patients in the Low utility Markov state |
$3,000 | Bhattacharyya et al. 15 Soler et al.20 |
NIH, National Institutes of Health; ESS, Endoscopic sinus surgery; Tx, treatment; OR, operating room; CSF, cerebrospinal fluid
III. Probabilities
All ESS perioperative complication probabilities were extracted from the medical literature. The literature search involved querying Ovid MEDLINE (1947-Aug 2012) for studies evaluating complications associated with ESS. The search terms: “endoscop$”, “sinus$”, “surgery”, and “complication$” were combined and produced an initial result of 408 studies. To input the highest level of evidence into the model13, the search was limited to meta-analyses or systematic reviews and yielded a total of 15 articles. The reference lists of all 15 studies were examined to ensure all relevant studies were captured. Data was extracted from four systematic reviews14-17 and probabilities are presented in Table 1.
Markov state entrance data was obtained from the prospective observational study (Clinicaltrials# NCT01332136) evaluating clinical outcomes of patients who self-selected management with either ESS or continued medical therapy for refractory CRS. Since it has been demonstrated that patients will self-select into either medical or ESS treatment arms based on their baseline level of QoL impairment18,19, we had to account for this potential confounding variable to prevent inaccurate health state entrance and transition probabilities. To obtain the baseline health state entrance probabilities, all patients were assumed to receive either ESS or medical therapy alone. Therefore, this removed the selection bias associated with patients self selecting into the medical therapy group with better baseline utility scores. Transition probabilities in the medical therapy group were calculated using patients with matched characteristics as those in the ESS group to ensure selection bias did not influence the year-to-year comparisons.
As of March 2013, a total of 442 patients were enrolled into both the ESS and medical therapy treatment arms. Following ESS, the proportion of patients entering into each of the three Markov states were: Low = 3.4%, Moderate = 24.1%, and High = 72.4%. Following continued medical therapy, the proportion of patients entering into each of the three Markov states were: Low = 16.1%, Moderate = 38%, and High = 45.9%.
Using patient level data (Clinicaltrials# NCT00799097)10, transition probabilities were generated for each of the three Markov states based on year-to-year movements of patients through each state (Table 2 and 3). The transition probabilities are supported by two recent studies which demonstrated that patients with refractory CRS receive stable long-term utility improvements following ESS20 and patients who have significantly reduced baseline QoL are unlikely to receive further improvements from ongoing medical therapy21.
Table 2.
Transition probabilities for patients with refractory CRS following ESS
| Time + 1 | ||||
|---|---|---|---|---|
| High | Moderate | Low | ||
| Time | High | 0.809 | 0.134 | 0.057 |
| Moderate | 0.857 | 0.143 | 0 | |
| Low | 0.761 | 0.239 | 0 | |
CRS, chronic rhinosinusitis; ESS, endoscopic sinus surgery
Table 3.
Transition probabilities for patients with refractory CRS treated with medical therapy alone
| Time + 1 | ||||
|---|---|---|---|---|
| High | Moderate | Low | ||
| Time | High | 0.882 | 0.118 | 0 |
| Moderate | 0 | 0.889 | 0.111 | |
| Low | 0 | 0.667 | 0.334 | |
CRS, chronic rhinosinusitis
IV. Costs
A US-based study by Bhattacharyya et al. reported the mean cost of uncomplicated outpatient ESS to be $7,726 (range $7,554 - $7,898)22. Based on a recent study from the perspective of the Canadian government, a cost of $3,510 was included in the sensitivity analysis23. The cost of ESS with major complication was obtained from the Healthcare Cost and Utilization Project (HCUP) database produced by the US Agency for Healthcare Research and Quality (AHRQ)24. Using the diagnostic-related group (DRG) code for sinus and mastoid procedures with major complication and comorbid conditions (MCC) (DRG #135) the mean charge was $57,449. Following cost-to-charge ratio (CCR) conversion, the mean cost of DRG #135 was $16,877 (Table 1). This cost was applied to all major complications requiring hospital admission such as CSF leak, orbital hematoma, and medial rectus injury.
The three potential postoperative complications included in this economic evaluation were: 1) Epistaxis, 2) Infection, and 3) CSF leak (Table 1). Postoperative complication costs were obtained from the Centers of Medicare and Medicaid Services (CMS) physician Fee Schedule using the corresponding CPT code25 and the HCUP database24. For this model a postoperative sinus infection would receive prednisone 30 mg for 10 days ($6.80) along with a broad spectrum antibiotic for 14 days ($302.45)26. The most expensive common oral antibiotic (moxifloxacin) prescribed for CRS was chosen in order to stack the costs against ESS.
Based on the reported annual cost of refractory CRS before and after ESS22 combined with the mean utility levels before and after ESS27, the moderate utility health state would cost an average of $2,449 per year and the high utility health state would cost an average of $1,118 per year for patients with refractory CRS. Since CRS-specific health care resource consumption is correlated to the severity of QoL reductions28, the low utility health state was assumed to cost more than the moderate health state (Table 1).
V. Discounting
An annual discount rate of 3.5% was applied to all future costs used in the reference case29 and the sensitivity analysis applied a discount rate of 0 and 6%30. The reference case did not discount effectiveness but two scenarios were included in the sensitivity analysis where both costs and effects were discounted at a rate of 3.5% and 6%.
VI. Sensitivity Analysis
Following current recommendations, we have performed several sensitivity analyses to test the influence of inherent data variability on the economic outcomes of this model. First we performed multiple analyses evaluating the change in discounting rates and time horizons8,29-31. Lastly, a multivariate probabilistic sensitivity analysis (PSA) using a Monte Carlo simulation with 15,000 scenarios was performed. For each parameter category, the following data distributions were applied: cost = gamma distribution, probabilities = beta distribution, and utilities = beta distribution. Probabilities from a chance node with 3 or more branches were assigned a dirichlet distribution to ensure all values were coherent. Each parameter in the model received a mean point estimate and standard error based on the study sample size32. Mean point estimates generated from smaller sample sizes possess high levels of parameter uncertainty and therefore received higher standard errors to test several plausible values in the Monte Carlo simulation. Results are presented in both a cost-effectiveness acceptability curve (CEAC) and ICER scatter plot.
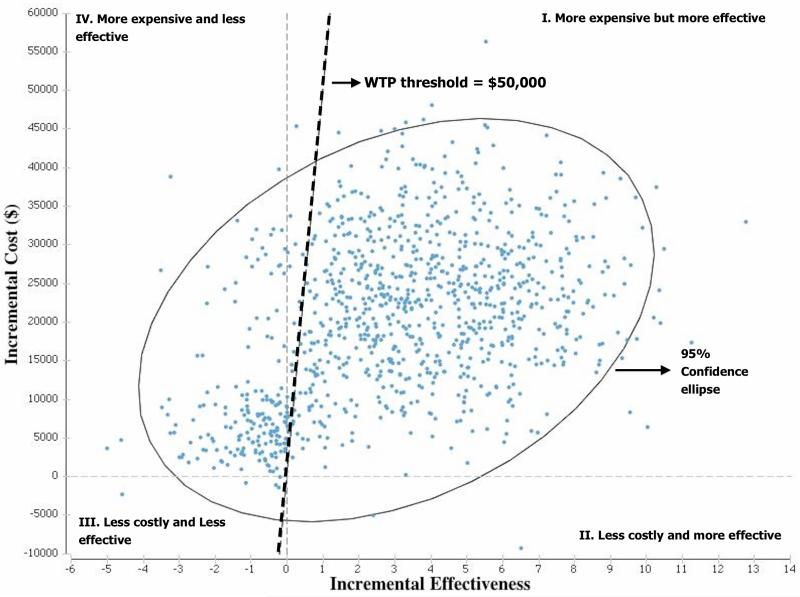
The CEAC is a technique used to graphically represent the uncertainty in an economic evaluation33. It is a very important outcome for policy makers since it provides the degree of certainty in an economic conclusion at several different willingness-to-pay (WTP) thresholds. The ICER scatterplot is a technique used to visually demonstrate the cost-effectiveness of all the different ICERs generated from the 15,000 iterations of the PSA. The ICERs are plotted onto the cost-effectiveness plane (CEP) which is divided into four quadrants34. Quadrant II ICERs are both cheaper and more effective therefore the dominant intervention. On the other hand, quadrant IV ICERs are more expensive and less effective therefore consider dominated and typically rejected. Decisions to accept the alternative intervention in quadrants I and III depend on the maximum ICER for which policy makers are willing to accept (i.e. willingness-to-pay threshold).
Results
I. Reference Case
The reference case managed a cohort of patients with refractory CRS for 30 years and demonstrated that the ESS strategy cost a total of $48,838.38 and produced a total of 20.50 QALYs. The medical therapy alone strategy cost a total of $28,948.98 and produced a total of 17.13 QALYs. The ICER for ESS versus medical therapy alone is $5,901.90 per QALY (Table 4).
Table 4.
Discount rate sensitivity analysis outcomes
| Scenario | Cost discount rate |
Effect discount rate |
Total Cost of ESS |
Total ESS QALYs |
Total Cost Medical therapy |
Total Medical Therapy QALYs |
ICER (ESS vs. Medical Therapy Alone) |
|---|---|---|---|---|---|---|---|
| 1 (reference case) |
3.5% | 0 | $48,838.38 | 20.50 | $28,948.98 | 17.13 | $5,901.90/QALY |
| 2 | 0 | 0 | $71,314.57 | 20.50 | $43,648.29 | 17.13 | $8,209.57/QALY |
| 3 | 6% | 0 | $39,579.92 | 20.50 | $22,813.95 | 17.13 | $4,975.07/QALY |
| 4 | 3.5% | 3.5% | $48,838.38 | 13.23 | $28,948.98 | 11.18 | $9,702.15/QALY |
| 5 | 6% | 6% | $39,579.92 | 10.23 | $22,813.95 | 8.71 | $11,030.24/QALY |
ESS, endoscopic sinus surgery; QALY, quality adjusted life year, ICER, incremental cost effectiveness ratio
II. Sensitivity Analysis
i). Discounting Sensitivity Analysis
Using both the NICE and WHO guidelines29,30, an additional four scenarios were considered which discount both costs and effects at different rates. The results demonstrate that ESS remains the most cost-effective decision with a maximum ICER of $11,030.24 per QALY when both costs and effects are discounted at 6% per year (Table 4).
ii). Time Horizon Sensitivity Analysis
The reference case assumed treatment duration of 30 years to cover the life span of an average patient cohort with refractory CRS. The time horizon sensitivity analysis from this model demonstrates that ESS becomes the most cost-effective intervention following the 3rd year after ESS with an ICER of $49,238.94 per QALY (Table 5).
Table 5.
Time horizon sensitivity analysis outcomes
| Years from Time = 0 |
Cost of ESS | Effectiveness of ESS (QALYs) |
Cost of Medical Therapy |
Effectiveness of Medical Therapy (QALYs) |
ICER (ESS vs. Medical Therapy Alone) |
|---|---|---|---|---|---|
| 1 | $10,176.53 | 0.74 | $2,044.19 | 0.70 | $203,308.50/QALY |
| 2 | $12,401.17 | 1.48 | $3,931.32 | 1.38 | $84,698.50/QALY |
| 3 | $14,542.88 | 2.22 | $5,679.87 | 2.04 | $49,238.94/QALY |
| 4 | $16,606.30 | 2.96 | $7,306.79 | 2.70 | $35,767.35/QALY |
| 5 | $18,593.60 | 3.69 | $8,826.19 | 3.34 | $27,906.89/QALY |
ESS, endoscopic sinus surgery; QALY, quality adjusted life year, ICER, incremental cost effectiveness ratio
iii). Multi-way: Probabilistic Sensitivity Analysis
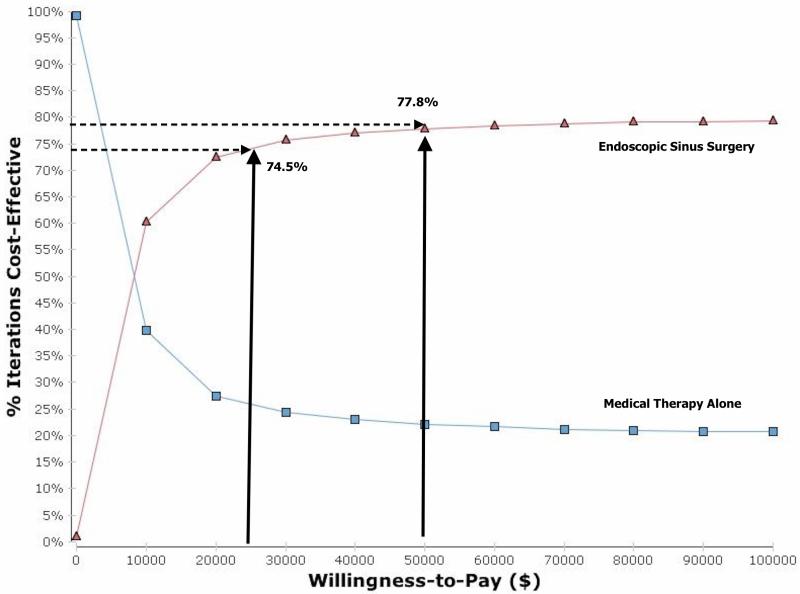
The CEAC is displayed in figure 3 and demonstrates that there is 74.5% and 77.8% certainty that the ESS strategy is the most cost-effective decision at a WTP threshold of $25,000 and $50,000 per QALY, respectively. When plotting the ICERs from the PSA onto the cost-effectiveness plane, it demonstrates that greater then 74% of individual ICER outcomes (blue dots) are below the $50,000 per QALY threshold (Figure 4).
Figure 3.
Cost effectiveness acceptability curve for ESS versus continued medical therapy for refractory CRS
Figure 4.
ICER scatterplot on the cost effectiveness plane
Discussion
This study evaluated the cost-effectiveness of managing a cohort of patients with refractory CRS with either ESS or continued medical therapy alone. Results from this state transition modeling-based economic evaluation suggest that managing refractory CRS patients with the ESS strategy is the most cost-effective intervention. The probabilistic sensitivity analysis demonstrated that ESS was the most cost-effective management option with greater then 74% certainty for any willingness to pay threshold greater than $25,000 per QALY.
Chronic rhinosinusitis is a highly-prevalent inflammatory disease of the nasal and sinus cavities which often reduces patient QoL and produces a significant financial burden on health systems around the globe35-38 and is associated with substantial productivity costs5. Current accepted practice dictates that CRS is initially treated with medical management, since the majority of patients will improve and require no further interventions35,36,39. Initial medical therapy typically includes topical high-volume sinonasal saline irrigations, topical intranasal corticosteroid sprays, short-courses of systemic antibiotics and systemic corticosteroids40-42. The challenge remains that despite best initial medical therapy, there is a fraction of patients who will fail to respond and continue to suffer with persistent symptoms and reduced QoL. Options for ongoing management of refractory CRS include either ESS or continued medical therapy. An estimated 240,000 ESS procedures are performed yearly in the US (and the rates appear to be increasing), but a decision to pursue surgery can be difficult given trade-offs in up-front costs and risks of surgery versus potential long-term improvements. Data from this study supporting cost-effectiveness of ESS for refractory CRS helps inform this decision and the robustness of findings applies to both governmental and third-party payer scenarios.
Results from the reference case and all sensitivity analyses suggest that when managing patients with refractory CRS, an ESS strategy was the more cost-effective intervention compared to continued medical therapy alone. However, despite the results from this economic evaluation, the outcomes must be taken into context given inherent study limitations. In an ideal situation, all data used in a model would be derived from meta-analyses or the economic evaluation would be performed along-side a large randomized controlled trial (RCT). An RCT evaluating ESS versus continued medical therapy would generate accurate patient level costs and effects; however, the feasibility of this RCT is low for several reasons. Ethical implications aside, it would be difficult to enroll patients and ask them to be randomly allocated to medical therapy in the face of strong effectiveness data supporting surgery. On the contrary, some patients may wish to avoid surgery regardless of the possibility of improved benefit given concerns over complications or perioperative discomfort. Additionally, blinding of patients as to whether surgery was performed or not would be impossible without attempts at a sham procedure. Since CRS is a non-terminal disease, the RCT would also require extensive follow-up (i.e. > 30 years) to accurately define long-term costs and effects without extrapolations. The paucity of published RCTs on CRS speaks to these difficulties and no large trials are currently underway or planned for the future. To overcome the lack of RCT data on this topic, we included the highest level of available evidence and patient-level data from two large NIH funded prospective observational studies43,44. For all variables which required an assumption, the highest quality available evidence was used to generate values and uncertainty was accounted for using broad ranges and large standard deviations. The sensitivity analysis failed to demonstrate that any of these parameters fundamentally changed the economic conclusions of this study.
For this model we assumed that refractory CRS was a single disease entity; however, it is likely that CRS represents a heterogeneous group of sinonasal inflammatory disorders whose endotypes are just beginning to be explored. It is likely that distinct subclasses of CRS will be defined in the future whose response to either medical therapy or surgery will differ compared to when the CRS cohort is considered as a single entity. If these subgroups are identified, then future economic evaluations will have to account for these differences to further refine the efficient management of this chronic inflammatory disease. However, despite inherent limitations with any model, this economic evaluation is strengthened by its long-term time horizon (30 years), patient-level data on health state transition probabilities, and robust sensitivity analyses including differing discount rates, time-horizons, and multivariate analysis.
Conclusion
Chronic rhinosinusitis is a common inflammatory disease which produces significant deleterious effects on patients and creates a substantial burden to the health care system. This cohort-style Markov decision tree model evaluated the cost-effectiveness of the ESS strategy compared to continued medical therapy alone for patients with refractory CRS. The purpose was to determine if the short-term increases in cost associated with performing ESS are justified in the long-term management of refractory CRS. When the CRS cohort is considered as one single entity, the results from this initial study suggest that employing an ESS treatment strategy to manage patients with refractory CRS is likely to be the most cost-effective intervention compared to continued medical therapy alone. Future studies will need to evaluate the role of ESS in specific CRS endotypes.
Acknowledgements
This was the thesis project for Dr. Luke Rudmik’s MSc in Health Economics from the London School of Economics (LSE). We would like to thank Dr. Caroline Rudisill-Michaelsen (LSE), Dr. Paul Dolan (LSE), and Dr. Michael Drummond (University of York) for their supervision during this project.
Funding/Support: Dr. T. L. Smith, Dr. Z. M. Soler, and J. Mace are supported by a grant from the National Institutes of Health (NIH: R01 DC005805).
Role of the Sponsor: The National Institutes of Health had no role in the preparation, review, or approval of this manuscript or decision to submit it for publication.
Footnotes
Potential Conflict(s) of Interest:
LR: None
ZMS: Grant support from the NIH/NIDCD
JCM: Grant support from the NIH/NIDCD
RJS: Consultant for BrainLAB, Olympus, United Allergy; Grant support from
Medtronic, Arthrocare, Intersect ENT, Optinose, NeilMed.
TLS: Consultant for Intersect ENT Inc. (Menlo Park, CA). Grant support from NIH/NIDCD
References
- 1.Anand VK. Epidemiology and economic impact of rhinosinusitis. The Annals of otology, rhinology & laryngology Supplement. 2004;193:3–5. doi: 10.1177/00034894041130s502. [DOI] [PubMed] [Google Scholar]
- 2.Chen Y, Dales R, Lin M. The epidemiology of chronic rhinosinusitis in Canadians. The Laryngoscope. 2003;113:1199–205. doi: 10.1097/00005537-200307000-00016. [DOI] [PubMed] [Google Scholar]
- 3.Soler ZM, Mace J, Smith TL. Symptom-based presentation of chronic rhinosinusitis and symptom-specific outcomes after endoscopic sinus surgery. American journal of rhinology. 2008;22:297–301. doi: 10.2500/ajr.2008.22.3172. [DOI] [PubMed] [Google Scholar]
- 4.Alt JA, Smith TL. Chronic rhinosinusitis and sleep: a contemporary review. International forum of allergy & rhinology. 2013;3:941–9. doi: 10.1002/alr.21217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rudmik L, Smith TL, Schlosser RJ, Hwang PH, Mace JC, Soler ZM. Productivity costs in patients with refractory chronic rhinosinusitis. The Laryngoscope. 2014 doi: 10.1002/lary.24630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhattacharyya N. Incremental health care utilization and expenditures for chronic rhinosinusitis in the United States. The Annals of otology, rhinology, and laryngology. 2011;120:423–7. doi: 10.1177/000348941112000701. [DOI] [PubMed] [Google Scholar]
- 7.Rudmik L, Drummond M. Health economic evaluation: important principles and methodology. The Laryngoscope. 2013;123:1341–7. doi: 10.1002/lary.23943. [DOI] [PubMed] [Google Scholar]
- 8.Husereau D, Drummond M, Petrou S, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS)--explanation and elaboration: a report of the ISPOR Health Economic Evaluation Publication Guidelines Good Reporting Practices Task Force. Value in health : the journal of the International Society for Pharmacoeconomics and Outcomes Research. 2013;16:231–50. doi: 10.1016/j.jval.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 9.Husereau D, Drummond M, Petrou S, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. BMJ. 2013;346:f1049. doi: 10.1136/bmj.f1049. [DOI] [PubMed] [Google Scholar]
- 10.Smith TL, Litvack JR, Hwang PH, et al. Determinants of outcomes of sinus surgery: a multi-institutional prospective cohort study. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery. 2010;142:55–63. doi: 10.1016/j.otohns.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arias E. United States life tables, 2007. National vital statistics reports : from the Centers for Disease Control and Prevention, National Center for Health Statistics. National Vital Statistics System. 2011;59:1–60. [PubMed] [Google Scholar]
- 12.Soler ZM, Rudmik L, Hwang PH, Mace JC, Schlosser RJ, Smith TL. Patient-centered decision making in the treatment of chronic rhinosinusitis. The Laryngoscope. 2013 doi: 10.1002/lary.24027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oxford Centre for Evidence-based Medicine (CEBM) - Levels of Evidence. 2009 http://www.cebm.net/index.aspx?o=1025
- 14.Ramakrishnan VR, Kingdom TT, Nayak JV, Hwang PH, Orlandi RR. Nationwide incidence of major complications in endoscopic sinus surgery. International forum of allergy & rhinology. 2012;2:34–9. doi: 10.1002/alr.20101. [DOI] [PubMed] [Google Scholar]
- 15.Stankiewicz JA, Lal D, Connor M, Welch K. Complications in endoscopic sinus surgery for chronic rhinosinusitis: a 25-year experience. The Laryngoscope. 2011;121:2684–701. doi: 10.1002/lary.21446. [DOI] [PubMed] [Google Scholar]
- 16.Dalziel K, Stein K, Round A, Garside R, Royle P. Endoscopic sinus surgery for the excision of nasal polyps: A systematic review of safety and effectiveness. American journal of rhinology. 2006;20:506–19. doi: 10.2500/ajr.2006.20.2923. [DOI] [PubMed] [Google Scholar]
- 17.Lindstrom DR, Toohill RJ, Loehrl TA, Smith TL. Management of cerebrospinal fluid rhinorrhea: the Medical College of Wisconsin experience. The Laryngoscope. 2004;114:969–74. doi: 10.1097/00005537-200406000-00003. [DOI] [PubMed] [Google Scholar]
- 18.Smith TL, Kern R, Palmer JN, et al. Medical therapy vs surgery for chronic rhinosinusitis: a prospective, multi-institutional study with 1-year follow-up. International forum of allergy & rhinology. 2013;3:4–9. doi: 10.1002/alr.21065. [DOI] [PubMed] [Google Scholar]
- 19.Smith TL, Kern RC, Palmer JN, et al. Medical therapy vs surgery for chronic rhinosinusitis: a prospective, multi-institutional study. International forum of allergy & rhinology. 2011;1:235–41. doi: 10.1002/alr.20063. [DOI] [PubMed] [Google Scholar]
- 20.Rudmik L, Mace J, Soler ZM, Smith TL. Long-term utility outcomes in patients undergoing endoscopic sinus surgery. The Laryngoscope. 2014;124:19–23. doi: 10.1002/lary.24135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith KA, Rudmik L. Impact of continued medical therapy in patients with refractory chronic rhinosinusitis. International forum of allergy & rhinology. 2014;4:34–8. doi: 10.1002/alr.21238. [DOI] [PubMed] [Google Scholar]
- 22.Bhattacharyya N, Orlandi RR, Grebner J, Martinson M. Cost burden of chronic rhinosinusitis: a claims-based study. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery. 2011;144:440–5. doi: 10.1177/0194599810391852. [DOI] [PubMed] [Google Scholar]
- 23.Au J, Rudmik L. The Cost of Outpatient Endoscopic SInus Surgery from the Perspective of the Canadian Government: A Time-Driven Activity Based Costing Approach. International forum of allergy & rhinology. 2013 doi: 10.1002/alr.21181. In press. [DOI] [PubMed] [Google Scholar]
- 24.HCUPnet [accessed Aug 2012];2012 http://hcupnetahrqgov/
- 25.CMS Physician Fee Schedule http://www.cms.gov/apps/physician-fee-schedule/
- 26.Redbook . Pharmacy’s fundamental reference. Thomson Healthcare; NJ: 2008. [Google Scholar]
- 27.Soler ZM, Wittenberg E, Schlosser RJ, Mace JC, Smith TL. Health state utility values in patients undergoing endoscopic sinus surgery. The Laryngoscope. 2011;121:2672–8. doi: 10.1002/lary.21847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang PC, Chu CC, Liang SC, Tai CJ. Cost-utility analysis for endoscopic sinus surgery. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery. 2004;130:31–8. doi: 10.1016/j.otohns.2003.07.006. [DOI] [PubMed] [Google Scholar]
- 29.NICE National Institute of Clinical Excellence: Guide to the methods of technology appraisal. 2008 http://wwwniceorguk/media/B52/A7/TAMethodsGuideUpdatedJune2008pdf
- 30.Murray CJ, Evans DB, Acharya A, Baltussen RM. Development of WHO guidelines on generalized cost-effectiveness analysis. Health economics. 2000;9:235–51. doi: 10.1002/(sici)1099-1050(200004)9:3<235::aid-hec502>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 31.Siegel JE, Weinstein MC, Russell LB, Gold MR. Recommendations for reporting cost-effectiveness analyses. Panel on Cost-Effectiveness in Health and Medicine. JAMA : the journal of the American Medical Association. 1996;276:1339–41. doi: 10.1001/jama.276.16.1339. [DOI] [PubMed] [Google Scholar]
- 32.Briggs AH, Weinstein MC, Fenwick EA, Karnon J, Sculpher MJ, Paltiel AD. Model parameter estimation and uncertainty: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force--6. Value in health : the journal of the International Society for Pharmacoeconomics and Outcomes Research. 2012;15:835–42. doi: 10.1016/j.jval.2012.04.014. [DOI] [PubMed] [Google Scholar]
- 33.Fenwick E, O’Brien BJ, Briggs A. Cost-effectiveness acceptability curves--facts, fallacies and frequently asked questions. Health economics. 2004;13:405–15. doi: 10.1002/hec.903. [DOI] [PubMed] [Google Scholar]
- 34.Black WC. The CE plane: a graphic representation of cost-effectiveness. Medical decision making : an international journal of the Society for Medical Decision Making. 1990;10:212–4. doi: 10.1177/0272989X9001000308. [DOI] [PubMed] [Google Scholar]
- 35.Desrosiers M, Evans GA, Keith PK, et al. Canadian clinical practice guidelines for acute and chronic rhinosinusitis. Allergy, asthma, and clinical immunology : official journal of the Canadian Society of Allergy and Clinical Immunology. 2011;7:2. doi: 10.1186/1710-1492-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fokkens WJ, Lund VJ, Mullol J, et al. EPOS 2012: European position paper on rhinosinusitis and nasal polyps 2012. A summary for otorhinolaryngologists. Rhinology. 2012;50:1–12. doi: 10.4193/Rhino12.000. [DOI] [PubMed] [Google Scholar]
- 37.Pleis JR, Ward BW, Lucas JW. Summary health statistics for U.S. adults: National Health Interview Survey, 2009. Vital and health statistics Series 10, Data from the National Health Survey. 2010:1–207. [PubMed] [Google Scholar]
- 38.Rudmik L, Smith TL. Quality of life in patients with chronic rhinosinusitis. Current allergy and asthma reports. 2011;11:247–52. doi: 10.1007/s11882-010-0175-2. [DOI] [PubMed] [Google Scholar]
- 39.Rosenfeld RM, Andes D, Bhattacharyya N, et al. Clinical practice guideline: adult sinusitis. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery. 2007;137:S1–31. doi: 10.1016/j.otohns.2007.06.726. [DOI] [PubMed] [Google Scholar]
- 40.Rudmik L, Hoy M, Schlosser RJ, et al. Topical therapies in the management of chronic rhinosinusitis: an evidence-based review with recommendations. International forum of allergy & rhinology. 2012 doi: 10.1002/alr.21096. [DOI] [PubMed] [Google Scholar]
- 41.Poetker DM, Jakubowski LA, Lal D, Hwang PH, Wright ED, Smith TL. Oral corticosteroids in the management of adult chronic rhinosinusitis with and without nasal polyps: an evidence-based review with recommendations. International forum of allergy & rhinology. 2012 doi: 10.1002/alr.21072. [DOI] [PubMed] [Google Scholar]
- 42.Soler ZM, Oyer SL, Kern RC, et al. Antimicrobials and chronic rhinosinusitis with or without polyposis in adults: an evidenced-based review with recommendations. International forum of allergy & rhinology. 2013;3:31–47. doi: 10.1002/alr.21064. [DOI] [PubMed] [Google Scholar]
- 43. [Accessed July. 14, 2014];Determinants of Surgical Outcomes for Chronic SInusitis: NCT00799097. 2010 at http://clinicaltrials.gov/ct2/show/NCT00799097?term=smith+rhinosinusitis&rank=3.
- 44. [Accessed July. 14, 2014];Determinants of Medical and Surgical Treatment Outcomes in Chronic Sinusitis: NCT01332136. 2013 at http://clinicaltrials.gov/ct2/show/NCT01332136?term=medical+therapy+sinus+surgery&rank=6.